Abstract
Carnivorous rainbow trout exhibit prolonged postprandial hyperglycemia when fed a diet exceeding 20% carbohydrate content. This poor capacity to utilize carbohydrates has led to rainbow trout being classified as “glucose-intolerant” (GI). The metabolic phenotype has spurred research to identify the underlying cellular and molecular mechanisms of glucose intolerance, largely because carbohydrate-rich diets provide economic and ecological advantages over traditionally used fish meal, considered unsustainable for rainbow trout aquaculture operations. Evidence points to a contribution of hepatic intermediary carbohydrate and lipid metabolism, as well as upstream insulin signaling. Recently, microRNAs (miRNAs), small noncoding RNAs acting as negative posttranscriptional regulators affecting target mRNA stability and translation, have emerged as critical regulators of hepatic control of glucose-homeostasis in mammals, revealing that dysregulated hepatic miRNAs might play a role in organismal hyperglycemia in metabolic disease. To determine whether hepatic regulatory miRNA networks may contribute to GI in rainbow trout, we induced prolonged postprandial hyperglycemia in rainbow trout by using a carbohydrate-rich diet and profiled genome-wide hepatic miRNAs in hyperglycemic rainbow trout compared with fasted trout and trout fed a diet devoid of carbohydrates. Using small RNA next-generation sequencing and real-time RT-PCR validation, we identified differentially regulated hepatic miRNAs between these groups and used an in silico approach to predict bona fide mRNA targets and enriched pathways. Diet-induced hyperglycemia resulted in differential regulation of hepatic miRNAs compared with fasted fish. Some of the identified miRNAs, such as miRNA-27b-3p and miRNA-200a-3p, are known to be responsive to hyperglycemia in the liver of hyperglycemic glucose-tolerant fish and mammals, suggesting an evolutionary conserved regulation. Using Gene Ontology term-based enrichment analysis, we identify intermediate carbohydrate and lipid metabolism and insulin signaling as potential targets of posttranscriptional regulation by hyperglycemia-regulated miRNAs and provide correlative expression analysis of specific predicted miRNA-target pairs. This study identifies hepatic miRNAs in rainbow trout that exhibit differential postprandial expression in response to diets with different carbohydrate content and predicts posttranscriptionally regulated target mRNAs enriched for pathways involved in glucoregulation. Together, these results provide a framework for testable hypotheses of functional involvement of specific hepatic miRNAs in GI in rainbow trout.
Keywords: glucose, liver, microRNA, nutrition, rainbow trout
INTRODUCTION
Rainbow trout is a carnivorous species that is considered to be “glucose intolerant” (GI) due to its poor utilization of carbohydrates and pronounced hyperglycemia following the ingestion of carbohydrates that exceed 20% of dietary macronutrients (3, 41, 42, 59). While the pronounced and prolonged high carbohydrate diet-induced hyperglycemia has led to the description of rainbow trout as GI (34), it is important to note that the long-term consequences of diet-induced hyperglycemia in rainbow trout may differ compared with well-described phenotypes of GI in diabetic mammals (78). While high-carbohydrate diets have been described to consistently increase the hepatosomatic index in rainbow trout (2, 28, 52), both inhibitory (2, 28) and stimulatory (52) effects on body weight gain have been described, as well as a lack of glucotoxic responses (52) associated with prolonged hyperglycemia in diabetic humans and animal models of diabetes (78).
Because of the rainbow trout’s prominent role as a fish model in comparative physiology (12) and its commercial importance in freshwater aquaculture (54), the investigation of the molecular and cellular basis of GI in different tissues has been a research focus over the last two decades (49, 60, 61). In the context of comparative physiology, the identification of mechanisms underlying rainbow trout GI at the molecular, cellular, tissue, and organismal level may yield important insights into the evolution of GI in this species. In the context of rainbow trout aquaculture today, increasing the utilization of dietary carbohydrates represents a sustainable and economical alternative to the traditionally used economically and ecologically unsustainable fish-meal based protein diets (50, 54).
Driven by these basic and applied research goals, investigators have put forward several hypotheses to explain molecular and cellular underpinnings of the GI phenotype in several tissues in rainbow trout. For example, evidence has been reported for a role for gastrointestinal absorption of digestible carbohydrates in diet-induced GI in rainbow trout, which may be higher compared with amino acids in trout (40, 71). Conversely, at the level of excretion, evidence points to an involvement of the kidney. At the resorption maximum for glucose, plasma glucose concentration is 22 mmol/L in rainbow trout (9), twice the estimated amount of the 11 mmol/L in healthy humans and matching the rate observed in diabetic humans (76), suggesting that increased resorption capacity of glucose in the rainbow trout kidney also contributes to the GI phenotype in rainbow trout.
When one considers the metabolic fate of postprandial circulating glucose in rainbow trout in between absorption and excretion by metabolic tracing studies (6, 8, 21, 40) and calculation of metabolic flux (23), it is evident that the liver plays a particularly important role in intermediary glucose metabolism following the ingestion of dietary carbohydrates. The liver participates in the systemic regulation of circulating glucose concentrations specifically via regulation of glucose uptake, glycolysis, and glycogen deposition (6, 21) or de novo lipogenesis (DNL) (8, 21) on the one hand and de novo gluconeogenesis on the other (23). Molecular components of these pathways and their regulation have been interrogated over the past decade and have led to integrative hypotheses to include cellular and molecular components that underlie the contribution of the described intermediary metabolic pathways to GI in rainbow trout. First, while the rainbow trout liver efficiently responds to dietary carbohydrates as evidenced by strong induction of glucokinase expression and activity (55), the transcript abundance and/or activity of gluconeogenic enzymes are not inhibited in response to high dietary carbohydrates (32, 56). This suggests a contribution of hepatic gluconeogenesis to rainbow trout GI. Second, induction of transcripts encoding transcription factors and enzymes involved in hepatic DNL in genetically selected high-fat rainbow trout lines (32, 41, 42, 56) and pharmacologically treated (metformin) rainbow trout (57) correlates with improved responses to diet-induced hyperglycemia. This suggests that DNL may be limiting glucose utilization and hence contribute to persistent postprandial hyperglycemia in rainbow trout.
In addition to these key glucose and lipid metabolic pathways investigated at the gene transcript and activity level, upstream analysis of hepatic insulin signaling pathway has also been investigated comparatively in rainbow trout liver in vitro (15, 35) and in vivo (13, 14). These studies have revealed that gene expression and activity of hepatic lipogenic (fatty acid synthase) and gluconeogenic (glucose-6-phosphatase) enzymes are mediated via target of rapamycin (Tor) signaling, which is more responsive to proteins and amino acids than carbohydrates. These results indicate that nutrient-dependent activation of insulin/Tor signaling and downstream regulation of gene expression of intermediary metabolic genes involved in glucose and lipid metabolism have evolved to respond to dietary proteins, rather than carbohydrates in this carnivorous species. While this hepatic regulation in response to dietary proteins ensures suitable production of lipids preferably used as fuel by rainbow trout muscles compared with carbohydrates (10, 39) and sufficient amounts of de novo produced glucose to sustain tissues with high requirements for carbohydrates, including the brain, kidney, and spleen (10, 39), the reviewed literature reveals that such hepatic molecular, cellular, and metabolite-level regulation may underlie rainbow trout GI and poor carbohydrate utilization.
The recent sequencing of the rainbow trout genome and its annotation (4, 66) now allow for additional layers of investigation into hepatic mechanisms of GI in this species, first by allowing a more precise characterization of (possibly subfunctionalized) paralogs present in salmonid genomes (42), and second by enabling the study of the involvement of molecular epigenetic mechanisms, including DNA methylation and histone modifications at the DNA level (41) and posttranscriptional mechanisms, especially the microRNA (miRNA or miR)-based mRNA transcript regulation via 3′-untranslated region (UTR) binding (30, 43, 46). Indeed, the lack of suppression of hepatic G6pc activity has been linked to the atypical induction of two g6pcb2 paralogs in rainbow trout in response to dietary carbohydrates, suggesting that paralogs retained following genome duplication events may contribute to GI in rainbow trout (41, 42). In addition to global hypomethylation, reduction in methylation of specific CpG sites in proximity to transcription state sites was determined for both g6pcb2 paralogs, suggesting a potential role for DNA methylation dynamics in the differential regulation of these paralogs (41).
In contrast, while a functional proof-of-principle study revealed a role for the liver-specific miRNA-122 in the regulation of postprandial circulating glucose in trout (44), possibly via reduced hepatic DNL capacity consistent with described effects in mammalian knockout models (74), the possible involvement of hepatic miRNAs in the GI phenotype in rainbow trout has not been systematically investigated at the transcriptome level following the publication of the rainbow trout genome. Given that hepatic miRNAs have emerged as important regulators of hepatic glucose metabolism (37, 48) and are indeed markers (26, 27, 36, 82) and functional contributors (22, 63, 65) of GI in mammalian models of metabolic disease by targeting intermediary metabolic pathways of glucose and lipid metabolism and/or upstream insulin signaling pathways (22, 63, 65), the current study aims to comparatively assess a possible role for hepatic miRNAs in GI in rainbow trout by a multitiered approach. First, we globally determine a possible involvement for hepatic miRNAs in diet-induced GI by profiling key components of the canonical miRNA biogenesis machinery in fasted trout compared with trout refed a diet devoid of carbohydrates and enriched in carbohydrates that induces GI. Second, to identify specific hepatic miRNAs that may be implicated in GI in rainbow trout, we identify differentially expressed hepatic miRNAs between fasted trout and two groups of trout: one fed a diet devoid of carbohydrates and the other fed a diet enriched in carbohydrates that induces GI, using a transcriptome level sequencing approach. Lastly, to infer possible functional posttranscriptional consequences at the genome-wide level, we use an in silico approach to predict whether differential expressed hepatic miRNAs target genes and enriched pathways relevant to GI in rainbow trout, and assess the activity of these pathways by using molecular markers. In doing so, we prioritize specific hepatic miRNAs for future functional study in the rainbow trout liver.
METHODS
Experimental Design, Diets, and Tissue Sampling
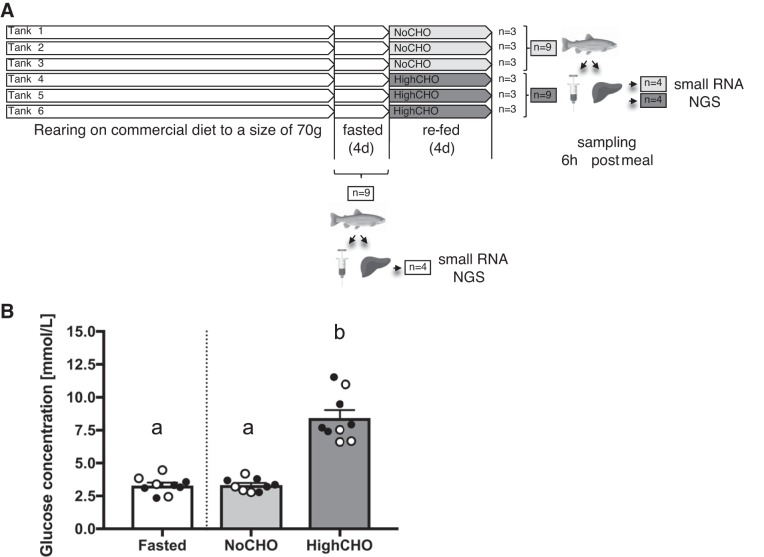
The liver samples used to profile miRNAs in the current study were obtained from an experiment originally described by Marandel et al. (41, 42). In brief, juvenile rainbow trout (70 g body mass) were distributed in six tanks (n = 15 fish per tank) and reared at 17°C in the INRA experimental facilities at Saint-Pée-sur-Nivelle, France. After 4 days of total starvation (Fasted), two fish per tank were euthanized. Fish were then fed twice a day at a ratio of 2.5% body weight for 4 days with one of two diets (Fig. 1A): a NoCHO (diet without carbohydrate) and a HighCHO (diet with ~30% carbohydrates), prepared at INRA, Saint-Pée-sur-Nivelle, France, as pressed pellets. The two diets were isolipidic and isoenergetic. Gelatinized starch was included as carbohydrate source, protein was provided by fishmeal, and dietary lipid by fish oil and fish meal. Inclusion of carbohydrates (30%) in the HighCHO diet was compensated for by a decreased dietary protein level (40%), which was still above the 37% protein requirement of rainbow trout (49a). No carbohydrates were added to the NoCHO diet, which contained 60% crude protein. A detailed description of diet composition can be found in Additional File 1. (Supplemental material for this article can be found at https://figshare.com/s/d2939b753fc65b1455e7.)
Fig. 1.
Overview of the experimental design, tissue sampling and experimental end points (A). Mean serum/plasma glucose concentration (+SE) in rainbow trout fasted for 2 days and in rainbow trout 6 h after being refed either a NoCHO diet (devoid of carbohydrates) or a HighCHO diet (containing 30% carbohydrates) (B). Diets used were isoenergetic. Individual data are represented by dots, with open circles representing samples from individual fish that correspond to randomly selected liver samples (n = 4) used for next-generation sequencing (NGS). Two separate one-way ANOVAs followed by Tukey’s post hoc (P < 0.05) tests revealed a significant induction of hyperglycemia 6 h after refeeding a carbohydrate-rich diet, both in the full sample set (F2,27 = 74.89, P < 0.01), as well as in the randomly selected subset of 4 samples per group (F2,12 = 18.27, P < 0.01). Different letters indicate significantly different glucose concentrations between groups.
Each dietary treatment was administered in three tanks, and neither body weight nor standard length changed between groups over the course of the experiment. Fish were euthanized 6 h after the last meal (n = 9 fish per diet, 3 per tank). Gut content of the sampled animals was systematically checked to confirm that the fish sampled had consumed the diet. Blood was removed from the caudal vein via heparinized syringes and centrifuged (3,000 g, 5 min). The plasma recovered was immediately frozen and kept at −20°C until analysis to confirm expected hyperglycemia in the HighCHO diet-fed group. The fresh liver of each fish was dissected and frozen immediately in liquid nitrogen and then kept at −80°C. A subset of n = 4 randomly selected samples per treatment group was used for miRNA profiling. The randomly selected samples maintained the different glucose profiles previously reported (Fig. 1B).
Ethics Approval and Consent to Participate
All investigations were conducted according to the guiding principles for the use and care of laboratory animals and in compliance with French and European regulations on animal welfare (Décret 2001-464, 29 May 2001 and Directive 2010/63/EU, respectively). Fish were killed by concussion/blow to the skull, and death was confirmed by exsanguination. No anesthetic was used to avoid bias in analysis of enzymatic activity. This protocol and the project as a whole were approved by the French National Consultative Ethics Committee.
Small RNA Next-generation Sequencing and Differential Expression Analysis
Total RNA was extracted by the Trizol method as previously described (41, 42), and the quality of total RNA was confirmed twice (before and after shipping of RNA samples) by RNA integrity numbers (RIN) >9.2 with an Agilent Technologies 2100 Bioanalyzer (Agilent, Mississauga, ON, Canada). Small RNA sequencing was performed by using services provided by LC Sciences (Houston, TX) using four randomly selected samples from each treatment group. Nucleotide fractions (15–50 nt) of small RNA were isolated from the total RNA by polyacrylamide gel electrophoresis and were ligated to a 30 nt adapter followed by a 50 nt adapter (TruSeq Small RNA Library Prep kit; Illumina, San Diego, CA). The small RNA ligated to the adaptors was reverse transcribed to cDNA, PCR amplified, and gel purified. The gel-extracted cDNA was used for library preparation, which was further used for cluster generation on Illumina's Cluster Station before sequencing using Illumina GAIIx. Raw sequence data were obtained from image data with Illumina's Sequencing Control Studio software version 2.8 (SCS v2.8) following real-time sequencing image analysis and base-calling by Illumina's Real-Time Analysis version 1.8.70 (RTA v1.8.70) and deposited in the National Center for Biotechnology Information (NCBI) Gene expression Omnibus (GEO) under accession number GSE112814 with specific files (n = 4) for fasted groups (GSM3084221-GSM3084224), refed NoCHO (GSM3084224-GSM3084228), and refed HighCHO (GSM3084229-GSM3084232).
Briefly, sequences were initially filtered with the proprietary LC Science ACGT10-miR v4.2 pipeline to remove low-quality sequences, low-complexity sequences, and sequences corresponding to common RNA families (mRNA, RFam, Repbase, piRNA), as described by Ma and colleagues (38). Retained high-quality sequences were then submitted to a bowtie search against all fish miRNA gene sequences deposited in miRbase v21.0 (1 mismatch allowed in the first 16 nt). In cases where unique sequences mapped to the miRbase-deposited fish miRNA genes, the obtained miRbase hits were subsequently mapped to the rainbow trout genome to determine genomic locations. Any alignment that had already previously been characterized as a genomic location for this specific microRNA in the rainbow trout genome (30) was identified as known rainbow trout miRNA (gp 1a). All miRNAs that were successfully aligned to miRbase deposited fish species miRNA genes, and had not been described in the rainbow trout miRNA repertoire but mapped to the rainbow trout genome, were retained as trout microRNAs (gp 1b). The remaining miRbase hits form both gp1a and gp1b that in addition to known miRNA loci also mapped to other rainbow trout genome loci were designated as gp1c.
High-quality sequences that could be matched to fish species miRNA genes in miRbase, which in turn could not be mapped to the rainbow trout genome, were further analyzed as follows: The high-quality short sequence was directly mapped onto the rainbow trout genome, and the locus further analyzed for its possibility to generate hairpin transcripts. This was achieved by retrieving 80 nt flanking sequences of mapped high-quality sequences. Retrieved sequence were then analyzed for their propensity to form hairpin structures by RNA-fold software (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi) with the following criteria: 1) ≤12 nt in one bulge in stem, 2) ≥16 bp in the stem region of the predicted hairpin, 3) ≤−15 kCal/mol cut-off of ∆G, 4) ≥50 hairpin length that contains up and down stems and hairpin loop, 5) a ≤200 nt hairpin loop length, 6) ≤4 nt in one bulge in the mature miRNA region, 7) ≤2 errors in one bulge in mature region, 8) ≤2 biased bulges in mature region, 9) ≤4 errors in the mature miRNA region, 10) ≥12 bp in the mature region of the predicted hairpin, 11) >80% of mature miRNA is in located in the stem. Sequences in located in loci predicted to form hairpins were grouped as gp2a, and sequences mapped to loci not predicted to form hairpins were designated as gp2b. High-quality sequences mapped to fish miRNA genes deposited in miRbase, which neither mapped to the rainbow trout genome as fish miRbase gene hit, nor as mature sequence, but had been identified as mature miRNA in rainbow trout were designated gp3a. Conversely, high-quality sequences mapped to fish miRNA genes deposited in miRbase, which neither mapped to the rainbow trout genome as fish miRbase gene hit, nor as mature sequence, and had not been identified as mature miRNA in rainbow trout were designated gp3b. Finally remaining sequences that could not be matched to miRbase fish species but could be mapped to the rainbow trout genome were again tested for the potential to form hairpin structures with flanking sequences. Sequences predicted to be able to form hairpins were designated as gp4a, sequences predicted to be unable to form hairpins were designated as gp4b. An overview of the bioinformatics pipeline and databases used are provided in Additional Files 2 and 3.
Differentially expressed miRNAs were identified with the statistical software R (version 3.2.2) package DESeq2 for one-way ANOVA comparison of all three treatment groups (fasted-NoCHO-HighCHO), and t-tests for specific between-group comparisons (fasted-NoCHO, fasted-HighCHO, NoCHO-HighCHO).
Relative mRNA quantification.
Total RNA was extracted by the Trizol method as previously described (41, 42), and the QuantiTect reverse transcriptase kit (Qiagen) was used to generate mRNA cDNA with total RNA according to the manufacturer’s protocol. The mRNA abundance levels of several specific targets (Table 1) were assessed by two-step real-time RT-PCR using the CFX96-Real-Time System-C1000 Thermal Cycler machine (Bio-Rad). Reactions were prepared in a total volume of 20 μL, containing 10 μL of SsoAdvanced Universal Inhibitor-Tolerant SYBR Green Supermix (Bio-Rad), 4 μL of diluted cDNA template, 0.5 μL of each specific forward and reverse primer at a concentration of 10 nM (IDT, Table 1), and 5 μL of H2O. Elongation factor 1 (ef1α) mRNA abundance was used to normalize mRNA gene expression (51). A noRT and noTemp reaction was included to control for DNA contamination in each assay. Standard curves were generated for each primer pair from serially diluted (1:2) pooled cDNA to determine amplification efficiency and R2 values. Following an enzyme activation step at 98°C (2 min), two steps were repeated for 40 cycles. These included a denaturation step at 95°C (20 s) and a combined annealing and extension step at primer-specific temperatures (Table 1). Following each run, melting curves were produced by gradual (0.5°C increase each 5 s) increase in temperature from 65 to 95°C and were monitored for single peaks suggesting specific amplicon production and absence of primer dimers. Specificity of newly designed primer pairs was furthermore confirmed by sequencing (OHRI, Ottawa, ON, Canada), and product sequences confirmed via NCBI Basic Local Alignment Search Tool search of the trout genome. The amplification efficiencies were between 92.3 and 112.8%, and the R2 was >0.98 (see Table 1). As for the miRNAs, normalized mRNA abundance (mRNA/ef1α) was calculated relative to the fasted group and presented as fold changes according to the ∆∆CT method (67). Data were analyzed with Prism Version 7 and transformed to fit normal distribution. In normally distributed data, Grubb’s outlier test was used to identify and remove possible single outliers in a treatment group. Data were subsequently analyzed by a one-way ANOVA followed by Tukey’s post hoc test. In cases where data did not meet normality and homoscedasticity criteria as assessed by Shapiro-Wilk and Bartlett’s test, data were analyzed by a Kruskal-Wallis test followed by Dunn’s multiple comparison test. Significance was determined at a P < 0.05 level.
Table 1.
Real-time RT-PCR assay primer sequences and reaction parameters
| Gene Target | Primer Pair (5′ to 3′) | Annealing Temperature, °C | Efficiency, % | R2 |
|---|---|---|---|---|
| Canonical microRNA biogenesis pathway | ||||
| drosha | F: GAGGAGTCGGTGAAGGAATG | 60 | 92.3 | 0.98 |
| GSONMT00009052001 | R: CATGTGGGAGAAGAGGGAGA | |||
| xpo5a | F: ACTCTCGAACACGGCTGATG | 59 | 103.3 | 0.99 |
| GSONMT00065065001 | R: CACACATATCAGCCACCGGT | |||
| xpo5b | F: AGTCAACTGGGTGGGGATTC | 55 | 108.6 | 0.98 |
| GSONMT00007401001 | R: TCCCACCTCAGACATGCTCA | |||
| ago2a | F: GTGGTGGGGTGGACTCATTT | 59 | 108.0 | 0.98 |
| GSONMT00060791001 | R: AAACCTTCAATCGGGCCCTT | |||
| ago2b | F: TGTCGCACGGTGTTAACATG | 58 | 101.3 | 0.99 |
| GSONMT00025973001 | R: AGCAATGCCGAGAAGAGCTA | |||
| miRNAs | ||||
| miR-200a-3p | TaqMan probe MIMAT0002981 | 60 | 98.1 | 0.97 |
| miR-27b-3p | TaqMan probe MIMAT0001797 | 60 | 102.8 | 0.99 |
| miR-122-5p | TaqMan probe MIMAT0001818 | 60 | 96.5 | 0.98 |
| Markers of hepatic lipid metabolism | ||||
| srebp1c | F: GACAAGGTGGTCCCAGTTGCT | 58 | 98.8 | 0.99 |
| n/a | R: CACACGTTAGTCCGCATCAC | |||
| fasn | F: TGATCTGAAGGCCCGTGTCA | 58 | 91.8 | 0.98 |
| n/a | R: GGGTGACGTTGCGTGGTAT | |||
| cpt1a | F: TCGATTTTCAAGGGTCTTCG | 57 | 103.1 | 0.98 |
| n/a | R: CACAACGATCAGCAAAGTGG | |||
| Other targets of interest | ||||
| ir | F: TCAGGAGCAGGAGGACTTTG | 50 | 96.8 | 0.98 |
| GSONMT00026581001 | R: GTGTGTGTACGGGACGTGTT | |||
| rac1 | F: CGACCACTGTCCTATCCACA | 54 | 112.8 | 0.98 |
| GSONMT00034847001 | R: TGATGGGGGTGAGTTTCTTC | |||
| glut9 | F: GTGTTCCTGGTGGTGTGTGT | 58 | 106.9 | 0.99 |
| GSONMT00066217001 | R: CTTGATGGCAAGTTCGTTCTC | |||
| adp-gk | F: GTTCTGCTGGCGTACTGGTT | 50 | 96.3 | 0.99 |
| GSONMT00073455001 | R: TTCATCCCCACTTTGCTTTC | |||
| soga1 | F: GCTGCACCAGTACACAGGAA | 58 | 92.8 | 0.99 |
| GSONMT00057063001 | R: GCAAAGCAGAAGGACAGGA | |||
| mfn1 | F: AGGGAGCTGGAGGGAGAAAT | 55 | 108.2 | 0.99 |
| GSONMT00027395001 | R: CCAAGACAGGCTCCAGTGAA | |||
| mfn2 | F: ACTGCTCCGGAATAAGGCTG | 55 | 98.4 | 0.99 |
| GSONMT00004526001 | R: GTGGTCTTTTCGCGGTCAAC | |||
| Fis | F: GGTTTGGTTGGCATGGCAAT | 55 | 103.2 | 0.99 |
| GSONMT00039888001 | R: TTCTCATCTTGGGGAACGGC | |||
| Reference genes | ||||
| ef1a | F: CATTGACAAGAGAACCATTGA | 56 | 102.6 | 0.97 |
| n/a | R: CCTTCAGCTTGTCCAGCAC | |||
F, forward; R, reverse, n/a, not available.
Quantitative miRNA quantification.
miRNA cDNA was synthesized by TaqMan MicroRNA Reverse Transcription Kit (Life Technologies, Burlington, ON, Canada) with 10 ng of total RNA per 15 μL RT reaction containing 0.15 μL 100 mM dNTPs (with dTTP), 1.00 μL MultiScribe Reverse Transcriptase 50 U/μL, 1.50 μL 10× Reverse Transcription Buffer, 0.19 μL RNase Inhibitor 20 U/ μL, 4.16 μL nuclease-free water, 5 μL total RNA, and 3 μL miRNA-specific 5× RT primer following the manufacturer’s protocol. Two-step real-time RT-PCR was used to validate the abundance of miR-122-5p, miR-27b-3p, miR-200a-3p, miR-21, miR-152, miR-722 using the CFX96-Real-Time System-C1000 Thermal Cycler machine (Bio-Rad, Mississauga, ON, Canada). Real-time RT-PCR reactions were prepared in a total volume of 20 μL per reaction, containing 1.00 μL TaqMan Small RNA Assay 20×, 1.33 μL Product from RT reaction, 10.00 μL TaqMan Universal PCR Master Mix (2×) with UNG, and 7.67 μL nuclease-free water. A no reverse transcriptase (noRT), where the RT was replaced with nuclease-free water, and a no template (noTemp), where product from RT reaction was replaced with nuclease-free water, were included during cDNA synthesis as controls for DNA contamination. Following an activation step at 50°C (2 min) to activate UNG and another activation step at 95°C (10 min) to activate the polymerase in the qPCR mix, two steps were repeated for 40 cycles including the denaturation at 95°C (15 s) and a combined annealing and extending step at 60°C (60 s). Assays were subsequently normalized by the NORMA-Gene approach as described by Heckman et al. (24) miRNA fold changes were calculated relative to the fasted group. Data were analyzed with Prism version 8 (GraphPad Software, La Jolla, CA) and transformed to fit normal distribution when necessary. In normally distributed data, Grubb’s outlier test was used to identify and remove possible single outliers in a treatment group. Data were subsequently analyzed by a one-way ANOVA followed by Tukey’s post hoc test. In cases were data did not meet normality and homoscedasticity criteria as assessed by Shapiro-Wilk and Bartlett’s test, data were analyzed by a Kruskal-Wallis test followed by Dunn’s multiple-comparison test. Significance was determined at a P < 0.05 level.
Protein Extraction and Cell Signaling Quantification
Fractions (~100 mg) of frozen livers (n = 4 per treatment group) were weighted into 1 mL of lysis buffer [150 mM NaCl, 10 mM Tris HCl, 1 mM EGTA, 1 mM EDTA (pH 7.4), 100 mM sodium fluoride, 4 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 1% Triton X-100, 0.5% NP-40-IGEPAL, and a protease inhibitor cocktail (Roche, Basel, Switzerland)] and homogenized using a Precellys-Cryolys (Bertin Instruments, Montigny-le-Bretonneux, France). We centrifuged the homogenates at 1,000 g for 15 min at 4°C and then recovered the supernatant to centrifuge again at 20 000 g for 30 min at 4°C. The resulting supernatant fractions were recovered and stored at −80°C. Protein concentrations were determined with the Bio-Rad protein assay kit (Bio-Rad Laboratories, Munich, Germany) with BSA (bovine serum albumin) as the standard. Lysates (20 μg of the total protein) were subjected to SDS-PAGE and wet-transfer blotting. Appropriate antibodies were obtained from Cell Signaling Technologies (Ozyme, Saint Quentin Yvelines, France). Anti-phospho-Akt (Ser473, #4060), anti-Akt (#9272), Anti-phospho-Tor (Ser2448, #5536), anti-Tor (#2972), Antiphospho-S6 (Ser235/236, #4858), anti-S6 (#2217) were used on the Western blots. All the antibodies have been shown to cross-react successfully with rainbow trout proteins of interest (13–16, 35, 44, 70). After being washed, membranes were incubated with an IRDye infrared secondary antibody (LI-COR Biosciences). The bands were visualized by infrared fluorescence using the odyssey Imaging System (LI-COR Biosciences) and quantified by Odyssey Infrared Imaging System software (version 3.0, LI-COR Biosciences). Data were analyzed with Prism version 7 and transformed to fit normal distribution. In normally distributed data, Grubb’s outlier test was used to identify and remove possible single outliers in a treatment group. Data were subsequently analyzed by a one-way ANOVA followed by Tukey’s post hoc test. In cases where data did not meet normality and homoscedasticity criteria as assessed by Shapiro-Wilk and Bartlett’s test, data were analyzed by a Kruskal-Wallis test followed by Dunn’s multiple-comparison test. Significance was determined at a P < 0.05 level.
In Silico miRNA-Target Analysis
To predict rainbow trout-specific mRNA targets of differentially regulated miRNAs, we utilized the miRanda package initially developed in the Enright laboratory (18). The miRanda algorithm places emphasis on seed match and free energy of the duplex structure, two of the most relevant aspects in miRNA-target interaction (1, 62). Among the available miRNA target prediction algorithms (20, 58), we chose the miRanda algorithm based on its increased sensitivity compared with other algorithms (77), as well as its previous application across several rainbow trout transcriptome-level miRNA target prediction studies (33, 46, 53), in an effort to facilitate comparison between studies. Since, increased sensitivity occurs at the cost of an increased rate of false-positive predictions, we used a stringent cut-off with a miRanda pairing score S > 140, and a free-energy score ΔG cut-off <−20, where S is the sum of single residue-pair match scores over the alignment trace, and ΔG is the free energy of duplex formation calculated by the Vienna package (29). Annotated 3′-UTRs were taken from the microTrout database (46).
Gene Ontology Enrichment, Pathway, and Subnetwork Enrichment Analysis
Gene Ontology (GO) enrichment analysis of mRNA targets predicted to be regulated by differentially expressed miRNAs is a widely used approach to infer possible biological consequence at the genome regulation level in rainbow trout and mammalian models (26, 33, 53). We performed GO enrichment analysis in JMP Genomics V8.1. Parametric analysis of gene expression (PAGE) was conducted to determine which GO terms were significantly enriched in the data set for each experiment based upon predicted targets of miRNAs. The rainbow trout genome was used as a background list for comparison. Genes were considered to be miRNA targets if transcript-miRNA predicted by the miRanda algorithm had cut-off values of S > 140 and ∆G < −20. The P value for each GO term was corrected by false discovery rate (FDR). To build pathways for gene targets, we imported the short list of genes within a group of GO categories (i.e., those related to “glucose metabolism”) into Pathway Studio 11.4 (Elsevier). The software uses known relationships (i.e., based on expression, binding, common pathways) between genes to create networks focused around a cell process. The miRNA targets were imported into the program using the “Name + Alias” and rainbow trout genes were mapped to their mammalian homologs. Lastly, the miRNA targets were subjected to a subnetwork enrichment analysis in Pathway Studio for “Cell Process.” This approach was used to achieve a general overview of cell processes that may be relevant to hepatic regulation of glucose and intermediary metabolism in general, those that may be controlled posttranscriptionally by the regulated miRNA network.
Correlative Analysis of miRNA-mRNA Targets
Specific mRNA targets belonging to enriched pathways of interest predicted to be regulated by differentially expressed miRNAs were quantified to elucidate a potential involvement in GI in response to HighCHO in rainbow trout and to determine miRNA-mRNA relationships correlatively. While not indicative of functional relationship, such correlations may aid to prioritize specific miRNA-mRNA target pairs for future functional studies. Correlation of expression between differentially expressed miRNAs and specific targets were assessed by Pearson coefficient and its significance computed in Prism Version 7 (GraphPad Software).
RESULTS
Transcripts of the miRNA Biogenesis Pathway are Responsive to High Dietary Carbohydrates In Rainbow Trout
Responsiveness to dietary carbohydrates of key enzymes of the miRNA biogenesis pathway was probed at the mRNA abundance level in rainbow trout liver (Fig. 2). Specifically, drosha (Fig. 2A), which exists as single locus in the rainbow trout genome (5), and the two salmonid paralogs of xpo5 (Fig. 2, B and C) and ago2 (Fig. 2, D and E) were quantified in rainbow trout that were fasted or refed with either a NoCHO and a HighCHO diet. The mRNA abundance of drosha (F2,24 = 6.459, P = 0.0057; Fig. 2A) was significantly induced postprandially in trout fed HighCHO (P = 0.0041) but not NoCHO diets (P = 0.1379). Conversely, both xpo5a (F2,24 = 5.332, P = 0.0121; Fig. 2B) and xpo5b (F2,24 = 3.995, P = 0.0318; Fig. 2C) were induced by both NoCHO (P = 0.0031 and P = 0.0004, respectively) and HighCHO diets (P = 0.0001 and P = 0.0001, respectively) compared with fasting; the HighCHO also led to a stronger induction of the xpo5b paralog compared with NoCHO diet (P = 0.0003). With regard to ago2 paralogs, ago2a (F2,24 = 3.615, P = 0.0424; Fig. 2D) was significantly induced in HighCHO diet-fed fish compared with fasted fish (P = 0.0368), while no induction was observed in NoCHO diet-fed fish compared with fasted fish (P = 0.6585). No change in mRNA abundance was observed between groups for the ago2b paralog (F2,23 = 0.7908, P = 0.1578; Fig. 2E).
Fig. 2.
Relative steady-state mRNA abundance (+SE) of hepatic canonical miRNA biogenesis components drosha (A), exportin 5 (B, C), and argonaute 2 (D, E) in rainbow trout 6 h after being refed either a NoCHO diet (devoid of carbohydrates) or a HighCHO diet (containing 30% carbohydrates) normalized to elongation factor 1α and expressed as relative fold change compared with rainbow trout fasted for 2 days. In cases where data were normally distributed and met the criteria of homoscedasticity, a one-way ANOVA followed by Tukey’s post hoc was used for analysis, whereas a Kruskal-Wallis test followed by Dunn’s multiple comparisons test was used in cases where data did not meet these criteria. A P value of P < 0.05 was used as cut-off for significant effects.
Small RNA Next-generation Sequencing and Identification of Hepatic miRNAs in Rainbow Trout
Of the overall ~96.18 M raw reads, 55.56 M read (57.8%) were excluded first due a lack of 3′-adaptor or 3′-ADT (~44.85 M or 46.6%) and second because of nucleotide size outside the targeted range of 15–32 nt (~10.7 M or 11.2%) after 3′-ADT sequence screening, resulting in ~40.60 M mappable reads, of which a combined 78% exhibit a size between 19 and 23 nt (Additional File 4). The Phred Score distribution of reads was larger than 30 (Additional File 4), indicating a probability of incorrect base calls of less than 1 in 1,000 nucleotides (or 99.9% accuracy). With regard to individual sample sequencing depth, a range between ~6.4 M and ~9.4 M reads per sample was obtained (Additional File 5). Following quality assurance of small RNA sequencing, the total ~40.6 M mappable reads was annotated in specific groups according to the described pipeline. In this process, a total of ~23.42 M or 57.7% of reads were annotated as 604 known miRNAs (group 1), while ~2.64 M or 4.9% of reads were annotated as 1,278 predicted miRNAs (groups 2–4), summarized in Additional File 6. A total of ~12.44 M or 38.9% of mappable reads were annotated as other RNA species or did not yield any hit (Additional File 7). Thus, we identified a total of 1886 unique miRNAs, whose specific sequences are available in Additional File 5.
Trout Hepatic miRNAome Responds More Strongly to Diets High in Carbohydrates than Diets Devoid of Carbohydrates
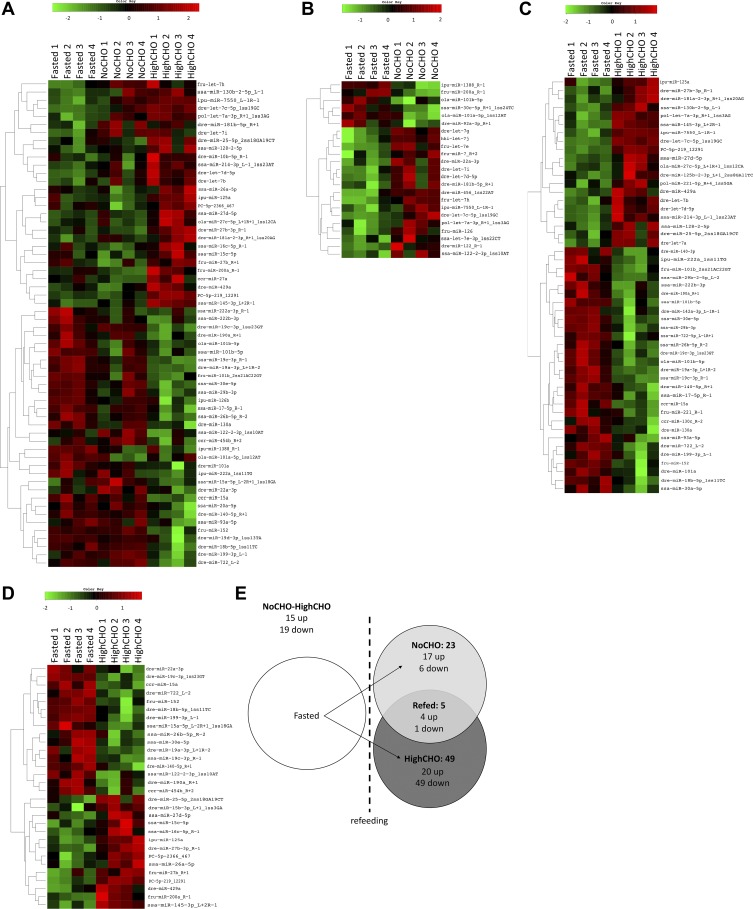
Differential hepatic miRNA abundance was analyzed globally between fasted, NoCHO, and HighCHO groups by one-way ANOVA (Fig. 3A), which identified a total of 60 differentially expressed candidates between all three experimental groups. Pair-wise comparisons investigating differentially expressed hepatic miRNA between fasted and NoCHO (Fig. 3B) and fasted and HighCHO (Fig. 3C) identified 23 (17 up, 6 down) differentially regulated miRNAs in response to NoCHO diet, and 49 (20 up 29 down) in response to HighCHO diet. When we compared NoCHO and HighCHO by pairwise comparison (Fig. 3D), 30 miRNAs (14 up, 16 down) were differentially regulated. A summary of differentially regulated miRNAs across all pairwise comparisons is shown in Fig. 3E. All individual miRNA sequences, their normalized count numbers, and their differential regulation for all comparisons displayed in Fig. 3 can be accessed in detail in Additional File 8.
Fig. 3.
Heat maps showing hierarchical clustering of differentially regulated miRNAs between Fasted, NoCHO, and HighCHO analyzed by one-way ANOVA (A) and between Fasted-NoCHO (B), Fasted-HighCHO (C), and NoCHO-HighCHO (D) analyzed by t-test. E: summary of differentially regulated miRNAs across all pairwise comparisons.
Real-time TaqMan RT-PCR Validation of Specific miRNAs
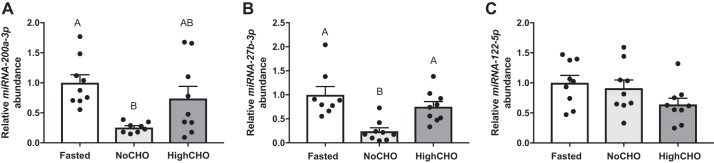
We quantified the expression of three specific miRNAs, miR-200a-3p (Fig. 4A), miRNA-27-3p (Fig. 4B), and hepatic miRNA abundance of miRNA-122-5p (Fig. 4C). Hepatic abundance of miRNA-200a-3p (F2,26 = 6.394 P < 0.01, Fig. 4A) was reduced in the NoCHO group (P = 0.0048) but not HighCHO group compared with the fasted group (P = 0.4187). A marginal, but nonsignificant increase in HighCHO compared with NoCHO was observed (P = 0.0764). Hepatic abundance of miRNA-27b-3p (F2,26 = 17.83, P < 0.01, Fig. 4B) was significantly decreased in response to NoCHO (P = 0.0001) but not HighCHO (P = 0.6181) compared with fasted fish, and significantly higher in HighCHO compared with NoCHO diets (P = 0.0003). No significant change in miRNA-122-5p was observed (F2,26 = 17.83, P > 0.05, Fig. 4C).
Fig. 4.
Relative steady-state miRNA abundance (+SE) of miRNA-200a-3p (A), miRNA-27b-3p (B), and miRNA-122-5p (C) in rainbow trout 6 h after being refed either a NoCHO diet (devoid of carbohydrates) or a HighCHO diet (containing 30% carbohydrates) normalized with NORMA-Gene and expressed as relative fold change compared with fasted group analyzed by real-time TaqMan RT-PCR assay (n = 9 samples per group). In cases where data were normally distributed and met the criteria of homoscedasticity, a one-way ANOVA followed by Tukey’s post hoc was used for analysis, whereas a Kruskal-Wallis test followed by Dunn’s multiple-comparisons test was used in cases where data did not meet these criteria. A P value of P < 0.05 was used as cut-off for significant effects.
GO Term Analysis of In Silico-predicted Targets of Differentially Regulated miRNAs Identifies Glucose and Intermediary Metabolic Pathways as Targets for Posttranscriptional Regulation in Response to Dietary Carbohydrates
By applying the miRanda algorithm used in microTrout (46), we identified specific 3′-UTR targets for all differentially regulated miRNAs in specific comparisons, the results of which can be consulted in Additional File 9. Using the GO term annotations based on genes containing these 3′-UTRs, we generated GO-term enrichment analysis to identify diet-dependent postranscriptionally regulated pathways in silico. The complete list of enriched GO terms and their probability of being enriched are contained in Additional File 10. GO terms can be overrepresented or underrepresented in a data set compared with a reference data set. The overrepresented GO terms are indicated as “0,” while the underrepresented GO terms are represented with “1” (Additional file 10). For figures and subsequent discussion, we focus exclusively on GO terms that were overrepresented in a predicted gene target list compared with the rainbow trout genome and are relevant to hepatic glucose and intermediary metabolism, summarized in Additional File 11. Specific miRNA-mRNA predictions within enriched metabolic pathways are listed in Additional File 12. A visual representation of immediate glucose metabolic pathways and their interactions predicted to be subject to posttranscriptional regulation by differential miRNAs between NoCHO and HighCHO groups obtained by SNEA analysis is shown in Additional Files 13 and 14.
Assessment of Hypotheses of Hepatic Cellular and Molecular Contributions to GI in Rainbow Trout
Following the identification of gluconeogenesis, lipogenesis, and insulin signaling as being regulated by differentially regulated miRNAs in response to dietary carbohydrates (Additional File 11), we assessed functional changes in these pathways using well-established rainbow trout markers at the molecular and cellular level. A lack of suppression of de novo gluconeogenesis, potentially linked to an induction of g6pc2 paralogs that are induced by HighCHO, had previously already been reported for our tissue samples at the transcript and enzyme activity level (41). We therefore used well-established general gene expression markers of rainbow trout DNL (13–15, 70) and protein markers of insulin signaling (13–16, 35, 70) to address the additional hypotheses that limited hepatic DNL and/or insulin signaling is associated with GI in rainbow trout fed excess carbohydrates.
Hepatic markers of DNL are not induced by the carbohydrate-rich diet.
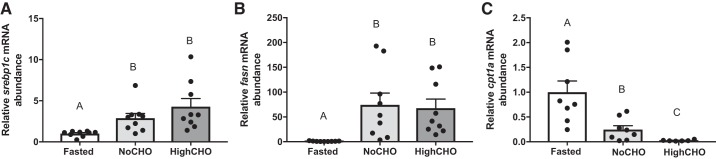
Compared with fasted rainbow trout, hepatic expression of the lipogenic transcription factor srebp1c (H2,27 = 15.62, P = 0.0004; Fig. 5A) and the lipogenic enzyme fatty acid synthase fasn (F2,24 = 47.49, P = 0.0001; Fig. 5B) was induced by refeeding, irrespective of dietary carbohydrate content: In the case of srebp1c, an induction of mRNA abundance was observed in both rainbow trout refed NoCHO (P = 0.0108) and HighCHO diets (P = 0.0005) compared with fasted trout, while no difference between diets was observed (P = 0.9999). Similarly, fasn (P = 0.0001) mRNA abundance increased in rainbow trout refed both NoCHO (P = 0.0001) and HighCHO diets, while no difference was found between refed dietary treatment groups (P = 0.9107). Conversely, while the mRNA abundance of the mitochondrial transport protein cpt1a (F2,19 = 4.814, P = 0.0203; Fig. 5C), the rate-limiting enzyme in fatty acid oxidation, was decreased in both NoCHO (P = 0.0028) and HighCHO (P = 0.0001) diet groups compared with fasted fish, the inhibition response was also diet dependent, as it was stronger in HighCHO-fed trout compared with the NoCHO fed trout (P = 0.0031).
Fig. 5.
Steady-state mRNA abundance (+SE) of general markers of hepatic lipid metabolism, including de novo lipogenesis (DNL) markers sterol regulatory element binding protein 1c (A), fatty acid synthase (B), and fatty acid oxidation pathway marker carnithine palmitoyltransferase 1a (C). Abundance of transcripts was measured in rainbow trout that were fasted and in rainbow trout 6 h after being refed either a NoCHO diet (devoid of carbohydrates) or a HighCHO diet (containing 30% carbohydrates) and normalized to elongation factor 1α and expressed as relative fold change compared with the fasted group. In cases where data were normally distributed and met the criteria of homoscedasticity, a one-way ANOVA followed by Tukey’s post hoc was used for analysis, whereas a Kruskal-Wallis test followed by Dunn’s multiple comparisons test was used in cases where data did not meet these criteria. A P value of P < 0.05 was used as cut-off for significant effects.
Activity of components of the hepatic insulin signaling pathway is increased by a carbohydrate-rich diet.
Activity of Akt (F2,12 = 5.44, P = 0.0283), assessed as the quantified ratio of Ser437 phosphorylated Akt and total Akt (Fig. 6A), significantly increased in response to refeeding the HighCHO diet (P = 0.0311), but not the NoCHO (P = 0.8423) diet. No difference was observed between groups refed with NoCHO and HighCHO diets (P = 0.0748). A similar pattern was observed in the Tor target Ser234/235 S6 phosphorylation (F2,12 = 7.10, P = 0.0144; Fig. 6B), which increased significantly in HighCHO-refed rainbow trout compared with fasted trout (P = 0.0149), but not in rainbow trout refed with NoCHO diet (P = 0.0512). Again, no differences between both refed groups were observed (P = 0.7138). Finally, the activity of Tor, as assessed by the ratio of Ser2448 phosphorylated and total Tor, did not change significantly between treatment groups (F2,12 = 0.53 P = 0.7222; Fig. 6C).
Fig. 6.
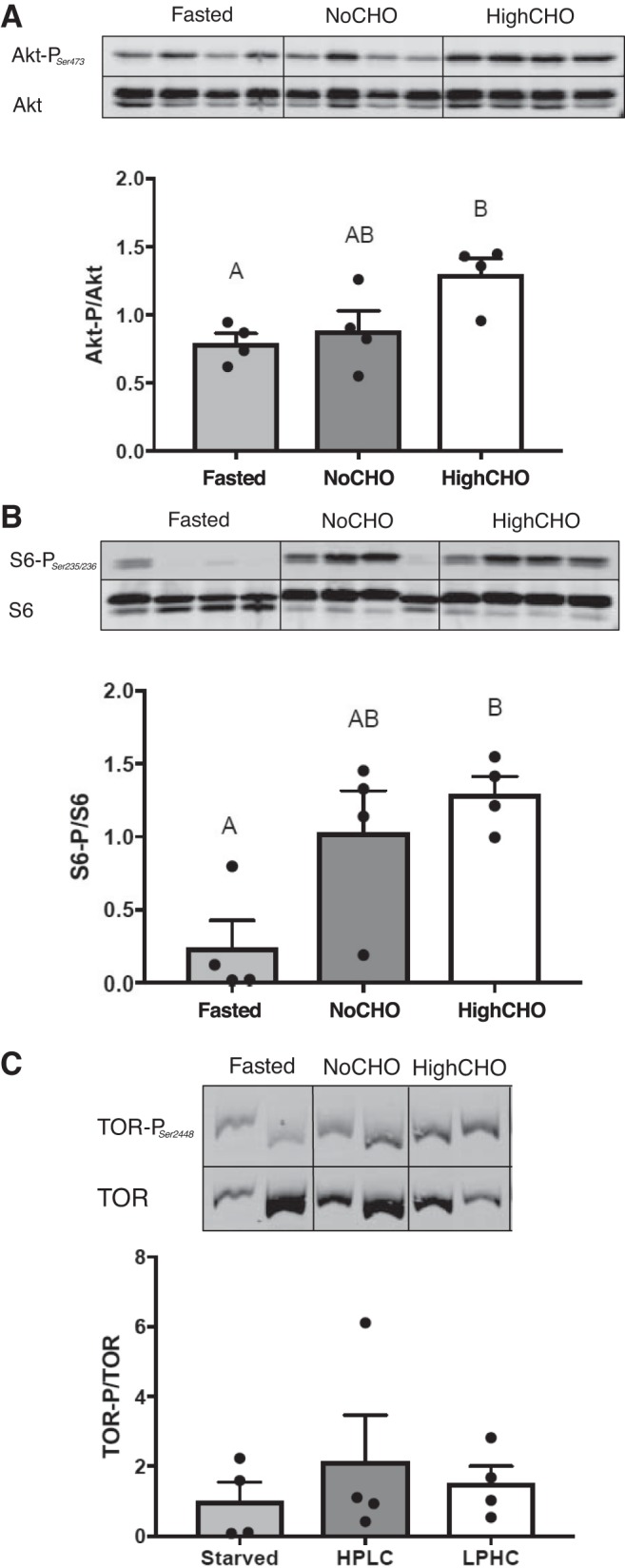
Hepatic insulin signaling pathway activity assessed by ratios of phosphorylated and total protein (+SE) of Akt (A), S6 (B), and Tor (C) based on densitometry-based quantification of four samples per group by Western blots. In cases where data were normally distributed and met the criteria of homoscedasticity, a one-way ANOVA followed by Tukey’s post hoc was used for analysis, whereas a Kruskal-Wallis test followed by Dunn’s multiple comparisons test was used in cases where data did not meet these criteria. A P value of P < 0.05 was used as cut-off for significant effects.
Additional targets of interest.
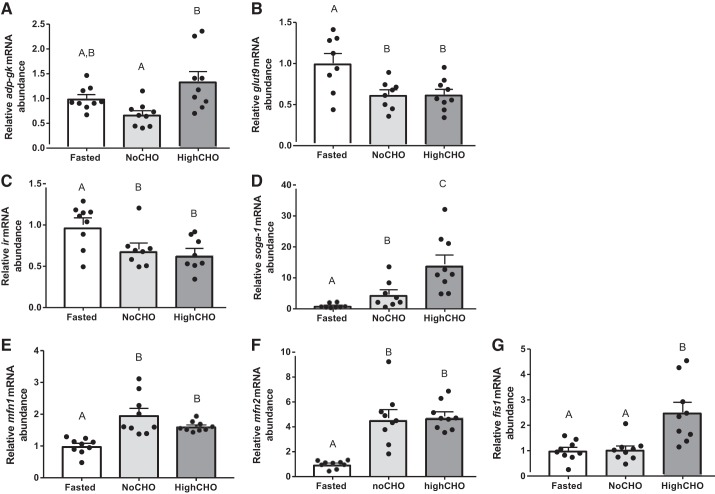
In addition to quantifying aforementioned hepatic markers of gluconeogenesis, DNL, and insulin signaling hypothesized to play a role in GI in rainbow trout, we quantified additional specific transcripts related to glucose metabolism-related pathways (Fig. 7, A and B) and insulin signaling pathways (Fig. 7, C and D), because they first belong to enriched pathways predicted to be targeted by differentially regulated miRNAs and second are individually predicted to be targeted by several differentially regulated miRNAs (Additional Files 9 and 11). Hepatic mRNA abundance of the adp-dependent glucokinase (adp-gk; F2,24 = 8.62, P = 0.015; Fig. 7A) and glucose transporter 9 (glut9; F2,22 = 6.495, P = 0.0061; Fig. 7B) changed significantly between groups. In the case of adp-gk, the only significant change between groups was observed between NoCHO and HighCHO-fed fish (P = 0.0041). With regard to glut9, refeeding either NoCHO (P = 0.0137) or HighCHO (P = 0.0120) significantly increased mRNA abundance compared with fasted fish, but there was no difference in expression between fish refed either diet (P = 0.9994). Hepatic mRNA abundance of the insulin receptor subunit ir (F2,22 = 5.456, P = 0.0119; Fig. 7C) exhibited a similar reduction following refeeding both the NoCHO diet (P = 0.0499) and HighCHO diet (P = 0.0147), without a significant difference between both refeeding groups (P = 0.8490).
Fig. 7.
Steady-state mRNA abundance (+SE) of adp-dependent glucokinase (A), glucose transporter 9 (B), insulin receptor (C), suppressor of glucose autophagy associated 1 (D), mitofusin 1 (E), mitofusin 2 (F), and mitochondrial fission protein 1 (G). In cases where data were normally distributed and met the criteria of homoscedasticity, a one-way ANOVA followed by Tukey’s post hoc was used for analysis, whereas a Kruskal-Wallis test followed by Dunn’s multiple comparisons test was used in cases where data did not meet these criteria. A P value of P < 0.05 was used as cut-off for significant effects.
We furthermore quantified transcripts related to organelle dynamics, specifically autophagy and mitochondrial dynamics, because they first have been linked to glucoregulation in mammals (19, 64, 68, 72, 73) and, in the case of autophagy, also in rainbow trout (69). Second, these genes have been predicted to be regulated by differentially expressed miRNAs both at the pathway enriched level (Additional File 11) and specific transcript level (Additional File 12). Expression of suppressor of glucose autophagy-regulated 1 (soga1; F2,22 = 25.12 P < 0.0001; Fig. 7D) was significantly induced by refeeding NoCHO (P = 0.0069) and HighCHO (P = 0.0001), with a significantly higher induction in HighCHO compared with NoCHO (P = 0.0046). Finally, expression of mitofusin 1 (mfn1; H2,27 = 17.75, P = 0.001; Fig. 7E) and mitofusin2 (mfn2; H2,27 = 17.40; Fig. 7F) increased with both NoCHO (P = 0.0003 and P = 0.0014) and HighCHO (P = 0.0029 and P = 0.0009) compared with fasted fish. In neither case was there differential expression between refed groups (P = 0.9999 and P = 0.9999). Conversely, mitochondrial fission protein 1 (fis1; F2,23 = 12.2, P = 0.0002, Fig. 7G) changed significantly only in response to HighCHO compared with both fasted (P = 0.0009) and NoCHO-fed (P = 0.0008) fish.
Correlative Analysis of Differentially Regulated Hepatic miRNAs and Specific Transcripts of GO Term Enriched Pathways
Predicted targets involved in gluconeogenesis, glucose metabolism, insulin signaling, and organelle dynamics.
Glucose metabolic pathways were predicted to be regulated by differentially regulated miRNAs in all comparisons (Additional File 11). Given that the gluconeogenesis pathway in general (Additional File 11) and g6pcb2 paralogs in particular (Additional File 12) were identified as target of miRNAs in silico (Fig. 8) and that this pathway had been linked to GI in rainbow trout (41, 42), we investigated a potential role of differentially regulated miRNAs on this pathway by specific correlative analysis (Table 2). While not a proof of a causative relationship, significant negative correlations between miRNAs and their predicted target transcripts may allow investigators to prioritize specific predicted miRNA-mRNA pairs for future mechanistic studies. With regard to gluconeogenic transcripts whose expression had been previously described for this study, we observed significant negative correlations of miRNAs that are differentially expressed between fasted and HighCHO groups, specifically between miR-27b-3p and the glucose-6-phosphatase paralog b1a (g6pcb1a), significant negative correlations between miR-126b-5p and miR-92a-3p and glucose-6-phosphatase paralog b2a (g6pcb2a), and a significant negative correlation between miR-101b-5p and fructose 1,6 bisphosphatase 1b1 (fbp1b1). With regard to glycolytic transcripts, whose expression had been described previously for samples used in this study (42), we identified significant negative correlations for the abundance of miR-126b-5p and glucokinase a (gcka) and miR-429a and glucokinase b (gckb) when comparing fasted and HighCHO-fed fish.
Fig. 8.
Graphical representation of predicted differential and overlapping targeting of g6pc paralogs by differentially regulated miRNAs identified in the current study. An identity matrix of glucose 6 phosphatase paralog 3′-untranslated regions is presented.
Table 2.
Correlation of expression of differentially regulated miRNAs and predicted targets involved in pathways relevant to hepatic regulation of glucose metabolism
| miRNA | Significantly Differentially Expressed | Pearson Correlation Coefficient | Significance | |
|---|---|---|---|---|
|
pck1 GSONMT00082468001 |
ssa-miR-27d-5p ssa-miR-27d-5p ssa-miR-27d-5p ola-miR-27c-5p_L+1R+1_1ss12CA ola-miR-27c-5p_L+1R+1_1ss12CA |
All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO ANOVA Fasted-HighCHO |
0.63 0.62 0.66 0.72 0.72 |
P < 0.05 n.s. n.s. P < 0.05 P < 0.05 |
|
pck2 GSONMT00059643001 |
ssa-miR-30e-5p ssa-miR-30e-5p ssa-miR-30e-5p ccr-miR-15a ccr-miR-15a ccr-miR-15a ssa-miR-30a-5p ssa-miR-30c-5p_R+1_1ss24TC |
All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO Fasted-HighCHO Fasted-NoCHO |
−0.41 −0.46 −0.43 −0.32 −0.22 −0.32 −0.28 0.24 |
n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. |
|
g6pca GSONMT00076843001 |
dre-miR-190a_R+1 dre-miR-190a_R+1 dre-miR-190a_R+1 fru-miR-221_R-1 fru-miR-221_R-1 ssa-miR-30e-5p ssa-miR-30e-5p ssa-miR-30e-5p ssa-let-7e-3p_1ss22CT ssa-miR-30a-5p |
All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO All groups (ANOVA) Fasted-HighCHO All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO Fasted-NoCHO Fasted-HighCHO |
−0.42 −0.29 −0.18 −0.21 −0.23 −0.13 −0.17 −0.25 −0.61 0.78 |
n.s. n.s n.s. n.s. n.s. n.s. n.s. n.s. n.s. P < 0.05 |
|
g6pcb1.a GSONMT00076841001 |
ccr-miR-27a dre-miR-27b-3p_R-1 dre-miR-27b-3p_R-1 dre-miR-27b-3p_R-1 dre-miR-142a-3p_L-1R-1 |
All groups (ANOVA) All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO Fasted-HighCHO |
−0.25 −0.42 −0.72 −0.42 0.03 |
n.s. n.s. P < 0.05 n.s. n.s. |
|
g6pcb1.b GSNOMT00066036001 |
none | n/a | n/a | n/a |
|
g6pcb2.a GSNOMT00013076001 |
dre-miR-181b-5p_R+1 dre-miR-181b-5p_R+1 dre-miR-190a_R+1 dre-miR-190a_R+1 dre-miR-190a_R+1 ipu-miR-1388_R-1 ipu-miR-1388_R-1 ipu-miR-222a_1ss11TG ipu-miR-222a_1ss11TG ssa-miR-222a-3p_R-1 ssa-miR-222b-3p ssa-miR-222b-3p ssa-miR-101b-5p ssa-miR-101b-5p ola-miR-101b-5p ola-miR-101b-5p ola-miR-101b-5p ssa-miR-128-2-5p ipu-miR-126b dre-miR-92a-3p_R+1 PC-5p-219_12291 |
All groups (ANOVA) Fasted-NoCHO All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO All groups (ANOVA) Fasted-NoCHO All groups (ANOVA) Fasted-HighCHO All groups (ANOVA) All groups (ANOVA) NoCHO-HighCHO All groups (ANOVA) Fasted-HighCHO All groups (ANOVA) Fasted-NoCHO Fasted-HighCHO All groups (ANOVA) All groups (ANOVA) Fasted-NoCHO NoCHO-HighCHO |
0.12 0.68 −0.45 −0.48 −0.52 −0.40 −0.62 −0.43 −0.49 −0.29 −0.43 −0.43 −0.47 −0.64 −0.41 −0.49 −0.59 0.66 −0.72 −0.73 0.76 |
n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. P < 0.05 P < 0.05 P < 0.05 P < 0.05 |
|
g6pcb2.b GSNOMT00014864001 |
dre-miR-181b-5p_R+1 dre-miR-181b-5p_R+1 dre-miR-190a_R+1 dre-miR-190a_R+1 dre-miR-190a_R+1 ipu-miR-1388_R-1 ipu-miR-1388_R-1 ipu-miR-222a_1ss11TG ipu-miR-222a_1ss11TG ssa-miR-222a-3p_R-1 ssa-miR-222b-3p ssa-miR-222b-3p dre-miR-456_1ss22AT PC-5p-219_12291 dre-miR-15b-3p_L+1_1ss3GA |
All groups (ANOVA) Fasted-NoCHO All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO All groups (ANOVA) Fasted-NoCHO All groups (ANOVA) Fasted-HighCHO All groups (ANOVA) All groups (ANOVA) Fasted-HighCHO Fasted-NoCHO NoCHO-HighCHO NoCHO-HighCHO |
0.23 0.78 −0.59 −0.60 −0.57 −0.38 −0.09 0.56 −0.64 −0.24 −0.48 −0.64 0.18 0.74 0.70 |
n.s. P < 0.05 n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s P < 0.05 P < 0.05 |
|
fbp1a GSNOMT00001932001 |
none | n/a | n/a | n/a |
|
fbp1b1 GSNOMT00063051001 |
dre-let-7c-5p_1ss19GC dre-let-7c-5p_1ss19GC dre-let-7c-5p_1ss19GC dre-miR-130a dre-miR-130a ola-miR-101b-5p ola-miR-101b-5p ssa-miR-101b-5p |
All groups (ANOVA) Fasted-NoCHO Fasted-HighCHO All groups (ANOVA) Fasted-HighCHO All groups (ANOVA) Fasted-NoCHO Fasted-NoCHO |
0.05 0.67 0.11 0.01 −0.05 −0.45 −0.86 0.71 |
n.s. n.s. n.s. n.s. n.s. n.s. P < 0.01 P < 0.05 |
|
fbp1b2 GSNOMT00015701001 |
ola-miR-101b-5p ola-miR-101b-5p ola-miR-101b-5p ssa-miR-101b-5p |
All groups (ANOVA) Fasted-NoCHO Fasted-HighCHO Fasted-HighCHO |
0.13 −0.03 0.07 0.39 |
n.s. n.s. n.s. n.s. |
|
gck1 GSONMG00033781001 gck2 GSONMG00012878001 |
ipu-miR-126b ipu-miR-126b ipu-miR-126b dre-miR-429a dre-miR-429a dre-miR-429a |
All groups (ANOVA) Fasted-NoCHO Fasted-HighCHO All groups (ANOVA) Fasted-NoCHO Fasted-HighCHO |
−0.56 −0.05 −0.71 −0.60 −0.42 −0.71 |
n.s. n.s. P < 0.05 n.s. n.s. P < 0.05 |
n/a, not available; n.s., Not significant; HighCHO, high-carbohydrate diet; NoCHO, no-carbohydrate diet.
Additional predicted targets involved in autophagy and mitochondria dynamics.
Our in silico analysis predicted pathways related to autophagy and mitochondria organelle dynamics as being targets of posttranscriptional regulation by differentially expressed miRNAs in response to dietary carbohydrates (Additional File 11). Because hepatic autophagy and mitochondrial dynamics have been linked to glucoregulation in mammals (19, 64, 68, 72, 73) and, in the case of autophagy, in rainbow trout (69), we quantified predicted target transcripts of both GO-enriched pathways and correlatively investigated miRNA-target expression patterns (Table 3): Correlations in transcript abundance were also investigated for additional targets of interest involved in glucose metabolism, insulin signaling, and organelle dynamics (Table 3). Significant negative correlations in expression were identified between miR-101-5p and a paralog of adp-dependent glucokinase (adp-gk), miR-30c-5p, and an insulin receptor (ir) paralog, miR-140 and suppressor of glucose autophagy associated1 (soga1), between let7a and mitofusin1 (mfn1), and between miR-15a-5p, miR-17-5p, miR-18b-5p, miR-152-5p, and mitofusin 2 (mfn2).
Table 3.
Correlation of expression of differentially regulated miRNAs and predicted targets involved in pathways of interest relevant to hepatic regulation of glucose metabolism
| miRNA | Significantly Differentially Expressed | Pearson Correlation Coefficient | Significance | |
|---|---|---|---|---|
| Glucose metabolism | ||||
|
glut9 GSONMT00066217001 |
ssa-miR-222a-3p_R-1 ssa-miR-222a-3p_R-1 ssa-miR-222b-3p pol-let-7a-3p_R+1_1ss3AG pol-let-7a-3p_R+1_1ss3AG dre-miR-722_L-2 dre-miR-722_L-2 dre-miR-722_L-2 |
All groups (ANOVA) Fasted-HighCHO All groups (ANOVA) All groups (ANOVA) Fasted-NoCHO All groups (ANOVA) Fasted-NoCHO Fasted-HighCHO |
0.20 0.15 0.33 −0.37 −0.63 0.42 0.04 0.50 |
n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. |
| adp-gk GSONMT00073455001 |
ssa-miR-214-3p_L-1_1ss23AT ssa-miR-214-3p_L-1_1ss23AT ssa-miR-27d-5p ssa-miR-27d-5p ssa-miR-27d-5p ola-miR-27c-5p_L+1R+1 1ss12CA ola-miR-27c-5p_L+1R+1_1ss12CA ola-miR-101b-5p ola-miR-101b-5p ola-miR-101b-5p ssa-miR-30c-5p_R+1_1ss24TC dre-miR-125b-2-3p_L+1_2ss8GA11TC |
All groups (ANOVA) Fasted-HighCHO All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO All groups (ANOVA) Fasted-HighCHO All groups (ANOVA) Fasted-NoCHO Fasted-HighCHO Fasted-NoCHO Fasted-HighCHO |
0.34 0.33 0.84 0.96 0.95 0.83 0.98 −0.27 0.59 −0.71 −0.55 0.77 |
n.s. n.s. P < 0.01 P < 0.01 P < 0.01 P < 0.01 P < 0.01 n.s. n.s. P < 0.05 n.s. P < 0.05 |
| Cell signaling | ||||
|
ir GSONMT00026581001 |
dre-miR-18b-5p_1ss11TC dre-miR-18b-5p_1ss11TC dre-miR-18b-5p_1ss11TC dre-miR-190a_R+1 dre-miR-190a_R+1 dre-miR-190a_R+1 ssa-miR-30e-5p ssa-miR-30e-5p ssa-miR-30e-5p ssa-miR-30c-5p_R+1_1ss24TC ssa-miR-30c-5p_R+1_1ss24TC ssa-miR-30a-5p ssa-miR-722-5p_L-1R+1 |
All groups (ANOVA) Fasted-HighCHO noCHO-HighCHO All groups (ANOVA) Fasted-HighCHO noCHO-HighCHO All groups (ANOVA) Fasted-HighCHO noCHO-HighCHO Fasted-NoCHO Fasted-HighCHO Fasted-HighCHO Fasted-HighCHO |
−0.03 0.04 0.02 −0.18 −0.12 −0.33 −0.01 0.00 0.00 −0.20 −0.74 0.12 −0.04 |
n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. n.s. <0.05 n.s. n.s. |
| Other pathways of interest | ||||
| Autophagy | ||||
| soga1 GSONMT00057063001 |
dre-miR-140-5p_R+1 dre-miR-140-5p_R+1 dre-miR-140-5p_R+1 dre-miR-214-5p_R+1 ssa-miR-214-3p_L-1_1ss23AT |
All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO ANOVA Fasted-HighCHO |
−0.80 −0.86 −0.77 0.07 0.34 |
P < 0.01 P < 0.01 P < 0.05 n.s. n.s. |
| Mitochondrial fusion | ||||
| fis1 GSONMT00039888001 |
fru-miR-200a_R-1 fru-miR-200a_R-1 fru-miR-200a_R-1 |
All groups (ANOVA) Fasted-NoCHO NoCHO-HighCHO |
0.20 −0.25 −0.18 |
n.s. n.s. n.s. |
| mfn1 GSONMT00027395001 |
dre-let-7d-5p dre-let-7d-5p dre-let-7d-5p dre-let-7c-5p_1ss19GC dre-let-7c-5p_1ss19GC dre-let-7c-5p_1ss19GC dre-let-7a ssa-miR-27d-5p ssa-miR-27d-5p ssa-miR-27d-5p ola-miR-27c-5p_L+1R+1_1ss12CA ola-miR-27c-5p_L+1R+1_1ss12CA fru-miR-7_R+2 |
All groups (ANOVA) Fasted-NoCHO Fasted-HighCHO All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO Fasted-HighCHO All groups (ANOVA) Fasted-HighCHO NoCHO-HighCHO All groups (ANOVA) Fasted-HighCHO Fasted-NoCHO |
0.74 0.89 0.93 0.57 0.94 −0.21 −0.72 −0.20 −0.54 −0.40 0.16 0.51 0.70 |
P < 0.01 P < 0.01 P < 0.01 n.s. P < 0.01 n.s. P < 0.05 n.s. n.s. n.s. n.s n.s n.s |
| mfn2 GSONMT00004526001 |
ssa-miR-214-3p_L-1_1ss23AT ssa-miR-214-3p_L-1_1ss23AT ssa-miR-16c-5p_R-1 ssa-miR-16c-5p_R-1 fru-miR-152 fru-miR-152 fru-miR-152 ccr-miR-15a ccr-miR-15a ccr-miR-15a ssa-miR-17-5p_R-1 ssa-miR-17-5p_R-1 dre-miR-18b-5p_1ss11TC dre-miR-18b-5p_1ss11TC dre-miR-18b-5p_1ss11TC ssa-miR-15b-5p ssa-miR-15b-5p dre-miR-181b-5p_R+1 ssa-miR-101b-5p ssa-miR-29b-2-5p_L-2 |
All groups (ANOVA) Fasted-HighCHO All groups (ANOVA) noCHO-HighCHO All groups (ANOVA) Fasted-HighCHO noCHO-HighCHO All groups (ANOVA) Fasted-HighCHO noCHO-HighCHO All groups (ANOVA) Fasted-HighCHO All groups (ANOVA) Fasted-HighCHO noCHO-HighCHO All groups (ANOVA) noCHO-HighCHO Fasted-NoCHO Fasted-HighCHO Fasted-HighCHO |
0.83 0.87 0.16 0.13 −0.57 −0.81 −0.53 −0.60 −0.89 −0.26 −0.54 −0.77 −0.53 −0.75 −0.40 −0.39 0.31 0.91 −0.75 −0.55 |
P < 0.01 P < 0.01 n.s n.s n.s P < 0.05 n.s. P < 0.05 P < 0.01 n.s. n.s. P < 0.05 n.s. P < 0.05 n.s. n.s. n.s. P < 0.01 P < 0.05 n.s. |
DISCUSSION
Increased Dietary Carbohydrates Upregulate Expression of Canonical miRNA Biogenesis Pathway Components in Rainbow Trout
Our initial probing of a global role for the miRNA biogenesis pathway in response to dietary carbohydrates revealed that at least one paralog of components probed at different levels of the pathway was responsive to dietary carbohydrates. This is in contrast to hepatic responses to diets lacking dietary carbohydrates, which resulted in an induction of xpo5 paralogs only. However, even with regard to xpo5, dietary carbohydrates induced a significantly higher mRNA abundance of the xpo5b paralog, suggesting an increase in pre-miRNA translocation out of the nucleus. Together, these data show that, at the level of gene expression, dietary carbohydrates concurrently induce the hepatic abundance of canonical miRNA biogenesis pathway components, suggesting a general stimulation of canonical miRNA biogenesis. As salmonids, rainbow trout are among the only species characterized to-date to possess paralogs of all components of this pathway with the exception of drosha (5). While their function in this species has not been probed, this pathway and its components are evolutionarily conserved (31), and recent siRNA-based functional studies targeting individual components of this pathway in a human cell line have revealed profound effects of drosha ablation on canonical miRNA biogenesis and only mild effects of xpo5 ablation (31). Interestingly, rainbow trout and Atlantic salmon Xpo5 protein paralogs display specific AA substitutions in key sites involved in pre-mRNA binding, suggesting sub- or neofunctionalization of these paralogs to differentially affect miRNA export (5). The current study is the first to identify differential regulation of both paralogs xpo5a and xpo5b in response to dietary carbohydrates at the gene expression level, lending further support to possible sub- or neofunctionalization of these paralogs in rainbow trout and salmonids in general. While a working hypothesis at this point, regulation the xpo5 paralogs may have contributed to differential miRNAs profiles between dietary groups via differential regulation of pri-miRNA nuclear export. It is important however to note that while isoenergetic diets were used that exclude secondary effects related to energy status, the increase in carbohydrates in the HighCHO diet was achieved by a corresponding reduction of protein content. It is therefore possible that the observed changes are related either to increased carbohydrate content and/or hyperglycemia or to a reduction in protein content and AA signaling. Future studies using different replacement of protein by undigestible carbohydrates to modulate protein content may be used to resolve this question.
In terms of nutritional regulation of key enzymes of the canonical miRNA biogenesis pathway, nutritional regulation of Drosha at the protein level and associated canonical miRNA biogenesis have recently been revealed to be dependent on signaling of the nutrient sensor mTor in C57/BL6 mice, which degrades Drosha to inhibit miRNA biogenesis under nutrient-rich conditions, while stabilizing Drosha to promote miRNA biogenesis under nutrient-poor conditions (79). While our current study only probed drosha mRNA abundance, it will be worthwhile to determine whether drosha and the miRNA pathway in trout respond to nutrient-poor conditions. In contrast to mammals, high dietary carbohydrate (and/or concurrent low protein content) represents poor nutrients for carnivorous rainbow trout, and the induction of drosha mRNA abundance and the canonical miRNA biogenesis pathway may represent a conserved mechanism to nutrient-poor conditions. The finding that activation of key components of the hepatic insulin pathway activity including upstream (Akt) and downstream (S6) components of Tor, but not Tor itself, increased in HighCHO-fed, but not NoCHO-fed trout compared with fasted trout in our study does suggest, however, that this mechanism may not be conserved between rainbow trout and C57/BL6 mice. However, whether Tor activation may play a role in observed significant induction of drosha and other components of the canonical miRNA biogenesis pathway in this study cannot be functionally answered, as experiments using pharmacological Tor inhibition in rainbow trout (4, 10, 39) would be needed to functionally link Tor signaling to the miRNA biogenesis pathway in rainbow trout.
The hepatic miRNAome is More Responsive to Carbohydrate Containing Diets Compared with Diets Devoid of Carbohydrates
In line with a more pronounced induction of miRNA biogenesis components in response to HighCHO diets compared with NoCHO diets, the global miRNAome is also more significantly altered with HighCHO diet compared with NoCHO diet. While the slightly higher number of upregulated miRNAs in trout refed a HighCHO diet, compared with trout fed a NoCHO diet, is indicative of an increase in miRNA biogenesis, an even stronger difference was observed for downregulated miRNAs, suggesting that in addition to increased miRNA biogenesis, specific hepatic miRNA processing, and/or miRNA turnover are also specifically increased by HighCHO diets. While components such as exonucleases are emerging to play a key role in miRNA turnover in mammals and are present in the trout genome (5), their functional roles remain poorly understood and appear to be very complex since they are, in contrast to the principal canonical miRNA biogenesis pathway, specific to individual miRNAs. Therefore, no general indexes of miRNA turnover could be quantified in our study.
The Regulated Hepatic miRNAome in Response to NoCHO and HighCHO after a Short-term Fast is Largely Different in Rainbow Trout
In addition to the described quantitative differences in the regulation of the hepatic miRNAome when comparing HighCHO and NoCHO refed trout compared with fasted trout, the miRNAome is also qualitatively different (Fig. 3E). Indeed, with the exception of four upregulated miRNAs (let7a-3p, let7c-5p, let7d-5p, miR-7550-5p) and one downregulated miRNA (miR-101b-5p) that significantly respond to refeeding irrespective of the nature of the diet when considering both pairwise comparisons between fasted fish and specific diets, all remaining miRNAs are differentially regulated. This pattern results in differential expression of 30 miRNAs when comparing HighCHO and NoCHO refed groups. Together, this suggests that the hepatic miRNAome is regulated more by specific diets than the process of refeeding isoenergetic meals itself. However, as previously stated, it is important to note that an increase in dietary carbohydrates is accompanied by an equivalent reduction of dietary protein content, and with the current experimental design it is impossible to dissociate the separate contribution of either parameter to the specific regulation of the hepatic miRNAome.
Identification of Evolutionarily Conserved and Divergent Regulation of Hepatic miRNAs in Response to Increased Circulating Glucose Concentrations
To assess whether the hepatic miRNA signature in GI rainbow trout in response to high dietary carbohydrate (30%)-induced hyperglycemia (~11 mmol) is largely species, class, or phenotype specific, we compared the hepatic miRNA profile obtained in our experiment to hepatic miRNA profiles identified in previous experiments. The first comparison included a recently published hepatic miRNA profiles in response to high dietary carbohydrates (50%) in blunt snout bream, Megalobrama amblycephala (47), which exhibited a significant but moderate increase in circulating glucose (~6 mmol), underlining its status as a glucose-tolerant species. The second comparisons included different genetic or pharmacological rodent models of hyperglycemia (26, 27, 36, 82): A GK rat strain exhibiting 25 mmol postprandial hyperglycemia compared with a normoglycemic BN strain (26, 27), C57BL/6 ob/ob mice, and streptozotocin-injected mice C57BL/6 mice exhibiting postprandial hyperglycemia at 13 and 19 mmol compared with normoglycemic C57BL/6 control mice (36), and BTBR ob/ob mice exhibiting hyperglycemia at 22 mmol compared with a normoglycemic B6 strain (82).
As shown in Fig. 9, we identified 11 miRNAs that include miRNA-27-3p, as being responsive to significant increases in circulating glucose in rainbow trout, blunt snout bream, and rodent models, revealing a core of miRNAs whose regulation may directly depend on circulating glucose across these species. Another subset of 11 miRNAs is regulated by high-carbohydrate diets and or increased circulating glucose in both rainbow trout and blunt snout bream with largely consistent directional expression changes, suggesting either dietary HighCHO-dependent responses or possible teleost-specific response to increased circulating glucose. These genes notably include the syn-expression of several components of the miR-17-92 cluster. A subset of 16 miRNAs were identified when comparing glucose-tolerant species displaying increases in circulating glucose, the blunt snout bream (47) and rodent models (26, 27, 36, 82), opening up the possibility that a lack of regulation of these miRNAs in rainbow trout may contribute to its GI phenotype. Together, these findings suggest complex species and/or phenotype-specific patterns of miRNA regulation. Future studies probing the functional role of identified target miRNAs in GI in rainbow trout will allow us to answer several questions. First, it will be informative to investigate whether specific miRNAs identified as being regulated in dietary carbohydrate-induced hyperglycemia across species may still contribute to differences in glucose tolerance based on rewiring of miRNA-target networks, which is estimated to differ by as much as 90% in targets between mammals and fish (43). Conversely, comparative identification of commonly regulated miRNA-target pairs with functions in glucoregulation may facilitate the identification of the biologically most relevant miRNA-mRNA target relationships in mammalian models, a major challenge in miRNA biology. Second, the identification of miRNAs that are specifically regulated in rainbow trout in response to HighCHO-induced glycemia will allow us to specifically and functionally probe the hypothesis that rainbow trout-specific miRNA regulation contributes to GI in this species. Third, by modulating specific expression of hepatic miRNAs that are only regulated in response to high dietary carbohydrates in GI species, but not rainbow trout, will allow us to test the hypothesis that, conversely, a lack of regulation of specific miRNAs in rainbow trout contributes to the GI phenotype in rainbow trout.
Fig. 9.
Venn diagram-based representation of differentially regulated hepatic miRNAs in response to diet-induced hyperglycemia in glucose-intolerant rainbow trout (current study), glucose-tolerant blunt snout bream (47) and rodent models of diet-induced hyperglycemia (26, 27, 36, 82). The identification of common differentially regulated hepatic miRNAs in hyperglycemic conditions between these species is indicated in intersections of the Venn diagram. Differentially regulated miRNAs in response to hyperglycemic conditions identified in rainbow trout, blunt snout bream, and mammalian models are indicated in the center of the Venn diagram. The directional change of specific miRNA expression within specific studies is indicated as follows: For each individual miRNA, an arrow to its left indicates the observed miRNA expression change observed in rainbow trout in the current study, an arrow to its right the observed expression changes in the glucose-tolerant blunt snout bream and an arrow located below each specific miRNA indicates its expression change observed in mammalian studies.
Enrichment of In Silico Predicted Targets of Differentially Expressed miRNAs Identifies Hepatic Metabolic and Cell Signaling Pathways Linked to GI in Rainbow Trout as Targets of Posttranscriptional Regulation
Glucose metabolism.
Several pathways related to glucose metabolism were predicted to be posttranscriptionally regulated by differentially expressed miRNAs in pairwise comparisons between all experimental groups (Additional File 11). Interestingly, gluconeogenesis is enriched in all comparisons, suggesting that miRNAs differentially regulate this pathway in response to both NoCHO and HighCHO. Functionally, differential regulation of gene expression and enzymatic activity of components of the gluconeogenic pathway have been previously described in the same samples and revealed a possible contribution of the atypical induction of hepatic g6pcb2 ohnologs in response to HighCHO to the lack of inhibition of gluconeogenesis (41). This differential regulation is, in turn, hypothesized to contribute to GI in rainbow trout (32, 41, 42, 56). Correlational analysis of specific miRNAs regulated in the HighCHO diet and their predicted target transcripts points to a possible role of miRNA-27-3p in regulating g6pcb1, but not g6pcb2 paralogs. A recent study investigating overexpression of miRNA-27b in the mouse liver revealed a significant decrease in G6PC and PCK protein levels, suggesting an evolutionarily conserved response (81). Conversely, significant negative correlations between miR-126b-5p and miR-92a-3p and glucose-6-phosphatase b2a (g6pcb2a) suggest a specific role for these miRNAs in the atypical regulation of g6pcb2 ohnologs. Together, these relationships raise the possibility that both rainbow trout g6pcb2 paralogs escape from evolutionary conserved posttranscriptional g6pc regulation and/or that the evolution of novel miRNA-dependent regulation of g6pcb2 paralogues may underlie their atypical regulation in response to dietary carbohydrates to contribute to GI in rainbow trout. Future functional studies using 3′-UTR luciferase reporter assays and miRNA modulation approaches in trout hepatocyte cell lines will be necessary to establish the first evidence of paralog-specific posttranscriptional regulation in salmonids and to functionally link these candidate miRNAs to paralog-specific regulation of gluconeogenic enzyme paralogs and their effect on de novo glucose production.
Lipid metabolism.
DNL-related GO terms were predicted as being enriched in the NoCHO (positive regulation of triglyceride biosynthetic process) and HighCHO (fatty acid biosynthesis), respectively, indicative of a differential, miRNA-dependent posttranscriptional regulation of the hepatic DNL pathway in response to either diet. Functional markers at the gene expression level were not indicative of a differential activation of DNL in response to NoCHO and HighCHO diets, suggesting that a lack of carbohydrate-dependent induction of DNL, which is observed in glucose tolerant mammals, may contribute to GI in rainbow trout. Interestingly, the most abundant hepatic miRNA detected in our study, miRNA-122, was significantly induced in the liver only in response to the protein-rich NoCHO diet but not the carbohydrate-rich HighCHO diet, confirming the observed acute postprandial induction of this hepatic miRNA in response to a commercial, protein rich diet (45). The induction of miRNA-122, which is lipogenic in trout as in mammals (17, 44), in response to dietary protein in the NoCHO, and the lack of induction in response to a HighCHO diet, is in line with reports that rainbow trout hepatic DNL is more responsive to dietary proteins than carbohydrates (13). Because miRNA-122 regulation not only stimulates hepatic lipogenic pathways, but also secondarily improves glucose tolerance in rainbow trout, likely by increasing glucose flux toward the ‘lipogenic sink’, suggests that this specific nutritional regulation of the most abundant, functionally conserved hepatic miRNA may play a role in GI tolerance in rainbow trout (7, 44). However, real-time RT-PCR did not validate an increase in miRNA-122 across all 9 samples, suggesting that the induction might have been observed only in a subset of n = 4 samples used for NGS small RNA sequencing.
Insulin signaling.
In contrast to a previous study that revealed a significant increase in activity of specific components (Akt, S6) of the hepatic insulin signaling in response to a diet with low, but not high CHO content in response to a single meal following a short-term fast, the current study of rainbow trout refed for 4 days, provides contrasting evidence for an induction of Akt and S6 activity in response to the HighCHO, but not a the NoCHO diet. Together, this finding may suggest a time-dependent acclimation of the hepatic insulin signaling pathway to excess dietary carbohydrates, refuting, at least under our current experimental conditions, the hypothesis that diminished hepatic insulin pathway signaling activity may be linked to GI in rainbow trout. Therefore, the reported enrichment of insulin signaling in general, and Akt and Tor signaling pathways in particular, in response to HighCHO (Additional File 11) may be indicative of miRNA-dependent adaptive changes in the hepatic insulin signaling pathway in response to HighCHO. Interestingly, an enrichment for insulin signaling was equally predicted in targets of differentially regulated hepatic miRNAs in the glucose-tolerant blunt snout bream (47) and GI rodent models (26), suggesting that posttranscriptional regulation of the insulin pathway in response to dietary carbohydrates is widespread in GI and glucose-tolerant fish species as well as rodent models (26). At the specific transcript level, we identified a significant correlation of miR-30c-5p family member and the profiled insulin receptor (ir) when comparing fasted and HighCHO groups, suggesting a possible posttranscriptional role for miR-30c-5p posttranscriptional regulation of ir under these conditions. However, because the abundance of this ir paralog was similarly decreased by refeeding irrespective of diet, it does not explain the differential activation observed for the insulin signaling pathway.
Additional predicted targets of differentially expressed miRNAs: organelle dynamics.
Additional hepatic pathways relevant to GI in rainbow trout that emerged as being regulated posttranscriptionally by differentially regulated miRNAs were related to specific hepatic organelle function, especially autophagy and mitochondrial dynamics, both of which have been linked to regulating systemic glucose homeostasis in trout (69) and/or in mammals (19, 64, 68, 72, 73). With regard to autophagy, analysis of transcript abundance of targeted markers involved in autophagy reveal an induction of a soga1, an inhibitor of autophagy and gluconeogenic AA-dependent de novo gluconeogenesis in response to HighCHO diets, indicative of an adaptive response to HighCHO diet-induced hyperglycemia. We identified a significant negative correlation in the expression of miR-140-5p and the soga1 paralog, across all experimental groups, raising the possibility of a general posttranscriptional control of soga1 and its regulated processes under different dietary conditions.
With regard to mitochondria fusion and fission dynamics, the induction of mfn1 and mfn2 paralogs did not differ between diets, in contrast to fis1, which was significantly induced in trout refed HighCHO compared with fasted and NoCHO-fed trout. Indeed, mitochondrial fission has been shown to be induced in response to oxidative stress and increased gluconeogenesis in a hepatoma cell line (25), suggesting that this process may play a role in HighCHO-induced GI in rainbow trout. However, significant negative correlations between miRNAs predicted to target these transcripts were only observed for mitofusins, but not fis1, suggesting that the differential regulation of fis1 is likely dependent on factors other than miRNAs.
Additional Considerations
The current study is the first to identify differential global and specific regulation of hepatic miRNAs in rainbow trout in response to refeeding and reveals that the regulation of the miRNA biogenesis pathway and the miRNAome in general is more dependent on carbohydrate and protein content than the process of refeeding itself. We furthermore show that the regulation of several identified hepatic miRNAs in response to increases in circulating glucose may be evolutionarily conserved in vertebrates. The current study furthermore predicts hepatic pathways relevant to GI in rainbow trout and especially identifies gluconeogenesis, de novo lipogenesis, and insulin signaling as being subjected to posttranscriptional regulation. Because these hepatic pathways have previously been linked to GI in rainbow trout, our study opens up the possibility to specifically probe hepatic miRNA-target pairs underlying GI in rainbow trout for future functional studies.
However, there are some limitations of the study that should be pointed out. First, it is important to note that while the study establishes important qualitative and quantitative differences related to macronutrient composition of the isoenergetic diets, it is not possible to specify whether the HighCHO diet changes are related to an increase in dietary carbohydrates and/or hyperglycemia or a decrease in dietary protein content, which is replaced in favor of increased carbohydrate content. The fact that several identified differentially regulated miRNAs in response to HighCHO diet have equally been shown to be induced in response increases in circulating glucose suggests that this subset of miRNAs is evolutionarily conserved in its direct response to circulating glucose. However, the possibility some miRNAs are differentially regulated by altered protein abundance remains a possibility. Diet preparation using nondigestible CHO to replace dietary protein content, as well as glucose injections, an/or in vitro studies of trout hepatocytes in response to medium glucose and AA will allow us to delineate the exact nutritional factors involved in this regulation further. Second, because of the large number of miRNA-mRNA target pairs, it remains challenging to infer specific biological consequences of differentially regulated miRNAs. Our study took advantage of the recently available rainbow trout genome resources (4, 43) to use an in silico approach to predict posttranscriptional regulation of transcripts at the genome level, similarly to previously published approaches in trout (33, 53), other fish species (47), and mammals (26). While not all 3′-UTRs are available, this approach represents a significant advancement compared with approaches used before genome publication, as previously discussed (46). The identification of particularly important individual miRNA-target relationships within these predicted pathways remains a particular challenge in miRNA biology, and expression between predicted miRNAs and targeted mRNA pairs remains correlative and now needs to be functionally tested. Despite a well-established negative posttranscriptional regulation as the principal mode of action of miRNAs, miRNA-mRNA target pairs do not necessarily correlate negatively. Several reasons account for this fact in addition to inherent limitations to in silico approaches (58). First, as in our study, samples analyzed for miRNAs and mRNAs are generally taken from a single time point, neglecting possible temporal factors in miRNA target interaction. Furthermore, miRNAs, in addition to acting to degrade mRNA abundance, may initially act at the protein level by inhibiting translation, thus without protein abundance data such effects may be missed (75). In general however, relatively good correlation in miRNA inhibition studies has been found at the protein and mRNA level, the inhibition of both of which is largely dependent on specific 3′-UTR seed binding sites (7, 17). Finally, miRNAs are one of several factors regulating gene expression, and indeed simple transcription factor and miRNA based gene regulation networks may result in negative or positive correlations, as well as stabilizing effect on mRNA abundance (11, 80). Nevertheless, the correlative expression between in silico predicted miRNA-mRNA pairs identifies interesting targets for functional in vitro assays in rainbow trout, which will delineate de facto regulation and metabolic functionality of specific proposed miRNA-mRNA target pairs in rainbow trout GI (43).
Conclusions
Overall, our study demonstrates that the hepatic miRNAs are more responsive to HighCHO diets compared with NoCHO diets in rainbow trout. This is evident from both end points related to the canonical pathway, as well as the number of differentially expressed miRNAs, which is higher in the HighCHO group compared with the NoCHO group. Specific comparative analyses identified a common set of miRNAs that increase in conditions of increased circulating glucose concentrations in GI rainbow trout, glucose-tolerant blunt snout bream, and rodent models of GI, as well miRNAs that are regulated under conditions of increased circulating glucose in both rainbow trout or glucose-tolerant species. These distinctions will aid in advancing our understanding of how specific miRNAs and/or evolutionary rewiring of miRNA-target networks may contribute to different phenotypes of glucose tolerance in the future. Genome-wide in silico analysis identified metabolic pathways (gluconeogenesis, DNL), cell signaling pathways (insulin signaling), and organelle dynamics (autophagy, mitochondrial fusion) as being subject to posttranscriptional regulation by miRNAs in response to HighCHO, providing specific targets for future studies probing the role of identified miRNAs in rainbow trout GI at a functional level in vitro.
GRANTS
This work benefited from the financial support of the INRA PHASE Department. This research was carried out under the E2S UPPA supported by the “Investissements d'Avenir” managed by ANR (ANR-16-IDEX-0002). L. Marandel received EU support under the Marie-Curie FP7 COFUND People Program through an AgreenSkills+ fellowship (#609398). D. J. Kostyniuk gratefully acknowledge funding through a MITACS Globalink grant (IT-10852), and J. A. Mennigen support from the Canadian Foundation for Innovation (#35859) and an NSERC Discovery grant (#147476). The funding agencies had no role in the design, execution or interpretation of the study.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.K., L.M., M.J., and K.D. performed experiments; D.J.K., L.M., M.J., K.D., R.F.d.S., D.Z., C.J.M., and J.A.M. analyzed data; D.J.K., L.M., C.J.M., and J.A.M. interpreted results of experiments; D.J.K., L.M., C.J.M., and J.A.M. prepared figures; D.J.K. and J.A.M. drafted manuscript; D.J.K., L.M., M.J., K.D., D.Z., C.J.M., S.P., and J.A.M. edited and revised manuscript; D.J.K., L.M., M.J., K.D., R.F.d.S., D.Z., C.J.M., S.P., and J.A.M. approved final version of manuscript; L.M., S.P., and J.A.M. conceived and designed research.
ACKNOWLEDGMENTS
Availability of data and material: The data sets are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beamish FW, Hilton JW, Niimi E, Slinger SJ. Dietary carbohydrate and growth, body composition and heat increment in rainbow trout (Salmo gairdneri). Fish Physiol Biochem 1: 85–91, 1986. doi: 10.1007/BF02290208. [DOI] [PubMed] [Google Scholar]
- 3.Bergot F. Effects of dietary carbohydrates and of their mode of distribution on glycaemia in rainbow trout (Salmo gairdneri richardson). Comp Biochem Physiol A Physiol 64: 543–547, 1979. doi: 10.1016/0300-9629(79)90581-4. [DOI] [Google Scholar]
- 4.Berthelot C, Brunet F, Chalopin D, Juanchich A, Bernard M, Noël B, Bento P, Da Silva C, Labadie K, Alberti A, Aury J-M, Louis A, Dehais P, Bardou P, Montfort J, Klopp C, Cabau C, Gaspin C, Thorgaard GH, Boussaha M, Quillet E, Guyomard R, Galiana D, Bobe J, Volff J-N, Genêt C, Wincker P, Jaillon O, Roest Crollius H, Guiguen Y. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat Commun 5: 3657, 2014. doi: 10.1038/ncomms4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Best C, Ikert H, Kostyniuk DJ, Craig PM, Navarro-Martin L, Marandel L, Mennigen JA. Epigenetics in teleost fish: From molecular mechanisms to physiological phenotypes. Comp Biochem Physiol B Biochem Mol Biol 224: 210–244, 2018. doi: 10.1016/j.cbpb.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Blasco J, Marimón I, Viaplana I, Fernández-Borrás J. Fate of plasma glucose in tissues of brown trout in vivo: effects of fasting and glucose loading. Fish Physiol Biochem 24: 247–258, 2001. doi: 10.1023/A:1014084313207. [DOI] [Google Scholar]
- 7.Boutz DR, Collins PJ, Suresh U, Lu M, Ramírez CM, Fernández-Hernando C, Huang Y, Abreu Rde R, Le S-Y, Shapiro BA, Liu AM, Luk JM, Aldred SF, Trinklein ND, Marcotte EM, Penalva LOF. Two-tiered approach identifies a network of cancer and liver disease-related genes regulated by miR-122. J Biol Chem 286: 18066–18078, 2011. doi: 10.1074/jbc.M110.196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brauge C, Corraze G, Médale F. Effects of dietary levels of carbohydrate and lipid on glucose oxidation and lipogenesis from glucose in rainbow trout, Oncorhynchus mykiss, reared in freshwater or in seawater. Comp Biochem Physiol A Physiol 111: 117–124, 1995. doi: 10.1016/0300-9629(95)98527-N. [DOI] [Google Scholar]
- 9.Bucking C, Wood CM. Renal regulation of plasma glucose in the freshwater rainbow trout. J Exp Biol 208: 2731–2739, 2005. doi: 10.1242/jeb.01668. [DOI] [PubMed] [Google Scholar]
- 10.Choi K, Weber J-M. Coping with an exogenous glucose overload: glucose kinetics of rainbow trout during graded swimming. Am J Physiol Regul Integr Comp Physiol 310: R493–R501, 2016. doi: 10.1152/ajpregu.00330.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cora’ D, Re A, Caselle M, Bussolino F. MicroRNA-mediated regulatory circuits: outlook and perspectives. Phys Biol 14: 045001, 2017. doi: 10.1088/1478-3975/aa6f21. [DOI] [PubMed] [Google Scholar]
- 12.Craig PM. Why trout? in Trout: From Physiology to Conservation (Polakof S, Moon TW, editors). Nova Science Publishers, 2013, p. 1–8. [Google Scholar]
- 13.Dai W, Panserat S, Kaushik S, Terrier F, Plagnes-Juan E, Seiliez I, Skiba-Cassy S. Hepatic fatty acid biosynthesis is more responsive to protein than carbohydrate in rainbow trout during acute stimulations. Am J Physiol Regul Integr Comp Physiol 310: R74–R86, 2016. doi: 10.1152/ajpregu.00281.2015. [DOI] [PubMed] [Google Scholar]
- 14.Dai W, Panserat S, Mennigen JA, Terrier F, Dias K, Seiliez I, Skiba-Cassy S. Post-prandial regulation of hepatic glucokinase and lipogenesis requires the activation of TORC1 signalling in rainbow trout (Oncorhynchus mykiss). J Exp Biol 216: 4483–4492, 2013. doi: 10.1242/jeb.091157. [DOI] [PubMed] [Google Scholar]
- 15.Dai W, Panserat S, Plagnes-Juan E, Seiliez I, Skiba-Cassy S. Amino Acids Attenuate Insulin Action on Gluconeogenesis and Promote Fatty Acid Biosynthesis via mTORC1 Signaling Pathway in trout Hepatocytes. Cell Physiol Biochem 36: 1084–1100, 2015. doi: 10.1159/000430281. [DOI] [PubMed] [Google Scholar]
- 16.Dai W, Panserat S, Terrier F, Seiliez I, Skiba-Cassy S. Acute rapamycin treatment improved glucose tolerance through inhibition of hepatic gluconeogenesis in rainbow trout (Oncorhynchus mykiss). Am J Physiol Regul Integr Comp Physiol 307: R1231–R1238, 2014. doi: 10.1152/ajpregu.00166.2014. [DOI] [PubMed] [Google Scholar]
- 17.Elmén J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjärn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, Kauppinen S. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res 36: 1153–1162, 2008. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol 5: R1, 2003. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezaki J, Matsumoto N, Takeda-Ezaki M, Komatsu M, Takahashi K, Hiraoka Y, Taka H, Fujimura T, Takehana K, Yoshida M, Iwata J, Tanida I, Furuya N, Zheng D-M, Tada N, Tanaka K, Kominami E, Ueno T. Liver autophagy contributes to the maintenance of blood glucose and amino acid levels. Autophagy 7: 727–736, 2011. doi: 10.4161/auto.7.7.15371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan X, Kurgan L. Comprehensive overview and assessment of computational prediction of microRNA targets in animals. Brief Bioinform 16: 780–794, 2015. doi: 10.1093/bib/bbu044. [DOI] [PubMed] [Google Scholar]
- 21.Felip O, Ibarz A, Fernández-Borràs J, Beltrán M, Martín-Pérez M, Planas JV, Blasco J. Tracing metabolic routes of dietary carbohydrate and protein in rainbow trout (Oncorhynchus mykiss) using stable isotopes ([13C]starch and [15N]protein): effects of gelatinisation of starches and sustained swimming. Br J Nutr 107: 834–844, 2012. doi: 10.1017/S0007114511003709. [DOI] [PubMed] [Google Scholar]
- 22.Fernández-Hernando C, Ramírez CM, Goedeke L, Suárez Y. MicroRNAs in metabolic disease. Arterioscler Thromb Vasc Biol 33: 178–185, 2013. doi: 10.1161/ATVBAHA.112.300144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haman F, Powell M, Weber JM. Reliability of continuous tracer infusion for measuring glucose turnover rate in rainbow trout. J Exp Biol 200: 2557–2563, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Heckmann L-H, Sørensen PB, Krogh PH, Sørensen JG. NORMA-Gene: a simple and robust method for qPCR normalization based on target gene data. BMC Bioinformatics 12: 250, 2011. doi: 10.1186/1471-2105-12-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández-Alvarez MI, Paz JC, Sebastián D, Muñoz JP, Liesa M, Segalés J, Palacín M, Zorzano A. Glucocorticoid modulation of mitochondrial function in hepatoma cells requires the mitochondrial fission protein Drp1. Antioxid Redox Signal 19: 366–378, 2013. doi: 10.1089/ars.2011.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera BM, Lockstone HE, Taylor JM, Ria M, Barrett A, Collins S, Kaisaki P, Argoud K, Fernandez C, Travers ME, Grew JP, Randall JC, Gloyn AL, Gauguier D, McCarthy MI, Lindgren CM. Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia 53: 1099–1109, 2010. doi: 10.1007/s00125-010-1667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera BM, Lockstone HE, Taylor JM, Wills QF, Kaisaki PJ, Barrett A, Camps C, Fernandez C, Ragoussis J, Gauguier D, McCarthy MI, Lindgren CM. MicroRNA-125a is over-expressed in insulin target tissues in a spontaneous rat model of Type 2 Diabetes. BMC Med Genomics 2: 54, 2009. doi: 10.1186/1755-8794-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilton JW, Atkinson JL. Response of rainbow trout (Salmo gairdneri) to increased levels of available carbohydrate in practical trout diets. Br J Nutr 47: 597–607, 1982. doi: 10.1079/BJN19820071. [DOI] [PubMed] [Google Scholar]
- 29.Hofacker IL. RNA secondary structure analysis using the Vienna RNA package. Curr Protoc Bioinformatics Chapter 12: Unit 12.2, 2004. doi: 10.1002/0471250953.bi1202s04. [DOI] [PubMed] [Google Scholar]
- 30.Juanchich A, Bardou P, Rué O, Gabillard J-C, Gaspin C, Bobe J, Guiguen Y. Characterization of an extensive rainbow trout miRNA transcriptome by next generation sequencing. BMC Genomics 17: 164, 2016. doi: 10.1186/s12864-016-2505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y-K, Kim B, Kim VN. Re-evaluation of the roles of DROSHA, Export in 5, and DICER in microRNA biogenesis. Proc Natl Acad Sci USA 113: E1881–E1889, 2016. doi: 10.1073/pnas.1602532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchner S, Panserat S, Lim PL, Kaushik S, Ferraris RP. The role of hepatic, renal and intestinal gluconeogenic enzymes in glucose homeostasis of juvenile rainbow trout. J Comp Physiol B 178: 429–438, 2008. doi: 10.1007/s00360-007-0235-7. [DOI] [PubMed] [Google Scholar]
- 33.Koganti PP, Wang J, Cleveland B, Ma H, Weber GM, Yao J. Estradiol regulates expression of miRNAs associated with myogenesis in rainbow trout. Mol Cell Endocrinol 443: 1–14, 2017. doi: 10.1016/j.mce.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan J, Rohner N. Sweet fish: Fish models for the study of hyperglycemia and diabetes. J Diabetes 11: 193–203, 2019. doi: 10.1111/1753-0407.12860. [DOI] [PubMed] [Google Scholar]
- 35.Lansard M, Panserat S, Plagnes-Juan E, Seiliez I, Skiba-Cassy S. Integration of insulin and amino acid signals that regulate hepatic metabolism-related gene expression in rainbow trout: role of TOR. Amino Acids 39: 801–810, 2010. doi: 10.1007/s00726-010-0533-3. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Chen X, Zhang H, Liang X, Xiang Y, Yu C, Zen K, Li Y, Zhang C-Y. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status. J Lipid Res 50: 1756–1765, 2009. doi: 10.1194/jlr.M800509-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynn FC. Meta-regulation: microRNA regulation of glucose and lipid metabolism. Trends Endocrinol Metab 20: 452–459, 2009. doi: 10.1016/j.tem.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Ma H, Weber GM, Hostuttler MA, Wei H, Wang L, Yao J. MicroRNA expression profiles from eggs of different qualities associated with post-ovulatory ageing in rainbow trout (Oncorhynchus mykiss). BMC Genomics 16: 201, 2015. doi: 10.1186/s12864-015-1400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magnoni L, Weber J-M. Endurance swimming activates trout lipoprotein lipase: plasma lipids as a fuel for muscle. J Exp Biol 210: 4016–4023, 2007. doi: 10.1242/jeb.007708. [DOI] [PubMed] [Google Scholar]
- 40.Mannerström M, Soivio A, Salama A. Intestinal absorption and tissue distribution of glucose and isoleucine in rainbow trout (Oncorhynchus mykiss). Aquacult Nutr 7: 229–235, 2001. doi: 10.1046/j.1365-2095.2001.00179.x. [DOI] [Google Scholar]
- 41.Marandel L, Lepais O, Arbenoits E, Véron V, Dias K, Zion M, Panserat S. Remodelling of the hepatic epigenetic landscape of glucose-intolerant rainbow trout (Oncorhynchus mykiss) by nutritional status and dietary carbohydrates. Sci Rep 6: 32187, 2016. doi: 10.1038/srep32187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marandel L, Seiliez I, Véron V, Skiba-Cassy S, Panserat S. New insights into the nutritional regulation of gluconeogenesis in carnivorous rainbow trout (Oncorhynchus mykiss): a gene duplication trail. Physiol Genomics 47: 253–263, 2015. doi: 10.1152/physiolgenomics.00026.2015. [DOI] [PubMed] [Google Scholar]
- 43.Mennigen JA. Micromanaging metabolism-a role for miRNAs in teleost energy metabolism. Comp Biochem Physiol B Biochem Mol Biol 199: 115–125, 2016. doi: 10.1016/j.cbpb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Mennigen JA, Martyniuk CJ, Seiliez I, Panserat S, Skiba-Cassy S. Metabolic consequences of microRNA-122 inhibition in rainbow trout, Oncorhynchus mykiss. BMC Genomics 15: 70, 2014. doi: 10.1186/1471-2164-15-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mennigen JA, Panserat S, Larquier M, Plagnes-Juan E, Medale F, Seiliez I, Skiba-Cassy S. Postprandial regulation of hepatic microRNAs predicted to target the insulin pathway in rainbow trout. PLoS One 7: e38604, 2012. doi: 10.1371/journal.pone.0038604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mennigen JA, Zhang D. MicroTrout: A comprehensive, genome-wide miRNA target prediction framework for rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol Part D Genomics Proteomics 20: 19–26, 2016. doi: 10.1016/j.cbd.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Miao L-H, Lin Y, Pan W-J, Huang X, Ge X-P, Ren M-C, Zhou Q-L, Liu B. Identification of Differentially Expressed Micrornas Associate with Glucose Metabolism in Different Organs of Blunt Snout Bream (Megalobrama amblycephala). Int J Mol Sci 18: E1161, 2017. doi: 10.3390/ijms18061161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirra P, Nigro C, Prevenzano I, Leone A, Raciti GA, Formisano P, Beguinot F, Miele C. The Destiny of Glucose from a MicroRNA Perspective. Front Endocrinol (Lausanne) 9: 46, 2018. doi: 10.3389/fendo.2018.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moon TW. Glucose intolerance in teleost fish: fact or fiction? Comp Biochem Physiol B Biochem Mol Biol 129: 243–249, 2001. doi: 10.1016/S1096-4959(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 49a.National Research Council Nutrient Requirements of Fish and Shrimp. Washington, DC: National Academies Press, 2011. doi: 10.17226/13039. [DOI] [Google Scholar]
- 50.Naylor RL, Hardy RW, Bureau DP, Chiu A, Elliott M, Farrell AP, Forster I, Gatlin DM, Goldburg RJ, Hua K, Nichols PD. Feeding aquaculture in an era of finite resources. Proc Natl Acad Sci USA 106: 15103–15110, 2009. doi: 10.1073/pnas.0905235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olsvik PA, Lie KK, Jordal A-EO, Nilsen TO, Hordvik I. Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Mol Biol 6: 21, 2005. doi: 10.1186/1471-2199-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page GI, Hayworth KM, Wade RR, Harris AM, Bureau DP. Non‐specific immunity parameters and the formation of advanced glycosylation end‐products (AGE) in rainbow trout, Oncorhynchus mykiss (Walbaum), fed high levels of dietary carbohydrates. Aquacult Res 30: 287–297, 1999. doi: 10.1046/j.1365-2109.1999.00330.x. [DOI] [Google Scholar]
- 53.Paneru BD, Al-Tobasei R, Kenney B, Leeds TD, Salem M. RNA-Seq reveals MicroRNA expression signature and genetic polymorphism associated with growth and muscle quality traits in rainbow trout. Sci Rep 7: 9078, 2017. doi: 10.1038/s41598-017-09515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panserat S, Kaushik SJ, Medale F. Rainbow trout as a model for nutrition and nutrient metabolism studies, in Trout: From Physiology to Conservation (Polakof S, Moon TW, editors). Nova Science Publishers, 2013, p. 131–151. [Google Scholar]
- 55.Panserat S, Médale F, Blin C, Brèque J, Vachot C, Plagnes-Juan E, Gomes E, Krishnamoorthy R, Kaushik S. Hepatic glucokinase is induced by dietary carbohydrates in rainbow trout, gilthead seabream, and common carp. Am J Physiol Regul Integr Comp Physiol 278: R1164–R1170, 2000. doi: 10.1152/ajpregu.2000.278.5.R1164. [DOI] [PubMed] [Google Scholar]
- 56.Panserat S, Plagnes-Juan E, Kaushik S. Nutritional regulation and tissue specificity of gene expression for proteins involved in hepatic glucose metabolism in rainbow trout (Oncorhynchus mykiss). J Exp Biol 204: 2351–2360, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Panserat S, Skiba-Cassy S, Seiliez I, Lansard M, Plagnes-Juan E, Vachot C, Aguirre P, Larroquet L, Chavernac G, Medale F, Corraze G, Kaushik S, Moon TW. Metformin improves postprandial glucose homeostasis in rainbow trout fed dietary carbohydrates: a link with the induction of hepatic lipogenic capacities? Am J Physiol Regul Integr Comp Physiol 297: R707–R715, 2009. doi: 10.1152/ajpregu.00120.2009. [DOI] [PubMed] [Google Scholar]
- 58.Peterson SM, Thompson JA, Ufkin ML, Sathyanarayana P, Liaw L, Congdon CB. Common features of microRNA target prediction tools. Front Genet 5: 23, 2014. doi: 10.3389/fgene.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillipps A, Tunison A, Brockway DR. The utilisation of carbohydrate by trout. Fisheries Research Bulletin NY 11: 1–44, 1948. [Google Scholar]
- 60.Polakof S, Mommsen TP, Soengas JL. Glucosensing and glucose homeostasis: from fish to mammals. Comp Biochem Physiol B Biochem Mol Biol 160: 123–149, 2011. doi: 10.1016/j.cbpb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 61.Polakof S, Panserat S, Soengas JL, Moon TW. Glucose metabolism in fish: a review. J Comp Physiol B 182: 1015–1045, 2012. doi: 10.1007/s00360-012-0658-7. [DOI] [PubMed] [Google Scholar]
- 62.Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA 10: 1507–1517, 2004. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rottiers V, Näär AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol 13: 239–250, 2012. [Erratum in Nat Rev Mol Cell Biol 13: 2012] 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol 11: 637–645, 2017. doi: 10.1016/j.redox.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saliani N, Montazersaheb S, Montasser Kouhsari S. Micromanaging Glucose Tolerance and Diabetes. Adv Pharm Bull 7: 547–556, 2017. doi: 10.15171/apb.2017.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Samy JKA, Mulugeta TD, Nome T, Sandve SR, Grammes F, Kent MP, Lien S, Våge DI. SalmoBase: an integrated molecular data resource for Salmonid species. BMC Genomics 18: 482, 2017. doi: 10.1186/s12864-017-3877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 68.Schrepfer E, Scorrano L. Mitofusins, from Mitochondria to Metabolism. Mol Cell 61: 683–694, 2016. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 69.Seiliez I, Belghit I, Gao Y, Skiba-Cassy S, Dias K, Cluzeaud M, Rémond D, Hafnaoui N, Salin B, Camougrand N, Panserat S. Looking at the metabolic consequences of the colchicine-based in vivo autophagic flux assay. Autophagy 12: 343–356, 2016. doi: 10.1080/15548627.2015.1117732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skiba-Cassy S, Lansard M, Panserat S, Médale F. Rainbow trout genetically selected for greater muscle fat content display increased activation of liver TOR signaling and lipogenic gene expression. Am J Physiol Regul Integr Comp Physiol 297: R1421–R1429, 2009. doi: 10.1152/ajpregu.00312.2009. [DOI] [PubMed] [Google Scholar]
- 71.Subramaniam M, Weber LP, Ching JC, Enns CB, Kilgour AB, Drew MD, Loewen ME. Species Differences in Gastrointestinal Tract Glucose Transporters between Rainbow Trout (Oncorhynchus mykiss) and Nile Tilapia (Oreochromis niloticus). FASEB J 30, Suppl: 760.20, 2016. [Google Scholar]
- 72.Theurey P, Rieusset J. Mitochondria-Associated Membranes Response to Nutrient Availability and Role in Metabolic Diseases. Trends Endocrinol Metab 28: 32–45, 2017. doi: 10.1016/j.tem.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Toledo M, Batista-Gonzalez A, Merheb E, Aoun ML, Tarabra E, Feng D, Sarparanta J, Merlo P, Botrè F, Schwartz GJ, Pessin JE, Singh R. Autophagy Regulates the Liver Clock and Glucose Metabolism by Degrading CRY1. Cell Metab 28: 268–281.e4, 2018. doi: 10.1016/j.cmet.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsai W-C, Hsu S-D, Hsu C-S, Lai T-C, Chen S-J, Shen R, Huang Y, Chen H-C, Lee C-H, Tsai T-F, Hsu M-T, Wu J-C, Huang H-D, Shiao M-S, Hsiao M, Tsou A-P. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest 122: 2884–2897, 2012. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilczynska A, Bushell M. The complexity of miRNA-mediated repression. Cell Death Differ 22: 22–33, 2015. doi: 10.1038/cdd.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilding JPH. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism 63: 1228–1237, 2014. doi: 10.1016/j.metabol.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 77.Witkos TM, Koscianska E, Krzyzosiak WJ. Practical Aspects of microRNA Target Prediction. Curr Mol Med 11: 93–109, 2011. doi: 10.2174/156652411794859250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan L-J. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res 2014: 137919, 2014. doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye P, Liu Y, Chen C, Tang F, Wu Q, Wang X, Liu C-G, Liu X, Liu R, Liu Y, Zheng P. An mTORC1-Mdm2-Drosha axis for miRNA biogenesis in response to glucose- and amino acid-deprivation. Mol Cell 57: 708–720, 2015. doi: 10.1016/j.molcel.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H-M, Kuang S, Xiong X, Gao T, Liu C, Guo A-Y. Transcription factor and microRNA co-regulatory loops: important regulatory motifs in biological processes and diseases. Brief Bioinform 16: 45–58, 2015. doi: 10.1093/bib/bbt085. [DOI] [PubMed] [Google Scholar]
- 81.Zhang W, Wang P, Chen S, Zhang Z, Liang T, Liu C. Rhythmic expression of miR-27b-3p targets the clock gene Bmal1 at the posttranscriptional level in the mouse liver. FASEB J 30: 2151–2160, 2016. doi: 10.1096/fj.201500120. [DOI] [PubMed] [Google Scholar]
- 82.Zhao E, Keller MP, Rabaglia ME, Oler AT, Stapleton DS, Schueler KL, Neto EC, Moon JY, Wang P, Wang I-M, Lum PY, Ivanovska I, Cleary M, Greenawalt D, Tsang J, Choi YJ, Kleinhanz R, Shang J, Zhou Y-P, Howard AD, Zhang BB, Kendziorski C, Thornberry NA, Yandell BS, Schadt EE, Attie AD. Obesity and genetics regulate microRNAs in islets, liver, and adipose of diabetic mice. Mamm Genome 20: 476–485, 2009. doi: 10.1007/s00335-009-9217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]