Summary
Environmental variation can have profound and direct effects on fitness, fecundity, and host–symbiont interactions. Replication rates of microbes within arthropod hosts, for example, are correlated with incubation temperature but less is known about the influence of host–symbiont dynamics on environmental preference. Hence, we conducted thermal preference (T p) assays and tested if infection status and genetic variation in endosymbiont bacterium Wolbachia affected temperature choice of Drosophila melanogaster. We demonstrate that isogenic flies infected with Wolbachia preferred lower temperatures compared with uninfected Drosophila. Moreover, T p varied with respect to three investigated Wolbachia variants (wMel, wMelCS, and wMelPop). While uninfected individuals preferred 24.4°C, we found significant shifts of −1.2°C in wMel‐ and −4°C in flies infected either with wMelCS or wMelPop. We, therefore, postulate that Wolbachia‐associated T p variation within a host species might represent a behavioural accommodation to host–symbiont interactions and trigger behavioural self‐medication and bacterial titre regulation by the host.
Introduction
Environmental variations through intrinsic (e.g., physiology, reproduction, metabolism) and extrinsic (e.g., food sources, predation risk, immunity) factors impose a strong impact on the fitness of all organisms (e.g., Levins, 1968; Endler, 1977, 1986; Fox et al., 2001). Temperature is one of the most important environmental abiotic factors that affect the physiology and life history traits in many organisms (Huey and Berrigan, 2001; Hoffmann, 2010; Bozinovic et al., 2011; Amarasekare and Savage, 2012). Ectotherms, such as terrestrial insects, depend on ambient conditions to maintain their body temperature within a thermoregulatory range (Angilletta et al., 2004). For example, thermal preference (T p) in Drosophila melanogaster, a dipteran model species of world‐wide distribution, varies with geography and elevation, and is thus potentially shaped by selection (Martin and Huey, 2008; Dillon et al., 2009; Garrity et al., 2010; Hoffmann and Sgrò, 2011; Huey et al., 2012; Rajpurohit and Schmidt, 2016). In addition, variation in temperature can have fundamental effects on ecological interactions among organisms and their symbiotic microbes. Titres of endosymbiotic Wolbachia bacteria are highly temperature‐dependent in various arthropod hosts. For example, some Wolbachia strains have increased replication rates at warmer temperatures (Clancy and Hoffmann, 1998; Hurst et al., 2000; Mouton et al., 2006; Correa and Ballard, 2012; Strunov et al., 2013a), while others are highly sensitive to heat stress (Van Opijnen and Breeuwer, 1999; Wiwatanaratanabutr and Kittayapong, 2009).
Endosymbionts of the genus Wolbachia are widespread and found in more than 50% of all investigated terrestrial and some aquatic insects (Zug and Hammerstein, 2012; Weinert et al., 2015; Sazama et al., 2017). Wolbachia have garnered extensive interest due to reproductive manipulations they can inflict on their hosts, i.e., inducing parthenogenesis, male killing, feminization, and cytoplasmic incompatibility (CI). By acting as reproductive parasites these bacteria boost their own transmission (reviewed by Werren et al., 2008). However, Wolbachia can also behave as facultative or obligate mutualists (reviewed by Zug and Hammerstein, 2015) by enhancing host fecundity and fitness (Dedeine et al., 2001; Hosokawa et al., 2010; Miller et al., 2010) and by providing protection against RNA viruses (Hedges et al., 2008; Teixeira et al., 2008; Moreira et al., 2009; Osborne et al., 2009). Several closely related genetic variants of Wolbachia have been isolated from natural and laboratory populations of D. melanogaster. wMel, wMelCS, and wMelPop, which represent three of the most well‐studied Wolbachia variants in D. melanogaster (Riegler et al., 2005), cause very weak, if any, CI in their native host (Hoffmann, 1988; Reynolds et al., 2003; Veneti et al., 2003; Fry et al., 2004; Yamada et al., 2007), but provide virus protection to varying degrees (Chrostek et al., 2013; Martinez et al., 2014). Both wMel and wMelCS infect natural populations of D. melanogaster. Historically, wMelCS existed globally at higher prevalence, but in the recent past wMel has almost completely replaced the more ancestral wMelCS strain in worldwide populations (Riegler et al., 2005; Nunes et al., 2008; Richardson et al., 2012; Ilinsky, 2013; Early and Clark, 2013). In contrast, wMelPop was isolated from a laboratory stock of D. melanogaster during a survey of genetic mutations and represents a pathogenic variant of wMelCS (Min and Benzer, 1997; Richardson et al., 2012; Chrostek et al., 2013). Depending on rearing temperature, wMelPop infections can lead to a strong reduction of host lifespan with respect to uninfected controls (Min and Benzer, 1997; McGraw et al., 2002; Reynolds et al., 2003; Chrostek et al., 2013). This detrimental effect is caused by over‐proliferation in host tissues, such as the brain, retina, and muscles (Min and Benzer, 1997; Strunov et al., 2013b). Importantly, not only wMelPop but also its natural predecessor wMelCS have significantly higher cellular densities and growth rates than wMel when assayed in the same fly genetic background at 25°C (Table 1 ; Chrostek et al., 2013). While high Wolbachia densities result in augmented antiviral protection, they also have negative effects by reducing their host's lifespan. Accordingly, it has been proposed that the higher titre – and hence more costly – wMelCS variant was replaced by the low‐titre wMel variant in natural D. melanogaster populations (Chrostek et al., 2013). Thereby, flies infected with the more recent wMel variant have higher fitness due to lower Wolbachia titres compared with flies infected with wMelCS. Alternatively, the highly protective wMelCS variant may have been replaced by wMel independent of the symbiont's capacity for virus resistance but because of better adaptation to viruses at the host level (Martins et al., 2014). In line with this hypothesis, a recent study failed to find correlations between RNA virus prevalence and Wolbachia frequency in natural populations of D. melanogaster (Webster et al., 2015). However, the main causalities explaining the well‐documented global almost complete replacement of wMelCS by wMel in worldwide populations of D. melanogaster remains elusive.
Table 1.
Comparison of strain type titre levels, growth rates and effects on host's lifespan at 25°C.
| Strain type | Relative amount of Wolbachia | Effects on host's lifespan |
|---|---|---|
| wMel | Lowest titre level and growth rate | No reduction |
| wMelCS | Approximately double the titre level compared with wMel and higher growth rate | Some reduction |
| wMelPop | Titre level 20 times higher compared with wMelCS | Reduction by approximately half |
Host–symbiont conflicts may arise from disparities between physiological requirements of Wolbachia and those of their hosts. For example, some insects induce behavioural fever (Louis et al., 1986) or behavioural chill (Fedorka et al., 2016) as an immune strategy to fight bacterial pathogen infections. Conversely, some bacterial symbionts are known to alter their host's thermal tolerance range in an adaptive manner (Russell and Moran, 2006; Dunbar et al., 2007; reviewed by Wernegreen, 2012). We, therefore, speculate that additional ecological and behavioural factors, such as host temperature preference, may play a pivotal role in determining Wolbachia prevalence and the dynamics of their strain replacement in natural D. melanogaster populations.
To test our hypothesis, we conducted laboratory‐based temperature preference assays using isogenic D. melanogaster w 1118 strains that are either uninfected (w‐) or infected with one of the three common Wolbachia strains wMel, wMelCS_b, and wMelPop (Teixeira et al., 2008; Chrostek et al., 2013) and determined if Wolbachia affects the temperature preference of its native host D. melanogaster. To this end, we built a custom thermal gradient apparatus and determined the temperature preference of replicated fly populations with varying Wolbachia infection statuses along the thermal gradient ranging from 17°C to 32°C. Our experiments demonstrate that the temperature preference of D. melanogaster is neither sex‐ nor age‐dependent but is highly dependent on the Wolbachia infection status and on the symbiont genotype. Our results provide compelling evidence that Wolbachia infections can affect host thermal preference behaviour, at least under strict laboratory conditions in D. melanogaster strains.
Results
To determine whether T p of adult D. melanogaster varies with Wolbachia infection status and Wolbachia genotype, we conducted lab‐based experiments using a custom‐built temperature gradient apparatus for assaying flies of the isogenic lab‐strain w 1118 that were either uninfected (w‐) or infected with one of the Wolbachia strains wMel, wMelCS, or wMelPop (Supporting Information Figs. S1–S4). We first investigated whether age (3–4, 5–7, or 10–14 days post‐eclosion) and Wolbachia infections or sex (males or females) and Wolbachia infections had an influence on T p by means of two‐way mixed‐effect Poisson regressions. We neither found significant effects of age or sex nor significant interactions of either factor with Wolbachia infections (see Fig. 1A and B and Table 2A, B; Supporting Information Fig. S5; Poisson regression: p > 0.05 for factors age and sex and both interaction terms respectively). In contrast, both two‐way regressions revealed highly significant effects of Wolbachia infections on T p (Poisson regression p < 0.001 for factor Wolbachia in both analyses). Since both aforementioned analyses were carried out on different subsets of the data which did not include all four infection types (w‐, wMel, wMelCS, and wMelPop), we further investigated all data jointly irrespective of sex and host age and evaluated the effect of symbiont genetic variation on T p by means of post‐hoc pairwise comparisons based on Tukey's honestly significant differences (HSD). We found that temperature preference of D. melanogaster strongly depended on (1) the infection status of the flies and (2) on the Wolbachia strain used for infections: Uninfected flies (w‐) exhibited the highest mean T p at 24.4°C (Median: 25°C; Mode: 26°C), while wMel‐infected flies preferred average temperatures at 23.2°C (Median: 24°C; Mode: 24°C), which is 1.2°C lower than uninfected (w‐) flies. In contrast, flies infected with wMelCS or wMelPop showed highly similar thermal preferences at 20.6°C and 20.5°C (Median: 19°C and Mode: 18°C for both) respectively, which were both approximately 4°C lower than to w‐ (see Fig. 1C, Tables 2C, and 3).
Figure 1.
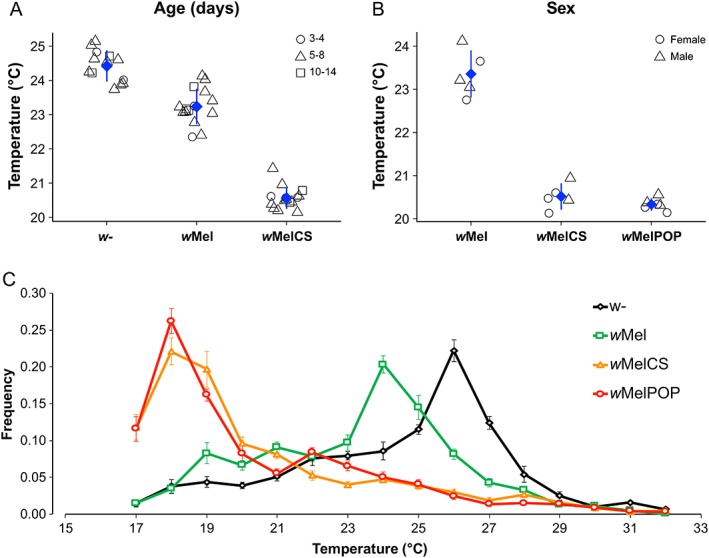
Thermal preference of Drosophila with and without Wolbachia infections. Panels A and B show average T p (blue diamonds) with respect to age (3–4, 5–7, or 10–14 days post eclosion, n = 4370 excluding flies infected with wMelPop) and sex (male or female; n = 1718, excluding uninfected flies) respectively. Each symbol represents the average T p for a replicate at a given factor level of either age (circle: 3–4 days, triangle: 5–8 days and square: 10–14 days) or sex (circle: females, triangle: males). Panel C shows line plots with relative proportions of flies observed at a given temperature. Each line represents the average proportion of flies which were either uninfected (w‐; black diamonds) or infected with wMel (green circles), wMelCS (orange triangles), or wMelPop (red squares). The error bars represent standard errors for average frequencies at a given temperature across all replicated experiments carried out for each infection type. We found that infected flies exhibit significantly lower thermal preference compared with uninfected flies. [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Table showing the results of three analyses based on generalized linear mixed models with a Poisson error structure to account for the statistical properties of count data.
| Analysis | Model | Factor | N | df | χ2 | p‐value |
|---|---|---|---|---|---|---|
| A | wol + age + wol × age | wol | 4370 | 6 | 119.71 | 1.87E‐23 |
| A | wol + age + wol × age | age | 4370 | 6 | 2.10 | 0.91 |
| A | wol + age + wol × age | wol × age | 4370 | 4 | 1.31 | 0.86 |
| B | wol + sex + wol × sex | wol | 1718 | 6 | 61.19 | 2.58E‐11 |
| B | wol + sex + wol × sex | sex | 1718 | 4 | 2.12 | 0.71 |
| B | wol + sex + wol × sex | wol × sex | 1718 | 3 | 1.90 | 0.59 |
| C | wol | wol | 5717 | 3 | 168.69 | 2.44E‐36 |
The columns show ID's for the different analyses (A‐C), the models, the individual factors and interactions tested, the samples size, the degrees of freedom for the χ2 test of the analysis of deviance, the χ2 value and the corresponding p‐value. Note that analyses with significant effects after Bonferroni correction (adjusted α = 0.017) are highlighted in bold.
Table 3.
Table showing z‐values from post‐hoc pairwise comparisons with Tukey's HSD for the factor Wolbachia (Analysis C; see section on ‘Experimental Procedures’) with four levels (non‐infected, wMel, wMelCS and wMelPop).
| w‐ | wMel | wMelCS | wMelPop | |
|---|---|---|---|---|
| w‐ | – | |||
| wMel | −6.76*** | – | ||
| wMelCS | −21.93*** | −15.49*** | – | |
| wMelPop | −21.5*** | −15.35*** | −0.6 | – |
Bold type indicates significance after Bonferroni correction (adjusted α′ = 0.017). *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
In this study, we, for the first time, investigated the relationship between temperature preference of D. melanogaster and Wolbachia infection under laboratory conditions. Using a custom‐built thermal gradient apparatus, we conducted temperature preference assays and showed that the T p of D. melanogaster is shifted to lower temperatures when flies are infected with Wolbachia. Uninfected D. melanogaster flies preferred an average temperature of 24.4°C, whereas wMel‐infected flies preferred 23.2°C and both wMelCS‐ and wMelPop‐infected flies preferred 20.6°C and 20.5°C respectively.
T p can vary significantly between populations of the same species (Matute et al., 2009; Rajpurohit and Schmidt, 2016) and can have profound effects on immune function, fitness, and fecundity (Huey and Berrigan, 2001; Martin and Huey, 2008; Hoffmann, 2010). Recent population analyses of Wolbachia and mitochondria from D. melanogaster have provided evidence that over the past few thousand years, the wMelCS variant is being globally replaced by the wMel‐variant (Riegler et al., 2005; Nunes et al., 2008; Richardson et al., 2012; Early and Clark, 2013). Rare cases of the wMelCS infection type were recently detected in the wild (Nunes et al., 2008; Ilinsky, 2013), thus replacement by wMel is still incomplete. Although the reason for the worldwide turn‐over remains elusive, it has been hypothesized that wMel, which persists in hosts at significantly lower densities than wMelCS at 25°C (Chrostek et al., 2013), has better adapted to D. melanogaster. Accordingly, wMel infections are less costly to the host compared with the more ancestral wMelCS variant (Chrostek et al., 2013; reviewed by Miller, 2013).
Insects can actively reduce or avoid costs of potentially fitness‐reducing symbionts or parasites by behavioural adjustments such as changing egg deposition (Kacsoh et al., 2013) or mating behaviour (reviewed by Wedell, 2013). We find compelling evidence for Wolbachia‐induced behavioural changes in host T p, which may provide an alternative explanation for the recent global replacement of wMelCS by wMel independent of density costs or anti‐viral effects: we propose that wMel is less costly for the host than wMelCS‐infections because flies harbouring wMel exhibit thermal preferences that are closer to uninfected flies under natural conditions compared with flies infected with wMelCS. Drosophila development is strictly temperature dependent (~ 14 days of egg‐to‐adult development at 20°C and 9 days at 24°C; Ashburner, 1989). Due to cooler thermal preference, infections with wMelCS may, thus, result in slower development and lead to longer generation times compared with wMel‐infected flies. Variance in generation times as a function of Wolbachia infections may, thus, have a substantial impact on fitness if wMel‐infected flies produce more generations per year resulting in higher net fecundity compared with flies infected with wMelCS.
Small fluctuations in temperature can cause considerable modifications to host–symbiont interactions (Blanford and Thomas, 1999). Pathogenicity of wMelPop is attributed to its active proliferation in host tissues at temperatures ≥ 19°C. The increase of wMelPop density confers strong anti‐viral protection but leads to a significant reduction in host lifespan at 25°C (Chrostek et al., 2013). However, at temperatures < 19°C, pathogenicity of wMelPop is eliminated (Reynolds et al., 2003). Similarly, but less dramatically wMelCS, the progenitor of wMelPop, is also costly by reducing host lifespan due to high symbiont densities at 25°C (Chrostek et al., 2013). We, therefore, speculate that the adjustment of lower temperature preference in D. melanogaster as a response to the wMelCS and wMelPop infections represents a physiological self‐medicating behaviour or behavioural chill (Fedorka et al., 2016) to attenuate the fitness costs associated with deleterious effects of Wolbachia over‐proliferation and high cell densities (Chrostek et al., 2013; Strunov et al., 2013a, b).
Wolbachia’s ability to provide anti‐viral protection to their hosts has emerged as the most promising approach to combatting insect‐vector borne pathogens that pose serious health risks to humans, such as dengue fever and Zika (Moreira et al., 2009; Iturbe‐Ormaetxe et al., 2011; Dutra et al., 2016). However, because the strength of anti‐viral protection is associated with higher Wolbachia densities (Chrostek et al., 2013; Martinez et al., 2014) and bacterial titres are a temperature sensitive trait (Hoffmann et al., 1990; Reynolds et al., 2003; Mouton et al., 2006; 2007; Bordenstein and Bordenstein, 2011; Correa and Ballard, 2012; Chrostek et al., 2013; Strunov et al., 2013a; Murdock et al., 2014; Versace et al., 2014), it is feasible that under certain thermal conditions such as lower environmental temperatures, Wolbachia‐induced virus protection could be attenuated or absent (Chrostek, 2014). Furthermore, our findings, as demonstrated in a highly inbred lab strain of D. melanogaster, need to be tested first in different host backgrounds, which are naturally or artificially infected with the endosymbiont.
In conclusion, we present experimental support for a potential ecological conflict between host and symbiont that may have profound effects on host physiology. Our results provide a novel conceptual platform from which to further investigate host temperature preference or behavioural chill, in other Wolbachia‐infected insect hosts. Future studies should examine if host temperature preference has a direct impact on Wolbachia density regulation. Additionally, it is important to determine any effects that host T p has on the strength of anti‐viral protection that Wolbachia provide to some hosts.
Experimental procedures
Fly lines
For all assays, we used D. melanogaster without Wolbachia (w‐) as well as flies infected with one of three genetic variants of the Wolbachia wMel‐strain; w‐, wMel, wMelCS_b, and wMelPop all set in the DrosDel w 1118 isogenic background, which were kindly provided by Luis Teixeira and previously described by Teixeira and colleagues (2008) and Chrostek and colleagues (2013).
We used biological replicates of approximately 30 flies per vial, independently rearing each vial of flies at 25°C, in a 12:12 light–dark cycle with constant 45% humidity. Flies were raised on Drosophila Formula 4‐24® Instant Medium (Carolina®, NC) that was supplemented with fresh yeast. Approximately equal numbers of male and female flies were used in each assay except for assays that explicitly tested sex‐class T p differences (see Supporting Information Table S1 and Supporting Information File 1). In addition to testing for sex‐class T p differences, we performed assays to test for age‐specific T p differences, thus all fly lines were segregated into three age‐classes – 3–4 days, 5–7 days, and 10–14 days post‐eclosion. Due to fitness costs to the host associated with infection by wMelPop at 25°C, possibly due to the onset of the life reducing phenotype (Min and Benzer, 1997) or increase in copy numbers of the Octomom repeat (Chrostek and Teixeira, 2015), our wMelPop‐infected fly line did not produce enough flies to conduct all three age‐class assays. Therefore, we excluded wMelPop from the statistical analyses of age‐specific effects (see Supporting Information Table S1 and the description of statistical analyses).
Genotyping of Wolbachia strains
Genome sections that contain hypervariable loci or hypervariable regions covering tandem repeats were used as genetic markers to differentiate Wolbachia strains and strain variants (O'Neill et al., 1992; Werren et al., 1995; Zhou et al., 1998; Riegler et al., 2012). To confirm Wolbachia‐infection status, we performed diagnostic PCR amplification using primers for a gene that encodes the Wolbachia surface protein, wsp (Jeyaprakash and Hoy, 2000) and for an intergenic region with 141 bp tandem repeats, VNTR‐141 loci (Riegler et al., 2005). The PCR reactions for wsp amplification were carried out in a total volume of 10 μl containing 2 μl Promega 5× Green GoTaq buffer, 4 mM Promega MgCl2, 0.8 μM of forward and reverse primers, 35 μM of each dNTP, 0.04 U Promega GoTaq DNA Polymerase and 1 μl of genomic DNA template. Diagnostic VNTR‐141 PCR reactions were each a total of 10 μl comprised of the following: 2 μl Promega 5x Green GoTaq buffer, 1.5 mM Promega MgCl2, 0.3 μM of forward and reverse primers, 35 μM of each dNTP, 0.04 U Promega GoTaq DNA Polymerase and 1 μl of genomic DNA template. PCR products were visualized on a 1% agarose gel. Presence/absence of the wsp signal and the size of the diagnostic VNTR‐141 locus confirmed their respective infection type (Riegler et al., 2012). The proper infection status of the wMelPop isoline was verified by assaying flies for early mortality at 29°C.
Thermal gradient apparatus
Temperature preference assays were performed using a custom made thermal gradient apparatus that allowed the flies to move in a three‐dimensional space (adapted from Rajpurohit and Schmidt, 2016; Supporting Information Fig. S2). An aluminium rod (length 74.93 cm, diameter 3.02 cm; Part #R31‐316 Metals Depot, Winchester, KY) was encased within a 58.76 cm long and 6.35 cm inside diameter polycarbonate tube, creating an enclosed chamber allowing for three‐dimensional movement. Constant voltage was applied to Peltier devices on each end of the aluminium rod to create a temperature gradient inside the thermal preference chamber. Temperatures along the gradient were measured at seven points that were 8.39 cm apart using K‐type thermocouples and two four‐channel thermocouple recorders. We recorded temperatures on the aluminium rod and inside polycarbonate tube surfaces (bottom, top, and mid‐point between the top and bottom surfaces; Supporting Information Fig. S3). The average temperatures from each thermocouple point on all surfaces from 57 different assays are depicted in Supporting Information Fig. S1. Mean temperatures increased linearly and ranged from 12°C at the coldest point to 40°C at the hottest point of the aluminium rod, 58.76 cm distance (Supporting Information Fig. S4). Along the aluminium rod, for every 4.2 cm from cold to hot, the temperature increased by 2°C. Temperatures along each of the measured polycarbonate tube surfaces (bottom, mid‐point, and top) increased 1°C every 4.2 cm from cold to hot. The gradient reached thermal stability after approximately 20 min and remained stable for at least 3 h. Assays were conducted once the device had attained thermal stability.
Thermal preference assays
All assays were conducted in a room with a constant temperature of 24°C and constant 40% humidity. During several trial runs, we established that 75–100 flies for each assay resulted in distributions along the thermal gradient that avoided over‐crowding in preferred temperature ranges, eliminating potential counting errors during analysis. Flies were introduced by aspiration into the thermal gradient chamber through a small hole located halfway along the top of the polycarbonate tube, where the temperature consistently averaged 25°C. Flies used for thermal preference assays were never anesthetized because of the strong effects from CO2 treatment on Drosophila behaviour (Barron, 2000). Each assay was conducted for 30 min. Between assays, the temperature gradient chamber was taken apart and thoroughly cleaned to avoid contamination from any pheromone particles. All aluminium parts were cleaned using 95% ethanol. Because ethanol and polycarbonate are chemically incompatible, the polycarbonate tube and end caps were cleaned using hot water and soap, followed by a 4‐min rinse with hot water to ensure that surfaces were free of soap residue.
Data collection
Using three GoPro HERO3+ cameras, we collected data for each assay in the form of digital images. To capture images of the entire thermal gradient and the flies within it, we mounted the cameras above, lateral to and below the apparatus, capturing images every 30 s for the duration of each treatment. Following Goda and colleagues (2014), treatment duration was 30 min to avoid any behavioural aberration from the desiccation and/or starvation of the flies. Images were analyzed using Adobe Photoshop CS6. All 60 images from each assay were reviewed, from which we determined that (a) the flies were highly active, retaining the ability to relocate as necessary, for the entire assay, and (b) after being introduced to the thermal gradient, actively flew around for up to 15 min before they settled on either the aluminium rod or polycarbonate tube surfaces. Therefore, we selected images for analysis of fly distribution at the 20‐min time point as representative of the 30‐min experiment. For each assay, we manually counted flies and marked the location of flies on a custom grid that delineated gradient surfaces and surface temperatures.
Statistical analyses
We calculated generalized linear mixed models (GLMM) with a Poisson error structure using the R (R Development Core Team, 2009) package lme4 (Bates et al., 2015) to account for the statistical properties of count data from flies observed at different temperatures. To test for significance of a given predictor variable, we compared the full model including all factors to a reduced model excluding the given factor by analysis of deviance with χ2 tests using the R function anova (see Supporting Information File 1 for full R code).
At first, we excluded flies infected with wMelPop, since we failed to obtain sufficient flies to test for age‐specific T p at all three age‐classes (3–4 days, 5–7 days, and 10–14 days post‐eclosion; Supporting Information Table S1) and tested for age‐ and Wolbachia‐specific differences in thermal preference with a two‐way GLMM of the form: T i = wol + age + wol × age + Rep + εi. Here, T is the continuous response variable ‘Temperature’, age is a nominal fixed factor with three levels each (age: 3–4 days, 5–7 days, and 10–14 days post‐eclosion), wol is a nominal fixed factor ‘Wolbachia’ with three levels (un‐infected, wMel, and wMelCS), wol × age is the interaction term, Rep is a nominal random factor ‘Replicate’ for replicate trials and εi is the error (Table 2A, Fig. 1A). In a complementary analysis, we removed all flies of the age class 3–4 days and repeated the abovementioned analysis including all Wolbachia strains on two age classes (5–7 days and 10–14 days post eclosion) only. This latter analysis yielded qualitatively similar results to the former analysis including all age classes without wMelPop (Supporting Information Table S2).
Next, we censored flies with undetermined sex status and excluded uninfected flies (w‐), since we failed to obtain sufficient replication to test for male‐specific T p for uninfected flies (Supporting Information Table S1). We then tested for sex‐ and Wolbachia‐specific differences in thermal preference with a two‐way GLMM of the form: T i = wol + sex + wol × sex + Rep + εI Here, T is the continuous response variable ‘Temperature’, sex is a nominal fixed factor with two levels (male and female), wol is a nominal fixed factor ‘Wolbachia’ with three levels (wMel, wMelCS, and wMelPop), wol × age is the interaction term, Rep is a nominal random factor ‘Replicate’ for replicate trials and εI is the error (Table 2B; Fig. 1B).
Finally, we included all flies, irrespective of age and sex status and tested for the effect of infection status and Wolbachia strain variation on thermal preference with a GLMM of the form: T i = wol + Rep + εi, where T is the continuous response variable ‘Temperature’, wol is a nominal fixed factor ‘Wolbachia’ with four levels (un‐infected, wMel, wMelCS, and wMelPop), Rep is the nominal random factor ‘Replicate’ and εi is the error (Table 2C; Fig. 1C). Here, we further tested for significant pair‐wise comparisons among the level of the factor ‘Wolbachia’ with Tukey's honestly significant difference (HSD) post‐hoc tests using the R package multcomp (Table 3). We conservatively applied Bonferroni corrections to the α threshold (α′ = 0.05/3 = 0.017) to account for multiple testing.
Supporting information
Appendix S1 Supporting Information
Fig. S1. Thermal gradient apparatus gradient depicting different temperature zones and fly dispersion (wMelPop).
Fig. S2. Schematic of the thermal gradient apparatus used for thermal gradient assays as adapted from Rajpurohit and Schmidt (2016). The polycarbonate tube and length of aluminium gradient within the tube were 58.76 cm and temperature was recorded with K‐type thermocouples.
Fig. S3. Average + 0.5°C (SD) temperatures from 18 runs that were recorded at each surface measured using k‐type thermocouples. There was a linear increase in temperature from cold to hot as measured at each of seven evenly spaced (8.39 cm).
Fig. S4. Plots showing linearity of temperature change for the different surfaces (a. aluminium rod, b. top, c. bottom, and mid‐point of the polycarbonate tube) as measured with K‐type thermocouples at regular intervals along the length of apparatus from the hottest end (H3) to the coldest (C3).
Fig. S5. Line plots showing the portion of flies observed at a given temperature for males and females in the left panel and age classes (young: 3–4 days or old: 10–14 days post eclosion) in the right panel. Each of the four subfigures shows the average proportion of flies with respect to different infection status (uninfected, wMel, wMelCS and wMelPop). For wMelPop infected flies only two age groups were tested. Error bars represent standard errors for average frequencies at a given temperature across all replicated experiments carried out for a given infection type and levels of the factors sex or age.
Table S1. Counts of flies and number of replicates (in parentheses) per sex and age class.
Table S2. Results of two‐way GLMM with independent factors age, Wolbachia and the interaction between them (see Table 2 for more detail).
Acknowledgements
The authors want to thank Luis Teixeira for providing the Drosophila melanogaster white‐isogenic DrosDel w 1118 lines infected with wMel, wMelCS_b and wMelPop. The authors declare no conflict of interest. This work was supported by the National Science Foundation award (0948041) ‘Cascades to Coast GK‐12: Enhancing STEM Education through Environmental Sustainability’ awarded to AMT and the FWF grant (P28255‐B22) from the Austrian Science Fund to WJM.
[Correction added on 19 October, 2018, after first online publication: the colour code description in the legend of Figure 1 is now corrected].
References
- Amarasekare, P. , and Savage, V. (2012) A framework for elucidating the temperature dependence of fitness. Am Nat 179: 178–191. 10.1086/663677. [DOI] [PubMed] [Google Scholar]
- Angilletta, M. J. , Steury, T. D. , and Sears, M. W. (2004) Temperature, growth rate, and body size in ectotherms: fitting pieces of a life‐history puzzle. Integr Comp Biol 44: 498–509. 10.1093/icb/44.6.498. [DOI] [PubMed] [Google Scholar]
- Ashburner, M. (1989) Drosophila: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Barron, A. D. (2000) Anaesthetising Drosophila for behavioural studies. J Insect Physiol 44: 439–442. 10.1016/S0022-1910(99)00129-8. [DOI] [PubMed] [Google Scholar]
- Bates, D. , Maechler, M. , Bolker, B. M. , and Walker, S. C. (2015) Fitting linear mixed‐effects models using lme4 . J Stat Softw 67: 1–48. 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Blanford, S. , and Thomas, M. B. (1999) Host thermal biology: the key to understanding host‐pathogen interactions and microbial pest control?. Agric For Entomol 1: 195–202. 10.1046/j.1461-9563.1999.00027.x. [DOI] [Google Scholar]
- Bordenstein, S. R. , and Bordenstein, S. R. (2011) Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia and cytoplasmic incompatibility. PLoS One 6: e29106 10.1371/journal.pone.0029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovic, F. , Bastías, D. , Boher, F. , Clavijo‐Baquet, S. , Estay, S. A. , and Angilletta, M. J., Jr. (2011) The mean and variance of environmental temperature interact to determine physiological tolerance and fitness. Physiol Biochem Zool 84: 543–552. 10.1086/662551. [DOI] [PubMed] [Google Scholar]
- Chrostek, E.A. (2014) Genomic and environmental factors influence Wolbachia‐Drosophila symbiosis. Chapter 5: Temperature dependence of Wolbachia‐conferred antiviral protection. Dissertation. Oeiras, Portugal: Instituto de Tecnologia Química e Biológica. https://run.unl.pt/handle/10362/14860
- Chrostek, E. , and Teixeira, L. (2015) Mutualism Breakdown by Amplification of Wolbachia Genes. PLoS Biol 13: e1002065 10.1371/journal.pbio.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrostek, E. , Marialva, M. S. P. , Esteves, S. S. , Weinert, L. A. , Martinez, J. , Jiggins, F. M. , and Teixeira, L. (2013) Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9: e1003896 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy, D. J. , and Hoffmann, A. A. (1998) Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia infected Drosophila simulans . Entomol Exp Appl 86: 13–24. http://doi.org10.1046/j.1570-7458.1998.00261.x. [Google Scholar]
- Correa, C. C. , and Ballard, J. W. O. (2012) Wolbachia gonadal density in female and male Drosophila vary with laboratory adaptation and respond differently to physiological and environmental challenges. J Invertebr Pathol 111: 197–204. 10.1016/j.jip.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Dedeine, F. , Vavre, F. , Fleury, F. , Loppin, B. , Hochberg, M. E. , and Boulétreau, M. (2001) Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci U S A 98: 6247–6252. 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon, M. E. , Wang, G. , Garrity, P. A. , and Huey, R. B. (2009) Thermal preference in Drosophila. J Therm Biol 34: 109–119. 10.1016/j.jtherbio.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar, H. E. , Wilson, A. C. C. , Ferguson, N. R. , and Moran, N. A. (2007) Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol 5: e96 10.1371/journal.pbio.0050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra, H. L. , Rocha, M. N. , Dias, F. B. , Mansur, S. B. , Caragata, E. P. , and Moreira, L. A. (2016) Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19: 771–774. 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early, A. M. , and Clark, A. G. (2013) Monophyly of Wolbachia pipientis genomes within Drosophila melanogaster: geographic structuring, titre variation, and host effects across five populations. Mol Ecol 22: 5765–5778. 10.1111/mec.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler, J. A. (1977) Geographic Variation, Speciation, and Clines. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- Endler, J. A. (1986) Natural Selection in the Wild. Princeton, NJ: Princeton University Press. [Google Scholar]
- Fedorka, K. M. , Kutch, I. C. , Collins, L. , and Musto, E. (2016) Cold temperature preference in bacterially infected Drosophila melanogaster improves survival but is remarkably suboptimal. J Insect Physiol 93–94: 36–41. 10.1016/j.jinsphys.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Fox, C. W. , Roff, D. A. , and Fairbairn, D. J. (2001) Evolutionary Ecology. Oxford: Oxford University Press. [Google Scholar]
- Fry, A. J. , Palmer, M. R. , and Rand, D. M. (2004) Variable fitness effects of Wolbachia infection in Drosophila melanogaster . Heredity 93: 379–389. 10.1038/sj.hdy.6800514. [DOI] [PubMed] [Google Scholar]
- Garrity, P. , Goodman, M. , Samuel, A. , and Sengupta, P. (2010) Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila . Genes Dev 24: 2365–2382. 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda, T. , Leslie, J. R. , and Hamada, F. N. (2014) Design and analysis of temperature preference behavior and its circadian rhythm in Drosophila . J Vis Exp 83: e51097 10.3791/51097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges, L. M. , Brownlie, J. C. , O'Neill, S. L. , and Johnson, K. N. (2008) Wolbachia and virus protection in insects. Science 322: 702 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. (1988) Partial cytoplasmic incompatibility between two Australian populations of Drosophila melanogaster . Entomol Exp Appl 48: 61–67. [Google Scholar]
- Hoffmann, A. A. (2010) Physiological climatic limits in Drosophila: patterns and implications. J Exp Biol 213: 870–880. 10.1242/jeb.037630. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. , and Sgró, C. M. (2011) Climate change and evolutionary adaptation. Nature 470: 479–485. 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. , Turelli, M. , and Harshman, L. G. (1990) Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans . Genetics 126: 933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa, T. , Koga, R. , Kikuchi, Y. , Meng, X. , and Fukatsu, T. (2010) Wolbachia as a bacteriocyte‐associated nutritional mutualist. Proc Natl Acad Sci U S A 107: 769–774. 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey, R. B. , and Berrigan, D. (2001) Temperature, demography, and ectotherm fitness. Am Nat 158: 204–210. 10.1086/321314. [DOI] [PubMed] [Google Scholar]
- Huey, R. B. , Kearney, M. R. , Krockenberger, A. , Holtum, J. A. M. , Jess, M. , W., and S. E. (2012) Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philos Trans R Soc Lond B Biol Sci 367: 1665–1679. 10.1098/rstb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst, G. D. , Johnson, A. P. , Schulenburg, J. H. , and Fuyama, Y. (2000) Male‐killing Wolbachia in Drosophila: a temperature‐sensitive trait with a threshold bacterial density. Genetics 156: 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilinsky, Y. (2013) Coevolution of Drosophila melanogaster mtDNA and Wolbachia genotypes. PLoS ONE 8: e54373 10.1371/journal.pone.0054373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe‐Ormaetxe, I. , Walker, T. , and O’ Neill, S. L. (2011) Wolbachia and the biological control of mosquito‐borne disease. EMBO Rep 12: 508–518. 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash, A. , and Hoy, M. A. (2000) Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty‐three arthropod species. Insect Mol Biol 9: 393–405. 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- Kacsoh, B. Z. , Lynch, Z. R. , Mortimer, N. T. , and Schlenke, T. A. (2013) Fruit flies medicate offspring after seeing parasites. Science 339: 947–950. 10.1126/science.1229625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins, R. (1968) Evolution in Changing Environments. Princeton, NJ: Princeton University Press. [Google Scholar]
- Louis, C. , Jourdan, M. , and Cabanac, M. (1986) Behavioral fever and therapy in a rickettsia‐infected Orthoptera . Am J Physiol 250: R991–R995. 10.1152/ajpregu.1986.250.6.R991. [DOI] [PubMed] [Google Scholar]
- Martin, T. L. , and Huey, R. B. (2008) Why "suboptimal" is optimal: Jensen's inequality and ectotherm thermal preferences. Am Nat 171: 102–118. 10.1086/527502. [DOI] [PubMed] [Google Scholar]
- Martinez, J. , Longdon, B. , Bauer, S. , Chan, Y. S. , Miller, W. J. , Bourtzis, K. , et al (2014) Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog 10: e1004369 10.1371/journal.ppat.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, N. E. , Faria, V. G. , Nolte, V. , Schlötterer, C. , Teixeira, L. , Sucena, É. , and Magalhães, S. (2014) Host adaptation to viruses relies on few genes with different cross‐resistance properties. Proc Natl Acad Sci U S A 111: 5938–5943. 10.1073/pnas.1400378111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute, D. R. , Novak, C. J. , and Coyne, J. A. (2009) Temperature‐based extrinsic reproductive isolation in two species of Drosophila . Evolution 63: 595–612. 10.1111/J.1558-5646.2008.00588.X. [DOI] [PubMed] [Google Scholar]
- McGraw, E.A ., Merritt, D.J ., Droller, J.N ., and O' Neill, S.L . (2002) Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci USA 99 : 2918–2923. 10.1073/pnas.052466499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, W. J. (2013) Bugs in transition: the dynamic world of Wolbachia in insects. PLoS Genet 9: e1004069 10.1371/journal.pgen.1004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, W. J. , Ehrman, L. , and Schneider, D. (2010) Infectious speciation revisited: impact of symbiont‐depletion on female fitness and mating behavior of Drosophila paulistorum . PLoS Pathog 6: e1001214 10.1371/journal.ppat.1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, K. T. , and Benzer, S. (1997) Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci U S A 94: 10792–10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, L. A. , Iturbe‐Ormaetxe, I. , Jeffery, J. A. , Lu, G. , Pyke, A. T. , Hedges, L. M. , et al (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139: 1268–1278. 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- Mouton, L. , Henri, H. , Bouletreau, M. , and Vavre, F. (2006) Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology 132: 49–56. 10.1017/S0031182005008723. [DOI] [PubMed] [Google Scholar]
- Mouton, L. , Henri, H. , Charif, D. , Boulétreau, M. , and Vavre, F. (2007) Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol Lett 3: 210–213. 10.1098/rsbl.2006.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, C. C. , Blanford, S. , Hughes, G. L. , Rasgon, J. L. , and Thomas, M. B. (2014) Temperature alters Plasmodium blocking by. Wolbachia Sci Rep 4: 3932 10.1038/srep03932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes, M. D. S. , Nolte, V. , and Schlötterer, C. (2008) Nonrandom Wolbachia infection status of Drosophila melanogaster strains with different mtDNA haplotypes. Mol Biol Evol 25: 2493–2498. 10.1093/molbev/msn199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, S. L. , Giordano, R. , Colbert, A. M. , Karr, T. L. , and Robertson, H. M. (1992) 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci U S A 89: 2699–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne, S. E. , Leong, Y. S. , O'Neill, S. L. , and Johnson, K. N. (2009) Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans . PLoS Pathog 5: e1000656 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2009) R: A Language and Environment for Statistical Computing. https://www.r‐project.org/
- Rajpurohit, S. , and Schmidt, P. S. (2016) Measuring thermal behavior in smaller insects: a case study in Drosophila melanogaster demonstrates effects of sex, geographic origin, and rearing temperature on adult behavior. Fly 10: 149–161. 10.1080/19336934.2016.1194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, K. T. , Thomson, L. J. , and Hoffmann, A. A. (2003) The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster . Genetics 164: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, M. F. , Weinert, L. A. , Welch, J. J. , Linheiro, R. S. , Magwire, M. M. , Jiggins, F. M. , and Bergman, C. M. (2012) Population genomics of the Wolbachia endosymbiont in Drosophila melanogaster . PLoS Genet 8: e1003129 10.1371/journal.pgen.1003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegler, M ., Sidhu, M ., Miller, W.J ., and O' Neill, S.L . (2005) Evidence for a global Wolbachia replacement in Drosophila melanogaster . Curr Biol 15 : 1428–1433. 10.1016/j.cub.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Riegler, M ., Iturbe‐Ormaetxe, I ., Woolfit, M ., Miller, W.J ., and O' Neill, S.L . (2012) Tandem repeat markers as novel diagnostic tools for high resolution fingerprinting of Wolbachia . BMC Microbiol 12 : S12 10.1186/1471-2180-12-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, J. A. , and Moran, N. A. (2006) Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc Biol Sci 273: 603–610. 10.1098/rspb.2005.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazama, E. J. , Bosch, M. J. , Shouldis, C. S. , Ouellette, S. P. , and Wesner, J. S. (2017) Incidence of Wolbachia in aquatic insects. Ecol Evol 7: 1165–1169. 10.1002/ece3.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunov, A. A. , Ilinskii, Y. Y. , Zakharov, I. K. , and Kiseleva, E. V. (2013a) Effect of high temperature on survival of Drosophila melanogaster infected with pathogenic strain of Wolbachia bacteria. Russ J Genet Appl Res 3: 435–443. 10.1134/S2079059713060099. [DOI] [Google Scholar]
- Strunov, A. , Kiseleva, E. , and Gottlieb, Y. (2013b) Spatial and temporal distribution of pathogenic Wolbachia strain wMelPop in Drosophila melanogaster central nervous system under different temperature conditions. J Invertebr Pathol 114: 22–30. 10.1016/j.jip.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Teixeira, L. , Ferreira, Å. , and Ashburner, M. (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster . PLoS Biol 6: e2 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Opijnen, T. V. , and Breeuwer, J. A. J. (1999) High temperatures eliminate Wolbachia, a cytoplasmic incompatibility inducing endosymbiont, from the two‐spotted spider mite. Exp Appl Acarol 23: 871–881. [DOI] [PubMed] [Google Scholar]
- Veneti, Z. , Clark, M. E. , Zabalou, S. , Karr, T. L. , Savakis, C. , and Bourtzis, K. (2003) Cytoplasmic incompatibility and sperm cyst infection in different Drosophila‐Wolbachia associations. Genetics 164: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace, E. , Nolte, V. , Pandey, R. V. , Tobler, R. , and Schlötterer, C. (2014) Experimental evolution reveals habitat‐specific fitness dynamics among Wolbachia clades in Drosophila melanogaster . Mol Ecol 23: 802–814. 10.1111/mec.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, C. L. , Waldron, F. M. , Robertson, S. , Crowson, D. , Ferrari, G. , Quintana, J. F. , et al (2015) The discovery, distribution, and evolution of viruses associated with Drosophila melanogaster . PLoS Biol 13: e1002210 10.1371/journal.pbio.1002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedell, N. (2013) The dynamic relationship between polyandry and selfish genetic elements. Philos Trans R Soc Lond B Biol Sci 368: 20120049 10.1098/rstb.2012.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert, L. A. , Araujo‐jnr, E. V. , Ahmed, M. Z. , Welch, J. J. , and Welch, J. J. (2015) The incidence of bacterial endosymbionts in terrestrial arthropods. Proc Biol Sci 282: 20150249 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernegreen, J. J. (2012) Mutualism meltdown in insects: bacteria constrain thermal adaptation. Curr Opin Microbiol 15: 255–262. 10.1016/j.mib.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren, J. H. , Zhang, W. , and Guo, L. R. (1995) Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc Biol Sci 281: 55–63. 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- Werren, J. H. , Baldo, L. , and Clark, M. E. (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6: 741–751. 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Wiwatanaratanabutr, I. , and Kittayapong, P. (2009) Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus . J Invertebr Pathol 102: 220–224. 10.1016/j.jip.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Yamada, R. , Floate, K. D. , Riegler, M. , and Neill, S. L. O. (2007) Male development time influences the strength of Wolbachia‐induced cytoplasmic incompatibility expression in Drosophila melanogaster. Genetics 177: 801–808. 10.1534/genetics.106.068486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W ., Rousset, F ., and O' Neil, S . (1998) Phylogeny and PCR‐based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci 265 : 509–515. 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug, R. , and Hammerstein, P. (2012) Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PloS One 7: e38544 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug, R. , and Hammerstein, P. (2015) Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol Rev Camb Philos Soc 90: 89–111. 10.1111/brv.12098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Fig. S1. Thermal gradient apparatus gradient depicting different temperature zones and fly dispersion (wMelPop).
Fig. S2. Schematic of the thermal gradient apparatus used for thermal gradient assays as adapted from Rajpurohit and Schmidt (2016). The polycarbonate tube and length of aluminium gradient within the tube were 58.76 cm and temperature was recorded with K‐type thermocouples.
Fig. S3. Average + 0.5°C (SD) temperatures from 18 runs that were recorded at each surface measured using k‐type thermocouples. There was a linear increase in temperature from cold to hot as measured at each of seven evenly spaced (8.39 cm).
Fig. S4. Plots showing linearity of temperature change for the different surfaces (a. aluminium rod, b. top, c. bottom, and mid‐point of the polycarbonate tube) as measured with K‐type thermocouples at regular intervals along the length of apparatus from the hottest end (H3) to the coldest (C3).
Fig. S5. Line plots showing the portion of flies observed at a given temperature for males and females in the left panel and age classes (young: 3–4 days or old: 10–14 days post eclosion) in the right panel. Each of the four subfigures shows the average proportion of flies with respect to different infection status (uninfected, wMel, wMelCS and wMelPop). For wMelPop infected flies only two age groups were tested. Error bars represent standard errors for average frequencies at a given temperature across all replicated experiments carried out for a given infection type and levels of the factors sex or age.
Table S1. Counts of flies and number of replicates (in parentheses) per sex and age class.
Table S2. Results of two‐way GLMM with independent factors age, Wolbachia and the interaction between them (see Table 2 for more detail).


