Abstract
Humans have bred different lineages of domestic dogs for different tasks such as hunting, herding, guarding, or companionship. These behavioral differences must be the result of underlying neural differences, but surprisingly, this topic has gone largely unexplored. The current study examined whether and how selective breeding by humans has altered the gross organization of the brain in dogs. We assessed regional volumetric variation in MRI studies of 62 male and female dogs of 33 breeds. Neuroanatomical variation is plainly visible across breeds. This variation is distributed nonrandomly across the brain. A whole-brain, data-driven independent components analysis established that specific regional subnetworks covary significantly with each other. Variation in these networks is not simply the result of variation in total brain size, total body size, or skull shape. Furthermore, the anatomy of these networks correlates significantly with different behavioral specialization(s) such as sight hunting, scent hunting, guarding, and companionship. Importantly, a phylogenetic analysis revealed that most change has occurred in the terminal branches of the dog phylogenetic tree, indicating strong, recent selection in individual breeds. Together, these results establish that brain anatomy varies significantly in dogs, likely due to human-applied selection for behavior.
SIGNIFICANCE STATEMENT Dog breeds are known to vary in cognition, temperament, and behavior, but the neural origins of this variation are unknown. In an MRI-based analysis, we found that brain anatomy covaries significantly with behavioral specializations such as sight hunting, scent hunting, guarding, and companionship. Neuroanatomical variation is not simply driven by brain size, body size, or skull shape, and is focused in specific networks of regions. Nearly all of the identified variation occurs in the terminal branches of the dog phylogenetic tree, indicating strong, recent selection in individual breeds. These results indicate that through selective breeding, humans have significantly altered the brains of different lineages of domestic dogs in different ways.
Keywords: canines, dogs, evolution, morphology, MRI, selective breeding
Introduction
A major goal of modern neuroscience is to understand how variation in behavior, cognition, and emotion relates to underlying neural mechanisms. A massive “natural experiment” in this arena has been right under our noses: domestic dogs. Humans have selectively bred dogs for different, specialized abilities—herding or protecting livestock, hunting by sight or smell, guarding property, or providing companionship. Significant breed differences in temperament, trainability, and social behavior are readily appreciable by the casual observer, and have also been documented quantitatively (Serpell and Hsu, 2005; Tonoike et al., 2015). Furthermore, recent genetic research indicates that this behavioral variation is highly heritable (MacLean et al., 2019).
This panoply of behavioral specializations must rely on underlying neural specializations. A small number of studies have investigated neural variation in dogs, including, for example, the effects of skull shape on brain morphology (Carreira and Ferreira, 2015; Pilegaard et al., 2017) and anatomical correlates of aggression (Jacobs et al., 2007; Våge et al., 2010). However, the neural underpinnings of behavioral differences between breeds remain largely unknown.
Most modern dog breeds were developed in an intentional, goal-driven manner relatively recently in evolutionary time; estimates for the origins of the various modern breeds vary between the past few thousand to the past few hundred years (Larson et al., 2012). This strong selection pressure suggests that brain differences between breeds may be closely tied to behavior. However, selection also occurred for outward physical appearance, including craniofacial morphology. This may have placed constraints on the internal dimensions of the skull, which in turn may have had secondary effects on brain morphology. There is substantial diversification of skull shape across dog breeds, and this has been linked to behavioral differences (Drake and Klingenberg, 2010; McGreevy et al., 2013). Alternatively, neuroanatomical variation may be explained primarily by body size rather than breed membership, with different breeds' brains representing minor, random, scaled-up or scaled-down variants of a basic species-wide pattern.
Any attempt to determine whether breeding for behavior has altered dog brains would have to be able to differentiate between these competing (and potentially interacting) hypotheses. A simple comparison of regional volumes would be insufficient for several reasons. First, a significant difference in the volume of, for example, the amygdala in pit bulls versus golden retrievers might seem intuitively meaningful, but to ascertain whether such a difference was truly the result of selection pressure on behavior, the phylogenetic structure of the dog family tree needs to be taken in to account to partition variance attributable to inheritance, and equal statistical priority needs to be given to the alternative hypotheses that observed variation in morphology. Second, and perhaps most importantly, a priori comparisons of regional gray matter volumes presuppose that experimenters can identify meaningful borders between regions. For highly conserved structures with clear anatomical boundaries, like the amygdala, this task is surmountable, but very little is known about the organization of higher-order cortical regions in dogs, and some complex behaviors that are the focus of selective breeding, like herding or interspecies communication, almost certainly rely on some of these areas. For this reason, even creating the regional outlines for a simple ROI analysis would be problematic.
Therefore, the current study took a totally data-driven, whole-brain, agnostic approach to assessing morphological variation across dog brains. Our goal was to determine whether significant nonrandom variation in brain anatomy exists across dogs and, if so, to differentiate between the competing and possibly interacting explanations for this variation.
Materials and Methods
Subjects
The dataset included T2-weighted MRI scans from 62 purebred dogs of 33 different breeds. These were grouped into 10 different breed groups as defined by American Kennel Club (AKC), which ostensibly represent groupings that were developed for similar behavioral specializations, such as herding or hunting. Table 1 lists the breed, breed group, and other data for all dogs included in the study.
Table 1.
Data for all dogs used in the study
| ID | Breed | Sex | Age (years) | Body mass (kg) | Cephalic index (from database) | Neuro-cephalic index | Brain volume (mm3) | Ostensible behavioral specialization/purpose |
|---|---|---|---|---|---|---|---|---|
| 1 | Basset hound | Male | 4.0 | 28.1 | 0.74 | 51.89 | 100070.10 | Scent hunting |
| 2 | Beagle | Male | 14.3 | 17.0 | 0.74 | 61.82 | 82750.29 | Scent hunting |
| 3 | Beagle | Male | 4.0 | 11.7 | 0.76 | 61.82 | 64887.65 | Scent hunting |
| 4 | Beagle | Male | ND | 28.5 | 0.85 | 61.82 | 23259.63 | Scent hunting |
| 5 | Beagle | Male | 4.0 | 8.3 | 0.82 | 61.82 | 66733.96 | Scent hunting |
| 6 | Beagle | Male | 1.7 | 28.5 | 0.78 | 61.82 | 65738.93 | Scent hunting |
| 7 | Bichon frise | Male | 9.0 | 9.3 | 0.80 | 61.51 | 61849.71 | Explicit companionship |
| 8 | Border collie | Male | 6.1 | 28.2 | 0.65 | 54.38 | 83215.10 | Herding |
| 9 | Border collie | Male | 5.6 | 20.6 | 0.65 | 54.38 | 81668.60 | Herding |
| 10 | Boston terrier | Male | 11.9 | 12.5 | 0.90 | 92.62 | 66301.82 | Explicit companionship |
| Vermin control | ||||||||
| Sport fighting | ||||||||
| 11 | Boston terrier | Male | 5.8 | 8.9 | 0.90 | 92.62 | 76426.61 | Explicit companionship |
| Vermin control | ||||||||
| Sport fighting | ||||||||
| 12 | Boxer | Male | 8.1 | 31.8 | 0.68 | 67.19 | 81555.33 | Guarding/protecting/sentinel work |
| Police/military work, war | ||||||||
| Sport fighting | ||||||||
| 13 | Boxer | Male | 5.0 | 34.2 | 0.67 | 67.19 | 80814.97 | Guarding/protecting/sentinel work |
| Police/military work, war | ||||||||
| Sport fighting | ||||||||
| 14 | Boxer | Female | 10.7 | 31.8 | 0.83 | 66.28 | 93337.26 | Guarding/protecting/sentinel work |
| Police/military work, war | ||||||||
| Sport fighting | ||||||||
| 15 | Boxer | Male | 9.3 | 40.8 | 0.70 | 67.19 | 82323.66 | Guarding/protecting/sentinel work |
| Police/military work, war | ||||||||
| Sport fighting | ||||||||
| 16 | Bulldog | Male | 1.0 | 16.8 | 0.74 | 90.18 | 63154.13 | Explicit companionship |
| Sport fighting | ||||||||
| 17 | Bulldog | Male | 4.4 | 30.0 | 0.77 | 90.18 | 80128.00 | Explicit companionship |
| Sport fighting | ||||||||
| 18 | Cavalier King Charles spaniel | Female | 0.5 | 3.2 | 0.81 | 76.77 | 55777.97 | Explicit companionship |
| 19 | Cavalier King Charles spaniel | Female | 0.5 | 14.5 | 0.92 | 76.77 | 64695.16 | Explicit companionship |
| 20 | Cocker spaniel | Female | 6.4 | 18.1 | 0.75 | 61.01 | 66708.41 | Bird retrieval |
| 21 | Dachshund | Female | 11.3 | 4.9 | 0.79 | 51.76 | 44076.29 | Vermin control |
| Scent hunting | ||||||||
| 22 | Dachshund | Female | 6.6 | 6.4 | 0.77 | 51.76 | 60492.56 | Vermin control |
| Scent hunting | ||||||||
| 23 | Dachshund | Male | 7.8 | 5.6 | 0.81 | 49.59 | 57168.79 | Vermin control |
| Scent hunting | ||||||||
| 24 | Dachshund | Female | 1.8 | 5.3 | 0.81 | 51.76 | 49716.87 | Vermin control |
| Scent hunting | ||||||||
| 25 | Doberman pinscher | Female | 4.7 | 29.8 | 0.62 | 46.96 | 80287.44 | Guarding/protecting/sentinel work |
| Police/military work, war | ||||||||
| 26 | English Pointer | Male | 7.3 | 27.3 | 0.74 | ND | 91448.24 | Bird retrieval |
| 27 | German short-haired pointer | Female | 6.2 | 27.0 | 0.73 | 48.30 | 75612.46 | Bird retrieval |
| 28 | Golden retriever | Male | 10.0 | 39.8 | 0.69 | 56.52 | 96010.49 | Bird retrieval |
| 29 | Golden retriever | Male | 6.0 | 42.2 | 0.70 | 56.52 | 96941.92 | Bird retrieval |
| 30 | Golden retriever | Male | 11.0 | 34.9 | 0.68 | 56.52 | 86438.69 | Bird retrieval |
| 31 | Greyhound | Female | 7.5 | 36.7 | 0.65 | 45.83 | 97610.47 | Sight hunting |
| 32 | Greyhound | Male | 3.8 | 37.1 | 0.65 | 46.84 | 97774.89 | Sight hunting |
| 33 | Greyhound | Female | 2.2 | 36.0 | 0.66 | 45.83 | 101969.38 | Sight hunting |
| 34 | Jack Russell terrier | Male | ND | 14.0 | 0.80 | 59.28 | 70125.35 | Vermin control |
| 35 | Keeshond | Male | 7.2 | 21.6 | 0.71 | 60.18 | 68766.94 | Explicit companionship |
| Guarding/protecting/sentinel work | ||||||||
| 36 | Labrador retriever | Male | 9.7 | 32.6 | 0.65 | 55.82 | 94762.33 | Bird retrieval |
| 37 | Labrador retriever | Female | 5.0 | 30.5 | 0.66 | 56.11 | 84161.70 | Bird retrieval |
| 38 | Lhasa apso | Female | 10.7 | 13.2 | 0.93 | ND | 58177.18 | Guarding/protecting/sentinel work |
| 39 | Lhasa apso | Female | 4.0 | 7.6 | 0.86 | ND | 58152.92 | Guarding/protecting/sentinel work |
| 40 | Maltese | Male | 6.6 | 6.0 | 0.81 | 65.29 | 46642.03 | Explicit companionship |
| 41 | Maltese | Male | 10.0 | 3.0 | 0.84 | 65.29 | 35280.20 | Explicit companionship |
| 42 | Maltese | Male | 5.5 | 6.6 | 0.77 | 65.29 | 46629.97 | Explicit companionship |
| 43 | Maltese | Male | 6.0 | 8.9 | 0.88 | 65.29 | 47610.27 | Explicit companionship |
| 44 | Maltese | Female | 6.0 | 2.0 | 0.92 | 68.83 | 28052.45 | Explicit companionship |
| 45 | Maltese | Female | 4.9 | 3.4 | 0.85 | 68.83 | 46330.73 | Explicit companionship |
| 46 | Miniature schnauzer | Male | 9.4 | 12.8 | 0.77 | 51.79 | 62053.63 | Vermin control |
| 47 | Miniature schnauzer | Female | 6.3 | 5.0 | 0.80 | 54.99 | 53517.22 | Vermin control |
| 48 | Old English sheepdog | Male | 3.7 | 33.1 | 0.69 | 54.39 | 80709.26 | Herding |
| 49 | Pit bull | Male | 2.1 | 27.1 | 0.72 | 69.96 | 80571.31 | Sport fighting |
| 50 | Siberian husky | Female | 3.0 | 18.1 | 0.67 | 55.17 | 62094.04 | Running/racing |
| 51 | Silky terrier | Male | 3.0 | 4.4 | 0.84 | 58.23 | 46832.08 | Vermin control |
| 52 | Springer spaniel | Female | 1.1 | 18.4 | 0.75 | 49.34 | 72442.26 | Bird retrieval |
| 53 | Standard poodle | Female | 7.9 | 22.6 | 0.73 | ND | 80235.75 | Bird retrieval |
| 54 | Weimaraner | Male | 3.3 | 48.4 | 0.66 | 49.05 | 110812.36 | Sight hunting |
| 55 | Welsh corgi | Male | 5.6 | 15.1 | 0.72 | 63.09 | 83234.19 | Herding |
| 56 | West highland white terrier | Male | 5.9 | 11.0 | 0.78 | 60.84 | 72254.08 | Vermin control |
| 57 | Wheaton terrier | Male | 7.0 | 19.2 | 0.71 | ND | 70234.47 | Guarding/protecting/sentinel work |
| Herding | ||||||||
| Vermin control | ||||||||
| Bird retrieval | ||||||||
| 58 | Whippet | Female | 15.5 | 13.6 | 0.72 | 50.60 | 71357.64 | Sight hunting |
| 59 | Yorkshire terrier | Female | 3.8 | 3.9 | 0.82 | ND | 45103.02 | Explicit companionship |
| Vermin control | ||||||||
| 60 | Yorkshire terrier | Male | 13.0 | 4.2 | 0.81 | ND | 45217.54 | Explicit companionship |
| Vermin control | ||||||||
| 61 | Yorkshire terrier | Male | 0.8 | 3.5 | 0.79 | ND | 38163.05 | Explicit companionship |
| Vermin control | ||||||||
| 62 | Yorkshire terrier | Male | 11.5 | 3.2 | 0.82 | ND | 51760.84 | Explicit companionship |
| Vermin control |
Dogs from mixed/unknown breeds were excluded from analyses that used breed group as an independent variable. Cephalic indices are sex- and breed-specific averages from a large public database (Stone et al., 2016).
ND, No data.
Image acquisition and preprocessing
T2-weighted MRI images were acquired on a 3.0 T GE Healthcare HDx MRI unit with a GE Healthcare 5147137–2 3.0T HD T/R Quad Extremity Coil. Images were opportunistically collected at the Veterinary Teaching Hospital at the University of Georgia at Athens from dogs that were referred for neurological examination but were not found to have any neuroanatomical abnormalities. All scans were re-reviewed by a board-certified veterinary neurologist before inclusion.
The preprocessing pipeline was implemented using the NiPype workflow engine (Gorgolewski et al., 2011). Both transverse-acquired and sagittally acquired images were available for each dog. Transverse-acquired images ranged from 0.234 mm2 in-plane resolution and 2.699 mm slice distance to 0.352 mm2 in-plane resolution and 3.499 mm slice distance. Sagittally acquired images ranged from 0.273 mm2 in-plane resolution and 3.200 mm slice distance to 0.430 mm2 in-plane resolution and 3.200 mm slice distance. To maximize the use of all available anatomical information, the transverse and sagittal images were combined as follows. First, we manually performed skull-stripping on the transverse image. Next, we determined the smallest ROI that completely covered the brain from the brain mask image. The transverse image and transverse brain mask were then cropped using the computed ROI coordinates. Then, the transverse images were resampled to produce isotropic voxels in all three dimensions, the sagittal image was resliced so that it was in the same orientation as the transverse images, and a rigid registration was computed from the sagittally acquired image to the original transverse image. The region containing the brain was then cropped in the sagittal image, and we then registered the smaller cropped sagittal image to the isotropically resampled transverse brain image using a rigid registration. Finally, the cropped transverse and sagittal images were then rescaled so that the robust mean intensity of both images was 100, the images were averaged together, and then the brain mask applied to this combined image. A general diagram illustrating the overall processing pipeline is included in Fig. 1-1 and a detailed NiPype registration workflow is included in Fig. 1-2. The accompanying registration code is available at https://gist.github.com/dgutman/a0e05028fab9c6509a997f703a1c7413.
Template creation
We produced a study-specific template representing the average brain morphology across the entire group, equally unbiased toward any particular image. This was accomplished using the buildtemplateparallel.sh script in the ANTS software package (Avants et al., 2009), which nonlinearly registers each image into a common spatial framework.
Experimental design and statistical analyses
Morphological analyses.
During nonlinear registration, a warp-field is produced that represents the mapping from the original image to the target image. The Jacobian of the warp-field represents the degree of warping that had to occur in each original image to bring it into alignment with the target image. To localize significant variation in gray matter morphology, we applied a one-sample t test on the demeaned log Jacobian determinant images. This was accomplished using FSL's randomize, a tool for Monte Carlo permutation testing on general linear models (Winkler et al., 2014). This analysis permutes the sign of the log Jacobian and tests the null hypothesis that variation from the mean is random and therefore symmetrically distributed and centered around zero. The resultant t-statistic image was thresholded at p < 0.05, after multiple-comparisons correction was performed using threshold-free cluster enhancement (Smith and Nichols, 2009).
To calculate neurocephalic index, we identified maximally distant points on the left–right, rostral–caudal, and dorsal–ventral axes; neurocephalic index was computed the ratio of brain width to brain length × 100.
Cephalic index is defined as the ratio of skull width to skull length × 100. For many scans in our database, the exterior of the skull was not visible, but a large database of skull measurements is publicly available (Stone et al., 2016). We computed male and female average cephalic indices separately for each breed and used these sex-specific, breed-average measures in our analyses.
To identify regional covariation in gray matter morphology, we used GIFT, a software package for MATLAB (Calhoun et al., 2001). GIFT's toolbox for source-based morphometry (SBM) (Xu et al., 2009) is a multivariate alternative to voxel-based morphometry (VBM). It uses independent components analysis to identify spatially distinct, distributed networks of regions that covary across individuals, and computes their statistical relationship to other categorical or continuous variables. T2-weighted images underwent bias field correction using ANTS's Atropos N4 tool (Avants et al., 2011) and segmentation into gray matter, white matter, and CSF using FSL's FAST tool (Zhang et al., 2001). Gray matter segmentations were warped to the study-specific template and modulated by their log Jacobian determinants to produce per-subject maps of the degree of morphological divergence from the study-specific group-average template. In other words, the input to SBM consisted of gray matter maps for each subject, where intensity at each voxel corresponded to the degree of deformation required to come into alignment with the template (i.e., the demeaned log Jacobians). The number of sources was estimated using Akaike's information criterion (AIC) (Akaike, 1974); the application of AIC in SBM is described in Xu et al. (2009). This procedure identified six components, each of which were thresholded at Z scores >1.96 or below −1.96. Multiple regression and ANOVA analyses were then used to compute the relationship of each component to AKC-defined breed groups, with the statistical threshold set at p < 0.05 after multiple comparisons correction.
Phylogenetic statistics.
Because comparative data may be nonindependent due to shared phylogenetic history, the assumptions of standard statistical methods may be violated (Harvey and Pagel, 1991). We therefore used phylogenetic comparative methods that account for phylogenetic nonindependence by including expected phylogenetic variance-covariance among species into the error term of generalized least-squares [phylogenetic generalized least squares (pGLS)] linear models (Rohlf, 2001). When quantifying linear models we additionally included a lambda parameter to account for phylogenetic signal (Pagel, 1997). To test for differences in statistical fit among linear models that include different parameters (e.g., the inclusion of grouping variables to test for differences among breed groups), we used least-squares phylogenetic analysis of covariance (pANCOVA) (Smaers and Rohlf, 2016; Smaers and Mongle, 2018). It should be noted that “phylogenetic” approaches such as pGLS and pANCOVA are interpreted in the same way as standard least-squares approaches. The only difference between standard and phylogenetic least-squares approaches is that the phylogenetic approaches weight data points according to phylogenetic relatedness (Rohlf, 2001).
We further investigated the relationship between morphological components and the phylogenetic tree by estimating the amount of change that occurs on each lineage using a multiple variance Brownian motion approach (Smaers et al., 2016; Smaers and Mongle, 2018). This approach estimates phenotypic change along individual lineages of a tree and has been shown to provide more accurate estimates than traditional ancestral estimation methods (Smaers and Mongle, 2017).
Last, we use multiregime Ornstein-Uhlenbeck (OU) approaches to estimate phylogenetic shifts in mean value directly from the data. This approach has become a standard approach in comparative biology to model trait change across a phylogeny. Specifically, this approach quantifies the evolution of a continuous trait X as dX(t) = α[θ − X(t)]dt + σdB(t) where σ captures the stochastic evolution of Brownian motion, α determines the rate of adaptive evolution toward an optimum trait value θ (90). This standard OU model has been modified into multiple-regime OU models allowing optima to vary across the phylogeny (Butler and King, 2004). Such multiregime OU models allow modeling trait evolution toward different “regimes” that each display a different mean trait value. In other words, these approaches allow estimating directly from the data where in a phylogeny a shift in mean value of a trait has occurred. To overcome inherent difficulties with optimizing OU parameters (Ho and Ané, 2014), several algorithmic improvements have been proposed. Here, we use the approach proposed by Khabbazian et al. (2016).
Results
Neuromorphological variation is plainly visible across breeds. Midline sagittal images from the raw, native-space scans of selected dogs are shown in Figure 1A. To provide a common spatial reference for measuring this variation, we created an unbiased, diffeomorphic template using the ANTS software package (Avants et al., 2009). This template represents the average brain for the entire dataset and is shown in Figure 1B.
Figure 1.
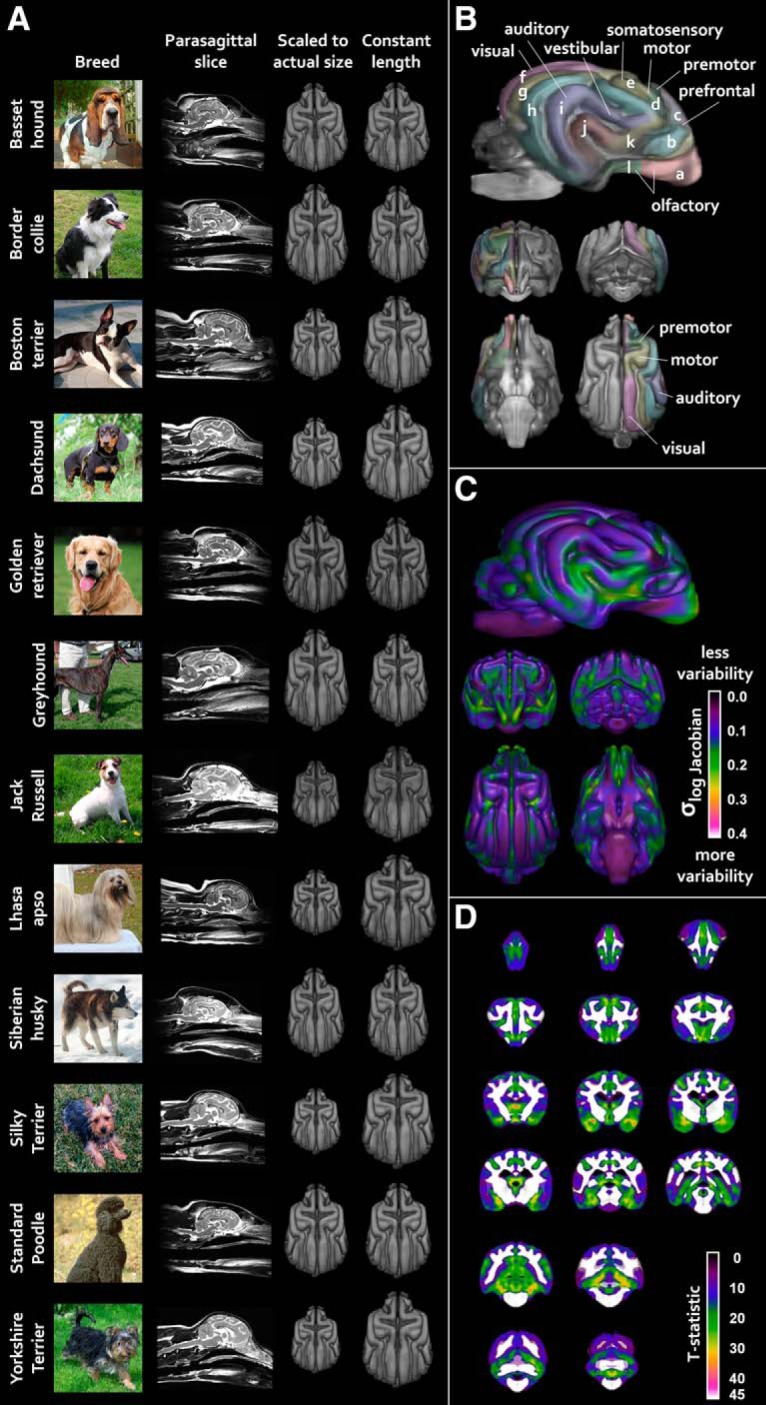
Neuroanatomical variation in domestic dogs. A, MRI images and 3D reconstructions of warped template from 10 selected dogs of different breeds. Images are public-domain photos from Wikimedia Commons. B, Unbiased group-average template for this dataset. See Figure 1-1, and Figure 1-2 for processing schematics. Neuroanatomical labels (based on Palazzi, 2011; Datta et al., 2012, Evans and de Lahunta, 2013) are as follows: (a) olfactory peduncle; (b) orbital (presylvian) gyrus; (c) proreal gyrus; (d) pre cruciate gyrus; (e) postcruciate gyrus; (f) marginal (lateral) gyrus; (g) ectomarginal gyrus; (h) suprasylvian gyrus; (i) ectosylvian gyrus; (j) sylvian gyrus; (k) insular cortex; and (l) piriform lobe. C, Brain-wide morphological variation, regardless of breed, as indexed by the SD of all dogs' Jacobian determinant images. D, A Monte Carlo permutation test on demeaned gray matter Jacobian determinant images revealed that much of gray matter shows significant deviation from group-mean morphology. Colored regions are all p < 0.05 after multiple-comparisons correction; t-statistic values are illustrated.
Conceptual schematic of neuroimaging analysis. Download Figure 1-1, TIF file (1MB, tif)
NiPype pipeline for merging axial and sagittal images from each dog before registration to the template. Code is available online at https://gist.github.com/dgutman/a0e05028fab9c6509a997f703a1c7413. Download Figure 1-2, TIF file (1.2MB, tif)
To visualize morphological variation in a more standardized manner, we nonlinearly warped the template to each dog's native-space image. This allowed us to examine breed variation in brain morphology and size with invariant contrast and resolution. We also additionally rescaled these images to have constant rostral-caudal lengths. This allowed us to more clearly visualize variation in morphology independent from variation in size. Both sets of scaled template images are shown in Figure 1A.
To carry out quantitative assessments of regional variation in gray matter morphology, we used the Jacobian determinants of the native-space-to-template spatial deformation fields to produce a variation intensity map. These fields represent a map of where and how much each dog's scan had to adjust to become aligned to the group-average template. The SD of these maps thus indexes the extent to which brain anatomy varies across individuals and is shown in Figure 1C.
To determine whether this variation was randomly distributed across the brain or focused in specific areas, we applied Monte Carlo permutation testing on the demeaned Jacobian determinant images. Importantly, this revealed that a large proportion of the brain shows significant gray matter morphological variation across subjects, as illustrated in Figure 1D.
Given these results, we next sought to determine what accounts for this variation by probing the extent to which it is related to body size, head shape, and/or breed group membership.
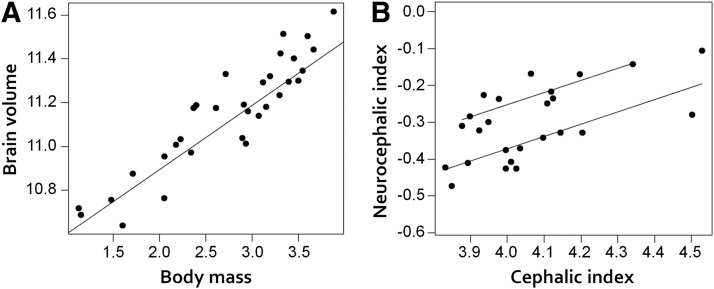
Figure 2A shows the relationship between brain volume and body mass. The scaling coefficient of this relationship [pGLS; b = 0.231, 95% confidence interval (CI) = 0.26–0.36] is significantly lower than that observed across most mammals (∼0.67), indicating the occurrence of more variation in body size relative to variation in brain size than would be expected. Importantly, using the tree structure from a recent large-scale genomic analysis (Parker et al., 2017), we were able to determine that the phylogenetic signal of the brain-body allometry is negative; that is, that variation present at the tree's terminal branches is not predicted by the deeper structure of the tree. If grade shifts in the brain–body allometry exist, then these would putatively show differences among different breeds. We tested this hypothesis by estimating putative grade shifts in the brain to body allometry directly from the data using an OU modeling approach (Khabbazian et al., 2016). This analysis revealed no grade shifts, thereby indicating that a one-grade allometry is the best explanation of the bivariate brain-to-body relationship.
Figure 2.
pGLS analyses on gross brain, body, and skull measurements. A, Brain volume versus body mass. B, Neurocephalic index vesus cephalic index. Plotted points represent breed averages, not individuals.
In mammals, head shape is commonly measured using cephalic index (also known as skull index), calculated as maximum head width divided by maximum head length. We were interested in the possibility that human-driven selection on external craniofacial morphology may have had on the internal dimensions of the skull. To assess this, we computed an analogous neurocephalic index for each dog (maximum internal cranial cavity length divided by maximum internal cranial cavity width). Figure 2B shows the relationship between neurocephalic and cephalic index. Cephalic index is a significant predictor of neurocephalic index (pGLS: b = 0.37, t = 3.70, p < 0.01). Also, here we questioned whether grade shifts in this allometry exist, putatively showing differences among breeds. This analysis revealed that the neurocephalic–cephalic allometry was thus best explained by a two-grade model (F = 31.19, p < 0.001). The breeds on the higher grade, with a greater neurocephalic index for a given cephalic index, were as follows: Basset hound, beagle, German short-haired pointer, dachshund, cavalier King Charles spaniel, springer spaniel, west highland white terrier, silky terrier, bichon frise, and maltese. Importantly, this grade difference in the neurocephalic to cephalic index aligns with a significant difference in body size (pANOVA: F = 9.73, p < 0.01; average body size 11 kg vs 23 kg in other breeds). Smaller-bodied dogs hereby have a higher neurocephalic index (more spherical brains) for a given cephalic index (external head shape).
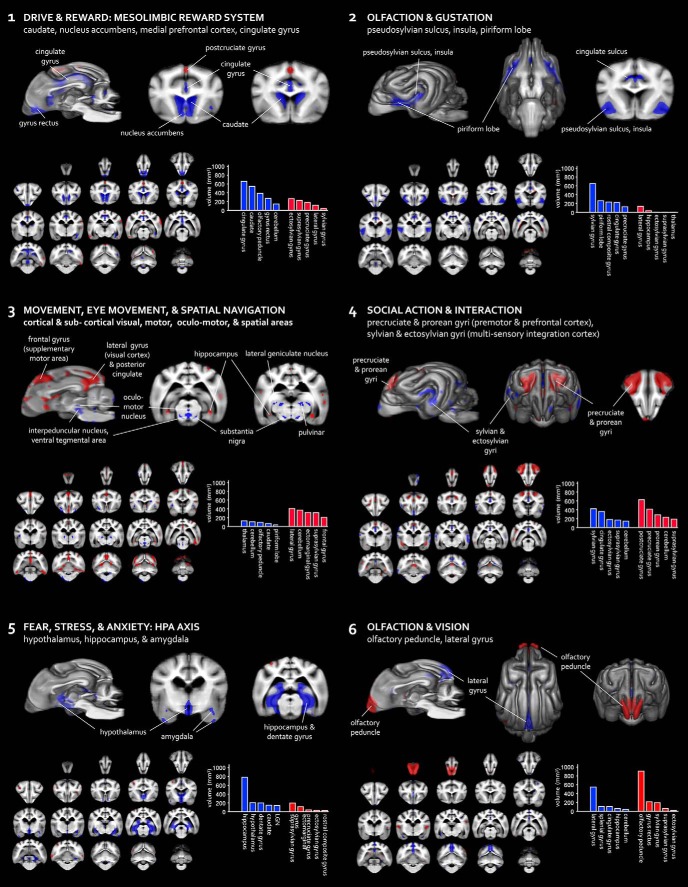
If variation in dog brain anatomy is unrelated to behavior, then variation should be randomly distributed across regions. Alternatively, if this variation represents heritable adaptations for behavior, then significant covariance should exist in separable, independent subnetworks of regions. To assess this, we performed source-based morphometry, a multivariate alternative to voxel-based morphometry which makes use of independent components analysis. This was accomplished using the GIFT software package (Xu et al., 2009). Results revealed six networks where regional volume covaried significantly across individuals. Figure 3 shows these networks, along with factor loadings for each breed group. Major anatomical constituents of each network are labeled. Additional research is needed to definitively link the function of each network to its adaptive role in response to behavior selection. However, we note putative roles that may serve as initial hypotheses for future research.
Figure 3.
Covarying regional networks in dog brain morphology. Independent components analysis revealed six regional networks where morphology covaried significantly across individuals. Red and blue regions are volumetrically anticorrelated: in individuals where red is larger, blue tends to be smaller, and vice versa. Graphs represent volumetric quantification of the top five anatomical constituents of each of the two portions of each component.
Network 1 includes the nucleus accumbens, dorsal and ventral caudate, cingulate gyrus, olfactory peduncle, and gyrus rectus (medial prefrontal cortex). These regions are part of or connected to the mesolimbic reward system, a network implicated in reward signaling related to reinforcement learning, incentive salience, and motivation broadly across species (Alcaro et al., 2007; O'Connell and Hofmann, 2011); in dogs, the caudate nucleus activates for both food reward and human social reward (Cook et al., 2016). Tentatively, this network might be relevant for social bonding to humans, training, and skill learning.
Network 2 involves brain regions involved in olfaction and gustation, including the piriform lobe, which contains olfactory cortex, and the insula and pseudosylvian sylcus, where the cortical representation of taste is located (Evans and de Lahunta, 2013). This component also involves regions of medial frontal cortex, which is involved in downstream or higher-order processing of chemosensation and shows activation in response to olfactory stimulation in awake but not sedated dogs (Jia et al., 2014). We propose that this network might support volitional (as opposed to instinctive) responses to olfactory and gustatory stimuli.
Network 3 includes a distributed network of subcortical regions that are involved movement, eye movement, vision, and spatial navigation, including the lateral geniculate nucleus, pulvinar, hippocampus, cerebellum, oculomotor nucleus, interpeduncular nucleus, ventral tegmental area, and substantia nigra. It also involves cortical regions, including the medial part of the frontal gyrus (supplementary motor area) and the lateral gyrus (visual cortex). Tentatively, this network may reflect a circuit involved in moving through the physical environment.
Network 4 involves higher-order cortical regions that may be involved in social action and interaction. The precruciate and prorean gyri house premotor and prefrontal cortex, respectively, while the gyrus rectus is part of medial prefrontal cortex. The expansion of frontal cortex has been linked to increased sociality in extant hyena species (Holekamp et al., 2007) and, notably, the prorean gyrus has been linked to the emergence of pack structure in canid evolution (Radinsky, 1969). The sylvian, ectosylvian, and suprasylvian gyri represent regions of lateral sensory cortex situated between gustatory, auditory, and somatosensory cortex (Evans and de Lahunta, 2013) and likely contain higher-order association areas related to sensation and perception. In domestic dog fMRI studies, multisensory activation in these regions has been observed during the presentation of dog and human faces and vocalizations (Cuaya et al., 2016; Andics et al., 2017; Thompkins et al., 2018).
Network 5 includes limbic regions that have a well established role in fear, stress, and anxiety, including the hypothalamus, amygdala, and hippocampus and adjacent dentate gyrus (for review, see Tovote et al., 2015). These regions are involved in the HPA axis, which regulates behavioral and endocrine responses to environmental stressors and threats. Some of these regions are also involved in other affective and instinctual processes, including mating, memory, and aggression (O'Connell and Hofmann, 2011).
Network 6 includes early sensory processing regions for olfaction and vision, including the olfactory peduncle and part of the lateral gyrus, which is the location of primary visual cortex (Evans and de Lahunta, 2013).
Next, we investigated the relationship between these components, total brain size, and skull morphology. A significant relationship with total brain volume was present for all but component 6, where it was marginal but did not meet significance (component 1: t = 3.663, p = 0.001; component 2: t = −2.608, p = 0.014; component 3: t = 6.219, p < 0.001; component 4: t = −6.325, p < 0.001; component 5: t = 3.938, p < 0.001; component 6: t = 1.845, p = 0.076). Components 3, 4, and 6 showed significant relationships with cephalic index, whereas component 1 was marginal (component 1: t = −1.945, p = 0.064; component 3: t = −2.165, p = 0.041; component 4: t = 2.411, p = 0.024; component 6: t = −2.171, p = 0.041; pGLS). Components 1, 3, 4, and 6 showed significant relationships with neurocephalic index (component 1: t = −2.258, p = 0.032; component 3: t = −3.823, p = 0.001; component 4: t = 7.066; p < 0.001; component 6: t = −2.890, p = 0.007, pGLS).
We also investigated the relationship between these covarying morphological components and the phylogenetic tree. If variation in brain organization mainly reflects the deep ancestry of the tree, with little relationship to recent behavioral specializations, then brain morphometry should be highly statistically dependent on phylogenetic structure (i.e., high phylogenetic signal). Conversely, if brain organization is strongly tied to selective breeding for behavioral traits, then morphological traits should be divorced from the structure of the tree (i.e., low phylogenetic signal). We observed the latter (Fig. 4). The majority of changes that occur in these components take place on the terminal branches of the phylogenetic tree.
Figure 4.
Relationship between morphologically covarying regional brain networks and phylogenetic tree. Circles indicate factor loading. (Phylogenetic tree is from Parker et al., 2017.)
Finally, we investigated whether these regionally covarying morphological networks were related to behavior. The AKC groups individual breeds into breed groups, but these breed groups change periodically and some groups contain breeds with disparate behavioral functions: for example, the nonsporting group includes both poodles and Shar-Peis. Therefore, rather using AKC breed groups, we identified each individual breed's ostensible behavioral specialization(s) as noted on the AKC website (www.akc.org). These were entered into in a multiple regression analysis using the GIFT Source Based Morphometry toolbox. Each of the six components showed significant correlation with at least one behavioral specialization (Fig. 5). The behavioral specialization associated with the most components (four of six) was explicit companionship, and the component associated with the most behavioral specializations (six of 10) was component 4, which involves regions involved in social action and interaction. Specific associations between associated brain networks and behavioral specializations are also apparent. For example, component 3, which involves regions involved in movement, eye movement, and spatial navigation, showed a significant correlation with sight hunting, whereas Network 2, which involves regions involved in olfaction and gustation, showed a significant correlation with scent hunting.
Figure 5.
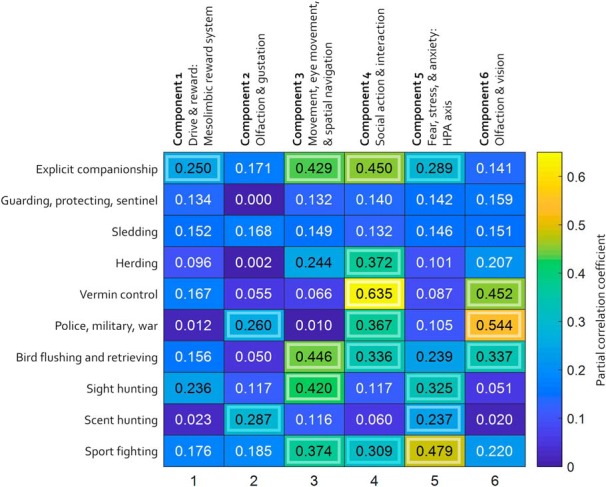
Relationship between morphologically covarying regional brain networks and ostensible behavioral specializations. Colors indicate partial correlation coefficients resulting from multiple regression analysis on source-based morphometry results. Outlined boxes are significant at p < 0.05.
Discussion
The current study took a comprehensive, data-driven, agnostic approach to investigating neuroanatomical variation in domestic dogs. We first questioned whether significant variation in dog brain morphology even exists. The answer is a clear “yes”: differences in gross brain anatomy are readily appreciable (Fig. 1A). This observation was further confirmed by a whole-brain, multiple-comparison-corrected, voxelwise statistical analysis (Fig. 1C,D). Having established this basic finding, we then went on to probe the relationship between multiple, potentially interacting factors that might be linked to this variation: the total size of the body or brain, the external and internal morphology of the skull, the structure of the dog phylogenetic tree, and the organization of internal brain networks.
Dogs show intraspecific variation in morphology to a degree rarely seen in nature. There is a 100-fold difference between the body mass of a Chihuahua (∼1 kg) and the body mass of a Great Dane (∼100 kg) (Sutter et al., 2008). However, we found that dog brain sizes do not scale commensurately to dog body sizes, as indicated by a relatively low scaling coefficient for the relationship between brain size and body mass. To appreciate this effect, consider the adjacent dachshund and golden retriever images in Figure 1A: the dachshund's brain takes up most of the available endocranial space, whereas the golden retriever shows noticeably larger sinuses. A phylogenetic analysis revealed that changes in relative brain size are not predicated by relatedness and are more likely the result of selection on specific terminal branches of the phylogenetic tree (i.e., individual breeds).
In comparative animal cognition research, total brain size is often used as a gross index of cognitive capacity. Several previous studies have investigated the relationship between dog body size and cognition or behavior, with apparently contradictory results (Helton and Helton, 2010; Stone et al., 2016; cf. Broadway et al., 2017). Additionally, a study that used a single scaling metric across breeds found that larger-brained (i.e., larger-bodied) dogs performed better on tests of executive function (Horschler et al., 2019). We found that larger dogs do tend to have larger brains, but that the brain to body allometry across breeds is low, indicating high variability in brain to body ratio across breeds (Fig. 2A). Furthermore, we found that a substantial amount of variation in internal dog brain morphology is related to total brain size, suggesting that evolutionary increases or decreases in relative brain volume may be driven by changes in specific groups of regions. Moreover, we found that these networks differed across breed groups. Therefore, shifts in relative brain size may be related to expansion or contraction of specific networks, potentially leading to the presence or absence of correlations between body size and behavior depending on the specific breeds or behaviors being studied.
We also found that selection for smaller body size has significantly influenced the internal morphology of the cranial cavity. For a given cephalic index, or exterior skull shape, smaller-bodied dogs have more spherical brains (Fig. 2B). This is consistent with a previous analysis linking foreshortening of the skull to ventral pitching of the brain and olfactory bulb, resulting in a more spherical brain (Roberts et al., 2010). We assessed the extent to which internal and exterior skull morphology were related to the covarying morphometric networks we identified. More networks showed a significant relationship with neurocephalic index than with cephalic index, suggesting that variation in brain morphology appears to be more tied to the internal morphology of the cranial cavity than to external craniofacial morphology, which is perhaps not surprising. Our results indicate that skull morphology is linked to the underlying anatomy of specific, different networks of brain regions; it is possible that this could underlie the reported associations between behavior and head shape (Gácsi et al., 2009; Helton, 2009; McGreevy et al., 2013). Not all networks showed a significant relationship with either cephalic index or neurocephalic index, indicating that variation in dog brain morphology is partially but not totally dependent on variation in skull morphology. Importantly, we cannot say from the current analyses whether variation in skull morphology drives variation in brain morphology, the reverse, or both.
In addition to these analyses of the gross external shape and size of the brain and skull, we also investigated internal brain organization. This was accomplished using source-based morphometry to identify maximally independent networks that explain the variation present in the dataset. We identified six such networks (Fig. 3). In the case of circuitry that is highly conserved across species, such as circuitry for reward and motivation or fear and anxiety, it is a safe bet that research on other species is a good indicator of the functional role of these systems in dogs. This cannot be assumed to be the case for circuits that involve higher-order cortical association areas. Particularly in the case of our network 4, it may be tempting to jump to conclusions about parallels with human cortical regions that are located in approximately the same location and are involved in similar tasks; for example, the fusiform face area, Wernicke's area, or the mirror system. However, it is important to remember that primates and carnivores diverged further back in time than primates and rodents: humans are more closely related to mice than to dogs. Our last common ancestor with dogs likely had a fairly smooth, simple brain (Kaas, 2011), and higher-order cortical association areas, along with whatever complex perceptual and cognitive abilities they support, have evolved independently in dogs and humans. Therefore, we stress that the functional roles of these networks, and their relationship to selection on behavior in specific breeds, should at this point still be considered an open question.
Having identified these six networks, we then investigated their relationship to the dog phylogenetic tree. We found that the majority of changes that occur in these components take place in the tree's terminal branches (i.e., individual breeds). This suggests that brain evolution in domestic dog breeds follows a “late burst model,” with directional changes in brain organization being primarily lineage specific. We also assessed whether these networks were related to selective breeding, as evidenced by the ostensible behavioral specialization(s) of each breed as noted by the AKC. In all six of the regionally covarying networks that we found, significant correlations were found with at least one behavioral specialization. Associations between brain networks and related behavioral specializations are apparent. For example, network 2, which involves regions that support higher-order olfactory processing, shows a significant correlation with scent hunting, whereas network 3, which involves regions that support movement, eye movement, and spatial navigation, shows a significant correlation with sight hunting. These findings strongly suggest that humans have altered the brains of different breeds of dogs in different ways through selective breeding.
It is important to note that the current study was performed on opportunistically acquired data. The dataset included different numbers of dogs from different breeds, and some breeds are not represented at all. We used permutation testing for statistical hypothesis testing, which is a nonparametric approach appropriate for differing group sizes, but it is still possible that different patterns of variation may have been obtained with a different sample makeup. Nonetheless, we expect the basic finding that this variation exists would remain.
Additionally, it should be noted that as dogs are increasingly bred to be house pets rather than working animals, selection on behavior is relaxing; significant behavioral differences have been found between working, show, and pet animals within a breed (Lofgren et al., 2014). To our knowledge, the dogs in the current study were all house pets. Therefore, the findings reported here should be taken as representative of the innate breed-typical adaptations to brain organization that emerge without the input of specific experience and may actually reflect relaxed or reduced versions of these adaptations. This might be akin to studying language circuitry in a lineage of language-deprived humans: humans almost certainly have some specialized “hard-wired” adaptations to this circuitry, but experience is required for the anatomical phenotype to fully emerge, and indeed it is difficult to consider language-related neural adaptations divorced from the context of language exposure and learning. Thus, future studies on purpose-bred dogs that are actively performing the tasks for which they are presumably adapted might expect to find additional or more pronounced neuroanatomical effects than we observed here.
These findings have relevance to both basic and applied science. First and foremost, our findings introduce neural variation in domestic dog breeds as a novel opportunity for studying the evolution of brain–behavior relationships. Dogs represent a “natural experiment” in behavioral selection that has been ongoing for thousands of years; it seems remarkable that attempts to observe the neurological results of this experiment have so far been fairly minimal. Our findings also have implications for the current proliferation of fMRI studies in pet dogs, which nearly always group together dogs of varying breeds. The current study suggests that this approach might not be ideal because there may be evolved breed differences in, for example, functional responses to stimuli or anatomical distribution of receptors. Consistent with this possibility, one study has already found that border collies and Siberian huskies respond significantly differently to intranasal oxytocin (Kovács et al., 2016). Additionally, on a practical level, our findings open the door to brain-based assessment of the utility of different dogs for different tasks. It might be possible, for example, to identify neural features that are linked to different breeds' specializations for specific behaviors, and to selectively breed or train dogs for enhanced expression of those neural features. Finally, on a philosophical level, these results tell us something fundamental about our own place in the larger animal kingdom: we have been systematically shaping the brains of another species.
Footnotes
This work was supported by the National Science Foundation–Division of Integrative Organismal Systems (Grant NSF-IOS 1457291). Additional support was provided by NIH OD P51OD11132 to the Yerkes National Primate Research Center. We appreciate the contributions of the veterinary and imaging staff at the UGA Veterinary Teaching Hospital.
The authors declare no competing financial interests.
References
- Akaike H. (1974) A new look at statistical nodel identification. IEEE Trans Autom Control 19:716–723. 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- Alcaro A, Huber R, Panksepp J (2007) Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev 56:283–321. 10.1016/j.brainresrev.2007.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andics A, Gácsi M, Faragó T, Kis A, Miklósi Á (2017) Voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Curr Biol 27:1248–1249. 10.1016/j.cub.2017.03.036 [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC (2011) Advanced normalization tools (ANTS). Insight j 2:1–35. Available at: http://scil.dinf.usherbrooke.ca/static/website/courses/imn530/ants.pdf. 10.1007/s12021-011-9109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Tustison NJ, Song G, Gee JC (2009) ANTS: Advanced Open-Source Tools for Normalization And Neuroanatomy. Penn Image Computing and Science Laboratory, University of Pennsylvania, Philadelphia, PA. [Google Scholar]
- Broadway MS, Samuelson MM, Christopher JL, Jett SE, Lyn H (2017) Does size really matter? Investigating cognitive differences in spatial memory ability based on size in domestic dogs. Behav Processes 138:7–14. 10.1016/j.beproc.2017.01.012 [DOI] [PubMed] [Google Scholar]
- Butler MA, King AA (2004) Phylogenetic comparative analysis: a modeling approach for adaptive evolution. American Naturalist 164:683–695. 10.1086/426002 [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001) A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151. 10.1002/hbm.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira LM, Ferreira A (2015) Anatomical variations in the pseudosylvian fissure morphology of brachy-, dolicho-, and mesaticephalic dogs. Anat Rec (Hoboken) 298:1255–1260. 10.1002/ar.23171 [DOI] [PubMed] [Google Scholar]
- Cook PF, Prichard A, Spivak M, Berns GS (2016) Awake canine fMRI predicts dogs' preference for praise vs food. Soc Cogn Affect Neurosci 11:1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuaya LV, Hernández-Pérez R, Concha L (2016) Our faces in the dog's brain: functional imaging reveals temporal cortex activation during perception of human faces. PLoS One 11:e0149431. 10.1371/journal.pone.0149431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R, Lee J, Duda J, Avants BB, Vite CH, Tseng B, Gee JC, Aguirre GD, Aguirre GK (2012) A digital atlas of the dog brain. PLoS One 7:e52140. 10.1371/journal.pone.0052140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AG, Klingenberg CP (2010) Large-scale diversification of skull shape in domestic dogs: disparity and modularity. Am Nat 175:289–301. 10.1086/650372 [DOI] [PubMed] [Google Scholar]
- Evans H, de Lahunta A (2013) Miller's anatomy of the dog. New York: Elsevier Saunders. [Google Scholar]
- Gácsi M, McGreevy P, Kara E, Miklósi A (2009) Effects of selection for cooperation and attention in dogs. Behav Brain Funct 5:31. 10.1186/1744-9081-5-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS (2011) Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PH, Pagel M (1991) The comparative method in evolutionary biology. New York, OUP. [Google Scholar]
- Helton WS. (2009) Cephalic index and perceived dog trainability. Behav Processes 82:355–358. 10.1016/j.beproc.2009.08.004 [DOI] [PubMed] [Google Scholar]
- Helton WS, Helton ND (2010) Physical size matters in the domestic dog's (Canis lupus familiaris) ability to use human pointing cues. Behav Proc 85:77–79. 10.1016/j.beproc.2010.05.008 [DOI] [PubMed] [Google Scholar]
- Ho LST, Ané C (2014) Intrinsic inference difficulties for trait evolution with Ornstein-Uhlenbeck models. Methods in Ecology and Evolution 5:1133–1146. 10.1111/2041-210X.12285 [DOI] [Google Scholar]
- Holekamp KE, Sakai ST, Lundrigan BL (2007) Social intelligence in the spotted hyena (Crocuta crocuta). Philos Trans R Soc Lond B Biol Sci 362:523–538. 10.1098/rstb.2006.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horschler DJ, Hare B, Call J, Kaminski J, Miklósi Á, MacLean EL (2019) Absolute brain size predicts dog breed differences in executive function. Anim Cogn 22:187–198. 10.1007/s10071-018-01234-1 [DOI] [PubMed] [Google Scholar]
- Jacobs C, Van Den Broeck W, Simoens P (2007) Neurons expressing serotonin-1B receptor in the basolateral nuclear group of the amygdala in normally behaving and aggressive dogs. Brain Res 1136:102–109. 10.1016/j.brainres.2006.11.096 [DOI] [PubMed] [Google Scholar]
- Jia H, Pustovyy OM, Waggoner P, Beyers RJ, Schumacher J, Wildey C, Barrett J, Morrison E, Salibi N, Denney TS, Vodyanoy VJ, Deshpande G (2014) Functional MRI of the olfactory system in conscious dogs. PLoS One 9:e86362. 10.1371/journal.pone.0086362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. (2011) Reconstructing the areal organization of the neocortex of the first mammals. Brain Behav Evol 78:7–21. 10.1159/000327316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabbazian M, Kriebel R, Rohe K, Ané C (2016) Fast and accurate detection of evolutionary shifts in Ornstein-Uhlenbeck models. Methods Ecology and Evolution 7:811–824. 10.1111/2041-210X.12534 [DOI] [Google Scholar]
- Kovács K, Kis A, Pogány Á, Koller D, Topál J (2016) Differential effects of oxytocin on social sensitivity in two distinct breeds of dogs (Canis familiaris). Psychoneuroendocrinology 74:212–220. 10.1016/j.psyneuen.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Larson G, Karlsson EK, Perri A, Webster MT, Ho SY, Peters J, Stahl PW, Piper PJ, Lingaas F, Fredholm M, Comstock KE, Modiano JF, Schelling C, Agoulnik AI, Leegwater PA, Dobney K, Vigne JD, Vilà C, Andersson L, Lindblad-Toh K (2012) Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc Natl Acad Sci U S A 109:8878–8883. 10.1073/pnas.1203005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren SE, Wiener P, Blott SC, Sanchez-Molano E, Woolliams JA, Clements DN, Haskell MJ (2014) Management and personality in labrador retriever dogs. Appl Anim Behav Sci 156:44–53. 10.1016/j.applanim.2014.04.006 [DOI] [Google Scholar]
- MacLean EL, Snyder-Mackler N, vonHoldt BM, Serpell JA (2019) Highly heritable and functionally relevant breed differences in dog behavior. Available from: https://www.biorxiv.org/content/10.1101/509315v1. [DOI] [PMC free article] [PubMed]
- McGreevy PD, Georgevsky D, Carrasco J, Valenzuela M, Duffy DL, Serpell JA (2013) Dog behavior co-varies with height, bodyweight and skull shape. PLoS One 8:e80529. 10.1371/journal.pone.0080529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA (2011) The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol 519:3599–3639. 10.1002/cne.22735 [DOI] [PubMed] [Google Scholar]
- Pagel M. (1997) Inferring evolutionary processes from phylogenies. Zoologica Scripta 26:331–348. 10.1111/j.1463-6409.1997.tb00423.x [DOI] [Google Scholar]
- Palazzi X. (2011) The beagle brain in stereotaxic coordinates. New York: Springer. [Google Scholar]
- Parker HG, Dreger DL, Rimbault M, Davis BW, Mullen AB, Carpintero-Ramirez G, Ostrander EA (2017) Genomic analyses reveal the influence of geographic origin, migration, and hybridization on modern dog breed development. Cell Rep 19:697–708. 10.1016/j.celrep.2017.03.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilegaard AM, Berendt M, Holst P, Møller A, McEvoy FJ (2017) Effect of skull type on the relative size of cereb cortex and lateral ventricles in dogs. Front Vet Sci 4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radinsky L. (1969) Outlines of canid and felid brain evolution. Ann N Y Acad Sci 167:277–288. 10.1111/j.1749-6632.1969.tb20450.x [DOI] [Google Scholar]
- Roberts T, McGreevy P, Valenzuela M (2010) Human induced rotation and reorganization of the brain of domestic dogs. PLoS One 5:e11946. 10.1371/journal.pone.0011946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlf FJ. (2001) Comparative methods for the analysis of continuous variables: geometric interpretations. Evolution 55:2143–2160. [DOI] [PubMed] [Google Scholar]
- Serpell JA, Hsu YA (2005) Effects of breed, sex, and neuter status on trainability in dogs. Anthrozoos 18:196–207. 10.2752/089279305785594135 [DOI] [Google Scholar]
- Smaers JB, Mongle CS (2018) evomap: R package for the evolutionary mapping of continuous traits. Available from: https://github.com/JeroenSmaers/evomap.
- Smaers JB, Mongle CS (2017) On the accuracy and theoretical underpinnings of the multiple variance Brownian motion approach for estimating variable rates and inferring ancestral states. Biological Journal of the Linnean Society 121:229–238. 10.1093/biolinnean/blx003 [DOI] [Google Scholar]
- Smaers JB, Mongle CS, Kandler A (2016) A multiple variance Brownian motion framework for the estimation of ancestral states and rates of evolution. Biological Journal of the Linnean Society 118:78–94. 10.1111/bij.12765 [DOI] [Google Scholar]
- Smaers JB, Rohlf FJ (2016) Testing species' deviation from allometric predictions using the phylogenetic regression. Evolution 70:1145–1149. 10.1111/evo.12910 [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Stone HR, McGreevy PD, Starling MJ, Forkman B (2016) Associations between domestic-dog morphology and behaviour scores in the dog mentality assessment. PLoS One 11:e0149403. 10.1371/journal.pone.0149403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter NB, Mosher DS, Gray MM, Ostrander EA (2008) Morphometrics within dog breeds are highly reproducible and dispute Rensch's rule. Mamm Genome 19:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompkins AM, Ramaiahgari B, Zhao S, Gotoor SSR, Waggoner P, Denney TS, Deshpande G, Katz JS (2018) Separate brain areas for processing human and dog faces as revealed by awake fMRI in dogs (Canis familiaris). Learn Behav 46:561–573. 10.3758/s13420-018-0352-z [DOI] [PubMed] [Google Scholar]
- Tonoike A, Nagasawa M, Mogi K, Serpell JA, Ohtsuki H, Kikusui T (2015) Comparison of owner-reported behavioral characteristics among genetically clustered breeds of dog (Canis familiaris). Sci Rep 5:17710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A (2015) Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16:317–331. [DOI] [PubMed] [Google Scholar]
- Våge J, Bønsdorff TB, Arnet E, Tverdal A, Lingaas F (2010) Differential gene expression in brain tissues of aggressive and non-aggressive dogs. BMC Vet Res 6:34. 10.1186/1746-6148-6-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. Neuroimage 92:381–397. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD (2009) Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum Brain Mapp 30:711–724. 10.1002/hbm.20540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imag 20:45–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Conceptual schematic of neuroimaging analysis. Download Figure 1-1, TIF file (1MB, tif)
NiPype pipeline for merging axial and sagittal images from each dog before registration to the template. Code is available online at https://gist.github.com/dgutman/a0e05028fab9c6509a997f703a1c7413. Download Figure 1-2, TIF file (1.2MB, tif)