Abstract
Although midazolam is a frequently used sedative in neonatal intensive care units, its use in preterm neonates has been off‐label. Recently, a new dosing advice for midazolam for sedation on intensive care units has been included in the label (0.03 mg/[kg·h] for preterm neonates <32 weeks and 0.06 mg/[kg·h] for neonates >32 weeks). Concentration‐time data of a prospective multicenter study (29 patients, median gestational age 26.7 [range 24.0‐31.1 weeks]) were combined with previously published data (26 patients, median gestational age 28.1 [range 26.3‐33.6 weeks]), and a population pharmacokinetic model describing the maturation of midazolam pharmacokinetics was developed in NONMEM 7.3. Clearance was 73.7 mL/h for a neonate weighing 1.1 kg and changed nonlinearly with body weight (exponent 1.69). Volume of distribution increased linearly with body weight and was 1.03 L for a neonate weighing 1.1 kg. Simulations of the newly registered dosing show considerable differences in steady‐state concentrations in preterm neonates. To reach similar steady‐state concentrations of 400 µg/mL (±100 µg/mL), a dose of 0.03 mg/(kg·h) is adequate for neonates ≥1 kg and ≤2 kg but would have to be reduced to 0.02 mg/(kg·h) (−33%) in neonates <1 kg and increased to 0.04 mg/(kg·h) (+33%) in neonates weighing >2 kg and ≤2.5 kg. The impact of the observed differences in exposure is difficult to assess because no target concentrations have yet been defined for midazolam, but the current analysis shows that one should be cautious in giving dosage advice based on historical data with a lack of reliable pharmacokinetic and effect data.
Keywords: drug metabolism, fetal medicine, neonatology, pharmacokinetics, pharmacometrics, population pharmacokinetics
Midazolam is 1 of the most frequently used sedatives in neonatal intensive care units,1, 2 and its use keeps on increasing.3 Besides acting as a sedative‐hypnotic, midazolam is also used for the treatment of refractory seizures and less‐frequently for anesthesia.1 Data on the pharmacodynamics of midazolam are very scarce. Jacqz‐Aigrain et al,4 Anand et al,5 and Arya and Ramji6 studied the sedative effect of midazolam in placebo‐controlled trials and concluded that it was superior to placebo. A recent meta‐analysis of these 3 studies points out the risk of potential severe side effects associated with the use of midazolam.3 In particular, Ng et al. report a higher incidence of adverse neurological events at 28 days postnatal age when midazolam is compared with morphine as observed in the study performed by Anand.5 This highlights that more research is urgently needed.
Concerning the pharmacokinetics of midazolam, an influence of maturation in preterm neonates is likely, as midazolam is mainly metabolized by CYP3A.7 CYP3A activity is known to be very low directly after birth and to increase rapidly during the first weeks of life, reaching values comparable to those in adults around the age of 1 year.8, 9 In accordance with this, the lowest clearance values have been reported in preterm neonates,10, 11 and these increase with age.12, 13, 14, 15 In addition to the influence of maturational status, severity of disease and inflammation may affect midazolam elimination in critically ill neonates and children.16, 17 No target concentration for the use of midazolam in preterm neonates has been defined because quantifying the relationship between plasma concentration and sedative effect has been difficult.18
Until recently, the use of midazolam in preterm neonates was off‐label because dosing information in this vulnerable population was lacking in the Summary of Product Characteristics.1, 3 Based on a review of the published literature, the drug label has recently been changed, and new dosing advice for midazolam sedation in preterm neonates treated in intensive care units has been included in the label. In the current label, for neonates with a gestational age of less than 32 weeks, lower infusion rates of 0.03 mg/(kg·h) are suggested, as compared with neonates with gestational ages above 32 weeks for whom 0.06 mg/(kg·h) is proposed.
In the current study we analyzed the midazolam concentration‐time data of a prospective multicenter study carried out in 4 neonatal intensive care units in the Netherlands. We have combined those data with the data on intravenous administration of midazolam in preterm neonates published earlier by de Wildt et al19 to develop a population pharmacokinetic model that describes the maturation of midazolam pharmacokinetics in preterm infants. Finally, we used this model to evaluate the performance of the newly registered midazolam dosing for preterm neonates.
Methods
Data and Studies
The Erasmus Medical Center ethics review board approved the protocols, and written informed consent from parents/legal guardians was obtained before study initiation. The Drug dosage Improvement NeOnates (DINO) study (study 1, MEC‐2014‐067, NL47409.078.14, ClinicalTrials.gov NCT02421068) prospectively studied a total of 9 drugs, including midazolam, routinely used in preterm infants born before 32 weeks of gestation. The primary objective was to develop pharmacokinetic and pharmacodynamic models from which evidence‐based individualized dosing regimens can be derived. From September 2014 to September 2017, 60 midazolam blood samples from 29 children (13 female, 16 male) were collected after intravenous administration in 4 participating Dutch level III neonatal intensive care units (Radboud University Medical Center Nijmegen, Maastricht University Medical Center Maastricht, Máxima Medical Center Veldhoven, and Sophia Children's Hospital Rotterdam). The study made use of sparse opportunistic sampling to reduce the burden on the individual child while the dose was determined by the treating physician. The indication for midazolam was either a stressful procedure or continuous sedation, and bolus doses as well as continuous infusions were administered (Table 1). Additional data (study 2, MEC‐171.586/1998/125) after intravenous administration were available from a previously published data set from the neonatal intensive care unit of the Sophia Children's Hospital in Rotterdam.19 This data set contained 172 samples from 26 neonates (16 female, 10 male) (Table 1) obtained 0.5, 1, 2, 4, 6, 12, and 24 hours after a single intravenous dose of 0.1 mg/kg over 30 minutes for stressful procedures. The combined data set thus consisted of 232 samples from 55 patients after intravenous administration for various doses and indications (Table 1).
Table 1.
Patient Characteristics of the DINO Study Data Set (Study 1) and the Shared Data Set (Study 2)
| DINO Study Data (Study 1) | de Wildt et al19 (Study 2) | Combined Data Set | |
|---|---|---|---|
| Patients | 29 | 26 | 55 |
| Samples (samples per neonate) | 60 (2.1) | 172 (6.6) | 232 (4.9) |
| Doses (doses per neonate) | 123 (4.2) | 26 (1) | 149 (2.7) |
| Doses for stressful procedures (infusion duration ≤30 min) | 59 (2.0) | 26 (1) | 85 (1.5) |
| Doses for continuous sedation (infusion duration >30 min) | 65 (2.2) | 0 (0) | 65 (1.2) |
| Female neonates, N (%) | 13 (45%) | 16 (62%) | 29 (53%) |
| Gestational age, wka | 26.7 (24.0–31.1) | 28.1 (26.3–33.6) | 27.3 (24.0–33.6) |
| Birth weight, kg29 , a | 0.84 (0.47–1.8) | 1.1 (0.75–1.6) | 0.95 (0.47–1.8) |
| Postnatal age (at day of inclusion), da | 18 (1–88) | 5.5 (3–11) | 9 (1–88) |
| Actual body weight, kg29 , a | 1.3 (0.6–4.3) | 1.0 (0.77–1.6) | 1.1 (0.6–4.3) |
Data provided as medians and ranges.
Analytic Methods
The plasma concentrations of the de Wildt et al19 data set were analyzed using gas chromatography with mass spectrometric detection. The lower limit of quantification was 1 µg/L. All samples were analyzed in duplicate, and the resultant mean concentration was used in the pharmacokinetic analysis. For further detail on the analytic method, the reader is referred to the original publication.19
Within the DINO study midazolam plasma samples were analyzed using liquid chromatography–tandem mass spectroscopy with electrospray ionization in the positive ionization mode. The lower limit of quantification for midazolam was 4 µg/L.20
Population Pharmacokinetic Analysis and Model Evaluation
The population pharmacokinetic analysis was performed using NONMEM version 7.3 (ICON Development Solutions, Ellicott City, Maryland), supported by Perl‐speaks‐NONMEM version 3.4.2 and Xpose version 4.3.5.21 Model development was performed in 4 steps: (1) selection of a structural model, (2) selection of an error model, (3) covariate analysis, and (4) internal validation of the model. The first‐order conditional estimation with interaction method was used throughout model development. In case of missing covariate information, the last value observed in the subject was carried forward. For body weight, linear interpolation between available measurements was performed. The objective function value (OFV) was used to compare models, in which a lower OFV indicated a better fit of the model to the data. A drop in OFV of more than 3.8 was considered statistically significant (P < .05) for structural model selection. Standard errors obtained from NONMEM and the confidence intervals of the bootstrap analysis in Perl‐speaks‐NONMEM (n = 1000) were used to evaluate the precision of the estimated parameters.
The covariates birth weight, actual body weight at each day in the neonatal intensive care units, gestational age, postnatal age, postmenstrual age (calculated using gestational age and postnatal age), and gender were evaluated using a stepwise covariate modeling procedure.22 For the covariate analysis, a significance level of P ≤ .01 was used for the forward inclusion and a significance level of ≤.001 for the backward elimination.
Key models as well as the final model were evaluated using goodness‐of‐fit plots and normalized prediction distribution errors23 based on 1000 simulations of the model and a bootstrap analysis based on 1000 samples of the data.
Evaluation of the Recently Registered Dosing Regimen
With use of the final model, 1000 simulations of the newly proposed dosing regimen were performed for 9 hypothetical preterm neonates weighing 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.25, or 2.5 kg. A continuous infusion of 0.03 mg/(kg·h) was administered over a duration of 72 hours as proposed by the label for preterm infants with a gestational age of less than 32 weeks. Steady‐state concentrations were evaluated for their ability to achieve the indicative target concentration of 400 µg/L as proposed by Ahsman et al24 while allowing for 10% (360‐440 µg/L) and 25% (300‐500 µg/L) variation in the population‐predicted steady‐state concentration, respectively.
Results
Population Pharmacokinetic Analysis and Model Evaluation
A 1‐compartment model described the available data best. Clearance was best predicted by actual body weight using a power function (P < .001; –116 points in OFV). Body weight was also identified as best predictor for volume of distribution (P < .01; −9 points in OFV). Because the estimated exponent for volume was close to 1, a linear influence of body weight on volume of distribution was tested for comparison. This led to an equally good fit (+0.2 points in OFV) and was therefore carried forward. No other covariates were found. More details on the covariate analysis can be found in Supplemental Table S1.
Figure 1 shows how clearance was found to change with body weight. The individual post hoc clearance estimates (black dots) are spread evenly around the population value for clearance (black line).
Figure 1.
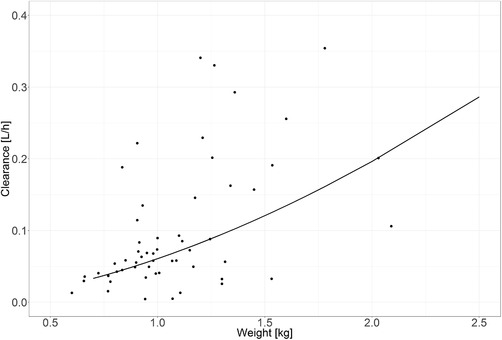
Clearance vs body weight function for the final model: the black line represents the population value for clearance according to the model; the black dots represent the individual post hoc clearance estimates.
The parameter estimates and their respective bootstrap estimates are provided in Table 2. Figure 2 shows the goodness‐of‐fit plots of the final model. Model evaluation showed no trends toward a model misspecification and no other remaining trends with respect to time, concentration, and gestational age. An absence of trends in the individual deviation of clearance and volume of distribution (η) versus covariate plots shows that covariates have been successfully implemented (Supplemental Figure S1). The normalized prediction distribution error shows that the variability in the observed data has been adequately described (Supplemental Figure S2).
Table 2.
Parameter Estimates of the Final Model and Their Corresponding Bootstrap Estimates
| Parameter | Final Model Estimate (RSE%) [Shrinkage] | Bootstrap Estimate (95%CI) |
|---|---|---|
| Fixed effects | ||
| CL [L/h] = CLp | 0.0737 (13%) | 0.0787 (0.0550–0.102) |
| CLi = CLp × (WTi/Median WT)ƟWT | ||
| ƟWT | 1.69 (10%) | 1.67 (0.406–2.47) |
| V [L] = Vp | ||
| Vi = Vp × (WTi/Median WT) | 1.03 (21%) | 1.11 (0.877–1.37) |
| Interindividual variability (η) | ||
| On CL [%] | 91.9 (15%) [8%] | 96.6 (67.5–128) |
| On V [%] | 67.2 (16%) [17%] | 72.7 (47.4–96.8) |
| Residual variability | ||
| Proportional (%) | 33.8 (16%) | 34.0 (28.6–39.4) |
| Additive (µg/L) | 0.218 (56%) | 0.283 (0.0800–0.585) |
CL indicates clearance; i, individual; p, population mean value of a parameter for an individual with body weight of 1.12 kg; RSE, residual standard error; V, central volume of distribution; WT, actual body weight (median WT = 1.12 kg); ƟWT, exponent for influence of WT on CL .
Bootstrap convergence rate was 83.1%.
Figure 2.
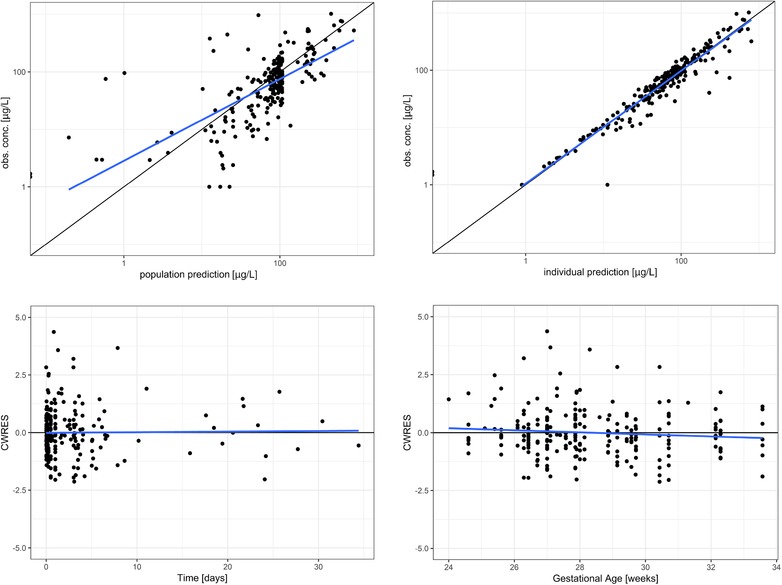
Goodness‐of‐fit plots for the final model. Top, population‐predicted (left) and individual predicted (right) concentrations vs observed values on a log scale. Bottom, conditional weighted residuals (CWRES) vs time on study (left) and gestational age (right).
Evaluation of the Recently Registered Dosing Regimen
Figure 3A illustrates concentration‐time profiles of preterm infants with different body weights when dosed according to the new dosing regimen (0.03 mg/[kg·h] for 72 hours). The dashed line represents a target concentration of 400 µg/L. The plots show that midazolam plasma concentrations in children with lower body weights are higher than those of children with higher body weights (Figure 3A). Seventy‐two hours after start of therapy the mean concentration was predicted to be 754 µg/L, 421 µg/L, and 262 µg/L for preterm infants with body weights of 0.5, 1.25, and 2.5 kg, respectively. At this time point 27.8%, 10.6%, and 5.4% of the individual simulated concentrations were above 1000 µg/L, and 5.7%, 19.6%, and 37.6% were below 200 µg/L, for a preterm infant weighing 0.5 kg, 1.25 kg, and 2.5 kg, respectively. This added up to a total of 11.6% and 23.2% of all simulated concentrations above 1000 µg/L and below 200 µg/L, respectively, from all simulations. Moreover, the time to reach steady state differed for different body weights. Whereas steady state is reached after 24 hours in neonates weighing 2 kg or more, it takes more than 48 hours in children weighing 1 kg or less and between 24 and 48 hours in children between 1 and 2 kg (Figure 3A).
Figure 3.
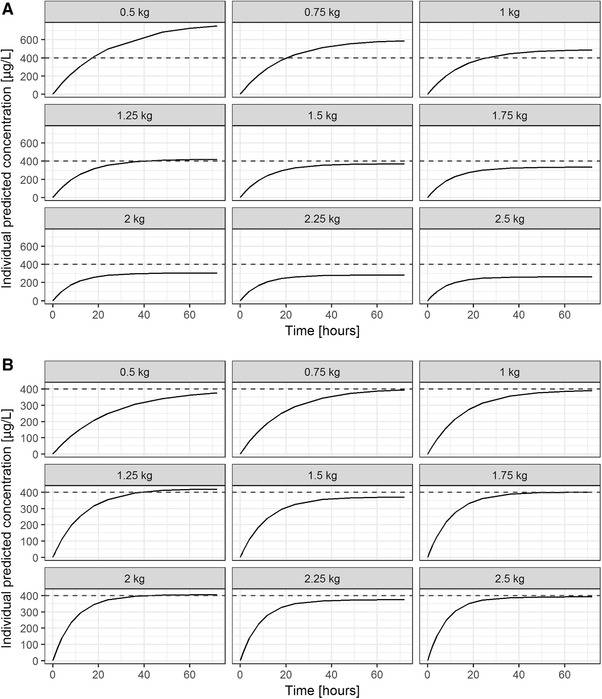
Population‐predicted midazolam concentration‐time profiles over 72 hours for preterm infants with body weights of 0.5 kg, 0.75 kg, 1 kg, 1.25 kg, 1.5 kg, 1.75 kg, 2 kg, 2.25 kg, and 2.5 kg; dashed lines represent indicative target concentration proposed by Ahsman et al24 for (A) a dose of 0.03 mg/(kg·h) according to the current label and (B) an adjusted dosing regimen to reach a concentration of 400 µg/L at 72 hours postdose.
The model predicts that a median steady‐state concentration of 400 µg/L (±10%) can be achieved by reducing the dose by 50% from 0.03 mg/(kg·h) to 0.015 mg/(kg·h) in preterm neonates weighing 0.5 kg, by 33% to 0.02 mg/(kg·h) in neonates weighing 0.75 kg, and to 0.024 mg/(kg·h) in neonates weighing 1 kg (Figure 3B). For preterm infants with a body weight of 1.25 kg or 1.5 kg, the proposed dose would be adequate. In preterm infants with a body weight of 1.75 kg, a dose increase of 20% (0.036 mg/kg) was suitable, whereas in neonates weighing 2 to 2.25 kg the dose had to be increased by 33% (0.04 mg/[kg·h]) and by 50% in a child weighing 2.5 kg (Figure 3B). If one would consider a target range between 300 µg/L and 500 µg/L (400 µg/L ± 25%) as adequate, a dose of 0.03 mg/(kg·h) would be adequate in neonates between 1 and 2 kg. This dose would have to be reduced by one third (0.02 mg/[kg·h]) in children below 1 kg and increased by one third in children weighing more than 2 kg and less or equal to 2.5 kg (0.04 mg/[kg·h], data not shown).
Discussion
In this study we successfully developed a population pharmacokinetic model for midazolam in preterm neonates with a gestational age of 24‐33 weeks on the basis of previously published data19 and newly available data from the prospective multicenter DINO study. The results of this study show that clearance nonlinearly increases with body weight (Figure 1), whereas central volume of distribution linearly increases with body weight (Table 2). Only due to the utilization of data from different sources was it possible to develop a population pharmacokinetic model of this drug. Although the data of de Wildt et al were very densely sampled, they only contained data on 1 intravenous bolus administration per patient.19 The DINO data made use of sparse opportunistic sampling, which allowed for the collection of the most informative data while putting the least burden on this vulnerable population. This practice resulted in a very low number of observations per administration, but patients were followed over a long period of time (up to 30 days) and received intravenous bolus administrations, continuous intravenous infusions, or both (Table 1). The combined data set thus contains sufficient information to describe the whole postnatal and gestational age range of preterm neonates, which can support identifying maturational processes and eventually aids dose finding.
So far, to our knowledge, only 2 population pharmacokinetic models including preterm neonates have been published. In 1994 Burtin et al10 studied 187 critically ill neonates with a gestational age of 26 to 42 weeks, a birth weight of 700 to 5200 g and a postnatal age of 0 to 10 days and found that clearance and volume of distribution were directly proportional to birth weight. They also found a categorical influence of gestational age, with children with a gestational age of 39 weeks or older having 1.6 times higher clearance values. In our analysis we did not find an additional influence of gestational or postnatal age after body weight was implemented, which may be explained by the correlation between age and weight and the fact that the range in gestational age was low in our data set. In 1999 Lee et al11 analyzed data of 60 mechanically ventilated preterm neonates with a gestational age of 24 to 31 weeks, a birth weight of 523 to 1470 g, and a postnatal age of 2 to 15 days. They found that clearance and volume of distribution were significantly lower in infants with a birth weight below 1 kg. We compared the 2 clearance × weight functions described in these publications with the function that we identified (Figure 4). Because the data of Burtin et al10 mainly included infants during the first week of life and the population of Lee et al had a median postnatal age of 4.5 days,11 we assumed that the current body weight was approximately equal to birth weight, as preterm infants do not gain weight during the first 10 days of life.25 This made us able to depict all clearance maturation functions in 1 figure, which shows that the estimates of previous population pharmacokinetic studies were in close agreement with our results. This is also true for the clearance estimate in the original publication of the de Wildt data, which was obtained using standard noncompartmental techniques.19 On the basis of these results and the successful model evaluation, the developed model is suitable for the data used (Table 2, Figure 2, Supplemental Figure S1) but should also be suitable to describe intravenous data from other preterm neonatal populations. The fact that body weight and not gestational age or birth weight was the best covariate to describe the data in our model is most likely related to the broader range in postnatal age and the longer observation period, resulting in body weight undergoing significant changes over time. With the low postnatal age range in the 2 previous studies,10, 11 it might have been difficult to detect an influence of postnatal maturation. Previous studies in older children have shown that clearance might be lower in children with systemic inflammation.16, 17 Because the data set of de Wildt et al did not contain information on inflammation, and C‐reactive protein values have been infrequently sampled in the DINO population,19 we were not able to test this covariate, but it seems possible that it might be able to explain some of the high interindividual variability in clearance.
Figure 4.
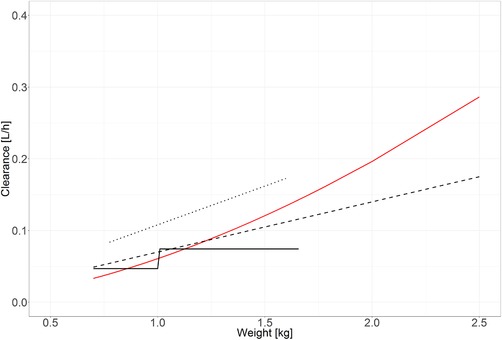
Comparison of clearance vs weight of the current analysis with literature; the red line represents the function of the current model; the solid black line represents the clearance estimates of Lee et al11; the dotted line represents the clearance estimates of de Wildt et al19; the dashed line represents the clearance estimates of Burtin et al.10 All estimates are displayed for the population ranges included in the respective studies only.
One of the major advantages of population pharmacokinetic modeling is that it offers the possibility of performing simulations based on the developed model.26 In this case we were able to review the recently proposed dosage advice for midazolam in preterm neonates. Our results show that when 1 fixed body weight–based dose is used for all preterm neonates up to 32 weeks of gestation, and another for preterm neonates above 32 weeks of gestation, steady‐state concentrations differ considerably. The steady‐state concentration after a 72‐hour infusion of 0.03 mg/(kg·h) was nearly 3 times as high for a child of 0.5 kg when compared with a child weighing 2.5 kg (754 vs 262 µg/L). Moreover, when performing our analysis, we were faced with the problem of defining the cutoff between the dosage advised below 32 weeks and that for above 32 weeks. We could not determine for how long after birth the dosage advice should be applicable. For example, if a child is born at 30 weeks of gestation and weighs 1.5 kg, should the dosage be changed from 0.03 mg/(kg·h) to 0.06 mg/(kg·h) if the child is 2 weeks old (postmenstrual age 32 weeks) and weighs 1.8 kg. Our developed model would not support such a sharp cutoff, because clearance increases gradually with body weight (Figure 3A). Also, the newly registered dosage does not give advice on suitable loading doses. With regard to the considerably long and weight‐dependent time to reach steady state, this seems desirable (Figure 3A).
No target concentration for the use of midazolam in preterm neonates has been defined as quantifying the relationship between plasma concentration and sedative effect. One publication related COMFORT scores to midazolam concentrations but did not find a significant correlation. In addition, the authors concluded that comediation might be of considerable influence to this relationship.18 Nevertheless, different authors have attempted to define indicative target concentrations. Jacqz‐Aigrain et al4 calculated their midazolam doses to obtain plasma concentrations between 200 and 1000 µg/L but without directly relating this target range to a specific effect. Although substantial variability was observed, only 2 of 46 infants with a gestational age of less than 32 weeks had a value above 1000 µg/L, and none had a value below 200 µg/L. The dosing schedule they used in this study was 0.06 mg/(kg·h) during the first 24 hours in all neonates and a dose reduction to 0.03 mg/(kg·h) from 24 hours onward in neonates younger than 33 weeks. Again, no correlation between sedation score and midazolam concentrations could be found. Ahsman et al proposed a target concentration of 400 µg/L for continuous infusions in term neonates because this was the median plasma concentration observed in their study. They did, however, assume that all of the patients included had been successfully titrated to their target sedation.24 To date, no clear concentration‐effect relationship for midazolam can be found; it is difficult to assess the impact of the observed differences. If we can assume a very broad target range of 200‐1000 µg/L, 65.2% of the simulated infants treated with the newly registered dosing regimen would be within this therapeutic window. Part of the reason for the rather large proportion of treated infants outside the therapeutic window is the large interindividual variability observed in the studied population, which has also been observed in the 2 other population pharmacokinetic models developed up to now.10, 11 This large unexplained interindividual variability may partly be caused by the use of 2 assays for quantification of midazolam and the limited (additional) covariate data available in our study, which did not enable us to study potentially interesting factors such as interacting comedication, ethnicity, severity of disease, and inflammation. Inflammation and disease severity have been reported to affect midazolam elimination in critically ill neonates and children16, 17 and thus may explain some of the variability. However, because the data set of de Wildt et al did not contain information on inflammation, and because C‐reactive peptide values have been infrequently sampled in the DINO population,19 we were not able to test this covariate. Physiologically based PK modeling, which can incorporate variation in CYP3A4 activity as a function of maturation or inflammation, can be used for simulations of the consequences of these variables. This approach has been used in pediatric PK assessments, including midazolam.27
When aiming for a comparable exposure in all preterm infants, the dosage of 0.03 mg/kg/h would have to be reduced in children weighing 1 kg or less and would have to be increased in children weighing 1.75 kg or more (Figure 3B). For a median child born at 33 weeks of gestation weighing around 2 kg,28 we would recommend an increase in dose by 33%, thus to 0.04 mg/kg/h and not to 0.06 mg/kg/h. The proposed dosing regimen could not be evaluated beyond a gestational age of 34 weeks because the population did not include individuals above that gestational age.
Conclusion
In conclusion, the newly registered dosage advice does not lead to uniform exposure in preterm infants resulting in considerable differences in typical steady‐state concentrations and time to reach steady‐state. The impact of this is difficult to assess, as no target concentrations have yet been defined for midazolam, but the current analysis shows that one should be cautious in giving dosage advice based on historical data with a lack of reliable pharmacokinetic and effect data.
DINO Study Group Members
K. Allegaert
P. Andriessen
D.M. Burger
K. Burggraaf
P. Degraeuwe
M. van Dijk
A.G.J. Engbers
R.B. Flint
R. de Groot
C.A.J. Knibbe
B.C.P. Koch
K.D. Liem
I.K.M. Reiss
S.H.P. Simons
D. Tibboel
S. Völler
Declaration of Conflict of Interest
All authors have no conflicts of interest to declare.
Data Accessibility Statement
The final data set and the accompanying code are available on request.
Supporting information
Supplemental Table 1. Details of Covariate Analysis
Supplemental Figure S2. Numerical prediction distribution errors of the final model: mean 0.04176 (standard error 0.059) and variance 0.8933 (standard error 0.079).
Supplemental Figure S1. Interindividual variability (η) on clearance (Left) and on volume of distribution (Right) vs relevant covariates (actual body weight, postnatal age, gestational age, and birth weight) for the final pharmacokinetic model.
Supporting Information
Acknowledgments
The DINO study and all accompanying research were funded by the Netherlands Organisation for Health Research and Development ZonMw (Grant 836011022).
References
- 1. Pacifici GM. Clinical pharmacology of midazolam in neonates and children: effect of disease—a review. Int J Pediatr. 2014;2014:309342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Wildt SN, Kearns GL, Sie SD, Hop WC, van den Anker JN. Pharmacodynamics of intravenous and oral midazolam in preterm infants. Clin Drug Invest. 2003;23(1):27‐38. [DOI] [PubMed] [Google Scholar]
- 3. Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev. 2017;1:CD002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jacqz‐Aigrain E, Daoud P, Burtin P, Desplanques L, Beaufils F. Placebo‐controlled trial of midazolam sedation in mechanically ventilated newborn babies. Lancet. 1994;344(8923):646‐650. [DOI] [PubMed] [Google Scholar]
- 5. Anand KJ, Barton BA, McIntosh N, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Arch Pediatr Adolesc Med. 1999;153(4):331‐338. [DOI] [PubMed] [Google Scholar]
- 6. Arya V, Ramji S. Midazolam sedation in mechanically ventilated newborns: a double blind randomized placebo controlled trial. Indian Pediatr. 2001;38(9):967‐972. [PubMed] [Google Scholar]
- 7. Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450 3A probe: I. In vitro‐in vivo correlations in liver transplant patients. J Pharmacol Exp Ther. 1994;271(1):549‐556. [PubMed] [Google Scholar]
- 8. Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. Expression of CYP3A in the human liver‐evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem. 1997;247(2):625‐634. [DOI] [PubMed] [Google Scholar]
- 9. Van Donge T, Mian P, Tibboel D, Van Den Anker J, Allegaert K. Drug metabolism in early infancy: opioids as an illustration. Expert Opin Drug Metab Toxicol. 2018;14(3):287‐301. [DOI] [PubMed] [Google Scholar]
- 10. Burtin P, Jacqz‐Aigrain E, Girard P, et al. Population pharmacokinetics of midazolam in neonates. Clin Pharmacol Ther. 1994;56(6 Pt 1):615‐625. [DOI] [PubMed] [Google Scholar]
- 11. Lee TC, Charles BG, Harte GJ, Gray PH, Steer PA, Flenady VJ. Population pharmacokinetic modeling in very premature infants receiving midazolam during mechanical ventilation: midazolam neonatal pharmacokinetics. Anesthesiology. 1999;90(2):451‐457. [DOI] [PubMed] [Google Scholar]
- 12. de Wildt SN, Kearns GL, Murry DJ, Koren G, van den Anker JN. Ontogeny of midazolam glucuronidation in preterm infants. Eur J Clin Pharmacol. 2010;66(2):165‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Wildt SN, de Hoog M, Vinks AA, van der Giesen E, van den Anker JN. Population pharmacokinetics and metabolism of midazolam in pediatric intensive care patients. Crit Care Med. 2003;31(7):1952‐1958. [DOI] [PubMed] [Google Scholar]
- 14. de Wildt SN, Kearns GL, Hop WC, Murry DJ, Abdel‐Rahman SM, van den Anker JN. Pharmacokinetics and metabolism of oral midazolam in preterm infants. Br J Clin Pharmacol. 2002;53(4):390‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harper KW, Collier PS, Dundee JW, Elliott P, Halliday NJ, Lowry KG. Age and nature of operation influence the pharmacokinetics of midazolam. Br J Anaesth. 1985;57(9):866‐871. [DOI] [PubMed] [Google Scholar]
- 16. Jacqz‐Aigrain E, Daoud P, Burtin P, Maherzi S, Beaufils F. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. Eur J Clin Pharmacol. 1992;42(3):329‐332. [DOI] [PubMed] [Google Scholar]
- 17. Vet NJ, Brussee JM, de Hoog M, et al. Inflammation and organ failure severely affect midazolam clearance in critically ill children. Am J Respir Crit Care Med. 2016;194(1):58‐66. [DOI] [PubMed] [Google Scholar]
- 18. de Wildt SN, de Hoog M, Vinks AA, Joosten KF, van Dijk M, van den Anker JN. Pharmacodynamics of midazolam in pediatric intensive care patients. Ther Drug Monit. 2005;27(1):98‐102. [DOI] [PubMed] [Google Scholar]
- 19. de Wildt SN, Kearns GL, Hop WC, Murry DJ, Abdel‐Rahman SM, van den Anker JN. Pharmacokinetics and metabolism of intravenous midazolam in preterm infants. Clin Pharmacol Ther. 2001;70(6):525‐531. [DOI] [PubMed] [Google Scholar]
- 20. Franken LG, Masman AD, de Winter BCM, et al. Hypoalbuminaemia and decreased midazolam clearance in terminally ill adult patients, an inflammatory effect? Br J Clin Pharmacol. 2017;83(8):1701‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol. 2013;2:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lindbom L, Pihlgren P, Jonsson EN. PsN‐Toolkit—a collection of computer intensive statistical methods for non‐linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241‐257. [DOI] [PubMed] [Google Scholar]
- 23. Comets E, Brendel K, Mentre F. Computing normalised prediction distribution errors to evaluate nonlinear mixed‐effect models: the npde add‐on package for R. Comput Methods Programs Biomed. 2008;90(2):154‐166. [DOI] [PubMed] [Google Scholar]
- 24. Ahsman MJ, Hanekamp M, Wildschut ED, Tibboel D, Mathot RA. Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin Pharmacokinet. 2010;49(6):407‐419. [DOI] [PubMed] [Google Scholar]
- 25. Anchieta LM, Xavier CC, Colosimo EA, Souza MF. Weight of preterm newborns during the first twelve weeks of life. Braz J Med Biol Res. 2003;36(6):761‐770. [DOI] [PubMed] [Google Scholar]
- 26. Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model‐based drug development. CPT Pharmacometrics Syst Pharmacol. 2012;1:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brussee JM, Vet NJ, Krekels EHJ, et al. Predicting CYP3A‐mediated midazolam metabolism in critically ill neonates, infants, children and adults with inflammation and organ failure. Br J Clin Pharmacol. 2018;84(2):358‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fenton TR, Nasser R, Eliasziw M, Kim JH, Bilan D, Sauve R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terrin G, Conte F, Oncel MY, et al. Paracetamol for the treatment of patent ductus arteriosus in preterm neonates: a systematic review and meta‐analysis. Arch Dis Child Fetal Neonatal Ed. 2016;101(2):F127‐F136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Details of Covariate Analysis
Supplemental Figure S2. Numerical prediction distribution errors of the final model: mean 0.04176 (standard error 0.059) and variance 0.8933 (standard error 0.079).
Supplemental Figure S1. Interindividual variability (η) on clearance (Left) and on volume of distribution (Right) vs relevant covariates (actual body weight, postnatal age, gestational age, and birth weight) for the final pharmacokinetic model.
Supporting Information
Data Availability Statement
The final data set and the accompanying code are available on request.


