Abstract
The predominant role of IL-6 in cancer is its key promotion of tumour growth. IL-6 binds IL-6 receptor (IL-6R) and the membrane-bound glycoprotein gp130. The complex I-6/IL-6R/gp130 starts the Janus kinases (JAKs) and signal transducer and activator of transcription 3 (STAT3) or JAK/STAT3 pathway. IL-6 R exits in two forms: a membrane-bound IL-6Rα subunit (mIL-6R) that participates in classic signalling pathway and soluble IL-6R subunit (sIL-6R) engaged in trans-signalling. The pro-tumour functions of IL-6 are associated with STAT3, a major oncogenic transcription factor that triggers up-regulation of target genes responsible for tumour cell survival. IL-6 combined with TGF-β induces proliferation of pathogenic Th17 cells. The anti-tumour function of IL-6 is the promotion of anti-tumour immunity. IL-6 trans-signaling contributed to transmigration of lymphocytes in high endothelial venules (HEV). Dendritic cell (DC) secreted IL-6 in the lymph node influences the activation, distribution and polarisation of the immune response. Elevated serum levels of IL-6 and increased expression of IL-6 in tumour tissue are negative prognostic marker for patients’ survival.
Keywords: Il-6, STAT3, Tumor microenvironment
Introduction
The cytokine interleukin-6 (IL-6) is a member of a group of cytokines that possess a four-helical structure [1]. It was described first as a B cell differentiation factor in 1986 [2], [3], [4]. IL-6 has various biological activities such as stimulation of the growth of tumour cells of murine plasmacytoma and human myeloma [5]. IL-6 also has an inhibitory effect on the antiviral antibody response [6]. Moreover, IL-6 is produced by several types of cells such as monocytes, macrophages, Kupffer cells [7], keratinocytes, endothelial cells, B cells and T cells [1].
The intracellular signaling is induced when the complex of IL-6 and IL-6 receptor (an 80-kDa ligand-binding chain IL-6Rα, CD126) binds the membrane glycoprotein 130 (gp 130) (a signal-transducing chain, IL-6Rb, CD130) [8], [9] that initiates the Janus kinases (JAKs) and signal transducer and activator of transcription (STAT) or JAKs / STAT pathway [8]. IL-6R is found in two forms, a transmembrane form mIL-6Rα, and a soluble form sIL-6R. IL-6 binds to both of these forms and subsequently interacts with the gp 130 to trigger downstream signal transduction and gene expression [7]. The gp130 lacks an intrinsic kinase domain, and therefore the members of the JAKs family, like JAK1, JAK2 and tyrosine kinase 2 (Tyk2), are linked to gp130 [5]. The complex of IL-6, IL-6R and gp130 phosphorylates the afore-mentioned kinases and later activates the cytoplasmic transcriptional factors as STAT1 and STAT3 [10]. Therefore, IL-6 activates transcriptional factors through IL-6R/gp130 complexes with following downstream effects [5].
The membrane-bound IL-6Rα subunit is located on the membrane of target cells. The second receptor subunit is the gp130 associated with mIL-6Rα/IL-6 that subsequently activates the “classic signaling pathway” [11]. The complex IL-6 / mIL-6Rα leads to dimerisation of gp130 and subsequent activation and phosphorylation of STAT3 via JAK. The classic signalling is realised during the early immune responses and activates acute-phase proteins like C-reactive protein (CRP) [9]. This “classic signalling” is accomplished on cells, expressing both the mIL-6R subunit and gp130 subunit. The latter is widely expressed, but the former is found only on hepatocytes, leukocytes and megakaryocytes [5], [9].
The second mechanism of induction of intracellular reaction is when IL-6 associates with soluble IL-6R (sIL-6R) and binds gp130 on cellular membranes that do not express mIL-6Rα. That process is defined as “trans-signalling” an alternative of classic signalling [12]. The presence of sIL-6R in the serum is a result of shedding of the mIL-6R from the cellular membranes induced by apoptosis and realised through a dis-integrin and a metalloproteinase 10 (ADAM10 or ADAM17) [13]. A second way of achieving sIL-6R is via differential splicing of IL-6 mRNA [12]. The shedding of the IL-6R is also initiated by CRP [14,15], or bacterial toxins [16]. The shedding of IL-6R is released from neutrophils at the beginning of the inflammatory process [9]. The presence of sIL-6R and IL-6 induces Th17 cells and is responsible for the balance between Th17 and T regulatory cells (Tregs) [17]. Therefore, IL-6 trans-signaling modulates the T cell response [18]. IL-6 trans-signalling is observed in many cell types such as epithelial cells, neutrophils, macrophages and T cells [9] and that the complex of IL-6 with the sIL-6R is associated with the cellular membrane gp130 [19] (Figure 1).
Figure 1.
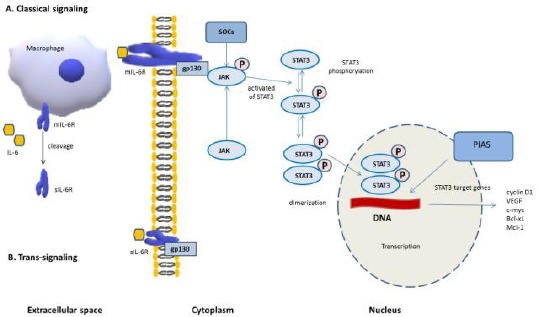
IL-6/JAK/STAT3 signaling; A) Classical-signaling: IL-6 binds to mIL-6 R, and interplays with membrane gp 130; B) Trans-signaling: sIL-6R cleaved from macrophage membranes binds to IL-6 and then the complex interplays with membrane gp130; Then the complex IL-6 / IL-6R / gp130 triggers the activation of JAK, and meanwhile the suppressor of cytokine signalling (SOCS) acts on JAKs and stops phosphorylation of gp130, STATs and the JAKs themselves. STAT3 (an oncogenic transcriptional factor) is activated by JAKs, phosphorylated and formed dimers (pSTAT3-pSTAT3). The dimerised pSTAT3 complex moves to nucleus and pSTAT3 complex trigger transcription of STAT3 target genes (cyclin D1, VEGF, c-myc, etc) through interaction with DNA. Cancer promotion is initiated. The protein inhibitors of activated STATs (PIAS) can suppress the transcription of STAT3 target genes
IL-6 up-regulates several acute-phase proteins such as CRP, fibrinogen, etc. [15], and IL-6 has both anti- and pro-inflammatory activities [9]. The anti-inflammatory functions are realized by the complex IL-6/mIL-6R and include activation of STAT3, followed by intestinal cell proliferation, inhibition of epithelial cell apoptosis and release of acute-phase proteins [19], [20]. The pro-inflammatory activities are realized by the complex IL-6 / sIL-6R and include activation of the immune system through recruitment of mononuclear cells (myeloid-derived suppressor cells – MDSC and macrophages), inhibition of T cell apoptosis and down-regulation of Treg differentiation [17], [21] (Figure 2).
Figure 2.
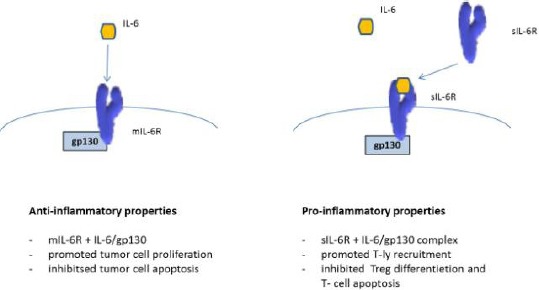
The dual role of IL-6 signalling: IL-6 classical signalling with anti-inflammatory properties and IL-6 trans-signalling with pro-inflammatory properties
During chronic inflammation, IL-6 induces proliferation of Th17 cells and inhibits the differentiation of Tregs [11]. The predominant cell type that secreted IL-6 during acute inflammation is monocyte/macrophage and in chronic inflammation – T lymphocyte [22]. IL-6 is also produced by endothelial cells, B cells, T cells, fibroblasts and some tumour cells [1]. IL-6 can be secreted by stromal fibroblasts in a mouse model of gastric cancer [23].
Colon tumours usually have a decreased expression of membrane-bound IL-6R in comparison to normal epithelial colon tissue. Nevertheless, the expression of ADAM17, associated with shedding of the IL-6R, is increased in tumours, and therefore the “trans-signalling” pathway is involved in colon carcinogenesis [24], [25].
Interleukin-6 and cancer development
Pro-tumour functions of IL-6
The predominant role of IL-6 in cancer is the promotion of tumour growth. The interaction of IL-6 and its receptor-activated JAKs with following induction/activation of STAT3 through tyrosine phosphorylation and subsequent transcription of target genes [9] is vital in cancer formation. In turn, IL-6 induces IL-6-dependent STAT3 activation, resulting in up-regulation of genes that promote the survival of cancer cells [26]. The target genes responsible for tumor cell survival (Bcl-2, survivin, Mcl-1), [27] proliferation (c-Myc, Cyclin D1, Cyclin B) [28], angiogenesis (VEGF) [29], metastasis (MMP2, MMP9) [30], [31], cell adhesion (ICAM-1, TWIST1), inflammation (IL-6, IL-17, IL-23, Cox2), and others [32]are influenced by IL-6 activities.
STAT3 is a major oncogenic transcription factor that is activated by the binding of IL-6 to the IL-6 receptor [25]. The first event is the binding of IL-6 to mIL-6R followed by gp130 dimerisation and trans-phosphorylation of STAT3 through tyrosine phosphorylation. Subsequently, STAT3 trans-locates to the nucleus in epithelial tumour cells, where STAT3 dimers bind DNA and modulate the expression of some target genes [22], [33]. Additionally, the IL-6 / STAT3 pathway blocks the maturation of dendritic cells (DCs), inhibits T cell activation [34] and maintains immunosuppression through MDSC and macrophages (tumour-associated macrophages – TAMs) [35].
IL-6 is also involved in the differentiation of monocytes to macrophages, downregulates apoptosis of T lymphocytes, and the production of Th2 cytokines [3], [36], [37].
Several molecules secreted by tumour cells, including IL-1β, TNF-α, IL-6 and TGF-β are considered to be promoters of Th17 differentiation from naïve CD4+ T cells. There exists evidence that Th17 cells increase in number in the tumour microenvironment (TME) [38]. In contrast, IFN-γ and IL-4, the main cytokines involved in Th1 and Th2 polarisation, respectively, negatively regulate Th17 differentiation [25], [38]. The pro-inflammatory cytokines, IL-6 and TNF-α, are produced in TME mostly by hematopoietic cells and also by tumour cells. They are tumour-promoting and further enhance nuclear factor kappa B (NF-kB) and STAT3 activation [39,40]. Moreover, IL-6 and IL-27 mediate signal transduction through STAT3 and STAT1 activation of Th17 and Treg differentiation [48]. IL-6 combined with TGF-β3 or TGF-β1 induce proliferation of pathogenic Th17 cells [42]. IL-6 and IL-27 both can initiate common signal transduction pathways in T cells [41] (Figure 3).
Figure 3.
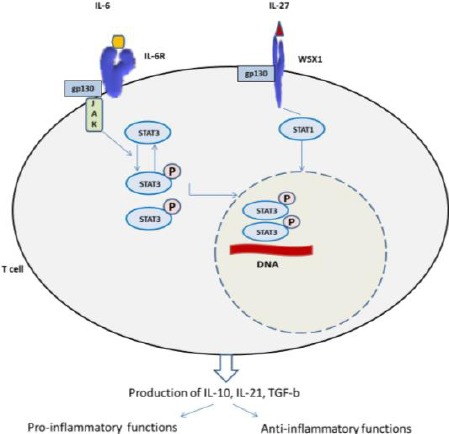
IL-6 and IL-27 trigger a common signal transduction pathway in T cell
STAT3 is an essential activator for Th17 cell proliferation [43], and on the other hand IL-6, a STAT3 activator, together with TGF-β increased the expression of main transcription factors RORα (human) and RORγt (mouse) for Th17 cell induction and IL-17 production [44], [45]. In contrast to STAT3 activation, STAT1 activation inhibits the development of Th17 cells [46]. Cytokines like IL-27 and IFN-γ are involved in the inhibition of Th17 development in a STAT1-dependent manner [5], [46]. Another cytokine that inhibits Th17 cells development is IL-2 in a STAT5 manner [47]. Therefore, the STAT family transcription factors, via the action of various cytokines, exert positive or negative influences on Th17 development. Interferon-regulatory factor 4 (IRF-4) exerts positive effect on Th17 cell appearance [48] and T-bet negatively influence the development of Th17 cells [49]. Treg helpers are mainly naturally occurring thymus-derived Tregs (nTregs) and TGF-β-induced Tregs (iTregs) [17]. Also, iTregs generate from naïve T cells in the periphery, after stimulation with TGF-β [50]. There are other T cells with regulatory functions including the CD8+ Tregs, Tr1 cells, and Th3 cells [51]. The balance between Th17 cells and Tregs is controlled by IL-6 that maintains immune homeostasis. TGF-β is important for Th17 and Treg cells differentiation, and it induces both Foxp3 and RORγt expression [52]. Therefore, IL-6 is considered to be a pro-inflammatory cytokine that promoted Th17 cell differentiation and inhibits Tregs development. The cytokine IL-17 has dual roles in TME, having pro- and anti-tumour activities [53] (Figure 4).
Figure 4.
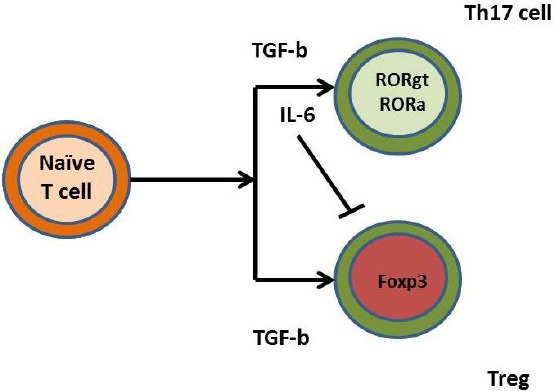
IL-6 maintains Th17/Treg balance. IL-6, together with TGF-β triggers Th17 differentiation from naïve T cells. On the other hand, IL-6 alone inhibits Treg differentiation triggered by TGF-β itself
IL-6 is a growth factor for human colon cancer cells, and inhibition of IL-6 signalling interferes with the growth of tumour cells [24]. In TME tumour-infiltrating lymphocytes (TILs) produce elevated levels of pro-tumorigenic cytokines such as IL-17A, IL-17F, IL-21, IL-22, TNF-α and IL-6 [40]. Some of the cytokines like IL-6 and TNF-α are also produced by tumour cells [20]. Colorectal cancer (CRC) cell lines - DLD-1 and HT-29, are affected by IL-6, TNF-α and IL-17 cytokines, and result in enhanced NF-kB and STAT3, which induce colorectal cancer cell growth [40].
IL-6 plays a major role in promoting proliferation of tumour cells and in inhibiting apoptosis via binding to IL-6Rα to the gp130. Following activation of JAK / STAT signalling pathway [54] namely of STAT1 and STAT3 [55] cancer initiation and proliferation occurs. Similarly, to TNF-α, IL-6 supports tumour development by induction of normal epithelial cells to convert into cancer stem-like cells [56]. STAT3 can mediate nuclear translocation of β-catenin. The nuclear co-expression of pSTAT3 and β-catenin is associated with poor survival of colon cancer patients [57]. IL-6 initiates tumorigenesis by hypermethylation of tumour suppressor genes or by hypomethylation of retrotransposon long interspersed nuclear element-1 (LINE-1) in oral squamous cell cancer [58]. IL-6 is a powerful (relevant) angiogenic factor, and its high levels correlate with that of VEGF in colorectal cancer [59,60]. Moreover, IL-6 initiates VEGF action in gastric cancer [61]. The secretion of IL-6 and subsequent STAT3 phosphorylation up-regulate some angiogenic mediators such as VEGF, VEGFR2 and neuropilin 2 [62].
In conclusion, IL-6 in the TME supports tumour development, metastasis and evasion from the effective anti-tumour immune response.
Anti-tumour functions of IL-6
The main anti-tumour function of IL-6 is the promotion of anti-tumour immunity [63], [64]. The analysis of many specimens of human tumours reveals that the immune contexture, defined by the type of immune cells, their activity, and distribution mainly in the invasive front, is a better prognostic factor as compared to histological staging and grading [63]. There is evidence that IL-6 trans-signalling is important in the initiation of T cell immune responses [65], [66]. Using trans-signalling IL-6 is a key cytokine in the modulation of anti-tumor immune response [67]. IL-6 maintains anti-tumour immunity at two main sites: first in the lymph nodes where lymphocyte priming takes place and second in tumour nests where IL-6 promotes the recruitment of effector T cells in TME [68].
In lymph nodes, dendritic cells (DCs) encounter tumour antigens. Also, naïve T cells and memory T cells enter lymph nodes through high endothelial venules (HEV). The polarisation interacts with the naïve T cells and initiates T cell polarisation [69], [70]. DCs secrete IL-6 in the lymph node that influences the activation, distribution and polarisation of the immune response [71].
In HEV, IL-6 trans-signaling acts on T lymphocytes to initiate tethering and rolling on the endothelial surface of HEV. Later the interaction between CCL21 on endothelial cells and CCR7 chemokine receptor on T lymphocytes initiates the chemokine activation that helps firm adhesion. The lymphocyte firm adhesion molecule 1 (LFA-1) binds to intercellular adhesion molecule 1 or 2 (ICAM-1 or ICAM-2) on endothelial cells and lymphocyte trans-endothelial migration in HEVs in lymph nodes or the tumour site [70], [72], [73]. IL-6 trans-signaling contributes to L-selectin-mediated and transmigration of lymphocytes to HEV [74]. Usually, tumour vessels had tortuous structure and express low levels of trafficking molecules such as ICAM-[66], [67]. Endothelial cells of tumour vessels and cancer-associated fibroblasts are the main producers of IL-6 at tumour sites [74]. Thus, injection of H-IL-6 induces high IL-6/sIL-6Rα concentration in TME. IL-6 trans-signaling increases CD8+ T cells trafficking into tumours and supports adoptive T cell transfer in adoptive cell therapy [67]. In mouse models the administration of H-IL-6 or application of systemic thermal therapy before adoptive CD8+ T cell transfer leads to enhanced tumour cell apoptosis and delay of tumour cell growth [67], [74].
The anti-tumour activities of IL-6 trans-signalling are used as basis for anti-tumour therapy. Thermal therapy is based on enhanced lymphocyte recruitment as response to febrile temperatures about 39.50 for periods up to 6 hours [66], [75]. The thermal stress leads to transient decrease in lymphocyte count with following increase of it in cancer patients with subsequent tumour restriction [76], [77].
Thermal therapy up-regulates gp130 on the endothelial cells in tumour microvessels [78] and thus supports IL-6/sIL-6Rα activity with following CD8+ T cell trafficking and recruitment into the tumour site [74], [78]. Taken together, the administration of H-IL-6 or thermal therapy could restrain cancer development when combined with adoptive CD8+ T cell vaccination [67], [78], [79].
Elevated levels of IL-6 and other serum biomarkers in cancer patients
Various biomarkers for the initiation and development of cancer exist. These biomarkers are associated mainly with inflammation and obesity [15], [80]. Chronic inflammation is related to colon carcinogenesis [68], [81]. It has been reported that cancer-associated inflammation determined disease progression and survival in CRC [82].
The existing meta-analysis shows that serum CRP and IL-6 levels could be associated with the risk of CRC development [33], [83], [84] but this is not useful for identifying colorectal adenomas [85]. TNF-α serum levels were studied in the risk of CRC development [86]. Another investigation report increased mRNA level of IL-6 that is predictive for colorectal cancer development with distant metastases [87]. Several CRC case-control studies show increased serum levels of CRP, TNF-α, IL-6 and IL-8 in colorectal adenoma and CRC patients [88]. Moreover, expression-enhancing polymer-phisms in the genes for IL-6, TNF-α, IL-1β and IL-8 are associated with increased risk for the development of colorectal cancer [89]. The increased release of IL-6 in the sera of CRC patients is associated with CEA-induced production of IL-6 by Kupffer cells, macrophages, lymphocytes and tumour cells [90]. Serum IL-6 > 10 pg/ml values are associated with higher incidence of CRC with distant metastasis and therefore can be an independent, negative prognostic marker for patients’ survival [91].
Adipose tissue is considered to be the largest endocrine tissue that secrets various cytokines such as IL-2, IL-6, IL-8, TNF-α, etc. [92]. IL-6 is a poor prognostic factor in obese patients with CRC [93], [94], [95], [96].
Clinical significance of tissue overexpression of IL-6 in CRC cancer tissue
Few studies address the immunohisto-chemical expression of IL-6 in CRC [97], [98], [99]. Some studies show overexpression of IL-6 in tumour tissue in glioblastoma [94], prostate cancer [43], renal cell cancer [57], gastric cancer [61] etc. Additionally, the expression of IL-6R and gp130 was investigated in tumour cells of CRC [97]. The overexpression of IL-6 in cancer tissue correlates to advanced stage, lymph node metastasis, and venous invasion [100], [101]. Therefore, IL-6 cancer cell expression can be a relevant marker of cancer progression.
In conclusion, IL-6 is mainly a pro-tumorigenic cytokine that triggers JAK / STAT3 activation with subsequent promotion of tumour cell growth and suppression of tumour cell apoptosis. IL-6 / STAT3 signalling regulates the balance between Th17 and Tregs in TME with immunosuppressive properties. The anti-tumour activity of IL-6is associated with modulation of T cell polarisation initiated by IL-6 secreting DCs and with the support of T lymphocyte recruitment in lymph nodes. A further investigation is necessary to elucidate the intimate mechanisms of IL-6 regulation.
Footnotes
Funding: This work was financially supported by the National Science Fund, Bulgarian, Research grant number KP-06-H23/2 from 17.12.2018 and Project by Trakia University Medical Faculty Research grant 2019/IL-6 and colorectal cancer
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Waldner MJ, Foersch S, Neurath MF. Interleukin-6 - a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8:1248–1253. doi: 10.7150/ijbs.4614. https://doi.org/10.7150/ijbs.4614 PMid:23136553 PMCid:PMC3491448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, et al. Complementary DNA, for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. https://doi.org/10.1038/324073a0 PMid:3491322. [DOI] [PubMed] [Google Scholar]
- 3.Walev I, Vollmer P, Palmer M, Bhakdi S, Rose-John S. Pore-forming toxins trigger shedding of receptors for interleukin 6 and lipopolysaccharide. Proc Natl Acad Sci USA. 1996;93:7882–7887. doi: 10.1073/pnas.93.15.7882. https://doi.org/10.1073/pnas.93.15.7882 PMid:8755571 PMCid:PMC38843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung Y-C, Chaen Y-L, Hsu C-P. Clinical significance of tissue expression of interleukin-6 in colorectal carcinoma. Anticancer Res. 2006;26:3905–3912. [PubMed] [Google Scholar]
- 5.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235, 238. doi: 10.1038/nature04753. https://doi.org/10.1038/nature04753 PMid:16648838. [DOI] [PubMed] [Google Scholar]
- 6.Chalaris A, Rabe B, Paliga K, Lange H, Laskay T, Fielding CA, et al. Apoptosis is a natural stimulus of IL-6R shedding and contributes to the pro-inflammatory trans-signaling function of neutrophils. Blood. 2007;110:1748–1755. doi: 10.1182/blood-2007-01-067918. https://doi.org/10.1182/blood-2007-01-067918 PMid:17567983. [DOI] [PubMed] [Google Scholar]
- 7.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. https://doi.org/10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 8.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. https://doi.org/10.1016/j.ccr.2009.01.002 PMid:19185844. [DOI] [PubMed] [Google Scholar]
- 9.Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp 130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. https://doi.org/10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Yi T, Kortylewsky M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-STAT3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. https://doi.org/10.1084/jem.20090207 PMid:19564351 PMCid:PMC2715087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano T. Interleukin 6 and its receptor:ten years later. Int Rev Immunnol. 1998;16:249–284. doi: 10.3109/08830189809042997. https://doi.org/10.3109/08830189809042997 PMid:9505191. [DOI] [PubMed] [Google Scholar]
- 12.Becker C, Fautini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR, Rose-John S, Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. https://doi.org/10.4161/cc.4.2.1413 PMid:15655344. [PubMed] [Google Scholar]
- 13.Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22(10):1517–1527. doi: 10.1038/sj.onc.1206226. https://doi.org/10.1038/sj.onc.1206226 PMid:12629515. [DOI] [PubMed] [Google Scholar]
- 14.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23(20):3550–3560. doi: 10.1038/sj.onc.1207383. https://doi.org/10.1038/sj.onc.1207383 PMid:15116091. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity:a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. https://doi.org/10.1038/nrc2734 PMid:19851315 PMCid:PMC4856025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Anguita A, Kakourou A, Tsilidis KK. Biomarkers of inflammation and immune function and risk of colorectal cancer. Curr Colorectal Cancer Rep. 2015;11:250–258. doi: 10.1007/s11888-015-0282-5. https://doi.org/10.1007/s11888-015-0282-5 PMid:26321888 PMCid:PMC4550652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlaykova T, Talve L, Hahka-Kemppinen M, Hernberg M, Muhonen T, Collan Y, Pyrhonen S. Immunohistochemically detectable Bcl-2 expression in metastatic melanoma:association with survival and treatment response. Oncology. 2002;62:259–268. doi: 10.1159/000059574. https://doi.org/10.1159/000059574 PMid:12065874. [DOI] [PubMed] [Google Scholar]
- 18.Ihle J, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. https://doi.org/10.1016/S0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Ye Y, Zhang H, Szmitkowski M, Makinen MJ, Li P, Xia D, Yang J, Wu Y, Wu H. Diagnostic and prognostic value of serum interleukn-6 in colorectal cancer. Medicine. 2016;92(2):e2502. doi: 10.1097/MD.0000000000002502. https://doi.org/10.1097/MD.0000000000002502 PMid:26765465 PMCid:PMC4718291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grivennikov S, Karin M. Dangerous liaisons:STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. https://doi.org/10.1016/j.cytogfr.2009.11.005 PMid:20018552 PMCid:PMC2834864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tacheva T, Chelenkova P, Dimov D, Chakarov I, Petkova R, Chakarov S, Vlaykova T. Frequency of the common promoter polymorphism MMP2-1306 C>T in a population from central Bulgaria. Biotechnology &Biotechnology Equipment. 2015;29(2):351–356. doi: 10.1080/13102818.2014.995411. https://doi.org/10.1080/13102818.2014.995411 PMid:26019651 PMCid:PMC4433921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PL, et al. IL-6 trans-signaling modulates TLR-dependent inflammatory responses via STAT3. J Immunol. 2011;186:1199–1208. doi: 10.4049/jimmunol.1002971. https://doi.org/10.4049/jimmunol.1002971 PMid:21148800. [DOI] [PubMed] [Google Scholar]
- 23.Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. https://doi.org/10.1016/S0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 24.De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di'Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F, Monteleone G, Stolfi C. Th-17 type cytokines, IL-6 and TNF-αsynergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493–3503. doi: 10.1038/onc.2014.286. https://doi.org/10.1038/onc.2014.286 PMid:25174402 PMCid:PMC4493653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry A, Rudra D, Trenting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+regulatory T cells control Th17 responses in a STAT3 dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. https://doi.org/10.1126/science.1172702 PMid:19797626 PMCid:PMC4408196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brústle A, Heink S, Huber M, Rosenplänter C, Stadelmann C, Yu P, Arpaia E, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. https://doi.org/10.1038/ni1500 PMid:17676043. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, et al. TGF-beta-induced Foxp3 Inhibits T(H) 17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. https://doi.org/10.1038/nature06878 PMid:18368049 PMCid:PMC2597437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naïve T cells to CD4+CD25+regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. https://doi.org/10.1084/jem.20030152 PMid:14676299 PMCid:PMC2194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Il'yasova D, Colbert LH, Harrris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. https://doi.org/10.1158/1055-9965.EPI-05-0316 PMid:16214925. [DOI] [PubMed] [Google Scholar]
- 30.Grivennikov S, Karin E, Terzic J, Mucida D, Yu G-Y, Vallabhapurapu S, et al. Il-6 and STAT3 signaling is required for survival of intestinal epithelial cells and colitis associated cancer. Cancer Cell. 2009;16:103–113. doi: 10.1016/j.ccr.2009.01.001. https://doi.org/10.1016/j.ccr.2009.01.001 PMid:19185845 PMCid:PMC2667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwasaki Y, Fujio K, Okamura T, Yamamoto K. Interleukin-27 in T cell Immunity. Int J Mol Sci. 2015;16:2851–2863. doi: 10.3390/ijms16022851. https://doi.org/10.3390/ijms16022851 PMid:25633106 PMCid:PMC4346869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cell down-regulate L-selectin expression on CD4+and CD8+T cells. J Immunol. 2009;183(2):937–944. doi: 10.4049/jimmunol.0804253. https://doi.org/10.4049/jimmunol.0804253 PMid:19553533 PMCid:PMC2800824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones GW, McLoughlin RM, Hammond VJ, Parker CR, Williams JD, Malhotra R, et al. Loss of CD4+T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J Immunol. 2010;184:2130–2139. doi: 10.4049/jimmunol.0901528. https://doi.org/10.4049/jimmunol.0901528 PMid:20083667. [DOI] [PubMed] [Google Scholar]
- 34.Kopf M, Baumann H, Freer G. Impaired immune and acute-phase responses in interleukin-6 deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. https://doi.org/10.1038/368339a0 PMid:8127368. [DOI] [PubMed] [Google Scholar]
- 35.Kraybill WG, Olenki T, Evans SS, Ostberg JR, O'Leary KA, Gibbs JF, et al. A phase I study of fever-range whole body hyperthermia (FR-WBH) in patients with advanced solid tumors:correlation with mouse models. Int J Hyperthermia. 2002;18(3):253–266. doi: 10.1080/02656730110116704. https://doi.org/10.1080/02656730110116704 PMid:12028640. [DOI] [PubMed] [Google Scholar]
- 36.Jones SA, Novick D, Horiuchi S, Yamamoto N, Szalai AJ, Fuller GM. C-reactive protein:a physiological activator of interleukin 6 receptor shedding. J Exp Med. 1999;189:599–604. doi: 10.1084/jem.189.3.599. https://doi.org/10.1084/jem.189.3.599 PMid:9927522 PMCid:PMC2192917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawada M, Seno H, Uenoyama Y, Sawabu T, Kanda N, Fukui H, Shimahara Y, Chiba T. Signal transducers and activators of transcription 3 activation is involved in nuclear accumulation of beta-catenin in colorectal cancer. Cancer Res. 2006;66:2913–2917. doi: 10.1158/0008-5472.CAN-05-3460. https://doi.org/10.1158/0008-5472.CAN-05-3460 PMid:16540637. [DOI] [PubMed] [Google Scholar]
- 38.Kakourou A, Koutsioumpa C, Lopez DS, Hoffman-Belton J, Bradwin G, Rifai N, Helzlsouer KJ, Platz EA, Tsilidis KK. Interleukin-6 and risk of colorectal cancer:results from the CLUE II cohort and a meta-analysis of prospective studies. Cancer Causes Control. 2015;26:1449–1460. doi: 10.1007/s10552-015-0641-1. https://doi.org/10.1007/s10552-015-0641-1 PMid:26220152 PMCid:PMC4763881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 40.Eldesoky A, Shouma A, Mosaad Y, Elhawary A. Clinical relevance of serum vascular endothelial growth factor and interleukin-6 in patients with colorectal cancer. Saudi J Gastroenterol. 2011;17(3):170–173. doi: 10.4103/1319-3767.80378. https://doi.org/10.4103/1319-3767.80378 PMid:21546718 PMCid:PMC3122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiuchi N, Nakajima K, Tchiba M, Fukada T, Narimatsu M, Mizuno K, et al. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med. 1999;189(1):63–73. doi: 10.1084/jem.189.1.63. https://doi.org/10.1084/jem.189.1.63 PMid:9874564 PMCid:PMC1887683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angell H, Galon J. From the immune contexture to the Immunoscore:the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25(2):261–267. doi: 10.1016/j.coi.2013.03.004. https://doi.org/10.1016/j.coi.2013.03.004 PMid:23579076. [DOI] [PubMed] [Google Scholar]
- 43.Appenheimer MM, Girard RA, Chen Q, Wang WC, Bankert KC, Hardison J, et al. Conservation of IL-6 trans-signaling mechanisms controlling L-selectin adhesion by fever-range thermal stress. Eur J Immunol. 2007;37(10):2856–2867. doi: 10.1002/eji.200636421. https://doi.org/10.1002/eji.200636421 PMid:17823890. [DOI] [PubMed] [Google Scholar]
- 44.Kinoshita H, Hirata Y, Nakagawa H, et al. Interleukin-6 mediates epithelial-stromal interactions and promotes gastric tumorigenesis. Plos ONE. 2013;8(4):e60914. doi: 10.1371/journal.pone.0060914. https://doi.org/10.1371/journal.pone.0060914 PMid:23593346 PMCid:PMC3625204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feurino LW, Zhang Y, Bharadwaj U, et al. IL-6 stimulates Th2 type cytokine secretion and upregulates VEGF and NRP-1 expression in pancreatic cancer cells. Cancer Biol Ther. 2007;6(7):1096–1100. doi: 10.4161/cbt.6.7.4328. https://doi.org/10.4161/cbt.6.7.4328 PMid:17568185. [DOI] [PubMed] [Google Scholar]
- 46.Klampfer L. Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets. 2011;11(4):451–464. doi: 10.2174/156800911795538066. https://doi.org/10.2174/156800911795538066 PMid:21247378 PMCid:PMC3540985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gasche JA, Hoffman J, Boland CR, Goel A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer. 2011;129(5):1053–1063. doi: 10.1002/ijc.25764. https://doi.org/10.1002/ijc.25764 PMid:21710491 PMCid:PMC3110561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gulubova M, Ananiev J, Ignatova M, Halacheva K. Pro-tumor and anti-tumor functions of IL-17 and of Th17 cells in tumor microenvironment. AMB. 2016;2(XLIII):68–79. https://doi.org/10.1515/amb-2016-0019. [Google Scholar]
- 49.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumors:impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. https://doi.org/10.1038/nrc3245 PMid:22419253. [DOI] [PubMed] [Google Scholar]
- 50.Klintrup K, Makinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41:2645–2654. doi: 10.1016/j.ejca.2005.07.017. https://doi.org/10.1016/j.ejca.2005.07.017 PMid:16239109. [DOI] [PubMed] [Google Scholar]
- 51.Bousso P, Robey E. Dynamics of CD8+T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4(6):579–585. doi: 10.1038/ni928. https://doi.org/10.1038/ni928 PMid:12730692. [DOI] [PubMed] [Google Scholar]
- 52.Kryczek I, Bonerjec M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Lin R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor microenvironment. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. https://doi.org/10.1182/blood-2009-03-208249 PMid:19470694 PMCid:PMC2723011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon KA, Kim SH, Oh SY, Lee S, Han J-Y, Kim KH, Goh RY, Choi HJ, Park KJ, Roh MS, Kim H-J, Kwon H-C, Lee JH. Clinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancer. BMC Cancer. 2010;10:203–211. doi: 10.1186/1471-2407-10-203. https://doi.org/10.1186/1471-2407-10-203 PMid:20465852 PMCid:PMC2886042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miki S, Iwano M, Miki Y, Yamamoto M, Tang B, Yokokawa K, Sonoda T, Hirano T, Kishimoto T. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989;250:607–610. doi: 10.1016/0014-5793(89)80805-1. https://doi.org/10.1016/0014-5793(89)80805-1. [DOI] [PubMed] [Google Scholar]
- 55.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. https://doi.org/10.1038/ni.2703 PMid:24048123 PMCid:PMC4118725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mikucki ME, Fisher DT, Ku AW, Appenheimer MM, Muhitch JB, Evans SS. Preconditioning thermal therapy:Flipping the switch on IL-6 for anti-tumour immunity. Int J Hyperthermia. 2013;29(5):464–473. doi: 10.3109/02656736.2013.807440. https://doi.org/10.3109/02656736.2013.807440 PMid:23862980 PMCid:PMC3893705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. Journal of immunology research. 2014:2014. doi: 10.1155/2014/149185. https://doi.org/10.1155/2014/149185 PMid:24901008 PMCid:PMC4036716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Formica V, Cereda V, Nardecchia A, Tesauro M, Roselli M. Immune reaction and colorectal cancer:friends or foes? Wourld J Gastroenterol. 2014;20(35):12407–12419. doi: 10.3748/wjg.v20.i35.12407. https://doi.org/10.3748/wjg.v20.i35.12407 PMid:25253941 PMCid:PMC4168074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, et al. Interleukin-2 signaling via STAT5 contains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. https://doi.org/10.1016/j.immuni.2007.02.009 PMid:17363300. [DOI] [PubMed] [Google Scholar]
- 60.Le JM, Vilcek J. Interleukin 6:a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989;61:588–602. https://doi.org/10.1007/978-1-4612-0485-5_7. [PubMed] [Google Scholar]
- 61.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. doi: 10.1016/j.ejca.2005.08.016. https://doi.org/10.1016/j.ejca.2005.08.016 PMid:16199153. [DOI] [PubMed] [Google Scholar]
- 62.Lee Y, Awasthi A, Josef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Haflet DA, et al. Induction and molecular signature of pathogenic Th17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. https://doi.org/10.1038/ni.2416 PMid:22961052 PMCid:PMC3459594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12(11):762–773. doi: 10.1038/nri3298. https://doi.org/10.1038/nri3298 PMid:23018291. [DOI] [PubMed] [Google Scholar]
- 64.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H) 17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. https://doi.org/10.1038/nature04754 PMid:16648837. [DOI] [PubMed] [Google Scholar]
- 65.Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121(10):3846–3859. doi: 10.1172/JCI44952. https://doi.org/10.1172/JCI44952 PMid:21926464 PMCid:PMC3195455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S-Y, Kang JW, Song X, et al. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signaling. 2013;25(4):961–969. doi: 10.1016/j.cellsig.2013.01.007. https://doi.org/10.1016/j.cellsig.2013.01.007 PMid:23333246 PMCid:PMC3595341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kimura A, Kishimoto T. IL-6:regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. https://doi.org/10.1002/eji.201040391 PMid:20583029. [DOI] [PubMed] [Google Scholar]
- 68.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. https://doi.org/10.1073/pnas.0705268104 PMid:17623780 PMCid:PMC1924582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol. 2014;26(1):38–47. doi: 10.1016/j.smim.2014.01.008. https://doi.org/10.1016/j.smim.2014.01.008 PMid:24602448 PMCid:PMC3970580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer:molecular features of adipose tissue. J Transl Med. 2016:14–33. doi: 10.1186/s12967-016-0772-5. https://doi.org/10.1186/s12967-016-0772-5 PMid:26801617 PMCid:PMC4722674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97(9):2727–2733. doi: 10.1182/blood.v97.9.2727. https://doi.org/10.1182/blood.V97.9.2727 PMid:11313264. [DOI] [PubMed] [Google Scholar]
- 72.Huang S-P, Wu M-S, Shun C-T, et al. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11(4):517–527. doi: 10.1007/BF02256101. https://doi.org/10.1007/BF02256101 PMid:15153787. [DOI] [PubMed] [Google Scholar]
- 73.Gulubova MV. Expression of cell adhesion molecules, their ligands and tumor necrosis factor αin the liver of patients with metastatic gastrointestinal carcinomas. Histochem J. 2002;34:67–77. doi: 10.1023/a:1021304227369. https://doi.org/10.1023/A:1021304227369 PMid:12365802. [DOI] [PubMed] [Google Scholar]
- 74.Gulubova MV. Collagen type IV, laminin, α-smooth muscle actin (αSMA), α1 and α6 integrins expression in the liver with metastases from malignant gastrointestinal tumors. Clin Exp Metastast. 2004;21:485–494. doi: 10.1007/s10585-004-3171-x. https://doi.org/10.1007/s10585-004-3171-x. [DOI] [PubMed] [Google Scholar]
- 75.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, et al. STAT3 and STAT4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. https://doi.org/10.4049/jimmunol.178.8.4901 PMid:17404271. [DOI] [PubMed] [Google Scholar]
- 76.Naugler WE, Karin M. The wolf in sheep's clothing:the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. https://doi.org/10.1016/j.molmed.2007.12.007 PMid:18261959. [DOI] [PubMed] [Google Scholar]
- 77.Meir EV, Sawamura Y, Diserens A, Hamou M, Tribolet N. Human glioblastoma cells release interleukin-6 in vivo and in vitro. Cancer Res. 1990;50:6683–6688. [PubMed] [Google Scholar]
- 78.Miller S, Senior PV, Prakash M, Apostolopoulis V, Sakkai S, Nurgali K. Leukocyte populations and IL-6 in the tumor microenvironment of an orthotopic colorectal cancer model. Acta Biochem Biophys Sin. 2016;48(4):334–341. doi: 10.1093/abbs/gmw002. https://doi.org/10.1093/abbs/gmw002 PMid:26893144 PMCid:PMC4886242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hope JC, Cumberbatch M, Fielding I, Dearman RJ, Kimber I, Hopkins SJ. Identification of dendritic cells as a major source of IL-6 in draining lymph nodes following skin sensitization of mice. Immunology. 1995;86(3):441–447. [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou B, Shu B, Yang J, Liu J, Xi T, Xing Y. C-reactive protein, interleukin-6 and the risk of colorectal cancer:a meta-analysis. Cancer Causes Control. 2014;25:1397–1405. doi: 10.1007/s10552-014-0445-8. https://doi.org/10.1007/s10552-014-0445-8 PMid:25053407. [DOI] [PubMed] [Google Scholar]
- 81.Monteleone G, Pallone F, Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci. 2012;13(9):11071–11084. doi: 10.3390/ijms130911071. https://doi.org/10.3390/ijms130911071 PMid:23109839 PMCid:PMC3472731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X, Liu S, Zhou Y. Circulating levels of C-reactive protein, interleukin-6 and tumor necrosis factor-αand risk of colorectal adenomas:a meta-analysis. Oncotarget. 2016;7(39):64371–64379. doi: 10.18632/oncotarget.11853. https://doi.org/10.18632/oncotarget.11853 PMid:27608842 PMCid:PMC5325449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83–89. doi: 10.1016/j.cytogfr.2011.02.003. https://doi.org/10.1016/j.cytogfr.2011.02.003 PMid:21377916. [DOI] [PubMed] [Google Scholar]
- 84.Okugawa Y, Miki C, Toiyama Y, Yasuda H, Yokoe T, Saigusa S, Hiro J, Tanaka K, Inoue Y. Loss of tumoral expression of soluble IL-6 receptor is associated with disease progression in colorectal cancer. Brit J Cancer. 2010;103:787–795. doi: 10.1038/sj.bjc.6605827. https://doi.org/10.1038/sj.bjc.6605827 PMid:20823887 PMCid:PMC2966622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173(6):3844–3854. doi: 10.4049/jimmunol.173.6.3844. https://doi.org/10.4049/jimmunol.173.6.3844 PMid:15356132. [DOI] [PubMed] [Google Scholar]
- 86.Groblewska MMB, Wereszczynska-Siemiatkowska U, Kedra B, Lukaszewicz M, Baniukiewicz A, Szmitkowski M. Serum interleukin 6 (IL-6) and C-reactive protein (CRP) in colorectal adenoma and cancer patients. Clin Chem Lab Med. 2008;46(10):1423–1428. doi: 10.1515/CCLM.2008.278. https://doi.org/10.1515/CCLM.2008.278 PMid:18844497. [DOI] [PubMed] [Google Scholar]
- 87.Yeh K-Y, Li Y-Y, Hsieh L-L, Lu C-H, Chou W-C, Liaw C-C, Tang R-P, Liao S-K. Analysis of the effect of interleukin-6 (IL-6) and soluble IL-6 receptor levels on survival of patients with colorectal cancer. Jpn J Clin Oncol. 2010;40(6):580–587. doi: 10.1093/jjco/hyq010. https://doi.org/10.1093/jjco/hyq010 PMid:20194250. [DOI] [PubMed] [Google Scholar]
- 88.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson M. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. https://doi.org/10.1084/jem.20052222 PMid:16880257 PMCid:PMC2118365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gunter M, Canzian F, Landi S, Chanock SJ, Sinha R, Rothman N. Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1126–1131. doi: 10.1158/1055-9965.EPI-06-0042. https://doi.org/10.1158/1055-9965.EPI-06-0042 PMid:16775170. [DOI] [PubMed] [Google Scholar]
- 90.Grivennikov S, Karin M. Autocrine IL-6 signaling:a key event in tumorigenesis? Cancer Cell. 2008;13:7–9. doi: 10.1016/j.ccr.2007.12.020. https://doi.org/10.1016/j.ccr.2007.12.020 PMid:18167335. [DOI] [PubMed] [Google Scholar]
- 91.Roncarolo MG, Gregori S, Battaglia M, Barchetta R, Flischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2005;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. https://doi.org/10.1111/j.0105-2896.2006.00420.x PMid:16903904. [DOI] [PubMed] [Google Scholar]
- 92.Miteva LD, Stanilov NS, Cirovski GM, Stanilova SA. Upregulation of Treg-related genes in addition with IL-6 showed the significant role for the distant metastasis in colorectal cancer. Cancer Microenvironment. 2017;10:69–76. doi: 10.1007/s12307-017-0198-5. https://doi.org/10.1007/s12307-017-0198-5 PMid:28868572 PMCid:PMC5750202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yehuda-Shnaidman E, Schwartz B. Mechanisms linking obesity, inflammation and altered metabolism to colon carcinogenesis. Obes Rev. 2012;13(12):1083–1095. doi: 10.1111/j.1467-789X.2012.01024.x. https://doi.org/10.1111/j.1467-789X.2012.01024.x PMid:22937964. [DOI] [PubMed] [Google Scholar]
- 94.Rose-John S. Coordination of interleukin-6 biology by membrane bound and soluble receptors. Adv Exp Med Biol. 2001;495:145–151. doi: 10.1007/978-1-4615-0685-0_19. https://doi.org/10.1007/978-1-4615-0685-0_19 PMid:11774558. [DOI] [PubMed] [Google Scholar]
- 95.Yamamoto K, Rose-John S. Therapeutic blockade of interleukin-6 in chronic inflammatory disease. Clin Pharmacol Ther. 2012;91:574–576. doi: 10.1038/clpt.2012.11. https://doi.org/10.1038/clpt.2012.11 PMid:22434029. [DOI] [PubMed] [Google Scholar]
- 96.Rose-John S. IL-6 trans-signaling via the soluble IL-6-receptor:importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8(9):1237–1247. doi: 10.7150/ijbs.4989. https://doi.org/10.7150/ijbs.4989 PMid:23136552 PMCid:PMC3491447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors:generation and biological function. Biochem J. 1994;300:281–290. doi: 10.1042/bj3000281. https://doi.org/10.1042/bj3000281 PMid:8002928 PMCid:PMC1138158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung YC, Chung YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83:222–226. doi: 10.1002/jso.10269. https://doi.org/10.1002/jso.10269 PMid:12884234. [DOI] [PubMed] [Google Scholar]
- 99.Zeng J, Tang Z-H, Liu S, Guo S-S. Clinicopathologcal significance of overexpression of interleukin-6 in colorectal cancer. Wourld J Gstroenterol. 2017;23(10):1780–1786. doi: 10.3748/wjg.v23.i10.1780. https://doi.org/10.3748/wjg.v23.i10.1780 PMid:28348483 PMCid:PMC5352918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Repasky EA, Evans SS, Dewhirst MW. Temperature Matters!And Why It Should Matter to Tumor Immunologists. Cancer Immunol Res. 2013;1(4):210–216. doi: 10.1158/2326-6066.CIR-13-0118. https://doi.org/10.1158/2326-6066.CIR-13-0118 PMid:24490177 PMCid:PMC3904378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer:harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. https://doi.org/10.1038/nri3191 PMid:22437939 PMCid:PMC6292222. [DOI] [PMC free article] [PubMed] [Google Scholar]


