Abstract
Background:
There is substantial evidence that many depressed individuals experience impaired executive functioning. Understanding the causes of executive dysfunction in depression is clinically important because cognitive impairment is a substantial contributor to functional impairment. This study investigated whether elevated levels of an inflammatory cytokine (interleukin-6 [IL-6]) and/or higher body mass index (BMI) concurrently and/or prospectively accounted for the relationship between depressive symptoms and impaired executive functioning in adolescents.
Methods:
A diverse, community sample of adolescents (N = 288; Mean age = 16.33; 51.4% female; 59.0% African-American) completed assessments of height and weight, IL-6, depressive symptoms, and self-report/behavioral measures of executive functioning (selective attention, switching attention) and future orientation annually over three years. Adolescents experiencing acute illness or medical conditions that affect inflammation were excluded from analyses. Path analysis within a structural equation modeling (SEM) framework simultaneously examined the concurrent and prospective relationships between BMI, IL-6, depressive symptoms, and the measures of cognitive functioning across three time-points.
Results:
Across all time-points, higher BMI was prospectively associated with higher levels of IL-6 and depressive symptoms, while higher levels of IL-6 were associated with worse performance on three behavioral and self-report measures of cognitive functioning. Higher depressive symptoms also were prospectively associated with elevated IL-6 and both higher depressive symptoms and a higher BMI predicted worse future executive functioning via increased IL-6.
Conclusions:
More severe depressive symptoms and increased BMI may disrupt executive functioning via elevated IL-6.
Introduction
Depression is associated with the greatest disease burden among all psychological disorders (Whiteford et al., 2013). The severe disease burden is largely attributable to the early onset, frequent recurrence, and substantial impairment associated with depressive disorders (Kessler et al., 2003, Burcusa and Iacono, 2007). Cognitive dysfunction is a clinical feature of depression, observed in both clinical and community samples (Rock et al., 2013, Snyder, 2013, Mac Giollabhui et al., 2018), that contributes substantially to functional impairment in depression, both in individuals who meet criteria for a depressive episode as well as those with remitted or subclinical depressive symptoms (Gotlib et al., 1995, Woo et al., 2016). Thus, understanding cognitive dysfunction in depression will advance our understanding of the overall course and etiology of depression as well as providing insight on a substantial contributor of functional impairment
Dysfunction across a broad range of cognitive functions, such as verbal memory and executive functioning, is a reliable correlate of depression in children, adolescents and adults (Rock et al., 2013, Snyder, 2013, Wagner et al., 2015). However, it also has been observed prior to first onset, in unaffected first-degree relatives of depressed individuals, and when depression has remitted (Rock et al., 2013, Scult et al., 2017, MacKenzie et al., 2018). Thus, it remains unclear whether, in addition to being a correlate of depression, cognitive dysfunction is a risk factor for depression, a consequence of depression, or whether both depression and cognitive dysfunction are caused by a common underlying process (Allott et al., 2016, Mac Giollabhui et al., 2018). It is likely that the inconsistent associations between cognitive dysfunction and depression observed in the literature are, in part, driven by the effect of specific clinical characteristics (e.g., persistent depression), comorbid conditions (e.g., substance use disorder), and risk factors (e.g., chronic stress). In particular, anxiety is highly comorbid with depression and substance use disorders (Clark and Watson, 1991, Grant et al., 2015, Grant et al., 2016), is associated with elevated peripheral inflammatory activity (Michopoulos et al., 2017), has been implicated in the up-regulation of inflammatory responses in depression (Slavich and Irwin, 2014), and could explain some of the variability in the association between depression and executive functioning (Levin et al., 2007). In order to advance our understanding of who will experience cognitive dysfunction in depression and under which conditions, a more mechanistic understanding is required (Carvalho et al., 2014).
Two putative mechanisms underpinning the association of depression and cognitive dysfunction are inflammation and body mass index (BMI). Sickness behaviors (e.g., anhedonia/social withdrawal) are common correlates of heightened inflammation as well as core characteristics of depression (Maes et al., 1995); indeed, when an inflammatory response is experimentally induced, depressive symptoms reliably follow (Dantzer, 2001). Meta-analyses report that depressed patients – or, at least, a subsample of depressed patients – exhibit consistently elevated inflammatory biomarkers, including: interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-α), and C-reactive protein (CRP) (Raison and Miller, 2011, Haapakoski et al., 2015, Kohler et al., 2017). However, the direction of the relationship between depression and inflammation also remains unclear, with some studies reporting that elevated inflammation prospectively predicts higher depressive symptoms (Stewart et al., 2009, Au et al., 2015, Moriarity et al., In Press) and others the reverse (Duivis et al., 2013, Zalli et al., 2016). Higher BMI also is associated with increased risk of depression across the lifespan (de Wit et al., 2010, Quek et al., 2017), however, as is the case with inflammation, the temporal relationship between obesity and depression remains unclear, with some evidence for a bidirectional association (Preiss et al., 2013).
There is considerable evidence linking both inflammation and obesity with cognitive dysfunction. Inflammatory biomarkers, particularly IL-6, IL-1beta, and TNF-α, disrupt neuronal processes (e.g., long-term potentiation, synaptic plasticity, and neurogenesis) and negatively impact learning, memory, and executive functioning (Reichenberg et al., 2001, McAfoose and Baune, 2009, Carvalho et al., 2014, Shields et al., 2017). Similarly, obesity is reliably associated with poor cognitive performance in children, adolescents, and adults (Liang et al., 2014, Prickett et al., 2015). Given that adipose tissue also can secrete cytokines and contributes to levels of inflammatory cytokines in systemic circulation, the relationship between obesity and cognition is thought to be mediated, at least in part, by inflammatory processes (Spyridaki et al., 2016). Although few studies have examined this longitudinally, prior research has reported that higher adiposity predicts impaired cognitive performance via elevated peripheral inflammation (Spyridaki et al., 2014).
Executive functioning refers to cognitive abilities needed to effortfully guide behavior toward a goal, especially in non-routine situations (Banich, 2009). Although consensus is lacking on a specific model of executive functioning (Friedman et al., 2008, Miyake and Friedman, 2012, Diamond, 2013), there is general consensus that it involves multiple cognitive processes, including inhibition, working memory, set-shifting, and planning (Packwood et al., 2011). Executive dysfunction is reliably observed during depressive episodes and when depression is in remission (Rock et al., 2013, Snyder, 2013); it is also associated with increased peripheral inflammation (Chang et al., 2012, Heringa et al., 2014, Huang et al., 2016) and BMI (Smith et al., 2011, Yang et al., 2018). However, no study to date has tested whether peripheral inflammatory cytokines or BMI are predictors of worse executive function in depression, particularly in a diverse community sample of largely unmedicated adolescents, who are less likely to be afflicted by some of the confounding/moderating factors in older adults (e.g., repeated depressive episodes, pre-clinical dementia-related cognitive decline (Misiak et al., 2018).
The Present Study
We used a structural equation model (SEM) framework to simultaneously examine the concurrent and prospective associations between peripheral inflammatory biomarkers, BMI, depressive symptoms, and executive functioning in a diverse sample of adolescents. These measures were obtained across three timepoints, thereby assessing if inflammation and BMI are two processes that can explain the association between depression and impaired executive functioning over time. Two measures of executive functioning (selective attention, switching attention) and one self-report questionnaire assessing future orientation, a construct reliant on executive functioning (D’Argembeau et al., 2010), were administered. It was hypothesized that a higher BMI would predict more severe depressive symptoms and worse cognitive functioning via elevated peripheral inflammatory activity. Although five inflammatory biomarkers were assayed (IL-6, IL-8, IL-10, TNF-α, and CRP), the main analyses primarily focus on IL-6 because it is the inflammatory cytokine most reliably associated with BMI (Amaral et al., 2015), depression, and executive dysfunction in humans (Kohler et al., 2017). Supplemental analyses investigated whether i) associations observed for depressive symptoms also were present for anxiety and substance use, ii) results differed for TNF-α, CRP, IL-8, and IL-10, iii) results were affected by sex and race, and iv) whether the reported results held after controlling for additional confounds.
Methods
Participants
Participants were drawn from a prospective, longitudinal study of adolescent-onset depression. A diverse community sample of 642 adolescents aged 12–13 years and their primary female caregivers were recruited from the Philadelphia area for the Adolescent Cognition and Emotion (ACE) Study. Inclusion criteria for the original study were: adolescents aged 12–13 years and their mothers were willing to participate, and that adolescents self-identified as Caucasian, African-American, or biracial (examining racial differences in depression was a goal of ACE). Exclusion criteria included if the adolescent/mother had insufficient English reading/speaking skills to complete assessments or a psychotic, developmental, or learning disorder.
From the 642 adolescents initially enrolled in the study, 307 agreed to participate in a supplementary section of the study, introduced four years after initial enrollment, to assess peripheral inflammation. From the 307 participants with blood assayed to date, IL-6 data were available for: 91 individuals at one assessment, 73 at two assessments, 73 at three assessments, 54 at four assessments, and 16 at five or six assessments. Following enrollment in this supplementary section of the study, the 307 participants took part in an average of 4.03 assessments (SD = 2.73) per person, with blood draws at 3.01 of these assessments (SD = 1.20), totaling 753 blood samples. From the 753 blood samples, 151 observations were removed either because CRP values were > 10 (indicative of a possible acute infection), participants reported a pertinent medical condition (autoimmune disease, diabetes, blood-clotting disorder), were pregnant at time of blood draw, or the observation at which the blood was drawn represented an individual’s fourth or fifth blood draw, leaving a total of 288 participants with 602 observations; time to first follow-up was 1.39 years (SD = .61) and to second follow-up was 1.01 years (SD = .51). Observations occurring at the fourth or subsequent blood draw were not included in analyses because of the small number of participants who had completed this number of blood collections.
Missing data analyses tested whether the analytic sample differed significantly from the complete ACE sample. The analytic sample did not differ significantly from the complete sample in the proportion of females, . However, the racial composition of the analytic sample differed significantly from what was expected based on the complete ACE sample, . There were fewer Caucasians present in the analytic sample (Standardized Residual = −1.6) and more African-Americans (Standardized Residual = 1.5) than expected. Similarly, a trend was observed for socioeconomic status (SES), , with fewer participants of low SES present in the analytic sample than anticipated (Standardized Residual = −1) and more participants not of low SES (Standardized Residual = 1).
Measures
Depressive Symptoms.
The Children’s Depression Inventory (CDI) is a valid, reliable self-report measure of current depressive symptoms in youth (Kovacs, 1992). The CDI consists of 27 items scored on a three-point scale ranging from zero to two. Items were summed, with higher scores indicating more severe depressive symptoms. The CDI was administered at every timepoint in the study and demonstrated consistent reliability; Cronbach’s α over time was consistently > .80.
Executive Attention.
Four subtests of the Test of Everyday Attention for Children (TEAch) and the Test of Everyday Attention (TEA) were administered assessing switching, sustained, divided and selective attention (Robertson et al., 1994, Manly et al., 2001). However, given the inadequate reliability of sustained and divided attention when transitioning from TEAch to the TEA and the focus of this study on executive functioning, only behavioral measures of selective and switching attention were used (Mac Giollabhui et al., 2018). Scaled scores, where a score of 10 is indicative of performance in the 50th percentile (SD = 3), are reported for each domain of attention, with higher scores indicating superior performance. The ‘Sky Search’ subtest is a non-linguistic measure of selective attention that controls for motor speed (in the TEAch), in which participants were asked to identify cases in which identical stimuli are paired together on a page. Different stimuli were used for the TEA and TEAch to improve the ecological validity for their respective age groups. Both speed and accuracy are encouraged and the outcome is based both on the participant’s ability to correctly identify targets and the speed with which they complete the task. Attentional switching is assessed in the TEAch using the ‘Creature Counting’ subtest and in the TEA using the ‘Elevator Counting’ subtest. Both tests measure the temporary slowing that is associated with switching from one task or mental set to another. Separate scores are computed for the speed and accuracy with which participants complete all test items. Both the TEA and TEAch are ecologically valid measures of attentional functioning that are extensively used in both research and clinical settings with demonstrable validity and reliability (Robertson et al., 1994, 1996, Manly et al., 2001).
Future Orientation.
The Future Orientation Scale (FOS) is a reliable and valid measure of the degree to which adolescents tend to perceive, anticipate, and plan for the future (Steinberg et al., 2009). Participants are presented with a series of contrasting statements with the word “BUT” between them (i.e., “Some people like to think about all the possible good and bad things that can happen before making a decision” BUT “Other people don’t think it’s necessary to think about every little possibility before making a decision”) and are asked to select the statement that best describes them. They then are asked to indicate whether the selected descriptor was really true or sort of true. Responses for each pair of statements then were coded on a 4-point Likert scale, ranging from really true for one descriptor to really true for the contrasting descriptor. The internal consistency of this measure in the current sample was adequate with Cronbach’s alpha ranging from .71-.82 across assessments.
Inflammation.
Blood (10 mL) was obtained primarily in the late afternoon to control for diurnal variation and collected via antecubital venipuncture by a certified phlebotomist. The blood was centrifuged to separate the plasma fraction (BD Hemogard with K2 EDTA) and stored at −80°C until the day of assay. Medication use, medical disorder status, time of last meal, time of day and participant’s BMI were recorded at each blood draw. Cytokines were quantified by multi-cytokine array (IL-6, IL-8, IL-10 and TNF-α) and high-sensitivity CRP (hsCRP) determined via singleplex assay using an electrochemiluminescence platform and a QuickPlex SQ 120 imager for analyte detection (Meso Scale Discovery, Gaithersburg, MD). The analytes were run in duplicate, with intra-assay coefficients varying from 1.94–4.38%. Values were referenced to a standard curve generated from seven calibrators with known concentrations. The lower limit of detection for cytokines was 0.1 pg/mL, with a dynamic range up to 2000 pg/mL. However, hsCRP is present in sera at higher concentrations, and thus, plasma was diluted to correspond to the standard curve. Values were converted to mg/L units and were quantified down to 0.1 mg/L (Breen et al., 2011).
Body Mass Index.
BMI was quantified as weight (kg), determined with an electronic scale, divided by height.
Demographics.
Age, sex, race, and SES were assessed via self-report. Mothers indicated whether their child received federally-subsidized school lunch, with receipt of free lunch assumed to indicate low SES.
Procedure
Participants completed two types of assessments. The first type was a comprehensive assessment scheduled to occur annually. During comprehensive assessments, participants completed the CDI, FOS, and interviewers conducted a behavioral assessment of attentional functioning (TEAch/TEA). Shorter six-month assessments also were scheduled in which participants completed measures of negative life events and the CDI assessing depressive symptoms. Blood draws could occur at either annual or six-month assessments.
Data Analysis
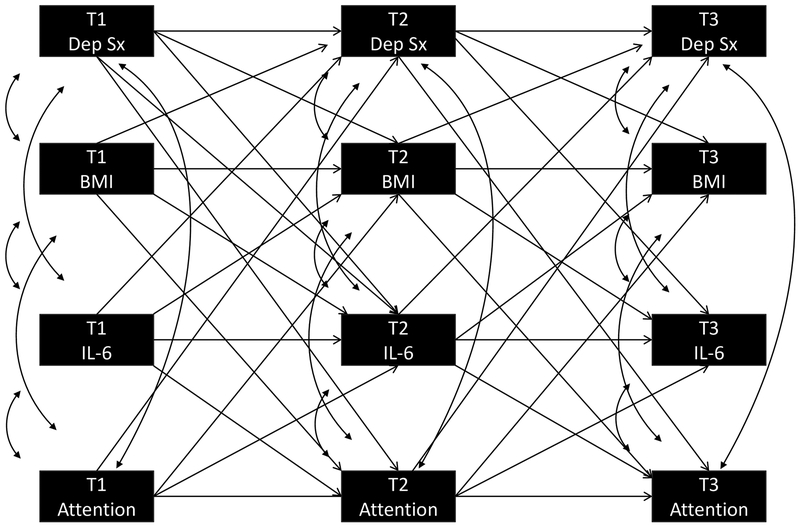
Analyses were conducted in Mplus (Version 7.4) and missing data were handled using Full Information Maximum Likelihood. Path analysis within a SEM framework simultaneously examined concurrent and prospective relationships between IL-6, BMI, depressive symptoms, and four measures of executive/cognitive functioning. All pathways are graphically presented in Figure 1, which consist of all concurrent, prospective, and auto-regressive pathways among the four variables in each model. Models assumed that the relationship between variables across all timepoints were equivalent (i.e., the relationship between Time 1 depression and Time 2 inflammation was the same as Time 2 depression and Time 3 inflammation) and, therefore, equality constraints were applied to associations between the same variables across different timepoints. Although sex and race were variables of theoretical interest given their association with BMI, inflammation, and depression, they were not included in analyses to limit the number of parameters being simultaneously estimated by the model. Model fit was estimated using: chi-square estimate of goodness of fit, comparative fit index (CFI), and root-mean-square error of approximation (RMSEA). The Chi-Square test of model fit was reported according to convention, but not interpreted given its limited utility in large samples (Cheung and Rensvold, 2002, Chen, 2007). For the CFI, “good” fit is indicated by a value >.90 and “excellent” fit by a value >.95. A RMSEA statistic between .05 and .10 is indicative of “good” fit and a value ≤.05 is indicative of “excellent” fit (Schermelleh-Engel et al., 2003). Finally, indirect pathways were tested, based on 5000 bootstrapped samples, where significant associations between variables of interest were observed. Supplemental analyses also investigated whether i) findings from the model predicting depressive symptoms also were evident when predicting anxiety symptoms and substance use, ii) the model would differ if different biomarkers was considered as predictors (i.e., TNF-α, CRP, IL-8, and IL-10), iii) the results were different if sex and race were considered, and finally iv) if the significance of the model was affected by considering several factors known to influence the predictor and outcome variables – complete details are provided as supplementary material.
Figure 1.
Path Analysis Examining Association of Depressive Symptoms, Cognitive Functioning, IL-6 and BMI.
T = Time; Dep Sx = Depressive Symptoms; BMI = Body Mass Index; IL-6 = Interleukin-6
Results
Bivariate correlations for the main study variables are presented in Table 1 for the 288 participants aged 16.34 (SD = 1.44) who were present at first blood draw. Four SEM-based path analyses were conducted for each of the following four cognitive variables sequentially considered as outcomes: selective attention, switching attention (timing), switching attention (accuracy), and future orientation.
Table 1.
Descriptive statistics and bivariate correlations of study variables for 288 participants at Time 1
| Measure | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1: T1 Age | −.01 | −.10 | −.02 | −.08 | −.10 | −.09 | −.07 | −.01 | −.23** | .10 |
| 2: Female | - | −.03 | −.01 | .10 | .14* | .25*** | −.08 | .09 | .04 | .02 |
| 3: Race | - | .40*** | .07 | .01 | .11 | .02 | −.04 | −.05 | −.22*** | |
| 4: Low SES | - | .02 | .13* | .06 | .11 | −.11 | −.17* | −.21** | ||
| 5: T1 BMI | - | .09 | .33*** | −.02 | −.07 | −.06 | −.10 | |||
| 6: T1 CDI | - | −.01 | −.01 | −.04 | −.09 | .06 | ||||
| 7: T1 IL6 | - | −.15* | −.06 | .12 | −.18* | |||||
| 8: T1 FOS | - | −.02 | −.05 | .13 | ||||||
| 9: T1 ATT | - | .15* | .17* | |||||||
| 10: T1 CCT | - | .21** | ||||||||
| 11: T1 CCA | - | |||||||||
| Mean | .51 | .59 | .46 | 23.77 | 7.23 | .55 | 2.89 | 10.59 | 9.25 | 10.00 |
| (SD) | .50 | .50 | .50 | 5.87 | 5.86 | .33 | .47 | 3.54 | 3.56 | 2.96 |
T1 Age=Age at baseline; ; SES=Socioeconomic status; Race: 1=African-American; BMI=Body Mass Index; CDI=Children’s Depression Inventory; FOS =Future Orientation Scale; T1: Time 1; ATT=TEA or TEAch Selective Attention; CCT=TEA or TEAch Switching Attention Timing; CCA=TEA or TEAch Switching Attention Accuracy
Probability
=p<.05;
= p<.01;
= p<.001
Model Fit
Model fit statistics are provided for each of the four models in Table 2. The Chi-Square Test of Model Fit was statistically significant, indicating that the observed data differed significantly from the expected for this model. For each of the models, both the CFI (>.90) and the RMSEA (≥.05 and ≤.10), indicated adequate model fit.
Table 2.
Model Fit For Each of the Four SEM Models
| Selective Attention | Switching Attention (Timing) | Switching Attention (Accuracy) | Future Orientation | |
|---|---|---|---|---|
| Chi-Squared Test of Model Fit | 110.43*** | 122.07*** | 112.39*** | 116.88*** |
| Comparative Fit Index | .93 | .92 | .92 | .92 |
| Root Mean Square Error of Approximation (95% CI) | .07(.05–.09) | .07(.06–.09) | .07(.05–.09) | .07(.06–.09) |
Probability
=p<.05;
= p<.01;
= p<.001
Concurrent and Prospective Associations between Depressive Symptoms, Cognitive Functioning, IL-6, and Body Mass Index
All of the pathways tested in the four models are presented in Figure 1. The pathways presented in the models can divided into three conceptual components: concurrent, prospective, and auto-regressive associations.
All pathways are reported in Table 3; a number of statistically significant patterns were evident in the results. Concurrent associations represent the partial correlation of any two variables constrained to be equal across all timepoints. Across all timepoints, higher levels of IL-6 were associated with worse selective attention, switching attention (accuracy), and future orientation. Higher depressive symptoms and higher BMI both were concurrently associated with worse switching attention (timing and accuracy, respectively). Across all models, higher BMI was concurrently associated with higher IL-6. Similarly, a number of significant findings were evident in the prospective associations of depressive symptoms, cognitive functioning, IL-6, and BMI. First, higher BMI prospectively predicted worse selective attention and switching attention (accuracy). Second, across all models, higher BMI predicted both more depressive symptoms and a higher level of IL-6. Finally, higher depressive symptoms prospectively predicted higher IL-6.
Table 3.
Concurrent and Prospective Associations For Each of the Four SEM Models
| Selective Attention | Switching Attention (Timing) | Switching Attention (Accuracy) | Future Orientation | |
|---|---|---|---|---|
| Concurrent Associations | ||||
| IL6 w/ COG | −.15* | .00 | −.13* | −.13* |
| BMI w/ COG | −.07 | −.10 | −.11* | −.05 |
| CDI w/ COG | −.03 | −.13* | .02 | −.03 |
| BMI w/ CDI | .10Ψ | .08Ψ | .08 | .08Ψ |
| IL6 w/ CDI | .01 | .00 | .01 | .00 |
| IL6 w/ BMI | .15*** | 15*** | .15*** | .16*** |
| Prospective Associations | ||||
| IL6 predicting to COG | .10 | −.05 | .02 | .01 |
| BMI predicting to COG | −.17** | −.08 | −.20** | −.03 |
| CDI predicting to COG | −.01 | −.12Ψ | .02 | −.08 |
| BMI predicting to CDI | .10* | .09Ψ | .11* | .10* |
| IL6 predicting to CDI | .02 | .01 | .02 | .01 |
| COG predicting to CDI | .05 | −.06 | .06 | −.02 |
| BMI predicting to IL6 | .33*** | .33*** | .33*** | .33*** |
| CDI predicting to IL6 | .10* | .10* | .11* | .10* |
| CDI predicting to BMI | −.05 | −.02 | −.02 | −.02 |
IL6=Interleukin-6; COG=Cognitive variable of interest; BMI=Body Mass Index; CDI=Children’s Depression Inventory
Probability:
=p<.10;
=p<.05;
= p<.01;
= p<.001
Indirect Effects: Body Mass Index on Executive Functioning via IL-6
Given the prospective associations observed between BMI and cognition, additional analyses were conducted using 5000 bootstrapped samples to test whether higher BMI predicted worse cognition indirectly via increased IL-6 levels in circulation, thereby determining whether baseline BMI predicted levels of circulating IL-6 in the future, which, in turn, predicted concurrent executive functioning. Indirect pathways via IL-6 were found when predicting to future orientation (b = −.02: 95% CI = −.001, −.05) and selective attention (b = −.21: 95% CI = −.07, −.41). No indirect pathway was observed for switching attention timing (b = −.01: 95% CI = −.15, .15) or accuracy (b = −.14: 95% CI = −.31, .001).
Given the prospective association of depression and IL-6, an indirect pathway also was tested to determine the influence of depression on cognitive functioning via inflammatory activity. Significant indirect effects were evident, including the effect of depressive symptoms on selective attention (b = −.06: 95% CI = −.02, −.17), switching attention (accuracy) (b = −.04: 95%CI = −.002, −.13), and future orientation (b = −.01: 95%CI = −.0001, −.02) via IL-6, but not for switching attention (timing) (b = −.01: 95%CI = −.05, .05). No indirect effects of BMI on prospective depressive symptoms via IL-6 were obtained.
Supplemental Analyses
Complete details on a series of additional analyses can be found in supplementary material. This statistical testing indicated that the effects reported above were specific to depressive symptomatology, and do not generalize to the future experience of anxiety symptoms nor to the initiations of substance use (see Supplementary Tables 1–2). The effects observed for IL-6 were not observed consistently in models examining TNF-α, CRP, IL-8, and IL-10, however similar associations were observed inconsistently; for example, higher levels of TNF-α were significantly associated with worse future orientation but not executive functioning (see Supplementary Tables 3–6). Finally, the conclusions did not change substantially when participant sex or ethnicity were considered, and the results held when additional control variables were added to the model (see Supplementary Table 7–10).
Discussion
In a diverse community sample of urban adolescents assessed annually over three years, we found that a higher BMI predicted increased future depressive symptoms as well as worse executive functioning/future orientation via higher levels of IL-6 in systemic circulation. Depressive symptoms also predicted an increase in peripheral IL-6. Significantly, all observed associations were unique to depressive symptoms and were not observed in models of anxious symptoms or substance use. Thus, these results suggest that higher depressive symptoms and greater BMI are two distinct pathways leading to worse cognition via a shared pathway of increased IL-6.
More severe depressive symptoms were concurrently and prospectively (trend-level) associated with worse switching attention and, moreover, prospectively predicted worse selective attention, switching attention (accuracy), and future orientation via elevated peripheral IL-6. The direct concurrent and prospective associations between depression and switching attention have been observed in analyses examining the entire ACE sample (Mac Giollabhui et al., 2018) and are consistent with previous papers reporting that depression is concurrently and prospectively associated with worse executive functioning (Rock et al., 2013, Snyder, 2013). A number of previous studies have shown that inflammation is associated with concurrent and prospective impairment across a number of different cognitive functions (Chang et al., 2012, Goldsmith et al., 2016). Studies that included control groups found that increased inflammation predicted worse cognition irrespective of diagnostic status (Krogh et al., 2014). Thus, depressed individuals experiencing chronic inflammatory activity may be more likely to experience disrupted cognitive functioning (Raison and Miller, 2011). Should inflammation be linked with cognitive dysfunction in a subset of depressed individuals, this may further explain the considerable heterogeneity in cognitive dysfunction observed in depression (Snyder, 2013).
Inflammation is both concurrently and prospectively associated with impaired cognition in medical and community samples (Reichenberg et al., 2001, Baune et al., 2008, Jenny et al., 2012, Li et al., 2014, Singh-Manoux et al., 2014, Huang et al., 2016), although there is considerable heterogeneity in the cognitive domains affected as well as the cytokines implicated. This study also found that IL-6 activity was associated with worse executive functioning/future orientation in a healthy adolescent sample. Supplementary analyses suggest that the association between cognitive functioning and inflammation is largely specific to IL-6 (of the cytokines assayed); it is unclear whether this finding will be replicated, however, animal research also has reported that the inflammatory effect on executive functioning is specific to IL-6 (Sparkman et al., 2006). It also should be kept in mind that IL-6 is pleiotropic with multiple origins, particularly adipocytes, and thus, peripheral assessment of IL-6 is not solely a measure of immune system activation. There was no evidence of a direct prospective association between inflammation and executive functioning; this may be due to the concurrent modelling of BMI alongside inflammation in all models. Instead, this study suggested that higher BMI prospectively predicts worse cognitive functioning via increased peripheral IL-6. Higher BMI has been consistently associated with a generalized pattern of cognitive dysfunction, including executive dysfunction, across the lifespan (Laitala et al., 2011, Liang et al., 2014, Prickett et al., 2015, Yang et al., 2018) and these findings support previous research hypothesizing that inflammation may be the mechanism underpinning an association between high BMI and cognitive dysfunction (Spyridaki et al., 2014, Spyridaki et al., 2016). There is considerable interest in understanding how BMI and poor cognitive functioning are related, with physical activity and poor diet quality identified as two putative mechanisms (Pistell et al., 2010, Esteban-Cornejo et al., 2015). Further research is needed to identify the mechanisms by which BMI leads to worse cognitive functioning in humans.
A concurrent association between inflammation and depression was not observed; however, higher depressive symptoms prospectively predicted higher IL-6 (supplementary analyses also observed that higher baseline TNF-α predicted increased future depressive symptoms). There is a relatively established body of research linking acute inflammation with depression (Maes et al., 1995, Dantzer, 2001) as well as evidence of a bi-directional association between inflammation and depression (Messay et al., 2012). However, it is likely that elevated inflammation only characterizes a sub-group of depressed individuals, and further, the majority of studies have been conducted in adult clinical samples, rather than an adolescent community sample where significantly lower levels of depression are observed (Kohler et al., 2017). Additionally, once control variables were introduced, this prospective association disappeared, which may point to a confounding factor (e.g., gender) that is underpinning the increases in both inflammation and depressive symptoms. Thus, it may be that the current sample is not sufficiently powered to observe an effect that is only present in a sub-sample of individuals with depression, or that a concurrent association between depression and inflammation is only present in individuals meeting criteria for MDD (Raison and Miller, 2011, Kohler et al., 2017).
These results should be interpreted in light of the limitations of this study. Inflammation was based on a single sera assessment and variability in food intake, medication status, and diurnal variation may decrease the precision of our estimates, although significant confounds (e.g., infection) were addressed. Although established measures of attentional functioning, the TEA/TEAch are not normed on large US adolescent samples. Thus, it is possible that the normed scores used in this study do not accurately capture age- and gender-normed performance. Moreover, it is unclear whether these results generalize to a clinical sample of depressed individuals, given that assessment relied on self-report questionnaires. Although the ACE sample is notable for its racial, gender, and socioeconomic diversity, the analytic sample was less likely to include Caucasian participants and participants of low SES compared to the entire ACE sample, which could potentially influence the generalizability of these findings. It should be noted that the SEM models presented in this paper were complex, and required estimation of a large number of parameters, which, even for a sizeable sample, increased the risk that the parameters estimated were biased. These limitations are mitigated by: direct and repeated measurement of peripheral inflammatory markers in a large, diverse cohort where cognitive functioning was assessed using reliable instruments across multiple modalities.
Conclusions
This study provides strong evidence that a higher BMI may index a pathway where higher levels of body fat lead to depression and cognitive dysfunction in adolescence. It also highlights that an inflammatory cytokine, IL-6, may play a mechanistic role linking higher BMI with impaired cognitive functioning. Moreover, it may point to a sub-type of depression that is characterized by both metabolic dysregulation and cognitive dysfunction. Importantly, these relationships were found to be largely specific to depression, rather than anxiety, and the significance of the statistical modeling held even when considering several potential confounding factors. Future research should investigate systematically whether BMI and IL-6 are associated with executive functioning impairment alone using established theoretically-based models of executive functioning.
Supplementary Material
Acknowledgements
None.
Financial support
This research was supported by National Institute of Mental Health Grants MH079369 and MH101168 awarded to Lauren B. Alloy and National Institute of Mental Health Grant MH096478 awarded to Lauren Ellman.
Footnotes
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Contributor Information
Naoise Mac Giollabhui, Department of Psychology, Temple University.
Dominika Swistun, Department of Psychology, University of Wisconsin-Madison
Susan Murray, Department of Psychology, Temple University
Daniel P Moriarity, Department of Psychology, Temple University
Marin M Kautz, Department of Psychology, Temple University
Lauren M Ellman, Department of Psychology, Temple University
Thomas M Olino, Department of Psychology, Temple University
Christopher L Coe, Department of Psychology, University of Wisconsin-Madison
Lyn Y Abramson, Department of Psychology, University of Wisconsin-Madison
Lauren B Alloy, Department of Psychology, Temple University
References
- Allott K, Fisher CA, Amminger GP, Goodall J & Hetrick S (2016). Characterizing neurocognitive impairment in young people with major depression: state, trait, or scar? Brain and Behavior 6, e00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral WZ, Krueger RF, Ryff CD & Coe CL (2015). Genetic and environmental determinants of population variation in interleukin-6, its soluble receptor and C-reactive protein: insights from identical and fraternal twins. Brain, Behavior, and Immunity 49, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au B, Smith KJ, Gariepy G & Schmitz N (2015). The longitudinal associations between C-reactive protein and depressive symptoms: evidence from the English Longitudinal Study of Ageing (ELSA). International Journal of Geriatric Psychiatry 30, 976–84. [DOI] [PubMed] [Google Scholar]
- Banich MT (2009). Executive function. The search for an integreated account. Current Directions in Psychological Science 18, 89–94. [Google Scholar]
- Baune B, Ponath G, Golledge J & Varga G (2008). Association between IL-8 cytokine and cognitive performance in an elderly general population—the MEMO-Study. Neurobiology of Aging 29, 937–44. [DOI] [PubMed] [Google Scholar]
- Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, Dibben O, Margolick JB, Bream JH, Sambrano E, Martinez-Maza O, Sinclair E, Borrow P, Landay AL, Rinaldo CR & Norris PJ (2011). Multisite comparison of high-sensitivity multiplex cytokine assays. Clinical and Vaccine Immunology 18, 1229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcusa SL & Iacono WG (2007). Risk for recurrence in depression. Clinical Psychology Review 27, 959–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Miskowiak KK, Hyphantis TN, Kohler CA, Alves GS, Bortolato B, PM GS, Machado-Vieira R, Berk M & McIntyre RS (2014). Cognitive dysfunction in depression - pathophysiology and novel targets. CNS & Neurological Disorders Drug Targets 13, 1819–35. [DOI] [PubMed] [Google Scholar]
- Chang HH, Lee IH, Gean PW, Lee SY, Chi MH, Yang YK, Lu RB & Chen PS (2012). Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain Behavior and Immunity 26, 90–5. [DOI] [PubMed] [Google Scholar]
- Chen FF (2007). Sensitivity of Goodness of Fit Indexes to Lack of Measurement Invariance. Structural Equation Modeling: A Multidisciplinary Journal 14, 464–504. [Google Scholar]
- Cheung GW & Rensvold RB (2002). Evaluating Goodness-of-Fit Indexes for Testing Measurement Invariance. Structural Equation Modeling: A Multidisciplinary Journal 9, 233–255. [Google Scholar]
- Clark LA & Watson D (1991). Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology 100, 316. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ortoleva C, Jumentier S & Van der Linden M (2010). Component processes underlying future thinking. Memory & Cognition 38, 809–819. [DOI] [PubMed] [Google Scholar]
- Dantzer R (2001). Cytokine-induced sickness behavior: where do we stand? Brain Behavior and Immunity 15, 7–24. [DOI] [PubMed] [Google Scholar]
- de Wit L, Luppino F, van Straten A, Penninx B, Zitman F & Cuijpers P (2010). Depression and obesity: A meta-analysis of community-based studies. Psychiatry Research 178, 230–235. [DOI] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology 64, 135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duivis HE, Vogelzangs N, Kupper N, de Jonge P & Penninx BW (2013). Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology 38, 1573–85. [DOI] [PubMed] [Google Scholar]
- Esteban-Cornejo I, Tejero-Gonzalez CM, Sallis JF & Veiga OL (2015). Physical activity and cognition in adolescents: A systematic review. Journal of Science and Medicine in Sport 18, 534–539. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP & Hewitt JK (2008). Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General 137, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Haroon E, Woolwine BJ, Jung MY, Wommack EC, Harvey PD, Treadway MT, Felger JC & Miller AH (2016). Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behavior and Immunity 56, 281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Lewinsohn PM & Seeley JR (1995). Symptoms versus a diagnosis of depression: differences in psychosocial functioning. Journal of Consulting and Clinical Psychology 63, 90. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B & Hasin DS (2015). Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, Zhang H, Smith SM, Pickering RP, Huang B & Hasin DS (2016). Epidemiology of DSM-5 Drug Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions–III. JAMA Psychiatry 73, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H & Kivimäki M (2015). Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behavior and Immunity 49, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringa S, Van den Berg E, Reijmer Y, Nijpels G, Stehouwer C, Schalkwijk C, Teerlink T, Scheffer P, van den Hurk K & Kappelle L (2014). Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population–the Hoorn study. Psychoneuroendocrinology 40, 108–118. [DOI] [PubMed] [Google Scholar]
- Huang YS, Guilleminault C, Hwang FM, Cheng C, Lin CH, Li HY & Lee LA (2016). Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine 95, e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, Ding J, Fried LP, Kritchevsky SB, Rifkin DE, Sarnak MJ & Newman AB (2012). Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. The Journals of Gerontology: Series A 67, 970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE & Wang PS (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289, 3095–105. [DOI] [PubMed] [Google Scholar]
- Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL & Carvalho AF (2017). Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatrica Scandinavica 135, 373–387. [DOI] [PubMed] [Google Scholar]
- Kovacs M (1992). The Children’s Depression Inventory. Multi-Health Systems, Inc.: North Tonawanda, NY. [Google Scholar]
- Krogh J, Benros ME, Jørgensen MB, Vesterager L, Elfving B & Nordentoft M (2014). The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behavior and Immunity 35, 70–76. [DOI] [PubMed] [Google Scholar]
- Levin RL, Heller W, Mohanty A, Herrington JD & Miller GA (2007). Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research 31, 211–233. [Google Scholar]
- Li X, Robertson CM, Yu X, Cheypesh A, Dinu IA & Li J (2014). Early postoperative systemic inflammatory response is an important determinant for adverse 2-year neurodevelopment-associated outcomes after the Norwood procedure. The Journal of Thoracic and Cardiovascular Surgery 148, 202–6. [DOI] [PubMed] [Google Scholar]
- Liang J, Matheson BE, Kaye WH & Boutelle KN (2014). Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. International Journal of Obesity 38, 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N, Olino TM, Nielsen J, Abramson LY & Alloy LB (2018). Is Worse Attention a Risk Factor for or a Consequence of Depression, or Are Worse Attention and Depression Better Accounted for by Stress? A Prospective Test of Three Hypotheses. Clinical Psychological Science, 2167702618794920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie LE, Uher R & Pavlova B (2018). Cognitive Performance in First-Degree Relatives of Individuals With vs Without Major Depressive Disorder: A Meta-analysis. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R & Desnyder R (1995). Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. Journal of Affective Disorders 34, 301–9. [DOI] [PubMed] [Google Scholar]
- Manly T, Anderson V, Nimmo-Smith I, Turner A, Watson P & Robertson IH (2001). The differential assessment of children’s attention: The Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. Journal of Child Psychology and Psychiatry 42, 1065–1081. [DOI] [PubMed] [Google Scholar]
- McAfoose J & Baune BT (2009). Evidence for a cytokine model of cognitive function. Neuroscience and Biobehavioral Reviews 33, 355–366. [DOI] [PubMed] [Google Scholar]
- Messay B, Lim A & Marsland AL (2012). Current understanding of the bi-directional relationship of major depression with inflammation . Biology of Mood & Anxiety Disorders 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ & Jovanovic T (2017). Inflammation in fear-and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak B, Beszlej JA, Kotowicz K, Szewczuk-Boguslawska M, Samochowiec J, Kucharska-Mazur J & Frydecka D (2018). Cytokine alterations and cognitive impairment in major depressive disorder: From putative mechanisms to novel treatment targets. Progress in Neuro-Psychopharmacology & Biological Psychiatry 80, 177–188. [DOI] [PubMed] [Google Scholar]
- Miyake A & Friedman NP (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions In Psychological Science 21, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Mac Giollabhui N, Ellman LM, Klugman J, Coe C, Abramson LY & Alloy LB (In Press). Inflammatory biomarkers differentially predict change in depressive symptoms over time in male and female adolescents. Clinical Psychological Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packwood S, Hodgetts HM & Tremblay S (2011). A multiperspective approach to the conceptualization of executive functions. Journal of Clinical and Experimental Neuropsychology 33, 456–470. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK & Bruce-Keller AJ (2010). Cognitive impairment following high fat diet consumption is associated with brain inflammation. Journal of Neuroimmunology 219, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss K, Brennan L & Clarke D (2013). A systematic review of variables associated with the relationship between obesity and depression. Obesity Reviews 14, 906–18. [DOI] [PubMed] [Google Scholar]
- Prickett C, Brennan L & Stolwyk R (2015). Examining the relationship between obesity and cognitive function: a systematic literature review. Obesity Research & Clinical Practice 9, 93–113. [DOI] [PubMed] [Google Scholar]
- Quek YH, Tam WWS, Zhang MWB & Ho RCM (2017). Exploring the association between childhood and adolescent obesity and depression: a meta-analysis. Obesity Reviews 18, 742–754. [DOI] [PubMed] [Google Scholar]
- Raison CL & Miller AH (2011). Is depression an inflammatory disorder? Current Psychiatry Reports 13, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A & Pollmacher T (2001). Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry 58, 445–52. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Ward T, Ridgeway V & Nimmo-Smith I (1994). The test of everyday attention: TEA Thames Valley Test Company Bury St. Edmunds, UK. [Google Scholar]
- Robertson IH, Ward T, Ridgeway V & Nimmo-Smith I (1996). The structure of normal human attention: The Test of Everyday Attention. Journal of the International Neuropsychological Society 2, 525–534. [DOI] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ & Blackwell a. D. (2013). Cognitive impairment in depression: A systematic review and meta-analysis. Psychological Medicine, 1–12. [DOI] [PubMed] [Google Scholar]
- Schermelleh-Engel K, Moosbrugger H & Müller H (2003). Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods of Psychological Research Online 8, 23–74. [Google Scholar]
- Scult MA, Paulli AR, Mazure ES, Moffitt TE, Hariri AR & Strauman TJ (2017). The association between cognitive function and subsequent depression: a systematic review and meta-analysis. Psychological Medicine 47, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Moons WG & Slavich GM (2017). Inflammation, self-regulation, and health: an immunologic model of self-regulatory failure. Perspectives on Psychological Science 12, 588–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A & Kivimaki M (2014). Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology 83, 486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM & Irwin MR (2014). From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychological Bulletin 140, 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Hay P, Campbell L & Trollor J (2011). A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obesity Reviews 12, 740–755. [DOI] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological Bulletin 139, 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL & Johnson RW (2006). Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. Journal of Neuroscience 26, 10709–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyridaki EC, Avgoustinaki PD & Margioris AN (2016). Obesity, inflammation and cognition. Current Opinion in Behavioral Sciences 9, 169–175. [Google Scholar]
- Spyridaki EC, Simos P, Avgoustinaki PD, Dermitzaki E, Venihaki M, Bardos AN & Margioris AN (2014). The association between obesity and fluid intelligence impairment is mediated by chronic low-grade inflammation. British Journal of Nutrition 112, 1724–1734. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E & Banich M (2009). Age differences in future orientation and delay discounting. Child Development 80, 28–44. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF & Kamarck TW (2009). A prospective evaluation of the directionality of the depression–inflammation relationship. Brain, Behavior, and Immunity 23, 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Müller C, Helmreich I, Huss M & Tadić A (2015). A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. European Child & Adolescent Psychiatry 24, 5–19. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJL & Vos T (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–86. [DOI] [PubMed] [Google Scholar]
- Woo YS, Rosenblat JD, Kakar R, Bahk WM & McIntyre RS (2016). Cognitive Deficits as a Mediator of Poor Occupational Function in Remitted Major Depressive Disorder Patients. Clinical Psychopharmacology and Neuroscience 14, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shields GS, Guo C & Liu Y (2018). Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neuroscience & Biobehavioral Reviews 84, 225–244. [DOI] [PubMed] [Google Scholar]
- Zalli A, Jovanova O, Hoogendijk WJ, Tiemeier H & Carvalho LA (2016). Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology 233, 1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.