Abstract
The human gut is home to a large and diverse microbial community, comprising about 1,000 bacterial species. The gut microbiota exists in a symbiotic relationship with its host, playing a decisive role in the host's nutrition, immunity and metabolism. Accumulating studies have revealed the associations between gut dysbiosis or some special bacteria and various cancers. Emerging data suggest that gut microbiota can modulate the effectiveness of cancer therapies, especially immunotherapy. Manipulating the microbial populations with therapeutic intent has become a hot topic of cancer research, and the most dramatic manipulation of gut microbiota refers to fecal microbiota transplantation (FMT) from healthy individuals to patients. FMT has demonstrated remarkable clinical efficacy against Clostridium difficile infection (CDI) and it is highly recommended for the treatment of recurrent or refractory CDI. Lately, interest is growing in the therapeutic potential of FMT for other diseases, including cancers. We briefly reviewed the current researches about gut microbiota and its link to cancer, and then summarized the recent preclinical and clinical evidence to indicate the potential of FMT in cancer management as well as cancer‐treatment associated complications. We also presented the rationale of FMT for cancer management such as reconstruction of intestinal microbiota, amelioration of bile acid metabolism, and modulation of immunotherapy efficacy. This article would help to better understand this new therapeutic approach for cancer patients by targeting gut microbiota.
Keywords: gut microbiota, dysbiosis, cancer, fecal microbiota transplantation, therapy
Abbreviations
- CDI
Clostridium difficile infection
- CRC
colorectal cancer
- CTLA‐4
cytotoxic T‐lymphocyte‐associated antigen 4
- FMT
fecal microbiota transplantation
- Fn
Fusobacterium nucleatum
- GVHD
graft‐vs.‐host disease
- HCC
hepatocellular carcinoma
- HSCT
hematopoietic stem cell transplantation
- PD‐1
programmed cell death protein 1
- PDAC
pancreatic ductal adenocarcinoma
- PD‐L1
programmed death ligand 1
- Sgg
Streptococcus gallolyticus subsp. Gallolyticus
- TLRs
Toll‐like receptors
Introduction
The human intestinal tract is inhabited by numerous microbes, and the number of microbial cells is roughly equivalent to that of cells in human body.1 The human intestine contains about 1,000 different species of known bacteria with the largest number of bacteria in colon.2 The bacterial populations inhabiting the gut differ greatly between individuals,2 depending on host specificities (such as genetics and lifestyle).
In recent decades, understanding of the role that intestinal microbial community plays in health and disease has increased.3, 4 The intestinal microbial community in a state of delicate balance is now widely recognized to maintain health. However, as the balance can be disrupted by various factors including host genetics, diet, antibiotics and stress, altered microorganisms potentially initiate and perpetuate different disorders.5 Various studies have shown that microbial alternations, characterized by a marked increase in the numbers of pathogens and a relative decrease in levels of beneficial bacteria, are connected with the development of gastrointestinal and extra‐gastrointestinal cancers.6, 7, 8, 9, 10, 11, 12, 13
Altering the gut microbiota is expected as a novel method to deal with diseases associated with intestinal dysbiosis. Potential routes to target intestinal microbiota community include diet, probiotics, prebiotics, antibiotics and fecal microbiota transplantation (FMT). FMT is defined as the transplantation of gut microbiota from healthy donors to sick patients via the upper or lower gastrointestinal route to restore intestinal microbial diversity.14, 15 FMT is recognized as the most innovative and dramatic method due to its ability to alter the recipients’ gut microbiota. The utilization of feces for the treatment of food poisoning or severe diarrhea was firstly recorded by a well‐known medical expert named Ge Hong approximately 1,700 years ago.16 FMT was firstly reported to treat severe pseudomembranous enterocolitis by Eiseman in 1958.17 Nevertheless, this practice was less used until the first documented case of Clostridium difficile infection (CDI) treated with FMT was reported in 1983 by Schwan.18 Currently, FMT has been approved as a clinical method for treating recurrent CDI by 2013 guidelines19 and its clinical effectiveness has reached approximately 90%.20 Moreover, accumulating data indicate that FMT proves beneficial for the treatment of inflammatory bowel diseases and intractable functional constipation, etc.21, 22 In addition, the observed intestinal dysbiosis in cancer leads to increasing interests in the potential of FMT for the management of cancer.
Fecal donors are either close relatives, family members or unrelated individuals. However, where possible, fecal material is best sourced from a healthy unrelated individual, from a centralized stool bank.23 To eliminate the risk of inadvertently transmitting infection, donors in preparation of FMT should be screened according to an established protocol.24 With regard to methods of preserving fecal materials, the frozen fecal material has the advantage of more convenient management.25 However, the bacterial diversity of frozen product seems lower than that of fresh material.26 In a recent double‐blind study of patients with CDI, the frozen fecal product had a lower efficacy compared to fresh material.27 As well as differences in recipient preparation methods, the routes of administration are also various. Fecal microbiota can be delivered via capsule, nasogastric tube, nasoduodenal tube, enema, or colonoscopy.28 Although endoscopic administration allows direct evaluation of intestinal mucosa, oral administration is accepted easily by patients due to higher satisfaction.29 Retention enema is cheap and safe, but it might be hard to retain the donor microbiota.30 The optimum route of administration has not yet been determined. A European consensus conference on FMT published in Gut strongly recommended the implementation of FMT centers,31 while Terveer et al. deemed that a centralized stool bank could ensure the safety of fecal materials, and permit the rest of the FMT procedures in local hospitals.32
In this review, we focused on gut microbiota in various cancers. We then summarized the current preclinical and clinical studies on the use of FMT for gastrointestinal and nongastrointestinal cancers as well as cancer treatment‐associated complications including CDI and radiation enteritis (Table 1).
Table 1.
Summary of studies of fecal microbiota transplantation in cancer management
| Cancers and treatment‐associated complications | Ref. | Publication year | Study type |
|---|---|---|---|
| Colorectal cancer | Rosshart et al.91 | 2017 | Experimental study |
| Wong et al.39 | 2017 | Experimental study | |
| Our study52 | 2017 | Experimental study | |
| Chronic liver disease | |||
| Nonalcoholic steatohepatitis | De et al.95 | 2014 | Experimental study |
| Zhou et al.100 | 2017 | Experimental study | |
| Alcoholic hepatitis | Llopis et al.96 | 2016 | Experimental study |
| Philips et al.103 | 2017 | Case report | |
| Ferrere et al.101 | 2017 | Experimental study | |
| Philips et al.102 | 2017 | Experimental study | |
| Chemical‐induced liver injury | Qin et al.97 | 2017 | Experimental study |
| Chronic hepatitis B | Ren et al.104 | 2017 | Experimental study |
| Liver cirrhosis | Bajaj et al.105 | 2018 | RCT |
| Hepatic encephalopathy | Kao et al.107 | 2016 | Case report |
| Wang et al.106 | 2017 | Experimental study | |
| Bajaj et al.108 | 2017 | RCT | |
| Hepatocellular carcinoma | Ma et al.93 | 2018 | Experimental study |
| Pancreatic cancer | Pushalkar et al.114 | 2018 | Experimental study |
| Melanoma | Gopalakrishnan et al.71 | 2018 | Experimental study |
| Cancer treatment‐associated complications | |||
| Recurrent CDI | Neemann et al.127 | 2012 | Case report |
| Kelly et al.123 | 2014 | Observational study | |
| Blackburn et al.124 | 2015 | Case report | |
| Trubiano et al.125 | 2015 | Case report | |
| Mittal et al.126 | 2015 | Case report | |
| de Castro et al.128 | 2015 | Case report | |
| Webb et al.129 | 2016 | Case report | |
| Innes et al.130 | 2017 | Case report | |
| Hefazi et al.122 | 2017 | Observational study | |
| Radiation enteritis | Cui et al.132 | 2017 | Experimental study |
| Gerassy‐Vainberg et al.131 | 2018 | Experimental study | |
| Graft‐versus‐host disease | Kakihana et al.135 | 2016 | Case report |
Abbreviations: RCT, Randomized controlled trial; CDI, Clostridium difficile infection.
Gut Microbiota and Cancer
During the past several years, the involvement of gut microbiota in carcinogenesis has been increasing recognized.9, 33 Microbial dysbiosis and individual bacteria in the gut can induce carcinoma or promote cancer process by activating tumorigenic pathway, inducing inflammation and damaging host DNA34, 35 (Fig. 1). Several bacteria possess or produce proteins that promote the separation of β‐catenin from E‐cadherin, activating β‐catenin signal pathway involved in carcinogenesis. Intestinal dysbiosis leads to a decrease in the production of bacteria‐derived short‐chain fatty acids. Intestinal dysbiosis exerts pro‐inflammatory effects, via microorganism‐associated molecular patterns by Toll‐like receptors (TLRs), increasing the cells’ production of pro‐inflammatory factors, thereby increasing carcinogenesis. Beyond inducing inflammation, many bacteria also have the ability to damage DNA through releasing specific metabolites, which in turn promote cancer progression. Surprisingly, specific microbiota species modulate the efficacy of cancer therapy,36 markedly influencing the clinical outcome of cancer patients. Hence, a better knowledge of the link between intestinal bacteria and cancer can provide opportunities to develop promising therapeutic and diagnostic strategies.
Figure 1.
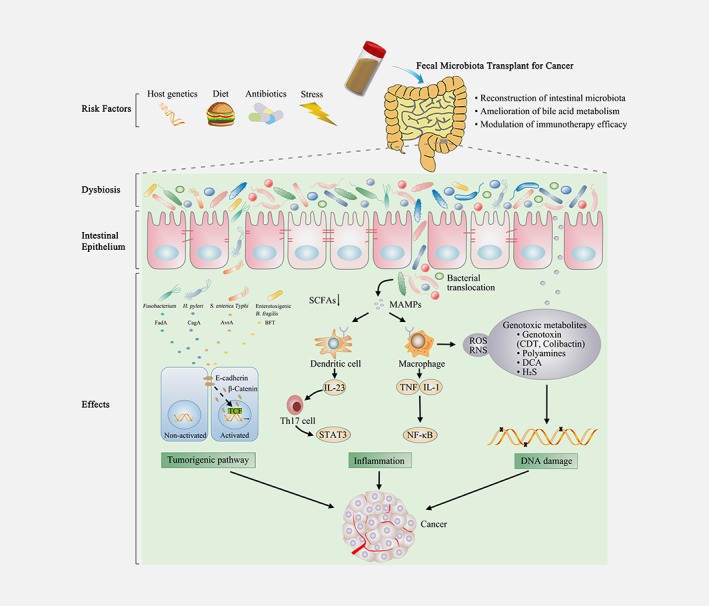
Management of cancer by fecal microbiota transplant. FMT represents a potential therapeutic strategy for cancer by reconstruction of intestinal microbiota, amelioration of bile acid metabolism and modulation of immunotherapy efficacy. Various factors such as host genetics, diet, antibiotics and stress could lead to alterations of gut microbiota, named as gut dysbiosis. Microbial dysbiosis and special bacteria in the gut are capable of affecting cancer development and progression via activating tumorigenic pathway, inducing inflammation and damaging host DNA. Special bacterial products, such as FadA toxin from Fusobacterium nucleatum, CagA protein from Helicobacter pylori, AvrA protein from S. enterica Typhi, and BFT from Enterotoxigenic Bacteroides fragilis can promote the separation of β‐catenin from E‐cadherin, which can trigger β‐catenin activation and contribute to tumorigenesis. The beneficial component in bacterial metabolites, such as SCFAs, is also decreased in microbial dysbiosis. Intestinal dysbiosis may be conducive to bacterial translocation, exerting pro‐inflammatory effects, which is mediated by MAMPs that activate TLRs in macrophages and dendritic cells. TLR signaling promotes the expression of the pro‐inflammatory factors, including IL‐23, TNF and IL‐1, thereby promoting carcinogenesis. Several microbial metabolites can directly or indirectly damage host DNA, fueling carcinogenesis. Special microbial toxins (CDT and colibactin) could directly induce DNA damage. Furthermore, gut bacteria also damage DNA indirectly via polyamines, DCA, ROS, RNS and H2S. FMT, fecal microbiota transplantation; BFT, Bacteroides fragilis toxin; SCFAs, short‐chain fatty acids; MAMP, microbe‐associated molecular pattern; TLR, Toll‐like receptor; IL‐23, interleukin 23; TNF, tumor necrosis factor; IL‐1, interleukin; Th17, T helper 17; STAT3, signal transducer and activator of transcription 3; NF‐κB, nuclear factor‐κB; CDT, cytolethal distending toxins; DCA, deoxycholic acid; H2S, hydrogen sulphide; RNS, reactive nitrogen species; ROS, reactive oxygen species. [Color figure can be viewed at wileyonlinelibrary.com]
Gut dysbiosis and cancer development
The alterations in gut microbiota composition have been implicated in the initiation and development of cancer of various tissues, including gastric cancer, colorectal cancer (CRC), hepatocellular carcinoma (HCC), pancreatic cancer, breast cancer, and melanoma. Recent studies have described the specific changes in the gut bacterial community in patients with cancers (such as gastric cancer or CRC) in comparison with healthy individuals.37, 38 The fecal microbiota from patients with CRC promoted tumorigenesis in germ‐free or conventional mice given a carcinogen,39 which showed the carcinogenic properties of the CRC microbiota. Accumulating epidemiological evidence supports the opinion that long‐term antibiotic exposures, known to change the composition and decrease the diversity of gut microbiota,40 increase the risk of CRC,41, 42, 43, 44 as well as gastric, pancreatic, lung, breast and prostate cancers.45 Consistent with this, long‐term antibiotic use was highly correlated with increased colorectal tumor progression in the Apc Min/+ mouse, a genetic model for human adenomatous polyposis.46 However, there is conflicting data about the association between antibiotics and risk of cancer. Oral administration of metronidazole could reduce Fusobacterium load and colorectal tumor growth in mice bearing a colon cancer xenograft.47 Moreover, antibiotic use could clear biofilms and eliminate microbial sulfide, and thereby protect the colon mucous barrier and prevent epithelial hyperproliferation.48, 49 Additionally, several studies have suggested that depletion of the gut microbiota upon exposure to an antibiotic cocktail could block intestinal tumorigenesis.50, 51, 52 It is possible that different antibiotic exposures (differ in dose or course) and subjects may lead to diverse variations in microbial community, which could result in distinct disease outcomes (Supporting Information, Table S1). Further investigations are required to elucidate the impact of antibiotic exposures on outcomes in cancer patients and its underlying mechanisms. With the deepening comprehension of gut dysbiosis, interest is growing rapidly worldwide in the application of microbiota‐target therapy for cancer.
Special microbial pathogens in cancer
It has been estimated that some microorganisms as etiological factors, such as Human papillomavirus, Helicobacter pylori (H. pylori) and Hepatitis B virus, account for about 20% of total cancers worldwide.53 Several bacterial species and their tumor‐promoting mechanisms have been investigated mostly on cell and animal levels, including production of toxic metabolites, alteration of intestinal microenvironment, induction of tumorigenic signaling pathways (Supporting Information, Table S2). For example, H. pylori is well known to contribute to the development of chronic gastritis and gastric carcinogenesis by secreting virulence factors and activating various tumor‐promoting signaling pathways.54, 55, 56, 57, 58 Enterotoxigenic Bacteroides fragilis, the producer of Bacteroides fragilis toxin, can induce intestinal inflammation and DNA damage, which participates in the pathogenesis of CRC.59 Streptococcus gallolyticus subsp. gallolyticus (Sgg), a Gram‐positive, opportunistic pathogen, is present in most colon tumor tissue compared to normal tissues in CRC patients.60 Sgg has also been shown to promote the development of colon tumor via the β‐catenin signaling pathway in mice given a carcinogen.61 Pathogenic Escherichia coli (E. coli) can produce many toxins including cyclomodulin, which is involved in tumorigenesis.62 Lately, gram‐negative oral commensal Fusobacterium nucleatum (Fn), which is enriched in colon tumor tissues compared to adjacent healthy tissues, has been reported to promote proliferation and invasion ability of tumor cells.63, 64, 65 Additionally, Fn induces cancer cell autophagy, thereby increasing chemotherapeutic drug resistance and tumor recurrence rate.66
The significant difference in gut microbiota composition between cancer patients and healthy individuals demonstrates diagnostic and prognostic potentials of special microbial pathogens in cancer. For examples, a significant stepwise increase of Fn abundance was found in healthy controls, colorectal adenoma patients and CRC patients, indicating its potential application value in early diagnosis of CRC.67 Combining the abundance of Fn and fecal immunochemical test could improve the accuracy and sensitivity in diagnosis of CRC and advanced adenoma.67, 68 In addition to the diagnostic utility, the amount of Fn in CRC tissue is associated with patient survival. Collectively, a better understanding of how special microbial pathogens elicit specific carcinogenesis may uncover valuable biomarkers for diagnosing and prognosticating cancer.
Gut microbiota and cancer therapy
Gut microbiota could influence cancer therapy efficacy. In 2013, Viaud et al. reported that gut microbiota modulated the therapeutic effect of the anti‐cancer immunomodulatory agent cyclophosphamide.69 Subcutaneous cancer‐bearing mice which were germ‐free or given antibiotics therapy to kill gram‐positive bacteria showed resistant to cyclophosphamide.69 Two bacterial species, Enterococcus hirae and Barnesiella intestinihominis, were identified to potentiate the antitumor efficacy of cyclophosphamide through engagement of immune responses.70
Several studies using melanoma‐bearing mice showed that the effectiveness of programmed cell death protein 1 (PD‐1) inhibitor was diminished under aseptic conditions,71 and improved effectiveness was observed in the presence of Bifidobacterium, which activated antigen‐presenting cells, thus promoting activated CD8+ T cells accumulation in the tumor microenvironment.72 MCA205 (mouse fibrosarcoma of C57BL background) sarcoma growth was controlled by anti‐cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA ‐4) therapy in specific pathogen free laboratory mice, compared to germ‐free or antibiotic‐treated mice.73 These studies highlight the impact of the intestinal microbiota on responses to cancer immunotherapy in mice.
Lately, corroborating these experimental results, clinical outcomes such as survival time to anti‐PD‐1 monoclonal antibodies were found to positively correlate with the relative abundance of Akkermansia, one of the most abundant bacteria in the ileum of healthy individuals.74 Microbiome encompasses microbiota genomes, microbial products and host environment.75 Transfer of the gut microbiome from cancer patients who responded to immunotherapy and oral supplementation of Akkermansia improved the efficacy of immunotherapy.74 Together, it is tempting to speculate that FMT is beneficial for the treatment of cancer.
FMT as a Possible Therapy for Various Type of Cancers and Cancer Treatment‐Associated Complications
FMT for digestive system cancers
Gastrointestinal cancers
Carcinogenesis of gastric cancers is associated with H. pylori and some oral microbiota including Fn, Parvimonas micra and Peptostreptococcus stomatis.76 Significant enrichment of Peptostreptococcus stomatis, Parvimonas micra, Streptococcus anginosus, Dialister pneumosintes, Slackia exigua, 38 Clostridium colicanis and Fn 77 and depletion of Helicobacterium 78 was observed in gastric cancer, and alterations in bacterial diversity and abundance in patients with gastric cancer revealed a dysbiotic microbial community with prediction potential.79 Recently, incremental data has demonstrated that eradication treatment for H. pylori could reduce the risk of gastric cancer.80, 81 Collectively, these studies indicate that gastric microbiota is involved in gastric carcinogenesis.With enormous microorganism at close proximity to the colonic epithelial cells, the involvement of gut microbiota in colorectal carcinogenesis is becoming clear. Indeed, some bacterial species can trigger the occurrence of CRC through toxic substance exposure, chronic inflammation, mucosal barrier injury and bacterial translocation. Pathogenic bacteria species, such as enterotoxigenic Bacteroides fragilis, can confer pro‐tumorigenic traits via producing harmful substances.82, 83, 84 Moreover, clinical studies reported significant shifts in intestinal microbiota composition between healthy individuals and those afflicted with CRC, showing a CRC‐specific bacterial signature.37, 85 Some bacteria (such as Lactobacillus, Bifidobacterium, etc.) were diminished, while others (such as Staphylococcaceae, Fusobacteria, Peptostreptococcus anaerobius, etc.) were augmented in stool samples from patients with CRC vs. healthy individuals. Analysis of fecal microbiota as a noninvasive tool might be used to improve detection accuracy of early CRC.86
There are several evidences that support a protective role of probiotics against CRC. As known butyrate producers, Clostridium butyricum and Bacillus subtilis could inhibit DMH‐induced colonic tumor in mice.87 Notably, another probiotic, Lactobacillus casei strain BL23 not only inhibited CRC in mice, but also counteracted gut dysbiosis induced by CRC.88 Additionally, recent clinical studies established that oral Bifidobacterium triple viable probiotics could improve gut dysbiosis and combat small intestinal bacterial overgrowth in CRC patients.89, 90
Our team identified the role of intestinal dysbiosis induced by deoxycholic acid (a carcinogenic secondary bile acid) in the development of CRC. We found that the transfer of feces from deoxycholic acid‐treated mice increased intestinal tumor development compared to untreated donor.52 Interestingly, the result has been verified in patients in a recent study, and the fecal microbiota from patients with CRC promoted intestinal tumor formation and lowered microbial abundance in germ‐free and conventional mice given a carcinogen.39 Moreover, Rosshart et al. reported that laboratory mice transplanted with intestinal microbiomes from wild mice showed better resistance to CRC and amelioration of inflammation, compared to control mice of their own bacteria,91 supporting the assumption that FMT could harbor a potential therapeutic ability for CRC.
Hepatocellular carcinoma
The liver is exposed to intestinal microbiota through the portal vein which delivers gut‐derived bacterial products or toxins, such as lipopolysaccharide and deoxycholic acid.6, 92 The close structural and functional interaction between the gut and the liver is defined as the gut‐liver axis. Liver diseases are often associated with intestinal dysbiosis, and it has been shown that gut bacterial metabolites could promote the development of chronic liver disease and HCC through gut‐liver axis.93, 94
Alteration of intestinal microbiota has been reported in liver disease, but the extent to which it is a cause is unknown. Microbiota transplantation from mice with high‐fat diet‐induced chronic liver damage revealed more liver injury in recipient mice.95 The stool from patients with severe alcoholic hepatitis increased the susceptibility to chronic alcoholic liver disease in mice.96 Microbial dysbiosis after penicillin or dextran sulfate sodium in rats aggravated hepatotoxicity of recipient mice.97 Moreover, colonization of Clostridium species, which could influence the metabolism of bile acids, increased liver tumor growth in mice with gram‐positive bacteria removed.93 These data provide direct evidence that microbial dysbiosis could directly contribute to liver disease.
There are several clinical studies regarding the use of probiotics as a novel and effective approach to treat or prevent chronic liver disease and HCC. Probiotic VSL#3, a combination of Bifidobacteria, lactobacilli and Streptococcus thermophilus, could short inpatient time for patients with liver cirrhosis and hepatic encephalopathy.98 A randomized controlled multicenter study involving 117 alcoholic hepatitis patients found that those who received probiotics treatment with Lactobacillus subtilis and Streptococcus faecium had lower level of serum lipopolysaccharide, compared to the placebo group.99
More recently, extensive research supports that FMT is showing promise as a therapy to control liver disease. FMT improved high‐fat diet‐induced liver injury and lipid metabolism along with increased gut microbiota diversity in mice.100 FMT from donor mice resistant to alcoholic liver disease could prevent alcohol‐induced liver injury.101 Moreover, FMT has already been used in human with chronic liver disease. A recent pilot study of patients with severe alcoholic hepatitis showed that FMT was associated with increased survival and resolved ascite.102 Philips et al. reported a case of a young male patient with corticosteroid nonresponsive severe alcoholic hepatitis in 2017.103 FMT led to rapid amelioration of appetite and hyperbilirubinemia. Notably, FMT was performed in 18 patients with persistent positive HBeAg.104 FMT was effective for these patients via inducing HBeAg clearance, suggesting that regulating intestinal microbiota might be beneficial to chronic hepatitis B treatment. A Phase I clinical trial demonstrated that FMT restored antibiotic‐induced microbial dysbiosis in patients with advanced liver cirrhosis.105 Even more, the effect of FMT on hepatic encephalopathy has been confirmed in both animal models and human beings. FMT alleviated cognitive function and prevented hepatic necrosis in animal models, thereby triggering improvement of hepatic encephalopathy.106 Kao et al. reported a significant improvement in serum ammonia and quality of life in a patient with hepatic encephalopathy after performing FMT.107 Bajaj et al. conducted a randomized clinical trial, which suggested that FMT has the potential to improve cognition and reduce hospitalizations in hepatic encephalopathy patients.108 Given the success of treating chronic liver disease, the benefit of FMT in patients with HCC deserves attention.
Pancreatic cancer
Recent studies have demonstrated that microbiota influences the development and treatment of pancreatic cancer.109 Evidence in mouse model manifested that lipopolysaccharide, which is generated from many gram‐negative bacteria, could promote pancreatic cancer formation via activating TLR4 in immune cells.110 In a recently published study, 76% of subjects were positive for intratumor bacteria in 113 humans with pancreatic ductal adenocarcinoma (PDAC).111 Some of these detected bacteria including Gammaproteobacteria could promote resistance to gemcitabine, a chemotherapeutic drug commonly used for PDAC, while antibiotic ciprofloxacin was able to abrogate the resistance.
Previous studies have shown the variation of oral microbial composition between healthy and pancreatic cancer individuals. Among pancreatic cancer groups, significant increases were noted in Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis, and significant decreases were observed in phylum Fusobacteria and genus Leptotrichia, suggesting the potential of oral microbiota to serve as a noninvasive and specific clinical diagnostic marker for pancreatic cancer.112 Moreover, the high abundance of Fusobacterium species in pancreatic cancer tissue was independently correlated with a worse prognosis,113 indicating that Fusobacterium species might become a promising prognostic parameter of pancreatic cancer. The transfer of the microbiota from mice with PDAC, but not healthy mice, accelerated tumor progression in germ‐free mice.114 Taken together, these studies revealed that microbiota‐based treatment might be useful to manage pancreatic cancer.
FMT for nondigestive system cancers
Breast cancer
Hill et al. first proposed a hypothesis about gut microbiota and the etiology of breast cancer in 1971, considering the similarity of colon and breast cancer in epidemiologic characteristics.115 By now, studies on the direct relationship between gut microbiota and breast cancer are rather limited. Goedert et al. analyzed differences between 48 pretreatment postmenopausal breast cancer patients and 48 healthy controls.116 Compared to controls, patients had significantly reduced alpha diversity and alterations in the composition of fecal microbiota. Studies have been dedicated to possible mechanisms, such as estrogen metabolism, immune regulation, obesity and so forth.117 Evidence from animal experiments suggests that modulation of the gut microbiota by probiotics can provide protection against breast cancer. For example, oral supplement with Lactobacillus acidophilus can delay the development of breast cancer by regulating anti‐tumor immune response.118 Further work is needed to elaborate the mechanism, and thus to manipulate gut microbiota with regards to management of breast cancer.
Melanoma
Recent evidence demonstrates that gut microbiota has implications for the progression and treatment of melanoma. Melanoma growth and its response to anti‐programmed death ligand 1 (PD‐L1) immunotherapy in two mouse facilities (JAX and TAC) harboring distinct gut microbial compositions were remarkably different.72 Through genomic analyses of the gut microbiota, Bifidobacterium was identified to facilitate the effects of PD‐L1 treatment.72 Lately, a study of 39 metastatic melanoma patients receiving immune checkpoint therapy also showed that there was a significant correlation between the content of microorganism and the response of immunotherapy.119 In the responders to cancer immunotherapy, Bacteroides thetaiotaomicron, Faecalibacterium prausnitzii and Holdemania filiformis were rich in their gut.119 The transfer of feces harvested from responding melanoma patients into mice established that FMT could enhance the effectiveness of immunotherapy to optimize the current therapies.71 A clinical study is testing the effect of FMT from PD‐1 responders into intestinal tracts of nonresponders in melanoma.120 Thus, FMT seems to be promising in enhancing antitumor immunity in melanoma patients by transferring a favorable gut microbiota.
FMT for cancer treatment‐associated complications
Clostridium difficile infection
Clostridium difficile is the most common cause of antibiotic‐associated diarrhea, leading to high morbidity and mortality in cancer patients. Both primary and recurrent CDI are not uncommon in patients with cancer owing to the fact that chemotherapy, frequent use of broad‐spectrum antibiotics, prolonged hospitalization, immunodepression and other factors can lead to the damage of normal gut microbiota.121 Obviously, FMT is an effective and acceptable procedure for the treatment of recurrent CDI and now recommended in clinical use. Recent research has demonstrated the effectiveness of FMT for clinical cure of recurrent CDI approximately 90%.20 Apart from the successful restoration of microbial diversity and bacterial metabolites, the regulation of bile acid metabolism is also one of the mechanisms of FMT for CDI.24
Although long‐term safety data are lacking, the benefit of FMT on CDI in cancer patients has been confirmed by clinical studies and case reports. Hefazi et al. investigated the influence of FMT for recurrent CDI in 23 cancer patients (mainly hematologic cancer) receiving cancer chemotherapeutic agents. It is compelling to observe that the effective rate was 86% without serious adverse reactions or infectious complications.122 Kelly et al. analyzed 80 immunocompromised patients who underwent FMT, and found that no infectious complications resulted from FMT.123 In addition, several clinical trials have been conducted and published about the successful utilization of FMT for diarrhea caused by Clostridium difficile in patients with T‐cell lymphocytic leukemia124 or B‐cell lymphoma.125, 126 Hematopoietic stem cell transplantation (HSCT) is the most effective and promising procedure for treating hematological malignancy. To our knowledge, the first case of successful application of FMT for severe CDI that was refractory to conventional treatment with antibiotics in an HSCT patient was reported in 2012.127 Then two simple case reports were published about FMT as the management of CDI refractory to conventional therapy,128, 129 showing that this approach is safe and effective in CDI after HSCT without infectious complications and other adverse effects while conventional therapy fails. The first case that before preparing for HSCT, FMT effectively solved the problem of pathogenic bacteria infection was reported in 2017. A male patient suffered from Philadelphia‐positive acute lymphoblastic leukemia and developed a severe infection (β‐lactamase‐producing E. coli, Clostridium difficile and carbapenemase‐producing Enterobacteriaceae) before preparing for HSCT. After receiving FMT, his infection symptoms improved.130
Radiation enteritis
Radiotherapy is one of the most successful cancer therapies, but it may give rise to severe tissue damage that limits its use. Small intestine epithelium has high sensitivity to radiation and is the major site of radiation‐induced injury due to frequent intestinal epithelial turnover.131 A shift in intestinal microbiota composition after radiotherapy was observed in mice.131, 132 FMT from irradiated mice to germ‐free mice exposed to radiation resulted in more severe radiation damage, compared to mice transplanted with naïve microbiota.131 Interestingly, transplantation of fecal microbiota from healthy mice significantly alleviated radiation‐induced gastrointestinal syndrome and improved the survival rate of irradiated mice.132 Therefore, FMT might be employed as a radioprotector in tumor radiotherapy to improve the prognosis.132
Graft‐versus‐host disease
In allogeneic HSCT, donor T cells attack host healthy tissues, resulting in graft‐vs.‐host disease (GVHD), which is the main cause of mortality associated with HSCT.133 A clinical study identified intestinal bacterial diversity as a new independent prognostic factor in allogeneic HSCT.134 Allogeneic HSCT led to impaired gut microbiota with decreased diversity, and patients with higher intestinal diversity had a better prognosis and prolonged survival time than patients with lower diversity.134 Successfully applying FMT to stem cell transplantation patients with intestinal acute GVHD was first reported by Kakihana in 2016.135 Of the four patients who underwent FMT, three achieved complete response, and one had a partial response. Targeted restoration of gut microbiota via FMT may present a novel ecological strategy for managing GVHD.
Safety of FMT
FMT has been designated as a biological drug by the U.S. Food and Drug Administration, and doctors need to submit an investigational new drug application so as to obtain permission to implement FMT for treating any disease or condition other than recurrent CDI.136 Offering FMT treatment is requested strictly, while the majority of existing literature indicating that it is not allowed in clinics without ethics approval. Because of the unidentified composition and pathogenicity of fecal bacteria, the safety of FMT remains controversial.137 Moreover, as an emerging treatment, FMT has not been applied for a long time, so it lacks a long‐term safety investigation. Consequently, it is quite indispensable to closely follow the patients after FMT and carefully recorded their condition. Our team conducted a systematic review among 1,089 patients receiving FMT in a total of 50 selected publications and found that serious side effects, such as death and virus infections, were not rare.138 Two cases of norovirus gastroenteritis were reported in FMT recipients, though the donor was innocent of the transmission.139 Although there are some encouraging success cases and clinical studies, the quality of evidence of FMT in cancer management remains generally low. High quality clinical data are still required to further investigate whether could be employed as a safe therapeutic intervention against cancer.
Conclusion and Perspective
The role of the intestinal microbiota and its relationship to carcinogenesis provide an unprecedented opportunity to explore new diagnostic and therapeutic applications for cancers. Strategically FMT is the most direct method to change the composition of gut microbiota. Case reports and series reveal the potential of FMT in alleviating various cancers linked to intestinal dysbiosis and cancer treatment‐associated complications. Additionally, FMT could enhance the efficacy of cancer immunotherapy, thus remarkably affect clinical outcomes. However, FMT has not been clearly studied in cancer management and large‐sample randomized controlled studies are urgently required to delineate the validity of FMT, especially focus on the long‐term consequences. With the rapid progress of gut microbiology, FMT might become a promising therapeutic strategy for cancers in the near future.
Supporting information
Table S1 Overview of the association between antibiotics and risk of colorectal cancer
Table S2 Summary of gastrointestinal cancers associated with specific gut pathogens
Acknowledgements
This study is supported by the grants (81570478 and 81741075) from the National Natural Science Foundation of China, the grant (17JCYBJC24900) from Tianjin Research Program of Application Foundation and Advanced Technology of China.
Conflict of interest: None.
References
- 1. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016;164:337–40. [DOI] [PubMed] [Google Scholar]
- 2. Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang L, Cao H, Liu L, et al. Activation of epidermal growth factor receptor mediates mucin production stimulated by p40, a lactobacillus rhamnosus GG‐derived protein. J Biol Chem 2014;289:20234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 2016;16:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao H, Liu X, An Y, et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep 2017;7:10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med 2016;22:1079–89. [DOI] [PubMed] [Google Scholar]
- 7. Mima K, Nakagawa S, Sawayama H, et al. The microbiome and hepatobiliary‐pancreatic cancers. Cancer Lett 2017;402:9–15. [DOI] [PubMed] [Google Scholar]
- 8. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016;375:2369–79. [DOI] [PubMed] [Google Scholar]
- 9. Arthur JC, Perez‐Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer‐inducing activity of the microbiota. Science 2012;338:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan S. Potential role of Escherichia coli DNA mismatch repair proteins in colon cancer. Crit Rev Oncol Hematol 2015;96:475–82. [DOI] [PubMed] [Google Scholar]
- 11. Khan S, Zakariah M, Rolfo C, et al. Prediction of mycoplasma hominis proteins targeting in mitochondria and cytoplasm of host cells and their implication in prostate cancer etiology. Oncotarget 2017;8:30830–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan S, Zakariah M, Palaniappan S. Computational prediction of mycoplasma hominis proteins targeting in nucleus of host cell and their implication in prostate cancer etiology. Tumour Biol 2016;37:10805–13. [DOI] [PubMed] [Google Scholar]
- 13. Khan S, Imran A, Khan AA, et al. Systems biology approaches for the prediction of possible role of chlamydia pneumoniae proteins in the etiology of lung cancer. PLoS One 2016;11:e0148530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013;368:407–15. [DOI] [PubMed] [Google Scholar]
- 15. Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011;9:1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang F, Luo W, Shi Y, et al. Should we standardize the 1,700‐year‐old fecal microbiota transplantation. Am J Gastroenterol 2012;107:1755–6. [DOI] [PubMed] [Google Scholar]
- 17. Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958;44:854–9. [PubMed] [Google Scholar]
- 18. Schwan A, Sjölin S, Trottestam U, et al. Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet 1983;2:845. [DOI] [PubMed] [Google Scholar]
- 19. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013;108:478–98. quiz 499. [DOI] [PubMed] [Google Scholar]
- 20. Konturek PC, Haziri D, Brzozowski T, et al. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra‐gastrointestinal diseases. J Physiol Pharmacol 2015;66:483–91. [PubMed] [Google Scholar]
- 21. Xu MQ, Cao HL, Wang WQ, et al. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J Gastroenterol 2015;21:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costello SP, Soo W, Bryant RV, et al. Systematic review with meta‐analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther 2017;46:213–24. [DOI] [PubMed] [Google Scholar]
- 23. Mullish BH, Quraishi MN, Segal JP, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018;67:1920–41. [DOI] [PubMed] [Google Scholar]
- 24. Kelly CR, Kahn S, Kashyap P, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 2015;149:223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Costello SP, Conlon MA, Vuaran MS, et al. Faecal microbiota transplant for recurrent Clostridium difficile infection using long‐term frozen stool is effective: clinical efficacy and bacterial viability data. Aliment Pharmacol Ther 2015;42:1011–8. [DOI] [PubMed] [Google Scholar]
- 26. Cui B, Feng Q, Wang H, et al. Fecal microbiota transplantation through mid‐gut for refractory Crohn's disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol 2015;30:51–8. [DOI] [PubMed] [Google Scholar]
- 27. Jiang ZD, Ajami NJ, Petrosino JF, et al. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridum difficile infection ‐ fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther 2017;45:899–908. [DOI] [PubMed] [Google Scholar]
- 28. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol 2014;48:693–702. [DOI] [PubMed] [Google Scholar]
- 29. Kao D, Roach B, Silva M, et al. Effect of Oral capsule‐ vs colonoscopy‐delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2017;318:1985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Distrutti E, Monaldi L, Ricci P, et al. Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J Gastroenterol 2016;22:2219–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017;66:569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Terveer EM, van Beurden YH, Goorhuis A, et al. Faecal microbiota transplantation in clinical practice. Gut 2018;67:196. [DOI] [PubMed] [Google Scholar]
- 33. Shahanavaj K, Gil‐Bazo I, Castiglia M, et al. Cancer and the microbiome: potential applications as new tumor biomarker. Expert Rev Anticancer Ther 2015;15:317–30. [DOI] [PubMed] [Google Scholar]
- 34. Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol 2017;14:527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lam SY, Yu J, Wong SH, et al. The gastrointestinal microbiota and its role in oncogenesis. Best Pract Res Clin Gastroenterol 2017;31:607–18. [DOI] [PubMed] [Google Scholar]
- 36. Zitvogel L, Ma Y, Raoult D, et al. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science 2018;359:1366–70. [DOI] [PubMed] [Google Scholar]
- 37. Zeller G, Tap J, Voigt AY, et al. Potential of fecal microbiota for early‐stage detection of colorectal cancer. Mol Syst Biol 2014;10:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coker OO, Dai Z, Nie Y, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018;67:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wong SH, Zhao L, Zhang X, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ‐free and conventional mice. Gastroenterology 2017;153:1621–33.e6. [DOI] [PubMed] [Google Scholar]
- 40. Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 2016;65:1906–15. [DOI] [PubMed] [Google Scholar]
- 41. Dik VK, van Oijen MG, Smeets HM, et al. Frequent use of antibiotics is associated with colorectal cancer risk: results of a nested case‐control study. Dig Dis Sci 2016;61:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boursi B, Haynes K, Mamtani R, et al. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol Drug Saf 2015;24:534–42. [DOI] [PubMed] [Google Scholar]
- 43. Wang JL, Chang CH, Lin JW, et al. Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case‐control study in patients with type 2 diabetes mellitus. Int J Cancer 2014;135:956–67. [DOI] [PubMed] [Google Scholar]
- 44. Cao Y, Wu K, Mehta R, et al. Long‐term use of antibiotics and risk of colorectal adenoma. Gut 2018;67:672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boursi B, Mamtani R, Haynes K, et al. Recurrent antibiotic exposure may promote cancer formation‐‐another step in understanding the role of the human microbiota. Eur J Cancer 2015;51:2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaur K, Saxena A, Debnath I, et al. Antibiotic‐mediated bacteriome depletion in ApcMin/+ mice is associated with reduction in mucus‐producing goblet cells and increased colorectal cancer progression. Cancer Med 2018;7:2003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bullman S, Pedamallu CS, Sicinska E, et al. Analysis of fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017;358:1443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johnson CH, Dejea CM, Edler D, et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab 2015;21:891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ijssennagger N, Belzer C, Hooiveld GJ, et al. Gut microbiota facilitates dietary heme‐induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad Sci USA 2015;112:10038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schulz MD, Atay C, Heringer J, et al. High‐fat‐diet‐mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 2014;514:508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sethi V, Kurtom S, Tarique M, et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology 2018;155:33–7.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cao H, Xu M, Dong W, et al. Secondary bile acid‐induced dysbiosis promotes intestinal carcinogenesis. Int J Cancer 2017;140:2545–56. [DOI] [PubMed] [Google Scholar]
- 53. Gagnaire A, Nadel B, Raoult D, et al. Collateral damage: insights into bacterial mechanisms that predispose host cells to cancer. Nat Rev Microbiol 2017;15:109–28. [DOI] [PubMed] [Google Scholar]
- 54. Wang F, Meng W, Wang B, et al. Helicobacter pylori‐induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196–202. [DOI] [PubMed] [Google Scholar]
- 55. Odenbreit S, Püls J, Sedlmaier B, et al. Translocation of helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2000;287:1497–500. [DOI] [PubMed] [Google Scholar]
- 56. Yong X, Tang B, Li BS, et al. Helicobacter pylori virulence factor CagA promotes tumorigenesis of gastric cancer via multiple signaling pathways. Cell Commun Signal 2015;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ricci V. Relationship between VacA toxin and host cell autophagy in helicobacter pylori infection of the human stomach: A few answers, many questions. Toxins (Basel) 2016;8(7):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mashima H, Suzuki J, Hirayama T, et al. Involvement of vesicle‐associated membrane protein 7 in human gastric epithelial cell vacuolation induced by helicobacter pylori‐produced VacA. Infect Immun 2008;76:2296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Boleij A, Hechenbleikner EM, Goodwin AC, et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis 2015;60:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boleij A, Tjalsma H. The itinerary of streptococcus gallolyticus infection in patients with colonic malignant disease. Lancet Infect Dis 2013;13:719–24. [DOI] [PubMed] [Google Scholar]
- 61. Kumar R, Herold JL, Schady D, et al. Streptococcus gallolyticus subsp. gallolyticus promotes colorectal tumor development. PLoS Pathog 2017;13:e1006440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bonnet M, Buc E, Sauvanet P, et al. Colonization of the human gut by E. coli and colorectal cancer risk. Clin Cancer Res 2014;20:859–67. [DOI] [PubMed] [Google Scholar]
- 63. Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll‐like receptor 4 signaling to nuclear factor‐κB, and up‐regulating expression of MicroRNA‐21. Gastroenterology 2017;152:851–66.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E‐cadherin/β‐catenin signaling via its FadA adhesin. Cell Host Microbe 2013;14:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abed J, Emgård JE, Zamir G, et al. Fap2 mediates fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor‐expressed gal‐GalNAc. Cell Host Microbe 2016;20:215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes Chemoresistance to colorectal cancer by modulating autophagy. Cell 2017;170:548–63.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xie YH, Gao QY, Cai GX, et al. Fecal clostridium symbiosum for noninvasive detection of early and advanced colorectal cancer: test and validation studies. EBioMedicine 2017;25:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wong SH, Tny K, Chow TC, et al. Quantitation of faecal fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut 2017;66:1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013;342:971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Daillère R, Vétizou M, Waldschmitt N, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide‐induced therapeutic immunomodulatory effects. Immunity 2016;45:931–43. [DOI] [PubMed] [Google Scholar]
- 71. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti‐PD‐L1 efficacy. Science 2015;350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA‐4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Routy B, Le CE, Derosa L, et al. Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- 75. Whiteside SA, Razvi H, Dave S, et al. The microbiome of the urinary tract‐‐a role beyond infection. Nat Rev Urol 2015;12:81–90. [DOI] [PubMed] [Google Scholar]
- 76. Dias‐Jácome E, Libânio D, Borges‐Canha M, et al. Gastric microbiota and carcinogenesis: the role of non‐helicobacter pylori bacteria ‐ a systematic review. Rev Esp Enferm Dig 2016;108:530–40. [DOI] [PubMed] [Google Scholar]
- 77. Hsieh YY, Tung SY, Pan HY, et al. Increased abundance of clostridium and fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci Rep 2018;8:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ferreira RM, Pereira‐Marques J, Pinto‐Ribeiro I, et al. Gastric microbial community profiling reveals a dysbiotic cancer‐associated microbiota. Gut 2018;67:226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shah MA. Gastric cancer: the gastric microbiota ‐ bacterial diversity and implications. Nat Rev Gastroenterol Hepatol 2017;14:692–3. [DOI] [PubMed] [Google Scholar]
- 80. Doorakkers E, Lagergren J, Engstrand L, et al. Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a Western population. Gut 2018;67:2092–2096. [DOI] [PubMed] [Google Scholar]
- 81. Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of Metachronous gastric cancer. N Engl J Med 2018;378:1085–95. [DOI] [PubMed] [Google Scholar]
- 82. Toprak NU, Yagci A, Gulluoglu BM, et al. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect 2006;12:782–6. [DOI] [PubMed] [Google Scholar]
- 83. Wu S, Powell J, Mathioudakis N, et al. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin‐8 through mitogen‐activated protein kinases and a tyrosine kinase‐regulated nuclear factor‐kappaB pathway. Infect Immun 2004;72:5832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 2009;15:1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tsoi H, Esh C, Zhang X, et al. Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in mice. Gastroenterology 2017;152:1419–33.e5. [DOI] [PubMed] [Google Scholar]
- 86. Yu J, Feng Q, Wong SH, et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non‐invasive biomarkers for colorectal cancer. Gut 2017;66:70–8. [DOI] [PubMed] [Google Scholar]
- 87. Chen ZF, Ai LY, Wang JL, et al. Probiotics clostridium butyricum and Bacillus subtilis ameliorate intestinal tumorigenesis. Future Microbiol 2015;10:1433–45. [DOI] [PubMed] [Google Scholar]
- 88. Jacouton E, Chain F, Sokol H, et al. Probiotic strain lactobacillus casei BL23 prevents colitis‐associated colorectal cancer. Front Immunol 2017;8:1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang JW, Du P, Gao J, et al. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am J Med Sci 2012;343:199–205. [DOI] [PubMed] [Google Scholar]
- 90. Liang S, Xu L, Zhang D, et al. Effect of probiotics on small intestinal bacterial overgrowth in patients with gastric and colorectal cancer. Turk J Gastroenterol 2016;27:227–32. [DOI] [PubMed] [Google Scholar]
- 91. Rosshart SP, Vassallo BG, Angeletti D, et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell 2017;171:1015–28.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Malhi H, Camilleri M. Modulating bile acid pathways and TGR5 receptors for treating liver and GI diseases. Curr Opin Pharmacol 2017;37:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ma C, Han M, Heinrich B, et al. Gut microbiome‐mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018;360(6391):eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Segura‐López FK, Güitrón‐Cantú A, Torres J. Association between helicobacter spp. infections and hepatobiliary malignancies: a review. World J Gastroenterol 2015;21:1414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. De Minicis S, Rychlicki C, Agostinelli L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology 2014;59:1738–49. [DOI] [PubMed] [Google Scholar]
- 96. Llopis M, Cassard AM, Wrzosek L, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016;65:830–9. [DOI] [PubMed] [Google Scholar]
- 97. Qin C, Zhang H, Zhao L, et al. Microbiota transplantation reveals beneficial impact of berberine on hepatotoxicity by improving gut homeostasis. Sci China Life Sci 2017. [in press]. [DOI] [PubMed] [Google Scholar]
- 98. Dhiman RK, Rana B, Agrawal S, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 2014;147:1327–37.e3. [DOI] [PubMed] [Google Scholar]
- 99. Han SH, Suk KT, Kim DJ, et al. Effects of probiotics (cultured lactobacillus subtilis/streptococcus faecium) in the treatment of alcoholic hepatitis: randomized‐controlled multicenter study. Eur J Gastroenterol Hepatol 2015;27:1300–6. [DOI] [PubMed] [Google Scholar]
- 100. Zhou D, Pan Q, Shen F, et al. Total fecal microbiota transplantation alleviates high‐fat diet‐induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep 2017;7:1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ferrere G, Wrzosek L, Cailleux F, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol‐induced liver injury in mice. J Hepatol 2017;66:806–15. [DOI] [PubMed] [Google Scholar]
- 102. Philips CA, Pande A, Shasthry SM, et al. Healthy donor fecal microbiota transplantation in steroid‐ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol 2017;15:600–2. [DOI] [PubMed] [Google Scholar]
- 103. Philips CA, Phadke N, Ganesan K, et al. Healthy donor faecal transplant for corticosteroid nonresponsive severe alcoholic hepatitis. BMJ Case Rep 2017;2017:bcr‐2017–222310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ren YD, Ye ZS, Yang LZ, et al. Fecal microbiota transplantation induces hepatitis B virus e‐antigen (HBeAg) clearance in patients with positive HBeAg after long‐term antiviral therapy. Hepatology 2017;65:1765–8. [DOI] [PubMed] [Google Scholar]
- 105. Bajaj JS, Kakiyama G, Savidge T, et al. Antibiotic‐associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology 2018;68(4):1549–1558. [DOI] [PubMed] [Google Scholar]
- 106. Wang WW, Zhang Y, Huang XB, et al. Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride‐induced acute hepatic dysfunction. World J Gastroenterol 2017;23:6983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kao D, Roach B, Park H, et al. Fecal microbiota transplantation in the management of hepatic encephalopathy. Hepatology 2016;63:339–40. [DOI] [PubMed] [Google Scholar]
- 108. Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 2017;66:1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Michaud DS. Role of bacterial infections in pancreatic cancer. Carcinogenesis 2013;34:2193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ochi A, Nguyen AH, Bedrosian AS, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med 2012;209:1671–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Geller LT, Barzily‐Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017;357:1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fan X, Alekseyenko AV, Wu J, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population‐based nested case‐control study. Gut 2018;67:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mitsuhashi K, Nosho K, Sukawa Y, et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015;6:7209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pushalkar S, Hundeyin M, Daley D, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov 2018;8:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hill MJ, Goddard P, Williams RE. Gut bacteria and aetiology of cancer of the breast. Lancet 1971;2:472–3. [DOI] [PubMed] [Google Scholar]
- 116. Goedert JJ, Hua X, Bielecka A, et al. Postmenopausal breast cancer and oestrogen associations with the IgA‐coated and IgA‐noncoated faecal microbiota. Br J Cancer 2018;118:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yang J, Tan Q, Fu Q, et al. Gastrointestinal microbiome and breast cancer: correlations, mechanisms and potential clinical implications. Breast Cancer 2017;24:220–8. [DOI] [PubMed] [Google Scholar]
- 118. Maroof H, Hassan ZM, Mobarez AM, et al. Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. J Clin Immunol 2012;32:1353–9. [DOI] [PubMed] [Google Scholar]
- 119. Frankel AE, Coughlin LA, Kim J, et al. Metagenomic shotgun sequencing and unbiased Metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia 2017;19:848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mullard A. Oncologists tap the microbiome in bid to improve immunotherapy outcomes. Nat Rev Drug Discov 2018;17:153–5. [DOI] [PubMed] [Google Scholar]
- 121. Kim JS, Ward KK, Shah NR, et al. Excess risk of Clostridium difficile infection in ovarian cancer is related to exposure to broad‐spectrum antibiotics. Support Care Cancer 2013;21:3103–7. [DOI] [PubMed] [Google Scholar]
- 122. Hefazi M, Patnaik MM, Hogan WJ, et al. Safety and efficacy of fecal microbiota transplant for recurrent Clostridium difficile infection in patients with cancer treated with cytotoxic chemotherapy: a single‐institution retrospective case series. Mayo Clin Proc 2017;92:1617–24. [DOI] [PubMed] [Google Scholar]
- 123. Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 2014;109:1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Blackburn LM, Bales A, Caldwell M, et al. Fecal microbiota transplantation in patients with cancer undergoing treatment. Clin J Oncol Nurs 2015;19:111–4. [DOI] [PubMed] [Google Scholar]
- 125. Trubiano JA, George A, Barnett J, et al. A different kind of "allogeneic transplant": successful fecal microbiota transplant for recurrent and refractory Clostridium difficile infection in a patient with relapsed aggressive B‐cell lymphoma. Leuk Lymphoma 2015;56:512–4. [DOI] [PubMed] [Google Scholar]
- 126. Mittal C, Miller N, Meighani A, et al. Fecal microbiota transplant for recurrent Clostridium difficile infection after peripheral autologous stem cell transplant for diffuse large B‐cell lymphoma. Bone Marrow Transplant 2015;50:1010. [DOI] [PubMed] [Google Scholar]
- 127. Neemann K, Eichele DD, Smith PW, et al. Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transpl Infect Dis 2012;14:E161–5. [DOI] [PubMed] [Google Scholar]
- 128. de Castro CG, Ganc AJ, Ganc RL, et al. Fecal microbiota transplant after hematopoietic SCT: report of a successful case. Bone Marrow Transplant 2015;50:145. [DOI] [PubMed] [Google Scholar]
- 129. Webb BJ, Brunner A, Ford CD, et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis 2016;18:628–33. [DOI] [PubMed] [Google Scholar]
- 130. Innes AJ, Mullish BH, Fernando F, et al. Faecal microbiota transplant: a novel biological approach to extensively drug‐resistant organism‐related non‐relapse mortality. Bone Marrow Transplant 2017;52:1452–4. [DOI] [PubMed] [Google Scholar]
- 131. Gerassy‐Vainberg S, Blatt A, Danin‐Poleg Y, et al. Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut 2018;67:97–107. [DOI] [PubMed] [Google Scholar]
- 132. Cui M, Xiao H, Li Y, et al. Faecal microbiota transplantation protects against radiation‐induced toxicity. EMBO Mol Med 2017;9:448–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Staffas A, Dsm B, van den Brink MR. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft‐versus‐host disease. Blood 2017;129:927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014;124:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kakihana K, Fujioka Y, Suda W, et al. Fecal microbiota transplantation for patients with steroid‐resistant acute graft‐versus‐host disease of the gut. Blood 2016;128:2083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Amirtha T. MICROBIOME RESEARCH. Banking on stool despite an uncertain future. Science 2016;352:1261–2. [DOI] [PubMed] [Google Scholar]
- 137. Olesen SW, Leier MM, Alm EJ, et al. Searching for superstool: maximizing the therapeutic potential of FMT. Nat Rev Gastroenterol Hepatol 2018;15:387–8. [DOI] [PubMed] [Google Scholar]
- 138. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One 2016;11:e0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol 2013;108:1367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Overview of the association between antibiotics and risk of colorectal cancer
Table S2 Summary of gastrointestinal cancers associated with specific gut pathogens


