Abstract
Purpose
This prospective study investigated the relationship between pregnancy-associated plasma protein A (PAPP-A) and human chorionic gonadotropin (hCG) and adverse pregnancy outcomes in the Iranian population.
Materials
Overall, 994 singleton pregnant mothers of 18–35-year old were referred for first-trimester screening tests, including PAPP-A and β-hCG, at the age of 6 days and 11–13 weeks, and were followed until the end of their pregnancy. The adverse pregnancy outcomes, PAPP-A, and β-hCG serum levels were recorded and analyzed. The sensitivity and specificity of the test were measured by calculating the area under the curve of receiver operating characteristic curve (ROC).
Results
The mean serum level of PAPP-A and β-hCG was 1.10 ± 0.69 and 1.09 ± 0.8 MoM, respectively. Pregnancy-associated plasma protein A, regardless of its percentile, showed a significant relationship with the incidence of preeclampsia, preterm birth, and fetal low birth weight (p < 0.001 for each). However, the relationship between PAPP-A and abortion was not significant (p > 0.05). According to ROC, the results indicated that PAPP-A had a significant relationship with the incidence of preeclampsia, preterm birth, and fetal low birth weight (p < 0.001). However, β-hCG levels showed no significant relationship with adverse pregnancy outcomes.
Conclusions
The result of this study revealed that lower level of PAPP-A and β-hCG could be a predictive factor in preterm labor. Also, this study indicated that PAPP-A measurements could be a screening test for adverse pregnancy outcomes, such as preeclampsia, low birth weight and preterm labor.
Keywords: PAPP-A, β-hCG, Preeclampsia, Preterm labor, Low birth weight, Abortion
Introduction
Pregnancy-associated plasma protein A (PAPP-A) is secreted by the placenta and enters directly into maternal circulation. The concentration of PAPP-A increases till the end of pregnancy and is detectable in maternal serum since the blastocyst implantation period. It is one of the principal markers measured in Down syndrome screening and should be performed between 11 and 14 weeks of gestational age. The serum markers for Down syndrome could influence pregnancy outcomes in the first and second trimesters [1]. Human chorionic gonadotropin (hCG) is the first hormone detected in pregnancy. It is produced by placenta, and its rapid rise in material serum or urine is an ideal marker for confirmation of conception [2]. As reported in the literature, maternal serum markers, such as PAPP-A and β-hCG, are related to adverse pregnancy outcomes, such as fetal growth restriction, preeclampsia, low birth weight, still birth, and preterm labor [3, 4].
Despite vast amount of research, none could prove their high sensitivity in detecting low- or high-risk pregnancy, according to adverse pregnancy outcomes [5, 6]. In spite of the relatively high adverse pregnancy outcomes in Iran, little research has been performed on such markers. On the other hand, the levels of these markers vary in different populations, the range of which should be detected [7]. The current research investigated the relationship between PAPP-A and hCG and adverse pregnancy outcomes in the Iranian population.
Methods and Materials
Study Population
This prospective analytical study was performed on singleton pregnant mothers of 18–35 years of age, whose first obstetrical visit was before 14 weeks of gestational age, at the health care centers of Qazvin province, Central region of Iran, during years 2016–2017. The participants were included in the study after information was provided by the researcher and an informed consent was obtained. The participants were excluded if they had diabetes, heart disease, hypertension, chromosomal abnormality, fetal anomaly, multiple pregnancy, and any other disease or if the tests were performed at any other laboratories, except the reference laboratories.
Study Design
Basic demographic data, including age, weight, height, parity, and gestational age, were recorded at the time of performing the screening tests. All the participants were referred to the reference laboratories for screening tests and reference radiology for nuchal translucency (NT) ultrasound scan, at the age of 11–13 weeks and 6 days. The method for measuring these markers in serum was the enzyme-linked immunosorbent assay (ELISA), which was the same in all the reference laboratories [8]. In order to compare the assessments, maternal age, weight and gestational age were used for standardizing PAPP-A results to multiple of the median (MOM) at the mentioned reference laboratories [9]. The results were recorded and all the pregnant mothers were followed up for prenatal care, according to the schedule released by the Iranian Ministry of Health.
Study Outcome
The participants were divided to three categories, based on their PAPP-A serum levels: (1) 5%; (2) between 5 and 95%, and (3) more than 95%. In each case, pregnancy outcome was followed up and fetal birth weight was recorded. Abortion before 22 weeks of gestational age, preeclampsia, preterm birth, and fetal weight (g) were charted in the questionnaire. Abortion was defined as unexpected pregnancy loss before 22 weeks of gestation. Preterm birth was defined as termination before 37 weeks. Preeclampsia was confirmed by systolic blood pressure of ≥ 140 mmHg and diastolic blood pressure of ≥ 90 mmHg plus proteinuria, as an objective criterion, presenting massive endothelial injury, defined as more than 300 mg proteinuria in the 24 h of urine collection or the ratio of urinary protein to creatinine equal or more than 0.3 or the detection of more than 30 mg protein in a random urine sample [10]. Postpartum follow-ups were performed by a resident midwife at each health care center.
Statistical Analysis
Data were evaluated with independent T test and Mann–Whitney in two-variable groups and analysis of variance (ANOVA) or Kruskal–Wallis in multivariate groups. Quantitative data were compared by Chi-square or Fisher’s exact test. The significance level was defined by p < 0.05. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the test were measured by calculating the area under the curve (AUC) of receiver operating characteristic curve (ROC) [10].
Result
At first, 1200 pregnant females were evaluated, out of whom 206 cases were excluded using exclusion criteria, and finally, 994 pregnant females participated in this research. Their age ranged from 14 to 44 years with 27.73 ± 6.12 as the average. Mean body mass index (BMI) was calculated as 25.73 ± 4.22 kg/m2. Overall, 539 (54.2%) participants were primiparous, whereas 455 (45. 7%) were multiparous. The range of fetal birth weight was 1.1–4.5 kg with average of 3.2 ± 0.46 kilograms (kg), out of which 60 (6.14%) fetuses had low birth weight. PAPP-A ranged from 0.05 to 4.99, and the mean was 1.1 ± 0.69 MoM. Mean β-hCG was 1.09 ± 0.8 with a range of 0.06–11.12 MoM. Finally, we recorded 899 (90.44%) term birth, 18 (1.81%) abortions, and 77 (7.74%) preterm births. Preeclampsia occurred in 73 (7.34%) cases. Age was the only demographic variable, which had a significant relationship with preterm birth and abortion (p = 0.017 and p < 0.001, respectively). Pregnancy-associated plasma protein A, regardless of its percentile, had a significant relationship with the incidence of preeclampsia, preterm birth, and fetal low birth weight (p < 0.001 for each). However, this relationship with abortion was not significant (p > 0.05) and β-hCG indicated no relationship with the mentioned adverse pregnancy outcomes (p > 0.05) (Table 1).
Table 1.
The relationship of demographic characteristics and β-hCG and PAPP-A with adverse pregnancy outcomes
| Preeclampsia | Preterm labor | Abortion | Low birth weight | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 73) | No (n = 921) | p value | Yes (n = 77) | No (n = 899) | p value | Yes (n = 18) | No (n = 976) | p value | Yes (n = 60) | No (n = 916) | p value | |
| Age | 28.89 ± 6.71 | 27.64 ± 6.07 | 0.209 | 28.51 ± 7.07 | 27.59 ± 5.94 | 0.017 | 31.94 ± 9.33 | 27.66 ± 6.03 | 0.000 | 28.78 ± 6.56 | 27.6 ± 5.99 | 0.568 |
| Parity | 0.321 | 0.373 | 0.091 | 0.314 | ||||||||
| Primiparous | 42 (7.8%) | 497 (92.2%) | 44 (8.2%) | 495 (91.8%) | 6 (1.1%) | 533 (98.9%) | 35 (6.6%) | 496 (93.4%) | ||||
| Multiparous | 31 (6.8%) | 424 (93.2%) | 33 (7.3%) | 422 (92.7%) | 11 (2.4%) | 444 (97.6%) | 25 (5.6%) | 419 (94.4%) | ||||
| BMI | 27.74 ± 4.92 | 25.57 ± 4.12 | 0.061 | 26.08 ± 4.60 | 25.69 ± 4.19 | 0.714 | 26.29 ± 4.01 | 25.72 ± 4.23 | 0.92 | 26.47 ± 4.38 | 25.68 ± 4.21 | 0.514 |
| PAPP-A | 0.61 ± 0.46 | 1.14 ± 0.69 | 0.000 | 0.77 ± 0.59 | 1.13 ± 0.69 | 0.000 | 0.96 ± 0.65 | 1.10 ± 0.69 | 0.491 | 0.78 ± 0.64 | 1.12 ± 0.69 | 0.000 |
| β-hCG | 1.18 ± 1.35 | 1.09 ± 0.74 | 0.839 | 1.17 ± 1.39 | 1.09 ± 0.73 | 0.156 | 0.92 ± 0.86 | 1.10 ± 0.80 | 0.112 | 1.32 ± 1.53 | 1.08 ± 0.72 | 0.764 |
The researchers categorized the participants to three groups based on their PAPP-A level as follows: first group PAPP-A ≤ 34 MOM (under 5th percentile), second group 0.35 ≤ PAPP-A ≤ 2.45 (among 5th–95th percentile), and the third group PAPP-A ≥ 2.46 or more than 95th percentile. None of the demographic variables had a significant relationship with PAPP-A in pregnant mothers (p > 0.05). The researchers observed a significant relationship between different PAPP-A percentiles and preeclampsia, preterm birth, and fetal low birth weight incidences (p < 0.001, p = 0.003, and p < 0.001, respectively). With regard to the β-hCG level, the researchers categorized the data to three groups as follows: The first β-hCG ≤ 0.34 MOM or under the 5th percentile; the second 0.35 ≤ β-hCG ≤ 2.49 MOM or between the 5th and 95th percentile; and the last group with β-hCG ≥ 2.5 MOM or over the 95th percentile. None of the demographic variables presented a significant relationship with β-hCG level in pregnant mothers (p > 0.05). The researchers observed a significant relationship between these categories and preterm birth and abortion incidence (p = 0.001 for each) (Table 2).
Table 2.
The relationship of different levels of β-hCG and PAPP-A with adverse pregnancy outcomes
| Conceptional outcomes | PAPP-A | β-hCG | ||||||
|---|---|---|---|---|---|---|---|---|
| ≥ 2.46 (n = 50) | 0.35–2.45 (n = 893) | ≤ 0.34 (n = 51) | p value | ≥ 2.5 (n = 50) | 0.35–2.49 (n = 892) | ≤ 0.34 (n = 52) | p value | |
| Preeclampsia | ||||||||
| Yes (n = 73) | 2 (2.7%) | 54 (74.0%) | 17 (23.3%) | < 0.001 | 5 (6.8%) | 64 (87.7%) | 4 (5.5%) | 0.755 |
| No (n = 921) | 48 (5.2%) | 839 (91.1%) | 34 (3.7%) | 45 (4.88%) | 828 (89.9%) | 48 (5.21%) | ||
| Preterm labor | ||||||||
| Yes (n = 77) | 4 (5.2%) | 62 (80.5%) | 11 (14.3%) | 0.003 | 4 (5.19%) | 65 (85.71%) | 8 (10.38%) | 0.001 |
| No (n = 899) | 45 (5%) | 816 (90.8%) | 38 (4.2%) | 44 (4.9%) | 815 (90.7%) | 40 (4.4%) | ||
| Abortion | ||||||||
| Yes (n = 18) | 1 (5.5%) | 15 (83.3%) | 2 (11.1%) | 0.446 | 2 (11.1%) | 12 (66.7%) | 4 (22.2%) | 0.001 |
| No (n = 976) | 49 (5.02%) | 878 (89.86%) | 49 (5.02%) | 48 (4.9%) | 880 (90.2%) | 48 (4.9%) | ||
| Low birth weight | ||||||||
| Yes (n = 60) | 4 (6.7%) | 46 (76.7%) | 10 (16.7%) | < 0.001 | 5 (8.33%) | 54 (90%) | 1 (1.66%) | 0.23 |
| No (n = 915) | 45 (4.9%) | 831 (90.8%) | 39 (4.3%) | 43 (4.7%) | 825 (90.2%) | 47 (5.1%) | ||
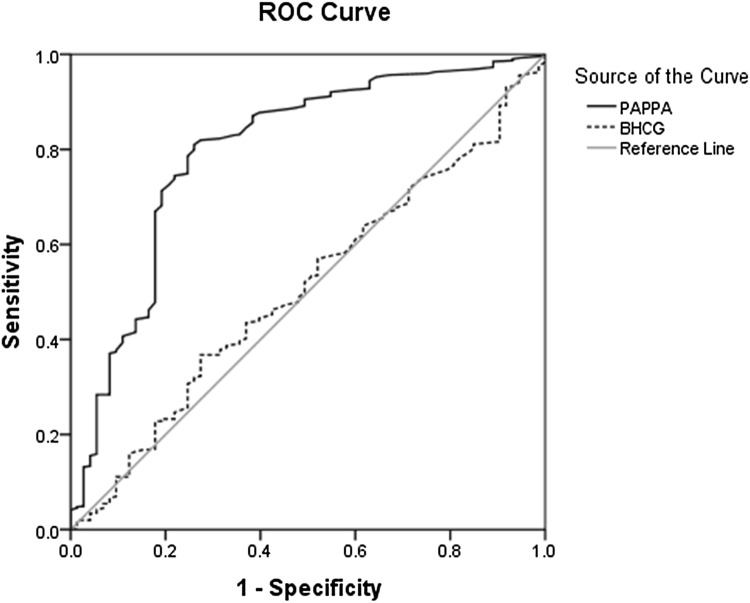
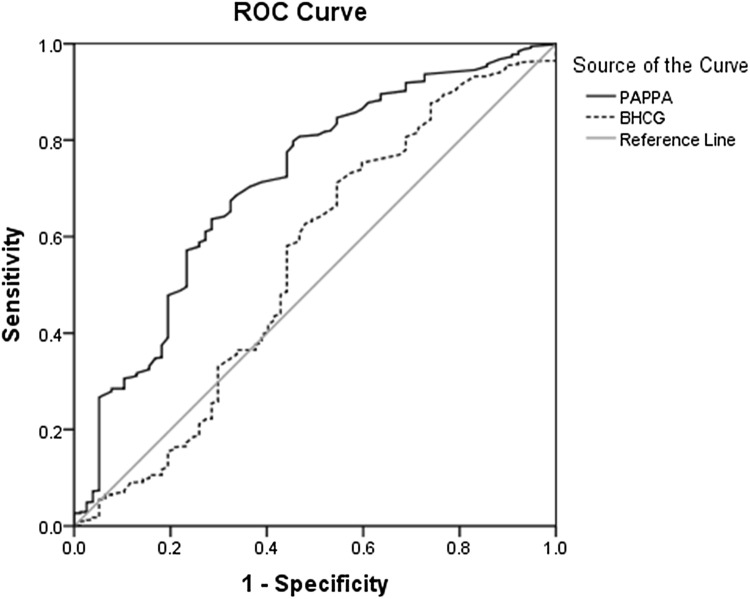
According to ROC, data analysis proved PAPP-A significance in preeclampsia predication with 81% sensitivity, 74% specificity, 23.6% PPV, and 97.5% NPV (AUC = 0.797, p < 0.001); however, the research showed no significant relationship between β-hCG level and preeclampsia prediction (AUC = 0.507, p = 0.839) (Fig. 1) (Table 3) with 0.674 sensitivity, 0.675 specificity, 84.92% PPV, and 95.88% NPV (AUC: 0.712, p < 0.001). However, β-hCG levels showed no significant relationship with preterm labor (AUC: 0.546, p < 0.181) (Fig. 2; Table 3).
Fig. 1.
Diagnostic value of PAPP-A for detecting of preeclampsia according to ROC
Table 3.
Diagnostic value of PAPP-A for detecting of adverse pregnancy outcomes according to ROC
| Cut off | y | D | Sensitivity | Specificity | PPV (%) | NPV (%) | AUC | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Preeclampsia | 0.585 | 0.55 | 0.103 | 0.81 | 0.74 | 23.6 | 97.5 | 0.797 | < 0.001 |
| Preterm labor | 0.745 | 0.349 | 0.211 | 0.674 | 0.675 | 84.92 | 95.88 | 0.712 | < 0.001 |
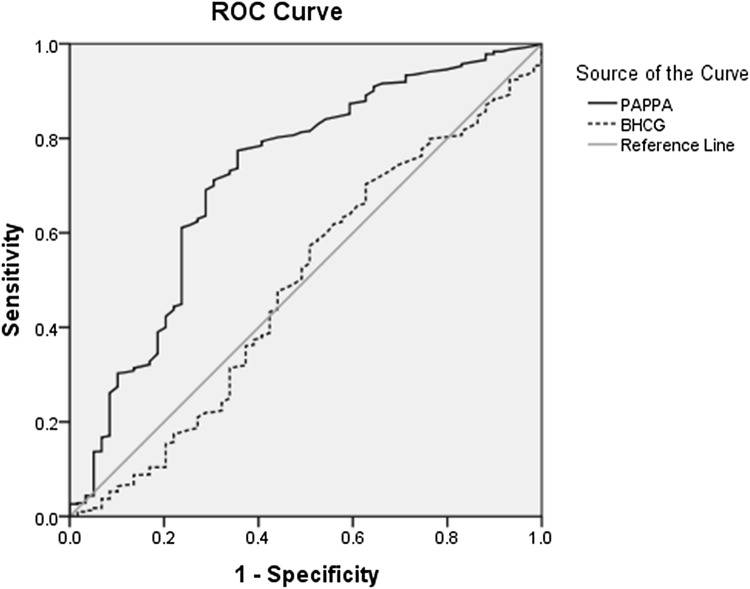
| Low birth weight | 0.705 | 0.406 | 0.176 | 0.711 | 0.70 | 13.4 | 97.1 | 0.717 | < 0.001 |
Fig. 2.
Diagnostic value of PAPP-A for detecting preterm labor according to ROC
Data analysis indicated that PAPP-A could predict low birth weight with 0.711 sensitivity, 0.70 specificity, 13.4% PPV, and 97.1% NPV (AUC: 0.717, p < 0.001), whereas β-hCG showed no significant difference associated with low birth weight (AUC: 0.488, p < 0.764) (Fig. 3; Table 3).
Fig. 3.
Diagnostic value of PAPP-A for detecting low birth weight according to ROC
Neither PAPP-A nor β-hCG had a significant relationship with abortion (p = 0.492, p = 0.112, AUC = 0.456, AUC = 0.388, respectively).
Discussion
This study was performed for the assessment of the relationship of first trimester, PAPP-A, and β-hCG with adverse pregnancy outcomes in Iran. The results indicated no significant relationship between demographic variables and material PAPP-A and β-hCG levels. In contrast, Sung et al. [11] only demonstrated a significant relationship between PAPP-A and maternal age, not with other demographic variables. In this study, 7.34% of the conceptions resulted in preeclampsia, whereas this was higher than the prevalence in Iran. Kharaghani et al. [12] estimated preeclampsia prevalence in Iran as 5% in a systematized meta-analysis of the literatures, until year 2014. This is variable in different countries around the world, such as 3.4% in the USA [13] and 3.2–12% in the Asia–pacific [14–16].
Preeclamptic Asian parturients suffer the worst outcome compared with other countries, as it has been reported that 10% of the Asian maternal mortalities are due to preeclampsia [12, 17]. This discloses the importance of searching for predictive factors of preeclampsia. The lower mean PAPP-A in preeclamptic parturients (0.61 ± 0.46) in comparison with non-preeclamptic (1.14 ± 0.69) revealed a significant relationship between the lower first-trimester PAPP-A and preeclampsia incidence. The results of studies conducted in the recent years in Iran, Canada, India, the USA, Finland, Turkey, and England indicate the same relationship as the current study [18–25]. However, Karahasanovic et al. [26] from Denmark and Gupta et al. [27] from India reported that although the PAPP-A level in preeclamptic parturients was lower than non-preeclamptics, this difference was not significant; this could be related to the research method, which was a case–control study. Furthermore, Morris et al. [4] planned a meta-analysis on 175,000 pregnancies and proved that lower level of PAPP-A has a relationship with preeclampsia (1.94 odds ratio). Allen et al. also estimated preeclampsia odds ratio in relation with lower PAPP-A levels, as 2.1, 2.6, and 1.6 with 95% CI in a meta-analysis on more than 65,000 parturients. They determined PAPP-A cutoff of 0.585 with 81% sensitivity and 74% specificity [28]. Compared to Yücel et al. [24] from Turkey, the current study indicated a higher sensitivity for this variable (81% vs. 63.41%). The former study in Iran suggested PAPP-A cutoff of 1.65 and AUC 0.793 with 60% sensitivity and 78% specificity [18].
This search indicated that mean β-hCG level was higher in preeclampsia parturient. Although the relationship was not significant, the results were similar to those of the current study [9]. However, Yliniemi, Karahasanovic, Keikkala, and their colleagues suggested lower β-hCG levels in preeclamptic mothers [22, 26, 29], which requires more studies in this field. The current research indicated that β-hCG is not a useful factor for predicting preeclampsia, which differs from the results of Keikkala et al.’s study [29], yet is related to Ozdamar’s study from Turkey [23]. This study revealed that lower PAPP-A and β-hCG levels had a relationship with preterm labor, which corresponds with Kirkegaard’s study from Denmark [30]. Researches carried out in India [27, 31], Thailand [7], the USA [32], and England [33] confirm the same results, whereas in studies from Turkey [34], PAPP-A level had a relationship with preterm labor, yet β-hCG did not. A meta-analysis on 175,000 conceptions revealed lower PAPP-A levels in relationship with preterm labor (0.2, 2.09) [4]. It was determined that PAPP-A cutoff of 0.745 (AUC: 0.712) had 67.4% sensitivity and 67.5% specificity for preterm birth predication; however, β-hCG was not useful for this prediction. The current results are similar to studies of Dane et al. [34] from Turkey and Cohen et al. [5] from the USA.
In this research, no relationship was detected among PAPP-A and β-hCG levels with abortion incidence, although incidence was higher with lower β-hCG. Therefore, PAPP-A and β-hCG are not useful indicators for abortion prediction. The results of Quattrocchi et al. [35] from Italia and Kaitu’u-Lino et al. [36] from Australia were in discordance with the current study and revealed significant lower PAPP-A and β-hCG levels in abortions.
According to the current study, lower PAPP-A levels correlated with lower birth weight, whereas β-hCG did not. The current results correspond with studies from Denmark [8], India [27], Thailand [7], Korea [11], Finland [37], Turkey [34], Germany [38], and England [33]. However, Karagiannis et al. [39] from England indicated that lower β-hCG also correlates with lower birth weight. This discordance could probably be due to the lower sample size of the current study.
The role of low levels of PAAPA for low birth weight prediction is so prominent that Baer et al. [40] proved that lower PAPP-A levels in all gestational weeks could predict low birth weight. The results of Morris et al.’s meta-analysis in 2017 detected that lower PAPP-A predicts low birth weight with 2.83 odds ratio [4]. Cutoff was 0.705 with AUC = 0.717, 70.1% sensitivity, and 71% specificity. In spite of β-hCG, PAPP-A seems appropriate for low birth weight prediction. This corresponded with the researches of Gundu et al. [31] from India and Cohen et al. [5] from the United States.
The limitation of this study was lack of a cohort or case–control format. Another weakness was lack of measurement of other markers as predictive factors. The current researchers suggest more meta-analyses in this field regarding the variable results around the world.
Conclusions
The result of this study revealed that lower PAPP-A and β-hCG levels could be predictive factors in preterm labor and lower PAPP-A levels would be useful in low birth weight and preeclampsia prediction. Also, this study indicated that PAPP-A measurements could be a screening test for adverse pregnancy outcomes, such as preeclampsia, low birth weight, and preterm labor.
Acknowledgements
This work was supported in part by Kowar Hospital Development Research Center, Qazvin University of Medical Sciences. We gratefully acknowledge the Zahra Sadat Mohammadi for assistance with statistical analysis.
Hamideh Pakniat
completed her Medical Doctorate (MD) at Azad University of Medical Sciences, Iran, in 1994 and Residency in Obstetrics and Gynecology at Qazvin University of Medical Sciences (QUMS), Iran, in 1999. She is Assistant Professor of QUMS. She teaches evidence-based practice, supervise residents and students dissertations. She received an award from the Iranian National Board of Obstetrics and Gynecology in 1999 and also a certificate and letter for the best invention in the 8th international exhibition of interventions in 2013. She has several publications in Persian and English to her credit. She has also done a film on Cesarean Section for the Ministry of Health’s medical training, and now she is member of the Virtual Education Committee of QUMS (movie site http://www.medtube.ir).
Compliance with Ethical Standards
Conflict of interest
The authors declared no conflict of interest.
Ethical Approval
The researchers were committed to the ethical guidelines of the Declaration of Helsinki, and approval for the study was obtained from the Ethical Committee and Institutional Review Board of Qazvin University of Medical Sciences. The data were gathered after obtaining the participants’ informed consents, considering confidentiality and cultural obligations.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Funding
This study was funded by Kowsar Research Center, Qazvin University of Medical Sciences.
Footnotes
Hamideh Pakniat is an Assistant Professor of Department of Obstetrics and Gynecology, Qazvin University of Medical Sciences at Qazvin, Iran. Atieh Bahman is a General Physician in gynecology oncology fellowship at the Department of Gynecologic Oncology, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Iman Ansari is a General Physician and Researcher at the Medical Students Research Committee of Shahed University, Tehran, Iran.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sweeting A, Park F, Hyett J. The first trimester: prediction and prevention of the great obstetrical syndromes. Best Pract Res Clin Obstet Gynaecol. 2015;29(2):183–193. doi: 10.1016/j.bpobgyn.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaides KH, Syngelaki A, Poon LC, et al. First-trimester contingent screening for trisomies 21, 18 and 13 by biomarkers and maternal blood cell-free DNA testing. Fetal Diagn Ther. 2014;35(3):185–192. doi: 10.1159/000356066. [DOI] [PubMed] [Google Scholar]
- 3.Cignini P, Savasta LM, Gulino FA, et al. Predictive value of pregnancy-associated plasma protein-A (PAPP-A) and free beta-hCG on fetal growth restriction: results of a prospective study. Arch Gynecol Obstet. 2016;293(6):1227–1233. doi: 10.1007/s00404-015-3947-z. [DOI] [PubMed] [Google Scholar]
- 4.Morris RK, Bilagi A, Devani P, et al. Association of serum PAPP-A levels in first trimester with small for gestational age and adverse pregnancy outcomes: systematic review and meta-analysis. Prenat Diagn. 2017;37(3):253–265. doi: 10.1002/pd.5001. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JL, Smilen KE, Bianco AT, et al. Predictive value of combined serum biomarkers for adverse pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol. 2014;181:89–94. doi: 10.1016/j.ejogrb.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Huynh L, Kingdom J, Akhtar S. Low pregnancy-associated plasma protein A level in the first trimester. Can Fam Physician. 2014;60(10):899–903. [PMC free article] [PubMed] [Google Scholar]
- 7.Luewan S, Teja-Intr M, Sirichotiyakul S, et al. Low maternal serum pregnancy-associated plasma protein-A as a risk factor of preeclampsia. Singap Med J. 2018;59(1):55–59. doi: 10.11622/smedj.2017034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen YB, Myrhøj V, Jørgensen FS, et al. First trimester PAPP-A2, PAPP-A and hCGβ in small-for-gestational-age pregnancies. Clin Chem Lab Med. 2016;54(1):117–123. doi: 10.1515/cclm-2015-0230. [DOI] [PubMed] [Google Scholar]
- 9.Jelliffe-Pawlowski LL, Baer RJ, Blumenfeld YJ, et al. Maternal characteristics and mid-pregnancy serum biomarkers as risk factors for subtypes of preterm birth. BJOG. 2015;122(11):1484–1493. doi: 10.1111/1471-0528.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saraswathy S, Sahai K, Yadav TP, et al. Evaluation of fetal hypermethylated RASSF1A in pre-eclampsia and its relationship with placental protein-13, pregnancy associated plasma protein-A and urine protein. Pregnancy Hypertens. 2016;6(4):306–312. doi: 10.1016/j.preghy.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Sung KU, Roh JA, Eoh KJ, et al. Maternal serum placental growth factor and pregnancy-associated plasma protein A measured in the first trimester as parameters of subsequent pre-eclampsia and small-for-gestational-age infants: a prospective observational study. Obstet Gynecol Sci. 2017;60(2):154–162. doi: 10.5468/ogs.2017.60.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kharaghani R, Cheraghi Z, Esfahani BO, et al. Prevalence of preeclampsia and eclampsia in Iran. Arch Iran Med. 2016;19(1):64–71. https://doi.org/0161901/AIM.0012. [PubMed]
- 13.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564. doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornton C, Dahlen H, Korda A, et al. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000–2008. Am J Obstet Gynecol. 2013;208:476.e1–476.e5. doi: 10.1016/j.ajog.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 15.Perveen S. Frequency and impact of hypertensive disorders of pregnancy. J Ayub Med Coll. 2014;26:518–521. [PubMed] [Google Scholar]
- 16.Pitakkarnkul S, Phaloprakarn C, Wiriyasirivaj B, et al. Seasonal variation in the prevalence of preeclampsia. J Med Assoc Thai. 2011;94:1293–1298. [PubMed] [Google Scholar]
- 17.Cripe S, O’Brien W, Gelaye B, et al. Perinatal outcomes of southeast asians with pregnancies complicated by gestational diabetes mellitus or preeclampsia. J Immigr Minor Health. 2012;14:747–753. doi: 10.1007/s10903-011-9537-7. [DOI] [PubMed] [Google Scholar]
- 18.Moslemi Zadeh N, Naghshvar F, Peyvandi S, et al. PP13 and PAPP-A in the first and second trimesters: predictive factors for preeclampsia? ISRN Obstet Gynecol. 2012;2012:263871. doi: 10.5402/2012/263871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crosley EJ, Durland U, Seethram K, et al. First-trimester levels of pregnancy-associated plasma protein A2 (PAPP-A2) in the maternal circulation are elevated in pregnancies that subsequently develop preeclampsia. Reprod Sci. 2014;21(6):754–760. doi: 10.1177/1933719113512532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patil M, Panchanadikar TM, Wagh G. Variation of PAPP-A level in the first trimester of pregnancy and its clinical outcome. J Obstet Gynaecol India. 2014;64(2):116–119. doi: 10.1007/s13224-013-0481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelliffe-Pawlowski LL, Baer RJ, Currier RJ, et al. Early-onset severe preeclampsia by first trimester pregnancy-associated plasma protein A and total human chorionic gonadotropin. Am J Perinatol. 2015;32(7):703–712. doi: 10.1055/s-0034-1396697. [DOI] [PubMed] [Google Scholar]
- 22.Yliniemi A, Makikallio K, Korpimaki T, et al. Combination of PAPP-A, fhCGβ, AFP, PlGF, sTNFR1, and maternal characteristics in prediction of early-onset preeclampsia. Clin Med Insights Reprod Health. 2015;9:13–20. doi: 10.4137/CMRH.S21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozdamar O, Gun I, Keskin U, et al. The role of maternal serumbeta-hCG and PAPP-A levels at gestational weeks 10 to 14 in the prediction of pre-eclampsia. Pak J Med Sci. 2014;30(3):568–573. doi: 10.12669/pjms.303.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yücel B, Gedikbasi A, Dündar O, et al. The utility of first trimester uterine artery Doppler, placental volume and PAPP-A levels alone and in combination to predict preeclampsia. Pregnancy Hypertens. 2016;6(4):269–273. doi: 10.1016/j.preghy.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 25.O’Gorman N, Wright D, Poon LC, et al. Accuracy of competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol. 2017;49(6):751–755. doi: 10.1002/uog.17399. [DOI] [PubMed] [Google Scholar]
- 26.Karahasanovic A, Sørensen S, Nilas L. First trimester pregnancy-associated plasma protein A and human chorionic gonadotropin-beta in early and late pre-eclampsia. Clin Chem Lab Med. 2014;52(4):521–525. doi: 10.1515/cclm-2013-0338. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Goyal M, Verma D, et al. Adverse pregnancy outcome in patients with low pregnancy-associated plasma protein-A: the Indian experience. J Obstet Gynaecol Res. 2015;41(7):1003–1008. doi: 10.1111/jog.12662. [DOI] [PubMed] [Google Scholar]
- 28.Allen RE, Rogozinska E, Cleverly K, et al. Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;182:194–201. doi: 10.1016/j.ejogrb.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Keikkala E, Koskinen S, Vuorela P, et al. First trimester serum placental growth factor and hyperglycosylated human chorionic gonadotropin are associated with pre-eclampsia: a case control study. BMC Pregnancy Childbirth. 2016;16(1):378. doi: 10.1186/s12884-016-1169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkegaard I, Uldbjerg N, Petersen OB, et al. PAPP-A, free β-hCG, and early fetal growth identify two pathways leading to preterm delivery. Prenat Diagn. 2010;30(10):956–963. doi: 10.1002/pd.2593. [DOI] [PubMed] [Google Scholar]
- 31.Gundu S, Kulkarni M, Gupte S, et al. Correlation of first-trimester serum levels of pregnancy-associated plasma protein A with small-for-gestational-age neonates and preterm births. Int J Gynaecol Obstet. 2016;133(2):159–163. doi: 10.1016/j.ijgo.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Goetzinger KR, Cahill AG, Kemna J, et al. First-trimester prediction of preterm birth using ADAM12, PAPP-A, uterine artery Doppler, and maternal characteristics. Prenat Diagn. 2012;32(10):1002–1007. doi: 10.1002/pd.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Antonio F, Rijo C, Thilaganathan B, et al. Association between first-trimester maternal serum pregnancy-associated plasma protein-A and obstetric complications. Prenat Diagn. 2013;33(9):839–847. doi: 10.1002/pd.4141. [DOI] [PubMed] [Google Scholar]
- 34.Dane B, Dane C, Batmaz G, et al. First trimester maternal serum pregnancy-associated plasma protein-A is a predictive factor for early preterm delivery in normotensive pregnancies. Gynecol Endocrinol. 2013;29(6):592–595. doi: 10.3109/09513590.2013.788626. [DOI] [PubMed] [Google Scholar]
- 35.Quattrocchi T, Baviera G, Pochiero T, et al. Maternal serum PAPP-A as an early marker of obstetric complications? Fetal Diagn Ther. 2015;37(1):33–36. doi: 10.1159/000365147. [DOI] [PubMed] [Google Scholar]
- 36.Kaitu’u-Lino TJ, Bambang K, Onwude J, et al. Plasma MIC-1 and PAPP-a levels are decreased among women presenting to an early pregnancy assessment unit, have fetal viability confirmed but later miscarry. PLoS ONE. 2013;8(9):e72437. doi: 10.1371/journal.pone.0072437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranta JK, Raatikainen K, Romppanen J, et al. Decreased PAPP-A is associated with preeclampsia, premature delivery and small for gestational age infants but not with placental abruption. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):48–52. doi: 10.1016/j.ejogrb.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Krauskopf AL, Knippel AJ, Verde PE, et al. Predicting SGA neonates using first-trimester screening: influence of previous pregnancy’s birthweight and PAPP-A MoM. J Matern Fetal Neonatal Med. 2016;29(18):2962–2967. doi: 10.3109/14767058.2015.1109622. [DOI] [PubMed] [Google Scholar]
- 39.Karagiannis G, Akolekar R, Sarquis R, et al. Prediction of small-for-gestation neonates from biophysical and biochemical markers at 11–13 weeks. Fetal Diagn Ther. 2011;29(2):148–154. doi: 10.1159/000321694. [DOI] [PubMed] [Google Scholar]
- 40.Baer RJ, Lyell DJ, Norton ME, et al. First trimester pregnancy-associated plasma protein-A and birth weight. Eur J Obstet Gynecol Reprod Biol. 2016;198:1–6. doi: 10.1016/j.ejogrb.2015.12.019. [DOI] [PubMed] [Google Scholar]