Abstract
Introduction
Regulatory B cells (Bregs), a novel subpopulation of B cells, are a significant area of research due to their immune regulatory function in the immunological response. Bregs have been reported to regulate acute inflammation and immunity through the production of anti-inflammatory cytokines.
Material and methods
A B cell subpopulation was identified using flow cytometric analysis in two different processes: 1) after preparation and storage of peripheral blood mononuclear cells (PBMCs) using Ficoll density gradient centrifugation from a human blood sample, 2) followed by isolation and storage of B cells through magnetic separation using a B cell isolation kit and MS column. ELISA assays were performed to observe the cytokine production of interkleukin 10 (IL-10) and transforming growth factor β1 (TGF-β1) by this novel B cell subpopulation.
Results
Double positive staining of CD5+CD1d+ Bregs represents (19.27 ±1.52) from PBMCs, (33.32 ±2.95) from B cells accordingly (n = 40). Through ELISA assays, it has been found that B cell subpopulation produces IL-10 (0.56 ±0.08) and TGF-β1 (0.90 ±0.12) (n = 40).
Conclusions
These methods should be able to facilitate progress in research on Bregs through the following steps: 1) the regulatory role may be observed in comparison with particular autoimmune diseases, inflammation, cancer, and immunologic responses to find out whether Breg alteration and/or cytokine production is altered as well in these disorders or conditions. 2) If the alteration of Bregs and cytokine production is significant along with the clinical correlation, a further in vitro study can be initiated with exposure of certain drugs to overcome the alteration of the cytokine production; then, an in vivo study can be initiated.
Keywords: Bregs, interkleukin 10, transforming growth factor β1, flow cytometry, ELISA
Introduction
B cells are white blood cells (WBCs) in vertebrates. B cells mature in the bone marrow [1] and are a critical element of the acquired immune system. B-cell maturation in the bone marrow is a dynamic and compound procedure involving a steady balance between cell proliferation and apoptotic selection. Due to this balance in the generation of functioning B cells that are liable for eliciting humoral immunity [2–5]. B cells are well recognised to organise immunological responses by antibody production, which represents the humoral arm of the acquired immune system. B cells are the first facilitators of antigen-specific immunological responses by producing antibodies and differentiating into memory cells that give long-standing immunity [6]. B cells positively modulate immunological responses and inflammation by antibody production and antigen presentation for optimal CD4+ T-cell activation [7, 8]. The antibody production enables optimal CD41 T-cell activation as B cells act as antigen-presenting cells and exert different regulatory roles in immunological responses. Nevertheless, specific B cells also negatively modulate the immunological responses through the production of modulatory cytokines and primarily interact with pathogenic T cells via cell-to-cell communication. These categories of B cells are termed “Bregs” [5]. Over the past decade, the functions of Bregs in repressing pathological immunological responses have been recognised extensively. In recent years, various studies in both mice and humans have established that Bregs repress inflammatory responses, predominantly through the secretion of interleukin 10 (IL-10) [9]. The regulatory influences of Bregs have been reported in numerous models of autoimmune diseases, inflammation, transplantation reactions, and anti-tumour immunity. Bregs can originate from a distinct B cell subpopulation [10]. Bregs have been determined to act as a suppressive function in the immune system. Bregs can inhibit other immune cells through cytokine production and antigen presentation, which gives them a function in the pathogenesis of cancers and autoimmune diseases [11]. Analysing the B cell subpopulation is a significant area of research due to its therapeutic relevance and role in the immunological response.
Bregs: evidence and origin
The concept of Bregs and a B-cell suppressive event was first noted in 1974, during delayed-type hypersensitivity reactions in guinea pigs [12, 13]. However, it took two decades until the concept of “suppressor B cells” received attention again. Added proof of this B cell modulatory phenotype was evaluated in 1996, which was observed in a murine autoimmune model of experimental autoimmune encephalitis (EAE) where the deficiency of B cells aggravated the inflammation [14]. Then in 1997, it was observed in chronic colitis where the disease worsened in the B-cell deficiency group, suggesting their repressive role in inflammation [15]. The presence of regulatory B cells (Bregs) was confirmed afterwards in 2000 using the term “regulatory B cell”, which described its repressive capability in inflammatory bowel disease [16]. Bregs were reintroduced as an IL-10-producing B cell subpopulation in 2002 [17]. Many attempts have been made in several studies, including mice [18–34] and humans [26, 35–38], at phenotypic identification and characterisation.
Significance statement
Bregs are critical in maintaining self-tolerance [39]. The essentiality of human Bregs in the upholding of immune homeostasis originates from various immune-associated pathologies, such as cancers, autoimmune diseases, and chronic infections that are frequently related to abnormalities in Breg numbers or function [40]. Moreover, to be more specific clinically, such as the role of Bregs in autoimmunity, defects in numbers and functions have been reported in multiple autoimmune diseases, including multiple sclerosis (MS), myasthenia gravis (MG), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and psoriasis [35, 41–45]. Inverse correlations were found between Breg numbers and functions, and disease severity status measured using severity scales, e.g, the Multiple Sclerosis Severity Score (MSSS), the Quantitative Myasthenia Gravis score (QMG), the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), the Rheumatoid Arthritis Severity Scale (RASS), the Psoriasis Area and Severity Index (PASI), etc. These inverse correlations may be due to the loss of immune suppression precipitated by Bregs, giving rise to inflammation, or the reduced Breg numbers and function are a consequence of chronic inflammation. Even though a modulatory function of B cells is well established, more research is required to learn more about their phenotypic identification and characterisation [46]. Knowing the roles of B cells and having the capability to trace an increase or decrease in B cell subpopulation levels can anticipate a useful method for understanding the immunological responses to certain diseases [47]. A constant attempt to perceive Breg biology in healthy controls will yield new possibilities to establish Breg immunotherapy [40].
B cells play a pivotal role in several autoimmune diseases, including patients with MS [48]. Therapeutic interventions targeting B cells have been a useful approach in MS, such as total B cell depletion therapy. The studies describing total B cell depletion therapy using ocrelizumab and rituximab have proven the clinical efficacy in MS [49–51]. B cell depletion might be improved for the treatment of MS, through an approach to deplete subsets of B cells selectively [52], such as in vivo depletion of Bregs. Specific subsets of B cells would give increased advantages over current total B cell depletion therapies [40]. Other therapeutic interventions target Bregs to develop Breg immunotherapy in MS, by B cell isolation of patient-derived PBMCs and in vitro Breg expansion, then adoptive transfer into the patient could suppress inflammation, or in vivo modulation to expand Bregs [40]. These could offer advanced perspectives for therapy of patients with MS.
Material and methods
Materials and equipment
Blood sample with anticoagulation (heparin), Ficoll, phosphate buffered saline (PBS), buffer (ultra-pure water – 1000 ml, NaCl – 8 g, KCl – 0.2 g, Na2HPO4 × 12 H2O – 3.58 g, KH2PO4 – 0.27 g, EDTA – 0.74 g, 0.5% BSA – 5 g), fetal bovine serum (FBS), RPMI-1640 medium, 1% penicillin/streptomycin/amphotericin B mixture, B cell isolation kit II, MS column and miniMACS separator, human IL-10 and TGF-β1 ELISA kit, standard ELISA microplate reader, PerCP CD19, FITC CD5 and PE CD1d antibodies, flow cytometer, water bath (37ºC), refrigerator, cell culture flasks, and dishes, centrifuges, centrifuge tubes, pipettes, haemocytometer.
Ethical consideration and approval, informed consent
The research was performed with the approval of the institutional review board (IRB) of the Hubei University of Medicine, Shiyan, Hubei 442000, China; and the project was evaluated and approved by the assessment panel. The purpose of this research was explained to the participants, and when the oral consent was understood and agreed, in front of a nurse, a consent form was provided in English and Chinese. This process was done to obtain the informed consent as well as the legal identification. The individuals who gave consent were included in the project.
Data collection
We involved all participants from Taihe Hospital of Hubei University of Medicine. We obtained 20 ml blood samples from 40 healthy controls, aged between 18 and 55 years of age. These participants did not receive any medication for cancers or autoimmune diseases.
Preparation and storage of PBMCs
First of all, a human peripheral blood sample was obtained in 20 ml disposable syringes and anticoagulation (heparin) was added. It was then gently diluted in PBS (Good Bio, China) and peripheral blood mononuclear cells (PBMCs) were isolated through Ficoll (TBD Sciences, China) gradient centrifugation based on the manufacturer’s protocol. A culture medium (RPMI-1640 medium + 10% FBS + 1% penicillin/streptomycin/amphotericin B mixture) was added, and then the sample was transferred to two different cell culture flasks using a pipette. Flask 1 was used for isolation and storage of B cells, and flask 2 was used for flow cytometric analysis of PBMCs.
Isolation and storage of B cells
After centrifugation, supernatant was discarded, and cell numbers (PBMCs) were determined using a haemocytometer. Then the B cells were isolated through magnetic separation based on the manufacturer’s protocol utilising an MS column and B cell isolation kit II (Miltenyi Biotec, USA). A culture medium (RPMI-1640 medium + 10% FBS + 1% penicillin/streptomycin/amphotericin B mixture) was added and then transferred to a cell culture flask using a pipette. The flask was used for flow cytometric analysis of B cells and ELISA assays.
Cell staining and flow cytometric analysis
After centrifugation, supernatant was discarded, and cell numbers (PBMCs or B cells) were determined using a haemocytometer. Then the cells were resuspended in buffer and cells were surface stained with CD19, CD5, and CD1d antibodies (BD Biosciences, USA). The cells were then incubated in a refrigerator at 4°C for 30 min, followed by two washing steps to remove any residual antibody from the cell suspension. The samples were interpreted using the flow cytometer BD FACSCanto II (BD Biosciences, USA), and the data were analysed by BD FACSDiva 8.0.1 software.
ELISA
Enzyme-linked immunosorbent assay (ELISA) was performed using a human IL-10 and TGF-β1 ELISA kit (Neobioscience, China) based on the manufacturer’s protocol. The absorbency was assessed by a microplate reader (Thermo Scientific, Finland). After completing the experiments, remaining reagents were put back in a refrigerator for temperature restoration.
Statistical analysis
Data were analysed using descriptive statistics in SPSS V25.0 and shown as mean ± SD.
Results
Phenotypic Identification of CD19+CD5+CD1d+ Bregs
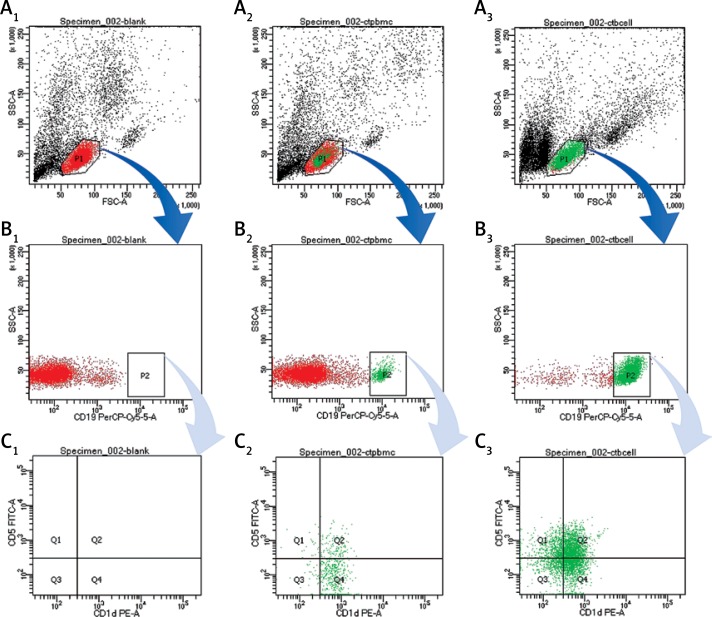
The CD19+CD5+CD1d+ Bregs were phenotypically identified using specific CD markers: CD19, CD5, CD1d which are the key markers for identifying Bregs [17, 41, 53–56]. Breg cell subpopulations were identified by double positive staining of CD5+CD1d+ Bregs from PBMCs and B cells (Figure 1). This novel CD19+CD5+CD1d+ Breg subpopulation in human peripheral blood was represented (n = 40; 19.27 ±1.52) from PBMCs (n = 40; 33.32 ±2.95) from isolated B cells accordingly. These mean values of Bregs identified here can be referred to as the normal ranges that are responsible for maintaining a dynamic balance through a certain level in healthy controls. Any alterations to the mean values may indicate marked defects.
Figure 1.
Analysis of CD19+CD5+CD1d+ Breg expression using flow cytometry from PBMCs and B cells in human peripheral blood. A1, B1, C1 – without staining of an antibody – shows forward scatter (FSC-A) measures cell size and side scatter (SSC-A) measures cell granularity; counting beads (black, red and green) and B cells gates are depicted; A2, B2, B3 – PBMCs with staining of CD19, CD5, CD1d antibody – B cells fating revealed a CD19+ population (green) and a CD19– population (red); A3, B3, C3 – B cells with staining of CD19, CD5, CD1d antibody – CD19+ B cells gating revealed CD5+CD1d+ (Q2) population; Bregs are defined as CD19+CD5+CD1d+
Characterisation of phenotypically identified CD19+CD5+CD1d+ Bregs
The characterisation of phenotypically identified CD19+CD5+CD1d+ Bregs was observed through ELISA assays using human IL-10 and TGF-β1 ELISA kits based on the manufacturer’s protocol. The IL-10 production represents (n = 40; 0.56 ±0.08), and TGF-β1 production represents (n = 40; 0.90 ±0.12) by these phenotypically identified CD19+CD5+CD1d+ Bregs. These mean values of cytokine production can be referred to as the functional properties of Bregs when maintaining a dynamic balance through a certain level in healthy controls. Alterations may disrupt the normal regulatory function.
Discussion
During immunoreaction, B cells manifested positive and negative modulatory influences [57]. The modulatory function of B cells in autoimmune diseases was demonstrated in 1996 in EAE [58]. Bregs’ (B10 cells) existence was confirmed after that by others [58, 59]; B cell subpopulations were found to be capable of influencing immunological tolerance [22, 28, 57, 58, 60, 61]. Bregs expressed a reduced and impaired role in most of the studies. The term “Bregs” was introduced by Matsushita, who established Bregs as an IL-10 secreting B cell subpopulation in 2002 [62]. Bregs carried out diverse mechanisms to regulate immunological responses and aimed for numerous unique immune cell types, such as DCs [63] and macrophages [64] as well as T helper (Th1 and Th2) cells [65]. It was found that Bregs were effective in repressing the expansion of CD4+CD25– T cells [66]. According to the present state of affairs concerning the phenotypes and markers of human Bregs [17, 67–70], they exhibit some of the same phenotypic properties as formerly defined markers in mice [35, 37]. In this study, we phenotypically identified Bregs using specific CD markers by double positive staining of CD5+CD1d+ Bregs in human peripheral blood. In most of the previous studies, Bregs were identified from PBMCs [17, 26, 35–38, 40–45, 53–56, 67–72]. In this study, we used both techniques which include Breg identification from PBMCs and isolated B cells. We observed that the percentage of Bregs was higher if identified from isolated B cells than from PBMCs. Therefore, the identification of isolated B cells provides greater specificity.
Nonetheless, of the variety of markers used to identify Bregs, the protective effects of Bregs are dependent on IL-10 [17, 37, 71, 72], a potent deactivator which limits the potency and time of inflammatory responses. Consequently, IL-10 secretion is still an essential criterion in the identification of Bregs. The leading role of IL-10 is to repress excessive inflammation and control homeostasis via various mechanisms. This mechanism involves hindering antigen presentation and co-stimulatory molecules CD80, CD86, and MHC class II expression through DCs and macrophages. The suppression of excessive inflammation and maintenance of homeostasis can also be regulated by inhibiting cytokine release by T cells in immunological responses and supporting Treg differentiation [73]. While IL-10 secretion remains the hallmark of Bregs’ negative modulatory role, other independent repressive mechanisms, e.g. involving TGF-β1, have been revealed in recent years. Secretion of TGF-β1 by B cells give rise to the support of Treg development in transplant tolerance, diabetes, allergic diseases, and colitis [74]. In this study, we also observed, through ELISA assays, that these novel CD19+CD5+CD1d+ Bregs produce anti-inflammatory cytokines IL-10 and TGF-β1 (see Figure 2 for Breg cell phenotype and character diagram based on the current state of knowledge and this study).
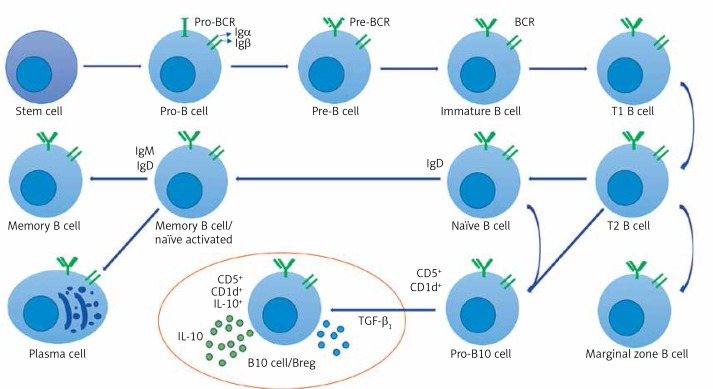
Figure 2.
Breg cell phenotype and character diagram based on the current state of knowledge. B cells express clonally distributed antigen presenting receptors (BCRs) that bind to one particular antigen. The mature B cell expresses both immunoglobulin M (IgM) and immunoglobulin D (IgD) [30, 79, 80]. A mature B cell can differentiate into several types of B cells, the most common being plasma and memory B cells [81, 82]. Bregs (also called B10 cells due to their IL-10 expression ability) produce anti-inflammatory cytokines IL-10 and TGF-β1, shown in the circled area (drawn from the current state of knowledge of Bregs and the conclusion of this study)
Interestingly, recent research suggests that Bregs can also directly approach the central nervous system (CNS) [31]. In addition, people living with type 1 diabetes express the lowest frequency of B10 cells in comparison with type 2 diabetes, adult latent autoimmune diabetes, and controls [75]. In SLE, Bregs are emerging as an essential player at the time of disease instigation. Adoptive shifting of splenic CD5+CD1dhi Bregs from wild-type mice expanded the time of endurance of CD19–/–mice in this lupus model [76]. In humans, SLE patients showed indistinguishable frequencies of circulating CD24hiCD38hi B cells, but had a remarkably lower IL-10+ proportion in comparison with controls [77]. Rheumatoid arthritis studies have also implied that Bregs are responsible for Tregs’ initial inducement [78]. In one of our recent studies, we identified a lower frequency of CD19+CD5+CD1d+ Bregs in MG and lower cytokine secretion compared to healthy controls. Based on QMG scoring techniques we have also observed that the lower the cytokine levels are, the more clinical symptoms the patient has [41]. Thus, the detailed protocol and methods presented here are extremely valuable and should be able to facilitate progress in research on various autoimmune diseases, cancer, inflammation, and immunologic responses.
In conclusion, Bregs are a significant area of research due to their immune regulatory function in the immunological responses through their potential cytokine production. Through flow cytometric analysis and ELISA assays of B cells of the human peripheral blood sample, either from PBMCs or isolated B cells, the regulatory role may be observed. The healthy controls can be compared with particular autoimmune diseases, inflammation, cancer, and immunologic responses to find out whether Breg alteration and/or cytokine production is altered in these disorders or conditions. If the alteration of Bregs and cytokine production is significant along with the clinical correlation, a further in vitro study can be conducted such as PBMC or B cell culture with exposure to certain drugs. If cytokine production improves, then an in vivo study can be initiated.
Acknowledgments
The authors thank Dr Afsarunnesa Syeda from Renmin Hospital of Hubei University of Medicine, China, for all the support; and appreciate the help of Ms Helia Garcia from Wright State University, USA for proofreading this work.
The study was supported by the national natural science foundation of China (no. 81671238).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Cooper MD. The early history of B cells. Nature Rev Immunol. 2015;15:191–7. doi: 10.1038/nri3801. [DOI] [PubMed] [Google Scholar]
- 2.Osmond DG. B cell development in the bone marrow. Semin Immunol. 1990;2:173–80. [PubMed] [Google Scholar]
- 3.Cooper MD. Exploring lymphocyte differentiation pathways. Immunol Rev. 2002;185:175–85. doi: 10.1034/j.1600-065x.2002.18515.x. [DOI] [PubMed] [Google Scholar]
- 4.Welner RS, Pelayo R, Kincade PW. Evolving views on the genealogy of B cells. Nature Rev Immunol. 2008;8:95–106. doi: 10.1038/nri2234. [DOI] [PubMed] [Google Scholar]
- 5.Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol. 2013;10:122–32. doi: 10.1038/cmi.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman-Genuino RM, Diener KR. Regulatory B cells in pregnancy: lessons from autoimmunity, graft tolerance, and cancer. Front Immunol. 2017;8:172. doi: 10.3389/fimmu.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–80. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tedder TF. Introduction: regulatory B cell special issue – making all the pieces fit. Int Immunol. 2015;27:467–70. doi: 10.1093/intimm/dxv047. [DOI] [PubMed] [Google Scholar]
- 9.Mauri C, Bosma A. Immune regulatory function of B cells. Ann Rev Immunol. 2012;30:221–41. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 10.Vitale G, Mion F, Pucillo C. Regulatory B cells: evidence, developmental origin and population diversity. Mol Immunol. 2010;48:1–8. doi: 10.1016/j.molimm.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Qian H, Liu Y, Duan L, Li Y, Shi G. The roles of regulatory B cells in cancer. J Immunol Res. 2014;2014:215471. doi: 10.1155/2014/215471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974;251:550–1. doi: 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- 13.Neta R, Salvin SB. Specific suppression of delayed hypersensitivity: the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J Immunol. 1974;113:1716–25. [PubMed] [Google Scholar]
- 14.Wolf SD, Dittel BN, Hardardottir F, Janeway CA. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–8. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med. 1997;186:1749–56. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol. 2000;12:597–605. doi: 10.1093/intimm/12.5.597. [DOI] [PubMed] [Google Scholar]
- 17.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 18.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–24.e8. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Bankoti R, Gupta K, Levchenko A, Stager S. Marginal zone B cells regulate antigen-specific T cell responses during infection. J Immunol. 2012;188:3961–71. doi: 10.4049/jimmunol.1102880. [DOI] [PubMed] [Google Scholar]
- 20.Blair PA, Chavez-Rueda KA, Evans JG, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–78. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 22.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan AR, Amu S, Saunders SP, et al. Ligation of TLR7 on CD19+CD1dhiB cells suppresses allergic lung inflammation via regulatory T cells. Eur J Immunol. 2015;45:1842–54. doi: 10.1002/eji.201445211. [DOI] [PubMed] [Google Scholar]
- 24.Klinker MW, Reed TJ, Fox DA, Lundy SK. Interleukin-5 supports the expansion of Fas ligand-expressing killer B cells that induce antigen-specific apoptosis of CD4+ T cells and secrete interleukin-10. PLoS One. 2013;8:e70131. doi: 10.1371/journal.pone.0070131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Wu Y, Song J, et al. Tolerogenic CX3CR1+B cells suppress food allergy-induced intestinal inflammation in mice. Allergy. 2013;68:1241–8. doi: 10.1111/all.12218. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto M, Baba A, Yokota T, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41:1040–51. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Moreau A, Blair PA, Chai J, et al. Transitional-2 B cells acquire regulatory function during tolerance induction and contribute to allograft survival. Eur J Immunol. 2014;45:843–53. doi: 10.1002/eji.201445082. [DOI] [PubMed] [Google Scholar]
- 28.Sheng JR, Quan S, Soliven B. CD1dhiCD5+B cells expanded by GM-CSF in vivo suppress experimental autoimmune myasthenia gravis. J Immunol. 2014;193:2669–77. doi: 10.4049/jimmunol.1303397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimomura Y, Mizoguchi E, Sugimoto K, et al. Regulatory role of B-1 B cells in chronic colitis. Int Immunol. 2008;20:729–37. doi: 10.1093/intimm/dxn031. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe R, Ishiura N, Nakashima H, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–9. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanaba K, Bouaziz J, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Yanaba K, Yoshizaki A, Asano Y, Kadono T, Tedder TF, Sato S. IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model. Am J Pathol. 2011;178:735–43. doi: 10.1016/j.ajpath.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M, Deng J, Liu Y, et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol. 2012;180:2375–85. doi: 10.1016/j.ajpath.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Deriaud E, Jiao X, Braun D, Leclerc C, Lo-Man R. Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J Exp Med. 2007;204:1107–18. doi: 10.1084/jem.20062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blair PA, Noreña LY, Flores-Borja F, et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Das A, Ellis G, Pallant C, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–35. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2010;117:530–41. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van de Veen W, Stanic B, Yaman G, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–12. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Tian X, Lu X, et al. Significant decrease in peripheral regulatory B cells is an immunopathogenic feature of dermatomyositis. Sci Rep. 2016;6:27479. doi: 10.1038/srep27479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauri C, Menon M. Human regulatory B cells in health and disease: therapeutic potential. J Clin Investig. 2017;127:772–9. doi: 10.1172/JCI85113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karim MR, Zhang H, Yuan J, Sun Q, Wang Y. Regulatory B cells in seropositive myasthenia gravis versus healthy controls. Front Neurol. 2017;8:43. doi: 10.3389/fneur.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knippenberg S, Peelen E, Smolders J, et al. Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naïve/memory Breg ratio during a relapse but not in remission. J Neuroimmunol. 2011;239:80–6. doi: 10.1016/j.jneuroim.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Bosma A, Abdel-Gadir A, Isenberg D, Jury E, Mauri C. Lipid-antigen presentation by CD1d+ B cells is essential for the maintenance of invariant natural killer T cells. Immunity. 2012;36:477–90. doi: 10.1016/j.immuni.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flores-Borja F, Bosma A, Ng D, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting Th1 and Th17 differentiation. Sci Transl Med. 2013;5:173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi M, Yanaba K, Umezawa Y, et al. IL-10-producing regulatory B cells are decreased in patients with psoriasis. J Dermatol Sci. 2016;81:93–100. doi: 10.1016/j.jdermsci.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Ray A, Dittel B. Mechanisms of regulatory B cell function in autoimmune and inflammatory diseases beyond IL-10. J Clin Med. 2017;6:12. doi: 10.3390/jcm6010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos M, Zhang W, White L, Whalley J, Hsu M. Überwachung von DNA-schäden an kontrollpunkten des zell-zyklus. BIOspektrum. 2011;17:200–2. [Google Scholar]
- 48.Memon AB, Javed A, Caon C, et al. Long-term safety of rituximab induced peripheral B-cell depletion in autoimmune neurological diseases. PLoS One. 2018;13:e0190425. doi: 10.1371/journal.pone.0190425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–34. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 50.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 51.Ellwardt E, Ellwardt L, Bittner S, Zipp F. Monitoring B-cell repopulation after depletion therapy in neurologic patients. Neurol Neuroimmunol Neuroinflamm. 2018;5:e463. doi: 10.1212/NXI.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark EA. How does B cell depletion therapy work, and how can it be improved? Ann Rheum Dis. 2005;64(Suppl 4):iv77–80. doi: 10.1136/ard.2005.042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Habib J, Deng J, Lava N, Tyor W, Galipaeu J. Blood B cell and regulatory subset content in multiple sclerosis patients. J Mult Scler. 2015;2 doi: 10.4172/2376-0389.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang M, Zeng G, Yang Q, et al. Anti-tuberculosis treatment enhances the production of IL-22 through reducing the frequencies of regulatory B cell. Tuberculosis. 2014;94:238–44. doi: 10.1016/j.tube.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, Zheng X, Zhang J, et al. CD19+CD1d+CD5+ B cell frequencies are increased in patients with tuberculosis and suppress Th17 responses. Cell Immunol. 2012;274:89–97. doi: 10.1016/j.cellimm.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Sun X. The expression and effect of helper IL-10- producing B cells in human minimal change nephrotic syndrome. Ann Clin Labor Res. 2016:4. doi: 10.21767/2386-5180.100079. [DOI] [Google Scholar]
- 57.Airoldi I. Heterogeneous expression of interleukin-18 and its receptor in B-cell lymphoproliferative disorders deriving from naive, germinal center, and memory B lymphocytes. Clin Cancer Res. 2004;10:144–54. doi: 10.1158/1078-0432.ccr-1026-3. [DOI] [PubMed] [Google Scholar]
- 58.Lang ML. How do natural killer T cells help B cells? Exp Rev Vaccines. 2009;8:1109–21. doi: 10.1586/erv.09.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benson M, Erickson L, Gleeson M, Noelle R. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr Opin Immunol. 2007;19:275–80. doi: 10.1016/j.coi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watkins NA, Gusnanto A, De Bono B, et al. A HaemAtlas: characterizing gene expression in differentiated human blood cells. Blood. 2009;113:e1–9. doi: 10.1182/blood-2008-06-162958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Novershtern N, Subramanian A, Lawton LN, et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell. 2011;144:296–309. doi: 10.1016/j.cell.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsushita T. Regulatory B cell and autoimmune disease. Japan J Clin Immunol. 2010;33:234–41. doi: 10.2177/jsci.33.234. [DOI] [PubMed] [Google Scholar]
- 63.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immunologic-responses during inflammation, autoimmunity, and cancer. Ann N York Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 64.Lehmann-Horn K, Kronsbein HC, Weber MS. Targeting B cells in the treatment of multiple sclerosis: recent advances and remaining challenges. Ther Adv Neurol Disord. 2013;6:161–73. doi: 10.1177/1756285612474333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jensen F, Muzzio D, Soldati R, Fest S, Zenclussen AC. Regulatory B10 cells restore pregnancy tolerance in a mouse model1. Biol Reprod. 2013;89:1–7. doi: 10.1095/biolreprod.113.110791. [DOI] [PubMed] [Google Scholar]
- 66.Giannoukakis N, Trucco M. A role for tolerogenic dendritic cell-induced B-regulatory cells in type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2012;19:279–87. doi: 10.1097/MED.0b013e328355461b. [DOI] [PubMed] [Google Scholar]
- 67.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–52. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong S, Puaux A, Chittezhath M, et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 69.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–9. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y, Chen Y, Li Z, et al. Role of IL-10-producing regulatory B cells in control of cerebral malaria in plasmodium berghei infected mice. Eur J Immunol. 2013;43:2907–18. doi: 10.1002/eji.201343512. [DOI] [PubMed] [Google Scholar]
- 71.Furuzawa-Carballeda J, Hernández-Molina G, Lima G, Rivera-Vicencio Y, Férez-Blando K, Llorente L. Peripheral regulatory cells immunophenotyping in primary Sjögren’s syndrome: a cross-sectional study. Arthritis Res Ther. 2013;15:R68. doi: 10.1186/ar4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daien CI, Gailhac S, Mura T, et al. Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheumatol. 2014;66:2037–46. doi: 10.1002/art.38666. [DOI] [PubMed] [Google Scholar]
- 73.Wang L, Qiu J, Yu L, Hu X, Zhao P, Jiang Y. Increased numbers of CD5+CD19+CD1dhighIL-10+ Bregs, CD4+Foxp3+ Tregs, CD4+CXCR5+Foxp3+ follicular regulatory T (TFR) cells in CHB or CHC patients. J Transl Med. 2014;12:251. doi: 10.1186/s12967-014-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008;64:187–99. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- 75.Matsushita T, Yanaba K, Bouaziz J, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–30. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Byrne SN, Halliday GM. B cells activated in lymph nodes in response to ultraviolet irradiation or by interleukin-10 inhibit dendritic cell induction of immunity. J Investig Dermatol. 2005;124:570–8. doi: 10.1111/j.0022-202X.2005.23615.x. [DOI] [PubMed] [Google Scholar]
- 77.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Ann Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 78.Ray A, Wang L, Dittel BN. IL-10-independent regulatory B-cell subpopulations and mechanisms of action. Int Immunol. 2015;27:531–6. doi: 10.1093/intimm/dxv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korniotis S, Gras C, Letscher H, et al. Treatment of ongoing autoimmune encephalomyelitis with activated B-cell progenitors maturing into regulatory B cells. Nat Commun. 2016;7:12134. doi: 10.1038/ncomms12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deng C, Xiang Y, Tan T, et al. Altered peripheral B-lymphocyte subpopulations in type 1 diabetes and latent autoimmune diabetes in adults. Diabetes Care. 2015;39:434–40. doi: 10.2337/dc15-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heinemann K, Wilde B, Hoerning A, et al. Decreased IL-10+regulatory B cells (Bregs) in lupus nephritis patients. Scand J Rheumatol. 2016;45:312–6. doi: 10.3109/03009742.2015.1126346. [DOI] [PubMed] [Google Scholar]
- 82.Carter NA, Vasconcellos R, Rosser EC, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569–79. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]