Abstract
Heart failure with preserved ejection fraction (HFpEF) currently represents approximately 50% of heart failure (HF) cases in the USA and is increasingly recognized as a leading cause of morbidity and mortality. Recent data suggest that the prevalence of HFpEF relative to HF with reduced ejection fraction (HFrEF) is increasing at a rate of 1% per year. With an aging population and increasing risk factors such as hypertension, obesity, and diabetes mellitus, HFpEF will soon be the most prevalent HF phenotype. Two-dimensional speckle-tracking echocardiography (STE) has been used to diagnose HFpEF specifically by focusing on the longitudinal systolic function of the left ventricle (LV). Yet there are many patients with HFpEF in whom there are no differences in LV global longitudinal systolic strain, but there are changes in left atrial function and structure. There are several proposed pathophysiological mechanisms for HFpEF such as endothelial dysfunction, interactions among proteins, signaling pathways, and myocardial bioenergetics. Yet only one specific therapy, mineralocorticoid receptor antagonist, spironolactone, is recommended as a treatment for patients with HFpEF. However, spironolactone does not address many of the pathophysiologic changes that occur in HFpEF, thus new novel therapeutic agents are needed. With the limited available therapies, clinicians should use STE to assess for the presence of this syndrome in their patients to provide effective diagnosis and management.
Keywords: Speckle tracking, Heart failure with preserved ejection fraction, Diagnostic testing
Introduction
With the aging population, the number of patients with heart failure is rising along with significant morbidity and mortality [1]. Heart failure (HF) is the most common diagnosis in patients 65 years or older in high-income nations [2]. It is expected that by the year 2030 there will be a 46% increase in the occurrence of HF with approximately 960,000 new cases each year. HF is often defined as a complex clinical syndrome related to functional or structural impairment of ventricular filling or ejection of blood [3, 4]. In 2017 the American College of Cardiology and the American Heart Association (ACC/AHA) established committees that continuously review, update, and modify guidelines for heart failure [5]. There are two major categories of heart failure that are based on systolic and diastolic dysfunction. If the left ventricular ejection fraction (LVEF) is below 40%, the patient typically has a dilated heart with systolic dysfunction and is diagnosed with HF with reduced ejection fraction (HFrEF). However, if the patient presents with a LVEF that is equal to or greater than 50%, there is irregularity in the filling properties, and the patient is diagnosed with HF with preserved ejection fraction (HFpEF) [6, 7]. The definition of HF has now expanded to include HFpEF as borderline (EF 40–49%) and HFpEF improved (EF > 50%). Approximately 50% of patients with this clinical syndrome have HFpEF because of the additional risk factors of aging, hypertension, obesity, and coronary artery disease [7, 8]. Thus, accurate and precise diagnostic assessment of the myocardium using echocardiography is essential.
Two-dimensional (2D) echocardiography is used for patients with HF to visually assess left ventricular (LV) function and deformation. Often the echocardiographic interpretations are subjective and result in semi-quantitative data. A more sophisticated software package called speckle-tracking echocardiography (STE) provides superior quantification of both regional and global LV systolic and diastolic function [9]. With new imaging and software capability, clinicians and researchers can better evaluate the left atrium (LA), which is a crucial measurement in HF [10]. The development in recent years of using STE to examine myocardial deformation and imaging the LA and LV has allowed more precise evaluation of the myocardium in patients with HFpEF. Early detection of diastolic dysfunction by STE would improve clinicians’ understanding and management of the signs and symptoms of HFpEF. In this review, we summarize the pathophysiology and clinical evaluation of HFpEF and the importance of the use of STE in these patients.
Pathophysiology of HFrEF and HFpEF
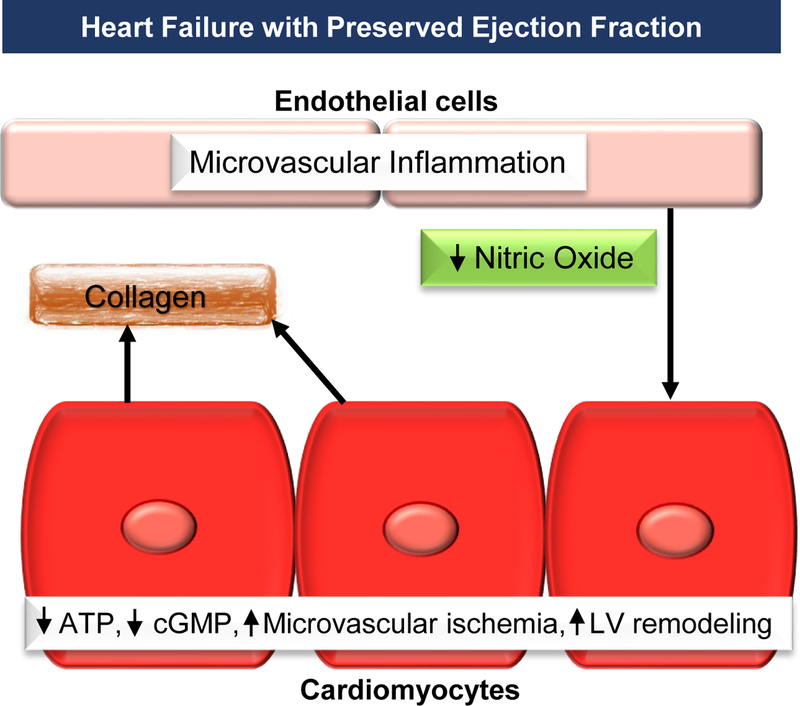
Many factors have been identified related to the mechanisms associated with HFpEF; however, there is no consensus on the exact pathophysiology. HFpEF is related to inflammation and endothelial dysfunction, abnormal ventricular-arterial coupling, chronotropic deficiencies, diastolic dysfunction, pulmonary hypertension, impaired systolic function, and particularly altered myocardial bioenergetics [11]. One mechanism of HFpEF that relates to endothelial dysfunction results in cardiomyocyte dysfunction, LV remodeling, and diastolic dysfunction. When microvascular inflammation and endothelial activation occur, there is a reduction in nitric oxide, adenosine triphosphate (ATP), and cyclic guanosine monophosphate (cGMP). This results in changes in titin with microvascular ischemia, LV remodeling, and fibrosis (Fig. 1).
Figure 1.
In heart failure with preserved ejection fraction there is microvascular inflammation that activates the endothelial to begin a cascade of adverse effects that leads to left ventricular dysfunction.
ATP, adenosine triphosphate; cGMP, cyclic guanosine monophosphate; LV, left ventricular.
Myocardial remodeling occurs in both HFrEF and HFpEF; however, in HFpEF there is no effective therapeutic strategy to review the remodeling. There are studies suggesting interactions among proteins, signaling pathways, and cardiac bioenergetics. When there is an increase in myocardial adenosine diphosphate (ADP) in the presence of diastolic Ca2+, there is diastolic dysfunction from actin-myosin interactions. In other words, an increase in myocardial Ca2+ sensitivity and stiffness reduces diastolic sarcomere length [12–14]. These elevated Ca2+ levels in HFpEF are not due to impaired regulatory proteins, Na+ gradient, or ion transporters [15].
The myocardium requires a tremendous amount of ATP and the heart has only enough stored ATP for three contractions. Thus, myocardial ATP supply and demand is crucial to maintain proper function. There is active relaxation within cardiac myocytes that requires the actin and myosin cross-bridges to disconnect with lower ADP cytosolic calcium concentrations and increased ATP concentration. Most of the ATP in the myocardium is derived from fatty acids coupled to mitochondrial oxidative phosphorylation [16]. Deficiencies in the cardiac energetics have been found in patients with HFpEF [17]. Several investigators postulate that HFpEF is related to inefficient or decreased ATP conversion, transfer, or concentration [18]. In HFpEF, there is a decrease in myocardial energy reserve associated with a decline in mitochondrial function [12, 19]. Recent studies suggest that in HFpEF, production of myocardial reactive oxygen species (ROS), damages mitochondria, which leads to deterioration of ATP production [20–22]. Thus, using targeted mitochondrial antioxidants may improve mitochondrial respiration and ATP production and reduce the symptoms of HFpEF [23–26].
Signs and Symptoms of HFpEF
According to population-based studies and registries, HFpEF patients are predominantly female and elderly, with high rates of comorbidities such as obesity, hypertension, chronic kidney disease, coronary artery disease (CAD), anemia, hyperlipidemia, diabetes mellitus, and atrial fibrillation (Table 1) [27–31]. Patients with HFpEF are as functionally limited as their counterparts with HFrEF, require frequent hospitalizations, and have generally poor quality of life [27, 30, 32, 33]. Survival of patients with HFpEF is poor and similar to that of patients with HFrEF; observational studies show a concerning 5-year survival of only 35 to 40% post-hospitalization for HF [27, 34].
Table 1.
Clinical characteristics and predisposing factors for heart failure with preserved ejection fraction
| Older Age | Atrial Fibrillation |
| Female | Cardiac Valve Disease |
| History of Hypertension | Infiltrative Processes Amyloidosis Hemochromatosis Sarcoidosis |
| Obesity | |
| Chronic Kidney Disease | |
| Coronary Artery Disease | |
| Anemia | Hypertrophic Cardiomyopathy |
| Hyperlipidemia | Pericardial Disease Constrictive Pericarditis |
| Diabetes | |
Patients with HFpEF present with signs and symptoms that are often indistinguishable from those with HFrEF. As with any patient who has a clinical picture consistent with HF, other entities should be considered such as pulmonary embolus, pneumonia, chronic obstructive pulmonary disease, and pneumothorax. Signs and symptoms of HFpEF are listed in Table 2.
Table 2.
The most common symptoms and signs of heart failure with preserved ejection fraction
| Shortness of Breath with Exertion or at Rest | Paroxysmal Nocturnal Dyspnea |
| Decreased Exercise Tolerance | Jugular Venous Distension |
| Fatigue | Rales |
| Chest Discomfort | Cardiac Gallop Sounds (S3, S4,) |
| Swelling in the Lower Extremities (Edema) | Hepatomegaly |
| Shortness of Breath Lying Flat (Orthopnea) | |
Diagnosis of HFpEF with Echocardiography
The diagnosis of HFpEF is challenging and often based on exclusion of HFrEF in subjects who clinically seem to have HF. Diagnostic testing in most patients relies on echocardiographic analysis (Table 3), although in some cases invasive hemodynamic testing is needed to confirm the diagnosis [35–38]. Exercise may demonstrate evidence of diastolic function that is not apparent at rest [36]. Realistically, invasive testing is not practical for most patients and will not be discussed in this review [39]. Patients with suspected HF should be referred for 2D transthoracic echocardiography to confirm the diagnosis and identify preserved or reduced ejection fraction. This includes those patients with elevated brain natriuretic peptide (BNP) levels or physical examination findings suggestive of HF.
Table 3.
Measurements from standard and speckle-tracking echocardiography.
| Standard Echocardiogram | Speckle Tracking |
|---|---|
| Ejection Fraction (%) | Reservoir |
| LVEDV | Global strain (%) |
| LVESV | Global strain rate (%) |
| LVMI | Conduit |
| LA Volume | Global strain (%) |
| LAVI | Global strain rate (%) |
| LA Ejection Fraction (%) | Booster Pump |
| Peak E-wave Velocity | Global strain (%) |
| Peak A-wave Velocity | Global strain rate |
| E/A ratio | Contraction strain index |
| Deceleration Time (DT) | Stiffness (mmHg) |
| Mitral A wave duration | LV Global Longitudinal Strain |
| Pulmonary venous flow assessment • Peak systolic pulmonary venous inflow velocity during ventricular systole (Smax) • Peak diastolic pulmonary venous inflow velocity during early phase of atrial diastole (Dmax) • Systolic/Diastolic ratio (S/D ratio) • Peak reversed systolic A wave during atrial contraction (AV max) • A wave duration (pulmonary A dur) |
|
| Septal e’ | |
| Lateral e’ | |
| Mean E/e’ | |
| IVRT |
A, late mitral inflow A wave (m/s); E, early mitral inflow E wave (m/s); e’, early diastolic mitral annular tissue velocity; IVRT, isovolumic relaxation time; LA, left atrial; LAVI, left atrial volume index; LV, left ventricular; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; LVMI, left ventricular mass index; %, percent; mmHg, millimeters of mercury.
LV diastolic function is determined by LV relaxation and compliance properties, which can be assessed non-invasively via Doppler techniques in the echocardiography laboratory [36, 37]. Traditional Doppler-derived assessments of diastolic function are obtained from transmitral and pulmonary venous recordings, but they are dependent upon loading conditions. Other parameters such as tissue Doppler echocardiography (TDE) have been developed that exhibit relatively linear properties and are independent of loading conditions. These modalities can be used to complement traditional Doppler methods for the assessment of diastolic function in subjects undergoing echocardiographic examination [38, 40–43].
The most important echocardiographic measurement for determining of diastolic dysfunction is the E/e’ ratio: E represents the peak velocity of transmitral flow in early diastole, as assessed by pulsed wave Doppler; and e’ represents either the early diastolic septal or lateral lengthening peak velocity of the mitral annulus, measured with tissue Doppler. While e’ is a reflection of the amount of blood entering the ventricle and mainly related to ventricular relaxation/LV filling pressures, E is felt to be an estimate of the maximum pressure differences between the LA and LV and therefore is primarily dependent on both ventricular relaxation and LA pressures [42].
E/e’ ratio is thought to reflect LA pressures and thus LV end-diastolic pressure. E/e’ is generally assumed to be less sensitive to preload than other echocardiographic indices of diastolic dysfunction and therefore yields more accurate estimations of filling pressures. Studies suggest that although E/e’ is a hallmark of diastolic dysfunction, it is not sensitive enough to detect HFpEF in outpatients with unexplained dyspnea in an early stage of the disease when impairments in diastole are less prominent. The absence of an elevated E/e’ therefore does not rule out the presence of diastolic dysfunction [44, 45].
Although assessment of diastolic function by echocardiography has been a key element of the diagnostic evaluation of HFpEF [36], it is recognized that these techniques have many limitations [39, 46]. Subjects may meet criteria for diastolic dysfunction but not have the clinical syndrome of HFpEF. Other patients may have clinical heart failure in the setting of preserved EF but do not meet criteria for the diagnosis of diastolic dysfunction. The algorithm for diastolic function assessment is largely empirical and based on expert opinion, and it may not apply to all patient subsets such as the elderly and patients with valve disease, arrhythmias, or other cardiac disease states. Additional modalities for evaluation of HFpEF are much needed and will be discussed later in this review.
Role of Brain Natriuretic Peptic in the Diagnosis and Management of HFpEF
BNP, also known as B-type natriuretic peptide, is a hormone secreted by cardiomyocytes in the heart ventricles in response to stretching caused by increased ventricular blood volume [47–50]. BNP or its precursor N-terminal pro b-type natriuretic peptide (NT-proBNP) may be elevated in the setting of either HFrEF or HFpEF, although these markers tend to be higher in HFrEF than HFpEF [51]. There is no identifiable BNP threshold that effectively distinguishes HFpEF from HFrEF [52]. BNP measurements are often not useful for obese patients with HFpEF because their BNP levels are usually very low [53]. Both markers are elevated with acute deterioration of HFpEF, but these assays are not specific for diagnosis.
Based on the latest information from the 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure [5], measurement of natriuretic peptide biomarkers is useful to support a diagnosis or exclusion of HF in patients presenting with dyspnea. In addition, per these guidelines, measurement of BNP or NT-proBNP is useful for establishing prognosis or disease severity in chronic HF and measurement of baseline levels of natriuretic peptide biomarkers and/or cardiac troponin on admission to the hospital is useful to establish a prognosis in acutely decompensated HF. During a hospitalization for HF, a pre-discharge natriuretic peptide level can be useful to establish a post-discharge prognosis [5].
Suggested values for diagnosis of acute HF are BNP >100 pg/mL or NT-proBNP >300 pg/mL [54, 55]. Of note, these values are independent predictors of adverse cardiovascular events in patients with HFpEF. For a given BNP level, the prognosis in patients with HFpEF is similar to that in patients with HFrEF, although criteria vary based on assay and consideration of sensitivity versus specificity. Age, gender, weight, and comorbidities can impact levels of these factors [53, 56].
Speckle Tracking Echocardiography (STE)
HFpEF is a malady of fatigue and exertional dyspnea in which STE plays a key role in both the evaluation and management of the syndrome [57]. The introduction of speckle tracking to echocardiography was initially reported in 2004 by Reisner et al., using global longitudinal strain [58]. Conventional electrocardiography continues to be useful in imaging cardiac and other structures; however, STE has become increasingly useful for the critical evaluation of myocardial function. Table 3 lists common measurements used in standard echocardiography and STE [59]. Usual measurements such as ejection fraction and E/A ratio are universal measures but are limited. Now with STE, clinicians can obtain more specific data related to strain of both the atria and ventricles. The applications of STE have expanded to include a multitude of disease processes with or without myocardial involvement such as amyloidosis [60], inflammatory diseases, rheumatoid arthritis, and neoplasia [61]. The focus of this review is the use of STE for individuals who have HFpEF.
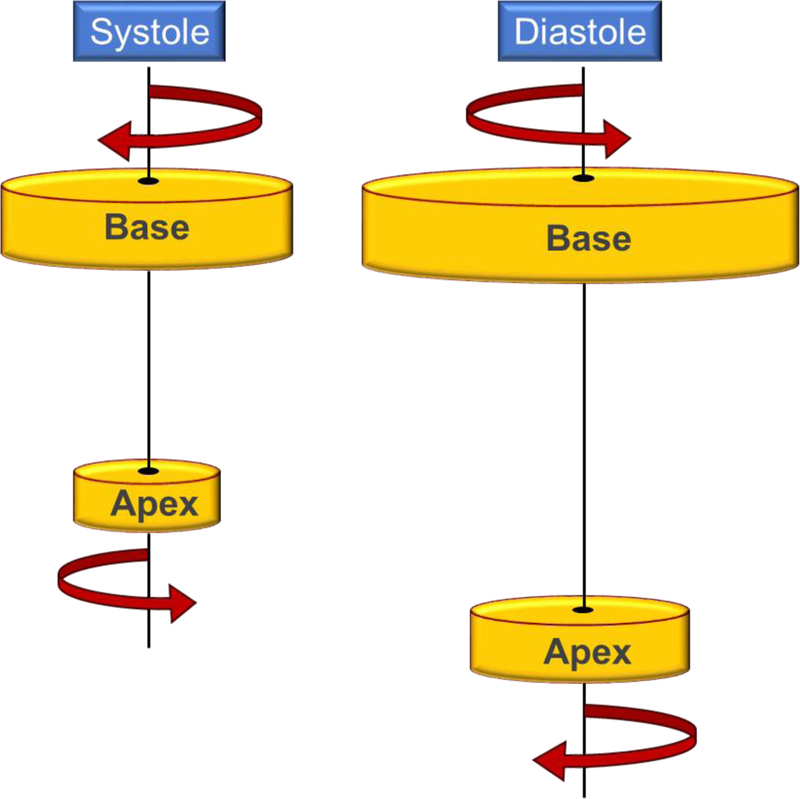
Understanding HFpEF requires an understanding of the anatomy of the myocardium that contributes uniquely to the heart’s ability to contract. There are myocardial spiral muscle layers, attached in angles from perpendicular to horizontal. The three inner layers spiral in an opposing direction to the outer three layers, with fourth connecting layer. Figure 2 is an illustration of ventricular torsion in relation to ventricular fiber arrangement. There is opposing rotation in the base and apex both in systole and diastole, comparable to the twisting and untwisting of a towel [62].
Figure 2.
An illustration of how the left ventricle rotates during systole and diastole.
STE generates backscatter myocardial images produced by an ultrasound beam that is tracked frame by frame, statistically matching the best analogous area. The images are obtained by a trained echocardiogram specialist who understands the need to acquire clear recordings of all the myocardial chambers. STE complements the conventional echocardiogram by using software that enables measure of rotation, twist, and torsion by degrees and torsional gradient. A typical cluster of STE measurements could include the reservoir, conduit, and booster pump, LA stiffness index, and both left and right atrial strain and LV longitudinal strain. A growing number of echocardiographic instruments have STE software for clinicians’ use [63].
Most STE hardware and software are 2D, which has length and height as its dimensions. More recently, three-dimensional STE is being performed that not only includes length, and height but also width (depth). One significant advantage of 3D STE is that automated images are available that have higher resolution and greater prognostic power for cardiac death, nonfatal myocardial infarction, stroke, and admission for heart failure [64, 65].
Role of Left Atrial Strain in Evaluating HFpEF with Speckle Tracking
Atrial strain is the study of the function of the LA, which cardiologists believe is imperative when evaluating patients with HFpEF. With STE, understanding myocardial displacement and deformation is important. Cardiac fiber displacement from a given position is distinguishable from myocardial deformation because displacement reflects a change in the myocardial fiber dimensions over the cardiac cycle. This is calculated as the change in the initial length (L0) from the final length (L), divided by initial length, strain Strain is positive if L increases, and negative if L decreases. While calculation of strain in one dimension is mathematically simple, measuring strain in two or three dimensions is substantially more complex [66]. The LA regulates LV filling by affecting reservoir, conduit, and booster pump function [67]. LA strain is load dependent, affected by LV function, and may be used to assess LV diastolic function and filling pressure. LA function improves as LA volume decreases, and atrial dysrhythmias increase as LA strain increases [68]. The LA strain has been shown to be a more accurate measure of adverse events than either LV or RV longitudinal strain. Measurements obtained using conventional echocardiography and STE have similar results. The LA functional parameters from conventional echocardiography are useful for the measurement of myocardial function. However, STE uses the atrial longitudinal strain that has the greatest predictive accuracy, sensitivity (73%), and specificity (70%) [69]. STE evaluation of LA strain and strain rate are superior to conventional parameters of atrial function. LA strain also serves as a biomarker of the presence and outcomes of cardiovascular disease [70].
A decrease in LA strain is associated with HFpEF. If the LA volume index is >34 mL/m2, there is greater incidence of adverse events, including mortality, HF, atrial fibrillation, and ischemic stroke. It appears that LA strain may be associated with LA fibrosis and may be useful in evaluating therapeutic agents [71]. Decreased LA reservoir strain has also been shown to correlate with increased cardiovascular hospitalization or death [72]. The use of LV strain patterns including LVEF, strain curves, and bull’s-eye plots can be utilized to diagnose the underlying cause of HFpEF and evaluate subsequent response to therapy such as ubiquinol and D-ribose [73].
When using STE, the reservoir, conduit, and booster pump functions must be accurately measured to insure proper diagnoses and treatment. LA dimensions must be measured at ventricular end-systole, without foreshortening. The normal reference ranges for STE are: reservoir (39%), conduit (23%), and contractile (booster) (17%) [74]. If patients with HFpEF have paroxysmal atrial fibrillation and primary arterial hypertension, they may have decreased reservoir, conduit, and booster pump LA functions. This may lead to HF and can therefore be an early marker of HFpEF that is not found in standard echocardiographic measures. Conduit and reservoir functions decline prior to diagnosis of LV diastolic dysfunction [75]. Impaired LA reservoir and pump function have been reported in HFpEF patients who have atrial stiffness and shortness of breath during exercise [76]. Other studies have compared STE on patients with HFpEF at rest and during exercise to evaluate prognostic parameters associated with increased risk of hospitalizations. In addition, exercise stress STE may provide an improved assessment of patients with HFpEF and direct treatment options to reduce symptom burden [77–79].
In summary, STE has been demonstrated to provide incremental data beyond traditional echocardiographic parameters and can be used to monitor and guide the clinical course for patients with HFpEF [80]. Global longitudinal strain is a useful and robust marker of systolic function. Measuring LA strain is extremely useful as a technique to support the evaluation in the underlying diagnosis, disease management, and prognosis of HFpEF. Studies have shown that deformation of strain and strain-rate imaging have been previously validated but have not yet been integrated into clinical cardiology practices [81]. However, accuracy of the findings from STE depends on obtaining high quality images from trained echocardiographic technicians [82].
Conclusion
STE is an imaging modality that should be used to assess patients with HFpEF to provide clinicians with essential data about the patient’s myocardial mechanics, deformation, and strain parameters. This more quantitative, objective measure of cardiac function would be useful as an early indicator and progression of HF. STE is a diagnostic tool that may be useful to assess cardiac abnormalities when made in the early stages of the disease process. Additionally, STE may be helpful to monitor the impact of treatments for patients with HFpEF and guide future prospective studies.
Highlights:
Heart failure with preserved ejection fraction (HFpEF) is a major health condition
There are various theories on the pathophysiology of HFpEF
HFpEF can be diagnosed by the evaluation of brain natriuretic peptide levels and echocardiography
Speckle-tracking echocardiography is able to assess myocardial measurements
Funding
Sponsoring Information: Supported by the Department of Health and Human Services, National Institutes of Health, National Institute on Aging (Grant Number: 1R01AG054486-01A1). Trial registration: ClinicalTrials.gov Identifier:
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There is no conflict of interest for any of the authors to declare.
References
- [1].Magnussen C, Blankenberg S. Biomarkers for heart failure: small molecules with high clinical relevance. J Intern Med 2018;283(6):530–43. [DOI] [PubMed] [Google Scholar]
- [2].Braunwald E The war against heart failure: the Lancet lecture. Lancet 2015;385(9970):812–24. [DOI] [PubMed] [Google Scholar]
- [3].Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Smith KR, Hsu CC, Berei TJ, Aldemerdash A, Hollis IB, Vardeny O, et al. PARADIGM-HF Trial: Secondary Analyses Address Unanswered Questions. Pharmacotherapy 2018;38(2):284–98. [DOI] [PubMed] [Google Scholar]
- [5].Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136(6):e137–e61. [DOI] [PubMed] [Google Scholar]
- [6].Abebe TB, Gebreyohannes EA, Tefera YG, Abegaz TM. Patients with HFpEF and HFrEF have different clinical characteristics but similar prognosis: a retrospective cohort study. Bmc Cardiovasc Disor 2016;16:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med 2016;375(19):1868–77. [DOI] [PubMed] [Google Scholar]
- [8].Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14(10):591–602. [DOI] [PubMed] [Google Scholar]
- [9].Opdahl A, Helle-Valle T, Skulstad H, Smiseth OA. Strain, strain rate, torsion, and twist: echocardiographic evaluation. Curr Cardiol Rep 2015;17(3):568. [DOI] [PubMed] [Google Scholar]
- [10].Parthenakis F, Vardas P. The Pivotal Role Of Studying The Left Atrium By Speckle Tracking In Heart Failure. Hellenic J Cardiol 2016;57(1):30–2. [DOI] [PubMed] [Google Scholar]
- [11].Webb J, Fovargue L, Tondel K, Porter B, Sieniewicz B, Gould J, et al. The Emerging Role of Cardiac Magnetic Resonance Imaging in the Evaluation of Patients with HFpEF. Curr Heart Fail Rep 2018;15(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rodrigues PG, Leite-Moreira AF, Falcao-Pires I. Myocardial reverse remodeling: how far can we rewind? Am J Physiol Heart Circ Physiol 2016;310(11):H1402–22. [DOI] [PubMed] [Google Scholar]
- [13].Sequeira V, Najafi A, McConnell M, Fowler ED, Bollen IAE, Wust RCI, et al. Synergistic role of ADP and Ca2+ in diastolic myocardial stiffness. J Physiol-London 2015;593(17):3899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, et al. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail 2014;7(1):104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Runte KE, Bell SP, Selby DE, Haussler TN, Ashikaga T, LeWinter MM, et al. Relaxation and the Role of Calcium in Isolated Contracting Myocardium From Patients With Hypertensive Heart Disease and Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2017;10(8):e004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rech M, Aizpurua AB, van Empel V, van Bilsen M, Schroen B. Pathophysiological understanding of HFpEF: microRNAs as part of the puzzle. Cardiovasc Res 2018;114(6):782–93. [DOI] [PubMed] [Google Scholar]
- [17].Phan TT, Abozguia K, Shivu GN, Mahadevan G, Ahmed I, Williams L, et al. Heart Failure With Preserved Ejection Fraction Is Characterized by Dynamic Impairment of Active Relaxation and Contraction of the Left Ventricle on Exercise and Associated With Myocardial Energy Deficiency. Journal of the American College of Cardiology 2009;54(5):402–9. [DOI] [PubMed] [Google Scholar]
- [18].Sequeira V, van de Velden J. The failing heart: an engine operating on “bad fuel”. Heart Metab 2016;71:37–9. [Google Scholar]
- [19].Luptak I, Sverdlov AL, Panagia M, Qin FZ, Pimentel DR, Croteau D, et al. Decreased ATP production and myocardial contractile reserve in metabolic heart disease. J Mol Cell Cardiol 2018;116:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mahmod M, Pal N, Rayner J, Holloway C, Raman B, Dass S, et al. The interplay between metabolic alterations, diastolic strain rate and exercise capacity in mild heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. J Cardiovasc Magn R 2018;20(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nickel A, Kohlhaas M, Maack C. Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol 2014;73:26–33. [DOI] [PubMed] [Google Scholar]
- [22].Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, et al. Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights Into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic Transl Sci 2018;3(5):575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Birk AV, Liu SY, Soong Y, Mills W, Singh P, Warren JD, et al. The Mitochondrial-Targeted Compound SS-31 Re-Energizes Ischemic Mitochondria by Interacting with Cardiolipin. J Am Soc Nephrol 2013;24(8):1250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ha CM, Wende AR. The Growing Case for Use of SGLT2i in Heart Failure: Additional Benefits of Empagliflozin in a HFpEF Rodent Model. JACC: Basic to Translational Science 2019;4(1):38–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sharma A, Fonarow GC, Butler J, Ezekowitz JA, Felker GM. Coenzyme Q10 and Heart Failure: A State-of-the-Art Review. Circ-Heart Fail 2016;9(4):e002639. [DOI] [PubMed] [Google Scholar]
- [26].Munzel T, Gori T, Keaney JF Jr., Maack C, Daiber A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J 2015;36(38):2555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of Heart Failure with Preserved Ejection Fraction in a Population-Based Study. New England Journal of Medicine 2006;355:260–9. [DOI] [PubMed] [Google Scholar]
- [28].Lam CSP, Carson PE, Anand IS, Rector TS, Kuskowski M, Komajda M, et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: The Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circulation: Heart Failure 2012;5(5):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lam CSP, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. European Journal of Heart Failure 2011;13(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circulation: Heart Failure 2013;6(2):184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: A report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. Journal of the American College of Cardiology 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- [32].Hoekstra T, Lesman-Leegte I, Van Veldhuisen DJ, Sanderman R, Jaarsma T. Quality of life is impaired similarly in heart failure patients with preserved and reduced ejection fraction. European Journal of Heart Failure 2011;13:1013–8. [DOI] [PubMed] [Google Scholar]
- [33].Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- [34].Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- [35].Huis in ‘t Veld AE, de Man FS, van Rossum AC, Handoko ML. How to diagnose heart failure with preserved ejection fraction: The value of invasive stress testing. Netherlands Heart Journal 2016;24:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal – Cardiovascular Imaging 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- [37].Oh JK, Hatle L, Jamil Tajik A, Little WC, Carolina N. Diastolic Heart Failure Can Be Diagnosed by Comprehensive Two-Dimensional and Doppler Echocardiography. Journal of the American College of Cardiolog 2006;47:500–6. [DOI] [PubMed] [Google Scholar]
- [38].Shin HW, Kim H, Son J, Yoon HJ, Park HS, Cho YK, et al. Tissue Doppler Imaging as a Prognostic Marker for Cardiovascular Events in Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. Journal of the American Society of Echocardiography 2010;23:755–61. [DOI] [PubMed] [Google Scholar]
- [39].Vaidya GN, Abramov D. Echocardiographic Evaluation of Diastolic Function Is of Limited Value in the Diagnosis and Management of HFpEF. J Card Fail 2018;24(6):392–6. [DOI] [PubMed] [Google Scholar]
- [40].Ho CY, Solomon SD. A clinician’s guide to tissue doppler imaging. Circulation 2006;113(10):e396–8. [DOI] [PubMed] [Google Scholar]
- [41].Kadappu KK, Thomas L. Tissue doppler imaging in echocardiography: Value and limitations. Heart Lung and Circulation 2015;24:224–33. [DOI] [PubMed] [Google Scholar]
- [42].Mitter SS, Shah SJ, Thomas JD. A Test in Context: E/A and E/e′ to Assess Diastolic Dysfunction and LV Filling Pressure. Journal of the American College of Cardiology 2017;69:1451–64. [DOI] [PubMed] [Google Scholar]
- [43].Srivastava PM, Burrell LM, Calafiore P. Lateral vs medial mitral annular tissue Doppler in the echocardiographic assessment of diastolic function and filling pressures: Which should we use? European Journal of Echocardiography 2005;6:97–106. [DOI] [PubMed] [Google Scholar]
- [44].Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, et al. Heart Failure With Preserved Ejection Fraction in Outpatients With Unexplained Dyspnea. A Pressure-Volume Loop Analysis. Journal of the American College of Cardiology 2010;55:1701–10. [DOI] [PubMed] [Google Scholar]
- [45].Santos M, Rivero J, McCullough SD, West E, Opotowsky AR, Waxman AB, et al. E/e′ ratio in patients with unexplained dyspnea lack of accuracy in estimating left ventricular filling pressure. Circulation: Heart Failure 2015;8:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Argulian E, Chandrashekhar Y, Shah SJ, Huttin O, Pitt B, Zannad F, et al. Teasing Apart Heart Failure with Preserved Ejection Fraction Phenotypes with Echocardiographic Imaging: Potential Approach to Research and Clinical Practice. Circulation Research 2018;122:23–5. [DOI] [PubMed] [Google Scholar]
- [47].Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. The American journal of cardiology 2012;110:870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Harada E, Mizuno Y, Kugimiya F, Shono M, Maeda H, Yano N, et al. B-Type Natriuretic Peptide in Heart Failure With Preserved Ejection Fraction — Relevance to Age-Related Left Ventricular Modeling in Japanese —. Circulation Journal 2017;81:1006–13. [DOI] [PubMed] [Google Scholar]
- [49].Kasahara S, Sakata Y, Yamauchi T, Onose T, Tsuji K, Abe R, et al. Comparable Prognostic Impacts of BNP Levels between HFpEF and HFrEF -A Report from the CHART-2 Study-. J Card Fail 2016;22:S211. [DOI] [PubMed] [Google Scholar]
- [50].Yoo B-S. L ifestyle Clinical Significance of B-type Natriuretic Peptide in Heart Failure. Journal of lifestyle medicine 2014;4:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kang SH, Park JJ, Choi DJ, Yoon CH, Oh IY, Kang SM, et al. Prognostic value of NT-proBNP in heart failure with preserved versus reduced EF. Heart 2015;101:1881–8. [DOI] [PubMed] [Google Scholar]
- [52].Van Veldhuisen DJ, Linssen GCM, Jaarsma T, Van Gilst WH, Hoes AW, Tijssen JGP, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. Journal of the American College of Cardiology 2013;61:1498–506. [DOI] [PubMed] [Google Scholar]
- [53].Meijers WC, Hoekstra T, Jaarsma T, van Veldhuisen DJ, de Boer RA. Patients with heart failure with preserved ejection fraction and low levels of natriuretic peptides. Neth Heart J 2016;24(4):287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Daniels LB, Maisel AS. Natriuretic Peptides. Journal of the American College of Cardiology 2007;50:2357–68. [DOI] [PubMed] [Google Scholar]
- [55].Martinez-Rumayor A, Richards AM, Burnett JC, Januzzi JL. Biology of the Natriuretic Peptides. American Journal of Cardiology 2008;101:S3–S8. [DOI] [PubMed] [Google Scholar]
- [56].AbouEzzeddine OF, French B, Mirzoyev SA, Jaffe AS, Levy WC, Fang JC, et al. From statistical significance to clinical relevance: A simple algorithm to integrate brain natriuretic peptide and the Seattle Heart Failure Model for risk stratification in heart failure. J Heart Lung Transplant 2016;35(6):714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Obokata M, Reddy YNV, Borlaug BA. The Role of Echocardiography in Heart Failure with Preserved Ejection Fraction: What Do We Want from Imaging? Heart Fail Clin 2019;15(2):241–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr 2004;17(6):630–3. [DOI] [PubMed] [Google Scholar]
- [59].Telles F, Marwick TH. Imaging and Management of Heart Failure and Preserved Ejection Fraction. Curr Treat Options Cardiovasc Med 2018;20(11):90. [DOI] [PubMed] [Google Scholar]
- [60].Di Bella G, Minutoli F, Mazzeo A, Stancanelli C, Gentile L, Baldari S, et al. Quantitative comparison between amyloid deposition detected by 99mTc-diphosphonate imaging and myocardial deformation evaluated by strain echocardiography in transthyretin related cardiac amyloidosis. Orphanet Journal of Rare Diseases 2015;10 (Suppl 1):47.25896868 [Google Scholar]
- [61].Naseem M, Samir S, Ibrahim IK, Khedr L, Shahba AA. 2-D speckle-tracking assessment of left and right ventricular function in rheumatoid arthritis patients with and without disease activity. J Saudi Heart Assoc 2019;31(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Blessberger H, Binder T. NON-invasive imaging: Two dimensional speckle tracking echocardiography: basic principles. Heart 2010;96(9):716–22. [DOI] [PubMed] [Google Scholar]
- [63].D’Hooge J, Barbosa D, Gao H, Claus P, Prater D, Hamilton J, et al. Two-dimensional speckle tracking echocardiography: standardization efforts based on synthetic ultrasound data. Eur Heart J Cardiovasc Imaging 2016;17(6):693–701. [DOI] [PubMed] [Google Scholar]
- [64].Baysan O, Ocakli EP, Saglam Y, Altuner TK. Advances in echocardiography: global longitudinal strain, intra-cardiac multidirectional flow imaging and automated 3d volume analysis. Heart Vessels and Transplantation 2018;2(4):113–22. [Google Scholar]
- [65].Celutkiene J, Plymen CM, Flachskampf FA, de Boer RA, Grapsa J, Manka R, et al. Innovative imaging methods in heart failure: a shifting paradigm in cardiac assessment. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure 2018;20(12):1615–33. [DOI] [PubMed] [Google Scholar]
- [66].Narang A, Addetia K. An introduction to left ventricular strain. Curr Opin Cardiol 2018;33(5):455–63. [DOI] [PubMed] [Google Scholar]
- [67].Cameli M, Lisi M, Righini FM, Mondillo S. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc Ultrasound 2012;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Donal E, Galli E, Schnell F. Left Atrial Strain A Must or a Plus for Routine Clinical Practice? Circ-Cardiovasc Imag 2017;10(10):e007023. [DOI] [PubMed] [Google Scholar]
- [69].Carluccio E, Biagioli P, Mengoni A, Francesca Cerasa M, Lauciello R, Zuchi C, et al. Left Atrial Reservoir Function and Outcome in Heart Failure With Reduced Ejection Fraction. Circ Cardiovasc Imaging 2018;11(11):e007696. [DOI] [PubMed] [Google Scholar]
- [70].Cameli M, Mandoli GE, Loiacono F, Dini FL, Henein M, Mondillo S. Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Heart Fail Rev 2016;21(1):65–76. [DOI] [PubMed] [Google Scholar]
- [71].Gan GCH, Ferkh A, Boyd A, Thomas L. Left atrial function: evaluation by strain analysis. Cardiovasc Diagn The 2018;8(1):29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imaging 2016;9(3): e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Marwick TH, Shah SJ, Thomas JD. Myocardial Strain in the Assessment of Patients With Heart Failure: A Review. JAMA Cardiol 2019;4(3):287–94. [DOI] [PubMed] [Google Scholar]
- [74].Pathan F, D’Elia N, Nolan MT, Marwick TH, Negishi K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr 2017;30(1):59–70 e8. [DOI] [PubMed] [Google Scholar]
- [75].Jarasunas J, Aidietis A, Aidietiene S. Left atrial strain - an early marker of left ventricular diastolic dysfunction in patients with hypertension and paroxysmal atrial fibrillation. Cardiovasc Ultrasound 2018;16(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Telles F, Nanayakkara S, Evans S, Patel HC, Mariani JA, Vizi D, et al. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21(4):495–505. [DOI] [PubMed] [Google Scholar]
- [77].Li M, Lu Y, Fang C, Zhang X. Correlation between myocardial deformation on three-dimensional speckle tracking echocardiography and cardiopulmonary exercise testing. Echocardiography 2017;34(11):1640–8. [DOI] [PubMed] [Google Scholar]
- [78].Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, et al. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol 2009;54(1):36–46. [DOI] [PubMed] [Google Scholar]
- [79].Wang J, Fang F, Wai-Kwok Yip G, Sanderson JE, Feng W, Xie JM, et al. Left ventricular long-axis performance during exercise is an important prognosticator in patients with heart failure and preserved ejection fraction. Int J Cardiol 2015;178:131–5. [DOI] [PubMed] [Google Scholar]
- [80].Luis SA, Pellikka PA. Is Speckle Tracking Imaging Ready for Prime Time in Current Echo Clinical Practice? Prog Cardiovasc Dis 2018;61(5–6):437–45. [DOI] [PubMed] [Google Scholar]
- [81].Leischik R, Littwitz H, Dworrak B, Garg P, Zhu M, Sahn DJ, et al. Echocardiographic Evaluation of Left Atrial Mechanics: Function, History, Novel Techniques, Advantages, and Pitfalls. Biomed Res Int 2015;2015:765921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Buggey J, Hoit BD. Left atrial strain: measurement and clinical application. Curr Opin Cardiol 2018;33(5):479–85. [DOI] [PubMed] [Google Scholar]