Abstract
Objective:
To determine how infant illness and parent demographics are associated with parent health-related quality of life (HRQL) during and 3 months after hospitalization in the neonatal intensive care unit (NICU). We hypothesized that parents of extremely preterm infants would report lower NICU HRQL than other parents, and that all parents would report improved HRQL after discharge.
Study design:
Prospective study of parent-infant dyads admitted to a level IV NICU for ≥14 days from 2016–2017. Parent HRQL before and 3 months post-discharge was measured using the PedsQL Family Impact Module. Multivariable regression was used to identify risk factors associated with HRQL differences during hospitalization and after discharge.
Results:
Of the 194 dyads, 167 (86%) completed the study (24% extremely preterm; 53% moderate-late preterm; 22% term). During the NICU hospitalization, parents of extremely preterm infants reported lower adjusted HRQL (−7 points, P = .013) than other parents. Post-discharge, parents of extremely preterm infants reported higher HRQL compared with their NICU score (+10 points, p=0.001). Tracheostomy (−13, p=0.006), home oxygen (−6, p=0.022), and readmission (−5, p=0.037) were associated with lower parent HRQL 3 months after discharge, adjusted for NICU HRQL score.
Conclusions:
Parents of extremely preterm infants experienced more negative impact on HRQL during the NICU hospitalization, and more improvement post-discharge than parents of other infants hospitalized in the NICU. Complex home care was associated with lower parent HRQL after discharge. The potential benefit of home discharge should be balanced against the potential negative impact of complex home care.
Keywords: health related quality of life, premature infants, NICU
The neonatal intensive care unit (NICU) is a stressful place for families.1,2 Parents in the NICU experience a markedly altered caregiving role which impacts their quality of life.3–5 At discharge, parents assume all caregiving needs for their infant, many of whom may have complex home health needs. Complex home care has also been associated with lower caregiver health-related quality of life (HRQL).6,7 The impact of child health on parent wellbeing is an important outcome in health care.8 Parents’ physical, emotional and psychological health affects their caregiving ability, which in turn affects child health.9–12 Therefore, understanding parent HRQL prior to and after NICU discharge is crucial to supporting at-risk families.
Quality of life research in neonatology has led to important insights on quality of life for child and young adult survivors of prematurity compared with full term controls.6,7,13–20 HRQL has been shown to be lower for parents of preterm infants than parents of healthy term infants after the NICU during infancy,11 continuing into early childhood; parent HRQL is influenced by the NICU hospitalization itself,21 later childhood morbidities,22–24 and parental factors such as coping, stress and mental health.3,11,21–29 HRQL for parents of full-term infants requiring NICU care has not been described. Also, the change in parent HRQL from NICU hospitalization through subsequent discharge has not been studied across a cohort of infants of all gestational ages and diagnoses. Understanding parent HRQL before and after the transition home could help target interventions to better support NICU discharge.
Our objective was to determine how NICU illness, parent demographics, and post-NICU healthcare utilization are associated with parent HRQL in the NICU and after discharge. We hypothesized that parents of extremely preterm infants would report lower HRQL in the NICU than parents of other infants, adjusted for illness and demographic factors, and that all parents would report higher HRQL after discharge compared with in the NICU.
METHODS
We conducted a prospective study among parents of infants hospitalized for ≥14 days in a level IV NICU from November 2016-July 2017. This NICU admits infants of all gestational ages requiring intensive care born at a co-located birth hospital, and receives patients transferred for medical and surgical subspecialty evaluation. The unit has single patient rooms, psychologists, case managers, social workers, and a family support coordinator. We excluded non-English speaking families because of personnel limitation in obtaining interviews; we also excluded non-biological parents who could not provide consent, infants previously discharged home, infants transferred to cardiac intensive care, or infants for whom death was imminent. Parents of multiples chose one child to enroll. If an infant died, the family was excluded from further assessments.
The primary outcome was parent HRQL, measured using the PedsQL Family Impact Module.30 This 36-item self-report tool assesses parent HRQL related to their child’s illness among eight domains: physical, emotional, social, and cognitive functioning; communication; worry; family relationships; and daily activities.30 A 5-point Likert scale is used; mean scores are transformed to a 0–100 scale with higher scores indicating higher HRQL. Based on pilot data from our NICU, we determined that a sample size of 214 patients would provide 80% power to detect a 5-point difference in between-group HRQL pre- versus post-discharge, accounting for 8–10 covariates, with 80% follow-up at 3 months.
The primary exposure was infant gestational age based on best obstetric estimate by groupings: extremely preterm, 23–28 weeks gestation; moderate-late preterm, 29–36 weeks; term, 37–42 weeks. We reviewed the chart for variables that would reflect NICU illness, co-morbidities, parent demographics and discharge medical needs across all gestational ages (variables shown in Table I). Insurance type was identified by chart review. Healthcare utilization after discharge was determined by chart review and confirmed with the parent. This included number of subspecialty appointments, emergency room visits, hospital readmissions and durable medical equipment.
Study procedure
Eligible parents were approached by a research assistant to complete questionnaires using a tablet into a secure database.31 Upon enrollment, parents answered demographic questions. Parents completed the PedsQL Family Impact Module within a few weeks of anticipated discharge, once the infant’s anticipated care needs were becoming clear. Our pilot work showed this timing to be critical to enrollment, as parents are often too stressed to participate in research immediately prior to going home. The timing of HRQL assessment was determined by weekly screening by the study team and confirmed with the primary clinical caregivers. A post-NICU discharge assessment was completed three months after discharge by phone or in person.
Statistical Analyses
We compared demographics, infant illness, and post-NICU healthcare utilization between gestational age groups. Next, we compared NICU and 3-month parent HRQL scores by demographics, infant illness, and post-NICU healthcare utilization. We also compared subdomain scores of the PedsQL between NICU and 3 months post-discharge by gestational age group. Between-group comparisons were performed with Kruskal-Wallis tests, Chi-squared or Fisher exact tests; within-group changes in HRQL were compared with paired-samples Wilcoxon signed-rank tests. Non-parametric comparisons were used to avoid assumptions about the HRQL distribution.
We calculated a clinically relevant change in HRQL from NICU to 3 months post-discharge. Because an illness-specific measure was not available, as an anchor-based method of determining clinically relevant change we asked parents to respond to the question “How sick is your child?” on a 5-point Likert scale at both time points.32,33 This question was chosen to reflect the parent’s perception of infant illness severity, which has been linked to perception of an infant’s future quality of life.34 We considered a clinically relevant change to be the median HRQL change for parents who responded that their child was at least one point different after discharge compared with the NICU. As an alternative distribution-based measure of change, we calculated twice the standard error of the mean of NICU HRQL scores.33 We determined whether the proportion of parents reporting a clinically significant change in HRQL was different for any parent or infant risk factor, using chi-squared or Fisher exact tests.
As the bivariate analyses were normally distributed, we developed multivariable linear regression models to assess associations between risk factors and parent HRQL. To assess NICU HRQL, we first developed a model including all covariates which had bivariate association with HRQL differences at a p value of <0.2. To assess HRQL 3 months after NICU discharge compared with the NICU, we adjusted for the parent’s NICU HRQL score, then entered all potentially significant risk factors that were identified on bivariate analysis. Because of the collinearity between related risk factors, we reduced each model to include only risk factors with a p value <0.2, also assessing for <15% change in effect size of the remaining factors before dropping a variable. In both models, moderate-late preterm infants (29–36 weeks) were used as the gestational age reference group as they had the highest HRQL scores at both time points. We assessed the assumptions of the linear regression model by examining whether the residuals from the final models were normally distributed as well as examining diagnostic plots. A p-value of <0.05 was accepted for statistical significance. STATA version 14 (College Station, TX) was used for analyses. This study was approved by the Medical College of Wisconsin Institutional Review Board.
RESULTS
We enrolled 214 parent-infant dyads. Twenty dyads were ineligible for follow-up due to death (n=8), social reasons (n=7), or ongoing hospitalization at the study conclusion (n=5 extremely-preterm patients with tracheostomy and long-term ventilation). Of the remaining 194 eligible dyads, 167 (86%) completed an interview three months after discharge (Figure 1; available at www.jpeds.com). Mothers were the primary respondents (88%). There were no significant differences between eligible subjects who completed follow-up and those lost to follow-up.
Figure 1.

(available online): Flow of participants through the study.
Of the study cohort, 52 (24%) of infants were born extremely preterm; 114 (53%) were moderate-late preterm; 48 (22%) were term. Table 1 shows infant and parent characteristics stratified by gestational age. Parents of extremely preterm infants were more likely to report black race and single parent households. At discharge, extremely preterm infants were more likely to be discharged with home oxygen, whereas term infants were more likely to be discharged with tube feedings. By 3 months, term infants had the highest proportion of hospital readmission. Emergency room visits and acute care visits were not different by gestational age groups. Most extremely preterm infants had diagnoses consistent with complications related to prematurity; most full-term infants had either a congenital anomaly or required neurological evaluation. (Table 2; available at www.jpeds.com)
Table 1.
Infant and parent characteristics of study sample by gestational age.
| ≤28 weeks n=52 | 29–36 weeks n=114 | ≥37 weeks n=48 | p | ||
|---|---|---|---|---|---|
| Parent Characteristics | |||||
| Age, years, median (IQR) | 31 (25–35) | 29 (26–33) | 30 (23–34) | 0.820 | |
| Race/ethnicity, n (%) | Black | 17 (33) | 25 (22) | 9 (19) | 0.042 |
| White | 27 (53) | 78 (68) | 34 (71) | ||
| Hispanic | 2 (4) | 4 (4) | 5 (10) | ||
| Other | 6 (10) | 7 (6) | 0 | ||
| Education, n (%) | Have not finished high school | 6 (12) | 3 (3) | 3 (6) | 0.170 |
| High school graduate | 25 (48) | 66 (58) | 23 (48) | ||
| College/technical school graduate | 21 (40) | 45 (39) | 22 (46) | ||
| Has a car, n (%) | 40 (77) | 93 (81) | 41 (85) | 0.550 | |
| Staying at Ronald McDonald House, n (%) | 14 (27) | 42 (37) | 12 (25) | 0.200 | |
| Single parent household | 8 (15) | 6 (5) | 2 (4) | 0.040 | |
| Number of siblings (for child), n (%) | 0 | 23 (44) | 34 (30) | 22 (46) | 0.200 |
| 1 | 13 (25) | 34 (30) | 9 (19) | ||
| >1 | 16 (31) | 46 (40) | 17 (35) | ||
| History of mental health concerns, n (%) | 16 (31) | 40 (35) | 18 (38) | 0.900 | |
| Public insurance, n (%) | 29 (56) | 63 (55) | 22 (46) | 0.044 | |
| Infant clinical characteristics | |||||
| Birth weight, grams, median (IQR) | 745 (581–1000) | 1805 (1468–2213) | 3113 (2735–3557) | <0.001 | |
| Male sex, n (%) | 21 (40) | 52 (46) | 28 (58) | 0.200 | |
| Congenital or chromosomal anomaly present, n (%) | 5 (10) | 32 (28) | 33 (69) | <0.001 | |
| Multiple gestation, n (%) | 11 (21) | 24 (21) | 1 (2) | 0.008 | |
| Cesarean section, n (%) | 31 (60) | 70 (61) | 15 (31) | <0.001 | |
| Outborn delivery, n (%) | 34 (65) | 32 (28) | 29 (60) | <0.001 | |
| Number of surgeries, median (IQR) | 1 (0–2) | 0 (0–1) | 1 (0–2) | <0.001 | |
| Number of consultants, median (IQR) | 4 (2–7) | 1 (0–3) | 3 (2–6) | <0.001 | |
| Required mechanical ventilation, n (%) | 27 (52) | 39 (34) | 19 (40) | 0.096 | |
| Ventilator days, median (IQR) | 59 (3–77) | 0 (0–1) | 1 (0–6) | <0.001 | |
| Received vasopressors, n (%) | 18 (35) | 10 (9) | 9 (19) | <0.001 | |
| Day of life at assessment, days, median (IQR) | 97 (67–140) | 20 (17–47) | 19 (16–40) | <0.001 | |
| Total length of stay, days, median (IQR) | 123 (87–177) | 42 (27–59) | 34 (23–47) | <0.001 | |
| Post-NICU healthcare utilization | n=36 | n=92 | n=39 | ||
| Home oxygen, n (%) | 20 (59) | 12 (13) | 6 (15) | <0.001 | |
| Home gastrostomy tube feedings, n (%) | 7 (20) | 11 (12) | 11 (28) | 0.080 | |
| Home nasogastric tube feedings, n (%) | 1 (3) | 0 | 5 (13) | <0.001 | |
| Tracheostomy, n (%) | 7 (13) | 3 (3) | 3 (6) | 0.004 | |
| Medications prescribed, n (%) | 0 | 8 (23) | 53 (58) | 19 (49) | 0.003 |
| 1–2 | 14 (40) | 27 (30) | 13 (33) | ||
| >2 | 13 (37) | 11 (12) | 7 (18) | ||
| Emergency department visits (at least 1), n (%) | 12 (33) | 25 (27) | 13 (33) | 0.680 | |
| Acute care visits (at least 1), n (%) | 10 (28) | 24 (26) | 10 (26) | 0.970 | |
| Hospital readmission (at least 1), n (%) | 9 (26) | 16 (17) | 18 (46) | 0.003 | |
Parent and infant characteristics of the study sample, by gestational age. Data in the table represents n (%) or median (IQR) as noted. P values indicate chi squared or Fisher’s exact tests for differences between proportions, and Kruskal-Wallis tests for differences in medians, as appropriate. Post-NICU healthcare utilization is calculated for the 167 infants with 3-month follow-up. There were no differences in other clinical characteristics between eligible infants who did and did not receive 3-month follow-up.
Table 2.
Common diagnoses for infants admitted to the NICU by gestational age.
| 23–28 weeks (n=52) | 29–36 weeks (n=114) | 37–43 weeks (n=48) | |||
|---|---|---|---|---|---|
| Diagnosis | n (%) | Diagnosis | n (%) | Diagnosis | n (%) |
| Moderate-severe BPD | 39 (75) | Moderate-severe BPD | 24 (21) | Genetic syndrome* | 11 (23) |
| PDA | 22 (42) | NEC | 9 (8) | Pulmonary hypertension | 7 (15) |
| Late-onset sepsis | 16 (31) | Genetic syndrome* | 9 (8) | Seizures | 7 (15) |
| Surgical ROP | 11 (21) | Other anomalies | 9 (8) | Hydrocephalus requiring reservoir or shunt | 7 (15) |
| GI anomalies (gastroschisis, bowel | Pulmonary anomaly | ||||
| Severe IVH | 7 (13) | atresia, TEF) | 8 (7) | (CDH, CPAM) | 6 (13) |
| NEC | 7 (13) | Pneumothorax | 7 (6) | GI anomaly (Bowel atresia, omphalocele) | 5 (10) |
| Surgical NEC | 5 (10) | Late-onset sepsis | 6 (5) | CNS anomaly (myelomeningocele, encephalocele) | 5 (10) |
| Congenital anomaly or genetic syndrome* | 5 (10) | Cardiac anomalies (VSD, TOF) | 6 (5) | ||
| Hydrocephalus requiring reservoir or shunt | 5 (4) | ||||
| PDA | 5 (4) | ||||
Table 2 illustrates the most common diagnoses encountered in each gestational age group, listed in order from most to least common, for all diagnoses present in at least 5 infants in that gestational age group.
Genetic syndrome refers to an identified syndrome confirmed by testing. 7 infants had Trisomy 21. The rest were individual identified syndromes consistent with the infant’s congenital anomalies; specific types not listed to protect patient confidentiality for infants with rare diseases in a single center. Other listed anomaly categories reflect infants without an identified genetic syndrome. An infant with multiple anomalies may be represented more than once.
Abbreviations and definitions: BPD, bronchopulmonary dysplasia defined as mild if required >21% after day of life 28, moderate if required <30% FiO2 at 36 weeks GA and severe if required ≥30% FiO2 and/or positive pressure at 36 weeks GA. PDA, patent ductus arteriosus that required medical therapy or surgical ligation; IVH, interventricular hemorrhage defined as grade 3 or grade 4 IVH (unilateral or bilateral); NEC, necrotizing enterocolitis defined as Bell’s stage 2 or greater. Late onset sepsis was defined as culture positive bacteremia, meningitis or urinary tract infection obtained > 3 days after birth and treated with antibiotics >5 days. GI, gastrointestinal; TEF, tracheo-esophageal fistula; VSD, ventricular septal defect; TOF, tetralogy of Fallot; CDH, congenital diaphragmatic hernia; CPAM, congenital pulmonary adenomatoid malformation; CNS, central nervous system
Demographic characteristics, NICU illness, and post-NICU healthcare utilization were associated with parent HRQL (Table 3). Demographic factors including Hispanic ethnicity, single parent household, and history of mental health concerns were associated with lower NICU HRQL; no demographic factor was associated with differences in 3-month HRQL. NICU illness variables associated with lower parent HRQL both in the NICU and at 3 months after discharge included gestational age, mechanical ventilation, multiple surgeries, and multiple consultants involved in care. Post-NICU healthcare utilization variables associated with lower parent HRQL included gastrostomy tubes, multiple prescribed medications, emergency department visits, and hospital readmissions.
Table 3.
Associations with parent HRQL in the NICU and 3 months post-discharge.
| n | NICU HRQL Median (IQR) | p | 3-month HRQL Median (IQR) | p | ||
|---|---|---|---|---|---|---|
| Parent Characteristics | ||||||
| Race/Ethnicity | Black | 32 | 74 (57–81) | 0.03 | 70 (61–82) | 0.229 |
| White | 116 | 69 (58–82) | 73 (61–85) | |||
| Hispanic | 8 | 50 (44–64) | 67 (45–71) | |||
| Other | 11 | 71 (63–89) | 74 (68–84) | |||
| Education | Didn’t graduate high school | 8 | 74 (62–80) | 0.112 | 74 (68–92) | 0.289 |
| Graduated high school | 82 | 75 (62–84) | 78 (64–88) | |||
| Graduated college | 77 | 65 (56–80) | 70 (57–80) | |||
| Single parent household | Yes | 10 | 53 (43–58) | 0.002 | 64 (57–77) | 0.129 |
| No | 157 | 70 (59–82) | 72 (62–84) | |||
| History of mental health disorder | Yes | 58 | 63 (52–78) | 0.007 | 70 (58–83) | 0.127 |
| No | 109 | 73 (61–85) | 74 (62–85) | |||
| Infant NICU Characteristics | ||||||
| Gestational age | ≤28 weeks | 36 | 60 (51–77) | 0.004 | 72 (63–83) | 0.007 |
| 29–36 weeks | 92 | 74 (61–85) | 76 (65–86) | |||
| ≥37 weeks | 39 | 67 (59–79) | 63 (56–80) | |||
| Major anomaly | Yes | 47 | 67 (56–81) | 0.336 | 68 (57–81) | 0.015 |
| No | 120 | 70 (56–81) | 75 (63–86) | |||
| Delivery location | Inborn | 96 | 73 (59–84) | 0.04 | 74 (63–85) | 0.182 |
| Outborn | 71 | 67 (53–79) | 69 (60–82) | |||
| Multiple surgeries | No | 94 | 76 (61–85) | 0.005 | 76 (64–88) | 0.002 |
| Yes | 73 | 65 (53–78) | 67 (58–81) | |||
| Multiple consultants | No | 102 | 76 (61–85) | <0.001 | 76 (65–87) | <0.001 |
| Yes | 65 | 63 (51–76) | 64 (57–79) | |||
| Mechanical ventilation | No | 102 | 76 (61–85) | <0.001 | 76 (65–88) | <0.001 |
| Yes | 65 | 62 (53–73) | 65 (58–81) | |||
| Vasopressors | No | 140 | 70 (58–83) | 0.15 | 72 (61–85) | 0.269 |
| Yes | 27 | 65 (58–79) | 69 (61–80) | |||
| Length of NICU stay | <28 days | 43 | 77 (63–85) | 0.04 | 72 (65–85) | 0.635 |
| 29–60 days | 66 | 69 (59–81) | 74 (58–84) | |||
| >60 days | 58 | 65 (53–79) | 71 (61–83) | |||
| Post Discharge Healthcare Utilization | ||||||
| Home oxygen | No | 124 | 73 (62–85) | 0.227 | ||
| Yes | 43 | 68 (60–81) | ||||
| Gastrostomy Tube | No | 137 | 74 (63–85) | 0.001 | ||
| Yes | 30 | 63 (51–76) | ||||
| Nasogastric Tube | No | 155 | 72 (61–84) | 0.551 | ||
| Yes | 9 | 68 (57–80) | ||||
| Tracheostomy present | No | 156 | 73 (62–84) | 0.070 | ||
| Yes | 9 | 64 (48–69) | ||||
| Medications prescribed | No | 100 | 75 (63–88) | 0.008 | ||
| Yes | 67 | 68 (60–81) | ||||
| Emergency Department visits | No | 113 | 74 (63–85) | 0.040 | ||
| Yes | 51 | 69 (58–81) | ||||
| Hospital readmissions | No | 121 | 76 (64–85) | <0.001 | ||
| Yes | 43 | 64 (56–76) | ||||
Comparisons with parent and infant NICU characteristics and median parent HRQL scores. NICU HRQL = parent health-related-quality of life, as measured by PedsQL Family Impact Module within a few weeks prior to neonatal intensive care unit discharge. Higher scores indicate higher HRQL. 3-month HRQL = parent health-related quality of life as reported 3 months after the infant’s NICU discharge. p values represent non-parametric comparisons of between-group differences at each time point. Additional variables not shown here included access to a car, number of siblings for the child, insurance status, multiple gestation, mode of delivery; these were not significant at p >0.2 and were dropped from further analysis.
When estimating a clinically significant change in parent HRQL based on response to the question “How sick is your child?” on a 5-point Likert scale, a 1-point difference in parents’ response corresponded to a median HRQL difference of 4 points on the PedsQL Family Impact Module (Table 4; available at www.jpeds.com). This anchor-based estimate of a 4-point difference in HRQL was more conservative than a distribution-based approach using twice the standard error of the mean (2.5-point difference). Using a 4-point difference in total PedsQL score as a clinically significant change in parent HRQL, Figure 2 shows the proportion of parents of infants in each gestational age group who reported a clinically significant change in HRQL between the NICU hospitalization and 3 months post-discharge. For parents of extremely preterm infants, 67% reported clinically significant improvement in HRQL at 3 months, compared with 31% of parents of term infants. Conversely, 22% of parents of extremely preterm infants reported lower HRQL after discharge, compared with 41% of parents of term infants (p <0.001). No other parent or infant risk factor was associated with significant bivariate differences in pattern of HRQL change over time.
Table 4.
Anchor-based calculation of clinically significant change in HRQL between NICU and 3 months post-discharge.
| Change in response to “How sick is your child?” | n | Median HRQL change |
|---|---|---|
| 3 points better | 11 | +8 |
| 2 points better | 27 | +5 |
| 1 point better | 39 | +4 |
| No change | 70 | +1 |
| 1 point worse | 13 | −1 |
Table 4 presents the median change in HRQL at 3 months post-discharge compared to the NICU associated with each interval change in a parent’s response to the question “How sick is your child?” on a 5-point Likert scale. Categories of change that reflected at least 5 respondents were included. This method has been used to anchor patient-reported outcomes in the absence of a numeric illness scale.
Figure 2.
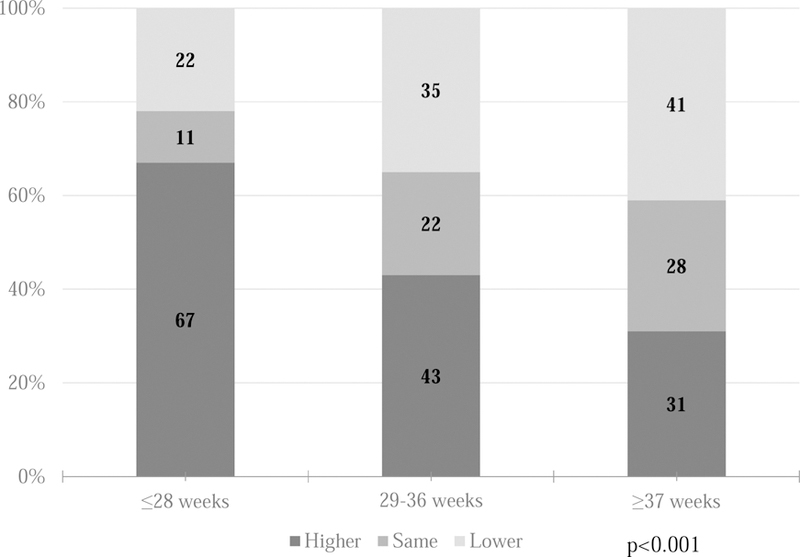
Clinically significant change in parent HRQL between NICU and 3 months post-discharge. Numbers in each colored box indicate the n of parents responding. P value was calculated by chi-squared tests. HRQL, health related quality of life; NICU, neonatal intensive care unit.
Analysis of HRQL sub-domains (Table 5; available at www.jpeds.com) revealed that during the hospitalization, parents of extremely preterm infants reported lower emotional functioning and lower worry scores compared with parents of later-gestation infants. At 3 months, parents of term infants reported lower emotional and cognitive functioning, communication and worry scores than parents of earlier-gestation infants. By 3 months post-hospitalization, parents of extremely preterm infants reported significant improvement in physical, emotional and cognitive functioning, and family relationships. In contrast, parents of term infants reported lower scores in cognitive functioning and family relationships after discharge, and no improvement in any subdomains.
Table 5.
Parent HRQL Subdomains between NICU and 3 Months Post-Discharge by Gestational Age.
| 23–28 weeks Median (IQR) | 29–36 weeks Median (IQR) | 37–42 weeks Median (IQR) | p | |
|---|---|---|---|---|
| NICU | ||||
| Physical Functioning | 58 (46–75) | 67 (54–79) | 67 (50–75) | 0.365 |
| Emotional functioning | 55 (43–70) | 70 (55–90) | 65 (45–80) | 0.007 |
| Social Functioning | 69 (50–88) | 75 (63–88) | 75 (53–91) | 0.222 |
| Cognitive Functioning | 70 (50–90) | 80 (60–95) | 70 (53–90) | 0.127 |
| Communication | 67 (50–83) | 75 (67–92) | 67 (54–83) | 0.065 |
| Worry | 60 (43–80) | 75 (55–90) | 55 (45–75) | <0.001 |
| Daily Activities | 58 (42–75) | 50 (42–75) | 50 (42–67) | 0.673 |
| Family Relationships | 75 (55–95) | 90 (65–100) | 80 (70–100) | 0.081 |
| | ||||
| 3 MONTHS | ||||
| Physical Functioning | 67 (63–79) | 71 (58–81) | 63 (50–75) | 0.124 |
| Emotional functioning | 80 (70–90) | 80 (70–90) | 70 (55–80) | 0.006 |
| Social Functioning | 75 (63–88) | 75 (63–94) | 69 (56–94) | 0.473 |
| Cognitive Functioning | 80 (63–98) | 75 (60–95) | 65 (50–90) | 0.020 |
| Communication | 75 (58–84) | 75 (67–96) | 67 (58–83) | 0.035 |
| Worry | 70 (55–80) | 80 (70–95) | 60 (45–80) | <0.001 |
| Daily Activities | 50 (33–71) | 58 (42–75) | 50 (33–67) | 0.161 |
| Family Relationship | 80 (65–100) | 80 (70–95) | 75 (60–90) | 0.316 |
| | ||||
| CHANGE | ||||
| Physical Functioning | +8 (−4 to +25) | +4 (−8 to +13) | 0 (−13 to +8) | 0.048 |
| Emotional functioning | +25 (0 to 40) | +5 (−5 to +25) | 0 (−15 to +15) | 0.003 |
| Social Functioning | +13 (−13 to +25) | 0 (−13 to +13) | 0 (−13 to +6) | 0.088 |
| Cognitive Functioning | +10 (0 to +25) | 0 (−10 to +10) | −5 (−10 to 5) | <0.001 |
| Communication | +8 (0 to 17) | 0 (−8 to +17) | 0 (−17 to +8) | 0.053 |
| Worry | +10 (0 to +20) | 0 (−10 to +10) | 0 (−10 to +10) | 0.098 |
| Daily Activities | 0 (−17 to +17) | 0 (−17 to +17) | −8 (−17 to +8) | 0.555 |
| Family Relationship | +10 (−3 to +25) | 0 (−15 to +5) | −5 (−20 to +0) | 0.001 |
Table 6 presents median (interquartile range) HRQL subdomain scores in the NICU and at 3 months, as well as the median (interquartile range) change from NICU to 3 months within individual patients. p values represent non-parametric Kruskal-Wallis tests between gestational age groups.
NICU, neonatal intensive care unit; HRQL, health related quality of life; IQR, interquartile range
Table 6 shows reduced regression models of factors associated with parent HRQL. During the NICU hospitalization, Hispanic ethnicity, single parent household, and history of a mental health concern were significantly associated with lower HRQL. Significant clinical risk factors included extreme prematurity, multiple subspecialty consultants, and use of mechanical ventilation. At 3 months, parents of extreme preterm infants reported an increase in HRQL compared with their score at enrollment. No demographic factor was significantly associated with change in HRQL following discharge home. Multiple gestation or having other children at home were not associated with differences in baseline HRQL or change in HRQL over time. Post-discharge healthcare utilization including home oxygen, tracheostomy, and inpatient readmission were significantly associated with lower HRQL. Full models including all risk factors that were significant in bivariate analysis did not change the direction or significance of the association between gestational age and HRQL. Residuals from the models were normally distributed.
Table 6.
Multivariable associations with parent HRQL during NICU and 3 months after discharge.
| Covariate | HRQL Coefficient | 95% LCI | 95% UCI | P value | |
|---|---|---|---|---|---|
| During NICU admission | Parent race/ethnicity | ||||
| Black | 4.0 | −1.5 | 9.5 | 0.152 | |
| White | 0.0 | (ref) | |||
| Hispanic | −11.1 | −20.9 | −1.3 | 0.026 | |
| Other | 6.7 | −1.7 | 15.0 | 0.116 | |
| Single parent household | −18.0 | −27.1 | −8.9 | <0.001 | |
| History of mental health disorder | −9.2 | −13.4 | −4.9 | <0.001 | |
| Gestational age | |||||
| 23–28 weeks | −7.0 | −12.6 | −1.4 | 0.015 | |
| 29–36 weeks | 0.0 | (ref) | |||
| 37–43 weeks | −1.0 | −6.3 | 4.4 | 0.721 | |
| Multiple surgeries | 4.4 | −1.2 | 9.9 | 0.122 | |
| Multiple consultants | −5.7 | −10.6 | −0.8 | 0.023 | |
| | |||||
| Mechanical ventilation | −7.2 | −11.7 | −2.6 | 0.002 | |
| | |||||
| 3 months after NICU discharge | Enrollment HRQL (every 1 point) |
0.6 |
0.4 |
0.7 |
<0.001 |
| Gestational age | |||||
| 23–28 weeks | 10.6 | 4.7 | 16.4 | <0.001 | |
| 29–36 weeks | 0.0 | (ref) | |||
| 37–43 weeks | −5.1 | −9.9 | −0.4 | 0.036 | |
| Mechanical ventilation in NICU | −3.5 | −7.7 | 0.7 | 0.101 | |
| Tracheostomy | −10.0 | −19.0 | −1.0 | 0.030 | |
| Readmission | −4.9 | −9.7 | −0.1 | 0.044 | |
| Home oxygen | −6.6 | −11.8 | −1.5 | 0.012 | |
Reduced linear regression models for multivariable associations with A) parent HRQL before NICU discharge; B) parent HRQL 3 months after NICU discharge, adjusted for NICU HRQL within the same parent. HRQL coefficient is the adjusted difference in total PedsQL Family Impact Module score. HRQL, health related quality of life; LCI, lower confidence interval; UCI, upper confidence interval; NICU, neonatal intensive care unit.
DISCUSSION
This study prospectively examined how demographics, NICU illness, and post-NICU healthcare utilization impact HRQL for parents of infants of all gestational ages requiring a prolonged NICU stay. Our major conclusions are that parents of extremely preterm infants experience the most negative impact on parent HRQL during the NICU hospitalization, and the most improvement after discharge. Demographic risk factors are associated with lower parent HRQL in the NICU, but these differences do not persist after NICU discharge. Post-discharge healthcare utilization including home oxygen, tracheostomy, and inpatient readmission is associated with lower parent HRQL 3 months after NICU discharge.
Parents of extremely preterm infants reported the lowest HRQL during the NICU hospitalization. Parenting an extremely preterm infant has been associated with higher incidence of stress and depressive symptoms, which are correlated with HRQL.2,35 Mothers of preterm infants have been reported to have lower HRQL at 8 weeks postpartum than parents of healthy term controls. Indeed, research on parent HRQL of term infants has been limited to those who did not require a NICU stay,36 or those with later childhood diagnoses.37 This study extends those findings by comparing parent HRQL for extremely preterm infants to that of other parents requiring an extended NICU stay.36 Term infants who are sick enough to require a two week NICU stay often have major anomalies, a known cause of psychological distress to parents.38 Nonetheless, extremely preterm infants experience the highest risk of mortality, comorbidities, and long LOS, even compared with critically ill term infants.39 Whether gestational age itself, the long LOS, or its associated comorbidities are responsible for the impact on parent HRQL is probably less important than recognizing the impact of extreme prematurity on parents.
After discharge, parents of extremely preterm infants reported the greatest improvement in HRQL. A Brazilian study of parents of preterm infants reported that HRQL improved over time.40 We found that this pattern was distinctly different than parents of later-gestation infants requiring an extended NICU stay. It seems unlikely that the higher HRQL for parents of extremely preterm infants results entirely from regression to the mean, because parents of term infants had a similarly low NICU HRQL score yet were more likely to experience lower HRQL after discharge. Although we adjusted for some measures of post-NICU healthcare utilization, sick term infants may have other unmeasured medical needs causing stress for parents. Also, neonatal care providers typically describe an array of medical and developmental outcomes for survivors of extreme prematurity, but in a heterogeneous term infant population, even less prognostic information may be available.41–44 Lakshmanan et al found that time off work, financial worry and social isolation were associated with lower parent HRQL in preterm infants; parents of term infants with extensive home care needs and uncertain prognosis may be impacted to an even greater extent.45 In addition, there were important differences in sub-domain HRQL changes between preterm and term parents, with preterm parents reporting the greatest increases in emotional functioning and family relationships compared with term parents. This may be due to better known support programs in place in the hospital and community for families of preterm children, and an overall better understanding of prematurity through programs such as March of Dimes; parents of sick term infants who may have less well-known diagnoses may also have fewer perceived support systems. Another key difference between parents of extremely preterm and term infants is that parents of term infants have a shorter NICU LOS. Witt et al found that longer NICU LOS in preterm infants was a risk factor for lower parent HRQL.22 In our cohort of infants of all gestational ages, we did not find that LOS was an additional risk factor for lower HRQL. In the post-discharge period, parents of sick term infants paradoxically may have less time for teaching and adaptation to the infant’s condition, which may contribute to a relative lack of discharge readiness and resulting lower HRQL post-discharge.46 Parents of preterm infants may have already mourned the prospect of having a healthy child during the hospitalization, whereas parents of term infants may have had less time to cope with the lost expectation of a healthy child. Future research is important to understand the specific psychosocial and educational needs of parents of term infants in the NICU.
We found several demographic factors associated with lower parent HRQL during hospitalization. These demographic factors impacted HRQL in the NICU but did not affect parents’ change in HRQL after discharge home. Single parents and parents with a history of mental health concerns may have delayed discharge to ensure adequate teaching and comfort with cares; mothers with a history of mental health disorders previously have reported less perceived discharge readiness.47–49 Our results suggest that high-risk parents may benefit more from earlier teaching and discharge planning when possible, rather than delaying discharge, if clinical criteria are met and adequate home support is available. Family-integrated care models and transition-to-home programs report improved outcomes and decreased parental stress.50–52 Future work in NICUs serving families with different demographics than ours, or in communities with different follow-up structures, will be important to understand the potential applications of these findings. Earlier discharge planning integrated with ongoing family education may be essential to optimizing both parent HRQL and infant safety.
Parents of children with more post-NICU healthcare utilization reported lower HRQL after discharge. Lakshmanan et al similarly found that readmissions and home technology were associated with lower parent HRQL in parents of preterm infants 2 years after discharge.45 Our study extends those findings by comparing NICU to post-NICU HRQL in the same parents over time. For parents of preterm infants, some of the potential negative impact of complex home care may be offset by the potential positive impact of home discharge. In a pediatric transplant population, positive pre-discharge hospital processes were associated with higher parent HRQL at home.53 Parents of preterm infants may uniquely benefit from strategies to expedite discharge, if empowered early to ensure safe transition home but the positive impact of home discharge needs to be balanced against the negative impact of complex home care.
Our study has important limitations. Our follow-up period was three months, so we did not capture parent HRQL changes in the long term. Parents of medically complex children may adapt to their child’s illness over time, resulting in higher HRQL; conversely, parents of preterm infants may experience lower HRQL later if school problems emerge.54 Defining clinically meaningful change in HRQL is difficult in the absence of a quantitative illness measure that changes with time.32 Anchor-based methods of assessing HRQL change have gained increasing importance in the literature, but to our knowledge have not been applied to caregiver HRQL previously. We did not include healthy newborn infants, although this has been done in prior studies of parents of preterm infants.11 Some demographic factors are hard to interpret; for example, staying at Ronald McDonald house implies both living far away and having the life flexibility to stay away from home. Previous history of a mental health concern was assessed via parent report rather than with a separate screening tool. Our study design of comparing parent HRQL after discharge to their own score during hospitalization may partially mitigate these issues, but future studies incorporating more detailed maternal health measures will be important for future research.
Acknowledgments
Supported by the National Institutes of Health (K23HL136525 [to J.L.]) and a Medical College of Wisconsin Presidential Faculty Scholar Award (to J.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- NICU
neonatal intensive care unit
- HRQL
health related quality of life
- LOS
length of stay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of this study were presented at the Pediatric Academic Societies annual meeting, May 5–8, 2018, Toronto, Canada.
References
- 1.Carter JD, Mulder RT, Darlow BA. Parental stress in the NICU: The influence of personality, psychological, pregnancy and family factors. Personality and mental health 2007;1(1):40–50. [Google Scholar]
- 2.Shaw RJ, Deblois T, Ikuta L, Ginzburg K, Fleisher B, Koopman C. Acute stress disorder among parents of infants in the neonatal intensive care nursery. Psychosomatics 2006;47(3):206–212. [DOI] [PubMed] [Google Scholar]
- 3.Amorim M, Alves E, Kelly-Irving M, Ribeiro AI, Silva S. Quality of life of parents of very preterm infants 4 months after birth: a mixed methods study. Health and quality of life outcomes 2018;16(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyatt GH, Cook DJ. Health status, quality of life, and the individual. Jama 1994;272(8):630–631. [PubMed] [Google Scholar]
- 5.Donohue PK, Maurin E, Kimzey L, Allen MC, Strobino D. Quality of life of caregivers of very low-birthweight infants. Birth September 2008;35(3):212–219. [DOI] [PubMed] [Google Scholar]
- 6.Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics March 2009;123(3):1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLean A, Townsend A, Clark J, et al. Quality of life of mothers and families caring for preterm infants requiring home oxygen therapy: a brief report. Journal of paediatrics and child health October 2000;36(5):440–444. [DOI] [PubMed] [Google Scholar]
- 8.Hodek J-M, von der Schulenburg J-M, Mittendorf T. Measuring economic consequences of preterm birth-Methodological recommendations for the evaluation of personal burden on children and their caregivers. Health economics review 2011;1(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatzmann J, Heymans HS, Ferrer-i-Carbonell A, van Praag BM, Grootenhuis MA. Hidden consequences of success in pediatrics: parental health-related quality of life--results from the Care Project. Pediatrics November 2008;122(5):e1030–1038. [DOI] [PubMed] [Google Scholar]
- 10.Henderson J, Carson C, Redshaw M. Impact of preterm birth on maternal well-being and women’s perceptions of their baby: a population-based survey. BMJ open October 8 2016;6(10):e012676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer LT, Salvator A, Guo S, Collin M, Lilien L, Baley J. Maternal psychological distress and parenting stress after the birth of a very low-birth-weight infant. JAMA March 3 1999;281(9):799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treyvaud K, Anderson V, Lee K, et al. parental Mental Health And Early Social-emotional Development Of Children Born Very Preterm: a147. Journal of paediatrics and child health 2010;46:46. [DOI] [PubMed] [Google Scholar]
- 13.Klassen AF, Landgraf JM, Lee SK, et al. Health related quality of life in 3 and 4 year old children and their parents: preliminary findings about a new questionnaire. Health and quality of life outcomes 2003;1(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saigal S, Feeny D, Rosenbaum P, Furlong W, Burrows E, Stoskopf B. Self-perceived health status and health-related quality of life of extremely low-birth-weight infants at adolescence. Jama 1996;276(6):453–459. [PubMed] [Google Scholar]
- 15.Saigal S, Rosenbaum PL, Feeny D, et al. Parental perspectives of the health status and health-related quality of life of teen-aged children who were extremely low birth weight and term controls. Pediatrics 2000;105(3):569–574. [DOI] [PubMed] [Google Scholar]
- 16.Viera CS, Medoff-Cooper B, de Mello DF, Monti LM. Brazilians Families of Preterm Child: Experiences in the Transition Period from NICU to Home. International Journal of Nursing 2016;3(2):39–45. [Google Scholar]
- 17.Schiariti V, Houbè JS, Lisonkova S, Klassen AF, Lee SK. Caregiver-reported health outcomes of preschool children born at 28 to 32 weeks’ gestation. Journal of Developmental & Behavioral Pediatrics 2007;28(1):9–15. [DOI] [PubMed] [Google Scholar]
- 18.Brady JM, Zhang H, Kirpalani H, DeMauro SB. Living with Severe Bronchopulmonary Dysplasia—Parental Views of Their Child’s Quality of Life. The Journal of Pediatrics 2019;207:117–122. [DOI] [PubMed] [Google Scholar]
- 19.Bjorkqvist J, Hovi P, Pesonen AK, et al. Adults who were born preterm with a very low birth weight reported a similar health-related quality of life to their term-born peers. Acta paediatrica February 2018;107(2):354–357. [DOI] [PubMed] [Google Scholar]
- 20.Natalucci G, Bucher HU, Von Rhein M, Borradori Tolsa C, Latal B, Adams M. Population based report on health related quality of life in adolescents born very preterm. Early Human Development 2017/January/01/ 2017;104:7–12. [DOI] [PubMed] [Google Scholar]
- 21.McGowan JE, Alderdice FA, Boylan J, et al. Neonatal intensive care and late preterm infants: Health and family functioning at three years. Early Human Development 2014/April/01/ 2014;90(4):201–205. [DOI] [PubMed] [Google Scholar]
- 22.Witt WP, Litzelman K, Spear HA, et al. Health-related quality of life of mothers of very low birth weight children at the age of five: results from the Newborn Lung Project Statewide Cohort Study. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation November 2012;21(9):1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McManus BM, Robert S, Albanese A, Sadek-Badawi M, Palta M. Racial disparities in health-related quality of life in a cohort of very-low-birth-weight 2- and 3-year-olds with and without asthma. Journal of epidemiology and community health July 2012;66(7):579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McManus BM, Robert SA, Albanese A, Sadek-Badawi M, Palta M. Racial disparities in health-related quality of life in a cohort of very low birthweight 2- and 3-year-olds with and without cerebral palsy. Developmental medicine and child neurology May 2011;53(5):467–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balakrishnan A, Stephens BE, Burke RT, et al. Impact of very low birth weight infants on the family at 3months corrected age. Early Human Development 2011/January/01/ 2011;87(1):31–35. [DOI] [PubMed] [Google Scholar]
- 26.Drotar D, Hack M, Taylor G, Schluchter M, Andreias L, Klein N. The impact of extremely low birth weight on the families of school-aged children. Pediatrics June 2006;117(6):2006–2013. [DOI] [PubMed] [Google Scholar]
- 27.Taylor HG, Klein N, Minich NM, Hack M. Long-term Family Outcomes for Children With Very Low Birth Weights. Archives of pediatrics & adolescent medicine 2001;155(2):155–161. [DOI] [PubMed] [Google Scholar]
- 28.Amorim M, Alves E, Kelly-Irving M, Ribeiro AI, Silva S. Quality of life of parents of very preterm infants 4 months after birth: a mixed methods study. Health and quality of life outcomes September 10 2018;16(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amorim M, Silva S, Kelly-Irving M, Alves E. Quality of life among parents of preterm infants: a scoping review. Quality of Life Research 2018/05/01 2018;27(5):1119–1131. [DOI] [PubMed] [Google Scholar]
- 30.Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL Family Impact Module: preliminary reliability and validity. Health and quality of life outcomes 2004;2:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Project REDCap. https://www.project-redcap.org/.
- 32.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Quality of Life Research 2002;11(3):207–221. [DOI] [PubMed] [Google Scholar]
- 33.Angst F, Aeschlimann A, Angst J. The minimal clinically important difference raised the significance of outcome effects above the statistical level, with methodological implications for future studies. Journal of Clinical Epidemiology 2017;82:128–136. [DOI] [PubMed] [Google Scholar]
- 34.Ferrand A, Gorgos A, Ali N, Payot A. Resilience Rather than Medical Factors: How Parents Predict Quality of Life of Their Sick Newborn. The Journal of pediatrics September 2018;200:64–70 e65. [DOI] [PubMed] [Google Scholar]
- 35.Baía I, Amorim M, Silva S, Kelly-Irving M, de Freitas C, Alves E. Parenting very preterm infants and stress in Neonatal Intensive Care Units. Early Human Development 2016/October/01/ 2016;101:3–9. [DOI] [PubMed] [Google Scholar]
- 36.Hill PD, Aldag JC. Maternal perceived quality of life following childbirth. Journal of Obstetric, Gynecologic, & Neonatal Nursing 2007;36(4):328–334. [DOI] [PubMed] [Google Scholar]
- 37.Levi RB, Drotar D. Health-related quality of life in childhood cancer: Discrepancy in parent–child reports. International Journal of Cancer 1999;83(S12):58–64. [DOI] [PubMed] [Google Scholar]
- 38.Fonseca A, Nazaré B, Canavarro MC. Parental psychological distress and quality of life after a prenatal or postnatal diagnosis of congenital anomaly: a controlled comparison study with parents of healthy infants. Disability and health journal 2012;5(2):67–74. [DOI] [PubMed] [Google Scholar]
- 39.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics September 2010;126(3):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moura M, Araújo C, Prado M, et al. Factors associated with the quality of life of mothers of preterm infants with very low birth weight: A 3-year follow-up study. Quality of Life Research 2017;26(5):1349–1360. [DOI] [PubMed] [Google Scholar]
- 41.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive Care for Extreme Prematurity — Moving beyond Gestational Age. New England Journal of Medicine 2008;358(16):1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agha MM, Williams JI, Marrett L, To T, Dodds L. Determinants of survival in children with congenital abnormalities: A long-term population-based cohort study. Birth Defects Research Part A: Clinical and Molecular Teratology 2006;76(1):46–54. [DOI] [PubMed] [Google Scholar]
- 43.Aite L, Zaccara A, Trucchi A, et al. When uncertainty generates more anxiety than severity: the prenatal experience with cystic adenomatoid malformation of the lung. Journal of Perinatal Medicine Vol 372009:539. [DOI] [PubMed] [Google Scholar]
- 44.Poley MJ, Stolk EA, Tibboel D, Molenaar JC, Busschbach JJV. Short term and long term health related quality of life after congenital anorectal malformations and congenital diaphragmatic hernia. Archives of disease in childhood 2004;89(9):836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lakshmanan A, Agni M, Lieu T, et al. The impact of preterm birth <37 weeks on parents and families: a cross-sectional study in the 2 years after discharge from the neonatal intensive care unit. Health and quality of life outcomes 2017;15(1):38–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith VC, Hwang SS, Dukhovny D, Young S, Pursley DM. Neonatal intensive care unit discharge preparation, family readiness and infant outcomes: connecting the dots. Journal Of Perinatology 03/14/online 2013;33:415. [DOI] [PubMed] [Google Scholar]
- 47.Sims DC, Jacob J, Mills MM, Fett PA, Novak G. Evaluation and development of potentially better practices to improve the discharge process in the neonatal intensive care unit. Pediatrics 2006;118(Supplement 2):S115–S123. [DOI] [PubMed] [Google Scholar]
- 48.McGowan EC, Du N, Hawes K, Tucker R, O’Donnell M, Vohr B. Maternal Mental Health and Neonatal Intensive Care Unit Discharge Readiness in Mothers of Preterm Infants. The Journal of pediatrics May 2017;184:68–74. [DOI] [PubMed] [Google Scholar]
- 49.Raffray M, Semenic S, Osorio Galeano S, Ochoa Marín SC. Barriers and facilitators to preparing families with premature infants for discharge home from the neonatal unit. Perceptions of health care providers. Investigacion y educacion en enfermeria 2014;32(3):379–392. [DOI] [PubMed] [Google Scholar]
- 50.Bracht M, O’Leary L, Lee SK, O’Brien K. Implementing family-integrated care in the NICU: a parent education and support program. Advances in Neonatal Care 2013;13(2):115–126. [DOI] [PubMed] [Google Scholar]
- 51.O’Brien K, Bracht M, Macdonell K, et al. A pilot cohort analytic study of Family Integrated Care in a Canadian neonatal intensive care unit. BMC Pregnancy and Childbirth 2013;13(1):S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, McGowan E, Tucker R, Glasgow L, Kluckman M, Vohr B. Transition Home Plus Program Reduces Medicaid Spending and Health Care Use for High-Risk Infants Admitted to the Neonatal Intensive Care Unit for 5 or More Days. The Journal of Pediatrics 2018;200:91–97.e93. [DOI] [PubMed] [Google Scholar]
- 53.Lerret SM, Weiss ME. How ready are they? Parents of pediatric solid organ transplant recipients and the transition from hospital to home following transplant. Pediatric transplantation 2011;15(6):606–616. [DOI] [PubMed] [Google Scholar]
- 54.Eiser C, Eiser JR, Mayhew AG, Gibson AT. Parenting the premature infant: balancing vulnerability and quality of life. Journal of Child Psychology and Psychiatry 2005;46(11):1169–1177. [DOI] [PubMed] [Google Scholar]
- 55.Ferrand A, Gorgos A, Ali N, Payot A. Resilience rather than medical factors: how parents predict quality of life of their sick newborn. The Journal of pediatrics 2018;200:64–70. e65. [DOI] [PubMed] [Google Scholar]


