Abstract
Background
Libman–Sacks endocarditis is a cardiac manifestation of systemic lupus erythematosus (SLE) and is characterized by non-bacterial verrucous vegetations, causing valvular stenosis and/or regurgitation. The effectiveness of immunosuppressive therapy for valve dysfunction due to Libman–Sacks endocarditis has not been reported.
Case summary
A 67-year-old woman with a history of chronic atrial fibrillation was emergently admitted with acute decompensated heart failure. Transoesophageal echocardiogram revealed severe mitral regurgitation (MR) due to oedematous thickening and poor coaptation of the medial edge of A2/P2 segments and the lateral edge of A3/P3 segments. Serial blood culture results were negative, suggesting bacterial infective endocarditis to be a less likely cause of valvular damage. Because the patient developed photosensitivity, livedo reticularis, and pancytopenia, Libman–Sacks endocarditis with rapidly progressive SLE was diagnosed on the basis of positive test results of anti-double-stranded DNA-IgG and its complement titer. Two months after, immunosuppressive therapy including corticosteroids, a transoesophageal echocardiogram revealed thinning of the degenerative mitral valve leaflets and a reduction of MR from severe to mild.
Discussion
Corticosteroid therapy for Libman–Sacks endocarditis reportedly increases the extent of fibrosis and scarring of the valve leaflets, resulting in worse valve function. In our patient, MR decreased from severe to mild after corticosteroid therapy. Because low-echoic thickening of the mitral valve leaflets suggested acute oedematous changes without scarring and fibrosis and other clinical symptoms of SLE rapidly progressed, early initiation of immunosuppressive therapy for Libman–Sacks endocarditis lead to a benign clinical course in our patient.
Keywords: Libman–Sacks endocarditis, Systemic lupus erythematosus, Mitral regurgitation, Immunosuppressive therapy, Case report
Learning points
Libman–Sacks endocarditis causes significant mitral regurgitation (MR) due to inflammatory changes in the mitral leaflets.
Immunosuppressive therapy improved severe MR with acute-phase degenerative changes in the mitral leaflets in a case of Libman–Sacks endocarditis.
Introduction
Libman–Sacks endocarditis, characterized by non-bacterial verrucous vegetations with systemic lupus erythematosus (SLE), was first described by Libman and Sacks in 1924.1 Libman–Sacks endocarditis may cause serious complications, such as superimposed bacterial endocarditis, thromboembolic events, valvular regurgitation, and/or stenosis requiring valvular surgery.2 The clinical presentation of Libman–Sacks valvular changes ranges from asymptomatic valvular thickening with oedematous changes to severe valvular dysfunction.3,4 Improvement in Libman–Sacks endocarditis-related mitral regurgitation (MR) after surgical repair or replacement has been documented5,6; however, to the best of our knowledge, the effectiveness of immunosuppressive therapy against these changes has not been reported. We describe a case of severe MR due to Libman–Sacks endocarditis that improved with immunosuppressive therapy.
Timeline
| 3 days ago | The patient developed dyspnoea. |
| Admission | The patient was emergently hospitalized for acute decompensated heart failure. |
| Day 10 | Heart failure was managed with non-invasive ventilatory support and medication. |
| Day 11 | Transthoracic echocardiography (TTE) revealed severe mitral regurgitation (MR). |
| Day 14 | Transoesophageal echocardiography (TOE) revealed oedematous thickening and malcoaptation of the medial edge of A2/P2 segments and the lateral edge of A3/P3 segments. |
| Day 15 | Photosensitivity and livedo reticularis developed, and pancytopenia gradually worsened. |
| Day 34 | Immunosuppressive therapy was initiated for rapidly progressive systemic lupus erythematosus. |
| Day 87 | Follow-up TTE and TOE revealed mild MR. |
Case presentation
A 67-year-old woman with a history of chronic atrial fibrillation and hypertension was admitted to our hospital for progressive dyspnoea. Upon arrival, she was tachypnoeic (respiratory rate: 28 b.p.m.), hypotensive (blood pressure: 88/58 mmHg), and tachycardiac (heart rate: 154 b.p.m.). Her oxygen saturation was 85% on room air. Physical examination revealed coarse crackling and wheezing in both lungs and a Levine IV/VI systolic murmur at the apex. An electrocardiogram revealed atrial fibrillation with a rapid ventricular response. Laboratory test results were normal, except for B-type natriuretic peptide levels of 514 pg/mL (<18.4 pg/mL), haemoglobin levels of 99 g/L (113–152 g/L), platelet count of 59 000/μL (130 000–369 000/μL), and albumin levels of 24 g/L (38–53 g/L). A chest radiograph showed severe pulmonary congestion with a butterfly shadow and an increased cardiothoracic ratio of 68%. No significant coronary stenosis was noted on coronary angiography. Non-invasive ventilatory support and intravenous furosemide and diltiazem administration improved her symptoms.
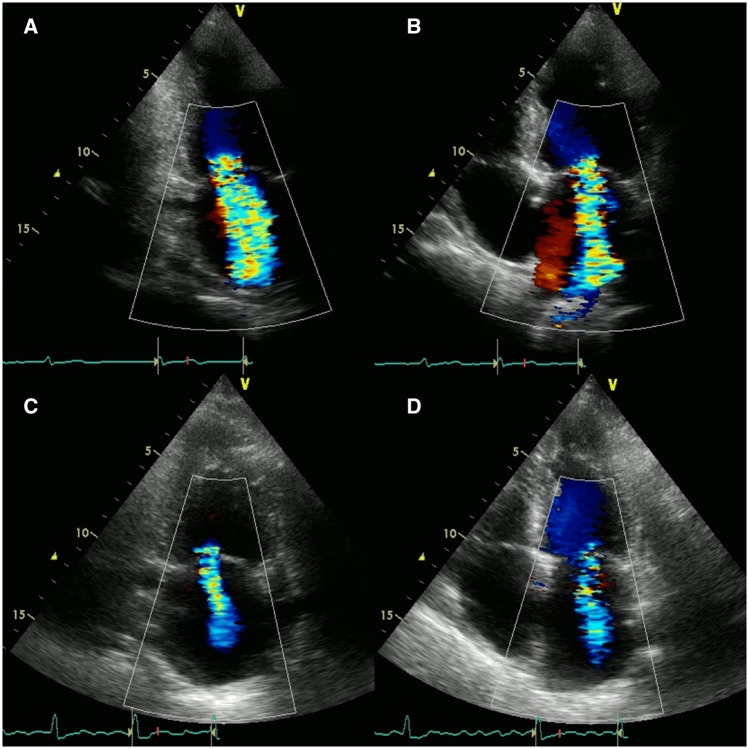
Transthoracic echocardiography (TTE) performed after the management of acute decompensated heart failure revealed preservation of left ventricular ejection fraction (60%) without regional wall motion abnormality. Left ventricular end-diastolic and end-systolic diameters were 49 mm and 30 mm, respectively. Severe MR was noted (regurgitant volume: 69 mL, effective regurgitant orifice area: 0.36 cm2) with degenerative changes in the mitral leaflets (Figure 1A and B).
Figure 1.
Transthoracic echocardiographic images at 11 days (pre-immunosuppressive therapy) showed severe mitral regurgitation (A and B). Transthoracic echocardiographic images at 87 days (post-immunosuppressive therapy) revealed significant reduction of mitral regurgitation with no obvious changes in ejection fraction and regional left ventricular wall motion (C and D).
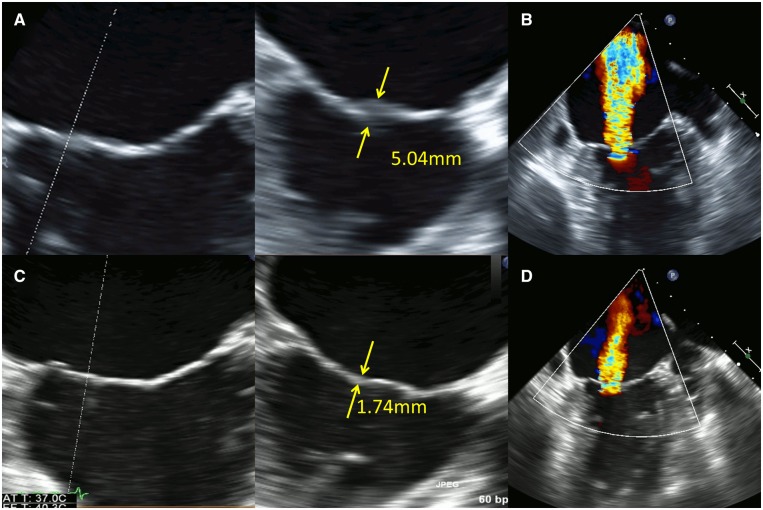
Transoesophageal echocardiography (TOE) revealed local low-echoic thickening of the medial edge of A2/P2 segments and the lateral edge of A3/P3 segments with poor coaptation of the mitral valve leaflets, causing severe MR (Figure 2A and B). No other orifice of MR was noted. The regurgitant volume was 61 mL, and the effective regurgitant orifice area was 0.37 cm2. Three-dimensional TOE (3D-TOE) images were acquired in multiple-beat full-volume mode. The vena contracta area was detected to be 0.42 cm2 using multiplanar reconstruction of 3D-TOE images. Significant tethering or prolapse of the leaflets was not found (tenting height: 3.6 mm, tenting area: 71.3 mm2). Furthermore, the possibility of bacterial endocarditis could not be ruled out on the basis of TTE and TOE and findings; however, blood cultures were obtained six times (two times each on three separate days) and showed negative results, suggesting that bacterial infection was a less likely cause of the degeneration of the mitral leaflets. According to the present guidelines for valvular disease,7 surgical, or percutaneous treatment for MR was indicated because of severe symptomatic MR with preserved ejection fraction.
Figure 2.
Transoesophageal echocardiographic images at 14 days (pre-immunosuppressive therapy) showed local low-echoic changes (thickness: 5.04 mm) of the medial edge of A2/P2 segments and the lateral edge of A3/P3 segments with poor coaptation of the mitral valve leaflets, causing severe mitral regurgitation (A and B). Transoesophageal echocardiographic images at 87 days (post-immunosuppressive therapy) elucidated the resolution of the oedematous changes in A2/P2 and A3/P3 segments and thinning of mitral valve leaflets (thickness: 1.74 mm) (C). Mitral regurgitation decreased from severe to mild (D).
The patient developed photosensitivity and livedo reticularis, and her pancytopenia gradually worsened [white blood cell count: 1900/μL (3500–9100/μL), red blood cell count: 3 400 000/μL (3 760 000–5 000 000/μL), and platelet count: 16 000/μL (130 000–369 000/μL) on the 16th hospital day] (Table 1). Results of immunological and autoantibody testing were as follows: anti-nuclear antibody titer, ×640 (≤×40); anti-cardiolipin beta-2 glycoprotein 1, 2.5 U/mL (≤3.5 U/mL); CH50, 19 U/mL (32–58 U/mL); C3, 59 mg/dL (86–160 mg/dL); C4, 6 mg/dL (17–45 mg/dL); and negative anti-Sm and positive anti-double-strand DNA-IgG. Based on these findings, the patient was diagnosed with Libman–Sacks endocarditis with rapidly progressive SLE.
Table 1.
Complete blood count and BNP level data
| 1 year ago | Day 0 | Day 15 | Day 58 | Day 87 | |
|---|---|---|---|---|---|
| Before admission | On admission | Pre-IST | Post-IST | TOE follow-up | |
| WBC count (/μL) (3500–9100/μL) | 7500 | 6900 | 1900 | 6000 | 5900 |
| RBC count (/μL) (3 760 000–5 000 000/μL) | 3 820 000 | 3 540 000 | 3 400 000 | 3 580 000 | 3 620 000 |
| Hb levels (g/L) (113–152 g/L) | 113 | 99 | 96 | 104 | 107 |
| Plt count (/μL) (130 000–369 000/μL) | 213 000 | 59 000 | 16 000 | 156 000 | 178 000 |
| BNP levels (pg/mL) (<18.4 pg/mL) | 55 | 514 | 162 | — | 86 |
BNP, B-type natriuretic peptide; Hb, haemoglobin; IST, immunosuppressive therapy; Plt, platelet; RBC, red blood cell; TOE, transoesophageal echocardiography; WBC, white blood cell.
Because the oedematous changes in the mitral valve leaflets were considered to be caused by Libman–Sacks endocarditis, surgery for MR was postponed. Prednisolone (60 mg/day), cyclosporine (2000 mg/day), and hydroxychloroquine sulfate (600 mg/day) administration gradually improved the symptoms and pancytopenia (Table 1). A follow-up TTE at 87 days showed significant reduction of MR with no obvious changes in ejection fraction and regional left ventricular wall motion (Figure 1C and D). Transoesophageal echocardiography at the same day revealed that the coaptation of the mitral valve had improved following the resolution of the oedematous changes in A2/P2 and A3/P3 segments (Figure 2C). Mitral regurgitation decreased from severe to mild (regurgitant volume: 32 mL, effective regurgitant orifice area: 0.17 cm2, vena contracta area: 0.21 cm2) (Figure 2D).
Discussion
Libman–Sacks endocarditis is a cardiac manifestation of SLE characterized by sterile and verrucous valvular lesions with a predisposition for the left-sided heart valves, especially the ventricular surface of the mitral valve.8,9 While a TTE study has reported that Libman–Sacks endocarditis occurs in approximately 11% of SLE patients, other studies using TOE have reported its higher prevalence (53–74%).8,10,11 Libman–Sacks-related valvular dysfunction ranges from a variable degree of inflammatory cell infiltration along with oedematous changes and fibrin deposits to end stage or healed forms with a fibrous plaque. The degree of progression is associated with SLE duration and activity.5 The pathogenesis involves the formation of fibrin–platelet thrombi due to inflammation, which organize and lead to fibrosis and scarring with subsequent valve dysfunction over time.12
While appropriate immunosuppressive therapy is essential to control SLE disease activity, corticosteroid therapy for Libman–Sacks-related valvular dysfunction reportedly results in increased fibrosis and scarring, followed by worsened valvular damage and dysfunction.6,9 Furthermore, in part due to mineralocorticoid effects, worsening MR with decompensated heart failure may occur after high-dose corticosteroid therapy.5,13 In our patient, MR decreased from severe to mild after immunosuppressive therapy including corticosteroids. Because low-echoic thickening of the mitral valve leaflets suggested acute oedematous changes without scarring and fibrosis and other symptoms of SLE progressed rapidly, immunosuppressive therapy was initiated early in our patient, which could be a reason for the benign clinical course of the disease.
While mitral valve surgery or percutaneous intervention is indicated for severe symptomatic MR,7,14,15 immunosuppressive therapy may be a possible treatment option for early-phase Libman–Sacks endocarditis-related valvular dysfunction.
Libman–Sacks endocarditis is a rare cause of degenerative MR. Immunosuppressive therapy including corticosteroids is a possible treatment option for early-phase Libman–Sacks endocarditis–related valvular dysfunction.
Patient perspective
Our patient was satisfied with the relief of symptoms due to reduction of mitral regurgitation following immunosuppressive therapy without the need for open-heart surgery or a percutaneous intervention.
Lead author biography

Kenichi Ishizu graduated from Kyoto University and started his career in Kobe City Medical Center General Hospital, Kobe, Japan. He is a general cardiology physician in the Department of Cardiovascular Medicine, Kokura Memorial Hospital, Kitakyushu, Japan, since 2018.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Libman E, Sacks B.. A hitherto undescribed form of valvular and mural endocarditis. Arch Intern Med 1924;33:701–737. [Google Scholar]
- 2. Bouma W, Klinkenberg TJ, van der Horst ICC, Wijdh-den Hamer IJ, Erasmus ME, Bijl M, Suurmeijer AJ, Zijlstra F, Mariani MA.. Mitral valve surgery for mitral regurgitation caused by Libman-Sacks endocarditis: a report of four cases and a systemic review of the literature. J Cardiothorac Surg 2010;5:13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takayama T, Teramura M, Sakai H, Tamaki S, Okabayashi T, Kawashima T, Yamamoto T, Horie M, Suzuki T, Asai T.. Perforated mitral valve aneurysm associated with Libman-Sacks endocarditis. Intern Med 2008;47:1605–1608. [DOI] [PubMed] [Google Scholar]
- 4. Mottram PM, Gelman JS.. Mitral valve thrombus mimicking a primary tumor in the antiphospholipid syndrome. J Am Soc Echocardiogr 2002;15:746–748. [DOI] [PubMed] [Google Scholar]
- 5. Hachiya K, Wakami K, Tani T, Yoshida A, Suzuki S, Suda H, Ohte N.. Double-valve replacement for mitral and aortic regurgitation in a patient with Libman-Sacks endocarditis. Intern Med 2014;53:1769–1773. [DOI] [PubMed] [Google Scholar]
- 6. Morin AM, Boyer AS, Nataf P, Gandjbakhch I.. Mitral insufficiency caused by systemic lupus erythematosus requiring valve replacement: three case reports and a review of the literature. Thorac Cardiovasc Surg 1996;44:313–316. [DOI] [PubMed] [Google Scholar]
- 7. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2786.28886619 [Google Scholar]
- 8. Roldan CA, Shively BK, Crawford MH.. An echocardiographic study of valvular heart disease associated with systemic lupus erythematosus. N Engl J Med 1996;335:1424–1430. [DOI] [PubMed] [Google Scholar]
- 9. Hojnik M, George J, Ziporen L, Shoenfeld Y.. Heart involvement (Libman Sacks endocarditis) in the antiphospholipid syndrome. Circulation 1996;93:1579–1587. [DOI] [PubMed] [Google Scholar]
- 10. Moyssakis I, Tektonidou MG, Vasilliou VA, Samarkos M, Votteas V, Moutsopoulos HM.. Libman-Sacks endocarditis in systemic lupus erythematosus: prevalence, associations, and evolution. Am J Med 2007;120:636–642. [DOI] [PubMed] [Google Scholar]
- 11. Roldan CA, Shively BK, Lau CC, Gurule FT, Smith EA, Crawford MH.. Systemic lupus erythematosus valve disease by transesophageal echocardiography and the role of antiphospholipid antibodies. J Am Coll Cardiol 1992;20:1127–1134. [DOI] [PubMed] [Google Scholar]
- 12. Chartash EK, Lans DM, Paget SA, Qamar T, Lockshin MD.. Aortic insufficiency and mitral regurgitation in patients with systemic lupus erythematosus and the antiphospholipid syndrome. Am J Med 1989;86:407–412. [DOI] [PubMed] [Google Scholar]
- 13. Hoffman R, Lethen H, Zunker U, Schöndube FA, Maurin N, Sieberth HG.. Rapid appearance of severe mitral regurgitation under high-dosage corticosteroid therapy in a patient with systemic lupus erythematosus. Eur Heart J 1994;15:138–139. [DOI] [PubMed] [Google Scholar]
- 14. Obadia J-F, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu J-N, Cormier B, Armoiry X, Boutitie F, Maucort-Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N.. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297–2306. [DOI] [PubMed] [Google Scholar]
- 15. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ.. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.