Abstract
Heart failure (HF) is associated with increased large conduit artery stiffness and afterload resulting in stiffening of the coronary arteries. Perivascular adipose tissue (PVAT) and advanced glycation end products (AGE) both promote arterial stiffness, yet the mechanisms by which coronary PVAT promotes arterial stiffness and the efficacy of exercise to prevent coronary stiffness are unknown. We hypothesized that both chronic continuous and interval exercise training would prevent coronary PVAT-mediated AGE secretion and arterial stiffness. Yucatan miniature swine were divided into four groups: control-sedentary (CON), aortic banded sedentary-heart failure (HF), aortic banded HF-continuous exercise trained (HF+CONT), and aortic banded HF-interval exercise trained (HF+IT). The left circumflex and right coronary arteries underwent ex vivo mechanical testing, and arterial AGE, elastin, and collagen were assessed. Coronary elastin elastic modulus (EEM) and elastin protein were lower and AGE was increased with HF compared with CON, which was prevented by both HF+CONT and HF+IT. Mouse aortic segments treated with swine coronary PVAT conditioned medium had lower EEM and elastin content and greater AGE secretion and arterial AGE accumulation in HF compared with CON, which was prevented by both HF+CONT and HF+IT. Aminoguanidine (AMG), an AGE inhibitor, prevented the reduction in EEM, arterial elastin content, and AGE accumulation in mouse aortic segments treated with PVAT conditioned medium in the HF group. Our data demonstrate efficacy for chronic continuous and interval exercise to prevent coronary artery stiffness via inhibition of PVAT-derived AGE secretion in a preclinical miniswine model of pressure overload-induced HF.
NEW & NOTEWORTHY Our findings show that chronic continuous and interval exercise training regimens prevent coronary artery stiffness associated with inhibition of perivascular adipose tissue-derived advanced glycation end products in a translational pressure overload-induced heart failure model potentially providing an effective therapeutic option for heart failure patients.
Keywords: continuous exercise, heart failure, inflammation, interval training, oxidative stress
INTRODUCTION
Stiffening of the large elastic arteries is an independent predictor of cardiovascular events, promotes hypertension, and is associated with incident heart failure (HF) (31). The increased afterload due to aortic stiffening and augmented pulse pressure contributes to the failure of the myocardium (6), in part, through increased coronary pulsatility contributing to both endothelial and microvascular dysfunction (15, 38). Recently, our laboratory reported increased coronary artery stiffness in an aortic-banded miniswine model of pressure overload-induced HF (15), indicating that the increased afterload also promotes coronary vascular dysfunction. Therefore, identifying mechanisms and interventions to reduce coronary artery stiffness may also alleviate additional stress on an already failing myocardium.
A key characteristic of arterial stiffening is remodeling of the extracellular matrix (ECM) within the vasculature. More specifically, reduction in elastin, increased collagen, and a greater abundance of advanced glycation end products (AGE) collectively promote vascular stiffening (29). AGE decreases arterial elastin and cross-links ECM proteins, in addition to influencing the artery through receptor of AGE (RAGE)-mediated cellular signaling mechanisms (45). Arterial AGE accumulation occurs in conditions where plasma glucose is elevated, such as diabetes. However, adipose tissue in nondiabetic conditions has also been shown to secrete AGE (45). Perivascular adipose tissue (PVAT) is an endocrine tissue shown to promote arterial dysfunction, including arterial stiffness (10, 40). These findings are significant, as recent evidence has shown that PVAT surrounding the coronary arteries, which is associated with increased inflammation, predicts cardiac mortality (33). Notably, both aging and HF promote PVAT oxidative stress and inflammation, which are important factors for AGE production in adipose tissue, suggesting that PVAT may be a novel source of AGE contributing to arterial stiffness (13, 15, 40). Currently, however, it is unknown whether PVAT secretes AGE to promote arterial stiffening with HF.
Both chronic continuous and interval exercise training programs are effective at reducing blood pressure and destiffening large elastic arteries such as the aorta (21, 50). The mechanisms by which exercise prevents and/or reverses central (aorta and carotid) arterial stiffness have been attributed to maintaining elastin and lowering collagen content (8, 14, 35) while suppressing oxidative stress and inflammation (17). Importantly, nonexercise interventions that reduce PVAT oxidative stress, inflammation, and AGE accumulation are associated with reductions in aortic stiffness (40). These findings collectively suggest that exercise may prevent coronary arterial stiffness by lowering PVAT-related AGE secretion. However, little is known about the efficacy of chronic continuous and interval exercise to prevent coronary artery stiffening in a translational large-animal HF model.
The aim of this study was to determine the influences of both chronic continuous and interval exercise training on coronary artery stiffness via PVAT-related AGE secretion in a preclinical miniswine model of pressure overload-induced HF. We hypothesized that both continuous and interval exercise training would prevent coronary artery stiffness associated with ECM remodeling, AGE accumulation, oxidative stress, and inflammation through a mechanism mediated by reduced PVAT-related AGE secretion.
METHODS
Aortic banding and exercise training protocols.
The animals in the present study are the same animals used in work from our laboratory recently published in the Journal of the American Heart Association (39) and the Journal of Applied Physiology (25, 38). Left ventricular (LV) hypertrophy/HF was induced by aortic banding for a period of 24 wk by methods previously published by our laboratory (23, 24, 34, 39, 51). Male Yucatan miniature swine (29–32 kg; 8 mo old) were assigned to four groups (n = 7 for all groups): sedentary control (CON), aortic banded sedentary (HF), aortic banded chronic continuous exercise trained (HF+CONT), and aortic banded chronic interval exercise trained (HF+IT). Aortic bands were placed around the ascending aorta (proximal to the brachiocephalic artery), and a systolic transstenotic gradient of ~70 mmHg was established [74 ± 2, 74 ± 2, and 72 ± 1 mmHg for HF, HF+IT, and HF+CONT, respectively; P = not significant (NS)] under anesthesia using phenylephrine (1–3 μg·kg−1·min−1 iv) to maintain a distal peripheral vascular mean aortic pressure of ~90 mmHg (90 ± 1, 91 ± 2, and 89 ± 1 mmHg for HF, HF+IT and HF+CONT, respectively; P = NS) at a heart rate of 100 beats/min (104 ± 5, 99 ± 8, and 106 ± 5 beats/min for HF, HF+IT and HF+CONT, respectively; P = NS) as previously reported (25, 38, 39).
Animals began exercise training after the development of LV hypertrophy, 2 mo after aortic banding as previously reported in these same animals (25, 38, 39). Swine were exercise trained on a treadmill, running 55 min/day, 3 days/week, for 17 wk with gradually increasing intensity according to the following previously published protocols: continuous exercise: 1) a 5-min warm-up at 1.5 mph, 2) 45 min at 2.5 mph, and 3) a 5-min cool-down at 1.5 mph (25, 39); interval exercise: 1) a 5-min warm-up at 2 mph, 2) six 5-min sessions at 3 mph with five 3-min intervals at 4 mph in between, and 3) a 5-min cool-down at 2 mph (11, 12, 34, 38). Animals were fed a standard diet averaging 15–20 g/kg once daily, and water was provided ad libitum. After the aforementioned protocols were completed, animals were euthanized by bilateral pneumothorax followed by exsanguination with removal of the heart after 15 min of 5% isoflurane anesthesia exposure. All animal protocols were in accordance with the Principles for the Utilization and Care of Vertebrate Animals Used in Testing Research and Training and were reviewed and approved by the University of Missouri Animal Care and Use Committee, conforming to National Institutes of Health (NIH) guidelines.
Mechanical stiffness testing.
Arterial stiffness was assessed as previously described (10, 15, 18, 40). The left circumflex (LCX) and right coronary (RCA) arteries were cleaned of the surrounding adipose tissue and cut into ~1.5-mm segments. The coronary arterial ring was placed in a preheated 37°C Ca2+- and Mg2+-free phosphate-buffered saline (PBS) solution in a myograph (DMT 620). The artery segment was stretched 1 mm every 3 min until mechanical failure. The elastic modulus was calculated from the stress-strain curve as previously described (10, 15, 18, 40). In brief, one-dimensional stress (t) was calculated as t = λL/2HD and strain (λ) was defined as λ = Δd/d(i), where L = one-dimensional load applied, H = wall thickness, D = length of vessel, Δd = change in diameter, and d(i) = initial diameter. Coronary diameter and wall thickness were assessed in histological sections, and length was measured under a dissecting microscope with calipers. The elastic modulus was determined as the greatest r2 value from the stress-strain curve as described previously (15). The elastin region, coinciding with the elastin elastic modulus (EEM), was determined as the transition point between the toe and heel regions of the stress-strain curve, and the collagen region, coinciding with the collagen elastic modulus (CEM), was the determined greatest r2 value before mechanical failure (15).
Coronary perivascular adipose conditioned medium experiments.
The conditioned medium study was performed as described previously by our laboratory (10, 13, 15). Briefly, adipose tissue surrounding the coronary artery was removed and cultured for 24 h in Dulbecco’s modified Eagle’s medium (DMEM) containing antibiotics (20 mg fat per 100 μL DMEM) at 37°C and 5% CO2. The coronary adipose conditioned medium (10% of total volume) was heat inactivated by being placed in a heat block for 30 min at 56°C. Young, 4- to 6-mo-old, wild-type C57BL/6 mice (The Jackson Laboratory) were euthanized by cardiac exsanguination while anesthetized with isoflurane (2%). Aortas without the surrounding adipose tissue were cultured in the coronary adipose conditioned medium with or without 1 mM AGE inhibitor aminoguanidine (AMG) for 72 h at 37°C and 5% CO2. The conditioned medium was changed daily. After treatment, aortic stiffness testing was performed. The University of Kentucky Animal Care and Use Committee reviewed and approved all protocols in this study, conforming to NIH guidelines.
Immunohistochemistry.
Immunohistochemistry was performed by standard procedures as previously described (10, 15). Segments of LCX, RCA, and mouse aortas were embedded with optimal cutting temperature compound and frozen. Arteries were cross-sectioned (8 μm), fixed on glass slides with acetone, and stained with the Dako Envison+ system horseradish peroxidase-diaminobenzidine kit (Agilent). The following primary antibodies were used: collagen (Abcam, ab21286), elastin (Abcam, ab21607), AGE (Abcam, ab23722), RAGE (Abcam, ab3611), nitrotyrosine (EMD, ab5411), NF-κB p65 (Abcam, ab7970), and scavenger receptor A (SRA, TransGenic KT022) were applied on slides separately and incubated at 4°C overnight with preoptimized dilutions (Table 1). The secondary horseradish peroxidase-conjugated labeled polymer was applied for 30 min at room temperature followed by diaminobenzidine application for ~2 min until appropriate darkness was achieved. Coronary PVAT was fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 5-μm thickness. Slides were deparaffinized with xylene, and proteinase K was used for antigen retrieval. Slides were stained with preoptimized diluted primary antibodies (Table 1). Images were acquired with a Nikon 80i microscope using ×4, ×10, and ×20 objectives for the swine coronary artery, mouse aorta, and adipose tissue, respectively. Densitometry analysis was performed by ImageJ (NIH) on the medial layer of the artery, and the data are presented as relative density in arbitrary units (AU). Additionally, amino acid sequence homology between the antibody and swine (Sus scrofa) sequences are reported for all antibodies (Table 2).
Table 1.
IHC primary antibody concentrations
| Primary Antibody | Coronary Artery | PVAT | Mouse Aorta |
|---|---|---|---|
| Collagen | 1:400 | ||
| Elastin | 1:300 | 1:200 | |
| AGE | 1:400 | 1:200 | 1:150 |
| RAGE | 1:200 | 1:150 | |
| Nitrotyrosine | 1:300 | 1:50 | 1:100 |
| NF-κB p65 | 1:300 | 1:50 | 1:100 |
| SRA | 1:100 | 1:50 |
Data are immunohistochemistry (IHC) primary antibody concentrations. AGE, advanced glycation end products; PVAT, perivascular adipose tissue; RAGE, receptor of AGE; SRA, scavenger receptor A.
Table 2.
Antibody specificity
| Primary Antibody | Sequence Homology |
|---|---|
| Collagen | 97% |
| Elastin | 80.7% (42) |
| NF-κB p65 | 100% |
| RAGE | 95% |
| SRA | Verified by other groups (30, 49) |
| AGE/nitrotyrosine | Species independent |
Comparison of antibody amino acid sequences to the amino acid sequences of swine (Sus scrofa). AGE, advanced glycation end products; RAGE, receptor of AGE; SRA, scavenger receptor A.
Adipose morphology.
PVAT surrounding coronary arteries was fixed in 4% paraformaldehyde, embedded in paraffin, and cross-sectioned (5 μm). After being deparaffinized, adipose tissue was stained with hematoxylin and eosin by standard procedures (10, 15, 40, 43). Digital images were acquired with a Nikon 80i microscope with a ×20 objective lens in an uncompressed tiff format. Adipocyte area (μm2) and diameter (μm) were averaged from at least 100 cells from three tissue sections for each sample with ImageJ (NIH). The data for each sample were averaged and used to calculate the mean for each group.
Enzyme-linked immunosorbent assay.
Pericoronary adipose tissue was removed and cultured in serum-free DMEM for 24 h at 37°C and 5% CO2 at a concentration of 20 mg fat/100 μL DMEM. AGE concentration in swine coronary perivascular adipose conditioned medium was assessed by enzyme-linked immunosorbent assay (ELISA) kit per the manufacturer’s protocol (Cell Biolabs, STA-817).
Cytokine array.
Cytokines in coronary PVAT conditioned medium were assessed with a Porcine Cytokine Antibody Array Kit (Abcam, ab197479). Ten cytokine targets (IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, granulocyte macrophage colony-stimulating factor, IFN-γ, transforming growth factor-β1, TNF-α) were assessed, and we report significant differences in IL-6 and IL-8 levels. All procedures were performed according to the product manual. A laser scanner equipped with Cy3 wavelength (Typhoon 9500; GE) was used to acquire fluorescence signals, and densitometry analysis was performed with ImageJ (NIH). Cytokine concentrations were calculated based on standard curves, and cytokines other than IL-6 and IL-8 were not detectable in the measurable range.
Statistical analysis.
All data analyses were performed with GraphPad Prism 7.0. Group mean comparisons for coronary stiffness, immunohistochemistry analyses, the ELISA and cytokine array were analyzed by one-way ANOVA. Coronary PVAT culture studies were analyzed by two-way ANOVA. Linear regression was used to examine the relationship between coronary elastin and AGE levels with the EEM. Fisher’s least significant difference post hoc analyses were performed when appropriate. All data are presented as means ± SE. Statistical significance is reported as P < 0.05.
RESULTS
LV remodeling and function.
A thorough investigation of myocardial remodeling and LV function was provided for the same animals used in the present study in work from our laboratory recently published in the Journal of Applied Physiology and Journal of the American Heart Association (25, 38, 39). In brief summary, these studies demonstrated the therapeutic efficacy of chronic exercise training on coronary vascular, isolated cardiomyocyte, and cognitive function in a translational pressure-overload model of HF with potential relevance to human HF. Lung weight, LV natriuretic peptide levels, global concentric hypertrophic remodeling (at the gross and cellular levels), and slope of the end-diastolic pressure-volume relationship (indicative of diastolic dysfunction) were increased in the HF group compared with CON. Body weight, ejection fraction (>50%), stroke volume, and end-diastolic pressure (7–9 mmHg) were the same in HF and CON animals. Considered together, these results are consistent with a scenario of HF with compensated resting systolic function (4). LV end-diastolic pressure may not be elevated early on in this type of HF (1, 5); thus our findings suggest that the severity of failure in the HF group may be in its initial stages. Body weight, ejection fraction (>50%), stroke volume, and end-diastolic pressure (8–9 mmHg) were similar in both exercise-trained groups compared with CON and HF animals. Increased lung weight and LV natriuretic peptide levels were attenuated in the exercise groups, but global concentric hypertrophic remodeling and the slope of the end-diastolic pressure-volume relationship were similar to levels reported in the HF group. Furthermore, chronic exercise training had a positive impact at both the organ and molecular levels in a number of different functional systems including isolated cardiomyocyte calcium handling and contractile function, large-conductance Ca2+-activated K+ (BKCa) channel-mediated coronary vascular function, and peripheral arterial stiffness and cognition. We refer the reader to the following published studies for specifics regarding the variables outlined above and other hemodynamic outcomes previously reported in these groups (25, 38, 39).
Exercise prevents coronary artery stiffness, ECM remodeling, oxidative stress, and inflammation.
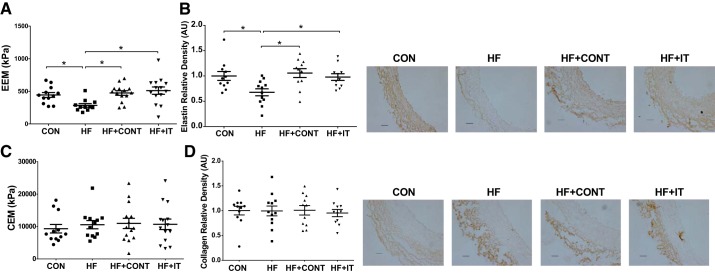
Compared with CON, the combined EEM of the LCX and RCA was lower in the HF group (284 ± 28 vs. 444 ± 38 kPa, P < 0.05; Fig. 1A), which was associated with reduced medial elastin protein content (P < 0.05; Fig. 1B). No differences were observed between the CON and HF groups for either the CEM (9,324 ± 1,319 vs. 10,563 ± 1,272 kPa, P > 0.05; Fig. 1C) or collagen content (P > 0.05; Fig. 1D). The 17-wk HF+CONT and HF+IT exercise training regimens prevented the decrease in coronary EEM (474 ± 38 and 511 ± 56 kPa, respectively, P < 0.05; Fig. 1A) and elastin content (P < 0.05; Fig. 1B), without influencing CEM or collagen content (P > 0.05; Fig. 1, C and D).
Fig. 1.
Coronary artery stiffness and arterial elastin and collagen content in swine. A and B: elastin elastic modulus (EEM; A) and elastin content (B) in coronary arteries of heart failure (HF) swine was prevented by exercise training. C and D: collagen elastic modulus (CEM; C) and collagen deposition (D) were not different between groups (n = 12–14/group). AU, arbitrary units. Data are means ± SE. *HF vs. control (CON), HF continuous exercise trained (HF+CONT), and HF interval exercise trained (HF+IT), P < 0.05. Data were analyzed with a 1-way ANOVA. Right: representative immunohistochemistry images of coronary artery showing medial elastin and collagen. Scale bars, 100 μm.
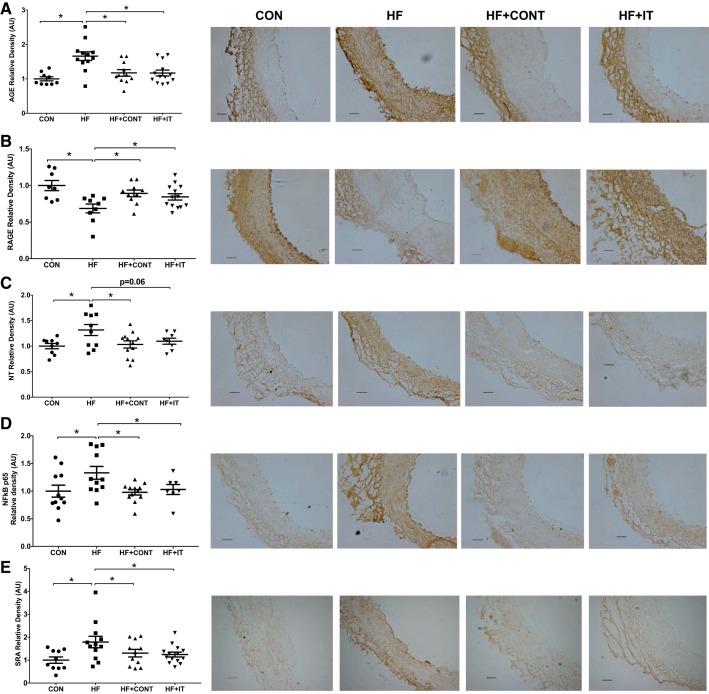
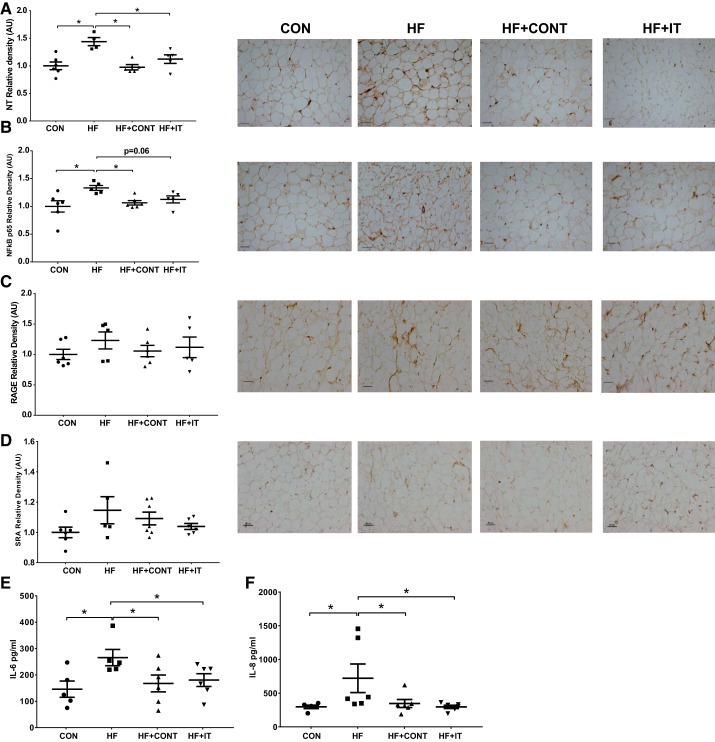
Greater coronary stiffness with HF was associated with concomitant increases in arterial AGE abundance, nitrotyrosine, NF-κB p65, and SRA relative to CON (P < 0.05; Fig. 2, A, C–E). Arterial RAGE was lower in the HF group compared with the CON group (P < 0.05; Fig. 2B). Both HF+CONT and HF+IT prevented the increase of AGE, nitrotyrosine, NF-κB p65, and SRA (P < 0.05; Fig. 2A, C–E) and the decrease in RAGE (Fig. 2B; P < 0.05). Correlation analyses demonstrate that coronary elastin content is positively (r = 0.31. P < 0.05) associated and coronary AGE accumulation is inversely associated (r = −0.28, P = 0.058) with the EEM. These findings demonstrate that a chronic continuous or interval exercise training regimen prevents coronary artery stiffness and vascular wall ECM remodeling in a preclinical miniswine model of pressure overload-induced HF.
Fig. 2.
Immunohistochemistry analysis of advanced glycation end products (AGE), receptor of AGE (RAGE), oxidative stress, and inflammation level of swine coronary arteries. A and C–E: greater AGE (A), nitrotyrosine (NT; C), NF-κB p65 (D), and scavenger receptor A (SRA; E) in heart failure (HF) compared with control (CON), which was attenuated by continuous (HF + CONT) and interval (HF + IT) exercise training. B: RAGE was lower in HF compared with CON, HF + CONT, and HF + IT (n = 6 or 7/group). AU, arbitrary units. Data are means ± SE. *HF vs. CON, HF+CONT, and HF+IT, P < 0.05. Data were analyzed with a 1-way ANOVA. Right: representative immunohistochemistry images of coronary artery showing AGE, RAGE, nitrotyrosine, NF-κB p65, and SRA alterations. Scale bars, 100 μm.
PVAT-related AGE secretion, arterial stiffening, and ECM remodeling are prevented by exercise training.
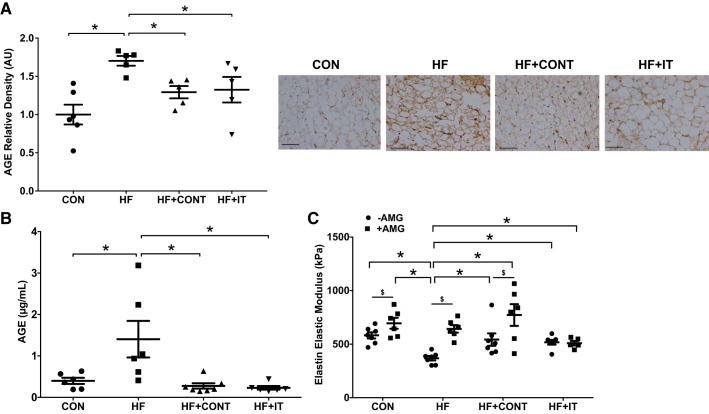
Compared with CON, HF had greater PVAT AGE abundance (P < 0.05; Fig. 3A) and concentration of AGE in PVAT conditioned medium (1.404 ± 0.44 vs. 0.39 ± 0.07 μg/mL, P < 0.05; Fig. 3B). To determine the influence of PVAT-derived AGE on arterial stiffness, mouse aortas were cultured with the conditioned medium and stiffness testing was performed. The EEM was reduced after exposure to HF compared with CON PVAT conditioned medium (368 ± 20 vs. 582 ± 29 kPa, P < 0.05; Fig. 3C). Both HF+CONT and HF+IT exercise attenuated or prevented the HF-related AGE accumulation in PVAT (P < 0.05; Fig. 3A), AGE secretion from PVAT (0.27 ± 0.06 and 0.23 ± 0.04 μg/mL, respectively, P < 0.05; Fig. 3B) and the PVAT-induced reduction in EEM (542 ± 58 and 518 ± 22 kPa, respectively, P < 0.05; Fig. 3C). The AGE inhibitor AMG prevented the PVAT-related reduction in EEM with HF (643 ± 35 kPa, P < 0.05; Fig. 3C). AMG also increased the EEM in CON and HF+CONT aortic segments (695 ± 50 and 772 ± 101 kPa, respectively, P < 0.05; Fig. 3C).
Fig. 3.
Advanced glycation end product (AGE) secretion from swine pericoronary adipose tissue and perivascular adipose tissue (PVAT)-derived AGE effects on aortic stiffness. A: greater pericoronary adipose tissue AGE accumulation in heart failure (HF) compared with control (CON), HF continuous exercise trained (HF+CONT), and HF interval exercise trained (HF+IT). B: AGE concentration in coronary PVAT conditioned medium from HF swine was higher compared with control and exercise training groups (n = 6 or 7/group). AU, arbitrary units. Data are means ± SE. *HF vs. CON, HF+CONT, and HF+IT, P < 0.05. Data in A and B were analyzed with a 1-way ANOVA. C: exposure to PVAT conditioned medium from HF swine coronary artery decreased mouse aortic elastin elastic modulus, which was prevented by the AGE inhibitor aminoguanidine (AMG) (n = 6/group). Data are means ± SE. *HF −AMG vs. CON ±AMG, HF+CONT ±AMG, and HF+IT ±AMG, P < 0.05; $−AMG vs. +AMG within group, P < 0.05. Data in C were analyzed with a 2-way ANOVA. Right: representative immunohistochemistry images of coronary PVAT showing AGE alterations. Scale bars, 100 μm.
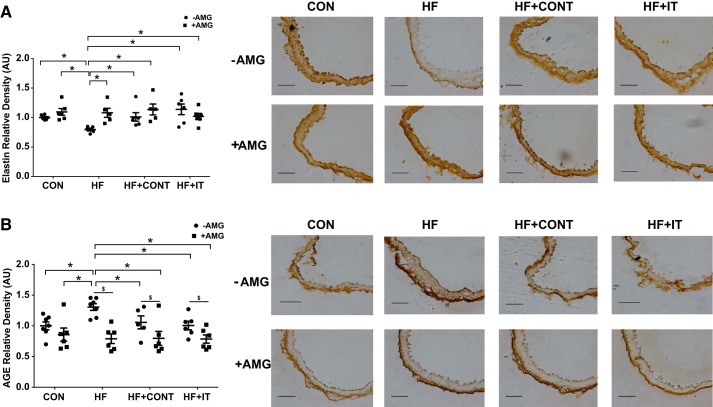
To determine whether coronary PVAT-derived AGE contributes to reductions in arterial elastin and AGE accumulation, mouse aortas treated with PVAT conditioned medium were analyzed. Elastin content was decreased (P < 0.05; Fig. 4A) and AGE was increased (P < 0.05; Fig. 4B) after culture in HF conditioned medium. HF+CONT and HF+IT exercise training regimens preserved elastin content and prevented AGE accumulation in arteries cultured in HF PVAT conditioned medium (Fig. 4; P < 0.05). AMG prevented the HF-induced reductions in arterial elastin (P < 0.05; Fig. 4A) and vascular AGE accumulation (P < 0.05; Fig. 4B). Arterial segments treated with AMG had lower nitrotyrosine and NF-κB p65 subunit expressions compared with segments not treated (P < 0.05, main effect of drug; Fig. 5). Collectively, these data indicate that AGE associated with PVAT promotes arterial stiffness via reductions in elastin content, which is prevented by exercise training of two different intensities.
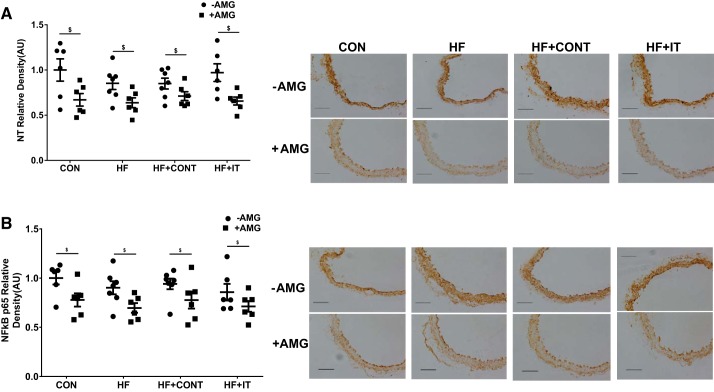
Fig. 4.
Influence of swine coronary adipose conditioned medium on mouse aortic extracellular matrix proteins. Exposure to conditioned medium from swine heart failure (HF) coronary adipose decreased mouse aorta elastin content (A) and increased advanced glycation end products (AGE; B) compared with control (CON), HF continuous exercise trained (HF + CONT) and HF interval exercise trained (HF + IT). Aminoguanidine (AMG) prevented both reduced elastin level and AGE accumulation (n = 6/group). AU, arbitrary units. Data are means ± SE. *HF −AMG vs. CON ±AMG, HF+CONT ±AMG, and HF+IT ±AMG, P < 0.05; $−AMG vs. +AMG within group, P < 0.05. Data were analyzed with a 2-way ANOVA. Right: representative immunohistochemistry images of mouse aorta showing medial elastin and AGE alterations. Scale bars, 100 μm.
Fig. 5.
Influence of swine coronary adipose conditioned medium on mouse aortic oxidative stress and inflammation. Nitrotyrosine (NT; A) and NF-κB p65 (B) were unchanged after culture in coronary adipose conditioned medium, but the advanced glycation end product inhibitor aminoguanidine (AMG) decreased NT and NF-κB p65 level in all groups (n = 6/group): control (CON), heart failure (HF), HF continuous exercise trained (HF+CONT), and HF interval exercise trained (HF+IT). AU, arbitrary units. Data are means ± SE. $−AMG vs. +AMG within group, P < 0.05. Data were analyzed with a 2-way ANOVA. Right: representative immunohistochemistry images of mouse aorta showing NT and NF-κB p65 level. Scale bars, 100 μm.
Exercise prevents coronary PVAT oxidative stress and inflammation.
Coronary PVAT nitrotyrosine and NF-κB p65 subunit were increased in HF compared with CON (P < 0.05; Fig. 6, A and B). No differences were observed for coronary PVAT RAGE (P > 0.05; Fig. 6C) or SRA (P > 0.05; Fig. 6D) between the CON and HF groups. Both exercise regimens prevented the increase in nitrotyrosine (P < 0.05; Fig. 6A). Furthermore, HF+CONT prevented NF-κB p65 subunit expression (P < 0.05; Fig. 6B), whereas reductions with HF+IT approached significance (P = 0.06; Fig. 6B). Compared with control, HF coronary PVAT also secreted greater concentrations of IL-6 (265.88 ± 30.85 vs. 146.02 ± 30.68 pg/mL) and IL-8 (720.93 ± 212.26 vs. 297.08 ± 25.60 pg/mL) in conditioned medium (P < 0.05; Fig. 6, E and F). In addition, both continuous and interval exercise training had lower IL-6 (167.74 ± 31.97 and 180.44 ± 24.11 pg/mL, respectively, P < 0.05; Fig. 6E) and IL-8 (347.02 ± 59.15 and 296.95 ± 24.10 pg/mL, respectively, P < 0.05; Fig. 6F) concentrations in PVAT conditioned medium. PVAT cell area and diameter were not influenced by HF or exercise training (Table 3).
Fig. 6.
Immunohistochemistry analysis of oxidative stress and inflammation in swine pericoronary adipose tissue and cytokine levels in swine perivascular adipose tissue (PVAT) conditioned medium. A and B: greater nitrotyrosine (NT; A) and NF-κB p65 (B) in heart failure (HF) was attenuated by both HF+CONT and HF+IT. CON, control; HF+CONT, HF continuous exercise trained; HF+IT, HF interval exercise trained. C and D: receptor of advanced glycation end products (RAGE; C) and scavenger receptor A (SRA; D) levels were unchanged in all groups. AU, arbitrary units. E and F: IL-6 (E) and IL-8 (F) secretion in HF conditioned medium was prevented by HF+CONT and HF+IT (n = 6/group). Data are means ± SE.*HF vs. CON, HF+CONT, and HF+IT, P < 0.05. Data were analyzed with a 1-way ANOVA. Right: representative immunohistochemistry images of coronary artery showing pericoronary adipose tissue expression of NT, NF-kB p65, RAGE and SRA. Scale bars, 100 μm.
Table 3.
Pericoronary adipose morphology
| CON | HF | HF+CONT | HF+IT | |
|---|---|---|---|---|
| Area, μm2 | 1,349 ± 99 | 1,218 ± 61 | 1,180 ± 49 | 1,347 ± 80 |
| Diameter, μm | 40.34 ± 1.38 | 38.49 ± 0.94 | 37.78 ± 0.80 | 40.08 ± 1.14 |
Data are means ± SE values for swine pericoronary adipose tissue morphology; n = 6 or 7/group. CON, control; HF, heart failure; HF+CONT, HF continuous exercise trained; HF+IT, HF interval exercise trained. Data were analyzed with a 1-way ANOVA.
DISCUSSION
In this study, we examined the efficacy of a 17-wk continuous or interval exercise training regimen to prevent coronary artery stiffness in a clinically relevant pressure-overload model of HF. The primary findings from this study include 1) both continuous and interval exercise training prevent coronary artery stiffness, with concomitant preservation of arterial elastin content, prevention of arterial AGE accumulation, and reduction in PVAT-mediated inflammatory cytokine release; 2) PVAT-related AGE secretion promotes arterial stiffness, which was prevented by both exercise interventions; and 3) PVAT is a source of AGE secretion contributing to decrements in coronary arterial elastin and AGE accumulation. These findings provide preliminary evidence for the efficacy of two clinically relevant exercise regimens to prevent coronary vascular stiffening with HF and identify PVAT-derived AGE secretion as a novel target to prevent arterial stiffening.
Exercise training prevents coronary stiffening and ECM remodeling.
A primary finding of the present study is that both chronic continuous and interval exercise training prevent coronary artery stiffness associated with pressure overload-induced HF. Although increased stiffening of large conduit arteries has been associated with end-organ damage, whether this relationship applies to the coronary arteries and myocardium remains to be elucidated. Recent studies have postulated that vascular dysfunction is a critical contributor to end-organ damage that occurs with HF (41, 46) and suggest coronary artery stiffening may alter microvascular and/or heart function. Indeed, we have previously shown that blood flow regulation, resistance artery vasomotor control, cardiomyocyte contractile function, and diastolic function are impaired in this same HF group of animals (25, 38, 39). Although our experimental approach does not allow us to definitively state cause versus effect, these outcomes are consistent with paradigms describing a similar process in large peripheral conduit arteries and other downstream organs such as the kidneys (26).
The prevention of increased coronary artery stiffness in this study complements recently published work from our group in these same animals that demonstrated that chronic exercise training also preserved coronary microvascular vasodilatory function via a BKCa channel-mediated mechanism (38) and prevented stiffening of the carotid artery (39). These data indicate that regular exercise can prevent pathological adaptations in the peripheral and coronary vasculature as well as all along the coronary vascular tree (i.e., both macro- and microvessels) in the presence of chronic pressure overload. Aerobic exercise training has been shown to reduce both central (aortic) and peripheral conduit artery stiffness in older and diseased adults (7, 22, 27), which is supported by animal models providing insight into the structural changes by which exercise attenuates arterial stiffening (8, 14, 35). Similar to these prior studies, our findings demonstrate an effect of exercise to preserve elastin content, prevent AGE accumulation, and reduce inflammation (3, 8, 35, 48). Notably, we extend these findings to demonstrate similar effects for both chronic continuous and interval exercise to prevent coronary stiffness and ECM remodeling. Our findings are supported by previous investigations demonstrating that continuous and interval exercise training prevent arterial stiffness in healthy and hypertensive subjects (21, 53). Because of the lack of effective treatment strategies for HF patients (57), our findings lend support for exercise training to lower coronary artery stiffness, which in combination with previous observations of improved coronary vascular conductance in these same animals (38) implies an overall effect of exercise to prevent increases in vascular resistance throughout the coronary arterial tree. Importantly, these data also show the efficacy of chronic exercise training as a therapeutic option for treating coronary vascular dysfunction in a setting of pressure overload-induced HF, using an intensity tolerable to HF patients.
The ECM proteins elastin and collagen are primarily responsible for contributing to mechanical stiffness of arteries (58). Our data indicate that HF promotes coronary artery stiffness by reducing arterial elastin content, without influencing collagen. Prior studies demonstrate that other pathological conditions, such as obesity and hyperlipidemia, as well as the elastin-deficient mouse promote arterial stiffness by reducing elastin content (10, 18, 52). As such, the elastin elastic modulus was decreased with HF, suggesting that in addition to lower elastin content arterial stiffness was increased. This is the first time to our knowledge that it has been shown that chronic continuous and interval exercise training programs of an intensity tolerable to HF patients prevent decrements in elastin content and arterial stiffness in the coronary vasculature of a preclinical model of pressure overload-induced HF. Previous investigations in the rat have indicated the potential for this effect in the aorta after exercise training, although these protocols used a higher training intensity and frequency compared with the present study (8, 35).
In addition to changes in the ECM, we observed greater AGE accumulation in the coronary artery of HF animals that was prevented by both exercise training protocols. Chronically increased plasma glucose level is a well-recognized factor contributing to AGE formation, and it is again interesting to note that the increase in coronary vascular AGE levels in the present study was observed in an experimental setting absent of metabolic comorbidities. This finding recapitulates recently published observations from our laboratory in the same swine model, which we previously attributed to increased oxidative stress related to chronic pressure overload (15). AGE cross-links proteins within the arterial wall, which includes elastin and collagen, to promote arterial stiffness (2, 54). Furthermore, exogenous AGE administered to rodents has been shown to promote elastin disruption and fragmentation that are associated with arterial stiffness (20). Thus the increased coronary AGE may contribute to the reductions in elastin content and arterial stiffening observed with HF. Elastin content and arterial stiffness were preserved with both continuous and interval exercise programs in this study, consistent with prior work related to aortic stiffness in aged Fischer 344 rats exercised daily at relatively low intensity (47, 48). Collectively, these data indicate that exercise training prevents coronary AGE accumulation, thereby preserving elastin content and preventing arterial stiffness in a translational swine model of HF.
In further support of the beneficial therapeutic effects of exercise, both continuous and interval training prevented pressure overload-induced increases in nitrotyrosine, NF-κB, and SRA in the coronary arteries, suggesting that the prevention of AGE accumulation by exercise also precludes the arterial oxidative stress and inflammatory response induced by HF. An important downstream effect of AGE is to signal via RAGE that, in turn, contributes to arterial oxidative stress and inflammation. As such, we observed an increase in nitrotyrosine abundance, NF-κB p65, and SRA proteins in the coronary arteries of HF swine. Oxidative stress and inflammation are important signaling mechanisms contributing to aortic stiffness both in animal models of aging and disease (18, 40) and in older adults (27, 44). More specifically, oxidative stress activates a proinflammation response via the NF-κB signaling pathway (32, 37), which in turn influences the ECM, leading to arterial stiffness (28). Our data suggest that AGE accumulation with HF increases nitrotyrosine, NF-κB, and SRA, reducing elastin content leading to arterial stiffness.
Coronary PVAT.
Continuous and interval exercise prevented coronary PVAT AGE expression and secretion, as well as the increased arterial stiffness with reduction of elastin content and AGE accumulation in the presence of the PVAT conditioned medium from HF swine. Notably, the present study extends our previous observations (15) by demonstrating the mechanistic involvement of PVAT-derived AGE in the reduced arterial elastin content and increased AGE accumulation in the mouse aorta experiments, providing evidence for this fat depot to promote ECM remodeling consistent with that observed in the coronary artery of HF swine. In support of this hypothesis, use of the AGE inhibitor AMG prevented the detrimental impact of PVAT conditioned medium from the swine HF group on mouse aortic stiffness and ECM remodeling. These results provide mechanistic evidence that chronic exercise training exerts its protective effect on pressure overload-induced coronary arterial stiffness mediated by a reduction in PVAT-related AGE secretion and associated oxidative stress and inflammation. Our findings imply that further interrogation regarding the potential of AGE inhibition as a therapeutic strategy to combat coronary arterial stiffness and dysfunction in HF is warranted.
In the present study, exercise training attenuated HF-related increases in coronary PVAT nitrotyrosine abundance, NF-κB p65 subunit expression, and IL-6/IL-8 secretion, indicating a potential integrative mechanism by which exercise prevents increased coronary arterial stiffness in a setting of experimental HF. Increased levels of these markers of oxidative stress and immune involvement in the HF group are consistent with the hypothesis that AGE formed in adipose tissue is a result of increased oxidative stress and inflammation (16, 45, 56). The increased PVAT IL-6 secretion is consistent with our previous findings in a hypercholesterolemic mouse model, which was shown to promote arterial stiffness (10). Oxidized lipid has also been shown to induce greater IL-8 accumulation (36), suggesting that PVAT nitrotyrosine abundance may be an important link to IL-8 secretion. Additionally, both NF-κB and IL-6 promote transcriptional activation of IL-8 that may also explain the increased secretion of this cytokine from PVAT (55). It is noteworthy, however, that PVAT conditioned medium from the HF group did not promote arterial oxidative stress and inflammation in the mouse aorta, yet inhibition of AGE did lower normal levels of these end points on a global fashion across all experimental groups, suggesting that other factors may promote vascular oxidative stress and inflammation.
Limitations.
The present study employed a mixed animal model approach (swine and mouse), which provided a scientific avenue yielding both translational and mechanistic insight into our findings. Although smaller in size, the main coronary arteries (e.g., the RCA and LCX) of swine are conduit vessels that have structural and functional properties similar to the mouse aorta, particularly when these traits are compared to the resistance vasculature (9, 19). In the present study, use of the mouse aorta was technically more practical and enhanced feasibility regarding successful experimental completion, in addition to laying the groundwork for future investigations using genetically modified mice to further elucidate mechanisms. Nevertheless, potential species and vessel differences require additional mechanistic interrogation to validate the translational relevance of the present approach. This investigation revealed the detrimental and therapeutic effects of HF and chronic exercise, respectively, on coronary PVAT inflammation (NF-kB) and immune cell infiltration (SRA). Importantly, other immune cell subpopulations (such as M1 and M2 macrophages) could have a significant impact on coronary PVAT phenotype, but the role of additional immune cell involvement could not be pursued in this study owing to a limited sample size. Finally, our findings indicate a potential integrative mechanism by which exercise training attenuates AGE-dependent increases in vascular stiffness by preserving a more physiological PVAT oxidative and immune phenotype. However, our experimental approach did not allow us to establish upstream mechanisms by which PVAT AGE levels were influenced by both pressure overload and chronic exercise.
In summary, these novel data provide evidence that both chronic continuous and interval exercise training prevent increased coronary artery stiffness and associated detrimental vascular ECM remodeling in a preclinical miniswine model of pressure overload-induced HF. Our data provide novel support regarding PVAT-derived AGE as a primary mechanism promoting arterial stiffness that can be therapeutically prevented by exercise training of intensity tolerable to a HF patient. Additional investigation is needed to further establish the importance of PVAT on coronary arterial function and cardiac health in a human HF patient population.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant RO1 HL-112998 (C. A. Emter, principal investigator).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.O., C.A.E., and B.S.F. conceived and designed research; A.O. performed experiments; A.O. and B.S.F. analyzed data; A.O., C.A.E., and B.S.F. interpreted results of experiments; A.O. and B.S.F. prepared figures; A.O., C.A.E., and B.S.F. drafted manuscript; A.O., T.D.O., C.A.E., and B.S.F. edited and revised manuscript; A.O., T.D.O., C.A.E., and B.S.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jan Ivey, Pamela Thorne, Melissa Cobb, Brian Ferguson, Jessica Hiemstra, and Daniel Dozier for considerable technical contributions, which were essential for the completion of the study. We also thank Gore for their generous gift of vascular Gore-Tex sleeves used in our aortic banding procedures.
REFERENCES
- 1.Andersen MJ, Borlaug BA. Invasive hemodynamic characterization of heart failure with preserved ejection fraction. Heart Fail Clin 10: 435–444, 2014. doi: 10.1016/j.hfc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21: 3–12, 2003. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Boor P, Celec P, Behuliak M, Grančič P, Kebis A, Kukan M, Pronayová N, Liptaj T, Ostendorf T, Sebeková K. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism 58: 1669–1677, 2009. doi: 10.1016/j.metabol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin 4: 23–36, 2008. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 3: 588–595, 2010. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutouyrie P, Laurent S, Girerd X, Benetos A, Lacolley P, Abergel E, Safar M. Common carotid artery stiffness and patterns of left ventricular hypertrophy in hypertensive patients. Hypertension 25: 651–659, 1995. doi: 10.1161/01.HYP.25.4.651. [DOI] [PubMed] [Google Scholar]
- 7.Collier SR, Kanaley JA, Carhart R Jr, Frechette V, Tobin MM, Hall AK, Luckenbaugh AN, Fernhall B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens 22: 678–686, 2008. doi: 10.1038/jhh.2008.36. [DOI] [PubMed] [Google Scholar]
- 8.Moraes-Teixeira JA, Félix A, Fernandes-Santos C, Moura AS, Mandarim-de-Lacerda CA, de Carvalho JJ. Exercise training enhances elastin, fibrillin and nitric oxide in the aorta wall of spontaneously hypertensive rats. Exp Mol Pathol 89: 351–357, 2010. doi: 10.1016/j.yexmp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Diego A, Pérez de Prado A, Cuellas C, Pérez-Martínez C, Gonzalo-Orden M, Altonaga JR, de Miguel A, Regueiro M, Ajenjo J, Sánchez-Lasheras F, Alvarez-Arenal A, Fernández-Vázquez F. [Instent restenosis related to vessel injury score degree. Are current experimental models valid for drug-eluting stents analysis?]. Rev Esp Cardiol 64: 745–751, 2011. doi: 10.1016/j.recesp.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Du B, Ouyang A, Eng JS, Fleenor BS. Aortic perivascular adipose-derived interleukin-6 contributes to arterial stiffness in low-density lipoprotein receptor deficient mice. Am J Physiol Heart Circ Physiol 308: H1382–H1390, 2015. doi: 10.1152/ajpheart.00712.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emter CA, Baines CP. Low-intensity aerobic interval training attenuates pathological left ventricular remodeling and mitochondrial dysfunction in aortic-banded miniature swine. Am J Physiol Heart Circ Physiol 299: H1348–H1356, 2010. doi: 10.1152/ajpheart.00578.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emter CA, Tharp DL, Ivey JR, Ganjam VK, Bowles DK. Low-intensity interval exercise training attenuates coronary vascular dysfunction and preserves Ca2+-sensitive K+ current in miniature swine with LV hypertrophy. Am J Physiol Heart Circ Physiol 301: H1687–H1694, 2011. doi: 10.1152/ajpheart.00610.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 13: 576–578, 2014. doi: 10.1111/acel.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588: 3971–3982, 2010. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleenor BS, Ouyang A, Olver TD, Hiemstra JA, Cobb MS, Minervini G, Emter CA. Saxagliptin prevents increased coronary vascular stiffness in aortic-banded mini swine. Hypertension 72: 466–475, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10993. [DOI] [PubMed] [Google Scholar]
- 16.Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, Nε-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem 271: 9982–9986, 1996. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 17.De la Fuente M, Hernanz A, Vallejo MC. The immune system in the oxidative stress conditions of aging and hypertension: favorable effects of antioxidants and physical exercise. Antioxid Redox Signal 7: 1356–1366, 2005. doi: 10.1089/ars.2005.7.1356. [DOI] [PubMed] [Google Scholar]
- 18.Garner T, Ouyang A, Berrones AJ, Campbell MS, Du B, Fleenor BS. Sweet potato (Ipomoea batatas) attenuates diet-induced aortic stiffening independent of changes in body composition. Appl Physiol Nutr Metab 42: 802–809, 2017. doi: 10.1139/apnm-2016-0571. [DOI] [PubMed] [Google Scholar]
- 19.Goodwill AG, Dick GM, Kiel AM, Tune JD. Regulation of coronary blood flow. Compr Physiol 7: 321–382, 2017. doi: 10.1002/cphy.c160016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossin N, Auger F, Niquet-Leridon C, Durieux N, Montaigne D, Schmidt AM, Susen S, Jacolot P, Beuscart JB, Tessier FJ, Boulanger E. Dietary CML-enriched protein induces functional arterial aging in a RAGE-dependent manner in mice. Mol Nutr Food Res 59: 927–938, 2015. doi: 10.1002/mnfr.201400643. [DOI] [PubMed] [Google Scholar]
- 21.Guimarães GV, Ciolac EG, Carvalho VO, D’Avila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res 33: 627–632, 2010. doi: 10.1038/hr.2010.42. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol 55: 235–239, 2005. doi: 10.2170/jjphysiol.S2116. [DOI] [PubMed] [Google Scholar]
- 23.Hiemstra JA, Gutiérrez-Aguilar M, Marshall KD, McCommis KS, Zgoda PJ, Cruz-Rivera N, Jenkins NT, Krenz M, Domeier TL, Baines CP, Emter CA. A new twist on an old idea part 2: cyclosporine preserves normal mitochondrial but not cardiomyocyte function in mini-swine with compensated heart failure. Physiol Rep 2: e12050, 2014. doi: 10.14814/phy2.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiemstra JA, Liu S, Ahlman MA, Schuleri KH, Lardo AC, Baines CP, Dellsperger KC, Bluemke DA, Emter CA. A new twist on an old idea: a two-dimensional speckle tracking assessment of cyclosporine as a therapeutic alternative for heart failure with preserved ejection fraction. Physiol Rep 1: e00174, 2013. doi: 10.1002/phy2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiemstra JA, Veteto AB, Lambert MD, Olver TD, Ferguson BS, McDonald KS, Emter CA, Domeier TL. Chronic low-intensity exercise attenuates cardiomyocyte contractile dysfunction and impaired adrenergic responsiveness in aortic-banded mini-swine. J Appl Physiol (1985) 124: 1034–1044, 2018. doi: 10.1152/japplphysiol.00840.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102: 203–210, 2000. doi: 10.1161/01.CIR.102.2.203. [DOI] [PubMed] [Google Scholar]
- 27.Jablonski KL, Donato AJ, Fleenor BS, Nowlan MJ, Walker AE, Kaplon RE, Ballak DB, Seals DR. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor κ B signalling. J Hypertens 33: 2477–2482, 2015. doi: 10.1097/HJH.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother 57: 195–202, 2003. doi: 10.1016/S0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 29.Johnson CP, Baugh R, Wilson CA, Burns J. Age related changes in the tunica media of the vertebral artery: implications for the assessment of vessels injured by trauma. J Clin Pathol 54: 139–145, 2001. doi: 10.1136/jcp.54.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Deug-Nam K, Prather RS, Lee K. Recombination Activating Gene 2 Gene Targeting Vector, Production of SCID-like Miniature Pigs by TALEN-Mediated Gene Targeting and Use Thereof. European Patent EP3068217A4, 2017.
- 31.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol 6: 19, 2013. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahabadi AA, Rassaf T. Imaging of coronary inflammation for cardiovascular risk prediction. Lancet 392: 894–896, 2018. doi: 10.1016/S0140-6736(18)31716-1. [DOI] [PubMed] [Google Scholar]
- 34.Marshall KD, Muller BN, Krenz M, Hanft LM, McDonald KS, Dellsperger KC, Emter CA. Heart failure with preserved ejection fraction: chronic low-intensity interval exercise training preserves myocardial O2 balance and diastolic function. J Appl Physiol (1985) 114: 131–147, 2013. doi: 10.1152/japplphysiol.01059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda M, Nosaka T, Sato M, Ohshima N. Effects of physical exercise on the elasticity and elastic components of the rat aorta. Eur J Appl Physiol Occup Physiol 66: 122–126, 1993. doi: 10.1007/BF01427052. [DOI] [PubMed] [Google Scholar]
- 36.N’Guessan DP, Riediger F, Vardarova K, Scharf S, Eitel J, Opitz B, Slevogt H, Weichert W, Hocke AC, Schmeck B, Suttorp N, Hippenstiel S. Statins control oxidized LDL-mediated histone modifications and gene expression in cultured human endothelial cells. Arterioscler Thromb Vasc Biol 29: 380–386, 2009. doi: 10.1161/ATVBAHA.108.178319. [DOI] [PubMed] [Google Scholar]
- 37.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 208: 417–420, 2011. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olver TD, Edwards JC, Ferguson BS, Hiemstra JA, Thorne PK, Hill MA, Laughlin MH, Emter CA. Chronic interval exercise training prevents BKCa channel-mediated coronary vascular dysfunction in aortic-banded miniswine. J Appl Physiol (1985) 125: 86–96, 2018. doi: 10.1152/japplphysiol.01138.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olver TD, Klakotskaia D, Ferguson BS, Hiemstra JA, Schachtman TR, Laughlin MH, Emter CA. Carotid artery vascular mechanics serve as biomarkers of cognitive dysfunction in aortic‐banded miniature swine that can be treated with an exercise intervention. J Am Heart Assoc 5: e003248, 2016. doi: 10.1161/JAHA.116.003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouyang A, Garner TB, Fleenor BS. Hesperidin reverses perivascular adipose-mediated aortic stiffness with aging. Exp Gerontol 97: 68–72, 2017. doi: 10.1016/j.exger.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 42.Piontkivska H, Zhang Y, Green ED, Elnitski L; NISC Comparative Sequencing Program . Multi-species sequence comparison reveals dynamic evolution of the elastin gene that has involved purifying selection and lineage-specific insertions/deletions. BMC Genomics 5: 31, 2004. doi: 10.1186/1471-2164-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 29: 1458–1464, 2009. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71: 1056–1063, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia 44: 129–146, 2001. [Erratum in Diabetologia 45: 293, 2002]. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 46.Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R, Mielniczuk LM. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail 9: e002562, 2016. doi: 10.1161/CIRCHEARTFAILURE.115.002562. [DOI] [PubMed] [Google Scholar]
- 47.Steppan J, Sikka G, Jandu S, Barodka V, Halushka MK, Flavahan NA, Belkin AM, Nyhan D, Butlin M, Avolio A, Berkowitz DE, Santhanam L. Exercise, vascular stiffness, and tissue transglutaminase. J Am Heart Assoc 3: e000599, 2014. doi: 10.1161/JAHA.113.000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, Nyhan SM, Lussman C, Gupta G, White AR, Daher JP, Shoukas AA, Levine BD, Berkowitz DE. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol 47: 565–572, 2012. doi: 10.1016/j.exger.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takenouchi T, Suzuki S, Shinkai H, Tsukimoto M, Sato M, Uenishi H, Kitani H. Extracellular ATP does not induce P2X7 receptor-dependent responses in cultured renal- and liver-derived swine macrophages. Results Immunol 4: 62–67, 2014. doi: 10.1016/j.rinim.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 18: 127–132, 1998. doi: 10.1161/01.ATV.18.1.127. [DOI] [PubMed] [Google Scholar]
- 51.Tee MW, Won S, Raman FS, Yi C, Vigneault DM, Davies-Venn C, Liu S, Lardo AC, Lima JA, Noble JA, Emter CA, Bluemke DA. Regional strain analysis with multidetector CT in a swine cardiomyopathy model: relationship to cardiac MR tagging and myocardial fibrosis. Radiology 277: 88–94, 2015. doi: 10.1148/radiol.2015142339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol 289: H1209–H1217, 2005. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Zhang T, Zhu W, Wu H, Yan S. Acute effects of continuous and interval low-intensity exercise on arterial stiffness in healthy young men. Eur J Appl Physiol 114: 1385–1392, 2014. doi: 10.1007/s00421-014-2869-y. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe M, Sawai T, Nagura H, Suyama K. Age-related alteration of cross-linking amino acids of elastin in human aorta. Tohoku J Exp Med 180: 115–130, 1996. doi: 10.1620/tjem.180.115. [DOI] [PubMed] [Google Scholar]
- 55.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res 14: 6735–6741, 2008. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 56.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res 93: 1159–1169, 2003. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 57.Zakeri R, Cowie MR. Heart failure with preserved ejection fraction: controversies, challenges and future directions. Heart 104: 377–384, 2018. doi: 10.1136/heartjnl-2016-310790. [DOI] [PubMed] [Google Scholar]
- 58.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25: 932–943, 2005. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]