Abstract
Sirtuins (Sirts) are implicated in regulating a myriad of biologic functions ranging from cell growth and metabolism to longevity. Here, we show that nuclear Sirt, Sirt6, and mitochondrial Sirt, Sirt3, regulate each other’s activity and protect the heart from developing diabetic cardiomyopathy. We found that expression of both Sirt6 and Sirt3 was reduced in cardiomyocytes treated with palmitate and in hearts of mice fed with a high-fat, high-sucrose (HF-HS) diet to develop obesity and diabetes. Conversely, whole-body overexpressing Sirt6 transgenic (Tg.Sirt6) mice were protected from developing obesity and insulin resistance when fed with the same HF-HS diet. The hearts of Tg.Sirt6 mice were also protected from mitochondrial fragmentation and decline of Sirt3, resulting otherwise from HF-HS diet feeding. Mechanistic studies showed that Sirt3 preserves Sirt6 levels by reducing oxidative stress, whereas Sirt6 maintains Sirt3 levels by up-regulating nuclear factor erythroid 2 (NF-E2)-related factor 2 (Nrf2)-dependent Sirt3 gene transcription. We found that Sirt6 regulates Nrf2-mediated cardiac gene expression in 2 ways; first, Sirt6 suppresses expression of Kelch-like ECH-associated protein 1 (Keap1), a negative regulator of Nrf2, and second, Sirt6 binds to Nrf2 and antagonizes its interaction with Keap1, thereby stabilizing Nrf2 levels in cardiomyocytes. Together, these studies demonstrate that Sirt6 and Sirt3 maintain each other’s activity and protect the heart from developing diabetic cardiomyopathy.—Kanwal, A., Pillai, V. B., Samant, S., Gupta, M., Gupta, M. P. The nuclear and mitochondrial sirtuins, Sirt6 and Sirt3, regulate each other’s activity and protect the heart from developing obesity-mediated diabetic cardiomyopathy.
Keywords: diabetes, insulin resistance, diabetic cardiomyopathy, NRF2, Keap1
Diabetes mellitus is an independent risk factor for the evolution of heart diseases. Clinical studies have suggested that the frequency of developing heart failure is 2 times greater in diabetic men and 5 times greater in diabetic women compared with age-matched controls (1). Patients with diabetes also experience worse clinical outcome following hypertension, myocardial infarction, and cardiac surgery (2, 3). The hallmarks of diabetic cardiomyopathy include insulin resistance, inflammation, cardiac hypertrophy, and fibrosis (4, 5). The molecular pathways that initiate these pathologic changes have not yet been fully understood, but it is generally believed that increased free fatty acid oxidation coupled with mitochondrial dysfunction leads to oxidative stress, and this triggers a vicious cycle that contributes to induction and progression of the disease process (6). Although no targeted therapy for treatment of diabetic cardiomyopathy is available, there is consensus that consistent exercise and calorie restriction help to mitigate consequences of diabetes and the evolution of diabetic cardiomyopathy, at least in animal models (7, 8). There is also evidence showing that these interventions provide health benefits by activating a family of enzymes called sirtuins (Sirts) (9, 10). These findings coupled with clinical reports showing that Sirt levels are reduced in patients with diabetes suggest that Sirt depletion could be part of the mechanisms involved in development of diabetic cardiomyopathy and subsequent heart failure (11, 12). However, the role of Sirts in pathophysiology of diabetic cardiomyopathy has not yet been completely understood.
Sirts are the NAD+-dependent deacylases, which regulate a myriad of biologic functions, ranging from cell growth and metabolism to longevity (13). The mammalian genome encodes 7 Sirt isoforms (Sirt1–7), which are localized in different subcellular compartments and regulate a multitude of cellular functions through post-translational modification of target proteins (13). Among them, Sirt3 is localized to mitochondria, where it controls almost every aspect of mitochondrial function by deacetylating and modifying enzymatic activities of mitochondrial proteins (14). A role of Sirt3 was demonstrated in regulating not only mitochondrial metabolism and ATP synthesis but also fusion-fission dynamics of mitochondria (15, 16). Other studies conducted to determine the roles of Sirt3 in maintaining the health of different organ systems have shown that Sirt3 activation blocks tumorigenesis, cardiac hypertrophy, lung fibrosis, scleroderma, and aging-associated hearing and vision loss (17–22). We have previously demonstrated that Sirt3 deficiency contributes to aging-associated fibrosis of multiple organs, including, heart, liver, and kidney (23). In rodent models of type II diabetes, Sirt3 levels have been shown to be decreased, and overexpression of Sirt3 protected mice from developing diabetes and metabolic syndrome (12). Furthermore, humans with a polymorphism in SIRT3 gene, which leads to reduced activity of the deacetylase, are shown to be highly vulnerable to develop metabolic syndrome with age (24). Consistent with this report, humans with variations in the SIRT3 promoter, which leads to increased activity of the enzyme, are shown to exhibit resistance to developing diabetes and enjoy an extended lifespan (25–27). There are also reports showing that interventions such as exercise and calorie restriction, which provide health benefits to patients with diabetes, activate SIRT3 (28, 29). Together, these studies have provided strong support for the role of SIRT3 to block development of diabetes. However, molecular factors regulating expression and maintenance of endogenous SIRT3 levels are not yet fully understood.
Another Sirt analog implicated in regulating metabolism and development of diabetes is Sirt6 (30). Sirt6 is localized to the nucleus, where it regulates chromatin remodeling, genome stability, and gene transcription (30). Sirt6 knockout mice develop severe degenerative changes associated with hypoglycemia and multisystemic accelerated aging phenotype and die at around 26 d after birth (31). The hypoglycemia of Sirt6-deficient mice was found to result from increased glucose uptake by the skeletal muscle tissue (31). However, similar changes in glucose metabolism were not observed when Sirt6 was deleted in a tissue-specific manner, suggesting that Sirt6 exerts tissue-specific effects. Sirt6 deletion in the liver resulted in fatty liver degeneration, whereas neuronal deletion of Sirt6 was shown to retard somatic growth of mice (32, 33). We reported that cardiac-specific deletion of Sirt6 produced cardiac hypertrophy and fibrosis associated with mitochondrial degeneration and lipid accumulation but no change in glucose uptake by the heart (34). Conversely, transgenic mice having cardiac-specific overexpression of Sirt6 are protected from developing pressure overload cardiac hypertrophy (34). Sirt6 has been shown to regulate activity of hypoxia-inducible factor-1 (Hif1α), activator protein 1 (AP1), and NF-kB and suppress the expression of inflammatory cytokines, thus blocking the aging-associated inflammatory response (31, 34, 35). Sirt6 was also shown to block expression of myostatin, a negative regulator of muscle growth, and thus prevented development of cachexia (36). Additionally, Sirt6 was shown to block activity of Insulin like growth factor (Igf)-protein kinase B (Akt) signaling and extend the lifespan of male mice by ∼15%, thus suggesting a role of Sirt6 in regulating the aging process (37).
In this study, we report that under diabetic conditions Sirt3 down-regulation is followed by down-regulation of Sirt6, and overexpression of one Sirt helps to maintain activity of the other. We also show that Sirt6 activation blocks insulin resistance, up-regulates Sirt3 gene transcription, and maintains health of mitochondria by stabilizing cardiac nuclear factor erythroid 2 (NF-E2)-related factor 2 (Nrf2) levels. These studies are the first to provide evidence that Sirt6 and Sirt3 mutually regulate each other’s activity to protect the heart from developing diabetic cardiomyopathy.
MATERIALS AND METHODS
Antibodies
The antibodies and conjugates used in this study were anti-SIRT3 (5490), anti-SIRT6 (12486), anti-insulin receptor substrate 1 (IRS1) (2382), anti–phosphorylated-IRS1(Ser307) (2381), anti-Nrf2 (127121), (Cell Signaling Technology, Danvers, MA, USA), anti–phosphorylated-IRS1(Tyr612) (Thermo Fisher Scientific, Waltham, MA, USA), anti–Kelch-like ECH-associated protein-1 (Keap1) (MAB3024; R&D systems, Minneapolis, MN, USA), anti-Mitofusin 1 (MFN1) (ABC41; MilliporeSigma, Burlington, MA, USA), anti-Fis1 (fission, mitochondrial 1) (AP1165; MilliporeSigma), anti-dynamin-related protein 1 (DRP1) (611112; BD Biosciences, San Jose, CA, USA), anti-transcription factor A, mitochondrial (TFAM) (sc-23588), anti-manganese superoxide dismutase (MnSOD) (06-984; MilliporeSigma), anti–heme oxygenase 1 (HO1) (MA1-112), anti-acetyl-lysine (06933; MilliporeSigma), anti–glutamate-cysteine ligase modifier subunit (GCLm) (PA5-29908), anti-optic atrophy 1 (OPA1) (612606; BD Biosciences), anti-tubulin (236-10501; Thermo Fisher Scientific), anti-actin (PAH-85271; Thermo Fisher Scientific). All anti-rabbit (sc-2077), anti-mouse (sc-2056), and anti-goat (sc-2096) horseradish peroxidase–conjugated secondary antibodies for Western blots were from Santa Cruz Biotechnology (Dallas, TX, USA). Anti–lysine-acetylated-MnSOD, anti–acetylated-lysine–oligomycin sensitivity conferring protein (OCSP) of mitochondrial ATP synthase, and anti-OSCP antibodies were provided by David Gius (Northwestern University, Evanston, IL, USA).
Adenovirus constructs
The Sirt3 and Sirt6 adenoviruses were purchased from Vector Biolabs (Malvern, PA, USA).
Animal care and generation of Sirt6-overexpressing transgenic mice
All animal protocols were reviewed and approved by the University of Chicago Institutional Animal Care and Use Committee. Hearts from diabetic (db/db) mice and their wild-type (WT) control mice were procured from Yan Chun Li’s laboratory (University of Chicago, Chicago, IL, USA). C57BL/6 control and whole-body overexpressing Sirt6 transgenic (Tg.Sirt6) female mice at 8 wk of age were fed either a high-fat, high-sucrose (HF-HS) diet (45% KCal from fat, 08811; Envigo, Huntingdon, United Kingdom) or chow diet for a 24-wk period.
Founder Tg.Sirt6 mice were generated by InGenious Targeting Laboratory (Ronkonkoma, NY, USA) as follows (Supplemental Fig. S1). The targeting vector (pROSA26-1), which carried Sirt6-FLAG cDNA was driven by Gt(ROSA)26Sor (ROSA26) promoter and controlled by a STOP cassette. The STOP cassette in the vector consisted of a splice acceptor and the floxed phosphoglycerokinase (PGK)/gb2neoPGKpolyA2xSV40pA sequence and was inserted in intron 1 of ROSA26 locus. Mouse Sirt6 cDNA with a C-terminal FLAG tag was cloned into the Micrococcus luteus 1 restriction (MluI) site of the vector immediately downstream of the STOP cassette. The Sirt6-FLAG sequence was followed by a BGHpA bovine growth hormone polyadenylation (BGHpA) sequence. To knock in Sirt6-FLAG sequence in the mouse ROSA26 locus, the targeting vector contained short (1.08 Kb) and long (4.34 Kb) homology arms with ROSA26 genomic sequence. The short homology arm was upstream of the STOP cassette, whereas the long homology arm was downstream of the BGHpA sequence (Supplemental Fig. S1). The 13.9-Kb targeting vector was linearized using SacII enzyme and electroporated into iTL BA1 (129/SvEv × C57BL/6) hybrid embryonic stem cells. Targeted cells were microinjected into C57BL/6 blastocysts. Resulting chimeras with a high percentage of agouti coat color were mated with WT C57BL/6N mice to generate filial generation 1 heterozygous offspring. To check for germline incorporation of Sirt6-FLAG transgene, offspring’s tail DNA was analyzed by PCR using the following set of primers: forward: 5′-TGCAACCCACAAAACATGAC-3′ and reverse: 5′-ACAATGCGATGCAATTTCC-3′. Transgenic mice yield a PCR product of 454 bp.
Whole-body Sirt6-FLAG–overexpressing mice were generated by crossing the ROSA26-Sirt6-FLAG transgenic mice with Pgk1-Cre mouse (Pgk1-Cre recombinase) (020811; The Jackson Laboratory, Bar Harbor, ME, USA), in which Cre expression was under the control of Pgk1 promoter. To genotype Cre+ progeny, the following set of primers was used: up-pgk1 (5′-GCTGTTCTCCTCTTCCTCATCTCC-3′) and IntCre-rev (5′-TCCATGAGTGAACGAACCTGGTCG-3′), which yields a PCR product of 500 bp.
Glucose uptake assay
Rat cardiomyoblast cell line (H9c2) was were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were plated at a density of 0.1 × 106 cells per well in a 12-well plate. After 6 h, cells were infected with Sirt3 and Sirt6 adenovirus for 24 h. Cells were treated with palmitate (Pal) for 24 h. For the glucose uptake experiment, all of the culture medium was removed from each well and replaced with 1 ml of glucose-free DMEM in the absence or presence of insulin. Cells were then incubated with fluorescent 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (NBDG) (50 μm) at 37°C with 5% CO2 for a period of 30 min. For flow cytometry analysis, the 2-NBDG uptake reaction was stopped by removing the incubation medium and washing cells twice with chilled PBS. Cells in each well were subsequently resuspended in 200 μl chilled HBSS and then given a final propidium iodide concentration of 1 μg/ml and maintained at 4°C for later flow cytometry analysis performed within 30 min. For each measurement, data from 10,000 single cell events were collected using a fluorescence-activated cell sorting (FACS) Calibur (BD Biosciences) flow cytometer (38).
Cell culture, transfection, and adenovirus infection
All cells were grown in DMEM (Thermo Fisher Scientific) supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum (complete growth medium). Cells were transfected with appropriate plasmids using Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. For all the adenovirus experiments, viruses were used at a multiplicity of infection of 10. For Pal treatment, 24 h after plating, cells were treated for 24 h either with vehicle (0.9% saline) or with Pal in serum-free medium. Before harvesting to immunoprecipitate, cells were treated for 1 h with 2 μM trichostatin A (MilliporeSigma) (16).
Pal solution preparation
Pal stock solution (100 mM) was prepared in 0.1 M NaOH along with heating at 70°C in a shaking water bath. In an adjacent water bath, at 55°C, a 10% (w/v) fatty acid-free bovine serum albumin (BSA) solution was prepared in double-distilled H2O. A stock solution [5 mM Pal/10% (w/v) BSA] was prepared by adding 50 μl of the 100 mM Pal solution dropwise to 950 μl of the 10% (w/v) BSA solution at 55°C, with mixing in between. The Pal/BSA complex solution was cooled to room temperature and filter sterilized using a 0.45-μm pore size membrane filter. The complex solution was stored at −20°C. The stored 5-mM Pal 10% (w/v) BSA stock solutions were heated for 15 min at 55°C and then added in DMEM to get the final working concentration of 200 or 500 µM (39).
Immunoblotting and immunoprecipitation analyses
Cell or heart ventricular tissue lysates were prepared in RIPA buffer [50 mM Tris-HCl (pH 7.5), 0.1% Nonidet P-40, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, and Sigma protease inhibitors and phosphatase inhibitors. Typically, 20–50 μg of protein lysates was used for immunoblots. Immunoprecipitation experiments using 500–1000 μg of total protein lysates were carried out using standard protocols.
Immunostaining
Cells (10,000 to 20,000) plated on glass coverslips were used for immunostaining. Cells were infected with mitochondrial matrix–targeted green fluorescent protein (Mt-GFP), SIRT3, SIRT6, or control adenovirus vector (Ad.) (multiplicity of infection, 10) as indicated for 24–48 h and then immunostained as previously described (16). Confocal microscopy and imaging analyses were done in the digital light microscopy core facility of the University of Chicago (Chicago, IL, USA).
Histology
Heart tissue from mice was fixed in neutral formalin, and sections of the tissues were processed and stained with Masson’s trichrome stain for detection of fibrosis. Imaging of stained sections was done using Pannoramic Viewer software (3dhistech, Budapest, Hungary), and quantitation was done using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Electron microscopy for heart samples
A portion of a heart tissue was harvested from the mice, fixed in the electron microscopy fixative per the standard protocol, and processed further at the transmission electron microscope core facility of the University of Chicago. Images were captured at 300 kV with FEI Tecnai F30 (Thermo Fisher Scientific) electron microscope equipped with a high-performance Gatan (Roper Technologies, Lakewood Ranch, FL, USA) charge-coupled device camera.
RNA isolation and real-time PCR analysis
Total RNA was isolated from cells and mouse hearts by using Trizol reagent (Thermo Fisher Scientific) as previously described (40). The residual genomic DNA was digested by incubating the RNA preparation with 0.5 U of RNase-free DNase-1 per microgram of RNA in a reaction buffer for 15 min at room temperature followed by heat inactivation at 90°C for 5 min. DNase-treated RNA (2 μg) was reverse transcribed by use of RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). The resultant cDNA was diluted 10-fold before PCR amplification. A reverse transcriptase minus reaction served as a negative control. The mRNA levels were measured by Sybr green real-time PCR.
Statistical analyses
All graphical data are presented as means ± sem. Statistical significance between 2 groups was determined using a 2-tailed, paired Student’s t test or 1-way analysis of variance (ANOVA) for more than 2 groups. Values of P < 0.05 were considered significant.
RESULTS
Sirt6 activation protects cardiomyocytes from insulin resistance
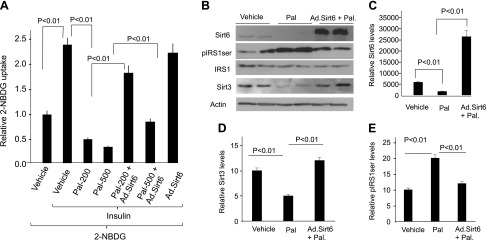
Many previous studies have reported that Pal treatment induces insulin resistance (1, 41–43). In order to develop an in vitro model for insulin resistance, we treated mouse H9c2 cardiomyocytes and neonatal rat cardiomyocytes with increasing doses of Pal (200 and 500 μM) for a total duration of 24 h. Glucose uptake was measured by monitoring transport of fluorescent analog 2-NBDG by FACS analysis. Our results show that Pal treatment induced insulin resistance in both models of cardiomyocytes (Fig. 1A). In these cells, we also measured expression of Sirt6 and Sirt3 by Western blot analysis, and we found that Pal treatment reduced expression of both Sirts (Fig. 1B–D). To test whether Sirt6 activation could alleviate insulin resistance, we transduced cardiomyocytes with Ad.Sirt6 or with an empty vector and then treated cells with Pal for 24 h. The results show that Sirt6 overexpression completely blocked Pal-induced insulin resistance of cardiomyocytes (Fig. 1A). In these cells, we also found that Sirt6 overexpression blocked the Pal-induced down-regulation of Sirt3, suggesting that Sirt6 has the ability to maintain expression of mitochondrial Sirt3 (Fig. 1B, D). Insulin resistance is largely contributed by defects in IRS1/PI3K pathway, which impairs the downstream mechanism of Akt-dependent glucose uptake. Ser307 phosphorylation of IRS1 reduces its ability to recruit PI3K, hence reducing its downstream activation (44). Consistent with this, we found that insulin resistance was associated with increased IRS1 serine (307) phosphorylation, and Sirt6 overexpression blocked this modification (Fig. 1B, E). These in vitro experiments disclosed 2 major findings: first, Sirt6 up-regulates mitochondrial Sirt3, and second, Sirt6 blocks phosphorylation of IRS1 and therefore insulin resistance of cardiomyocytes.
Figure 1.
Sirt6 blocks Pal-mediated insulin resistance of cardiomyocytes. A) H9c2 cardiomyocytes were overexpressed with Ad.Sirt6 or an empty vector. Cells were grown in DMEM with or without insulin and treated with Pal for 24 h. Glucose uptake was measured by estimating transport of fluorescent analog 2-NBDG by FACS analysis. Values are means ± se; n = 6. B) Western blot analysis showing expression levels of different proteins 24 h after Pal treatment of cells. Results are shown for duplicate samples. C–E) Densitometric quantitation of the Western blots; means ± se; n = 3.
During nutritional overload, Sirt3 decline precedes down-regulation of Sirt6
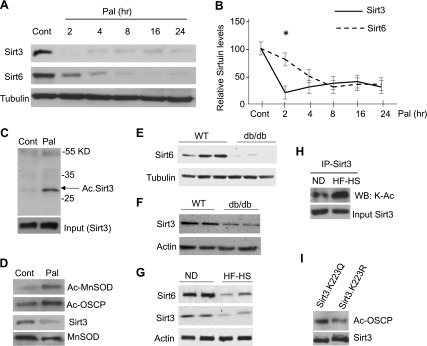
Pal treatment induces insulin resistance by causing mitochondrial dysfunction and increased reactive oxygen species (ROS) synthesis (43). Similar mitochondrial impairments have been shown to develop in conditions of Sirt3 deficiency (23). We therefore asked whether loss of Sirt3 could precede down-regulation of Sirt6. For this experiment, we harvested Pal-treated cardiomyocytes at different time points ranging from 2 to 24 h, and protein expression was analyzed by Western blotting. We found that, whereas Sirt3 levels were depleted at 2 h, complete Sirt6 down-regulation occurred post–8 h of Pal treatment of cells (Fig. 2A, B). Pal treatment is likely to increase the concentration of acetyl-CoA in mitochondria and induce nonenzymatic acetylation of mitochondrial proteins (45). A recent study has shown that Sirt3 acetylation decreases its stability (46). To test whether Sirt3 was acetylated after 2 h of treatment with Pal, we immunoprecipitated Sirt3 from Pal-treated cells and analyzed its acetylation by Western blotting. The results show that Sirt3 was hyperacetylated in Pal-treated cardiomyocytes (Fig. 2C). We also confirmed loss of Sirt3 activity at 2 h of Pal treatment by measuring acetylation of Sirt3-target proteins, MnSOD and OSCP (Fig. 2D). Thus, these results indicate that Sirt3 depletion precedes down-regulation of Sirt6 in Pal-treated cardiomyocytes.
Figure 2.
Under conditions of excess nutrition, Sirt3 decline precedes down-regulation of Sirt6. A) H9c2 cardiomyocytes were treated with Pal (200 µM) for different time durations. Sirt3 and Sirt6 expression was analyzed by Western blotting. B) Quantitation of the Western blots. Note: Sirt3 down-regulation precedes down-regulation of Sirt6. Means ± se; n = 4. C) Sirt3 was immunoprecipitated after 2-h treatment of H9c2 cells with Pal, and protein acetylation was analyzed by Western blotting using anti–Ac-lysine antibody. D) The same protein lysate was subjected to Western blotting using specific antibodies to determine acetylation of MnSOD and OSCP. E–G) Western blots showing down-regulation of Sirt6 (E) and Sirt6 (F) in the hearts of db/db mice and mice fed an HF-HS diet (G) for 24 wk. H) Sirt3 was immunoprecipitated from hearts of mice fed a normal diet (ND) or HF-HS diet, and protein acetylation was analyzed by Western blotting using anti–Ac-K antibody. I) H9c2 cells were overexpressed with plasmids expressing mutant Sirt3 having K223 mutated to Q or to R. The acetylation of the Sirt3 target protein OSCP was analyzed by use of a specific anti–Ac-OSCP antibody. Ac, acetylated; Cont., control; IP, immunoprecipitation; WB, Western blot.
To validate these in vitro findings in vivo, we measured Sirt6 and Sirt3 levels in 2 different animal models of diabetes, db/db mice and mice fed an HF-HS diet for 24 wk. In both cases, cardiac Sirt6 and Sirt3 levels were down-regulated (Fig. 2E–G). We also measured acetylation of Sirt3 in diabetic hearts and found that, same as in Pal-treated cardiomyocytes, Sirt3 was highly acetylated in diabetic hearts of mice fed a HF-HS diet (Fig. 2H). By mass spectrometry and mutational analyses, we found that Sirt3 was acetylated at Lys223, which leads to decreased deacetylase activity of Sirt3 in diabetic hearts (Fig. 2I and Supplemental Fig. S2). These results thus demonstrate that under diabetic conditions acetylation impairs Sirt3 activity.
Both Sirt6 and Sirt3 are capable of maintaining each other’s activity
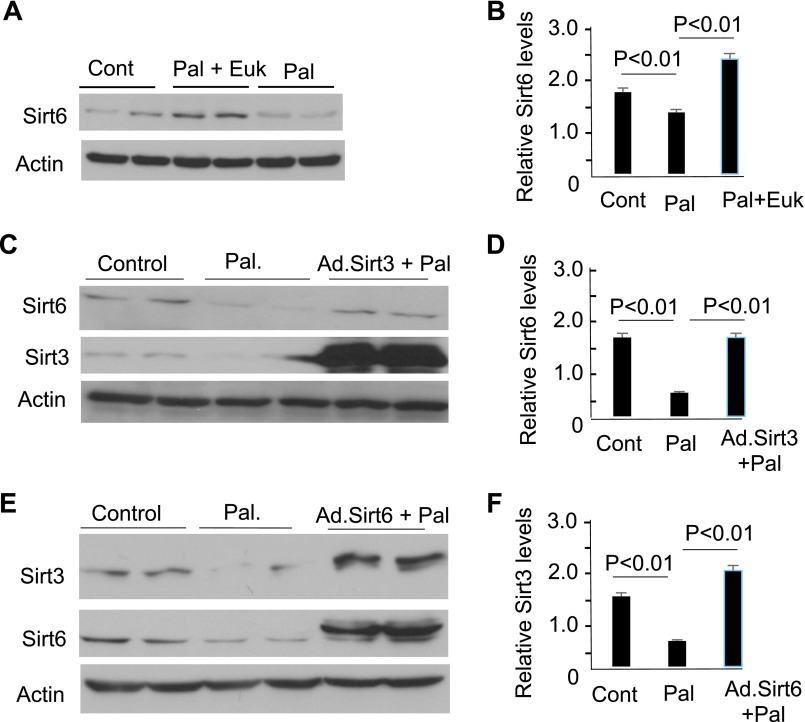
Because Sirt3 depletion impairs mitochondrial function leading to increased ROS synthesis, we asked whether a rise in cellular ROS levels could be contributing to loss of Sirt6. As shown in Fig. 3A, B, pretreatment of cardiomyocytes with an ROS scavenger EUK134 (MnSOD and catalase mimetic) prevented Pal-mediated down-regulation of Sirt6, suggesting that increased oxidative stress contributed to loss of Sirt6. We then reasoned that, if this is true, then by maintaining Sirt3 levels we should be able to maintain cardiac Sirt6 levels. The results presented in Fig. 3C, D, show that this was indeed the case. Furthermore, not only Sirt3 overexpression up-regulated Sirt6 but also Sirt6 overexpression up-regulated expression of Sirt3 (Fig. 3E, F), thus indicating that these 2 Sirts help to maintain each other’s activity in cardiomyocytes.
Figure 3.
Sirt3 and Sirt6 mutually regulate each other’s activity. A) Cardiomyocytes were treated with Pal (200 µM) in the absence or presence of the antioxidant Euk134. Sirt6 expression was analyzed by Western blotting. B) Quantitation of the Western blots. Means ± se; n = 3. C, E) H9c2 cells were overexpressed with Ad.Sirt3 (C) or Ad.Sirt6 (E) vectors and treated with Pal (200 µM) for 24 h. Protein expression was analyzed by Western blotting using specific antibodies. D, F) Quantitation of the Western blots. Means ± se; n = 4.
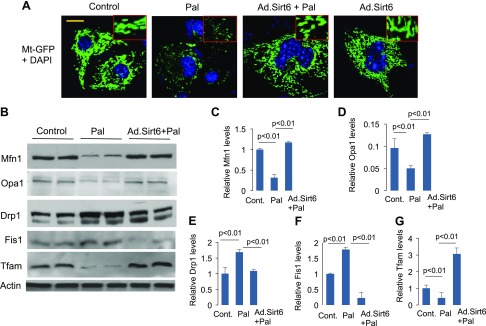
We have previously reported that Sirt3 deficiency promotes fragmentation of mitochondria (16), whereas others have reported that mitochondrial fragmentation induces insulin resistance (47). We therefore asked whether Sirt6 alleviates Pal-induced insulin resistance by mitigating mitochondrial fragmentation. To this end, we overexpressed cardiomyocytes with Ad.Mt-GFP along with Ad.Sirt6 vectors, followed by treatment of cells with Pal for 24 h. Mitochondrial morphology was monitored by confocal microscopy and by analyzing expression of proteins regulating mitochondrial fusion-fission dynamics. As shown in Fig. 4A, we found that Pal treatment caused mitochondrial fragmentation, whereas Sirt6 overexpression preserved the normal tubular shape of mitochondria, even when cells were treated with Pal, suggesting that Sirt6 promotes mitochondrial fusion (Fig. 4A). Consistent with these results, we found reduced levels of Mfn1 and Opa1 (proteins involved in mitochondrial fusion) and increased expression of Drp1 and Fis1 (proteins involved in mitochondrial fission) in Pal-treated cardiomyocytes. Sirt6 overexpression blocked the Pal-mediated changes in the expression of these proteins (Fig. 4B–F). Additionally, we found reduced expression of Tfam (a marker of mitochondrial density) in Pal-treated cells, which was again elevated by overexpressing cells with Ad.Sirt6 (Fig. 4B, G). These results thus indicate that Sirt6 blocked Pal-mediated degeneration of the mitochondrial population.
Figure 4.
Sirt6 blocks Pal-induced mitochondrial fragmentation. A) Confocal images of representative cardiomyocytes overexpressed with Ad.Sirt6 and treated with Pal for 24 h. Mitochondrial population was visualized by overexpressing cells with Ad.Mt-GFP vector (green). Position of nuclei was identified by DAPI staining (blue). Inset displays mitochondrial morphology in enlarged image. Scale bar, 2 µm. B) Cell lysate of cardiomyocytes was analyzed by Western blotting using protein-specific antibodies. C–G) Quantitation of the Western blots. Mean ± se; n = 3. Cont., control.
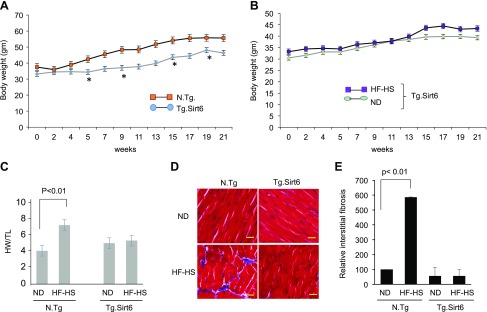
Tg.Sirt6 mice are protected from developing diabetic cardiomyopathy
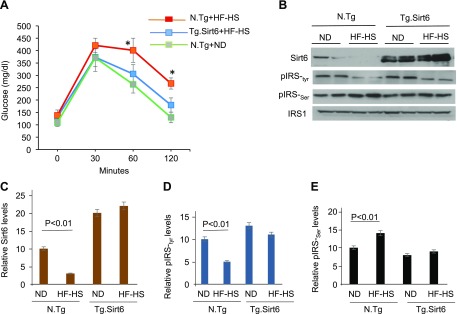
To test whether Sirt6 activation can protect the heart from developing diabetic cardiomyopathy, we generated a Tg.Sirt6 mouse (Supplemental Fig. S1). Control nontransgenic (N.Tg) and Tg.Sirt6 mice were fed with a normal diet or an HF-HS Western diet for 24 wk, and their weight gain was measured weekly. We found that Tg.Sirt6 mice fed with an HF-HS diet did not gain similar body weight as N.Tg mice did with age (Fig. 5A). When we compared age-dependent weight gain of Tg.Sirt6 mice fed a normal diet and an HF-HS diet, we found no significant change up to 13 wk of feeding. Thereafter, weight of HF-HS–fed mice was slightly higher at 15 and 17 wk, which again tended to come back to the weight range of mice fed a normal diet, suggesting that Sirt6 overexpression resisted developing HF-HS diet–induced obesity in mice (Fig. 5B). We also found that N.Tg mice fed an HF-HS diet developed cardiac hypertrophy (heart weight:tibia length ratio) and fibrosis, whereas Tg.Sirt6 mice maintained on the same diet did not develop these changes (Fig. 5C–E). To determine the effect of Sirt6 on insulin resistance, we performed a glucose tolerance test. Glucose tolerance was significantly decreased after HF-HS feeding in N.Tg control mice compared with mice fed a normal diet. In agreement with our in vitro results, Tg.Sirt6 mice fed a normal chow or HF-HS diet displayed similar glucose tolerance, suggesting that Sirt6 activation mitigated insulin resistance induced by HF-HS diet feeding (Fig. 6A). To get additional support for these results, we analyzed Sirt6 levels in the hearts and skeletal muscles of these mice by Western blotting. Consistent with our in vitro results, we found that WT mice fed an HF-HS diet had significantly reduced expression of Sirt6 both in skeletal muscle (unpublished results) and hearts when compared with normal chow–fed mice (Fig. 6B, C). We also found a significant increase in IRS1 serine (307) and decreased IRS1 tyrosine (612) phosphorylation (markers of insulin resistance) in the hearts of HF-HS–fed control (N.Tg) mice but not in Tg.Sirt6 mice (Fig. 6B, D, E), thus confirming that Sirt6 activation protects the heart from developing insulin resistance.
Figure 5.
Tg.Sirt6 mice are protected from developing diabetic cardiomyopathy. N.Tg controls and Tg.Sirt6 mice were fed an HF-HS diet for 24 wk. A) Age-dependent body weight gain in N.Tg and Tg.Sirt6 mice. B) Weight gain of Tg.sirt6 mice fed either a normal diet (ND) or HF-HS diet. C) Mice were fed a ND or HF-HS diet for 24 wk, and their heart weight/tibia length (HW/TL) ratio, a marker of hypertrophy, was measured. Values are means ± se; n = 7. D) Heart sections were stained with Masson’s trichrome (blue) to visualize interstitial myocardial fibrosis. Scale bars, 20 μM. E) Quantitation of myocardial fibrosis in different groups of mice. Values are means ± se; n = 5.
Figure 6.
Tg.Sirt6 mice are protected from developing insulin resistance. A) Glucose tolerance test was carried out in different groups of mice. Values are means ± se; n = 5. *P < 0.01. B) Western analysis of the heart lysate of different groups of mice. C–E) Quantitation of the Western blots. Mean ± se; n = 3. ND, normal diet.
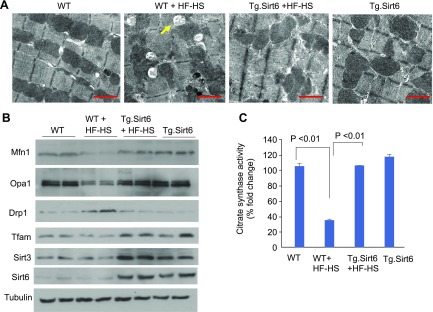
To examine the role of Sirt6 on mitochondrial integrity, we performed an ultrastructural examination of the heart tissue using transmission electron microscopy. WT mice fed with an HF-HS diet showed increased accumulation of lipid droplets in cardiomyocytes than normal chow–fed mice (Fig. 7A). HF-HS diet also caused focal damage in the heart muscle characterized by mitochondrial clustering (Fig. 7A). However, cardiac sections of Tg.Sirt6 mice fed a HF-HS diet were ultrastructurally indistinguishable from WT or Sirt6-overexpressing mice fed a normal diet, suggesting that Sirt6 activation helped to preserve mitochondrial morphology when challenged with excess nutrition (Fig. 7A). In the hearts of these mice, we also measured expression of proteins involved in regulating fusion-fission dynamics of mitochondria. Same as in Pal-treated cardiomyocytes, we found reduced expression of Mfn1, Opa1, and Tfam and increased expression of Drp1 in the heart lysate of mice fed an HF-HS diet compared with mice on the normal diet (Fig. 7B). However, similar changes were not found in the heart lysate of Tg.Sirt6 mice fed an HF-HS diet (Fig. 7B). We also analyzed the activity of citrate synthase, a mitochondrial enzyme involved in the first step of the tricarboxylic acid cycle. Citrate synthase catalyzes the condensation of acetate and oxaloacetate to form citrate and hence is a key marker for mitochondrial function. We found that HF-HS diet feeding caused significant reduction in citrate synthase activity in WT control mice (Fig. 7C). Similar results were noticed in the hearts of Sirt6 knockout mice (unpublished results). However, Tg.Sirt6 mice fed a HF-HS diet maintained citrate synthase activity comparable to WT mice on the same diet (Fig. 7C). Together, these results suggest that Sirt6 overexpression mitigates HF-HS diet–induced mitochondrial damage and helps to maintain mitochondrial health.
Figure 7.
Heart mitochondria of Tg.Sirt6 mice are preserved post–HF-HS feeding. A) Electron micrographs showing mitochondrial damage and lipid accumulation (arrow) in the hearts of WT controls but not in Tg.Sirt6 mice fed a HF-HS diet for 24 wk. Scale bars, 1 µM. B) Western blot analysis of the heart lysate showing preservation of proteins regulating mitochondrial fusion and down-regulation of fission proteins in HF-HS–fed Tg.Sirt6 mice. C) Enzymatic activity of citrate synthase in the hearts of different groups of mice. Values are means ± se; n = 5.
Sirt6 stabilizes Nrf2 and up-regulates expression of Nrf2-dependent mitochondrial genes
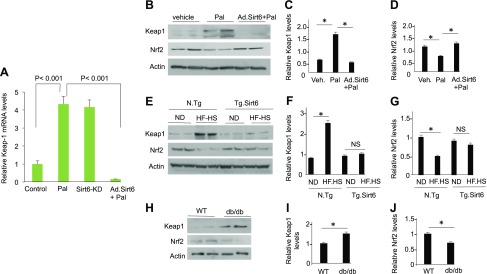
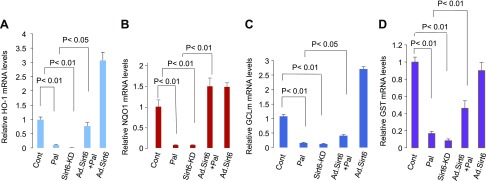
Knowing that Sirt6 helps to maintain mitochondrial health and up-regulates Sirt3, we asked whether Sirt6 can regulate Nrf2-dependent mitochondrial gene expression. Nrf2 is a key transcription factor that regulates expression of many nuclear encoded antioxidant genes (including Sirt3) needed for maintaining health of mitochondria (48, 49). One of the major mechanisms regulating Nrf2 activity is its binding to the protein Keap1. Under physiologic conditions, Nrf2 binds to Keap1 both in the cytoplasm and in the nucleus. In the nucleus, Keap1 binding promotes export of Nrf2 to the cytoplasm, whereas in the cytoplasm, Keap1 promotes association of Nrf2 with cul3-Rbx1-E3 Cullin3-Ring-Box 1-E3 ubiquitin ligase, which degrades Nrf2, thus suppressing transcription of Nrf2-dependent genes (50, 51). To delineate whether Sirt6 regulates Nrf2-dependent gene transcription in cardiomyocytes, we first determined expression of Nrf2 and Keap1 genes under conditions in which Sirt6 levels were amended. We found that in Pal-treated cardiomyocytes as well as in the Sirt6 knockdown (Sirt6KD) cells, Keap1 mRNA was increased by nearly 4-fold (Fig. 8A), but Nrf2 mRNA was not (unpublished results). Consistent with the change in Keap1 mRNA, Keap1 protein levels were increased in Pal-treated cells as well (Fig. 8B, C). Furthermore, increased Keap1 protein levels corresponded with decreased Nrf2 levels, both in Pal-treated cardiomyocytes and in hearts of mice fed a HF-HS diet (Fig. 8B–G). We also found increased Keap1 and decreased Nrf2 levels in hearts of db/db mice (Fig. 8H–J). Overexpression of cardiomyocytes with Ad.Sirt6 blocked Pal-mediated rise of Keap1 levels, and this again corroborated with increased levels of Nrf2 (Fig. 8B–D). Similarly, Tg.Sirt6 mice fed a HF-HS diet showed no increase in Keap1 levels, and that corroborated with preserved Nrf2 levels (Fig. 8E–G). To validate these results further, we measured expression of selected Nrf2 target mitochondrial genes [e.g., HO1, NAD(P)H quinone oxidoreductase 1, GCLm, and glutathione S-transferase]. As shown in Fig. 9, expression (mRNA) of these target genes was significantly decreased in Pal-treated cardiomyocytes as well as in Sirt6KD cells. Overexpression of cardiomyocytes with Ad.Sirt6 prevented Pal-mediated decrease of all these Nrf2 target genes. These results thus suggested that Sirt6 stabilizes Nrf2 levels in cardiomyocytes, leading to increased expression of Nrf2-dependent target genes.
Figure 8.
Sirt6 overexpression blocks excess nutrition–mediated down-regulation of Nrf2. A) RT-PCR analysis showing Keap1 mRNA levels in Pal-treated and Sirt6KD cardiomyocytes. Means ± se; n = 5. B, E, H) Western analysis showing Keap1 and Nrf2 levels in Pal-treated cardiomyocytes (B), hearts of N.Tg and Tg.Sirt6 mice fed a normal diet (ND) or HF-HS diet (E), and db/db mice hearts (H). The actin blot of loading control used in B is the same used in Fig. 1B because these panels were generated from the same blot. C, D, F, G, I, J) Quantitation of the Western blots. NS, not significant. Means ± se; n = 3. *P < 0.01.
Figure 9.
Sirt6 overexpression up-regulates expression of Nrf2 target genes. A–D) RT-PCR analysis showing mRNA expression of different Nrf2-target genes in Pal-treated cardiomyocytes, with or without expression of Ad.Sirt6, and in the Sirt6KD cells. Cont., control; GST, glutathione S-transferase; NQO1, NAD(P)H quinone oxidoreductase 1. Values are means ± se; n = 5.
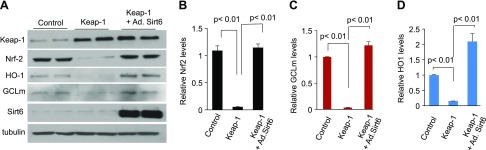
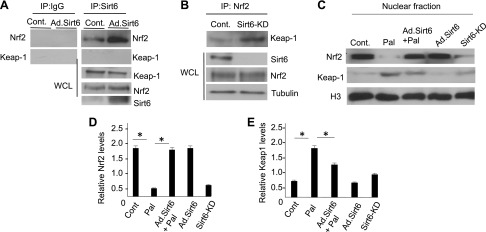
We next asked what the mechanism behind the ability of Sirt6 to stabilize Nrf2 levels in cardiomyocytes could be. We posited that at least 2 possibilities exist: first, Sirt6 indirectly preserves Nrf2 levels by suppressing expression of Keap1, and second, Sirt6 stabilizes Nrf2 by preventing its binding to Keap1. To test the first possibility, cardiomyocytes were overexpressed with vectors synthesizing Keap1 and/or Sirt6, and protein expression was analyzed by Western blotting. We found that when cells were overexpressed with Keap1 alone, expression of Nrf2 and its target genes was blocked, but this did not occur when cells were overexpressed with both Keap1 and Sirt6 together, suggesting that Sirt6 counteracted the negative effect of Keap1 on Nrf2 (Fig. 10). To address the second possibility, whether Sirt6 interferes with binding of Keap1 to Nrf2, we tested interaction of Sirt6 with Nrf2 and/or Keap1. The immunoprecipitation analysis showed that Nrf2 was pulled down, but not Keap1, with beads carrying Sirt6, thus indicating that Nrf2 binds to Sirt6 in cardiomyocytes (Fig. 11A). We did another experiment in which lysate of control and Sirt6KD cells was subjected to immunoprecipitation with Nrf2 antibody. The results showed that, whereas Keap1 was efficiently pulled down with Nrf2 from Sirt6-deficient cells, it was poorly precipitated from control cells expressing Sirt6, suggesting that Sirt6 interferes with binding of Nrf2 to Keap1 and thereby preserves Nrf2 levels (Fig. 11B). To determine whether Sirt6 regulates nuclear Nrf2 levels, we prepared a nuclear fraction of cells and determined Nrf2 levels by Western blotting. As expected, we found decreased nuclear Nrf2 levels in Pal-treated cells as well as in Sirt6KD cells, whereas it was preserved in the Sirt6-overexpressing cells, thus suggesting that Sirt6 stabilizes nuclear Nrf2 levels by counteracting its binding to Keap1 (Fig. 11C–E).
Figure 10.
Sirt6 overexpression antagonizes Keap1-mediated down-regulation of Nrf2. A) Cardiomyocytes were overexpressed with Keap1 with or without Ad.Sirt6, and protein expression was analyzed by Western blotting. B–D) Quantitation of protein levels in different groups of cardiomyocytes. Values are the meas + se of 4 Western blots.
Figure 11.
Sirt6 binds to Nrf2 and antagonizes Nrf2-Keap1 interaction in cardiomyocytes. A) Sirt6 was immunoprecipitated (IP) from control and Sirt6-overexpressing cardiomyocytes, and the precipitated beads were analyzed by Western blotting. B) Nrf2 was IP from the control and Sirt6KD cells, and the presence of Keap1 on beads was analyzed by Western blotting. C) Western analysis showing expression levels of Nrf2 and Keap1 in the nuclear fraction of cells. Histone H3 served as loading control. D, E) Quantification of the Western blots. Cont, control; WCL, whole cell lysate. Means ± se; n = 4. *P < 0.01.
Sirt6 up-regulates Sirt3 expression via activating Nrf2-dependent gene transcription
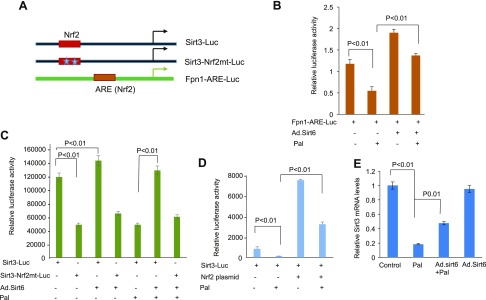
In a recent study, Satterstrom et al. (49) showed that transcription of Sirt3 is regulated by 2 Nrf2 binding sites present on its gene promoter. To test whether Sirt6 regulates cardiac gene transcription via Nrf2 binding sites on the promoter, we first analyzed expression of an Nrf2-sensitive promoter [ferroportin, (Fpn1)] reporter plasmid (Fig. 12A). We overexpressed H9c2 cells with Fpn1-luciferase plasmid and Ad.Sirt6, and treated them with Pal. As shown in Fig. 12B, Pal treatment reduced the activity of Fpn1 promoter-reporter gene, which was counteracted by Sirt6. We also found that Sirt6 activated the basal expression of Fpn1 gene promoter, suggesting that Sirt6 activates transcription of Nrf2-sensitive genes (Fig. 12B). Having known that Sirt6 regulates Nrf2-dependent gene transcription in cardiomyocytes, we then examined the effect of Sirt6 on the Sirt3 promoter. As shown in Fig. 12C, Sirt6 activated expression of Sirt3-Luc plasmid when Nrf2 sites on the promoter were intact but not when both Nrf2 sites were mutated (Sirt3-Nrf2mt-Luc). Pal treatment of cells reduced the activity of Sirt3-promoter-reporter plasmid, which was again blocked when cells were overexpressed with Sirt6 (Fig. 12C). We also analyzed the direct effect of Nrf2 on the activity of Sirt3-promoter-reporter plasmid. For this experiment, H9c2 cells were cotransfected with the Sirt3-promoter-reporter plasmid and an Nrf2-overexpressing plasmid. As expected, Nrf2 overexpression activated the Sirt3 gene promoter and also counteracted the negative effect of Pal on this promoter, thus suggesting that Pal treatment down-regulates Sirt3 expression not only by acetylation of the protein (as shown in Fig. 2C) but also by suppressing the activity of Nrf2-dependent Sirt3 gene transcription (Fig. 12D). To get additional support for the role of Sirt6 in regulating the Sirt3 gene transcription, we analyzed expression of Sirt3 mRNA by RT-PCR analysis. The results showed that Pal treatment significantly reduced the Sirt3 mRNA levels, which were restored when cells were overexpressed with Sirt6 (Fig. 12E). Collectively, these results indicate that Sirt6 up-regulates expression of Sirt3 gene by activating its Nrf2-dependent gene transcription.
Figure 12.
Sirt6 up-regulates Nrf2-dependent Sirt3 gene transcription. A) Schematic representation of different plasmids. B–D) Cardiomyocytes were transfected with different combinations of plasmids and treated with vehicle or Pal (200 µM) for 18 h. Luciferase reporter (Luc) gene activity was determined by using a luciferase assay kit. Means ± se; n = 4–6. E) RT-PCR analysis showing Sirt3 mRNA levels in different groups of cardiomyocytes. Mean ± se; n = 4.
DISCUSSION
Diabetic cardiomyopathy is a significant contributor of morbidity and mortality associated with diabetes and metabolic syndrome. Many previous studies have shown that Sirts play a significant role in regulating cellular metabolism and development of diabetes (9, 13). This study was undertaken to determine the role of Sirt6 in protecting a diabetic heart from developing cardiomyopathy. Our data presented in this study disclose 3 important findings: 1) Under conditions of excess nutrition, both Sirt6 and Sirt3 are down-regulated. Further, Sirt6 down-regulation follows loss of Sirt3 and increased ROS synthesis from mitochondria. 2) Sirt3 overexpression prevents down-regulation of Sirt6, and inversely, Sirt6 blocks down-regulation of Sirt3. 3) In cardiomyocytes, Sirt6 down-regulates Keap1 expression as well as inhibits Keap1 binding to Nrf2 and thus stabilizes Nrf2 and activates transcription of Nrf2-dependent genes, including Sirt3. Together, these studies demonstrate existence of an interdependent regulatory mechanism between Sirt3 and Sirt6, which helps to maintain the health of mitochondria and protect the heart from developing cardiomyopathy in settings of diabetes.
Mechanism behind down-regulation of Sirt3 and Sirt6 during diabetes
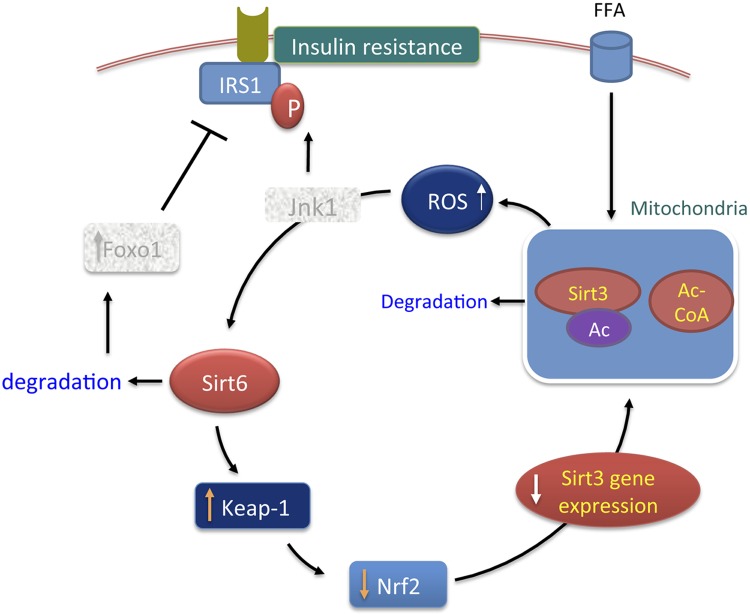
One unexpected finding of this study was that under conditions of nutritional overload, overexpression of mitochondrial Sirt3 protected the levels of nuclear Sirt6. Sirt3 is considered a mitochondrial fidelity protein because it plays a critical role in regulating many different aspects of mitochondrial functions (14). In cardiomyocytes, Sirt3 has been shown to regulate ATP and ROS synthesis from mitochondria as well as to maintain the integrity of mitochondrial DNA (15, 22, 52). In this study, because down-regulation of Sirt6 was blocked by treating cells with an MnSOD and catalase mimetic, we deduce that increased synthesis of ROS from mitochondria during diabetes contributed to loss of Sirt6. The heart mostly utilizes glucose and free fatty acids to meet its high ATP demands. Under physiologic conditions, glucose metabolism contributes to nearly 30% of ATP supply, whereas the rest of the ATP demand of the heart is met by oxidation of free fatty acids (53, 54). Under diabetic conditions, as glucose utilization is impaired, cardiomyocytes become exceedingly dependent upon oxidation of free fatty acids by mitochondria as a source of energy (53). Excess free fatty oxidation generates a high concentration of acetyl-CoA in the matrix of mitochondria, which could reach millimolar concentrations. Recent evidence suggests that acetylation of mitochondrial proteins is a nonenzymatic reaction, and that depends on the abundance of acyl-Co metabolites (45, 55). It is therefore likely that increased acetylation of mitochondrial proteins, including Sirt3, in diabetic hearts results from excess concentration of acetyl-CoA in mitochondria. Consistent with our results, increased acetylation of liver mitochondrial proteins has been reported under metabolic stress conditions such as long-term high-fat diet feeding and alcohol consumption (46, 56). One recent study has also demonstrated that, in obese and aged mice, liver Sirt3 is acetylated, and that this modification decreased its activity and stability (46). Based on these reports, we take that initial loss of Sirt3 in Pal-treated cardiomyocytes and hearts of HF-HS diet–fed mice results from acetylation of Sirt3 in mitochondria, which could be a first step in the scheme of evolution of diabetic cardiomyopathy. Later, as mitochondrial dysfunction and ROS synthesis leads to down-regulation of Sirt6 (which regulates Sirt3 gene transcription), Sirt6 depletion also exaggerates the loss of Sirt3 under conditions of nutritional overload (Fig. 13).
Figure 13.
Model illustrating interdependent regulation of Sirt3 and Sirt6 and the mechanisms via which Sirt6 blocks development of diabetic cardiomyopathy. Under diabetic conditions, heart becomes dependent on free fatty acids (FFA) as an energy source. Increased FFA oxidation overloads mitochondria with acetyl-CoA (Ac-CoA), which causes acetylation and inactivation of multiple proteins, including Sirt3. Mitochondrial dysfunction promotes ROS synthesis, which causes down-regulation of Sirt6 and induces insulin resistance via activation of multiple downstream mechanisms (e.g., Jnk1). Sirt6 down-regulation up-regulates Keap1 expression and reduces Nrf2 levels, leading to suppression of the Sirt3 gene transcription. Sirt6 down-regulation also activates Forkhead box O1 (Foxo1), which suppresses IRS1 expression. Thus, initial mitochondrial dysfunction subsequently develops a vicious cycle, which further down-regulates Sirt3 and Sirt6 and induces insulin resistance, leading to development of diabetic cardiomyopathy. In this scheme, Sirt6 activation can reverse this process by promoting mitochondrial health and/or by suppressing expression of Foxo1. Jnk1 and Foxo1 are presented in shaded boxes because these molecules are not studied in this study; rather, they are taken from previous studies to explain the possible mechanism. Ac, acetylated; P, Phosphorylated.
Our data indicate that loss of nuclear Sirt6 results from buildup of oxidative stress in cardiomyocytes. Although we have not explored the mechanism underlying down-regulation of Sirt6 by oxidative stress, several mechanisms could be postulated. In a previous study, we have shown that Sirt3 depletion and subsequent increased ROS synthesis from mitochondria overly activate Akt signaling in cardiomycytes, which causes development of cardiac hypertrophy (22). In cancer cells, overactivation of Akt has been shown to phosphorylate Sirt6 at Ser338 residue, which leads to ubiquitination and protein degradation (57). Another possibility could be activation of Jnk1 by increased cellular ROS synthesis. Sirt6 has been shown to possess multiple sites for Jnk1-mediated phosphorylation (58). Although, the consequences of Jnk1-mediated phosphorylation on stability of Sirt6 have not yet been reported, an earlier report showed that such a modification degrades Sirt1 (59). Further studies are needed to delineate the precise mechanism contributing to Sirt6 degradation under conditions of oxidative stress of cardiomyocytes.
How Sirt6 regulates expression of Sirt3
Sirt6 is a repressor of gene expression (30). It does not activate gene transcription directly, but it can do so by neutralizing effects of other repressors. In many previous studies, Sirt6 has been shown to regulate gene transcription by deacetylation of Histone H3 lysine 9 acetylated (HeK9Ac) and suppressing interaction of transcription factors such as Hif1α, c-Jun, and NF-kB to the gene promoters (30). In this study, results obtained from transient transfection assays indicated that Sirt6 activates Sirt3 gene promoter by coactivating other factors. One of the key transcription factors regulating expression of nuclear encoded mitochondrial genes (including Sirt3) is Nrf2 (48, 49). In this study, we found that Nrf2 levels were decreased in hearts of mice fed an HF-HS diet, whereas they were elevated in Sirt6-overexpressing hearts, suggesting that Sirt6 protects the cellular Nrf2 levels. However, we found no change in Nrf2 mRNA in Sirt6-depleted or -overexpressing cells, indicating that Nrf2 regulation by Sirt6 is a post-translational event. One of the major mechanisms regulating Nrf2 activity is its association with the protein Keap1 (60). Nrf2 binds to Keap1 in the cytoplasm as well as in the nucleus. In the nucleus, Keap1 binding promotes export of Nrf2 to the cytoplasm, thus suppressing the Nrf2-dependent gene transcription. In the cytoplasm, Keap1 binds to Nrf2 together with cul3-Rbx1-E3 ubiquitin ligase, which degrades Nrf2 (50, 51). Our studies show that Sirt6 stabilizes Nrf2 levels by operating at 2 different levels: first, Sirt6 suppresses the expression of Keap1, thereby reducing its interaction with Nrf2, and second, Sirt6 binds to Nrf2 and interferes with its binding to Keap1 and therefore Nrf2 export from the nucleus. Abundance of nuclear Nrf2 then increases the transcription of Nrf2-dependent genes, including Sirt3. To the best of our knowledge, this is the first report showing that Sirt6 controls the expression of Keap1, thereby maintaining Nrf2 levels. Consistent with our results, Sirt6 has been shown to protect human mesenchymal stem cells from oxidative stress–mediated cell death by coactivating Nrf2 (61).
The role of Sirt6 in mitigating insulin resistance
Insulin resistance is defined as decreased insulin-dependent glucose uptake by cardiomyocytes (5, 53). Several mechanisms have been proposed to explain the evolution of insulin resistance, including lipid accumulation and mitochondrial dysfunction, which generate ROS and inhibit IRS and Akt (62). In this pathway, one of the intermediate steps is activation of Jnk1, which phosphorylates serine residues of IRS1, leading to suppression of the insulin signaling. In the skeletal muscle, increased ROS synthesis from mitochondria was shown to cause skeletal muscle insulin resistance via activation of Jnk1 (12). Another mechanism proposed for insulin resistance is overt activation of Forkhead box O1 (Foxo1) in cardiomyocytes. Battiprolu et al. (63) have demonstrated that Foxo1 expression is highly up-regulated in the hearts of mice subjected to develop diabetes by feeding a high-fat diet. This study also showed that increased Foxo1 activity suppresses expression of IRS1 and phosphorylation of Akt and promotes lipid accumulation resulting in insulin resistance (63). In a previous study, we reported that Sirt6 depletion causes increased expression of Foxo1 in cardiomyocytes, and cardiac-specific overexpression of Sirt6 blocks this activation (34). This report, together with the findings reported in this study showing that whole-body Sirt6 overexpression reduces lipid accumulation, promotes mitochondrial health, and reduces ROS synthesis, indicates that Sirt6 can block insulin resistance via utilizing multiple mechanisms. Our findings also indicate that whole-body Sirt6 overexpression protects mice from developing HF-HS diet–mediated obesity, which further strengthens our observation regarding the ability of Sirt6 to block insulin resistance. Consistent with our findings, Yang et al. (64) reported that knockdown of Sirt6 abrogated the insulin-sensitizing activity of the antidiabetic drug rosigilitazone, thus underscoring the role of Sirt6 in mitigating the consequences of diabetes. Taken together, these findings highlight Sirt6 as a therapeutic target for developing drugs to combat obesity and diabetes.
In summary, our data presented in this study demonstrate a central role of Sirt6 in maintaining mitochondrial health, abrogating insulin resistance, and protecting the heart from developing diabetic cardiomyopathy. In previous studies, 3 Sirt analogs, Sirt1, Sirt3, and Sirt6, have been shown to be activated by endurance exercise and calorie restriction. These 3 analogs are also implicated in extending, directly or indirectly, health and lifespan of experimental animals (24, 35, 37, 65). There is also a report showing that Sirt1 binds to the Sirt6 promoter and regulates its gene transcription (32). Our findings showing that Sirt6 and Sirt3 regulate each other’s activity highlight the existence of an inter-Sirt network for mutual regulation of each other’s activity, which might be crucial for coordinating cellular metabolism and maintaining health of the organism.
TABLE 1.
Primers used for real-time PCR
| Gene | Primer sequence, 5′–3′ |
|
|---|---|---|
| Forward | Reverse | |
| HO1 | TGCTCAACATCCAGCTCTTTGA | GCAGAATCTTGCACTTTGTTGCT3 |
| NQO1 | AGGCTGGTTTGAGCGAGT | ATTGAATTCGGGCGTCTGCTG |
| GCLm | CTGCTAAACTGTTCATTGTAGG | CTATTGGGTTTTACCTGT |
| Gst | TGAACTCCTCTACCATGTGGAAGA | TCTGGCTGCCAGGTTGAAG |
| Sirt3 | GCTGCCAGCAAGGTTCTTAC | CCTTTCCACACCCTGGACTA |
| Sirt6 | GGCTACGTGGATGAGGTGAT | GGCTTGGGCTTATAGGAACC- |
| Nrf2 | CCATTTACGGAGACCCACCGC | GCCCAAGTCTTGCTCCAGCTC |
| Keap1 | TGGGCGTGGCAGTGCTCAAC | GCCCATCGTAGCCTCCTGCG- |
Gst, glutathione S-transferase; NQO1, NAD(P)H quinone oxidoreductase 1.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank the investigators who provided plasmids and technical help in doing experiments of this study. Expression plasmid for Keap1 was obtained from Prof. John D. Hayes (University of Dundee, Dundee, United Kingdom). Ad.Mt-GFP was provided by Paul Schumacker (Northwestern University, Chicago, IL, USA). The Sirt3 promoter-reporter plasmids and the Nrf2 expression plasmid were kindly provided by M. Haigis (Harvard Medical School, Boston, MA, USA) and H. Ardehali (Northwestern University), respectively. Mass spectroscopy analysis was performed by Dr. Don Wolfgehar (University of Chicago Chicago, IL, USA). This study was supported by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute RO1 Grants HL136712 and HL143488 (to M.P.G.). The authors declare no conflicts of interest.
Glossary
- Ad
adenovirus vector
- Akt
protein kinase B
- BSA
bovine serum albumin
- DRP1
dynamin-related protein 1
- FACS
fluorescence-activated cell sorting
- Foxo1
Forkhead box O1
- Fpn1
ferroportin
- GCLm
glutamate-cysteine ligase modifier subunit
- H9c2
rat cardiomyoblast cell line
- HF-HS
high-fat, high-sucrose
- HO1
heme oxygenase 1
- IRS1
insulin receptor substrate 1
- Keap1
Kelch-like ECH-associated protein 1
- MFN1
Mitofusin 1
- MnSOD
manganese superoxide dismutase
- Mt-GFP
mitochondrial matrix–targeted green fluorescent protein
- NBDG
(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose
- Nrf2
nuclear factor erythroid 2 (NF-E2)-related factor 2
- N.Tg
nontransgenic
- OPA1
optic atrophy 1
- OSCP
oligomycin sensitivity conferring protein
- Pal
palmitate
- Pgk
phosphoglycerokinase
- ROS
reactive oxygen species
- ROSA26
Gt(ROSA)26Sor
- Sirt
sirtuin
- Sirt6KD
Sirt6 knockdown
- TFAM
transcription factor A, mitochondrial
- Tg.Sirt6
Sirt6 transgenic
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. Kanwal performed nearly all Western blots presented in this study and wrote the first draft of the manuscript; V. B. Pillai did in vivo study with mice fed with HF-HS diet and assisted in the writing of the manuscript; S. Samant performed protein acetylation and protein-interaction studies; M. Gupta performed tissue culture and PCR studies; and M. P. Gupta coordinated with different members of the team, supervised the entire study, and prepared final draft of the manuscript.
REFERENCES
- 1.Kannel W. B., McGee D. L. (1979) Diabetes and cardiovascular disease. The Framingham study. JAMA 241, 2035–2038 [DOI] [PubMed] [Google Scholar]
- 2.Haffner S. M., Lehto S., Rönnemaa T., Pyörälä K., Laakso M. (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339, 229–234 [DOI] [PubMed] [Google Scholar]
- 3.King H., Aubert R. E., Herman W. H. (1998) Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 21, 1414–1431 [DOI] [PubMed] [Google Scholar]
- 4.Boden G. (2011) Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 18, 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boudina S., Abel E. D. (2007) Diabetic cardiomyopathy revisited. Circulation 115, 3213–3223 [DOI] [PubMed] [Google Scholar]
- 6.Alrob O. A., Sankaralingam S., Ma C., Wagg C. S., Fillmore N., Jaswal J. S., Sack M. N., Lehner R., Gupta M. P., Michelakis E. D., Padwal R. S., Johnstone D. E., Sharma A. M., Lopaschuk G. D. (2014) Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc. Res. 103, 485–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolinsky V. W., Dyck J. R. (2011) Calorie restriction and resveratrol in cardiovascular health and disease. Biochim. Biophys. Acta 1812, 1477–1489 [DOI] [PubMed] [Google Scholar]
- 8.Houtkooper R. H., Pirinen E., Auwerx J. (2012) Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarente L. (2006) Sirtuins as potential targets for metabolic syndrome. Nature 444, 868–874 [DOI] [PubMed] [Google Scholar]
- 10.Guarente L. (2011) Franklin H. Epstein Lecture: sirtuins, aging, and medicine. N. Engl. J. Med. 364, 2235–2244 [DOI] [PubMed] [Google Scholar]
- 11.Bao J., Scott I., Lu Z., Pang L., Dimond C. C., Gius D., Sack M. N. (2010) SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity. Free Radic. Biol. Med. 49, 1230–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jing E., Emanuelli B., Hirschey M. D., Boucher J., Lee K. Y., Lombard D., Verdin E. M., Kahn C. R. (2011) Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc. Natl. Acad. Sci. USA 108, 14608–14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haigis M. C., Guarente L. P. (2006) Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 20, 2913–2921 [DOI] [PubMed] [Google Scholar]
- 14.Pillai V. B., Sundaresan N. R., Gupta M. P. (2014) Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ. Res. 114, 368–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn B. H., Kim H. S., Song S., Lee I. H., Liu J., Vassilopoulos A., Deng C. X., Finkel T. (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 105, 14447–14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samant S. A., Zhang H. J., Hong Z., Pillai V. B., Sundaresan N. R., Wolfgeher D., Archer S. L., Chan D. C., Gupta M. P. (2014) SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol. Cell. Biol. 34, 807–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akamata K., Wei J., Bhattacharyya M., Cheresh P., Bonner M. Y., Arbiser J. L., Raparia K., Gupta M. P., Kamp D. W., Varga J. (2016) SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget 7, 69321–69336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bindu S., Pillai V. B., Kanwal A., Samant S., Mutlu G. M., Verdin E., Dulin N., Gupta M. P. (2017) SIRT3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial DNA damage. Am. J. Physiol. Lung Cell. Mol. Physiol. 312, L68–L78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H. S., Patel K., Muldoon-Jacobs K., Bisht K. S., Aykin-Burns N., Pennington J. D., van der Meer R., Nguyen P., Savage J., Owens K. M., Vassilopoulos A., Ozden O., Park S. H., Singh K. K., Abdulkadir S. A., Spitz D. R., Deng C. X., Gius D. (2010) SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 17, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J. B., Kubota S., Ban N., Yoshida M., Santeford A., Sene A., Nakamura R., Zapata N., Kubota M., Tsubota K., Yoshino J., Imai S. I., Apte R. S. (2016) NAMPT-mediated NAD(+) biosynthesis is essential for vision in mice. Cell Reports 17, 69–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Someya S., Yu W., Hallows W. C., Xu J., Vann J. M., Leeuwenburgh C., Tanokura M., Denu J. M., Prolla T. A. (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143, 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundaresan N. R., Gupta M., Kim G., Rajamohan S. B., Isbatan A., Gupta M. P. (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Invest. 119, 2758–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundaresan N. R., Bindu S., Pillai V. B., Samant S., Pan Y., Huang J. Y., Gupta M., Nagalingam R. S., Wolfgeher D., Verdin E., Gupta M. P. (2015) SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3β. Mol. Cell. Biol. 36, 678–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirschey M. D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D. B., Grueter C. A., Harris C., Biddinger S., Ilkayeva O. R., Stevens R. D., Li Y., Saha A. K., Ruderman N. B., Bain J. R., Newgard C. B., Farese R. V., Jr., Alt F. W., Kahn C. R., Verdin E. (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellizzi D., Rose G., Cavalcante P., Covello G., Dato S., De Rango F., Greco V., Maggiolini M., Feraco E., Mari V., Franceschi C., Passarino G., De Benedictis G. (2005) A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics 85, 258–263 [DOI] [PubMed] [Google Scholar]
- 26.Lanza I. R., Short D. K., Short K. R., Raghavakaimal S., Basu R., Joyner M. J., McConnell J. P., Nair K. S. (2008) Endurance exercise as a countermeasure for aging. Diabetes 57, 2933–2942; erratum: 61, 2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose G., Dato S., Altomare K., Bellizzi D., Garasto S., Greco V., Passarino G., Feraco E., Mari V., Barbi C., BonaFe M., Franceschi C., Tan Q., Boiko S., Yashin A. I., De Benedictis G. (2003) Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp. Gerontol. 38, 1065–1070 [DOI] [PubMed] [Google Scholar]
- 28.Edgett B. A., Hughes M. C., Matusiak J. B., Perry C. G., Simpson C. A., Gurd B. J. (2016) SIRT3 gene expression but not SIRT3 subcellular localization is altered in response to fasting and exercise in human skeletal muscle. Exp. Physiol. 101, 1101–1113 [DOI] [PubMed] [Google Scholar]
- 29.Qiu X., Brown K., Hirschey M. D., Verdin E., Chen D. (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 12, 662–667 [DOI] [PubMed] [Google Scholar]
- 30.Tasselli L., Zheng W., Chua K. F. (2017) SIRT6: novel mechanisms and links to aging and disease. Trends Endocrinol. Metab. 28, 168–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mostoslavsky R., Chua K. F., Lombard D. B., Pang W. W., Fischer M. R., Gellon L., Liu P., Mostoslavsky G., Franco S., Murphy M. M., Mills K. D., Patel P., Hsu J. T., Hong A. L., Ford E., Cheng H. L., Kennedy C., Nunez N., Bronson R., Frendewey D., Auerbach W., Valenzuela D., Karow M., Hottiger M. O., Hursting S., Barrett J. C., Guarente L., Mulligan R., Demple B., Yancopoulos G. D., Alt F. W. (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 [DOI] [PubMed] [Google Scholar]
- 32.Kim H. S., Xiao C., Wang R. H., Lahusen T., Xu X., Vassilopoulos A., Vazquez-Ortiz G., Jeong W. I., Park O., Ki S. H., Gao B., Deng C. X. (2010) Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 12, 224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwer B., Schumacher B., Lombard D. B., Xiao C., Kurtev M. V., Gao J., Schneider J. I., Chai H., Bronson R. T., Tsai L. H., Deng C. X., Alt F. W. (2010) Neural sirtuin 6 (Sirt6) ablation attenuates somatic growth and causes obesity. Proc. Natl. Acad. Sci. USA 107, 21790–21794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundaresan N. R., Vasudevan P., Zhong L., Kim G., Samant S., Parekh V., Pillai V. B., Ravindra P. V., Gupta M., Jeevanandam V., Cunningham J. M., Deng C. X., Lombard D. B., Mostoslavsky R., Gupta M. P. (2012) The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 18, 1643–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawahara T. L., Michishita E., Adler A. S., Damian M., Berber E., Lin M., McCord R. A., Ongaigui K. C., Boxer L. D., Chang H. Y., Chua K. F. (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136, 62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samant S. A., Kanwal A., Pillai V. B., Bao R., Gupta M. P. (2017) The histone deacetylase SIRT6 blocks myostatin expression and development of muscle atrophy. Sci. Rep. 7, 11877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanfi Y., Naiman S., Amir G., Peshti V., Zinman G., Nahum L., Bar-Joseph Z., Cohen H. Y. (2012) The sirtuin SIRT6 regulates lifespan in male mice. Nature 483, 218–221 [DOI] [PubMed] [Google Scholar]
- 38.Kanwal A., Singh S. P., Grover P., Banerjee S. K. (2012) Development of a cell-based nonradioactive glucose uptake assay system for SGLT1 and SGLT2. Anal. Biochem. 429, 70–75 [DOI] [PubMed] [Google Scholar]
- 39.Cheng Q., Dong W., Qian L., Wu J., Peng Y. (2011) Visfatin inhibits apoptosis of pancreatic β-cell line, MIN6, via the mitogen-activated protein kinase/phosphoinositide 3-kinase pathway. J. Mol. Endocrinol. 47, 13–21 [DOI] [PubMed] [Google Scholar]
- 40.Pillai V. B., Samant S., Sundaresan N. R., Raghuraman H., Kim G., Bonner M. Y., Arbiser J. L., Walker D. I., Jones D. P., Gius D., Gupta M. P. (2015) Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat. Commun. 6, 6656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sears B., Perry M. (2015) The role of fatty acids in insulin resistance. Lipids Health Dis. 14, 121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X. L., Zhang L., Youker K., Zhang M. X., Wang J., LeMaire S. A., Coselli J. S., Shen Y. H. (2006) Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes 55, 2301–2310 [DOI] [PubMed] [Google Scholar]
- 43.Nakamura S., Takamura T., Matsuzawa-Nagata N., Takayama H., Misu H., Noda H., Nabemoto S., Kurita S., Ota T., Ando H., Miyamoto K., Kaneko S. (2009) Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J. Biol. Chem. 284, 14809–14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sykiotis G. P., Papavassiliou A. G. (2001) Serine phosphorylation of insulin receptor substrate-1: a novel target for the reversal of insulin resistance. Mol. Endocrinol. 15, 1864–1869 [DOI] [PubMed] [Google Scholar]
- 45.Wagner G. R., Hirschey M. D. (2014) Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol. Cell 54, 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwon S., Seok S., Yau P., Li X., Kemper B., Kemper J. K. (2017) Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J. Biol. Chem. 292, 17312–17323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jheng H. F., Tsai P. J., Guo S. M., Kuo L. H., Chang C. S., Su I. J., Chang C. R., Tsai Y. S. (2012) Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 32, 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruni F., Polosa P. L., Gadaleta M. N., Cantatore P., Roberti M. (2010) Nuclear respiratory factor 2 induces the expression of many but not all human proteins acting in mitochondrial DNA transcription and replication. J. Biol. Chem. 285, 3939–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satterstrom F. K., Swindell W. R., Laurent G., Vyas S., Bulyk M. L., Haigis M. C. (2015) Nuclear respiratory factor 2 induces SIRT3 expression. Aging Cell 14, 818–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Z., Wu T., Zhao F., Lau A., Birch C. M., Zhang D. D. (2011) KPNA6 (Importin alpha7)-mediated nuclear import of Keap1 represses the Nrf2-dependent antioxidant response. Mol. Cell. Biol. 31, 1800–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Z., Zhang S., Chan J. Y., Zhang D. D. (2007) Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol. Cell. Biol. 27, 6334–6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pillai V. B., Bindu S., Sharp W., Fang Y. H., Kim G., Gupta M., Samant S., Gupta M. P. (2016) Sirt3 protects mitochondrial DNA damage and blocks the development of doxorubicin-induced cardiomyopathy in mice. Am. J. Physiol. Heart Circ. Physiol. 310, H962–H972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.An D., Rodrigues B. (2006) Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 291, H1489–H1506 [DOI] [PubMed] [Google Scholar]
- 54.Rodrigues B., Cam M. C., McNeill J. H. (1998) Metabolic disturbances in diabetic cardiomyopathy. Mol. Cell. Biochem. 180, 53–57 [PubMed] [Google Scholar]
- 55.Wagner G. R., Bhatt D. P., O'Connell T. M., Thompson J. W., Dubois L. G., Backos D. S., Yang H., Mitchell G. A., Ilkayeva O. R., Stevens R. D., Grimsrud P. A., Hirschey M. D. (2017) A class of reactive acyl-CoA species reveals the non-enzymatic origins of protein acylation. Cell Metab. 25, 823–837.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fritz K. S., Galligan J. J., Hirschey M. D., Verdin E., Petersen D. R. (2012) Mitochondrial acetylome analysis in a mouse model of alcohol-induced liver injury utilizing SIRT3 knockout mice. J. Proteome Res. 11, 1633–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thirumurthi U., Shen J., Xia W., LaBaff A. M., Wei Y., Li C. W., Chang W. C., Chen C. H., Lin H. K., Yu D., Hung M. C. (2014) MDM2-mediated degradation of SIRT6 phosphorylated by AKT1 promotes tumorigenesis and trastuzumab resistance in breast cancer. Sci. Signal. 7, ra71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miteva Y. V., Cristea I. M. (2014) A proteomic perspective of Sirtuin 6 (SIRT6) phosphorylation and interactions and their dependence on its catalytic activity. Mol. Cell. Proteomics 13, 168–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao Z., Zhang J., Kheterpal I., Kennedy N., Davis R. J., Ye J. (2011) Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. J. Biol. Chem. 286, 22227–22234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dinkova-Kostova A. T., Abramov A. Y. (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 88 (Pt B), 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan H., Guan D., Liu X., Li J., Wang L., Wu J., Zhou J., Zhang W., Ren R., Zhang W., Li Y., Yang J., Hao Y., Yuan T., Yuan G., Wang H., Ju Z., Mao Z., Li J., Qu J., Tang F., Liu G. H. (2016) SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 26, 190–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abel E. D. (2004) Insulin signaling in heart muscle: lessons from genetically engineered mouse models. Curr. Hypertens. Rep. 6, 416–423 [DOI] [PubMed] [Google Scholar]
- 63.Battiprolu P. K., Hojayev B., Jiang N., Wang Z. V., Luo X., Iglewski M., Shelton J. M., Gerard R. D., Rothermel B. A., Gillette T. G., Lavandero S., Hill J. A. (2012) Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J. Clin. Invest. 122, 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang S. J., Choi J. M., Chae S. W., Kim W. J., Park S. E., Rhee E. J., Lee W. Y., Oh K. W., Park S. W., Kim S. W., Park C. Y. (2011) Activation of peroxisome proliferator-activated receptor gamma by rosiglitazone increases sirt6 expression and ameliorates hepatic steatosis in rats. PLoS One 6, e17057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Satoh A., Brace C. S., Rensing N., Cliften P., Wozniak D. F., Herzog E. D., Yamada K. A., Imai S. (2013) Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 18, 416–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.