Abstract
Obesity originates from an imbalance between caloric intake and energy expenditure that promotes adipose tissue expansion, which is necessary to buffer nutrient excess. Patients with higher visceral fat mass are at a higher risk of developing severe complications such as type 2 diabetes and cardiovascular and liver diseases. However, increased fat mass does not fully explain obesity’s propensity to promote metabolic diseases. With chronic obesity, adipose tissue undergoes major remodeling, which can ultimately result in unresolved chronic inflammation leading to fibrosis accumulation. These features drive local tissue damage and initiate and/or maintain multiorgan dysfunction. Here, we review the current understanding of adipose tissue remodeling with a focus on obesity-induced adipose tissue fibrosis and its relevance to clinical manifestations.
Introduction
There are two morphologically distinct types of adipose tissue: white adipose tissue (WAT) and brown/beige adipose tissue (BAT). WAT is the predominant type and critically regulates whole-body energy homeostasis by acting as a key energy reservoir for the other organs. Efficient storage in times of food abundance is fundamental for survival during food shortage. Thus, the evolution of multicellular organisms has led to the development of specialized cells or organs that function to store nutrient excess as lipids, highly energy-dense nutrients (9 kcal/g versus 4 kcal/g for protein and carbohydrate). Excess caloric intake and low physical activity tips the energy balance into storage mode, and within the white adipocyte, free fatty acids (FFAs) are esterified into triglycerides that are packed into lipid droplets coated with regulatory proteins, ensuring lipid storage or mobilization (1).
In humans, WAT is organized as two major fat depots, subcutaneous and visceral, the latter of which surrounds the internal organs. In obesity, a high deposition of visceral adipose tissue is associated with increased risk of developing cardiometabolic diseases, such as type 2 diabetes, and their severe complications, whereas obese patients with predominantly subcutaneous fat storage may have a reduced risk of metabolic diseases (2–4) or at least exhibit delayed complications. However, the underlying mechanisms controlling the depot-specific growth of adipose tissue are not yet elucidated.
In adult mice, the paired gonadal fat depots around the ovaries (periovarian) or the testes (epididymal) found within the abdominal cavity are studied as a model of visceral adipose tissue in addition to the mesenteric, retroperitoneal, and perirenal fat (5). The inguinal depots in the anterior and upper portion of the hind limbs are representative of subcutaneous adipose tissue in mice (5).
In addition to its energy-storing function, the adipose tissue displays critical endocrine functions. Leptin (6, 7), adiponectin (8–10), and a myriad of other peptidic mediators or lipid metabolites derived from adipocytes or from the stroma (called adipokines) exert critical endocrine functions that maintain energy balance by targeting the central nervous system and/or by modulating the metabolic activities of peripheral organs. The role of these molecules has been extensively reviewed (11, 12).
By contrast to WAT, BAT is found subcutaneously in specific locations mostly in newborns and in smaller amounts in adults and functions primarily as a thermogenic organ owing to the presence of multilocular adipocytes enriched with mitochondria and uncoupling protein 1 (UCP1) (13). BAT might also have a secretory role (14). Similarly, the development of beige adipose tissue in the subcutaneous or visceral WAT in response to cold or β3-adrenergic stimulation is referred to as “browning” (15, 16). Several studies associated brown/beige adipose tissue activity with protection against obesity and metabolic disease development (17).
In response to excess energy, while the amount of activity of brown/beige fat is reduced (18, 19), WAT depots undergo a massive expansion to buffer the nutrient overload. With chronic obesity, which is eventually accompanied by periods of weight variation, WAT depots display continual remodeling. It is now recognized that inflammatory cell accumulation and activation within the WAT mediate at least some aspects of obesity-related morbidity, such as insulin resistance (20). Besides immune cell accumulation, prolonged positive energy balance induces adipocyte hypertrophy and neovascularization. In addition, extracellular matrix accumulation resulting in adipose tissue fibrosis participates in adipose tissue dysfunction. Inflamed and fibrotic WAT depots become deleterious for proper energy storage and endocrine functions, resulting in altered local and systemic metabolic control. We here review the current knowledge on obesity-induced WAT fibrosis and its local and systemic consequences.
Adipocytes and progenitors in adipose tissue growth
In obesity, the growth of the adipose mass is mediated by adipocyte hypertrophy (enlarged adipocytes) or hyperplasia (increased cell number). Both types of expansion are regulated by the local environment and genetic factors, although the molecular factors favoring one or the other pathway are largely unknown. A growing body of evidence indicates that these processes are closely associated with the maintenance of adipose tissue homeostasis. Adipocyte hyperplasia, in general, is more metabolically favorable than increased adipocyte size (21), as enlarged adipocytes exhibit numerous necrotic-like abnormalities such as ruptured plasma membranes, dilated endoplasmic reticulum, cell debris in the extracellular space, and the appearance of small lipid droplets in the cytoplasm (22, 23). In addition to these morphological abnormalities, hypertrophic adipocytes are dysfunctional, with increased expression and secretion of proinflammatory cytokines, including TNF-α, IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), and acute-phase serum amyloid A proteins, among others (24). Basal lipolysis is also elevated in these cells (25, 26), increasing the leakage of FFAs to ectopic locations. As a consequence, hypertrophic adipocytes and associated features contribute to the loss of tissue homeostasis and can promote or at least maintain insulin resistance when initiated (24, 26). On the other hand, hyperplastic expansion of the adipose tissue requires the proliferation and differentiation of precursor cells (also called progenitors or adipose tissue progenitor cells [APCs]) that reside within the adipose tissue stroma, since mature adipocytes are postmitotic cells (27–29). In rodents, lineage tracing studies have revealed that the mode of adipose depot expansion in obese mice occurs in a depot- and sex-dependent manner, suggesting the importance of sex hormones in driving the energy storage mode (30–32).
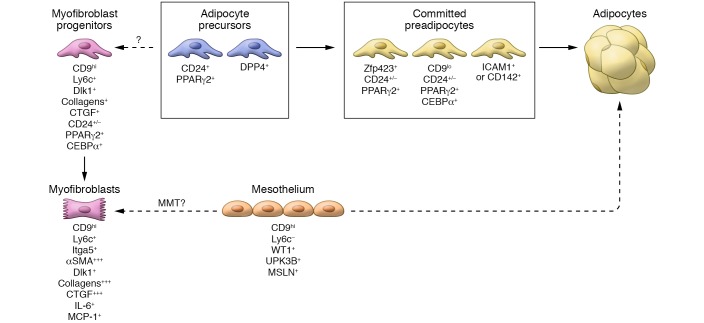
To identify WAT progenitors, studies tracing PPARγ-expressing cells revealed an adipocyte lineage tightly associated with the adipose vasculature (33). Concomitantly, Friedman’s group established a strategy to purify an enriched cell population prone to adipogenesis from the stromal vascular fraction of adipose tissue (27). Combining the use of various antibodies previously reported as mesenchymal stem cell antigens to target cell surface epitopes, they delineated a cell population with a strong adipogenic potential that exhibited Sca1, CD34, CD29, and PDGFRα expression (27, 34). The progenitors also coexpressed PDGFRβ (35, 36). Interestingly, this population is not homogeneous, and subpopulations with specific features could be discriminated (Figure 1). For instance, CD24+ precursors exhibit stem cell–like properties that play a role in the maintenance or the growth of local adipocyte precursors in line with the local microenvironment (27, 37). Specifically, the C2H2 zinc finger protein 423–expressing (Zfp423+) precursors constitute a subpopulation of progenitors with high adipogenic potential (38, 39).
Figure 1. Heterogeneity among progenitors in the adipose tissue.
Adipose tissue progenitors (or precursors), often located in the vicinity of the vascular network, constitute a heterogeneous population. They can be discriminated through their capacity to differentiate into mature adipocytes and also by their level of commitment into the adipocyte differentiation program. The application of flow cytometry using various markers as well as single-cell RNA sequencing has enabled the identification of multiple cell populations. The CD9hi progenitors exhibited very limited adipogenic capacity with a high propensity for the production of extracellular matrix components. CD9hi progenitors include mesothelial cells, whose contribution in adipose tissue remodeling is currently unresolved. Further investigations are still needed to establish the relationship between these various populations of progenitors. In addition, a better understanding of the critical functional determinants and whether acquired phenotypes are reversible is needed.
Since the identification of specific markers allowing the tracking of adipose tissue progenitors within the adipose tissue, APCs have received considerable attention and have been classified as precursors undergoing adipocyte differentiation linked not only to developmental (40), homeostatic, and obesogenic (32, 37, 38) conditions, or beige adipogenesis in response to cold or β3-adrenergic stimulation (41, 42), but also to fibrosis accumulation (43).
In humans, adipocyte turnover was quantified by analysis of the integration of atmospheric 14C into genomic DNA. This work proposed that adipocyte hyperplasia occurs from birth to early adulthood (20 years of age). By contrast, adipocyte renewal represents only 10% of adipocytes each year in adults (44), indicating that adipocyte hyperplasia is limited in adults. However, precursors undergoing adipogenesis in vitro have been identified (45), and findings support that different subpopulations of precursors do exist in humans as in mouse models. Adipocyte precursors are enriched in CD34+, PDGFRα+, CD45– (a leukocyte marker), and CD31– (an endothelial cell–specific marker) cell populations derived from the stromal vascular fraction of WAT. Various degrees of adipogenesis can also be observed in populations expressing CD36 (encoding the primary cellular fatty acid translocase) (46) and Msca1 (mesenchymal stromal cell antigen-1), which are induced during adipogenesis (47, 48). Importantly, small adipocyte size is associated with insulin sensitivity (49), and a better understanding of pathways controlling the production of new adipocytes (to limit adipocyte hypertrophy) could be of interest to limit the deleterious effects of obesity.
Suboptimal angiogenesis and low-grade inflammation in obese WAT
Fat mass expansion requires the formation of new blood vessels (angiogenesis), which develop from those preexisting within the adipose tissue. To stimulate angiogenesis, growing adipocytes produce many angiogenic factors, such as leptin, VEGF, FGF-2, hepatocyte growth factor (HGF), insulin-like growth factor (IGF), placental growth factor (PLGF), VEGF-C, heparin-binding epidermal growth factor (HB-EGF), and angiopoietins. APCs also secrete high levels of angiogenic factors, including VEGF, HGF, and FGF-2 (50). Consequently, antiangiogenic agents were first believed to represent encouraging therapeutic options to limit fat mass expansion during obesity. However, analysis of the mechanisms underlying the pathological events associated with obesity suggests that over the course of obesity, capillary density and function fail to meet the demands for adipose growth. Most interestingly, promoting angiogenesis favors healthy adipose tissue expansion with enhanced adipogenesis and reduced adipose tissue inflammation and fibrosis (51–55). In humans, endothelial cells isolated from obese adipose tissue exhibit phenotypic alterations with increased expression of inflammatory and senescence-related genes (56). Moreover, a GWAS analysis revealed a link between angiogenesis gene loci and insulin resistance markers, supporting the notion that suboptimal vascularization could be of importance in maintaining insulin resistance in obesity (57).
Local inflammation is another critical alteration observed in obese adipose tissue depots. A potential mechanism to explain this finding is that impaired vascularization precipitates local hypoxia, leading to adipocyte necrosis that favors the infiltration of proinflammatory leukocytes (58). A seminal study highlighted a causative link between adipose tissue inflammation and insulin resistance, albeit in rodents (59). This finding was later substantiated in humans with the discovery that macrophages accumulate in obese subjects’ adipose tissue (60). Since then, macrophages have been shown to critically control adipose tissue inflammation and favor the onset of insulin resistance, one of the major obesity comorbidities. Concomitantly, other innate and adaptive immune cells (e.g., T cells, B cells, NK and NKT cells, eosinophils) are modulated in obesity (61) and insulin-resistant states (62).
Overall, the relationship between adipose tissue inflammation and metabolic alterations is better established in mouse models than in human conditions. As such, overfeeding experiments in humans have shown that insulin resistance can be rapidly induced without substantial modification of systemic or tissue inflammation (63). These observations have raised questions regarding the kinetics of inflammatory events in humans and whether they are involved in the development or maintenance of metabolic diseases. Nevertheless, the identification of key inflammatory mediators is of interest to understand and eventually control adipose tissue remodeling over the course of obesity. We have, for example, characterized the phenotype of PAFR–/– mice, which are deficient in platelet-activating factor receptor (PAFR). The platelet-activating factor (PAF) is a glycerophospholipid able to initiate acute inflammation through activation of its G protein–coupled receptor (64). Activation of PAFR in adipose tissue promotes leukocyte recruitment and stimulates local production of proinflammatory cytokines such as TNF-α, IL1-β, and IL-6. PAFR–/– mice are protected against WAT inflammation and exhibit improved insulin sensitivity, albeit with increased adiposity and without any change in calorie intake (65). Thus, the phenotype of PAFR–/– mice, similarly to mice lacking leptin while overexpressing adiponectin (66) or other mouse models (67–69), illustrates the beneficial impact of limiting local inflammation while maintaining storage capacity in the adipose tissue. This increase or maintenance of storage capacity limits ectopic fat deposition and insulin resistance, as well as related complications.
Adipose tissue fibrosis identifies unhealthy remodeling in obesity
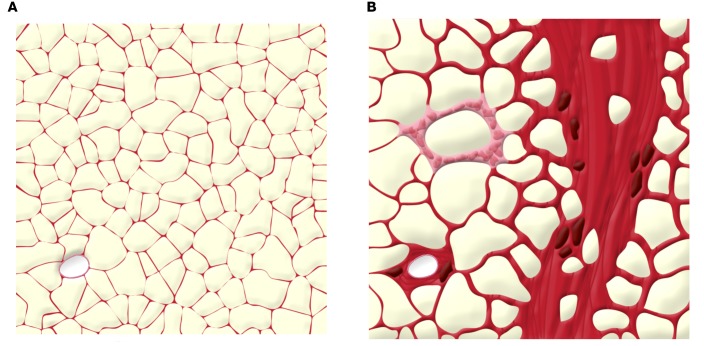
Unresolved inflammation is often associated with altered tissue remodeling in a number of pathological states and often progresses to fibrosis as a result of the persistence of inflammatory stress. Fibrosis is characterized by excessive extracellular matrix (ECM) component deposition, a dysfunctional process that ultimately causes severe disturbances in organ functions. Ten years ago, major changes in the expression of genes encoding ECM were described in the adipose tissue during obesity (70, 71). The ECM is a noncellular component of all tissues that is essential for tissue morphogenesis, differentiation, and homeostasis and includes structural (collagens) and adhesion proteins (fibronectin) as well as proteoglycans (biglycan, decorin), which preserve tissue architecture (72). The ECM is considered as a reservoir of secreted growth factors, cytokines, and proteases whose availability is highly regulated during ECM remodeling (73) while being particularly crucial for maintaining structural integrity of adipocytes and playing pivotal roles during adipogenesis (74–76). In obese human adipose tissue, collagens accumulate around the adipocytes to form pericellular fibrosis, or, alternatively, collagen fibers can be organized as fibrotic bundles of various thicknesses containing few adipocytes isolated from the rest of the parenchyma (Figure 2).
Figure 2. Illustration of adipose tissue fibrosis in obese subjects.
Obesity increases collagen deposition and myofibrocyte distribution in adipose tissue. (A) A section of adipose tissue with no fibrosis. (B) In contrast, fibrotic adipose tissue contains fibrosis-forming collagen bundles that trap adipocytes. Fibrosis can also be observed around a blood vessel (lower left) and around a crown-like structure with macrophages and inflammatory cells.
Loss of adipose tissue plasticity in obesity
Studies in humans and rodents indicate that fibrosis alters adipose tissue plasticity. Several findings support the concept that the global upregulation of ECM constituents may represent a physical constraint to adipose tissue expansion (69, 77). In mice, the predominantly expressed collagen mRNAs in adipose tissue encode type I, IV, and VI collagens, the latter being the most abundantly expressed. In collagen VI–null ob/ob mice, adipose tissue mass increases as a result of uninhibited volume expansion of individual adipocytes. As in mouse models discussed above, this increased adiposity is associated with lowered local inflammation together with a global improvement of metabolism in obese animals relative to collagen VI–expressing mice (69). Accumulation of ECM in adipose tissue might thus contribute to a failure to adequately limit the expansion in fat mass. This hypothesis is corroborated by other studies, such as those using mice lacking the pericellular collagenase MT1–matrix metalloproteinase (MT1-MMP). MT1-MMP inactivation results in the formation of a rigid network of collagen fibrils, which compromises adipocyte differentiation and lipid accumulation (77). Similarly, inhibition of lysyl oxidase (LOX), an enzyme involved in collagen cross-linking, dampens metabolic dysfunctions and WAT inflammation in obese mice (78). In human adipose tissue, decreased adipocyte size is detected in regions with fibrosis as compared with fibrosis-free cells (71). The development of a prototypic tool (AdipoScan) based on elastography shows that adipose tissue fibrosis is associated with significant change in tissue stiffness (79).
To further investigate the functional impact of increased interstitial fibrosis, the consequences of collagen deposition for adipocyte biology have been examined. Mature adipocytes, cultured in a peptide hydrogel containing decellularized material isolated from adipose tissue of obese subjects, showed that fibrosis itself induces adipocyte functional alterations similar to those observed in obese adipose tissue, such as decreased lipolysis and adipokine secretion and increased proinflammatory cytokines production. Those effects appear to be dependent on mechanosensitive signaling involving integrin and Yes-associated protein (YAP)/transcriptional enhancer–associated domain (TEAD) pathways (80).
Consistently, numerous studies have linked ECM deposition to modified adipose tissue metabolic and endocrine functions (81–84). Pasarica et al. reported that type VI collagen gene expression is elevated in obese subjects, and obese subjects with high collagen VI display increased adipose tissue inflammation (82). Spencer et al. also described an association between collagen VI, fibrosis, and alternative activation of macrophages in the adipose tissue of insulin-resistant subjects (83).
In addition to the deleterious impact of fibrosis on adipose tissue functions, subcutaneous adipose tissue fibrosis measured in severe obesity is associated with resistance to weight loss induced by bariatric surgery (71, 79).
In the future, use of scoring of adipose tissue fibrosis could be of interest to adapt the medical standard of care of obese patients in order to optimize patient follow-up and outcomes in obesity treatment (85).
Pathways perpetuating adipose tissue fibrosis
In various organs, the common underlying mechanism leading to fibrosis involves the generation and the proliferation of myofibroblasts producing excessive amounts of ECM components (86). In adipose tissue derived from obese humans, fibroblastic α–smooth muscle actin–positive (αSMA+) cells are enriched in fibrotic bundles (Figure 2 and ref. 71). TGF-β1 usually represents the prototypic inducer of profibrotic myofibroblast differentiation from all precursor cell types (87). However, a combination of fibrogenic signals, increased in the fibrotic adipose tissue (43), cooperates to sustain the adipose tissue fibrotic transformation. TGF-β1 (88) and activin A (89, 90) belong to the TGF-β superfamily and promote activation of SMAD2/3 transcription factors (91). Activation of SMAD2/3 modulates the expression of several profibrotic genes (91), including collagens, ECM-remodeling enzymes such as matrix metalloproteinases (92), or the integrin transmembrane receptor, activation of which can perpetuate fibrotic signaling (93). In addition, multiple studies, using activation or inhibition approaches, show that platelet-derived growth factor-α (PDGFα) is another critical profibrotic signal that binds tyrosine kinase receptors such as PDGFRα and PDGFRβ for an important contribution to the proliferative phenotype of fibrosis-producing cells (94–97). PDGFRβ was also found expressed on adipose progenitors, but its exact role in adipose tissue needs to be clarified, though its profibrotic activity was described in the liver (98). Moreover, connective tissue growth factor (CTGF), a secreted matricellular protein, can affect multiple signaling pathways that contribute to the persistence of fibrosis. CTGF is indeed involved in ECM remodeling and deposition, myofibroblast activation, cell adhesion, and migration (80, 99).
Emerging knowledge has provided the necessary information to start deciphering the molecular networks involved in the fibrotic process (Figure 3). During obesity, the production of various endogenous activators of TLR4 is augmented (including LPS, tenascin C, HMGB1, and fetuin-A) (100–104). Such activation of TLR4 on bone marrow–derived cells mediates the development of obesity-associated adipose tissue fibrosis (88). This suggests that obesity-induced adipose tissue inflammation (105) involves TLR4 activation on leukocytes, which in turn may secrete factors critical for the promotion of fibrogenesis. For example, stimulation of TLR4 potentiates PDGFRα signaling (106) and TGF-β production (88), and adipose tissue fibrosis is dampened upon treatment with a neutralizing anti–TGF-β antibody (88). In addition, macrophage-inducible C-type lectin (also known as Mincle, Clec4e, or Clecsf9) is induced in macrophages by LPS via TLR4; during obesity, Mincle is localized to macrophages within crown-like structures (CLSs) in adipose tissue. CLSs represent a site of interaction between adipocytes and macrophages that facilitates scavenging of residual lipid droplets, and increased CLS formation is associated with insulin resistance in humans (22, 107). Interestingly, Mincle-KO mice are protected against obesity-induced CLS formation and adipose tissue fibrosis (108). Transcriptomic profiling of macrophages showed that Mincle modulates macrophage functions important for the control of ECM production, fibroblast proliferation, and ECM degradation, further suggesting that macrophages may be of importance in myofibroblastic transformation with ECM deposition (108). Additionally, macrophages participate in fibrosis clearance through collagen uptake and lysosomal degradation involving the collagen receptors mannose receptor 1 (Mrc1) and urokinase plasminogen activator receptor–associated protein (Endo180, also known as Mrc2) (109), or milk fat globule epidermal growth factor 8 (Mfge8), a secreted glycoprotein that binds collagen to target it for removal from the ECM (110). Nonetheless, the involvement of other bone marrow–derived cell types in this process cannot be ruled out (111).
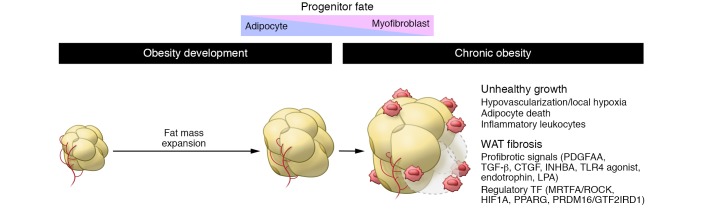
Figure 3. Adipose tissue fibrosis in obese subjects.
With chronic obesity, WAT depots undergo continual remodeling, becoming pathological. Combined with unadapted vascularization promoting hypoxia and unresolved inflammation, alteration of the equilibrium between the myofibroblast and the adipogenic fate of adipose progenitors is important in the unhealthy growth of adipose tissue. Various signals and transcription factors were found to be important in controlling the fate of progenitors.
Limited vascular outgrowth is also of importance in unhealthy adipose tissue remodeling, since it results in hypoxia. In adipose tissue, HIF1α links the hypoxic milieu to fibrosis and inflammation (78, 112). Overexpression of HIF1α promotes a transcriptional program associated with the induction of ECM proteins and inflammation. Conversely, the selective inhibition of HIF1α using an inhibitor or induction of WAT-specific dominant-negative Hif1a in obese mice alleviates WAT fibrosis and inflammation (112).
Progenitors in fibrosis: adipogenic versus myofibroblastic precursors
In fibrotic organs, the excessive deposition of ECM, a defining feature of fibrosis, starts with cells that are sensitive to profibrotic stimuli acquiring a myofibroblastic phenotype. Myofibroblasts are characterized by de novo expression of αSMA, formation of cellular stress fibers, and abundant production of ECM proteins and autocrine growth factor that maintains cell proliferation and survival (113). Some studies suggest that in mouse models of renal, hepatic, or pulmonary fibrosis, myofibroblasts could arise from the differentiation of local epithelial cells via epithelial-mesenchymal transition (114). However, this view is now challenged by strong evidence from lineage tracing studies in various organs highlighting that myofibroblasts originate from local mesenchymal cells (115, 116). As such, ADAM12+ and Gli1+ cells were identified as a minimal subset originating myofibroblasts (115, 116).
In injured muscle or in muscular dystrophy, bipotent fibro/adipogenic progenitors have been shown to give rise to adipocytes and collagen-producing cells that compromise muscle function. These progenitors were identified with markers very similar to those found on adipogenic progenitors isolated from WAT (117–119).
In adipose tissue fibrosis accumulation, PDGFRα+ progenitors are identified as the main contributors to ECM production. PDGFRα+ progenitors were initially identified for their ability to differentiate into white adipocytes (34). However, in fibrotic adipose tissue, we and others observed that PDGFRα+ cells produce the highest levels of fibrosis markers such as collagens as compared with adipocytes, endothelial cells, or macrophages, and they accumulate in fibrotic adipose tissue (43, 88).
This bipotent capacity to differentiate into adipocyte or myofibroblast suggests that heterogeneity among adipose tissue progenitors could be pathologically relevant. Accordingly, the expression level of CD9, a surface marker protein whose expression was coregulated with fibrosis markers (70), defines two progenitor populations. In lean mouse adipose tissue, PDGFRα+CD9hi progenitors are driven toward ECM production, whereas PDGFRα+CD9lo cells are committed to adipogenesis. In the fibrotic WAT, the PDGFRα+CD9hi subset accumulates, while the PDGFRα+CD9lo population is lost. These observations in mice were further translated by observations in visceral fat (i.e., omental adipose tissue) from severely obese subjects. CD9 expression also defines two populations among the CD34+CD45–CD31– cells. An increased frequency of CD9hi over CD9lo progenitors is also observed together with omental adipose tissue fibrosis in these subjects with severe obesity with or without diabetes. Interestingly, the number of CD9hi progenitors was also associated with glucose control in this population.
Furthermore, boosting PDGFRα-mediated profibrotic signaling in PDGFRα+ progenitors (94, 120, 121) favored the accumulation of CD9hi over CD9lo cells. This phenotypic switch was concomitant with collagen deposition, reduced fat accretion, and local insulin resistance, supporting a direct local role for PDGFRα+ progenitors in WAT metabolic alterations (43).
The use of single-cell RNA sequencing to examine the transcriptional profile of individual cells enables exploration of the cellular diversity in different adipose tissue microenvironments. An elegant study confirmed the fibrotic fate of CD9hi progenitors and added a layer of complexity, showing that CD9hi progenitors also include mesothelial cells (122). Mesothelial cells form a monolayer, known as the mesothelium, that covers internal organs. In some circumstances, they are shown to be able to undergo mesothelial-mesenchymal transition to acquire a myofibroblastic phenotype with secretion of inflammatory mediators and ECM components (123, 124). However, to date, the exact role of mesothelial cells in visceral adipose tissue homeostasis remains unelucidated, especially in the context of energy imbalances.
Several recent studies now depict great heterogeneity among adipose progenitors, along with functional differences. In adipose tissue, these progenitors also produce chemokines and cytokines, suggesting that they may be involved in obesity-induced WAT fibrosis and the orchestration of adipose tissue inflammation (122, 125). Accordingly, among progenitor cells, those that exert a regulatory function on immunocyte expansion can be distinguished from adipocyte precursors. Through the production of IL-33, IL-33+ progenitor subsets function to control the accumulation of regulatory T cells in the adipose tissue, suggesting progenitors as an important orchestrator of the tissular immunological response (126). In addition, IL-33 was shown to be a positive inducer of fibrosis in lung and liver (127).
In addition to heterogeneity in function, progenitor subsets are defined along a developmental hierarchy with specific location. DPP4+ progenitors located in the reticular interstitium surrounding the adipose depot give rise to precursors committed to adipocyte fate as ICAM1+ and CD142+ preadipocytes distributed between the mature adipocytes in the fat depot cells (128). Notably, in a separate work, a subpopulation identified as CD142+ displays adipogenesis-regulating properties, as these cells can suppress adipocyte formation in a paracrine manner without adipogenic potential (35). Interestingly, TGF-β signaling functions to maintain DPP4+ progenitor identity and to inhibit the adipogenic transformation of DPP4+ and CD142+ cells (128).
Our current understanding is that adipose tissue progenitors can undergo differentiation toward either adipogenic or fibrogenic cell programs. Thus, a switch in progenitor orientation toward either the adipocyte or fibrotic lineage might be instrumental in the adipose tissue response to obesogenic conditions. This orientation is probably critical in cell fate determination, and fibrosis orientation may alter the healthy expansion of adipose tissue with a reduced ability to form new fat cells. In agreement with this view in which adipogenesis and fibrogenesis are interconnected, limiting of the precursor adipogenic phenotype through PPARγ deletion or PDGFRα activation favors the fibrotic transformation of WAT (21, 94), and results in a maladaptive response of the adipose tissue to obesogenic stress. These observations also argue for an interplay between the adipogenic and the myofibroblastic fate.
Generation of white adipocytes is not solely connected to the fibrotic pathway. Adipose progenitors can also form beige adipocytes (16, 41), a process named adipose beiging (wherein WAT develops characteristics of metabolically active BAT), and compelling evidence supports that adipose beiging and fibrosis are opposing pathways. As such, the PRDM16 transcriptional complex not only activates brown/beige fat development (17), but also potently represses adipose tissue fibrosis through its direct interaction with GTF2IRD1. Interestingly, this phenomenon is independent of UCP1’s uncoupling function (129).
In addition, PRDM16-dependent metabolic signals arising from adipocytes regulate progenitor fate, blocking fibrosis while enhancing beige adipogenesis (130). The transcription factor MRTFA has also consistently been highlighted as another critical inducer of progenitor fibrotic fate (131) with critical roles in beige adipogenic orientation (132) and improvement of the metabolic health of adipose tissue.
An understanding of adipose tissue fibrosis resolution is still needed
Antiobesity treatments mostly rely on approaches limiting caloric intake in order to promote weight loss. In severe obesity, bariatric surgery is a therapeutic procedure that efficiently leads to drastic weight loss, amelioration of low-grade inflammation, and even diabetes resolution in some (but not all) patients along with reduction of cardiovascular risks. Surprisingly, analysis of human WAT revealed that weight loss induced by surgery is accompanied by increased deposition of ECM (133), suggesting that weight loss has a profound impact on tissue remodeling. Other studies have confirmed these findings and suggest that adipose tissue inflammation could be of importance in this process, as leukocyte and macrophage infiltration remained after loss of fat mass (134, 135) and could then sustain the lack of fibrosis resolution. The functional consequences of this WAT remodeling deserve further attention, as this process could favor/potentiate tissue metabolic deteriorations in patients who frequently experience weight loss and rebound.
Conclusions and perspectives
With excess energy pressure, expandability and remodeling appear to be critical adipose tissue functions for clinical outcomes (136). WAT fibrosis accumulation, characterized by pathological remodeling and reductions in adipose expandability, is considered to be an aggravating factor in obesity and associated metabolic diseases. As a solution to control the balance between adipose tissue expandability and fibrosis, adipose progenitors have become a target of interest. This is highlighted by the fact that intradermal adipose-derived myofibroblasts remain multipotent and can be reprogrammed during wound healing to generate fat cells (137, 138). More studies are necessary to delineate the critical pathways controlling adipocyte and myofibroblast balance in subcutaneous and visceral fat. Deeper understanding of these pathways would lay the groundwork to develop new therapeutic strategies to maintain (or rescue) adipose tissue plasticity in order to break the deleterious link between obesity and associated metabolic dysfunctions.
Acknowledgments
We acknowledge the Fondation pour la Recherche Medicale (DEQ20120323701), the French National Agency of Research (Adipofib and Captor programs), the research program CAPES (Coordenação de Aperfeiçooamento de Pessoal de Nível Superior)/COFECUB (Comité Français d’Évaluation de la Coopération Universitaire et Scientifique avec le Brésil), the French clinical program CRC-fibroTA, the European Foundation for the Study of Diabetes, the Société Française de Nutrition, the Association Française d’Etude et de Recherche sur l’Obésité (AFERO), the Benjamin Delessert institute, and the RHU-CARMMA program. We acknowledge Timothy Swartz for his critical editing of the manuscript.
Version 1. 09/09/2019
Electronic publication
Version 2. 10/01/2019
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2019;129(10):4032–4040.https://doi.org/10.1172/JCI129192.
Contributor Information
Laís Bhering Martins, Email: laisbmnutri@gmail.com.
Karine Clément, Email: karine.clement2@gmail.com.
References
- 1.Marcelin G, Chua S. Contributions of adipocyte lipid metabolism to body fat content and implications for the treatment of obesity. Curr Opin Pharmacol. 2010;10(5):588–593. doi: 10.1016/j.coph.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74(4):761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 3.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 5.Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol. 2018;221(pt suppl 1):jeb162958. doi: 10.1242/jeb.162958. [DOI] [PubMed] [Google Scholar]
- 6.de Luca C, et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115(12):3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297(6):E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamauchi T, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 9.Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014;25(7):348–355. doi: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8(2):93–100. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest. 2019;129(10):4041–4049. doi: 10.1172/JCI129193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 14.Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol. 2017;13(1):26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27(3):234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vishvanath L, et al. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 2016;23(2):350–359. doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen P, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156(1-2):304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitner BP, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci U S A. 2017;114(32):8649–8654. doi: 10.1073/pnas.1705287114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016;17(11):691–702. doi: 10.1038/nrm.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YS, Wollam J, Olefsky JM. An integrated view of immunometabolism. Cell. 2018;172(1–2):22–40. doi: 10.1016/j.cell.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao M, et al. De novo adipocyte differentiation from Pdgfrβ+ preadipocytes protects against pathologic visceral adipose expansion in obesity. Nat Commun. 2018;9(1):890. doi: 10.1038/s41467-018-03196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Giordano A, et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 2013;54(9):2423–2436. doi: 10.1194/jlr.M038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jernås M, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20(9):1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 25.Berger JJ, Barnard RJ. Effect of diet on fat cell size and hormone-sensitive lipase activity. J Appl Physiol. 1999;87(1):227–232. doi: 10.1152/jappl.1999.87.1.227. [DOI] [PubMed] [Google Scholar]
- 26.Wueest S, Rapold RA, Rytka JM, Schoenle EJ, Konrad D. Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia. 2009;52(3):541–546. doi: 10.1007/s00125-008-1223-5. [DOI] [PubMed] [Google Scholar]
- 27.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135(2):240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 28.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4(4):263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen ED, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4(4):611–617. doi: 10.1016/S1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 30.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SM, et al. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014;20(6):1049–1058. doi: 10.1016/j.cmet.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeffery E, et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 2016;24(1):142–150. doi: 10.1016/j.cmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang W, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15(3):302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwalie PC, et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature. 2018;559(7712):103–108. doi: 10.1038/s41586-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 36.Tabula Muris Consortium. et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018;562(7727):367–372. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17(4):376–385. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta RK, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15(2):230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta RK, et al. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464(7288):619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudak CS, et al. Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 2014;8(3):678–687. doi: 10.1016/j.celrep.2014.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15(4):480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013;18(3):355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcelin G, et al. A PDGFRα-mediated switch toward CD9high adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab. 2017;25(3):673–685. doi: 10.1016/j.cmet.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 45.Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab. 2014;19(1):8–20. doi: 10.1016/j.cmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2009;50(suppl):S86–S90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao H, et al. CD36 is a marker of human adipocyte progenitors with pronounced adipogenic and triglyceride accumulation potential. Stem Cells. 2017;35(7):1799–1814. doi: 10.1002/stem.2635. [DOI] [PubMed] [Google Scholar]
- 48.Estève D, et al. Human white and brite adipogenesis is supported by MSCA1 and is impaired by immune cells. Stem Cells. 2015;33(4):1277–1291. doi: 10.1002/stem.1916. [DOI] [PubMed] [Google Scholar]
- 49.Arner E, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59(1):105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117(9):2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujisawa S, Kadoma Y. Mechanisms of action of (meth)acrylates in hemolytic activity, in vivo toxicity and dipalmitoylphosphatidylcholine (DPPC) liposomes determined using NMR spectroscopy. Int J Mol Sci. 2012;13(1):758–773. doi: 10.3390/ijms13010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wree A, et al. Adipokine expression in brown and white adipocytes in response to hypoxia. J Endocrinol Invest. 2012;35(5):522–527. doi: 10.3275/7964. [DOI] [PubMed] [Google Scholar]
- 53.Michailidou Z, et al. Increased angiogenesis protects against adipose hypoxia and fibrosis in metabolic disease-resistant 11β-hydroxysteroid dehydrogenase type 1 (HSD1)-deficient mice. J Biol Chem. 2012;287(6):4188–4197. doi: 10.1074/jbc.M111.259325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.An YA, et al. Angiopoietin-2 in white adipose tissue improves metabolic homeostasis through enhanced angiogenesis. Elife. 2017;6:e24071. doi: 10.7554/eLife.24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robciuc MR, et al. VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab. 2016;23(4):712–724. doi: 10.1016/j.cmet.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pellegrinelli V, Rouault C, Veyrie N, Clément K, Lacasa D. Endothelial cells from visceral adipose tissue disrupt adipocyte functions in a three-dimensional setting: partial rescue by angiopoietin-1. Diabetes. 2014;63(2):535–549. doi: 10.2337/db13-0537. [DOI] [PubMed] [Google Scholar]
- 57.Shungin D, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 60.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrante AW. The immune cells in adipose tissue. Diabetes Obes Metab. 2013;15(suppl 3):34–38. doi: 10.1111/dom.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 63.Tam CS, et al. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes. 2010;59(9):2164–2170. doi: 10.2337/db10-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishii S, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187(11):1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menezes-Garcia Z, et al. Lack of platelet-activating factor receptor protects mice against diet-induced adipose inflammation and insulin-resistance despite fat pad expansion. Obesity (Silver Spring) 2014;22(3):663–672. doi: 10.1002/oby.20142. [DOI] [PubMed] [Google Scholar]
- 66.Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117(9):2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yanagida K, et al. The Gα12/13-coupled receptor LPA4 limits proper adipose tissue expansion and remodeling in diet-induced obesity. JCI Insight. 2018;3(24):97293. doi: 10.1172/jci.insight.97293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Okuno Y, et al. Oxidative stress inhibits healthy adipose expansion through suppression of SREBF1-mediated lipogenic pathway. Diabetes. 2018;67(6):1113–1127. doi: 10.2337/db17-1032. [DOI] [PubMed] [Google Scholar]
- 69.Khan T, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29(6):1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henegar C, et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008;9(1):R14. doi: 10.1186/gb-2008-9-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Divoux A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59(11):2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Datta R, Podolsky MJ, Atabai K. Fat fibrosis: friend or foe? JCI Insight. 2018;3(19):122289. doi: 10.1172/jci.insight.122289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Connor KC, Song H, Rosenzweig N, Jansen DA. Extracellular matrix substrata alter adipocyte yield and lipogenesis in primary cultures of stromal-vascular cells from human adipose. Biotechnol Lett. 2003;25(23):1967–1972. doi: 10.1023/B:BILE.0000004386.08923.ab. [DOI] [PubMed] [Google Scholar]
- 75.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35(3 pt 2):657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 76.Grandl G, Müller S, Moest H, Moser C, Wollscheid B, Wolfrum C. Depot specific differences in the adipogenic potential of precursors are mediated by collagenous extracellular matrix and Flotillin 2 dependent signaling. Mol Metab. 2016;5(10):937–947. doi: 10.1016/j.molmet.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125(3):577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 78.Halberg N, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29(16):4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdennour M, et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab. 2014;99(3):898–907. doi: 10.1210/jc.2013-3253. [DOI] [PubMed] [Google Scholar]
- 80.Pellegrinelli V, et al. Human adipocyte function is impacted by mechanical cues. J Pathol. 2014;233(2):183–195. doi: 10.1002/path.4347. [DOI] [PubMed] [Google Scholar]
- 81.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pasarica M, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94(12):5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spencer M, et al. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299(6):E1016–E1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guglielmi V, et al. Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr Diabetes. 2015;5:e175. doi: 10.1038/nutd.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bel Lassen P, et al. The FAT score, a fibrosis score of adipose tissue: predicting weight-loss outcome after gastric bypass. J Clin Endocrinol Metab. 2017;102(7):2443–2453. doi: 10.1210/jc.2017-00138. [DOI] [PubMed] [Google Scholar]
- 86.Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol. 2013;229(2):298–309. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scharenberg MA, et al. TGF-β-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J Cell Sci. 2014;127(pt 5):1079–1091. doi: 10.1242/jcs.142075. [DOI] [PubMed] [Google Scholar]
- 88.Vila IK, et al. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 2014;7(4):1116–1129. doi: 10.1016/j.celrep.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 89.Keophiphath M, Achard V, Henegar C, Rouault C, Clément K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23(1):11–24. doi: 10.1210/me.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zaragosi LE, et al. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes. 2010;59(10):2513–2521. doi: 10.2337/db10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walton KL, Johnson KE, Harrison CA. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol. 2017;8:461. doi: 10.3389/fphar.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7(2):193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reed NI, et al. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med. 2015;7(288):288ra79. doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iwayama T, et al. PDGFRα signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev. 2015;29(11):1106–1119. doi: 10.1101/gad.260554.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu E, et al. Comprehensive dissection of PDGF-PDGFR signaling pathways in PDGFR genetically defined cells. PLoS One. 2008;3(11):e3794. doi: 10.1371/journal.pone.0003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abdollahi A, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201(6):925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kocabayoglu P, et al. β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J Hepatol. 2015;63(1):141–147. doi: 10.1016/j.jhep.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5(suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 101.Catalán V, et al. Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J Clin Endocrinol Metab. 2012;97(10):E1880–E1889. doi: 10.1210/jc.2012-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gunasekaran MK, et al. Inflammation triggers high mobility group box 1 (HMGB1) secretion in adipose tissue, a potential link to obesity. Cytokine. 2013;64(1):103–111. doi: 10.1016/j.cyto.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 103.Nativel B, et al. Soluble HMGB1 is a novel adipokine stimulating IL-6 secretion through RAGE receptor in SW872 preadipocyte cell line: contribution to chronic inflammation in fat tissue. PLoS One. 2013;8(9):e76039. doi: 10.1371/journal.pone.0076039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trepanowski JF, Mey J, Varady KA. Fetuin-A: a novel link between obesity and related complications. Int J Obes (Lond) 2015;39(5):734–741. doi: 10.1038/ijo.2014.203. [DOI] [PubMed] [Google Scholar]
- 105.Orr JS, Puglisi MJ, Ellacott KL, Lumeng CN, Wasserman DH, Hasty AH. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes. 2012;61(11):2718–2727. doi: 10.2337/db11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Coin PG, Lindroos PM, Bird GS, Osornio-Vargas AR, Roggli VL, Bonner JC. Lipopolysaccharide up-regulates platelet-derived growth factor (PDGF) alpha-receptor expression in rat lung myofibroblasts and enhances response to all PDGF isoforms. J Immunol. 1996;156(12):4797–4806. [PubMed] [Google Scholar]
- 107.Cancello R, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55(6):1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 108.Tanaka M, et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat Commun. 2014;5:4982. doi: 10.1038/ncomms5982. [DOI] [PubMed] [Google Scholar]
- 109.Madsen DH, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol. 2013;202(6):951–966. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Atabai K, et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119(12):3713–3722. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol Cell Biol. 2013;33(5):904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 114.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18(8):1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 116.Kramann R, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16(1):51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 119.Uezumi A, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124(pt 21):3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 120.Olson LE, Soriano P. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16(2):303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun C, Berry WL, Olson LE. PDGFRα controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development. 2017;144(1):83–94. doi: 10.1242/dev.135962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hepler C, et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. Elife. 2018;7:e39636. doi: 10.7554/eLife.39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mutsaers SE. The mesothelial cell. Int J Biochem Cell Biol. 2004;36(1):9–16. doi: 10.1016/S1357-2725(03)00242-5. [DOI] [PubMed] [Google Scholar]
- 124.Yáñez-Mó M, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348(5):403–413. doi: 10.1056/NEJMoa020809. [DOI] [PubMed] [Google Scholar]
- 125.Kaplan JL, et al. Adipocyte progenitor cells initiate monocyte chemoattractant protein-1-mediated macrophage accumulation in visceral adipose tissue. Mol Metab. 2015;4(11):779–794. doi: 10.1016/j.molmet.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spallanzani RG, et al. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol. 2019;4(35):eaaw3658. doi: 10.1126/sciimmunol.aaw3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kotsiou OS, Gourgoulianis KI, Zarogiannis SG. IL-33/ST2 axis in organ fibrosis. Front Immunol. 2018;9:2432. doi: 10.3389/fimmu.2018.02432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Merrick D, et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science. 2019;364(6438):eaav2501. doi: 10.1126/science.aav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hasegawa Y, et al. Repression of adipose tissue fibrosis through a PRDM16-GTF2IRD1 complex improves systemic glucose homeostasis. Cell Metab. 2018;27(1):180–194.e6. doi: 10.1016/j.cmet.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang W, et al. A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab. 2019;30(1):174–189.e5. doi: 10.1016/j.cmet.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin JZ, Rabhi N, Farmer SR. Myocardin-related transcription factor a promotes recruitment of ITGA5+ profibrotic progenitors during obesity-induced adipose tissue fibrosis. Cell Rep. 2018;23(7):1977–1987. doi: 10.1016/j.celrep.2018.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell. 2015;160(1–2):105–118. doi: 10.1016/j.cell.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Y, et al. Accumulation and changes in composition of collagens in subcutaneous adipose tissue after bariatric surgery. J Clin Endocrinol Metab. 2016;101(1):293–304. doi: 10.1210/jc.2015-3348. [DOI] [PubMed] [Google Scholar]
- 134.Zamarron BF, et al. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes. 2017;66(2):392–406. doi: 10.2337/db16-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schmitz J, et al. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol Metab. 2016;5(5):328–339. doi: 10.1016/j.molmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48(6):1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67(4):1062–1073. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Plikus MV, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science. 2017;355(6326):748–752. doi: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]