Abstract
Background
The aim of this feasibility study was to assess the diagnostic performance of an electronic nose (e‐nose) as a noninvasive diagnostic tool in detecting locoregional recurrent and/or second (or third) primary head and neck squamous cell carcinoma (HNSCC) after curative treatment.
Methods
Using an e‐nose (Aeonose, The eNose Company, Zutphen, The Netherlands), breath samples were collected from patients after curative treatment of an HNSCC with a locoregional recurrence or second (or third) primary tumor (N = 20) and from patients without evidence of recurrent disease (N = 20). Analyses were performed utilizing artificial neural networking based on patterns of volatile organic compounds.
Results
A diagnostic accuracy of 83% was observed in differentiating follow‐up patients with locoregional recurrent or second (or third) primary HNSCC from those without evidence of disease.
Conclusion
This study has demonstrated the feasibility of using an e‐nose to detect locoregional recurrent and/or second (or third) primary HNSCC.
Keywords: diagnostics, electronic nose, head and neck squamous cell carcinoma, recurrent head and neck cancer, volatile organic compounds
1. INTRODUCTION
Head and neck cancer (HNC) has a major impact on global health, being the seventh most common cancer worldwide.1 The vast majority of HNCs are squamous cell carcinoma (HNSCC). Despite recent advances in diagnostic approaches and treatment modalities for HNC, the 5‐year survival rates have improved only marginally in the past decades.2 In part, this can be attributed to the high rates of locoregional recurrence and second primary HNSCC. Several authors report a 10% to 50% rate of recurrence,3, 4 approximately 75% of which found within 2 years after curative treatment. The probability of developing a second primary HNSCC within 5 years after initial diagnosis is approximately 20%.5, 6
Diagnosing locoregional recurrence and/or second primary HNSCC has proven to be challenging. Tumor recurrence and complications following radiotherapy, one of the main (adjuvant) treatment modalities, often present with identical clinical symptoms. Furthermore, oncological treatment often severely alters both anatomy and physiology. Consequently, it is difficult to assess the indication for an endoscopic procedure solely by clinical examination and diagnostic imaging. In addition, pathologic differentiation between (radio) necrosis and tumor can be difficult. As a result, less than half of endoscopic procedures correctly differentiate a recurrent HNSCC from postirradiation complications at first attempt, with relatively high rates of false‐negative biopsies.7, 8 It is therefore necessary to improve the diagnostic approach for previously treated patients with HNSCC with suspected locoregional recurrence and/or second primary HNSCC. The need to develop new strategies is urgent, in that early diagnosis could lead to higher survival rates and fewer futile endoscopic procedures under general anesthesia.
A promising diagnostic and screening tool for this purpose is volatile organic compounds (VOCs) analysis. VOCs are gaseous products of both physiological and pathological processes in the human body. Disease is often associated with altered metabolism, resulting in a modified VOC output with a distinctive fingerprint.9 Several techniques have been used to assess VOCs. One combines gas chromatography with mass spectrometry; another, called electronic nose technology (e‐nose), is based on pattern recognition rather than component identification.10 VOCs have been found in feces, urine, headspace of microorganism cultures, and exhaled breath. The compounds have been associated with respiratory,11 urogenital,12 and neurological disease,13 as well as with malignancies of the lung,14, 15 colorectal,14, 16 and head and neck origin.14, 17, 18, 19, 20
The present study used an Aeonose (The eNose Company, Zutphen, the Netherlands), a low‐cost, rapid, portable, handheld, and noninvasive diagnostic tool for VOC pattern recognition in breath samples. Using this device, our group illustrated the ability of an e‐nose to differentiate healthy patients from patients with primary HNSCC18 and lung carcinoma21 and to discriminate patients with primary HNSCC from those with bladder cancer, colon carcinoma, and lung carcinoma.14, 19 To our knowledge, no other studies have been performed to investigate the use of e‐nose technology in diagnosing recurrent and/or second primary HNSCC.
The aim of this feasibility study was to determine the diagnostic performance of an e‐nose as a noninvasive diagnostic tool in detecting locoregional recurrent and/or second (or third) primary HNSCC after prior curative treatment.
2. MATERIALS AND METHODS
2.1. Participants
This study was conducted at a tertiary care referral hospital: the Maastricht University Medical Center. Patients with histopathologically or cytologically confirmed locoregional recurrent and/or second (or third) primary HNSCC were included along with a follow‐up group (ie, patients who had previously been curatively treated for HNSCC and showed no evidence of recurrent disease). All enrolled patients without evidence of recurrent HNSCC at the time of e‐nose measurement remained tumor free at least up till the end of the study, with a minimal follow‐up duration of 6 months. Only patients with HNSCC originating from the oral cavity, oropharynx, hypopharynx, or larynx were included. Locoregional recurrence was defined as a newly diagnosed HNSCC at a distance of less than 2 cm from the primary tumor site or in an adjacent regional lymph node after a disease‐free period of 6 months or more but less than 2 years. A tumor arising farther than 2 cm from a primary tumor site or after a disease‐free period of more than 2 years was considered as a second primary HNSCC. A tumor arising less than 6 months after curatively intended treatment was considered as residual disease and therefore such patients were not included in the present study.22 This study protocol is approved by the METC. Project registration number 11407. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Exclusion criteria were: age under 18, current tracheostomy, supplemental oxygen, current carcinoma in situ, having had any treatment for current tumor or a history of any other form of cancer. Patients unable to complete an e‐nose measurement or to endure a nose clip were excluded as well. Follow‐up patients having had any oncological treatment within 6 months prior to measurement were excluded to rule out any adverse short‐term effects of treatment on VOC output. A past history of cutaneous squamous‐cell or basal‐cell carcinoma was permitted due to the fact that the vast majority of such cases represent localized disease with an extremely low incidence of metastasis.23
Tumor characteristics and medical history were gathered from the clinical records. TNM stages were established by an experienced head and neck tumor board using the American Joint Committee on Cancer Staging Manual (seventh edition). Patients' smoking habits were documented. Their smoking history was reported in pack years, which were calculated using an online pack year calculator.24 Smoking cessation was defined as no smoking for at least 1 month. Side effects or adverse effects during or shortly after measurement were documented. Oral informed consent was obtained from all patients. The study protocol was approved by the local medical ethics committee in accordance with the Declaration of Helsinki.
2.2. Study design
All patients were asked to inhale and exhale through the e‐nose for 5 minutes. Before starting the measurement, they were instructed to breathe through the device to familiarize themselves with the procedure. To prevent entry of nonfiltered air, a nose clip was placed on the nose. Participants were instructed to close their lips over the mouthpiece at all times, not to talk, and, if possible, not to sneeze or cough during the procedure. Measurements of patients not meeting these standards were not used for analysis.
E‐nose measurements took place in accordance with “standard cancer care” based on national guidelines for diagnostic routines in patients with cancer. To eliminate potential interference in the diagnostic process, no individual e‐nose outcomes were given to the participants or the medical practitioners. The results were labeled “sick” or “healthy”.
We then determined the diagnostic performance of an e‐nose in detecting locoregional recurrent and/or second (or third) primary HNSCC after prior curative treatment. This was achieved by analysis of the e‐nose measurements, whereby breath patterns of follow‐up patients without evidence of disease were compared to follow‐up patients with histopathologically or cytologically confirmed locoregional recurrent or second (or third) primary HNSCC. To reduce the long‐term effects of oncological treatment on the analysis of e‐nose measurements, participants with recurrent or second (or third) primary HNSCC were randomly matched to patients (without evidence of disease during measurement) who had received similar oncologic treatments for prior HNSCC. This was accomplished by means of “case‐control matching” analysis performed with a statistical software package of IBM SPSS Statistics for Windows, Version 24.0 (Armonk, New York: IBM Corp.). The matching resulted in a cohort of follow‐up patients with locoregional recurrent or second (or third) primary HNSCC (N = 20) and follow‐up patients without evidence of disease (N = 20), whereby the variances in received oncological treatment modalities, were equal for the two groups.
2.3. Materials
The e‐nose used in this study (Aeonose; The eNose Company, Zutphen, the Netherlands), harbors thee micro hotplate metal‐oxide sensors (AS‐MLV sensors, Applied Sensors GmbH). During measurement, the hotplates are heated and cooled in 32 steps, accurately regulating temperature between 260°C and 340°C. Although the sensors are exposed to exhaled air, temperature‐dependent reduction and oxidation (redox) reactions of VOCs on the metal‐oxide surface affect the conductivity of the sensors. The registered changes in conductivity represent a unique VOC pattern. A full measurement procedure lasts about 15 minutes, of which 5 minutes are spent on inhalation and exhalation. The remaining time is used for sensor regeneration and detecting possible low‐concentrated VOCs (for a more detailed discussion on this point‐of‐care device, see van Hooren et al).19 To eliminate possible device‐related confounding factors, two Aeonoses (serial numbers 309 and 362) were used in this study.
2.4. Statistical analysis
Differences in baseline characteristics were determined using independent sample t test, Fisher's exact Test, and Mann Whitney U test. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 24.0 (Armonk, New York: IBM Corp.).
Each e‐nose measurement yields 64 (temperature values) times and 36 (measurement cycles) times 3 (sensors) data points, generating a multiway data set consisting of conductivity values. After preprocessing, the data are compressed using a TUCKER3‐like solution generating low‐dimensional vectors for each measurement. Subsequently, these vectors, representing unique VOC patterns, are used to train an artificial neural network (ANN). The combination of several preprocessing techniques, vector lengths, and neural network topologies results into several models for separating patients who are“sick” from “healthy.” A model showing proper diagnostic accuracy was selected. Preprocessing, data compression, and ANN analysis have been integrated in a proprietary software package (Aethena; The eNose Company, Zutphen, the Netherlands). “Leave‐10%‐out” cross‐validation was applied to prevent fitting of the data on artifacts instead of breath profile classifiers. All data were labeled either “sick” (ie, follow‐up patient with recurrent, second, or third HNSCC) or “healthy” (ie, follow‐up patient without evidence of disease) when processed by Aethena.
The individual predictive values (ranging from −1 to 1) were presented in a scatterplot and subsequently used to assemble a receiver operating characteristics curve (ROC curve).
3. RESULTS
A total of 40 patients were enrolled in this study. The collection of breath samples did not result in any adverse effects requiring medical attention. The study included follow‐up patients with HNSCC without evidence of disease (N = 20) and follow‐up patients with histopathologically or cytologically confirmed locoregional recurrent or second (or third) primary HNSCC (N = 20). Baseline characteristics are shown in Tables 1 and 2. A significant difference in mean age was observed between the two groups.
Table 1.
Baseline characteristics (demographics and features of first tumor)
| Parameter | Follow‐up without HNSCC | Follow‐up with HNSCC | P values |
|---|---|---|---|
| No. of patients | 20 | 20 | |
| Age (mean years ± SD) | 62 ± 9 | 69 ± 10 | .02a |
| Male sex (%) | 14 (70) | 17 (85) | .45b |
| Currently smokingc (%) | 8 (40) | 6 (30) | .74b |
| Pack years (median) | 31 | 45 | .71d |
| Missing | 0 | 2 | |
| Location of first tumor | .50b | ||
| Oral cavity (%) | 7 (35) | 4 (20) | |
| Oropharynx (%) | 6 (30) | 7 (35) | |
| Hypopharynx (%) | 0 | 2 (10) | |
| Larynx (%) | 7 (35) | 7 (35) | |
| Stage of first tumor | .97b | ||
| I (%) | 6 (30) | 4 (22) | |
| II (%) | 3 (15) | 2 (11) | |
| III (%) | 4 (20) | 4 (22) | |
| IV (%) | 7 (35) | 8 (44) | |
| Missing | 0 | 2 | |
| History of oncologic treatment | .99b | ||
| Radiotherapy (%) | 5 (25) | 5 (25) | |
| Surgery (%) | 5 (25) | 5 (25) | |
| (Chemo)radiotherapy (%) | 4 (20) | 4 (20) | |
| Surgery and (chemo) radiotherapy (%) | 3 (15) | 3 (15) | |
| Combination of above (%) | 3 (15) | 3 (15) | |
| Days after treatment (median ± range) | 663 ± 2508 | 964 ± 7569 | .27d |
| Missing | 1 | 1 |
Independent samples t test.
Fisher's exact test.
Defined as no smoking for at least 1 month.
Mann‐Whitney U test.
Table 2.
Baseline characteristics (features of current tumor)
| N (%) | |
|---|---|
| Location of current tumor | |
| Oral cavity | 7 (35) |
| Oropharynx | 4 (20) |
| Hypopharynx | 1 (5) |
| Larynx | 7 (35) |
| Regional lymph node | 1 (5) |
| Stage of current tumor | |
| I | 3 (17) |
| II | 2 (11) |
| III | 4 (22) |
| IV | 9 (50) |
| Unknowna | 2 |
| Type of follow‐up tumor | |
| Local recurrence | 5 (25) |
| Regional recurrence | 1 (5) |
| Second primary | 12 (60) |
| Third primary | 2 (10) |
In two patients, the existence of metastatic disease was unknown.
Follow‐up patients with locoregional recurrent or second (or third) primary HNSCC were compared to follow‐up patients without evidence of disease. Figure 1 displays a scatterplot of individual predicted values as calculated by ANN on the basis of e‐nose measurements. To obtain the best possible diagnostic values, the threshold was set to −0.06. Individual predicted values above this threshold were classified as positive, and values below this threshold were classified as negative for recurrent or second (or third) primary HNSCC. Substantial variances in individual predicted values were observed; approximately 80% of the predictive values were located between −0.5 and 0.5.
Figure 1.
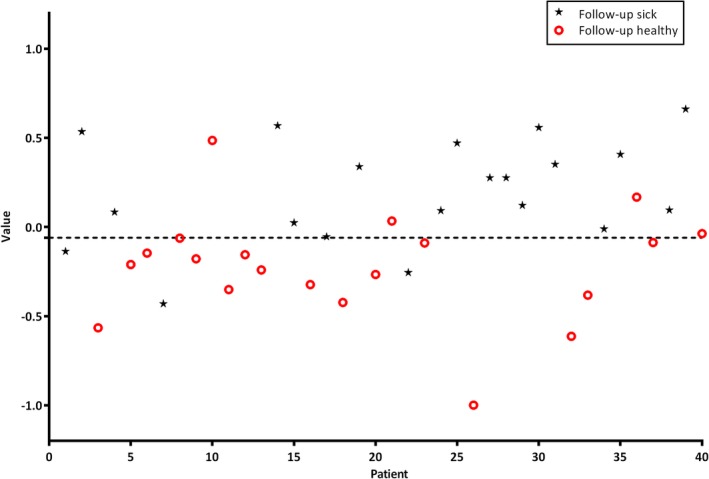
Scatterplot of individual predictive values of each patient (threshold −0.06). Values >−0.06 are scored as being positive for cancer. The black asterisks are follow‐up patients with histopathologically confirmed head and neck cancer and the red circles represent healthy tumor‐free follow‐up patients [Color figure can be viewed at wileyonlinelibrary.com]
A sensitivity (SE), specificity (SP), positive predictive value, negative predictive value, and overall diagnostic accuracy of respectively 85%, 80%, 81%, 84%, and 83% were achieved. The corresponding ROC curve, with an area under the curve (AUC) of 0.85, is presented in Figure 2.
Figure 2.
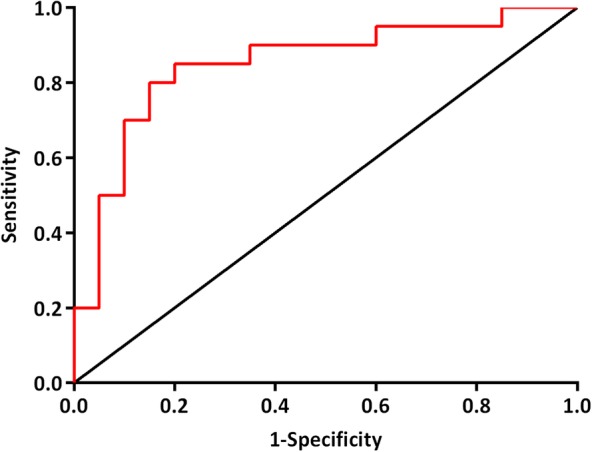
ROC curve: diagnostic performance of an e‐nose to discriminate between follow‐up patients with and without HNSCC; AUC = 0.85. Abbreviations: AUC, area under the curve; HNSCC, head and neck squamous cell carcinoma; ROC, receiver operating characteristics [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In this feasibility study, we examined the ability of an e‐nose to discriminate between follow‐up patients with locoregional recurrent or second (or third) primary HNSCC, on the one hand, and follow‐up patients without evidence of disease, after prior curative treatment, on the other. To attenuate the effects of oncological treatment on e‐nose VOC output, participants were randomly matched based on oncological treatment undergone previously. The substantial variances in individual predicted values indicate that, besides having (had) malignant disease and oncologic treatment, a complex interplay of multiple factors contributes to VOC output, as visualized in Figure 1. Nonetheless, our results show high diagnostic accuracies in differentiating follow‐up patients with from those without recurrent or second (or third) primary HNSCC. Our findings illustrate the feasibility of using an e‐nose for diagnosing recurrent and second (or third) primary HNSCC after prior oncological treatment.
In the present study, participants with locoregional recurrent HNSCC as well as participants with second (or third) primary HNSCC were analyzed together. Combining these groups could possibly mispresent the true diagnostic performance of an e‐nose in detecting either local or regional recurrence or second (or third) primary HNSCC. However, during the diagnostic work‐up of follow‐up patients suspected of HNSCC, the first concern of the medical practitioners is to detect (any) HNC rather than to discriminate between local or regional recurrence and second primary tumors. General practice would benefit most from an e‐nose that could detect follow‐up patients with HNSCC and discern those without malignant disease, a performance outcome that is in line with the present study design.
The ability of an e‐nose to discriminate follow‐up patients with locoregional recurrent or second primary HNSCC from those without disease might depend on previous oncologic treatment. There is evidence suggesting that irradiated normal tissue is subjected to persistent injury at molecular and cellular level (eg, oxidative stress, hypoxia, and inflammation), resulting in metabolic derangements25 and complications of radiotherapy (eg, mandibular osteoradionecrosis).26 Furthermore, recent studies propose the possibility of a self‐sustaining immunologic response to irradiated normal tissue, similar to an autoimmune reaction, following radiotherapy.25, 27, 28 Such a response has the potential to modify VOC output in irradiated patients. This may imply that separate predictive models based on prior treatment modalities need to be constructed in order to achieve the best possible diagnostic accuracies by means of an e‐nose. No studies have been conducted yet to evaluate the association between long‐term metabolic and immunogenic alterations following radiotherapy in relation to VOC output. Further research is needed in order to gain more insight in their potential role in VOC metabolism.
This is the first study to describe the potential role of an e‐nose in diagnosing locoregional recurrent or second (or third) primary HNSCC. A recent systematic review evaluated the diagnostic accuracy of 18FDG‐PET and 18FDG‐PET/CT in detecting locoregional recurrent HNSCC at least 12 months following curative treatment. In their article, the authors found a pooled SE and SP of 92% and 91%, respectively, of which the latter significantly increased with time after primary treatment.29 Similar diagnostic accuracies were found for 18FDG‐PET/MRI.30 A recent review described the potential role of apparent diffusion coefficients using MRI with diffusion‐weighted imaging. That review reported sensitivities and specificities ranging from 85% to 95% and 69% to 100%, respectively, for the detection of locoregional HNSCC at least 3 months after initial treatment.31 Nonetheless, these imaging techniques have disadvantages that should not be ignored: the use of ionizing radiation and/or contrast medium, limited use within the first weeks after radiotherapy, and high costs. Narrow‐band imaging (NBI) during flexible transnasal endoscopy is a relatively new optical method that is potentially suitable for detecting recurrent HNSCC. Different studies report promising diagnostic accuracies, with sensitivities and specificities ranging from 88% to 100% and 92% to 98%, respectively.32, 33, 34 However, it should be kept in mind that diagnostic performance is dependent on the clinicians' experience. In addition, for reliable examination of the laryngeal mucosa, direct laryngoscopy under general anesthesia is required. Furthermore, a regional lymph node recurrence and distant metastasis cannot be detected by NBI. An e‐nose might be useful in overcoming the common disadvantages of modern imaging techniques and NBI. Potentially, the device could be used to identify patients suspected of recurrent or second primary HNSCC who could benefit from examination under general anesthesia (with biopsies taken). Moreover, the e‐nose as a rapid, real‐time, and low‐cost diagnostic procedure might be particularly useful as a screening tool in primary health care and/or in less developed countries.
This is the first study to illustrate the diagnostic performance of an e‐nose in diagnosing locoregional recurrent or second (or third) primary HNSCC. An e‐nose seems to have potential as a rapid, real‐time, and noninvasive tool for diagnosing recurrent or second (or third) primary HNSCC. A larger study, including a blinded group for validation, would be needed to determine whether an e‐nose can be incorporated in the follow‐up of patients with HNSCC.
5. LIMITATIONS
This feasibility study has some limitations due to its design, and the results have to be interpreted accordingly. Due to matching, half of the participants were follow‐up patients without evidence of disease whose history of oncological treatment was similar to that of follow‐up patients with malignant disease. As a result, the group of follow‐up patients without evidence of disease may not be an authentic representation of this population in a tertiary care hospital.
A possible limitation of this study is related to the use of ANN to determine the diagnostic performance of an e‐nose. The models created by this technique could have been based partially on artifacts that are not directly related to malignant disease. The level of alcohol consumption and/or (history of) alcohol abuse was not documented, and the mean age differed significantly between the two groups, possibly contributing to artifacts. Cross‐validations were done to reduce the influence of these issues but cannot exclude it entirely.
The group of patients with recurrent or second (or third) primary HNSCC was relatively small, possibly restricting the potential of ANN to calculate the predictive values of both groups. The vast majority of follow‐up patients with malignant disease had second (or third) primary HNSCC, making the results less applicable for detection of locoregional recurrence of HNSCC. Also, due to the small number of participants in the current study, local and regional recurrences of HNSCC were not analyzed separately, thereby possibly limiting the diagnostic potential of an e‐nose. Furthermore, a subgroup analysis for patients having stage I/II tumors might be relevant for the clinical use of the e‐nose. However, the number of patients diagnosed with stage I/II tumors in our study population was not sufficient to create a reliable model with the ANN.
None of the patients without malignant disease received a diagnostic work‐up to exclude cancerous disease, as no clinical symptoms were present at the time of sample collection.
6. CONCLUSION
This is the first study to illustrate the potential of an e‐nose as a noninvasive diagnostic tool in the follow‐up of patients with HNSCC. With a diagnostic accuracy of 83%, an e‐nose is regarded as playing a feasible role in detecting locoregional recurrent or second (or third) primary HNSCC, after prior curative treatment. A larger study, including a blinded group for validation, is needed to determine whether an e‐nose could be incorporated in the follow‐up of patients with HNSCC.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
R.M.G.E.v.d.G., K.W.K., and B.K. designed the present study. R.M.G.E.v.d.G., J.C.A.H., and M.R.A.v.H. gathered the data. R.M.G.E.v.d.G. and J.C.A.H. were responsible for writing the manuscript. K.W.K. and B.K. helped writing the manuscript and played a supervising during this study.
ACKNOWLEDGMENTS
Each author has participated sufficiently in the present study. Authors of this article had access to all study data, are responsible for all contents of the article, and had authority over manuscript preparation and the decision to submit the manuscript for publication. All patients involved in this study gave oral informed consent to participation. We thank The eNose Company, Zutphen, the Netherlands, for supplying the Aeonoses, including software packages, filters, and mouthpieces free of charge.
van de Goor RMGE, Hardy JCA, van Hooren MRA, Kremer B, Kross KW. Detecting recurrent head and neck cancer using electronic nose technology: A feasibility study. Head & Neck. 2019;41:2983–2990. 10.1002/hed.25787
REFERENCES
- 1. Rettig EM, D'Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am. 2015;24(3):379‐396. [DOI] [PubMed] [Google Scholar]
- 2. Braakhuis BJ, Leemans CR, Visser O. Incidence and survival trends of head and neck squamous cell carcinoma in The Netherlands between 1989 and 2011. Oral Oncol. 2014;50(7):670‐675. [DOI] [PubMed] [Google Scholar]
- 3. Argiris A, Harrington KJ, Tahara M, et al. Evidence‐based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2017;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eckardt A, Barth EL, Kokemueller H, Wegener G. Recurrent carcinoma of the head and neck: treatment strategies and survival analysis in a 20‐year period. Oral Oncol. 2004;40(4):427‐432. [DOI] [PubMed] [Google Scholar]
- 5. Do KA, Johnson MM, Doherty DA, et al. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States). Cancer Causes Control. 2003;14(2):131‐138. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz LH, Ozsahin M, Zhang GN, et al. Synchronous and metachronous head and neck carcinomas. Cancer. 1994;74(7):1933‐1938. [DOI] [PubMed] [Google Scholar]
- 7. Zbaren P, Weidner S, Thoeny HC. Laryngeal and hypopharyngeal carcinomas after (chemo)radiotherapy: a diagnostic dilemma. Curr Opin Otolaryngol Head Neck Surg. 2008;16(2):147‐153. [DOI] [PubMed] [Google Scholar]
- 8. Brouwer J, Bodar EJ, de Bree R, et al. Detecting recurrent laryngeal carcinoma after radiotherapy: room for improvement. Eur Arch Otorhinolaryngol. 2004;261(8):417‐422. [DOI] [PubMed] [Google Scholar]
- 9. Amann A, Mochalski P, Ruzsanyi V, Broza YY, Haick H. Assessment of the exhalation kinetics of volatile cancer biomarkers based on their physicochemical properties. J Breath Res. 2014;8(1):016003‐016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haick H, Broza YY, Mochalski P, Ruzsanyi V, Amann A. Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev. 2014;43(5):1423‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dragonieri S, Pennazza G, Carratu P, Resta O. Electronic nose Technology in Respiratory Diseases. Lung. 2017;195(2):157‐165. [DOI] [PubMed] [Google Scholar]
- 12. Roine A, Veskimae E, Tuokko A, et al. Detection of prostate cancer by an electronic nose: a proof of principle study. J Urol. 2014;192(1):230‐234. [DOI] [PubMed] [Google Scholar]
- 13. Dragonieri S, Quaranta VN, Carratu P, et al. An electronic nose may sniff out amyotrophic lateral sclerosis. Respir Physiol Neurobiol. 2016;232:22‐25. [DOI] [PubMed] [Google Scholar]
- 14. van de Goor RMGE, Leunis N, van Hooren MRA, et al. Feasibility of electronic nose technology for discriminating between head and neck, bladder, and colon carcinomas. Eur Arch Otorhinolaryngol. 2017;274(2):1053‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kort S, Brusse‐Keizer M, Gerritsen JW, van der Palen J. Data analysis of electronic nose technology in lung cancer: generating prediction models by means of Aethena. J Breath Res. 2017;11(2):026006. [DOI] [PubMed] [Google Scholar]
- 16. Di Lena M, Porcelli F, Altomare DF. Volatile organic compounds as new biomarkers for colorectal cancer: a review. Colorectal Dis. 2016;18(7):654‐663. [DOI] [PubMed] [Google Scholar]
- 17. Hakim M, Billan S, Tisch U, et al. Diagnosis of head‐and‐neck cancer from exhaled breath. Br J Cancer. 2011;104(10):1649‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leunis N, Boumans M‐L, Kremer B, et al. Application of an electronic nose in the diagnosis of head and neck cancer. Laryngoscope. 2014;124(6):1377‐1381. [DOI] [PubMed] [Google Scholar]
- 19. van Hooren MRA, Leunis N, Brandsma DS, Dingemans A‐MC, Kremer B, Kross KW. Differentiating head and neck carcinoma from lung carcinoma with an electronic nose: a proof of concept study. Eur Arch Otorhinolaryngol. 2016;273(11):3897‐3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmutzhard J, Rieder J, Deibl M, et al. Pilot study: volatile organic compounds as a diagnostic marker for head and neck tumors. Head Neck. 2008;30(6):743‐749. [DOI] [PubMed] [Google Scholar]
- 21. van de Goor R, van Hooren M, Dingemans AM, Kremer B, Training KK. Validating a portable electronic nose for lung cancer screening. J Thorac Oncol. 2018;13(5):676‐681. [DOI] [PubMed] [Google Scholar]
- 22. Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2010;11:9. [DOI] [PubMed] [Google Scholar]
- 23. Nguyen‐Nielsen M, Wang L, Pedersen L, et al. The incidence of metastatic basal cell carcinoma (mBCC) in Denmark, 1997‐2010. Eur J Dermatol. 2015;25(5):463‐468. [DOI] [PubMed] [Google Scholar]
- 24. Masters N, Tutt C. Smoking Pack Years. Smoking Pack Years [Website]. 2010. http://www.smokingpackyears.com/. Accessed September 21, 2017.
- 25. Citrin DE, Mitchell JB. Mechanisms of Normal tissue injury from irradiation. Semin Radiat Oncol. 2017;27(4):316‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Danielsson D, Brehwens K, Halle M, et al. Influence of genetic background and oxidative stress response on risk of mandibular osteoradionecrosis after radiotherapy of head and neck cancer. Head Neck. 2016;38(3):387‐393. [DOI] [PubMed] [Google Scholar]
- 27. Schaue D, McBride WH. T lymphocytes and normal tissue responses to radiation. Front Oncol. 2012;2:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brush J, Lipnick SL, Phillips T, Sitko J, McDonald JT, McBride WH. Molecular mechanisms of late normal tissue injury. Semin Radiat Oncol. 2007;17(2):121‐130. [DOI] [PubMed] [Google Scholar]
- 29. Sheikhbahaei S, Taghipour M, Ahmad R, et al. Diagnostic accuracy of follow‐up FDG PET or PET/CT in patients with head and neck cancer after definitive treatment: a systematic review and meta‐analysis. AJR Am J Roentgenol. 2015;205(3):629‐639. [DOI] [PubMed] [Google Scholar]
- 30. Schaarschmidt BM, Heusch P, Buchbender C, et al. Locoregional tumour evaluation of squamous cell carcinoma in the head and neck area: a comparison between MRI, PET/CT and integrated PET/MRI. Eur J Nucl Med Mol Imaging. 2016;43(1):92‐102. [DOI] [PubMed] [Google Scholar]
- 31. van der Hoorn A, van Laar PJ, Holtman GA, Westerlaan HE. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with head and neck tumors, a systematic review and meta‐analysis. PLoS One. 2017;12(5):e0177986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piazza C, Cocco D, De Benedetto L, Bon FD, Nicolai P, Peretti G. Role of narrow‐band imaging and high‐definition television in the surveillance of head and neck squamous cell cancer after chemo‐ and/or radiotherapy. Eur Arch Otorhinolaryngol. 2010;267(9):1423‐1428. [DOI] [PubMed] [Google Scholar]
- 33. Zabrodsky M, Lukes P, Lukesova E, Boucek J, Plzak J. The role of narrow band imaging in the detection of recurrent laryngeal and hypopharyngeal cancer after curative radiotherapy. Biomed Res Int. 2014;2014:175398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin YC, Watanabe A, Chen WC, Lee KF, Lee IL, Wang WH. Narrowband imaging for early detection of malignant tumors and radiation effect after treatment of head and neck cancer. Eur Arch Otorhinolaryngol. 2010;136(3):234‐239. [DOI] [PubMed] [Google Scholar]


