Abstract
Background
Histoplasma capsulatum is an extremely rare cause of prosthetic valve endocarditis (PVE) and can present with non-specific symptoms leading to a delay in diagnosis with unfavourable outcomes.
Case summary
A 65-year-old male patient with a history of a bioprosthetic aortic valve replacement and non-obstructive coronary artery disease was admitted for altered mentation, failure to thrive, and a 20-pound unintentional weight loss over the past 4 months. Upon examination, he was lethargic but afebrile and haemodynamically stable. A late peaking ejection murmur was heard on exam. Skin exam was significant for embolic phenomenon involving the extremities. Inflammatory markers and serum calcium were elevated. A bedside echocardiogram showed severe obstruction across the aortic valve prosthesis. Two years prior, he had an echocardiogram with a normal functioning prosthesis. Routine blood cultures were negative and serologic screening was unrevealing. Urine Histoplasma antigen screen was positive on hospital day 3 and on hospital day 10, fungal blood cultures were positive for H. capsulatum. Unfortunately, the patient died shortly afterwards as a result of multiorgan failure from embolic manifestations of the infection.
Discussion
Based on our patient's findings and those of previously reported cases in the literature, H. capsulatum PVE should be strongly considered in patients from endemic areas with non-specific symptoms and negative routine blood cultures.
Keywords: Case report, Endocarditis, Histoplasma capsulatum, Prosthetic valve endocarditis, Fungal endocarditis
Learning points
Histoplasma capsulatum is an extremely rare cause of prosthetic valve endocarditis and can present with non-specific symptoms.
A high index of suspicion and early medical and surgical interventions are critical in the management to improve mortality rates.
Urine and serum antigen detection, PCR, and in situ hybridization have improved diagnostic capabilities, with sensitivities approaching 90% and are important in evaluating patients with suspected H. capsulatum infections.
Introduction
Fungi are the causative agents in ∼5–7% of all cases of prosthetic valve endocarditis (PVE), with the large majority of cases due to Candida species.1 In contrast, Histoplasma capsulatum is an extremely rare cause of PVE and can present with non-specific symptoms.2 These factors, coupled with negative or delayed positive blood cultures (including both ‘routine' and ‘fungal' cultures), are responsible for delayed diagnosis (median, 7 weeks) in most cases.3 A 40-year institutional experience of fungal PVE at Mayo Clinic included only 3 (14%) of 21 cases due to H. capsulatum.4 Due to the rarity of the syndrome, PVE due to H. capsulatum has been difficult to fully characterize. We, therefore, report an extremely rare case of H. capsulatum endocarditis involving a prosthetic aortic valve 6 years after valve placement. A high index of suspicion and early medical and surgical interventions are critical in he management of this life-threatening infection in an effort to improve mortality rates.
Case presentation
A 65-year-old male patient from Minnesota, USA was brought to the emergency department by his family with a 4-month history of failure to thrive, altered sensorium, and fever. During this time, he had lost over 20 pounds. He had a history of severe bicuspid aortic valve stenosis that required bioprosthetic aortic valve replacement 6 years prior, cerebrovascular accident with residual deficits, non-obstructive coronary artery disease, and medication non-compliance.
Timeline
| Time | Events |
|---|---|
| 21 April 2017 | Anorexia; urinary urgency and frequency with history of poor glucose control (haemoglobin A1C 12%). |
| 26 September 2017 | Altered sensorium, weakness, and weight loss; subjective fevers with night sweats and urinary symptoms. Computed tomographic (CT) of the abdomen reveals an enlarged spleen with interval development of infarcts involving 50% of the spleen. |
| 12 October 2017 | Altered sensorium and metabolic derangements. Echocardiogram shows interval development of severe aortic bioprosthetic valve dysfunction/stenosis, significant calcification, and mild prosthetic regurgitation. Routine blood cultures obtained, which were subsequently negative for growth. |
| 13 October 2017 | Transoesophageal echocardiogram reveals bulky thickening of the prosthetic aortic valve leaflets with mobile components prolapsing into the ventricular side of the valve. Echolucency in the posterior aortic root concerning for perivalvular abscess. Patient initiated on empiric antibiotics. |
| 14 October 2017 | Dental radiograph revealed periapical lucency suspicious for abscess. Extraction of remaining teeth performed. Histoplasma urine antigen is positive. Patient initiated on intravenous liposomal amphotericin B. |
| 17 October 2017 | Patient develops acute kidney injury. Positron emission tomography/CT findings are consistent with abscess along the posterior margin of the aortic root. Antifungal therapy was discontinued. |
| 18 October 2017 | Coronary CT angiography revealed extensive vegetations on the aortic side of the prosthesis; calcification and severe disease of distal left main and proximal left anterior descending artery (LAD) with poor distal LAD targets for bypass. |
| 19 October 2017 | Progressively worsening kidney injury, worsening mentation, and embolic phenomenon in extremities. |
| 21 October 2017 | Fungal blood cultures obtained on day 2 are positive for Histoplasma capsulatum. |
| 30 October 2017 | Patient expires. Request for post-mortem declined by family. |
Physical examination
The patient was afebrile (37.3°C) and normotensive (124/78 mmHg) with a heart rate of 88 b.p.m. Evaluation revealed a chronically ill, cachectic male. Oral inspection revealed multiple decayed and non-restorable mandibular teeth with associated gingival inflammation. There were no obvious signs of parulis, draining abscesses, or fluctuant masses. Cardiovascular examination revealed a well-healed midline sternotomy, normal heart sounds, and regular rate and rhythm. There was a 3/6, late-peaking systolic ejection murmur heard at the upper sternal border radiating to the carotids bilaterally. The dorsum of the right foot and multiple toes were dusky—concerning for systemic emboli (Figure 1).
Figure 1.
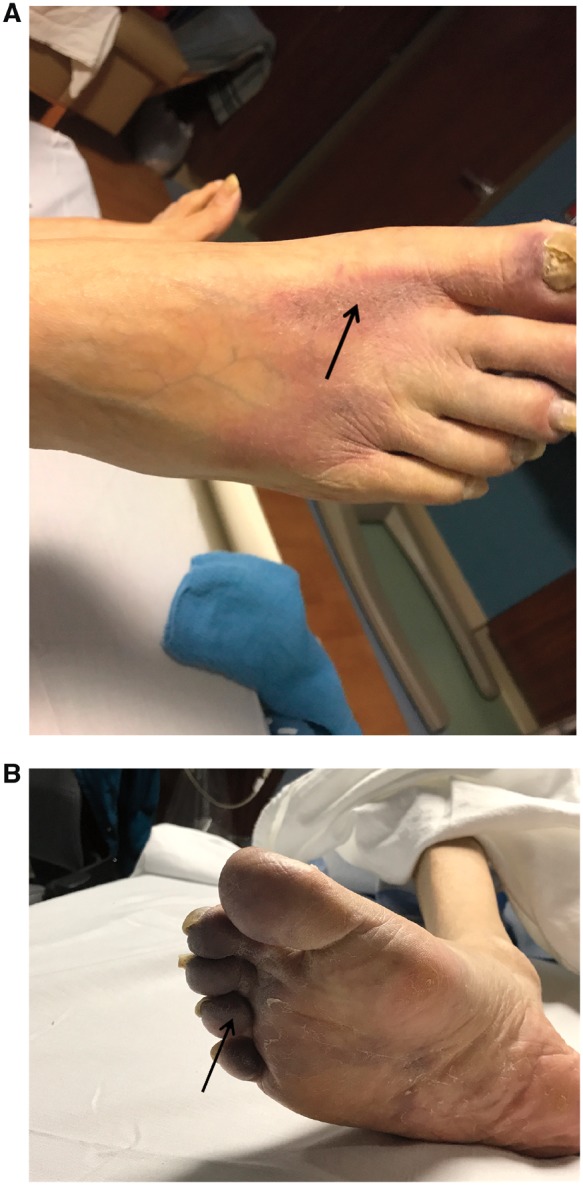
Lower extremity photograph with dusky appearance concerning for systemic embolization (A, B).
Diagnostic assessment and interventions
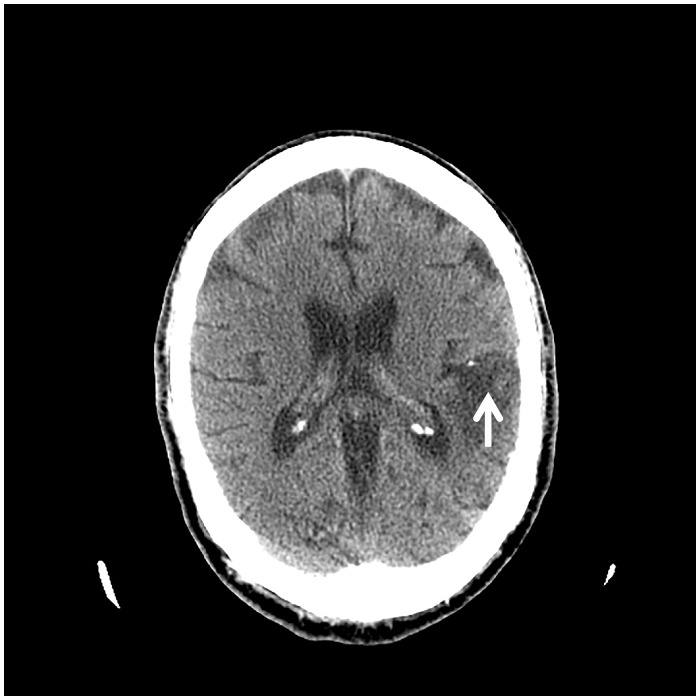
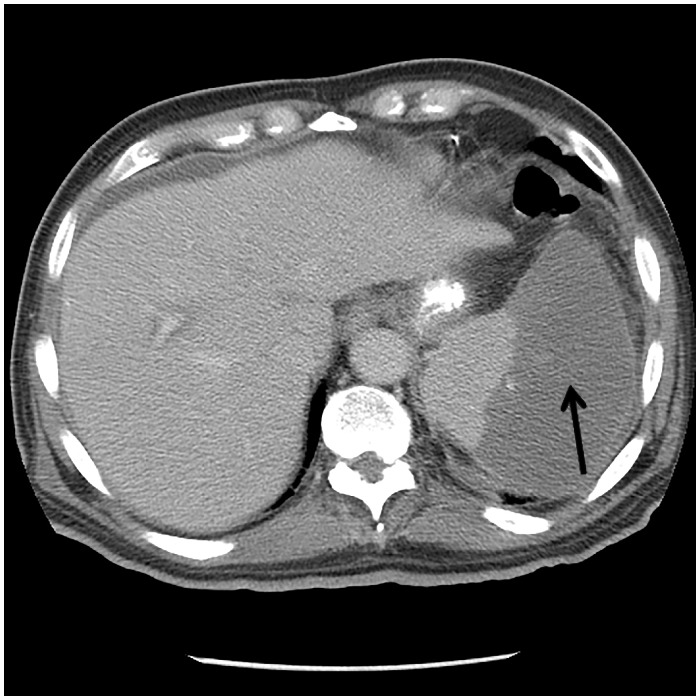
Laboratory evaluation revealed multiple abnormalities including normocytic anaemia and thrombocytopenia; and elevated acute phase reactants: ferritin 1143 μg/L (normal 24–336 μg/L); erythrocyte sedimentation rate 67 mm/h (normal 0–22 mm/h); and C-reactive protein 101 mg/L (normal ≤ 4.9 mg/L). Serum troponin T was elevated at 0.253 ng/mL (normal <0.01 ng/mL); creatinine 2.0 mg/dL (baseline 1 mg/dL); and calcium 15.8 mg/dL (normal 8.8–10.3 mg/dL). Computed tomographic (CT) of the head was negative for acute intracranial processes; however, remote infarcts involving the left parietal lobe and right occipital lobe were observed (Figure 2). Computed tomographic of the abdomen and pelvis revealed splenic infarcts involving approximately 50% of the spleen (Figure 3). Routine blood cultures, Coxiella PCR (blood), Bartonella, and Brucella serology results were all negative. Coccidioides enzyme immunoassay (EIA, blood) was negative; however, Blastomyces EIA (blood) was ‘equivocal’ and automatically reflexed to immunodiffusion screening, which was negative.
Figure 2.
Computed tomography of the head showing remote infarcts involving the left parietal lobe.
Figure 3.
Computed tomography of the abdomen showing a moderately enlarged spleen with new wedge-shaped region of abnormal low attenuation involving 50% of the spleen - consistent with an infarct.
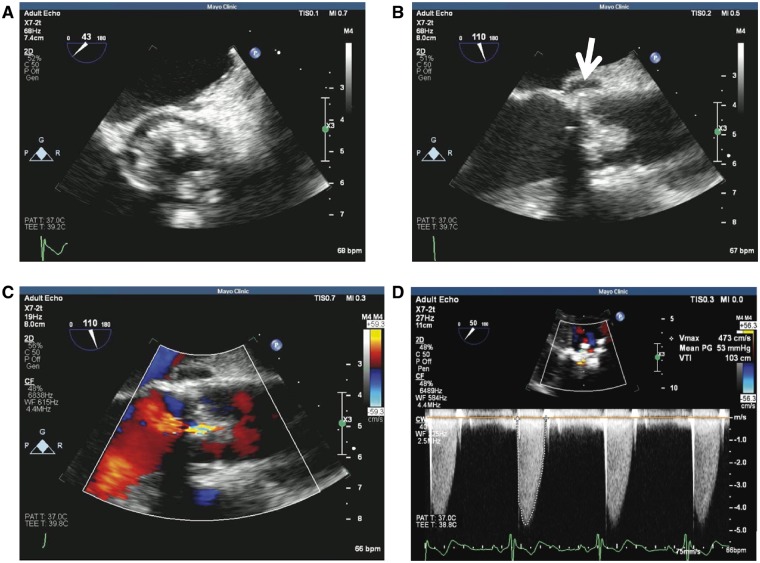
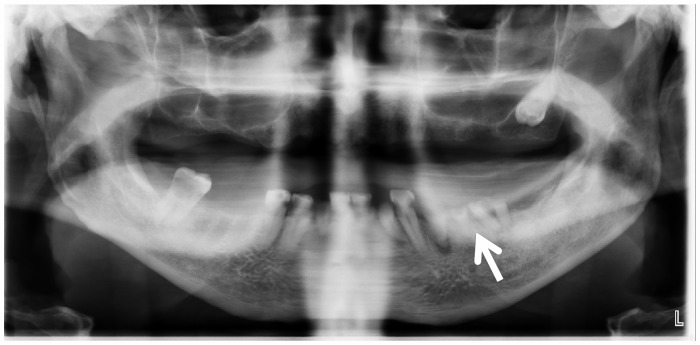
Transthoracic echocardiogram demonstrated severe stenosis/obstruction of the aortic valve prosthesis, nodular calcifications affecting the prosthetic leaflets, and mild prosthetic regurgitation. The mean Doppler gradient through the aortic valve prosthesis was 51 mmHg with an aortic valve prosthetic orifice area by Doppler of 0.92 cm2. Transoesophageal echocardiogram showed bulky thickening of the bioprosthetic aortic valve leaflets with a 6 mm × 4 mm mobile echo density prolapsing into the ventricular side of the valve. A 5 mm × 10 mm lucency in the posterior aortic root was noted, concerning for perivalvular abscess (Figure 4A–D). Dental panoramic radiograph revealed periapical lucency around a tooth suspicious for abscess (Figure 5) prompting oral surgery evaluation.
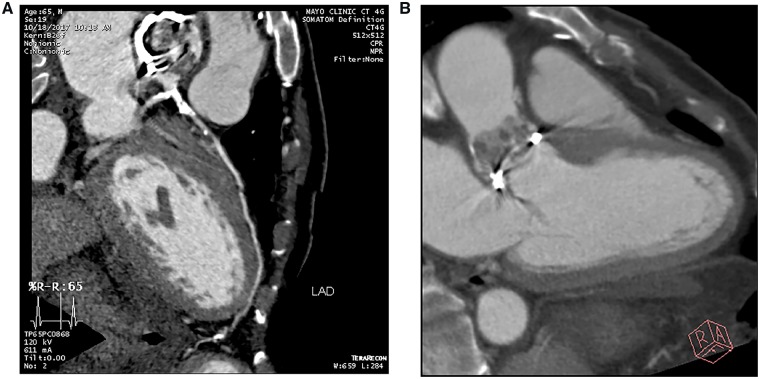
Figure 4.
Transoesophageal echocardiogram of the bioprosthetic valve. (A) Bulky thickening of the prosthetic aortic valve leaflets in short axis. (B) Aortic valve in long axis view showing bulky thickening and echolucency of the aortic root concerning for abscess (arrow). (C and D) Colour flow assessment and Doppler profile of aortic valve prosthesis showing severe stenosis.
Figure 5.
Dental panoramic radiograph: periapical lucency about tooth 21, suspicious for abscess.
Empiric antibiotics with intravenous vancomycin and ceftriaxone was initiated. On day 3, urine Histoplasma antigen result was reported positive (5.89 ng/mL; normal ≤ 0.10 ng/mL). Antifungal therapy with intravenous liposomal amphotericin B was initiated and a cardiovascular surgery consult was obtained for definitive treatment of fungal endocarditis. Blood cultures for fungal/mycobacterial organisms that were obtained on day 2 returned positive for H. capsulatum 10 days after admission.
Follow-up and outcomes
Given the patient’s comorbid conditions, concern for surgical risk, and proximity of aortic root abscess to the coronary ostia; further evaluation of the aortic root and coronary ostia was performed with CT angiography (Figure 6A). This revealed extensive vegetations on the aortic side of the prosthetic aortic valve (Figure 6B) and severe coronary artery disease with extensive calcifications, but no definite perivalvular extension of infection. Fludeoxyglucose (FDG) positron emission tomography images showed a focal area of increased FDG uptake just posterior to the suture ring of the aortic valve, concerning for an abscess posterior to the valve. After two doses of the intravenous liposomal amphotericin, the patient suffered worsening renal function and antifungal therapy was discontinued. Following a multidisciplinary care conference, the family opted for initiation of comfort measures and the patient expired shortly afterwards.
Figure 6.
Cardiac CT angiogram. (A) Severe atherosclerotic disease involving the distal left main coronary artery and proximal LAD with extensive calcification. The distal LAD does not have adequate targets for bypass. (B) Extensive vegetations, primarily on the aortic side of the bioprosthetic aortic valve, with no definite perivalvular extension. PET images (not shown) showed a focal area of increased uptake concerning for an abscess posterior to the valve.
Discussion
The diagnosis of infective endocarditis (IE) is straightforward in patients with ‘classic’ manifestations described by Osler and other historic experts in the field. Of note, ‘classic’ cases of IE with characteristic clinical findings of IE are less commonly seen today.5 In patients with non-specific symptoms, a diagnostic strategy that is both sensitive and specific becomes crucial to reduce the likelihood of devastating outcomes.6 Common presenting symptoms in patients with H. capsulatum include fever, anorexia, respiratory symptoms, malaise, night sweats, and weight loss. Unfortunately, these findings are not specific and it is not unusual for patients to manifest symptoms for weeks to months before a diagnosis is confirmed.
Other clinical signs associated with histoplasmosis include skin lesions, lymphadenopathy, pulmonary nodules, hepatosplenomegaly, pallor, petechiae, and embolic phenomenon.3Histoplasma capsulatum infection is endemic in certain areas of North, Central, and South America, Africa, and Asia. In the USA, most cases have occurred within the Ohio and Mississippi River valleys. The diagnosis of Histoplasma endocarditis remains challenging because most of the commonly used automated blood culture systems do not support growth of the organism. Moreover, blood cultures are often discarded after a ∼5 day incubation period, which would not be sufficient in duration for growth of H. capsulatum in most cases. In addition, unlike the high-grade bacteraemia seen in most cases of IE due to untreated Gram-positive cocci, the fungaemia associated with untreated H. capsulatum IE may not be high grade.1 Over the last few decades, the use of urine and serum antigen detection as well as PCR and in situ hybridization has improved diagnostic capabilities, with sensitivity of antigen detection assay approaching 90%.3,7
A multicentre case series of 14 cases of H. capsulatum in the USA between 2003 and 2012 described features associated with H. capsulatum IE. In this study, the mean age at presentation was 65.6 ± 16.2 years; patients had multiple comorbidities and non-specific clinic presentations. In 71% of cases, patients were male with infection involving a prosthetic aortic valve;3 importantly, our patient was 65 years of age, male sex, and had a prosthetic aortic valve.
Echocardiography is central in the diagnosis and management of patients with IE and should be performed when IE has suspected.6 Classic findings on echocardiogram that should raise the suspicion for fungal infections include bulky and mobile vegetations, vegetations greater than 1 cm, and occurring within the first year of surgery.4 These findings, however, are reflective of fungal infection due to Candida species, which account for the large majority of fungal endocarditis cases. The extreme rarity of endocarditis due to histoplasmosis limits the echocardiographic characterization of this syndrome.
Current guidelines recommend a two-phase approach to the management of patients with H. capsulatum endocarditis. The approach consists of (i) infection control with a combination of a parenteral antifungal agent, usually an amphotericin B containing product, and (ii) prompt valve surgery in most cases of fungal IE (Class I; LOE B).6
Histoplasma capsulatum is a rare cause of endocarditis and thus has been difficult to fully characterize. Nevertheless, features including older age, male sex, and presence of an aortic prosthetic valve, as seen in our patient and 10 of 14 cases previously described in the literature, may be a characteristic presentation. This diagnosis should be considered in (routine blood) culture-negative endocarditis and appropriate laboratory screening should be included in the evaluation of patients from endemic areas of histoplasmosis. A high index of suspicion, prompt institution of medical therapy, surgical intervention, and long-term oral antifungal suppressive therapy are important to hopefully improve patient outcome.
Lead author biography

Dr Rosalyn O. Adigun, MD, PharmD, is a 2019 graduate of the Cardiovascular Diseases Fellowship Programme at Mayo Clinic, Rochester, MN, USA and will begin an Advanced Cardiovascular Imaging (Echocardiography) Fellowship at Mayo Clinic. She is also completing a Postdoctoral Master's Degree Programme in Clinical and Translational Science through the Mayo Clinic Graduate School of Biomedical Sciences. Her research interests include the diagnostic and prognostic implications of cardiovascular imaging in various cardiovascular diseases with an area of interest in Advanced Heart Failure and Cardiac Transplantation.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Bhatti S, Vilenski L, Tight R, Smego RA Jr.. Histoplasma endocarditis: clinical and mycologic features and outcomes. J Infect 2005;51:2–9. [DOI] [PubMed] [Google Scholar]
- 2. Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007;20:115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riddell J, Kauffman CA, Smith JA, Assi M, Blue S, Buitrago MI, Deresinski S, Wright PW, Drevets DA, Norris SA, Vikram HR, Carson PJ, Vergidis P, Carpenter J, Seidenfeld SM, Wheat LJ.. Histoplasma capsulatum endocarditis: multicenter case series with review of current diagnostic techniques and treatment. Medicine (Baltimore) 2014;93:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boland JM, Chung HH, Robberts FJL, Wilson WR, Steckelberg JM, Baddour LM, Miller DV.. Fungal prosthetic valve endocarditis: Mayo Clinic experience with a clinicopathological analysis. Mycoses 2011;54:354–360. [DOI] [PubMed] [Google Scholar]
- 5. Prendergast BD. The changing face of infective endocarditis. Heart 2006;92:879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA.. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. A scientific statement for healthcare professionals from the American Heart Association. Circulation 2015;132:1435–1486. [DOI] [PubMed] [Google Scholar]
- 7. Pierrotti LC, Baddour LM.. Fungal endocarditis, 1995–2000. Chest 2002;122:302–310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.