Abstract
Background
Survivors of childhood cancer are at risk of neurocognitive impairment, emotional distress and poor health-related quality of life (HRQOL); however, the effect of race/ethnicity is understudied. We aimed to identify race/ethnicity-based disparities in neurocognitive, emotional and HRQOL outcomes among survivors of childhood cancer.
Methods
Self-reported measures of neurocognitive function, emotional distress (BSI-18), and HRQOL (SF-36) were compared between minority (Hispanic [n=821], non-Hispanic black [NHB: n=600]) and non-Hispanic white (NHW: n=12,287) survivors from the Childhood Cancer Survivor Study, median age 30.9 years (range 16.0-54.1). Using a sample of 3,055 siblings, the magnitude of same race/ethnicity survivor-sibling differences were compared between racial/ethnic groups, adjusting for demographic and treatment characteristics and current socioeconomic status (SES).
Results
No clear pattern of disparity in neurocognitive outcomes by race/ethnicity was observed. The magnitude of the survivor-sibling difference in mean score for depression was greater in Hispanics than NHWs (3.59 vs. 1.09, p=0.004). NHBs and Hispanics had greater survivor-sibling differences in HRQOL than NHWs for mental health (NHB: −5.78 vs −0.69, p=0.001; Hispanic: −3.87 vs. −0.69, p=0.03) and social function (NHB: −7.11 vs −1.47, p<0.001; Hispanic: −5.33 vs. −1.47, p=0.001). NHBs had greater survivor-sibling differences in physical subscales of HRQOL than NHWs. Findings were, in general, not attenuated by current SES.
Conclusion
Although no pattern of disparity in neurocognitive outcomes was observed, differences across many HRQOL outcomes among minorities compared to NHWs, not attenuated by current SES, were identified. This suggests further research into environmental and sociocultural factors during and immediately after treatment is needed.
Keywords: Pediatric, Cancer, Survivor, Race, Ethnicity, Disparity, Psychological
Precis:
Disparities across many domains of health-related quality of life among minority survivors of childhood cancer compared to non-Hispanic whites were identified and are not fully explained by socioeconomic status. Fortunately, no pattern of differences in neurocognitive outcomes by race/ethnicity were identified.
INTRODUCTION
Given the improvement in survival of childhood cancer observed over the last five decades, the number of pediatric cancer survivors living in the United States is predicted to exceed 500,000 by 2020.1 However, survival comes at a cost, including increased risk for late mortality and chronic health conditions.2,3 Childhood cancer survivors are known to be at increased risk of neurocognitive impairment compared to siblings, with 20-40% of survivors having measurable deficits.4,5 Additionally, certain populations of survivors have been found to be at increased risk for poor emotional and health-related quality of life (HRQOL) outcomes.6-10
Risk factors for neurocognitive impairment, emotional distress and poor HRQOL are reasonably well established. Survivors treated at a younger age or who received CNS-directed chemotherapy or cranial radiation have consistently been found to be at higher risk for neurocognitive impairment.4,5,11 Risk factors for emotional distress include a brain tumor diagnosis, adolescent age at diagnosis, more intensive treatment, female sex, and remaining unmarried.5,7,10 Similarly, female sex, lower socioeconomic status (SES), major medical problems or prior treatment with cranial radiation are risk factors for poor HRQOL.6-8 However, the impact of race and ethnicity on these outcomes is not established, attributable largely to the small numbers of minority survivors who have been systematically assessed.4,6
The demographics of the United States are shifting and by 2044 over 50% of individuals are expected to belong to a racial/ethnic background other than non-Hispanic white (NHW).12 In both the general population and childhood cancer survivors, minorities report lower income, education and rates of health insurance coverage compared to NHWs.13-17 Racial and ethnic differences in overall and event-free survival of childhood cancer are not fully explained by differences in disease biology or pharmacogenetics and, likely, are in part due to socioeconomic and sociocultural factors.16 Evaluation of racial and ethnic disparities in childhood cancer survivors focused on late mortality and chronic health condition outcomes has identified that, although differences exist, they are abrogated after adjusting for SES differences between groups.17,18 However, there is a paucity of literature published on the effects of race and ethnicity on long-term psychological outcomes of childhood cancer survivors. The successful expansion of the Childhood Cancer Survivor Study (CCSS) to include survivors diagnosed 1970-1999 provides a unique opportunity for evaluating the impact of race and ethnicity on neurocognitive, emotional and HRQOL outcomes of childhood cancer survivors.
METHODS
Population
The CCSS is a retrospective cohort study with longitudinal follow-up of survivors of childhood cancer treated at 31 institutions in the US and Canada. Study eligibility included diagnosis of cancer before age 21 years, initial treatment between January 1, 1970 and December 31, 1999, and alive at five years after diagnosis of leukemia, CNS malignancy, Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft tissue sarcoma, or bone tumor representing approximately 20% of US children diagnosed with cancer during this time-period. A random sample of siblings of CCSS participants served as a comparison population. The cohort methodology, study design and characteristics have been described in detail previously.19,20
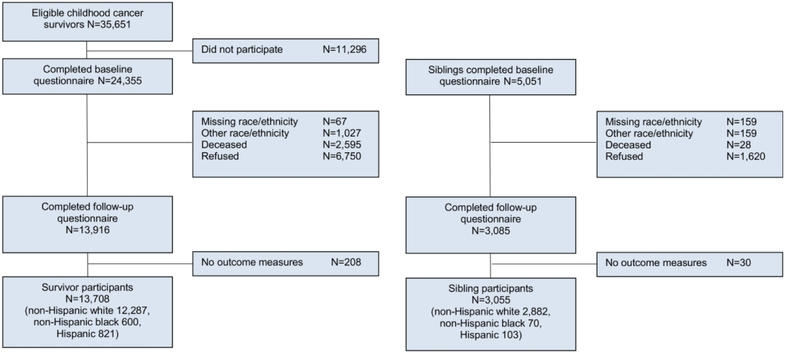
The CCSS was approved by institutional review boards at participating centers. Participants provided informed consent for the study and release of information from medical records. For eligible survivors, cancer diagnosis and treatment information were obtained from medical records at the treating institution. All participants completed a baseline questionnaire assessing demographic and health condition outcomes. Race/ethnicity status was obtained through self-report of white, black, American Indian or Alaska Native (AIAN), Asian or Pacific Islander (API), or other categories, with the option to write in their race. Hispanic ethnicity was reported through a separate yes/no question. For this study, excluding small numbers of AIAN (N=142), API (N=365) or other (N=520), participants were grouped into three mutually exclusive race/ethnicity populations: NHW, NHB and Hispanics. The recent expansion of the CCSS cohort to include those diagnosed 1987-1999 more than doubled the number of NHB and Hispanic survivor participants. A proxy (parent, spouse, next of kin) completed the baseline questionnaire for survivors who died more than five years after diagnosis, were under age 18, or unable to complete the questionnaire. Study questionnaires are available at http://ccss.stjude.org. Survivors and siblings with complete race/ethnicity information on baseline questionnaire and who completed a subsequent follow-up questionnaire that included assessment of psychological outcomes were considered eligible for this analysis (Table 1, Figure 1).
Table 1.
Demographic and treatment characteristics among survivors of childhood cancer and siblings.
| Characteristic | Survivors, n(%) | Siblings, n(%) | ||||||
|---|---|---|---|---|---|---|---|---|
| White, NH (n=12,287) |
Black, NH (n=600) |
Hispanic (n=821) |
P | White, NH (n=2882) |
Black, NH (n=70) |
Hispanic (n=103) |
P | |
| Sex | 0.03 | 0.84 | ||||||
| Male | 6139 (49.8) | 298 (48.8) | 373 (45.4) | 1311 (45.5) | 31 (44.3) | 44 (42.7) | ||
| Female | 6148 (50.2) | 302 (51.2) | 448 (54.6) | 1571 (54.5) | 39 (55.7) | 59 (57.3) | ||
| Age at diagnosis (years) | <0.001 | |||||||
| 0-4 | 4709 (40.2) | 240 (41.1) | 308 (39.5) | |||||
| 5-9 | 2733 (23.3) | 134 (23.9) | 233 (29.3) | |||||
| 10-14 | 2663 (20.2) | 142 (22.5) | 156 (17.8) | |||||
| 15-20 | 2182 (16.3) | 84 (12.5) | 124 (13.4) | |||||
| Year of diagnosis | <0.001 | |||||||
| 1970-79 | 3842 (28.4) | 114 (16.7) | 155 (16.4) | |||||
| 1980-89 | 5313 (41.6) | 274 (42.3) | 322 (36.7) | |||||
| 1990-99 | 3132 (30.1) | 212 (41.0) | 344 (46.9) | |||||
| Age at follow-up (years) | <0.001 | 0.03 | ||||||
| <25 | 2649 (22.6) | 150 (26.3) | 201 (26.6) | 544 (18.9) | 21 (30.4) | 29 (28.2) | ||
| 25-34 | 5227 (44.2) | 271 (45.3) | 369 (45.6) | 956 (33.3) | 25 (36.2) | 37 (35.9) | ||
| 35-44 | 3734 (28.2) | 159 (25.5) | 227 (25.2) | 1022 (35.6) | 17 (24.6) | 30 (2.1) | ||
| 45-54 | 676 (5.0) | 20 (2.9) | 24 (2.6) | 338 (11.8) | 6 (8.7) | 6 (5.8) | ||
| ≥55 | 11 (0.4) | 0 (0.0) | 1 (1.0) | |||||
| Cancer diagnosis | <0.001 | |||||||
| Acute lymphoblastic leukemia | 3216 (33.0) | 133 (31.4) | 232 (37.8) | |||||
| Acute myeloid leukemia | 421 (3.1) | 20 (2.9) | 48 (5.1) | |||||
| Other leukemia | 118 (0.9) | 5 (0.7) | 13 (1.4) | |||||
| Astrocytoma | 1279 (9.4) | 54 (7.9) | 87 (9.2) | |||||
| Medulloblastoma, PNET | 468 (3.5) | 34 (5.0) | 29 (3.1) | |||||
| Other CNS tumors | 331 (2.4) | 23 (3.4) | 21 (2.2) | |||||
| Hodgkin lymphoma | 1569 (11.6) | 67 (9.8) | 106 (11.2) | |||||
| Non-Hodgkin lymphoma | 992 (7.3) | 45 (6.6) | 54 (5.7) | |||||
| Kidney tumors | 1097 (8.1) | 96 (14.1) | 67 (7.1) | |||||
| Neuroblastoma | 864 (6.4) | 32 (4.7) | 57 (6.0) | |||||
| Soft tissue sarcoma | 919 (6.8) | 48 (7.1) | 38 (4.0) | |||||
| Ewing sarcoma | 377 (2.8) | 3 (0.4) | 16 (1.7) | |||||
| Osteosarcoma | 575 (4.2) | 40 (5.9) | 51 (5.4) | |||||
| Other bone tumors | 61 (0.5) | 0 (0.0) | 2 (0.2) | |||||
| Cranial radiation (maxTD, Gy) | 0.002 | |||||||
| None | 7902 (70.3) | 341 (68.7) | 536 (73.1) | |||||
| >0-<20† | 1121 (10.5) | 49 (13.6) | 64 (9.9) | |||||
| 20-<30 | 969 (8.2) | 32 (5.6) | 54 (7.7) | |||||
| 30- <50 | 335 (2.7) | 6 (1.1) | 15 (1.8) | |||||
| ≥50 | 1037 (8.2) | 63 (11.1) | 65 (7.6) | |||||
| Intravenous methotrexate, g/m2 | <0.001 | |||||||
| None | 9060 (75.2) | 361 (65.5) | 584 (75.4) | |||||
| <4.3 | 1265 (11.7) | 53 (11.4) | 81 (12.8) | |||||
| ≥4.3 | 1094 (13.2) | 73 (23.1) | 74 (11.8) | |||||
| Intrathecal methotrexate, mg/m2 | 0.02 | |||||||
| None | 7756 (62.6) | 347 (62.1) | 503 (60.6) | |||||
| <230 | 2602 (24.7) | 103 (24.7) | 145 (22.7) | |||||
| ≥230 | 900 (12.7) | 32 (13.3) | 75 (16.7) | |||||
| Systemic corticosteroid | 0.60 | |||||||
| None | 6697 (53.4) | 301 (52.6) | 436 (51.3) | |||||
| Prednisone only | 4510 (43.6) | 178 (44.6) | 301 (46.2) | |||||
| Any dexamethasone | 383 (3.0) | 16 (2.8) | 22 (2.5) | |||||
| Health insurance status | <0.001 | <0.001 | ||||||
| Yes | 11021 (90.3) | 478 (79.5) | 696 (85.8) | 2630 (91.6) | 61 (87.1) | 83 (81.4) | ||
| No | 1182 (9.7) | 116 (20.5) | 123 (14.2) | 241 (8.4) | 9 (12.9) | 19 (18.6) | ||
| Household income ($‡) | <0.001 | 0.003 | ||||||
| <20,000 | 1036 (10.1) | 115 (27.6) | 105 (15.8) | 125 (4.8) | 5 (10.6) | 8 (9.1) | ||
| 20-39,999 | 1464 (14.2) | 109 (23.0) | 144 (21.5) | 242 (9.2) | 4 (8.5) | 11 (12.5) | ||
| 40-59,999 | 1670 (15.9) | 89 (17.7) | 127 (18.4) | 313 (12.0) | 10 (21.3) | 12 (13.6) | ||
| 60-79999 | 1560 (14.5) | 62 (13.2) | 101 (14.7) | 346 (13.2) | 7 (14.9) | 17 (19.3) | ||
| 80-99999 | 1279 (11.9) | 31 (5.8) | 59 (8.5) | 327 (12.5) | 8 (17.0) | 15 (17.0) | ||
| ≥100,000 | 3741 (33.5) | 63 (12.8) | 158 (21.1) | 1266 (48.3) | 13 (27.7) | 25 (28.4) | ||
| Educational attainment | <0.001 | <0.001 | ||||||
| <High school graduate or GED | 564 (4.6) | 42 (7.8) | 52 (6.1) | 110 (3.8) | 5 (7.1) | 3 (2.9) | ||
| High school graduate | 1778 (14.2) | 128 (20.9) | 147 (18.4) | 342 (11.9) | 14 (20.0) | 12 (11.7) | ||
| Any college/post-high school training | 4041 (33.0) | 264 (43.1) | 334 (40.0) | 925 (32.1) | 30 (42.9) | 52 (50.5) | ||
| College or post-graduate degree | 5871 (48.2) | 163 (28.2) | 287 (35.5) | 1504 (52.2) | 21 (30.0) | 36 (35.0) | ||
| Employment | <0.001 | 0.69 | ||||||
| Unable to work | 985 (7.8) | 95 (15.1) | 96 (11.4) | 41 (1.4) | 0 (0.0) | 2 (1.9) | ||
| Unemployed | 1398 (11.4) | 81 (13.6) | 113 (13.7) | 284 (9.9) | 8 (11.4) | 13 (12.6) | ||
| Employed/student | 9904 (80.8) | 424 (71.2) | 612 (75.0) | 2557 (88.7) | 6 (88.6) | 88 (85.4) | ||
| Major medical condition | 0.33 | 0.93 | ||||||
| Yes | 3409 (26.4) | 167 (26.6) | 251 (28.5) | 236 (8.2) | 5 (7.1) | 9 (8.7) | ||
| No | 8878 (73.6) | 433 (73.4) | 570 (71.5) | 2646 (91.8) | 65 (92.9) | 94 (91.3) | ||
Analyses including percentages were weighted to account for under-sampling of acute lymphoblastic leukemia survivors in latter era (1987–1999)
Direct cranial radiation doses (not including stray/scatter).
Adjusted to 2016-dollar value. NH=non-Hispanic; maxTD=maximum tumor dose
Figure 1.
Study population
Outcome Measures
The CCSS Neurocognitive Questionnaire (CCSS-NCQ) was designed to assess self-reported neurocognitive symptoms in childhood cancer survivors sensitive to therapeutic exposures and has been previously validated in the CCSS cohort.21 It contains four subscales including task efficiency, emotional regulation, organization, and memory derived from a 25-item questionnaire. Participants were asked to report the degree to which they experienced any of 25 specific problems over the past 6 months using a Likert scale from 1 to 3. Scores were converted to standardized T-scores so that the overall sibling cohort had a mean=50 and standard deviation (SD) of 10. Scores were reported as a continuous variable where higher scores indicate worse neurocognitive function.
The Brief Symptom Inventory-18 (BSI-18) is an 18-item survey that measures symptoms of emotional distress over the past week and has been validated in the CCSS.22,23 A summary score, the Global Severity Index (GSI), and three subscale scores for anxiety, depression and somatic complaints were reported as continuous variables where higher scores indicate more emotional distress. The Medical Outcomes Short Form-36 (SF-36) is a 36 item-survey used to evaluate HRQOL based on questions about the previous four weeks.24 There are two summary scales (mental, physical) and eight subscales representing various aspects of well-being where lower scores indicate poor HRQOL. The SF-36 has been validated in childhood cancer survivors and utilized in the CCSS.6,25 For both the BSI-18 and the SF-36, scores were reported as standardized T-scores with a general population mean=50 and SD=10. Participants <18 years of age were excluded from analyses utilizing the BSI-18 and CCSS-NCQ. Proxy reports were included in analyses utilizing the SF-36 and CCSS-NCQ because of the observable nature of the symptoms assessed but excluded from the analysis of emotional distress utilizing the BSI-18.
Cancer Therapeutic Exposures and Additional Factors
Cancer diagnosis and treatment data were abstracted from medical records including systemic and intrathecal (IT) methotrexate exposure, corticosteroid exposure and cranial radiation therapy (CRT), defined as maximum tumor dose (maxTD) of radiotherapy to one of four brain quadrants.19,26 The maxTD for each brain segment was determined by summing the total prescribed dose from all overlapping treatment fields; the maxTD was applied only if ≥50% of a given brain segment was within the field(s). Demographic and socioeconomic characteristics including age at questionnaire completion, sex, current household income, education level and insurance status were available from the questionnaires. The presence of a severe/disabling or life-threatening medical condition (grade 3-4), scored applying the Common Terminology Criteria for Adverse Events (CTCAE, version 4.03, National Cancer Institute) intended for scoring both acute and chronic conditions in patients and survivors of cancer, was included.27
Statistical Analysis
Descriptive statistics from the time of last contact for survivors and siblings were calculated. Comparisons of the primary outcomes in the form of standardized T-scores were made between siblings and survivors stratified by CRT exposure, within each racial/ethnic group, using multiple linear regression, adjusting for sex, age at follow-up, era of diagnosis, methotrexate exposure (intravenous and IT), corticosteroid exposure and presence of any CTCAE grade 3-4 chronic medical condition. Modifications by generalized estimating equations (GEE) were used to account for possible within-family correlation between survivors and siblings from the same family. To assess disparities between minority populations and NHWs the magnitude of the survivor-sibling differences for NHBs and Hispanics were compared with the survivor-sibling differences for NHWs, using the same regression method. This was done to standardize, on a population level, for SES differences that existed at the time of diagnosis based on the assumption that survivors and siblings of the same race/ethnicity group experienced similar SES exposures. To assess whether current SES attenuated differences in emotional and HRQOL outcomes, variables for education level, household income and health insurance status at the time of follow-up were added to the model in a subsequent step. The results from these regression analyses were reported as estimated means, differences in means and associated standard errors (SE) with p-values of the differences obtained from the robust variance of GEE.
RESULTS
Comparisons of Survivors and Siblings
A total of 13,708 five-year survivors and 3,055 siblings completed the baseline and follow-up questionnaires (Figure 1). The median age at diagnosis for survivors was 7.2 years (range 0.0-21.0) and the median age at follow-up survey was 30.9 years (range 16.0-54.1) for survivors and 33.4 years (range 9.6-58.4) for siblings. NHB (n=600, 4.4%) and Hispanic (n=821, 6.0%) survivors were more likely than NHW survivors to report a household income <$20,000 (27.6%, 15.8%, 10.1% respectively) and less likely to have health insurance (79.5%, 85.8%, 90.3%) or obtain a college degree (28.2%, 35.5%, 48.2%, Table 1). NHB survivors were more likely than Hispanic or NHW survivors to have been diagnosed with a kidney tumor (14.1%, 7.1%, 8.1% respectively) or have received a cumulative intravenous methotrexate dose ≥4.3 g/m2 (23.1%, 11.8%, 13.2%) while all other cancer diagnosis and treatment variables differed between groups by <5%.
Neurocognitive Outcomes
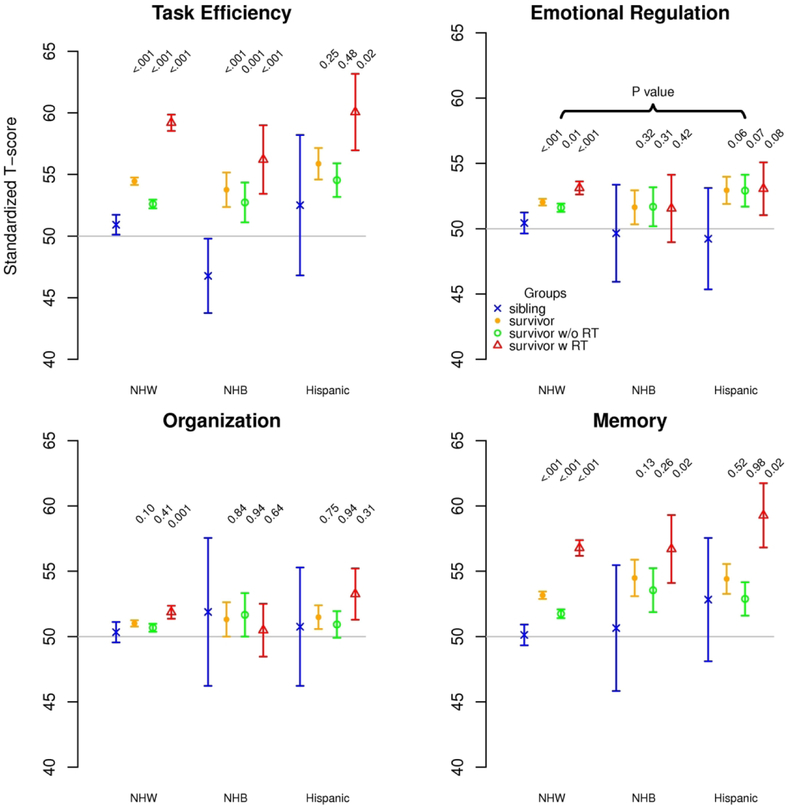
Within racial/ethnic groups, survivors were more likely than siblings to have higher scores for task efficiency (NHW, NHB; Hispanic who received CRT), emotional regulation (NHW only) and memory (NHW; NHB and Hispanic who received CRT; Figure 2A). For task efficiency, the comparison of the magnitude of the survivor-sibling difference among NHB survivors with that among NHW survivors overall (7.00 vs. 3.54; p=0.04; Table 2) and among those who did not receive CRT (5.96 vs. 1.68; p=0.02) achieved statistical significance. However, for the remaining neurocognitive subscales, the magnitude of the survivor-sibling differences among NHBs and Hispanics was not greater than that observed among NHWs, overall or among those exposed to CRT.
Figure 2.
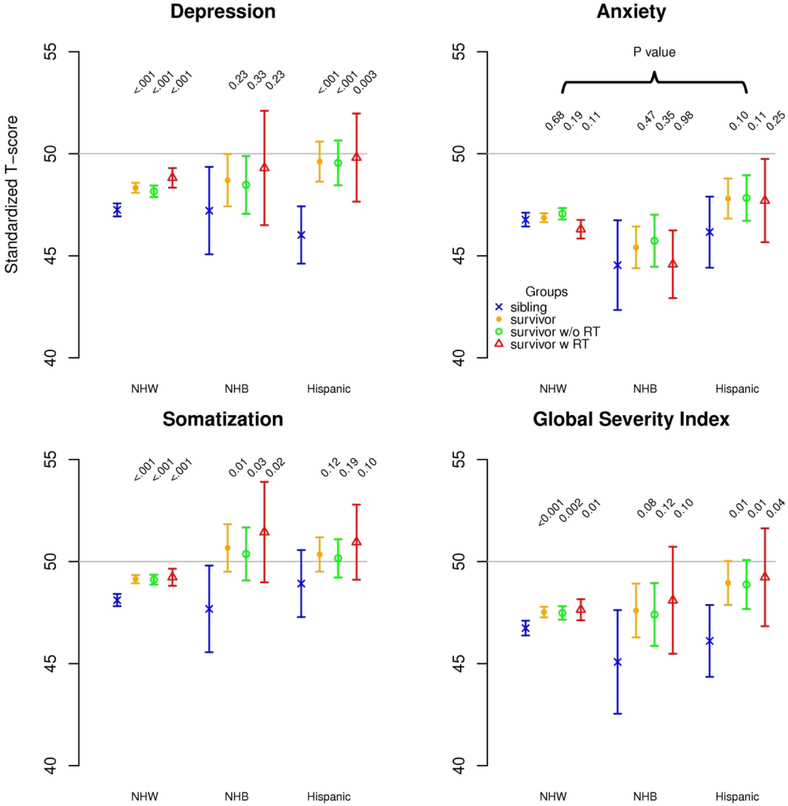
(A) Neurocognitive outcomes of survivors and siblings within each racial/ethnic group as standardized T-scores, sibling mean=50 SD=10. (B) Emotional (BSI-18) outcomes of survivors and siblings within each racial/ethnic group as standardized T-scores, general population mean=50 SD=10. For both outcomes, higher scores indicate worse function; P-values compare each survivor group to same race/ethnicity sibling group adjusted for sex, age at follow-up, year at diagnosis, methotrexate exposure (intravenous and intrathecal), corticosteroid exposure and any CTCAE grade 3-4 chronic medical condition. NHW=non-Hispanic white, NHB=non-Hispanic black, RT=cranial radiation therapy
Table 2.
Neurocognitive outcomes across racial/ethnic groups of childhood cancer survivors.
| White, non-Hispanic | Black, non-Hispanic | Hispanic | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | P | Mean | P | P* | Mean | P | P* | |
| Task efficiency | ||||||||
| Sibling | 50.92 | 46.77 | 52.51 | |||||
| Survivor | 54.45 | 53.76 | 55.87 | |||||
| Difference from sibling | 3.54 | <0.001 | 7.00 | <0.001 | 0.04 | 3.36 | 0.25 | 0.95 |
| Survivor no CRT | 52.60 | 52.73 | 54.54 | |||||
| Difference from sibling | 1.68 | <0.001 | 5.96 | 0.001 | 0.02 | 2.04 | 0.48 | 0.90 |
| Survivor with CRT | 59.20 | 56.21 | 60.06 | |||||
| Difference from Sibling | 8.28 | <0.001 | 9.45 | <0.001 | 0.57 | 7.55 | 0.02 | 0.83 |
| Emotional regulation | ||||||||
| Sibling | 50.44 | 49.65 | 49.23 | |||||
| Survivor | 52.03 | 51.64 | 52.94 | |||||
| Difference from sibling | 1.59 | <0.001 | 1.99 | 0.32 | 0.85 | 3.72 | 0.06 | 0.29 |
| Survivor no CRT | 51.61 | 51.68 | 52.91 | |||||
| Difference from sibling | 1.16 | 0.006 | 2.03 | 0.31 | 0.67 | 3.68 | 0.07 | 0.22 |
| Survivor with CRT | 53.12 | 51.55 | 53.06 | |||||
| Difference from sibling | 2.68 | <0.001 | 1.90 | 0.42 | 0.74 | 3.83 | 0.08 | 0.60 |
| Organization | ||||||||
| Sibling | 50.33 | 51.88 | 50.75 | |||||
| Survivor | 51.00 | 51.31 | 51.48 | |||||
| Difference from sibling | 0.68 | 0.10 | −0.57 | 0.84 | 0.67 | 0.73 | 0.75 | 0.98 |
| Survivor no CRT | 50.67 | 51.66 | 50.92 | |||||
| Difference from sibling | 0.34 | 0.41 | −0.22 | 0.94 | 0.85 | 0.17 | 0.94 | 0.94 |
| Survivor with CRT | 51.86 | 50.48 | 53.25 | |||||
| Difference from Sibling | 1.53 | 0.001 | −1.40 | 0.64 | 0.33 | 2.50 | 0.31 | 0.70 |
| Memory | ||||||||
| Sibling | 50.12 | 50.65 | 52.83 | |||||
| Survivor | 53.16 | 54.48 | 54.41 | |||||
| Difference from sibling | 3.04 | <0.001 | 3.83 | 0.13 | 0.76 | 1.58 | 0.52 | 0.55 |
| Survivor no CRT | 51.74 | 53.54 | 52.88 | |||||
| Difference from sibling | 1.63 | <0.001 | 2.89 | 0.26 | 0.63 | 0.05 | 0.98 | 0.53 |
| Survivor with CRT | 56.78 | 56.70 | 59.27 | |||||
| Difference from Sibling | 6.66 | <0.001 | 6.05 | 0.02 | 0.82 | 6.44 | 0.02 | 0.93 |
P=p-value comparing survivors to same race/ethnicity siblings; P*=p-value comparing survivor-sibling difference between racial/ethnic groups, referenced to non-Hispanic white survivor-sibling difference.
Adjusted for sex, age at follow-up, year at diagnosis, methotrexate exposure (intravenous and intrathecal), corticosteroid exposure and any CTCAE grade 3-4 chronic medical condition.
Emotional Outcomes
Within racial/ethnic groups, survivors were more likely than siblings to have higher scores for depression (NHW, Hispanic), somatization (NHW, NHB) and the global severity index (NHW, Hispanic; Figure 2B). The survivor-sibling difference in mean score for depression, adjusted for clinical and demographic characteristics, was significantly larger for Hispanics compared to NHWs, overall (3.59 vs. 1.09, p=0.004) and among those not exposed to CRT (3.52 vs. 0.91, p=0.004). Although the survivor-sibling differences among the group exposed to CRT was greater among Hispanics than NHWs (3.78 vs 1.57), it did not achieve statistical significance (p=0.09; Table 3). No significant survivor-sibling differences in emotional distress were observed when NHBs were compared to NHWs.
Table 3.
Emotional outcomes across racial/ethnic groups of childhood cancer survivors.
| White, non-Hispanic | Black, non-Hispanic | Hispanic | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | P | Mean | P | P* | Mean | P | P* | |
| Depression | ||||||||
| Sibling | 47.25 | 47.21 | 46.02 | |||||
| Survivor | 48.33 | 48.70 | 49.61 | |||||
| Survivor-sibling difference | 1.09 | <0.001 | 1.49 | 0.23 | 0.75 | 3.59 | <0.001 | 0.004 |
| Survivor no CRT | 48.16 | 48.47 | 49.55 | |||||
| Survivor-sibling difference | 0.91 | <0.001 | 1.26 | 0.33 | 0.79 | 3.52 | <0.001 | 0.004 |
| Survivor with CRT | 48.82 | 49.30 | 49.81 | |||||
| Survivor-sibling difference | 1.57 | <0.001 | 2.09 | 0.23 | 0.77 | 3.78 | 0.003 | 0.09 |
| Anxiety | ||||||||
| Sibling | 46.77 | 44.54 | 46.16 | |||||
| Survivor | 46.86 | 45.41 | 47.80 | |||||
| Survivor-sibling difference | 0.09 | 0.68 | 0.87 | 0.47 | 0.52 | 1.64 | 0.10 | 0.13 |
| Survivor no CRT | 47.06 | 45.73 | 47.83 | |||||
| Survivor-sibling difference | 0.29 | 0.19 | 1.19 | 0.35 | 0.48 | 1.67 | 0.11 | 0.19 |
| Survivor with CRT | 46.30 | 44.58 | 47.70 | |||||
| Survivor-sibling difference | −0.47 | 0.11 | 0.04 | 0.98 | 0.71 | 1.54 | 0.25 | 0.14 |
| Somatization | ||||||||
| Sibling | 48.11 | 47.68 | 48.92 | |||||
| Survivor | 49.14 | 50.67 | 50.35 | |||||
| Survivor-sibling difference | 1.03 | <0.001 | 3.00 | 0.01 | 0.11 | 1.43 | 0.12 | 0.67 |
| Survivor no CRT | 49.11 | 50.37 | 50.16 | |||||
| Survivor-sibling difference | 1.00 | <0.001 | 2.70 | 0.03 | 0.18 | 1.24 | 0.19 | 0.80 |
| Survivor with CRT | 49.23 | 51.44 | 50.95 | |||||
| Survivor-sibling difference | 1.12 | <0.001 | 3.76 | 0.02 | 0.11 | 2.03 | 0.10 | 0.47 |
| Global severity index | ||||||||
| Sibling | 46.74 | 45.08 | 46.11 | |||||
| Survivor | 47.52 | 47.60 | 48.95 | |||||
| Survivor-sibling difference | 0.78 | <0.001 | 2.52 | 0.08 | 0.23 | 2.85 | 0.005 | 0.05 |
| Survivor no CRT | 47.48 | 47.40 | 48.87 | |||||
| Survivor-sibling difference | 0.74 | 0.002 | 2.33 | 0.12 | 0.30 | 2.76 | 0.009 | 0.06 |
| Survivor with CRT | 47.64 | 48.10 | 49.23 | |||||
| Survivor-sibling difference | 0.90 | 0.005 | 3.02 | 0.10 | 0.25 | 3.12 | 0.04 | 0.14 |
P=p-value comparing survivors to same race/ethnicity siblings; P*=p-value comparing survivor-sibling difference between racial/ethnic groups, referenced to non-Hispanic white survivor-sibling difference.
Adjusted for sex, age at follow-up, year at diagnosis, methotrexate exposure (intravenous and intrathecal), corticosteroid exposure and any CTCAE grade 3-4 chronic medical condition.
Health-Related Quality of Life
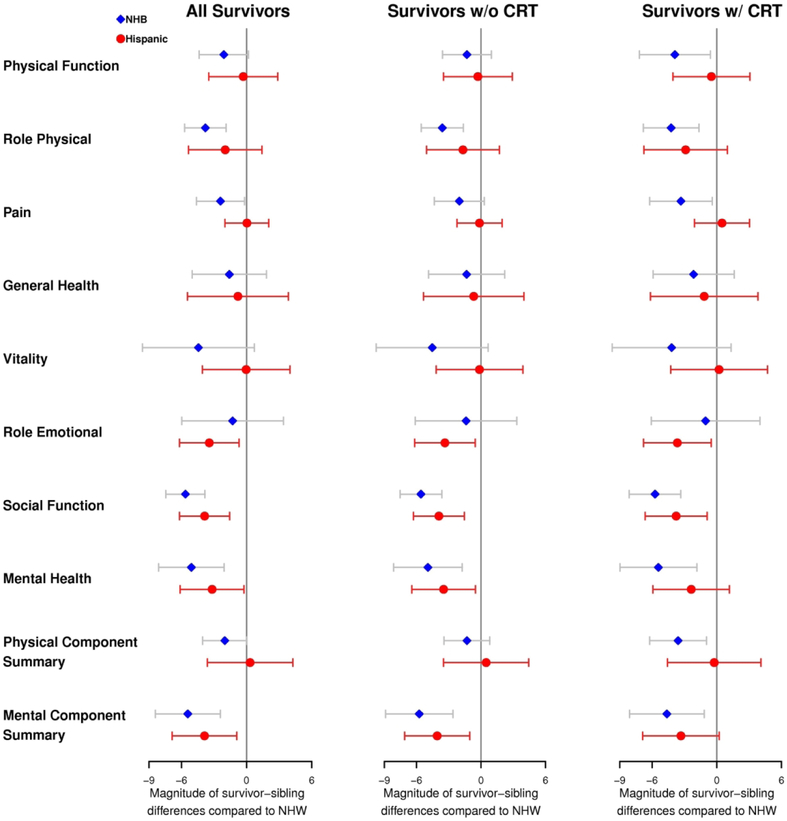
Survivor-sibling differences in many HRQOL domains were greater for minority survivors than NHWs (Figure 3, Supplemental Table 1). Specifically, the survivor-sibling difference in mean score for social function was significantly greater for both NHBs (−7.11 vs. −1.47, p=<0.001) and Hispanics (−5.33 vs. −1.47, p=0.001) compared to NHWs, overall and among those exposed to CRT (−7.70 vs. −1.97, p=<0.001 for NHBs and −5.75 vs. −1.97, p=0.01 for Hispanics). For mental health, the survivor-sibling difference in mean score was greater in magnitude for Hispanics (−3.87 vs. −0.69, p=0.03) and NHBs (−5.78 vs. −0.69, p=0.001) when compared to NHWs overall and among those exposed to CRT for NHBs only (−6.26 vs. −0.84, p=0.003). Among survivors exposed to CRT, survivor-sibling differences in mean score were greater in magnitude for NHBs compared to NHWs for physical function (−7.58 vs −3.69, p=0.02) and pain (−2.33 vs. 0.99, p=0.03). Additional differences were observed on the role physical and role emotional subscales as well as the mental and physical component summaries.
Figure 3.
Health-related quality of life outcomes. Magnitude of the survivor-sibling difference in mean score for Hispanic and non-Hispanic black (NHB) compared to survivor-sibling difference for non-Hispanic white (NHW). CRT=cranial radiation therapy
Finally, current SES was added to the models to assess whether it mediated observed disparities in emotional and HRQOL outcomes (Supplemental Table 2). This addition abrogated previously observed disparities in the magnitude of survivor-sibling differences for pain in NHBs and for mental health in Hispanics. However, the observed disparities in the magnitude of survivor-sibling differences between minority groups and NHWs remained significant, with little attenuation, for depression among Hispanics and most HRQOL subscales including social function for Hispanics and NHBs, role emotional for Hispanics, and mental health, role physical and physical function for NHBs.
DISCUSSION
In this study, we did not identify consistent differences in neurocognitive outcomes by race or ethnicity. However, for depression and many domains of HRQOL outcomes, the magnitude of the differences between minority survivors and siblings was significantly larger than that of NHW survivors and siblings. Further, although differences in current socioeconomic indicators including household income, insurance status and education were observed between groups, they did not account for these disparities. Taken together, these findings suggest unmeasured environmental and sociocultural factors, potentially including chronic stressors or ability to reintegrate into their community following cancer treatment, differentially impact minority survivors during and after therapy for childhood cancer and may place them at risk for poor emotional and quality of life outcomes.
Survivor-sibling differences in HRQOL were greater for minority survivors than NHWs across multiple domains. Both Hispanics and NHBs had significantly larger gaps between survivors and siblings for mental health scores across a variety of domains compared to NHWs. NHBs also reported disparities in physical well-being compared to NHWs. Multiple studies in survivors of adult-onset cancers, primarily breast cancer, have previously reported that black survivors report worse physical and functional well-being after cancer therapy than white survivors,28,29 even after adjustment for socioeconomic and demographic factors.30 To our knowledge, ours is the first study to report this disparity also exists in adult survivors of childhood cancers. Additionally, Hispanic survivors of breast cancer have been found to have no difference in physical function scores compared to NHWs,30 but have reported more distress and poorer social and mental HRQOL compared to NHW survivors.28 In a large meta-analysis of psychosocial outcomes in survivors of adult-onset cancers, distress, depression and social and mental HRQOL were found to be worse in Hispanics compared to NHWs and were largely unchanged after adjustment for socioeconomic status.31 These reported disparities in HRQOL of minority survivors of adult-onset cancers are generally consistent with our findings in minority survivors of childhood cancers.
With regard to emotional distress, the magnitude of the survivor-sibling difference for depression was larger for Hispanics compared to NHWs. However, all survivor and sibling groups, including Hispanics, reported mean scores for depression and the GSI that were at or below the expected population norm of 50. This is concordant with prior reports from the CCSS observing that although survivors report more emotional distress than siblings, both groups score lower than community norms, indicating less symptoms of emotional distress than the general population.7,9 Consistent with our findings in survivors, a recent study in pediatric cancer patients found no difference in reported symptoms of depression or anxiety in black patients or their caregivers compared to white.32 In the overall US population, both Hispanics and NHBs reported higher rates of depressive symptoms than NHWs; however, after accounting for poverty, population rates of depression did not differ significantly by race or ethnicity.33
Fortunately, there were no overall disparities in neurocognitive outcomes among minority survivors compared to NHW survivors; however, the magnitude of reported survivor-sibling differences in task efficiency for NHBs compared to NHWs was significant. In assessing the implications of this finding, we must observe that the absolute mean score for task efficiency comparing NHB and NHW survivors did not differ substantially in magnitude overall (53.76 NHB vs 54.45 NHW) or among survivors not-exposed to CRT (52.73 NHB vs 52.60 NHW). Therefore this finding would not be expected to represent a clinically significant difference in attention or processing speed. However, the sibling score for task efficiency in NHB (46.77) was substantially below the anticipated standardized sibling mean of 50, and below that of NHW and Hispanic siblings, suggesting that NHB siblings report above average function in task efficiency. This finding should be confirmed in future studies before any further conclusions can be drawn.
Prior studies evaluating race and health outcomes in the general population and adult-onset cancer patients have observed greater levels of psychological distress in participants reporting race-related stress, specifically everyday discrimination, which is reported more frequently in blacks than whites.34,35 Additional general stressors such as financial stress or stress from life events negatively affect health outcomes in the general population where, although blacks report these stressors more often, it appears that whites were more adversely impacted by them.35 Among survivors of childhood cancer, financial hardship has been observed to negatively impact HRQOL.36 The most significant predictors of financial hardship included lower household income and lower educational attainment, both social determinants that are disproportionately experienced by minority survivors compared to NHWs. While both NHBs and Hispanics may experience more race or ethnicity related stressors, all survivors and many siblings experience general stress related to their cancer experience and many go on to experience financial stress or chronic stress related to comorbidities. Thus, the specific cause of the differences in HRQOL for minorities compared to NHWs is unclear and further study into the effects of stressors on psychological outcomes of childhood cancer survivors is needed.
To our knowledge, this is the largest evaluation of psychological outcomes of minority survivors of childhood cancer to date, including detailed history of cancer therapy and a comparison population of siblings. The utilization of siblings to determine the “gap” between survivors and their same race/ethnicity siblings allowed us to account for differences in environment between racial/ethnic groups that may have been present prior to diagnosis, throughout therapy and into survivorship that were not otherwise captured in the available dataset. However, we did not have access to any direct measures of socioeconomic status at the time of diagnosis or surrogate measures, such as parental education. Additional limitations to consider when interpreting findings include that all neurocognitive and emotional outcomes were self-reported, and the number of minority siblings available for the comparison groups is still relatively small, which may have led to inadequate power to identify differences in neurocognitive outcomes. We must consider the possibility of participation bias leading to decreased generalizability of our findings to all childhood cancer survivors. We have previously reported baseline participation rates for survivors diagnosed 1987-1999 by race/ethnicity including 67% among Hispanic, 57% among NHB and 70% among NHW survivors and observed superior participation rates in the CCSS compared to other survey-based cancer survivor studies.17 Further, although rates of follow-up completion among non-deceased participants differed across racial/ethnic groups (NHW=69%, NHB=45%, Hispanic=50%), attrition does not appear to differ, overall, by socioeconomic indicators. (Supplemental Tables 3 and 4) For these reasons, our research is important to both draw attention to the disparities identified in emotional and HRQOL outcomes of childhood cancer survivors from racial and ethnic minorities and also motivate innovative strategies for comprehensive investigation of race/ethnicity specific outcomes and associated risk factors in future research.
In conclusion, although differences in long-term neurocognitive outcomes by race or ethnicity were not identified, disparities in health-related quality of life outcomes years after cancer therapy appear widespread in minority survivors. This may suggest that, the stressors a survivor experiences, their ability to cope, access to social support networks, and the expectations they or their community have for employment, financial independence, physical function or emotional adjustment after cancer therapy differ between racial and ethnic groups, leading to the reported health-related quality of life disparities. Clinicians should be aware that minority survivors of childhood cancer appear to represent a high-risk population for poor HRQOL outcomes and the need for annual, long-term follow-up including a comprehensive psychological assessment, which is recommended for all survivors,37 should be emphasized among this group. Further research to validate these results and evaluate the role of differential stressors, supports and expectations between minority childhood cancer survivors and NHWs is needed to better understand these findings and develop intervention strategies to close the identified gaps in minority survivor quality of life.
Supplementary Material
Acknowledgments
Funding: Supported by the National Cancer Institute grant CA55727 (PI: Gregory Armstrong.). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant CA21765 and the American Lebanese-Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest Disclosures: All authors declare no conflict of interest in relation to the work described.
REFERENCES
- 1.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19(12):1590–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010; 102(12):881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2396–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2008;17(2):435–446. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;12(11):1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zebrack BJ, Zevon MA, Turk N, et al. Psychological distress in long-term survivors of solid tumors diagnosed in childhood: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2007;49(1):47–51. [DOI] [PubMed] [Google Scholar]
- 10.Michel G, Rebholz CE, von der Weid NX, Bergstraesser E, Kuehni CE. Psychological distress in adult survivors of childhood cancer: the Swiss Childhood Cancer Survivor study. J Clin Oncol. 2010;28(10):1740–1748. [DOI] [PubMed] [Google Scholar]
- 11.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31(35):4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colby SO, Ortman JM. Projections of the size and composition of the U.S. Population: 2014 to 2060. Washington D.C.: U.S. Census Bureau;2015. [Google Scholar]
- 13.Mishel L, Bivens J, Gould E, Shierholz H. The State of Working America, 12th Edition. An Economic Policy Institute Book. Ithaca, N.Y.: Cornell University Press;2012. [Google Scholar]
- 14.Barnett JC, Berchick ER. Health Insurance Coverage in the United States: 2016. Washington, DC: U.S. Government Printing Office;2017. [Google Scholar]
- 15.Aud S, Fox M, KewalRamani A(2010). Status and Trends in the Education of Racial and Ethnic Groups(NCES 2010-015) U.S. Department of Education, National Center for Education Statistics. Washington, DC: U.S. Government Printing Office. [Google Scholar]
- 16.Bhatia S Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56(6):994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatia S, Gibson TM, Ness KK, et al. Childhood cancer survivorship research in minority populations: A position paper from the Childhood Cancer Survivor Study. Cancer. 2016;122(15):2426–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q, Leisenring WM, Ness KK, et al. Racial/ethnic differences in adverse outcomes among childhood cancer survivors: the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(14):1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. [DOI] [PubMed] [Google Scholar]
- 20.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenzik KM, Huang IC, Brinkman TM, et al. The Childhood Cancer Survivor Study-Neurocognitive Questionnaire (CCSS-NCQ) revised: item response analysis and concurrent validity. Neuropsychology. 2015;29(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derogatis L. Brief Symptom Inventory (BSI) 18: Administration, scoring, and procedures manual. Minneapolis, MN: NCS Pearson, Inc.; 2000. [Google Scholar]
- 23.Recklitis CJ, Parsons SK, Shih MC, Mertens A, Robison LL, Zeltzer L. Factor structure of the brief symptom inventory--18 in adult survivors of childhood cancer: results from the childhood cancer survivor study. Psychol Assess. 2006;18(1):22–32. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 25.Reulen RC, Winter DL, Lancashire ER, et al. Health-status of adult survivors of childhood cancer: a large-scale population-based study from the British Childhood Cancer Survivor Study. Int J Cancer. 2007;121(3):633–640. [DOI] [PubMed] [Google Scholar]
- 26.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1Pt2):141–157. [DOI] [PubMed] [Google Scholar]
- 27.Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events Version 4.03. National Cancer Institute, Bethesda, MD. [Google Scholar]
- 28.Blinder VS, Griggs JJ. Health disparities and the cancer survivor. Semin Oncol. 2013;40(6):796–803. [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro LC, Samuel CA, Reeder-Hayes KE, Wheeler SB, Olshan AF, Reeve BB. Understanding racial differences in health-related quality of life in a population-based cohort of breast cancer survivors. Breast Cancer Res Treat. 2016;159(3):535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luckett T, Goldstein D, Butow PN, et al. Psychological morbidity and quality of life of ethnic minority patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2011;12(13):1240–1248. [DOI] [PubMed] [Google Scholar]
- 32.Ramsey LH, Graves PE, Howard Sharp KM, Seals SR, Collier AB, Karlson CW. Impact of race and socioeconomic status on psychological outcomes in childhood cancer patients and caregivers. [published online ahead of print (January 8, 2019)]. J Pediatr Hematol Oncol. [DOI] [PubMed] [Google Scholar]
- 33.Pratt LA, Brody DJ. Depression in the U.S. household population, 2009-2012. NCHS data brief, no172. Hyattsville, MD: National Center for Health Statistics;2014. [PubMed] [Google Scholar]
- 34.Merluzzi TV, Philip EJ, Zhang Z, Sullivan C. Perceived discrimination, coping, and quality of life for African-American and Caucasian persons with cancer. Cultur Divers Ethnic Minor Psychol. 2015;21(3):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335–351. [DOI] [PubMed] [Google Scholar]
- 36.Huang IC, Bhakta N, Brinkman TM, et al. Determinants and consequences of financial hardship among adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. J Natl Cancer Inst. 2019;111(2):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 5.0. Monrovia, CA: Children’s Oncology Group; October 2018; Available on-line:www.survivorshipguidelines.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.