Abstract
Aims: Pulmonary arterial hypertension (PAH) is a progressive lethal disease with a known gender dimorphism. Female patients are more susceptible to PAH, whereas male patients have a lower survival rate. Initial pulmonary vascular damage plays an important role in PAH pathogenesis. Therefore, this study aimed at investigating the role of gender in activation of apoptosis/necrosis-mediated signaling pathways in PAH.
Results: The media collected from pulmonary artery endothelial cells (PAECs) that died by necrosis or apoptosis were used to treat naive PAECs. Necrotic cell death stimulated phosphorylation of toll-like receptor 4, accumulation of interleukin 1 beta, and expression of E-selectin in a redox-dependent manner; apoptosis did not induce any of these effects. In the animal model of severe PAH, the necrotic marker, high mobility group box 1 (HMGB1), was visualized in the pulmonary vascular wall of male but not female rats. This vascular necrosis was associated with male-specific redox changes in plasma, activation of the same inflammatory signaling pathway seen in response to necrosis in vitro, and an increased endothelial–leukocyte adhesion in small pulmonary arteries. In PAH patients, gender-specific changes in redox homeostasis correlated with the prognostic marker, B-type natriuretic peptide. Males had also shown elevated circulating levels of HMGB1 and pro-inflammatory changes.
Innovation: This study discovered the role of gender in the initiation of damage-associated signaling in PAH and highlights the importance of the gender-specific approach in PAH therapy.
Conclusion: In PAH, the necrotic cell death is augmented in male patients compared with female patients. Factors released from necrotic cells could alter redox homeostasis and stimulate inflammatory signaling pathways.
Keywords: pulmonary hypertension, gender difference, necrosis, inflammation
Introduction
Pulmonary arterial hypertension (PAH) is a fatal disease characterized by increased pulmonary vascular resistance and right ventricle (RV) hypertrophy. There is strong sexual dimorphism in all types of PAH, with the female-to-male ratio varying between 2:1 and 4:1 (7, 44). Female sex hormones and their metabolites have been postulated to be responsible for the development of PAH in female patients (36, 41). However, animal data suggest that estrogens exert a protective effect on the pulmonary vasculature, the so-called “estrogen paradox” (14, 53). This protection could explain the better survival prognosis for female patients compared with male patients. Indeed, the male gender has been reported to be one of the primary independent predictors of death (18). However, no specific molecular pathways responsible for the poor survival rates associated with the male gender have been identified. Nevertheless, elucidation of these mechanisms is critical as they will allow the development of differential therapeutic approaches for male and female patients.
Innovation.
The innovation of this study is several folds. It establishes the novel connection between the male gender and the higher level of necrotic cell death in pulmonary arterial hypertension (PAH), discovers the gender-specific redox alterations in plasma of animals and humans with PAH, and provides the molecular mechanisms that could be responsible for the poor survival prognosis of male patients with PAH. Most importantly, our results suggest the presence of a whole new area, gender-specific redox biology, which should be further investigated and taken into account to address the needs of each gender, in PAH and beyond.
Inflammatory and immune responses play an important role in the pathogenesis of PAH (37, 49). The initial vascular injury that results in the release of damage-associated molecular patterns (DAMPs), including high mobility group box 1 (HMGB1), is known to initiate pro-inflammatory signaling and, thus, contribute to the initiation and progression of PAH (6, 47). For example, the binding of HMGB1 to toll-like receptor 4 (TLR4) induces endothelial cell activation (32), recruitment and activation of leukocytes (13) and maintains a cross-talk between innate and adaptive response (59). Importantly, the extracellular release of HMGB1 and TLR4 activation was confirmed to happen specifically in cells dying by necrosis. Therefore, an extracellular HMGB1 is used as a specific marker of tissue necrosis (23). In apoptotic cells, the histone-like nuclei protein HMGB1 remains irreversibly attached to apoptotic chromatin (8) and is secreted outside the cells in low amounts and in the preoxidized state that is incapable of interacting with TLR4 (65).
It has been recently suggested that male and female genders possess a different predisposition to the type of cell death in response to damage-inducing stimuli. Experiments performed in bone marrow-derived macrophages isolated from mice and treated with hydrogen peroxide (25), renal cells isolated from spontaneously hypertensive rats (42), and our unpublished observations with mouse lung endothelial cells treated with Antymycin A, an inhibitor of complex III of the mitochondrial electron transport chain, show that cells isolated from male patients are more likely to die by necrosis compared with cells isolated from female patients. This discovery allowed us to hypothesize that the highly progressive form of PAH seen in male patients may be explained by the increased levels of necrotic cell death in pulmonary vasculature in response to initial damage.
Since the intracellular environment is known to contain a high amount of thiols, the rupture of necrotic cells will result in a free release of these high- and low-molecular-weight antioxidants to the extracellular space (33). Therefore, necrosis could shift the redox balance of the extracellular environment toward more reduced. HMGB1 is known to be a redox-sensitive protein (56). The reduction of HMGB1 cysteine residues leads to its sustained activation and enhances the interaction with TLR4 (55). In contrast, oxidation or mutation of Cys106 inhibits the binding of HMGB1 to TLR4, preventing activation of an immune response (65). We hypothesized that if the vascular cell damage occurs in the form of necrosis, this could shift the extracellular environment redox balance toward being more reduced and prolong HMGB1 activity, which would result in ongoing inflammatory signaling. Therefore, we designed a study aimed at evaluating the level of necrotic cell death and the activation of HMGB1-mediated inflammation in male and female patients by using the Sugen/hypoxia animal model and plasma samples obtained from PAH patients.
Results
Damage-associated factors released from necrotic cells are pro-inflammatory and redox dependent
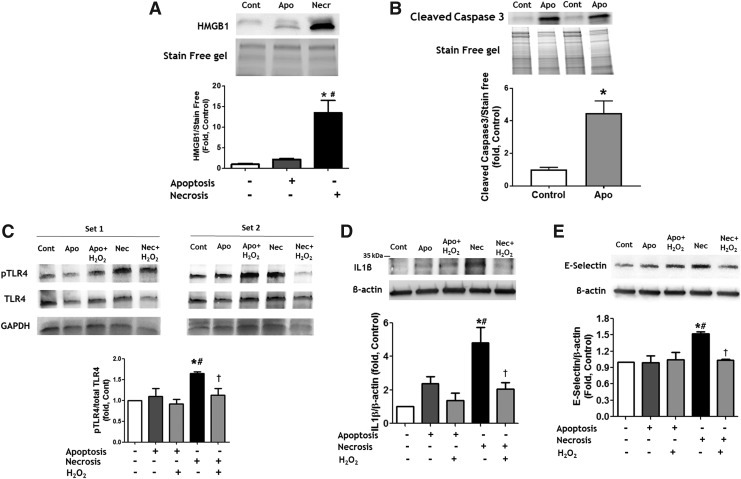
It has been previously described that gender is capable of mediating the type of cell death in response to initial cell damage. Thus, cells from female patients were found to possess a higher probability of dying by apoptosis, whereas death in cells from male patients was more likely to occur via necrosis (25, 42). However, the consequences of this difference are not well described or understood. In this study, we evaluated whether factors released from necrotic and apoptotic cells are capable of initiating distinct cell responses in intact pulmonary artery endothelial cells (PAECs) in vitro. For this purpose, we induced apoptosis in PAECs by exposing them to 2% oxygen for 24 h, whereas necrosis was initiated by three freeze-thaw cycles as published (5). The necrotic cell death was validated by measuring the release of HMGB1, an established marker of necrotic cell death, in cell media (Fig. 1A and Supplementary Fig. S1A). Apoptosis was confirmed by visualizing the signal from cleaved Caspase 3 (Fig. 1B and Supplementary Fig. S1B).
FIG. 1.
Reduced factors released from necrotic cells induce pro-inflammatory signaling and activation of intact endothelial cells. (A, B) Apoptosis was induced by incubation of PAEC in 2% of oxygen for 24 h, necrosis—by few free/thaw cycles. Necrosis but not apoptosis induced an accumulation of HMGB1 in cell culture media (A). Apoptosis induced Caspase 3 cleavage (B). The conditioned media collected from apoptotic or necrotic PAEC were used to treat intact PAEC. Factors released from necrotic, but not apoptotic cells induce phosphorylation of TLR4 (C), increase production of IL1β (D) and expression of E-selectin (E) in intact PAEC. The effects were attenuated when necrotic media were pretreated with H2O2 to induce oxidation of factors released from necrotic cells. Pretreatment with H2O2 did not induce any significant changes in PAEC treated by apoptotic media. Data are mean ± SEM, N = 3 per group (A); N = 4 per group (B–E).*p < 0.05 versus Control; #p < 0.05 versus apoptotic cells or media; †p < 0.05 versus reduced necrotic media in one-way ANOVA. ANOVA, analysis of variance; H2O2, hydrogen peroxide; HMGB1, high mobility group box 1; IL1β, interleukin 1 beta; PAEC, pulmonary artery endothelial cell; TLR4, toll-like receptor 4; SEM, standard error of the mean.
Next, we exposed the naive PAECs to the conditioned media collected from apoptotic and necrotic cells and found that treatment with necrotic, but not apoptotic media had induced an activation of TLR4 (Fig. 1C and Supplementary Fig. S1C) and an accumulation of the pro-inflammatory cytokine interleukin 1 beta (IL1β) (Fig. 1D and Supplementary Fig. S1D), which is known to be a downstream event of TLR4 stimulation (28). Treatment with cell media collected from necrotic cells has also increased the expression of E-selectin in PAECs (Fig. 1E and Supplementary Fig. S1E), suggesting that factors released from cells that died by necrosis, but not apoptosis, could initiate endothelial cell activation and increase endothelial–leukocyte adhesion. Importantly, pretreatment of cell media with hydrogen peroxide to induce oxidation of factors released from dying cells attenuated all these effects (TLR4 activation, IL1β accumulation, and E-selectin expression). This result highlights the importance of the redox status of the factors released from necrotic cells. These factors are capable of initiating pro-inflammatory responses in a reduced form, whereas their oxidation attenuates or completely prevents their pro-inflammatory activity.
Pulmonary hypertension is associated with an increased level of necrosis in male but not female rats
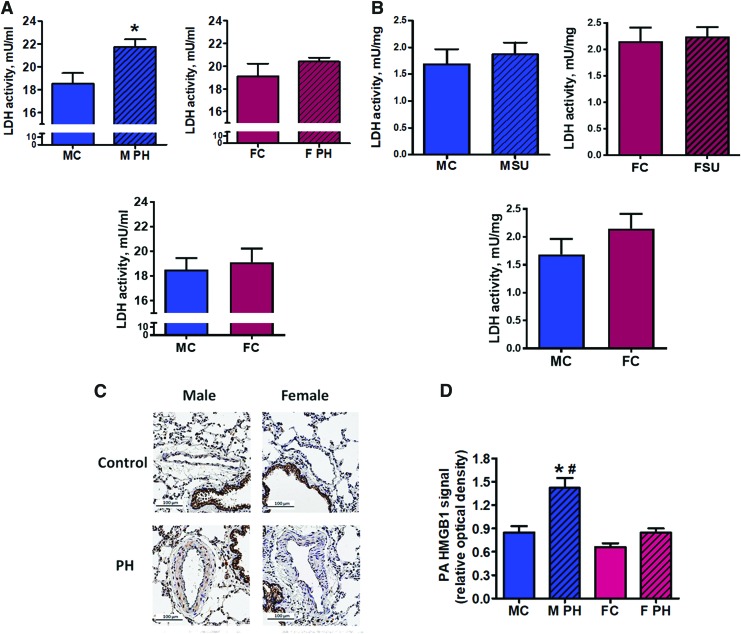
Sugen/hypoxia model has been previously reported to be the most human-relevant animal model of PAH due to its ability to reproduce the main histological changes in the pulmonary vasculature, such as the formation of plexiform and concentric lesions in small pulmonary arteries (PAs) (1). In this study, we used the plasma and tissue samples from rats for which the hemodynamic data have been already reported (40). According to these data, the severity of the disease, based on the level of right ventricle peak systolic pressure, was comparable between the male and female rats. However, males showed a significantly higher level of perivascular inflammation and fibrosis. In this study, we aimed at determining the particular molecular mechanisms responsible for the pro-inflammatory phenotype associated with the male gender. Plasma collected at the end of a 14-week study from male and female PAH rats and gender-matched healthy Controls was used to analyze plasma lactate dehydrogenase (LDH) activity as a measure of necrotic cell death. Only male rats with PAH possessed a significant increase in circulating LDH activity compared with healthy controls; whereas in females, the LDH activity remained unchanged (Fig. 2A). Importantly, the level of LDH activity in lung tissue remained at control level in both genders (Fig. 2B), confirming that the increase in plasma LDH activity cannot be explained by a gender-specific activation of LDH in lungs. We conclude that PAH development is associated with an increased risk of necrotic cell death, but only in males.
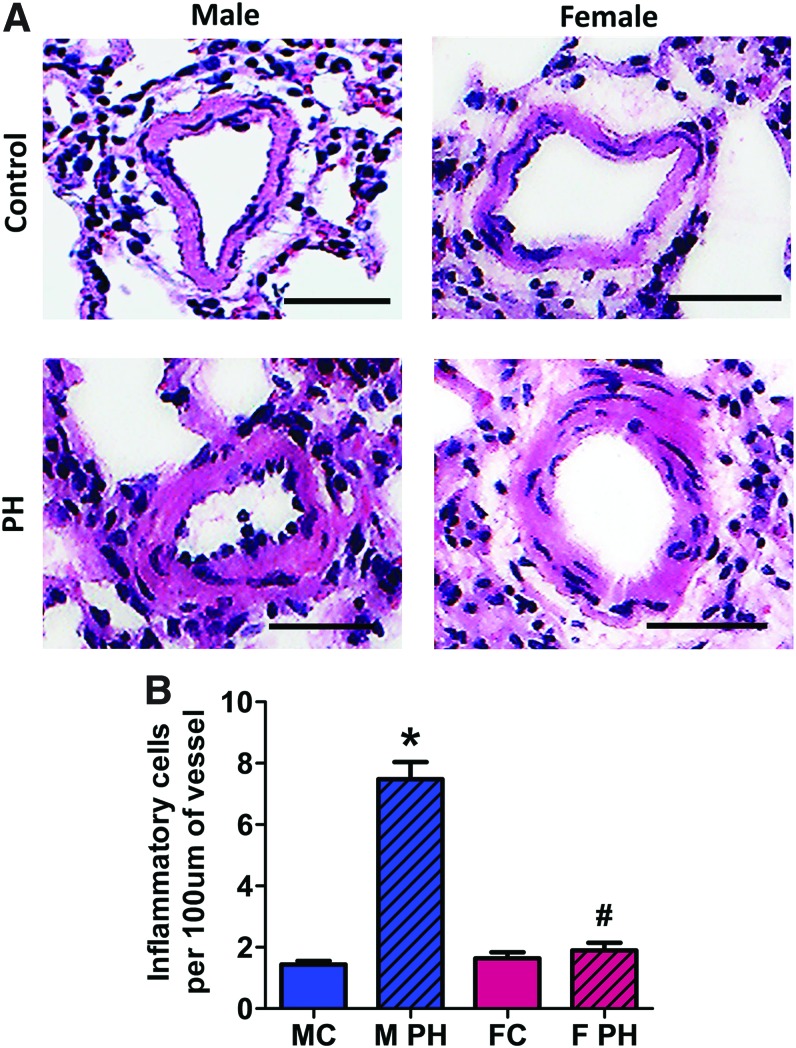
FIG. 2.
An increased level of necrosis is associated with pulmonary hypertension and male gender. Pulmonary hypertension induced an increase in the plasma level of LDH activity as a marker of tissue necrosis in male but not female rats (A) top left graph—males; top right graph—females; bottom graph—healthy controls; LDH activity in lungs show no difference in either gender compared with controls (B) top left graph—males; top right graph—females; bottom graph—healthy controls. Data are mean ± SEM, N = 5–7 per group. *p < 0.05 versus male control rats. p Value calculated by using unpaired t-test. The signal from extra-nuclear (non-co-localized with nuclei) HMGB1, an established marker of necrotic cell death, was visualized (C) and quantified (D) in the vascular wall of pulmonary arteries in Control and PAH male and female rats. Although PAH induced a strong PA vascular remodeling, a significant accumulation of extra-nuclear signal from HMGB1 was evidenced in male rats only (D). Data are mean ± SEM, N = 9–11 PA were analyzed for each rat with N = 6 rats in each group. *p < 0.05 versus male control rats; #p < 0.05 versus female with PAH rats in one-way ANOVA. MC indicates male control group; M PH—male PH group; FC—female control group; F PH—female PH group; LDH, lactate dehydrogenase; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension. Color images are available online.
The initial damage of the pulmonary vasculature caused by high pressure, shear stress, increased reactive oxygen species (ROS) production, and inflammation is strongly implemented in PAH pathogenesis (9, 48) and may result in necrotic changes of the intima and media. To confirm that an observed increase in plasma necrotic marker was due to increased necrosis in pulmonary vessels, we performed immunohistochemical analysis and evaluated the level of necrosis in PAs from Control and PAH animals. The extra-nuclear HMGB1 signaling was confirmed to be a specific marker of tissue necrosis (23), as apoptotic cells are known to retain HMGB1 irreversibly attached to apoptotic chromatin (8, 50). By normalizing the brown staining from HMGB1 to the blue hematoxylin counterstain that stained nuclei, we were capable of quantifying the extra-nuclear HMGB1 signal as a marker of vascular necrosis (Fig. 2C and D and Supplementary Fig. S2). We found that only males with PAH had a significant increase in the level of extra-nuclear HMGB1 in the PA wall.
Although our results confirm the accumulation of extra-nuclear HMGB1 specifically in male rats with PAH, we cannot completely delineate whether the increase in HMGB1 signal is due to the extracellular release of HMGB1 by necrotic cells on the loss of cell integrity, or it represents the translocation of HMGB1 to the cytosol during the programmed necrosis (or necroptosis). However, even the initial translocation of HMGB1 to the cytoplasm during necroptosis was shown to follow with the release of HMGB1 into the extracellular milieu (66). Thus, we propose that the increased HMGB1 found specifically in the pulmonary vascular wall of male rats with PAH will eventually induce HMGB1-mediated signaling in males.
Pulmonary hypertension induces plasma redox changes in male rats
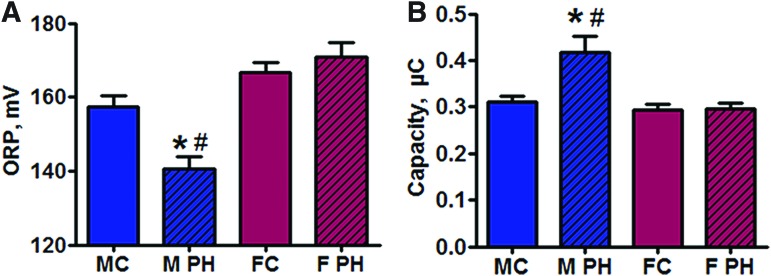
To evaluate whether the increased necrotic cell death is associated with any changes in the plasma redox balance, two primary plasma redox parameters—oxidation–reduction potential (ORP) and Cap—were assessed by using RedoxSYS Diagnostic System as previously described (54). We found that male rats with PAH possessed a significant decrease in plasma ORP, which represents the current balance between total oxidants and reductants in a biological sample (Fig. 3A) and had an elevated total antioxidant capacity, a measure of overall antioxidant reserve (Fig. 3B). There were no significant changes in plasma redox balance in females with PAH. We conclude that in males PAH shits the redox status of the plasma toward being more reduced. These changes in the plasma redox balance could prolong the activity of circulating HMGB1 released from necrotic cells.
FIG. 3.
Pulmonary hypertension-induced reductive shift is selectively associated with male gender. Two primary redox parameters—ORP (A) and Cap (B)—were measured in the plasma of control rats and rats with PAH. In male rats, a PAH-induced reductive shift in plasma was confirmed by decreased ORP and increased Cap. Data are mean ± SEM. (A) N = 12 for MC and M PH groups; N = 10 for FC; N = 8 for F PH; (B) N = 10 for MC, M PH, and FC; N = 8 for F PH. *p < 0.05 versus male control rats; #p < 0.05 versus female with PAH rats in one-way ANOVA. MC indicates male control group; M PH—male PH group; FC—female control group; F PH—female PH group. Cap, total antioxidant capacity; ORP, oxidation–reduction potential. Color images are available online.
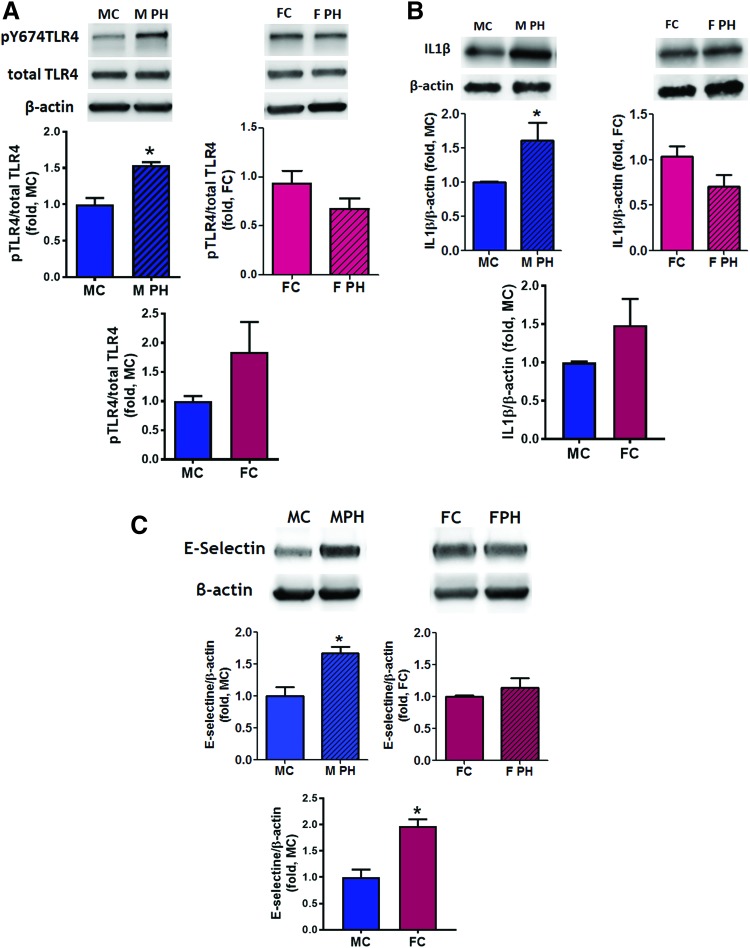
Activation of pro-inflammatory signaling in the lungs of male animals
DAMP molecules (such as HMGB1, S100 proteins, and serum amyloid A) released from dying cells are known to initiate pro-inflammatory signaling, in particular, due to their ability to directly bind to TLR4 (10). This binding is known to be redox sensitive (10, 55). Therefore, we investigated whether increased vascular necrosis and the shift of the plasma redox balance toward being more reduced stimulated activity of TLR4-mediated signaling in male animals. We found that phosphorylation of TLR4, which recently was reported as an important determinant of TLR4 activation (31), was significantly increased in the lungs of male rats with PAH compared with control males (Fig. 4A and Supplementary Fig. S3A). No difference was observed in the amount of phosphorylated TLR4 in female rats. There was also a strong accumulation of cytokine IL1β in pulmonary tissue of male, but not female rats with PAH (Fig. 4B and Supplementary Fig. S3B) and increased expression of E-selectin, also known as endothelial–leukocyte adhesion molecule 1 (ELAM-1, Fig. 4C and Supplementary Fig. S3C). As E-selectin is responsible for cell adhesion and is expressed only in activated endothelial cells (21), we further determined whether its increased expression in the male PAH group is associated with an enhanced endothelial–leukocyte interaction in these animals. The small PAs of male PAH rats showed a strong adhesion of leukocytes to the intima layer (Fig. 5A, B). In contrast, no endothelial–leukocyte adhesion was seen in a diseased female group.
FIG. 4.
Activation of pro-inflammatory signaling in male rats with PAH. In male, but not female rats, PAH development is associated with an activation of pro-inflammatory signaling and endothelial cells activation. The pulmonary tissue of male and female control rats and rats with PAH was used to measure the level of TLR4 phosphorylation as a marker of TLR4 activation (A) top left graph—males; top right graph—females; bottom graph—healthy controls, accumulation of pro-inflammatory cytokine IL1β (B) top left graph—males; top right graph—females; bottom graph—healthy controls and a protein level of E-selectin as a measure of pulmonary endothelial cell activation by cytokines (C) top left graph—males; top right graph—females; bottom graph—healthy controls. For all three measurements, there was a significant increase in pro-inflammatory signaling only in males with PAH; whereas in females, all pro-inflammatory markers were found to be comparable to the levels seen in control nonhypertensive rats. Data are mean ± SEM; (A, B) N = 4 for all groups; (C) N = 4 for MC, M PH, and FC; N = 5 for F PH group. *p < 0.05 versus male control rats. p Value calculated by using unpaired t-test. MC indicates male control group; M PH—male PH group; FC—female control group; F PH—female PH group. Color images are available online.
FIG. 5.
Pulmonary hypertension mediates leukocyte–endothelial cells adhesion in small pulmonary arteries of male rats. An increased level of endothelial–leukocyte adhesion molecule (E-selectin) expression in male PAH group correlated with an increase in the actual adhesion of leukocytes to endothelial layers in small PA of male rats. No increase in the leukocyte–endothelial interaction was found in female rats. (A) Representative images for each group (Control and PAH of both genders), (B) Quantification of leukocytes adhered to the endothelium of small PA. Data are mean ± SEM, N = 40 per group. *p < 0.05 versus male control rats; #p < 0.05 versus female with PAH rats in one-way ANOVA. Marker corresponds to 50 μM. MC indicates male control group; M PH—male PH group; FC—female control group; F PH—female PH group. Color images are available online.
Circulating HMGB1 and plasma redox changes in male and female PAH patients
As a DAMP molecule, HMGB1 is released from cells dying by necrosis, whereas apoptotic cells are reported either to release no HMGB1 (8, 50) or to release oxidized and inactive HMGB1 (26). To evaluate whether the PAH patients have the gender difference in the ability to release HMGB1, we compared the plasma levels of HMGB1 in patients with diagnosed PAH (PAH patient cohort), in patients diagnosed with non-PAH lung diseases (lung patient cohort), and in patients with acute and chronic heart diseases (heart patient cohort). The median values for age for all cohorts are presented in Table 1, the pulmonary hemodynamic parameters for male and female PAH patients are presented in Table 2, and the clinical data for lung and heart patient cohorts are presented in Tables 3 and 4 correspondingly. Although there were no significant changes in clinical parameters between PAH males and females, the plasma levels of HMGB1 in male patients with PAH was significantly elevated compared with either lung or heart patients (Fig. 6A and Supplementary Fig. S4A-C). No significant difference in the amount of circulating HMGB1 was found in the female groups.
Table 1.
Demographic Characteristics of Patient Cohorts
| Patient cohort | Age | Sex, females |
|---|---|---|
| PAH | 58 (47–70) | 51 (65) |
| Lung diseases | 63 (51–73) | 16 (22) |
| Heart diseases | 74 (69–79) | 9 (17) |
The data presented as median (IQR).
IQR, interquartile range; PAH, pulmonary arterial hypertension.
Table 2.
Clinical Characteristics of Pulmonary Arterial Hypertension Cohort
| Females | N | Males | N | |
|---|---|---|---|---|
| mPAP (mmHg)a | 40 (20–48) | 51 | 36 (32–43) | 14 |
| PVR (WU)a | 4.6 (3.0–6.7) | 50 | 4.7 (3.7–6.2) | 14 |
| RVCI (L/min · m2)a | 3.3 (2.8–3.8) | 51 | 3.1 (2.5–3.4) | 14 |
| RVSVi (mL/m2)b | 30 (27–41) | 29 | 31 (17–48) | 7 |
| RVEF (%)b | 40 (28–49) | 29 | 39 (24–41) | 7 |
| 6MWD (m) | 352 (270–399) | 35 | 274 (194–398) | 7 |
| BNP (pg/mL) | 84 (38–214) | 48 | 73 (35–174) | 14 |
| WBC ( × 109/L) | 6.4 (5.2–7.7) | 50 | 6.9 (5.4–9.8) | 14 |
The data presented as median (IQR).
Derived from right heart catheterization.
Derived from cardiac magnetic resonance imaging.
6MWD, six-minute walk distance; BNP, B-type natriuretic peptide; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; RVCI, right ventricle cardiac index; RVEF, right ventricular ejection fraction; RVSVi, indexed right ventricular stroke volume; WBC, white blood cell.
Table 3.
Clinical Characteristics of Lung Cohort
| Females | N | Males | N | |
|---|---|---|---|---|
| mPAP (mmHg)a | 15 (12–18) | 16 | 20 (13–26) | 6 |
| PVR (WU)a | 2.1 (1.4–2.3) | 15 | 1.5 (0.9–1.9) | 6 |
| RVCI (L/min · m2)a | 3.0 (2.6–3.3) | 15 | 3.2 (3.0–3.9) | 6 |
| BNP (pg/mL) | 32 (13–50) | 15 | 37 (18–100) | 6 |
| WBC ( × 109/L) | 6.1 (5.2–6.8) | 15 | 6.2 (4.9–10.6) | 6 |
The data presented as median (IQR).
Derived from right heart catheterization.
Table 4.
Clinical Characteristics of Heart Cohort
| Females | N | Males | N | |
|---|---|---|---|---|
| BNP (pg/mL) | 102 (36–205) | 9 | 223 (43–1106) | 8 |
| WBC ( × 109/L) | 9 (4.6–12.2) | 9 | 6.9 (6–9.7) | 8 |
The data presented as median (IQR).
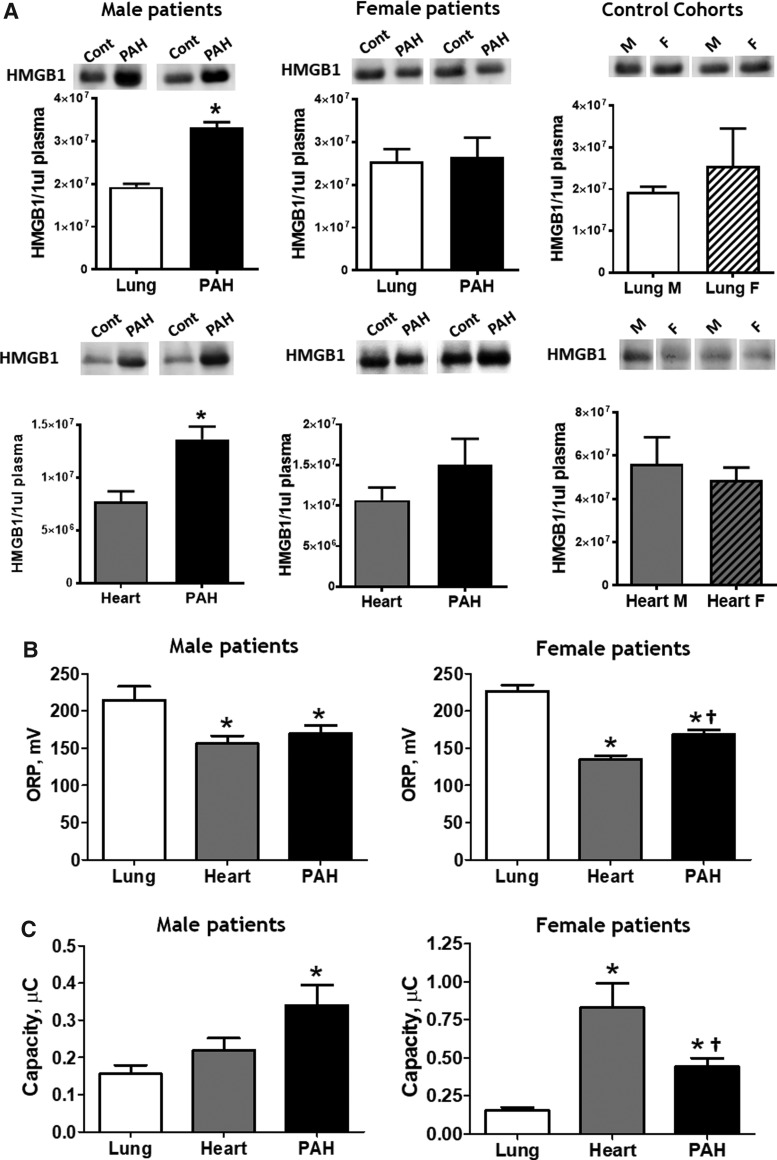
FIG. 6.
Male patients with PAH have elevated levels of circulating HMGB1 and reductive stress. The HMGB1 signal produced by 1 μL of plasma was quantified by using Western blot analysis and compared within each gender between PAH and lung cohorts [(A), three upper panels] and PAH and heart cohorts [(A), three lower panels]. Only male PAH patients had increased HMGB1 plasma levels compared with control cohorts. Data are mean ± SEM. Upper panel—N = 4 for lung male group; N = 6 for PAH male group; N = 10 for lung female group; N = 8 for PAH female group; lower panel—N = 7 for all groups. *p < 0.05 versus lung or heart cohort. p Value calculated by using unpaired t-test. ORP (B) and Cap (C) were measured in the plasma samples of control cohorts and patients with PAH. Patients with non-PAH lung diseases showed evidence of strong oxidative stress, whereas patients with non-PAH heart diseases had reductive changes. Females with PAH had more oxidized redox parameters compared with the heart cohort, whereas male patients with PAH possessed equal (ORP) or even more reduced (Cap) plasma redox changes. Data are mean ± SEM, (B, C) N = 5 for lung; N = 8 for heart; N = 14 for PAH in male groups; N = 16 for lung; N = 9 for heart; N = 51 for PAH in female groups. *p < 0.05 versus lung cohort, †p < 0.05 versus heart cohort. p Value calculated by using one-way ANOVA (Bonferroni's multiple-comparison test).
To investigate whether the increased level of plasma HMGB1 correlated with an altered redox balance, we measured the plasma redox parameters in male and female patients from all three cohorts as described in Figure 3A and B. Interestingly, there was a significant increase in the ORP levels and a decrease in Cap, or “oxidative shift,” in patients with non-PAH lung diseases compared with either PAH or heart cohorts (Fig. 6B, C). These data are in strong accordance with the previous studies that reported oxidative stress as an important mediator of pulmonary diseases (34). However, when we compared the redox parameters between PAH and “heart” patients, we found an evident gender difference in the PAH cohort. The PAH female patients had significantly more oxidized plasma compared with gender-matched heart patients. In the male groups, the levels of plasma ORP were comparable, whereas the antioxidant capacity in PAH patients was even increased, although not significant. These data that show a difference between PAH and heart cohorts in females but not in males are of the highest importance. Patients with heart diseases are known to have ongoing myocardial damage, even in the case of chronic heart disease (43, 51), and to have massive necrotic damage during acute events. We found that the plasma parameters in males with PAH are slightly more reduced, although nonsignificantly, compared with the “heart” cohort, which confirms the reductive changes in the PAH group and suggests severe vascular damage (especially based on the levels of circulating HMGB1, Fig. 6A).
Correlation between plasma redox parameters and RV dysfunction in male and female PAH patients
In this experiment, we evaluated whether the changes in redox parameters between male and female PAH patients correlate with RV dysfunction, the primary survival determinant in patients with PAH. Our results confirm that in female patients the level of brain natriuretic peptide (B-type natriuretic peptide [BNP]), an established marker of heart failure, significantly correlated with an increase in ORP and a decrease in Cap, which are the signs of the oxidative changes in plasma (Fig. 7). In contrast, in male PAH patients, the elevated BNP production significantly correlated with a decrease in ORP and an increase in Cap, which corresponds to the more reduced environment (Fig. 7). In particular, in female patients, an increase of ORP on 1 mV or a decrease of Cap on 1 μC produces a 1% or 40% upregulation of BNP level correspondingly; in males, every 1 mV of ORP decrease or every 1 μC of Cap increase resulted in a 2% or 81% rise of BNP level correspondingly. Importantly, although first considered simply as a marker of RV dysfunction, BNP was later shown to strongly correlate with pulmonary hemodynamics and functional impairment in PAH patients (29). Besides, BNP possesses mitogenic, hypertrophic, and pro-inflammatory properties, and it may be directly involved in the pathogenesis of PAH. Since the elevated plasma BNP levels are associated with increased mortality and a decrease in BNP levels is associated with improved survival (12), the factors that determine BNP levels in plasma may be involved in patient survival. The discovered relationship between plasma redox status and BNP levels, which is distinctive for each gender, is of high importance.
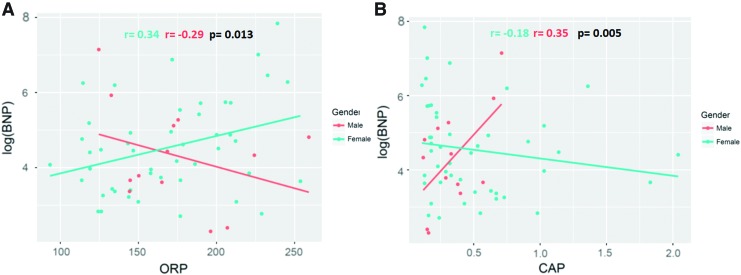
FIG. 7.
PAH patients show a gender difference in correlation between plasma redox parameters and markers of right ventricle dysfunction. Scatter plots showing the correlation between ORP—(A) and Cap—(B) and a biomarker of cardiac failure, BNP, in PAH patients. Adjustments are made to gender, age, and PAH therapy. The analysis indicates that the plasma redox environment significantly impacts BNP in PAH patients (p < 0.1). Moreover, this impact is significantly different with the interaction of genders (Pearson coefficients for ORP r = 0.34 [females] and r = −0.29 [males], p = 0.013, Pearson coefficients for Cap r = −0.18 [females] and r = 0.35 [males], p = 0.005). N = 50 for female PAH patients and N = 13 for male PAH patients. BNP, B-type natriuretic peptide. Color images are available online.
White blood cells in male and female PAH patients
Male gender is known to be associated with an enhanced inflammatory response (11). In Figure 8A, we plotted the distribution of white blood cells (WBCs) in male and female PAH patients and marked the mean values for age-matched healthy individuals (solid line), as previously published (30). According to these data, PAH induces more than two times increase in mean WBC levels in males compared with females (mean WBC is 8.4/nL ±4.7 in PAH males vs. 4.3/nL ±5.7 in age-matched [50–59 years old] healthy male individuals (30) and 6.5/nL ±1.8 in PAH females vs. 4.6/nL ±6.0 in age-matched [50–59 years old] healthy female individuals). Moreover, 21.4% of male patients and only 4% of females had an amount of WBC beyond the control values (4–10/nL), suggesting that PAH-induced inflammatory response is more associated with the male gender than with the female gender. The absolute amount of WBC in PAH males was also found to be significantly increased compared with PAH females (Fig. 8B).
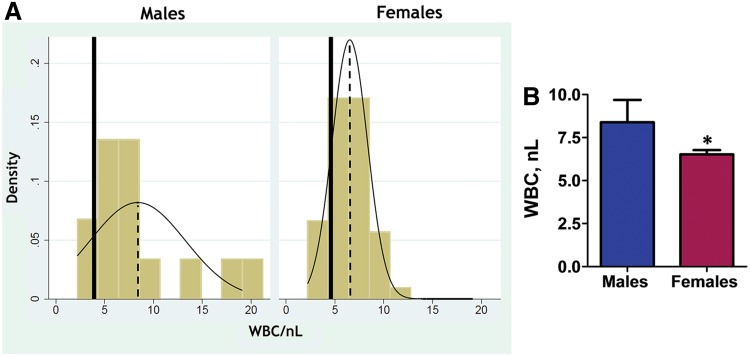
FIG. 8.
Gender difference in circulating WBC in patients with PAH. (A) PAH is associated with an increase in WBC compared with healthy individuals. The distribution of WBC values is shown as yellow columns. The dashed lines represent the mean WBC value. The solid lines show the mean WBC for the age-matched healthy individuals, as previously published (30). The inflammatory response is more profound in PAH male patients, whereas distribution in PAH females is more homogeneous and shows only a mild increase in WBC. (B) The levels of WBC in PAH male and female blood samples. N = 14 for male and N = 50 for female PAH patients. *p < 0.05 versus male PAH patients. p Value calculated by using unpaired t-test. WBC, white blood cell. Color images are available online.
Discussion
The important role of gender in PAH is known for many years (16). Although female sex hormones and their metabolites have been described to exert anti-inflammatory and protective properties on the pulmonary vasculature (60), PAH has a paradoxically high predominance in females. Nevertheless, females have a better survival prognosis compared with males. A few recent clinical studies revealed that the male gender has been associated with poorer survival (52), predisposition to develop RV failure (24) and serves as one of the primary independent predictors of death (22). However, there is no clear mechanistic explanation on why males have a more intense form of PAH.
Interestingly, not only for PAH but also for many acute inflammatory diseases, male gender is associated with more severe inflammation, poorer prognosis and considered an independent prognostic factor for infection-induced mortality (11). Not being a classic inflammatory disease, PAH still has a strong inflammatory component that is directly associated with disease development and progression (38). Our research group has recently compared the development of PAH in male and female rats by using the most human-relevant Sugen/hypoxia model of PAH. In this study, we have discovered two distinct gender-associated phenotypes of PAH: an inflammation of pulmonary vascular wall and RV fibrosis associated with male gender and an excessive level of pulmonary vascular remodeling in females (40).
Damage of the pulmonary vascular cells is a very well-established pathological mechanism directly involved in the initiation and progression of PAH. Thus, apoptosis inhibitors (19, 35) or blockade/genetic deletion of apoptosis-inducing factors completely prevent PAH development (63) in different PAH models. Moreover, antagonizing of apoptosis in PAECs reversed an already established PAH (58). It has been previously reported that in response to damaging stimuli males and females are prone to different types of cell death (25, 42), with cells from males being more likely to die by necrosis, and those from females—by apoptosis. However, the particular role of necrosis in the pathogenesis of PAH was never investigated. Based on our results, we propose that necrotic cell death can contribute to the higher intensity of inflammatory response in males.
Indeed, the environment inside the cell is known to be highly reduced due to the large amounts of intracellular antioxidants, such as thiols. During necrosis, which is a passive process of releasing the cellular content through the hole on the plasma membrane, these thiols appear in the normally oxidized extracellular space. Accumulation of antioxidants in the extracellular milieu would shift the redox status toward more reduced. HMGB1 and some other DAMPs are known to be redox sensitive, as they maintain their ability to bind to the DAMP receptors only in a reduced state and lose this activity when becoming oxidized (26). Oxidation of HMGB1 was even suggested to contribute to the resolution of inflammation (26). In contrast, reduced HMGB1 would prolong stimulation of the downstream inflammatory pathways.
Such a precise tuning of HMGB1 activity by the redox state of the extracellular environment allows to adequately control the duration and intensity of the initiated inflammatory response. Usually, HMGB1 released to the highly oxidative extracellular space produces only short signaling followed by a quick inactivation (Fig. 9). However, under the circumstances of chronic or severe necrotic cell death, a more reduced extracellular environment prolongs HMGB1 activity. This sustained ability of HMGB1 to bind to TLR4 will extend TLR4-mediated signaling and downstream inflammatory events. Interestingly, it has been recently discovered that DAMP-mediated stimulation of TLRs in monocytes and other inflammatory cells activates the cystine/cysteine redox cycle, which consists of uptake of oxidized free cysteine from the extracellular environment and secretion of large amounts of reduced free cysteine outside the cell (46). Activation of this cycle will not only additionally reduce the extracellular environment but also create a self-accelerating and persistent reductive stress, thus promoting inflammation (Fig. 9).
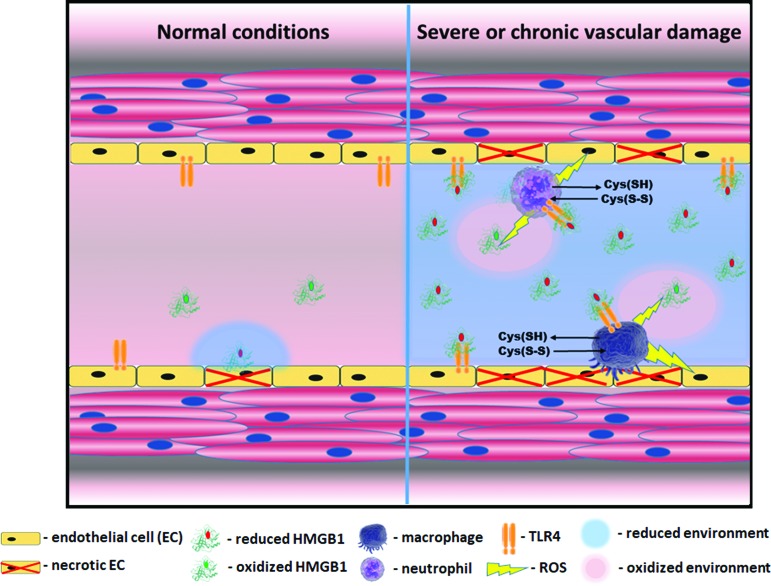
FIG. 9.
Necrosis mediated activation of vascular inflammation. HMGB1 is active in its reduced state, whereas oxidation inhibits its activity. Therefore, the duration of HMGB1 signaling depends on the redox status of the extracellular environment, where it gets released on cell death. In a healthy state (left part of a scheme), even in a case of occasional necrosis, oxidized state of the extracellular environment ensures a quick inhibition of HMGB1. However, if the level of necrosis is relatively high like it was found in males, the local extracellular environment shifts to become more reduced due to the release of high amounts of intracellular glutathione and other thiols (right part of a scheme). This reduced environment prolongs HMGB1-mediated signaling. The reduced HMGB1 binds to a TLR4 receptor of endothelial cells and induces their activation. Active endothelial cells release cytokines, which attract macrophages and leukocytes to the site of damage and express the adhesive molecules that promote the recruitment of inflammatory cells. Activated macrophages and neutrophils, by producing ROS, oxidize HMGB1, but at the same time may additionally injure endothelial cells and, thus, promote damage-mediated inflammation. Moreover, activation of pattern recognition receptors such as TLR4 and RAGE induces further propagation of reductive stress by activation of cystine/cysteine redox cycle and secretion of large amounts of reduced free cysteine outside the cell (45). RAGE, receptor for advanced glycation end products; ROS, reactive oxygen species. Color images are available online.
In this study, we provide direct evidence that necrosis, but not apoptosis induces activation of the TLR4/IL1β axis. The cells treated by necrotic media also show an increased expression of ELAM, E-selectin. Since expression of E-selectin directly depends on the presence of cytokines, and first of all IL1β, this response may be considered a straight signaling pathway (HMGB1/TLR4/IL1β/E-Selectin) recruiting the inflammatory cells to the site of damage. Importantly, this activation was significantly attenuated by pre-oxidizing the media, which support the hypothesis that the redox status of the factors released from dying cells determines their ability to activate TLR4. This finding is also in agreement with the previously reported critical role of the redox environment in mediating TLR/IL1β signaling (46). Thus, it has been noticed that antioxidants and exogenous reducing agents significantly activate this pathway and enhance the secretion of IL1β.
By using the rat Sugen/Hypoxia PAH model and plasma samples collected from PAH patients we confirmed that only male gender shows an increased level of necrotic cell death. In rats, this necrotic damage was associated with an increased level of circulating LDH and an elevated signaling from extra-nuclear HMGB1 in the PAs. Although the last finding confirms that necrotic damage occurs in lungs, the circulating LDH could also reflect the damage of RV that is known to be more severe in males (40). This gender-specific damage mediates a reductive shift in plasma redox homeostasis of males, strong activation of TLR4/IL1β/E-selectin axis, and an increased endothelial–leukocyte interaction in small PAs. It has been previously reported that circulating E-selectin is a strong independent predictor of mortality in PAH patients (3). Therefore, we propose that inflammation-mediated activation of endothelial cells may contribute to the poor survival prognosis in males.
In humans, the redox status of the plasma collected from PAH patients was compared with two other patient cohorts—with non-PAH lung diseases and with acute and chronic heart diseases (Fig. 6B, C). These “lung” and “heart” patient cohorts that served as controls in this study were specifically chosen to evaluate whether the redox parameters of patient plasma will allow distinguishing PAH that affects both lungs and heart from non-PAH lung or non-PAH heart diseases. It is well established that non-PAH lung diseases are strongly associated with oxidative stress (34). Overproduction of ROS was shown to be implemented in the pathogenesis of acute lung injury (20), chronic obstructive pulmonary disease (61), pulmonary fibrosis (4), and asthma (17). We found that in accordance to these published observations plasma samples from the “lung” cohort had the markers of developed oxidative stress—high ORP levels and depleted antioxidant capacity. In contrast, acute and chronic heart diseases are associated with ongoing myocardial damage (43, 51). This damage may explain the more reduced plasma parameters of patients from the “heart” cohort. Compared with these two cohorts, the redox status of plasma collected from patients with PAH, which is considered a combination of oxidative stress and vascular damage, appears somewhat in the middle between “lung” and “heart” cohorts. Moreover, there was a distinct difference between PAH male and female patients. As expected, males showed a more reduced plasma profile similar to the “heart” patients, and females that had more oxidized plasma samples. Based on these data, we believe that the redox analysis of plasma could become a useful tool in diagnosing PAH, especially in females that show a distinct difference in the plasma redox status compared with patients with heart or lung diseases. In males, the additional measurements of plasma HMGB1 levels may help to distinguish PAH (Fig. 6A).
Correlation of the redox parameters with BNP, an established marker of RV dysfunction, revealed that the redox status of the plasma could also serve as a prognostic marker in PAH (Fig. 7). Thus, in male patients, the reductive changes in plasma significantly correlated with the levels of BNP, a marker of RV failure. In contrast, in females, BNP levels increased in response to oxidative stress. Although the future research with larger patient cohorts requires to fully validate our discoveries, our results suggest that two genders are considerably more different in their ability to tolerate the redox stress than it was assumed earlier. Females seem to be more sensitive to oxidative conditions, whereas males are less tolerable to the reduced environment. The reductive changes that can occur in response to hypoxia, impaired mitochondrial respiration (2), insufficiency of nicotinamide adenine dinucleotide phosphate oxidases (67), or hyper-activation of the nuclear factor-erythroid 2 p45-related factor 2 system (57) have been already noticed to contribute to the disease, including PAH. This contribution could be more essential for males.
In conclusion, the mechanisms responsible for the manifestation of gender difference in PAH have been under close investigation for decades. Until recently, the contribution of sex hormones was the primary direction of this research. Recently, the importance of genetic factors in female predisposition to PAH was also highlighted. Thus, it was shown that Y chromosome-specific transcription factor Sex-determining region Y positively regulates the BMPR2 promoter (64). Given that downregulation of BMPR2 signaling is tightly associated with PAH pathogenesis, the factors that upregulate BMPR2 could become protective. We believe that we have discovered an additional very novel aspect of gender difference that was not described earlier. In this study, we established a connection between the male gender and the higher level of necrotic cell death in response to PAH. We also proposed the importance of this augmented necrotic cell death in the activation of inflammatory pathways. We believe that our results provide a novel approach to the problem of gender disparity in PAH that have to be taken into account to develop adequate therapeutic strategies and improve the survival prognosis, especially in males.
Materials and Methods
Rat model of pulmonary hypertension
Sprague Dawley rats (200–250 g) were obtained from Charles River (Wilmington, MA). The animals were kept in a standard 12-h light-dark cycle and received standard rodent food and water ad lib. All experimental procedures were approved by Augusta University's IACUC. To induce PAH, male and female rats either received the vehicle (Control group) or were injected with a single dose of the vascular endothelial growth factor receptor 2 antagonist, SU5416, 20 mg/kg subcutaneous (PAH group) as previously published (40). The PAH rats were placed in a hypoxic chamber (O2 10% ± 0.5%) for 4 weeks, then returned to normoxic conditions, and allowed to develop PAH for an additional 10 weeks. The control rats were kept under normoxic conditions for the duration of the study (14 weeks). At the end of the study, the animals were anesthetized (Inactin, 100 mg/kg intraperitoneal), the chest was opened, and the lungs were flushed with saline (0.9% sodium chloride) via a needle inserted into the RV to remove the blood from pulmonary vessels. The left lung from all animals was fixed in formalin and embedded in paraffin. The right lung was quick-frozen and stored at −80°C for future biochemical analysis. The pulmonary and systemic hemodynamic parameters for control male and female rats and rats with occlusive-angioproliferative PAH used in this study were reported in a previous publication (40).
Human subjects
Three different cohorts—(i) patients with a diagnosis of the World Health Organization Group I PAH (PAH cohort), (ii) patients who were diagnosed with non-PAH lung diseases (lung cohort), and (iii) patients who were diagnosed with acute and chronic heart diseases (heart cohort)—were prospectively recruited from the University of Arizona (UA). The lung cohort consists of patients with dyspnea who were initially suspected of PAH, but not confirmed based on RV catheterization. The heart cohort consists of patients diagnosed with heart diseases, including ischemic, atherosclerotic heart disease, myocardial infarction, heart failure, cardiomyopathies, and arrhythmias. All subjects provided written consent to participate in this study with the approval of the UA institutional human subjects review board. Blood samples were drawn during outpatient clinical visits or RV catheterization. Care was taken to standardize blood sample collection, preparation, and storage at −80°C as previously described (15).
Leukocyte–endothelial adhesion in small PAs
The left lung from all animals was fixed in formalin and embedded in paraffin. A series of 4-μM lung tissue sections were stained with hematoxylin and eosin. The transversely sectioned small PAs were digitally captured at 200 × by using a Motic BA410 microscope with Moticam pro 282A camera and analyzed by using Motic Images Plus 2.0 software. Ten random images from each animal were used. For each PA, the number of adhered leukocytes was counted and normalized on the perimeter of the artery that was measured by tracing the artery using ImageJ software.
Immunohistochemistry
Histology was performed by HistoWiz, Inc. (histowiz.com) using standard operating procedures and fully automated workflow. Immunohistochemistry was performed on a Bond Rx autostainer (Leica Biosystems) with heat-mediated epitope retrieval by using standard protocols. Antibodies used were rabbit polyclonal HMGB1 primary antibody (Abcam, Cambridge, United Kingdom; 18256, 1:1000). Bond Polymer Refine Detection (Leica Biosystems) was used according to the manufacturer's protocol. Sections were then counterstained with hematoxylin, dehydrated, and film coverslipped by using a TissueTek-Prisma and Coverslipper (Sakura). Whole-slide scanning (40 × ) was performed on an Aperio AT2 (Leica Biosystems). Ten to twelve random PAs per animal (n = 6 per each group) were deconvoluted into brown and blue color by using FIJI Image J software for hematoxylin/3,3′-diaminobenzidine DAB staining. The signal per area for HMGB1 staining was analyzed by an investigator who was blinded to the origin or treatment by tracing the vascular wall of each PA by using Image J as described (39) for both brown and blue fields using the same tracing. To calculate the optical density, the mean signal from the traced vascular wall was used in the formulae (Log10[Maximal Mean signal/Traced Mean signal]). To normalize signal from extra-nuclear HMGB1, the brown signal (HMGB1) was divided by the blue signal (nuclei).
Cell culture
Ovine PAECs were isolated and identified, as previously described (27, 62). Cells between passages 5–7 were maintained in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum (GenClone; #25–514H), antibiotics (Gibco™ Penicillin-Streptomycin, #15140122) at 37°C with 5% CO2–95% air. To induce apoptosis, PAEC were incubated at hypoxic conditions (O2 2% ± 0.5%) for 24 h. For necrosis induction cells underwent 3–4 freeze/thawing cycles. Media collected from apoptotic or necrotic cells were either directly used to incubate untreated PAECs or pretreated with hydrogen peroxide (H2O2, 1 mM) for 10 min. To eliminate the excess of H2O2, the media were incubated with purified catalase (Sigma, St. Louis, MO; #C1345 diluted to 100 U/mL) for another 15 min. The naive PAECs were incubated with normal cell media or with pretreated media and nonpretreated with H2O2 conditioned media collected from apoptotic and necrotic cells. To measure TLR4 activation, cells were incubated with apoptotic/necrotic media for 2 h, to assess the expression of IL1β and e-Selectin—for 16 h.
Western blot analysis
Lung tissues and cells were lysed as previously described (39). Lung lysates, cell lysate, conditioned cell culture media, and patient plasma samples were electrophoretically separated and transferred by using PowerPac™ Universal power supply and Trans-Blot Turbo transferring system (Bio-Rad Laboratories, Inc.). Membranes were probed by using anti-pY674TLR4 (ThermoFisher Scientific, Waltham, MA; #PA5-23263), anti-TLR4 (Santa Cruz Biotechnology, Dallas, TX; #sc-293072), anti-IL1β, anti-E-Selectin (Proteintech Group, Inc., Rosemont, IL; #16806-1-AP and #20894-1-AP correspondingly), anti-cleaved Caspase 3 (Cell Signaling Technology, Inc., Danvers, MA; #9661), and anti-HMGB1 (Abcam) antibodies. The reactive bands were visualized by using chemiluminescent procedures, recorded by using a Li-Cor Odyssey FC Imager, and analyzed by using IS Image Studio 5.0 software. The protein loading was normalized by re-probing with β-actin (Santa Cruz Biotechnology; #sc-47778), GAPDH (Cell Signaling Technology, Inc.; #2118), or total sample protein by using 4–20% Mini-PROTEAN® TGX Stain-Free™ Protein Gels (Bio-Rad Laboratories, Inc., Hercules, CA; #4568094) as previously described (45), or normalized per plasma volume. Some membranes were stripped and re-probed for more than one protein.
Redox parameters evaluation
ORP and total antioxidant capacity (Cap) were measured in 30 μL of patient or rat plasma samples electrochemically by using RedoxSys® Diagnostic System (Aytu BioScience, Inc., Englewood, CO) as described in the manufacturer's protocol. Briefly, the plasma ORP was measured by a galvanostat-based reader against the reference electrode. This results in the static ORP value. After that, the reader applied a small current sweep to oxidize the sample. This resulted in the exhaustion of antioxidants, and antioxidant capacity of the sample was calculated reflecting the amount of electrons applied to the sample. This technology is based on the well-established potentiometric titration method for OPR measurement and scaled for the small volume of biological samples. The method integrates all antioxidants and oxidants and is better suited for oxidative/reductive condition evaluation than glutathione oxidation/reduction measurements.
Plasma LDH activity
LDH activity in rat lung and plasma samples was measured by using LDH activity colorimetric assay kit (BioVision, Inc., Milpitas, CA; #K726–500) according to the manufacturer's protocol.
Statistical analysis
An analysis of the correlation between plasma redox parameters (reduction potential [ORP], Cap, and plasma level of BNP) in the PAH patient cohort was performed by using univariate multiple regression models. Log-transformed values of BNP are used in the linear models to reduce skewness and comply with assumptions of linear regression. The model was adjusted to assess whether these impacts persist in the presence of potential confounders such as gender, age, and PAH therapy. Other statistical calculations were performed by using the GraphPad Prism V. 4.01 software. The means ± standard error of the mean were calculated, and significance was determined by either the unpaired t-test or analysis of variance (ANOVA). For ANOVA, Newman-Keuls post hoc testing was utilized. A value of p < 0.05 was considered significant.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. S.M. Black for providing rat lung samples, PAEC line, and equipment. This work was supported by NIH grants R01HL133085 (O.R.), Arizona Health Sciences Center Career Development Award (O.R.), R01HL132918 (R.R.), and Scientist Development Grant (14SDG20480354) from the American Heart Association National Office (R.R.).
Abbreviations Used
- ANOVA
analysis of variance
- BNP
B-type natriuretic peptide
- Cap
total antioxidant capacity
- DAMP
damage-associated molecular pattern
- ELAM-1
endothelial–leukocyte adhesion molecule 1
- H2O2
hydrogen peroxide
- HMGB1
high mobility group box 1
- IL1β
interleukin 1 beta
- IQR
interquartile range
- LDH
lactate dehydrogenase
- mPAP
mean pulmonary artery pressure
- ORP
oxidation–reduction potential
- PA
pulmonary artery
- PAEC
pulmonary artery endothelial cell
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- PVR
pulmonary vascular resistance
- ROS
reactive oxygen species
- RV
right ventricle
- RVCI
right ventricle cardiac index
- SEM
standard error of the mean
- TLR4
toll-like receptor 4
- UA
University of Arizona
- WBC
white blood cell
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, and Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Aleshin VA, Artiukhov AV, Oppermann H, Kazantsev AV, Lukashev NV, and Bunik VI. Mitochondrial impairment may increase cellular NAD(P)H: resazurin oxidoreductase activity, perturbing the NAD(P)H-based viability assays. Cells 4: 427–451, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amabile N, Heiss C, Chang V, Angeli FS, Damon L, Rame EJ, McGlothlin D, Grossman W, De Marco T, and Yeghiazarians Y. Increased CD62e(+) endothelial microparticle levels predict poor outcome in pulmonary hypertension patients. J Heart Lung Transplant 28: 1081–1086, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, and Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med 103: 1245–1256, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Basu S, Binder RJ, Suto R, Anderson KM, and Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12: 1539–1546, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, and Bauer PM. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med 18: 1509–1518, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, and McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 142: 448–456, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Bianchi ME. and Manfredi A. Chromatin and cell death. Biochim Biophys Acta 1677: 181–186, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Boucherat O, Vitry G, Trinh I, Paulin R, Provencher S, and Bonnet S. The cancer theory of pulmonary arterial hypertension. Pulm Circ 7: 285–299, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carta S, Castellani P, Delfino L, Tassi S, Vene R, and Rubartelli A. DAMPs and inflammatory processes: the role of redox in the different outcomes. J Leukoc Biol 86: 549–555, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Casimir GJ. and Duchateau J. Gender differences in inflammatory processes could explain poorer prognosis for males. J Clin Microbiol 49: 478; author reply 478–479, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Casserly B. and Klinger JR. Brain natriuretic peptide in pulmonary arterial hypertension: biomarker and potential therapeutic agent. Drug Des Devel Ther 3: 269–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dauphinee SM. and Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest 86: 9–22, 2006 [DOI] [PubMed] [Google Scholar]
- 14. de Jesus Perez VA. Making sense of the estrogen paradox in pulmonary arterial hypertension. Am J Respir Crit Care Med 184: 629–630, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Desai AA, Zhou T, Ahmad H, Zhang W, Mu W, Trevino S, Wade MS, Raghavachari N, Kato GJ, Peters-Lawrence MH, Thiruvoipati T, Turner K, Artz N, Huang Y, Patel AR, Yuan JX, Gordeuk VR, Lang RM, Garcia JG, and Machado RF. A novel molecular signature for elevated tricuspid regurgitation velocity in sickle cell disease. Am J Respir Crit Care Med 186: 359–368, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dresdale DT, Schultz M, and Michtom RJ. Primary pulmonary hypertension. I. Clinical and hemodynamic study. Am J Med 11: 686–705, 1951 [DOI] [PubMed] [Google Scholar]
- 17. Dworski R. Oxidant stress in asthma. Thorax 55 Suppl 2: S51–S53, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Escribano-Subias P, Blanco I, Lopez-Meseguer M, Lopez-Guarch CJ, Roman A, Morales P, Castillo-Palma MJ, Segovia J, Gomez-Sanchez MA, and Barbera JA. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J 40: 596–603, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, Inman M, and Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest 119: 1298–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fink MP. Role of reactive oxygen and nitrogen species in acute respiratory distress syndrome. Curr Opin Crit Care 8: 6–11, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Granger DN. and Senchenkova E. Inflammation and the Microcirculation. Morgan & Claypool Life Sciences: San Rafael, CA, 2010 [PubMed] [Google Scholar]
- 22. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, and Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Ilmakunnas M, Tukiainen EM, Rouhiainen A, Rauvala H, Arola J, Nordin A, Makisalo H, Hockerstedt K, and Isoniemi H. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver Transpl 14: 1517–1525, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, Kawut SM, Bogaard HJ, Boonstra A, and Vonk Noordegraaf A. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest 145: 1230–1236, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jog NR. and Caricchio R. Differential regulation of cell death programs in males and females by poly (ADP-ribose) polymerase-1 and 17beta estradiol. Cell Death Dis 4: e758, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, and Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29: 21–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelly LK, Wedgwood S, Steinhorn RH, and Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol 286: L984–L991, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Latz E, Xiao TS, and Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol 13: 397–411, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leuchte HH, Holzapfel M, Baumgartner RA, Ding I, Neurohr C, Vogeser M, Kolbe T, Schwaiblmair M, and Behr J. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 43: 764–770, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Mahlknecht U. and Kaiser S. Age-related changes in peripheral blood counts in humans. Exp Ther Med 1: 1019–1025, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Wahl LM, Fenton MJ, and Vogel SN. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem 282: 16042–16053, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Menghini R, Campia U, Tesauro M, Marino A, Rovella V, Rodia G, Schinzari F, Tolusso B, di Daniele N, Federici M, Zoli A, Ferraccioli G, and Cardillo C. Toll-like receptor 4 mediates endothelial cell activation through NF-kappaB but is not associated with endothelial dysfunction in patients with rheumatoid arthritis. PLoS One 9: e99053, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ottaviano FG, Handy DE, and Loscalzo J. Redox regulation in the extracellular environment. Circ J 72: 1–16, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Park HS, Kim SR, and Lee YC. Impact of oxidative stress on lung diseases. Respirology 14: 27–38, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Partovian C, Adnot S, Raffestin B, Louzier V, Levame M, Mavier IM, Lemarchand P, and Eddahibi S. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am J Respir Cell Mol Biol 23: 762–771, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Pugh ME. and Hemnes AR. Development of pulmonary arterial hypertension in women: interplay of sex hormones and pulmonary vascular disease. Womens Health (Lond) 6: 285–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pullamsetti SS, Savai R, Janssen W, Dahal BK, Seeger W, Grimminger F, Ghofrani HA, Weissmann N, and Schermuly RT. Inflammation, immunological reaction and role of infection in pulmonary hypertension. Clin Microbiol Infect 17: 7–14, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Rabinovitch M, Guignabert C, Humbert M, and Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115: 165–175, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rafikova O, Rafikov R, Kangath A, Qu N, Aggarwal S, Sharma S, Desai J, Fields T, Ludewig B, Yuan JX, Jonigk D, and Black SM. Redox regulation of epidermal growth factor receptor signaling during the development of pulmonary hypertension. Free Radic Biol Med 95: 96–111, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rafikova O, Rafikov R, Meadows ML, Kangath A, Jonigk D, and Black SM. The sexual dimorphism associated with pulmonary hypertension corresponds to a fibrotic phenotype. Pulm Circ 5: 184–197, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rafikova O, Srivastava A, Desai AA, Rafikov R, and Tofovic SP. Recurrent inhibition of mitochondrial complex III induces chronic pulmonary vasoconstriction and glycolytic switch in the rat lung. Respir Res 19: 69, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rafikova O, Tipton AJ, Baban B, and Sullivan JC. Inhibition of necrosis attenuates blood pressure and renal inflammation in male SHR with no effect in females. Hypertension 62: A253, 2013 [Google Scholar]
- 43. Rayment NB, Haven AJ, Madden B, Murday A, Trickey R, Shipley M, Davies MJ, and Katz DR. Myocyte loss in chronic heart failure. J Pathol 188: 213–219, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, Levy PC, Reid LM, Vreim CE, and Williams GW. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 107: 216–223, 1987 [DOI] [PubMed] [Google Scholar]
- 45. Rivero-Gutierrez B, Anzola A, Martinez-Augustin O, and de Medina FS. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal Biochem 467: 1–3, 2014 [DOI] [PubMed] [Google Scholar]
- 46. Rubartelli A, Gattorno M, Netea MG, and Dinarello CA. Interplay between redox status and inflammasome activation. Trends Immunol 32: 559–566, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Sadamura-Takenaka Y, Ito T, Noma S, Oyama Y, Yamada S, Kawahara K, Inoue H, and Maruyama I. HMGB1 promotes the development of pulmonary arterial hypertension in rats. PLoS One 9: e102482, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sakao S, Tatsumi K, and Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 10: 95, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, and Schermuly RT. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 897–908, 2012 [DOI] [PubMed] [Google Scholar]
- 50. Scaffidi P, Misteli T, and Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Setsuta K, Seino Y, Ogawa T, Ohtsuka T, Seimiya K, and Takano T. Ongoing myocardial damage in chronic heart failure is related to activated tumor necrosis factor and Fas/Fas ligand system. Circ J 68: 747–750, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, and Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest 141: 363–373, 2012 [DOI] [PubMed] [Google Scholar]
- 53. Sharma S, Sun X, Kumar S, Rafikov R, Aramburo A, Kalkan G, Tian J, Rehmani I, Kallarackal S, Fineman JR, and Black SM. Preserving mitochondrial function prevents the proteasomal degradation of GTP cyclohydrolase I. Free Radic Biol Med 53: 216–229, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stagos D, Goutzourelas N, Ntontou AM, Kafantaris I, Deli CK, Poulios A, Jamurtas AZ, Bar-Or D, and Kouretas D. Assessment of eccentric exercise-induced oxidative stress using oxidation-reduction potential markers. Oxid Med Cell Longev 2015: 204615, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tang D, Billiar TR, and Lotze MT. A Janus tale of two active high mobility group box 1 (HMGB1) redox states. Mol Med 18: 1360–1362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang Y, Zhao X, Antoine D, Xiao X, Wang H, Andersson U, Billiar TR, Tracey KJ, and Lu B. Regulation of posttranslational modifications of HMGB1 during immune responses. Antioxid Redox Signal 24: 620–634, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, and Hayes JD. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med 88: 108–146, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, Gera L, Farkas L, Rabinovitch M, Zamanian RT, Inayathullah M, Fridlib M, Rajadas J, Peters-Golden M, Voelkel NF, and Nicolls MR. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 5: 200ra117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tipping PG. Toll-like receptors: the interface between innate and adaptive immunity. J Am Soc Nephrol 17: 1769–1771, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Umar S, Rabinovitch M, and Eghbali M. Estrogen paradox in pulmonary hypertension: current controversies and future perspectives. Am J Respir Crit Care Med 186: 125–131, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Eeden SF. and Sin DD. Oxidative stress in chronic obstructive pulmonary disease: a lung and systemic process. Can Respir J 20: 27–29, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wedgwood S. and Black SM. Molecular mechanisms of nitric oxide-induced growth arrest and apoptosis in fetal pulmonary arterial smooth muscle cells. Nitric Oxide 9: 201–210, 2003 [DOI] [PubMed] [Google Scholar]
- 63. White K, Dempsie Y, Caruso P, Wallace E, McDonald RA, Stevens H, Hatley ME, Van Rooij E, Morrell NW, MacLean MR, and Baker AH. Endothelial apoptosis in pulmonary hypertension is controlled by a microRNA/programmed cell death 4/caspase-3 axis. Hypertension 64: 185–194, 2014 [DOI] [PubMed] [Google Scholar]
- 64. Yan L, Cogan JD, Hedges LK, Nunley B, Hamid R, and Austin ED. The Y chromosome regulates BMPR2 expression via SRY: a possible reason ‘Why’ fewer males develop PAH. Am J Respir Crit Care Med 198: 1581–1583, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, and Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 107: 11942–11947, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang H, Rivera Z, Jube S, Nasu M, Bertino P, Goparaju C, Franzoso G, Lotze MT, Krausz T, Pass HI, Bianchi ME, and Carbone M. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A 107: 12611–12616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yu Q, Lee CF, Wang W, Karamanlidis G, Kuroda J, Matsushima S, Sadoshima J, and Tian R. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. J Am Heart Assoc 3: e000555, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.