Abstract
The blistering disease recessive dystrophic epidermolysis bullosa (RDEB) is caused by mutations in the gene encoding collagen VII (COL7), which forms anchoring fibrils that attach the epidermis to the dermis. Cutaneous gene therapy to restore COL7 expression in RDEB patient cells has been proposed, and cultured epithelial autograft containing COL7-modified keratinocytes was previously tested in clinical trials. Because COL7 in normal skin is expressed in both fibroblasts and keratinocytes, cutaneous gene therapy using a bilayer skin substitute may enable faster restoration of anchoring fibrils. Hypothetically, COL7 expression in either dermal fibroblasts or epidermal keratinocytes might be sufficient for functional anchoring fibril formation in a bilayer skin substitute. To test this, engineered skin substitutes (ESS) were prepared using four combinations of normal + RDEB cells: (1) RDEB fibroblasts + RDEB keratinocytes; (2) RDEB fibroblasts + normal keratinocytes; (3) normal fibroblasts + RDEB keratinocytes; and (4) normal fibroblasts + normal keratinocytes. ESS were incubated in vitro for 2 weeks prior to grafting to full-thickness wounds in immunodeficient mice. Biopsies were analyzed in vitro and at 1, 2, or 3 weeks after grafting. COL7 was undetectable in ESS prepared using all RDEB cells (group 1), and macroscopic blistering was observed by 2 weeks after grafting in ESS containing RDEB cells. COL7 was expressed, in vitro and in vivo, in ESS prepared using combinations of normal + RDEB cells (groups 2 and 3) or all normal cells (group 4). However, transmission electron microscopy revealed structurally normal anchoring fibrils, in vitro and by week 2 in vivo, only in ESS prepared using all normal cells (group 4). The results suggest that although COL7 protein is produced in engineered skin when cells in only one layer express the COL7 gene, formation of structurally normal anchoring fibrils appears to require expression of COL7 in both dermal fibroblasts and epidermal keratinocytes.
Keywords: recessive dystrophic epidermolysis bullosa, engineered skin substitute, collagen VII, anchoring fibril, basement membrane, fibroblast, keratinocyte
Introduction
The inherited blistering disease recessive dystrophic epidermolysis bullosa (RDEB), characterized by lack of adhesion of the epidermis to the dermis, is caused by mutations in the collagen VII gene (COL7A1)1. Type VII collagen protein (COL7) is secreted by both fibroblasts and keratinocytes to form anchoring fibrils that attach the dermis to the basement membrane zone1. Absence of functional anchoring fibrils results in fragile skin that easily shears and blisters. RDEB affects approximately 25,000 people worldwide, and is associated with a 70% risk of death by age 40 due to manifestations of the disease, including metastatic squamous cell carcinoma (SCC)2. The absence of collagen VII has been linked to changes in the extracellular microenvironment that promote development of SCC3. Thus, there is an urgent need to develop therapies that restore collagen VII expression to reduce skin blistering as well as decrease SCC incidence in patients with RDEB.
There is currently no cure for RDEB, and no truly effective treatment beyond management of symptoms. The demand for long-term solutions has fueled investigations into advanced therapies, including cell and protein-based therapies, as well as gene therapies2,4,5. Multiple preclinical studies exploring gene therapy approaches for epidermolysis bullosa have been published, and a limited number of clinical trials have been undertaken6–8. In an ex vivo gene therapy trial, retroviral COL7A1 gene transfer was used for genetic modification of autologous keratinocytes, which were transplanted to RDEB patient wounds as cultured epithelial autograft (CEA)8. Results in four RDEB patients, who were each treated with six COL7A1-modified epidermal grafts, indicated that all 24 grafts were well tolerated and no serious adverse reactions were observed8. Collagen VII expression and improved healing were observed in a majority of grafts at 3 months after transplantation, but the response was variable and declined over the 1-year observation period8. Coincident with this, anchoring fibrils were observed in 71% of biopsies at 3 months, but only in 33% of biopsies at 6 months and 25% of biopsies at 12 months8. It was speculated that the decline in healing over time may have resulted from a reduced number of stem cells in the patient skin biopsies, or that the corrected cells in the grafts were out-competed by uncorrected cells in the wound bed8,9.
A recent report described successful cutaneous gene therapy for a 7-year-old patient with junctional epidermolysis bullosa (JEB)7. The patient had a homozygous mutation in the gene encoding laminin β3 (LAMB3), which is a subunit of laminin-332, an essential part of the dermal–epidermal junction that links anchoring fibrils to the basement membrane. Autologous keratinocytes, modified by retroviral gene transfer to express a wild type LAMB3 transgene, were cultured on fibrin and transplanted to wounds as epidermal sheets. Grafting of the genetically modified CEA resulted in stable closure of wounds covering approximately 80% of the patient’s total body surface area (TBSA). A difference between the results observed in this study7, which involved transplantation of LAMB3-modified keratinocytes for wound closure in a patient with JEB, and the previous study involving grafting of COL7A1-modified keratinocytes in RDEB patients8, may be due to the fact that COL7A1 is expressed by both keratinocytes and fibroblasts whereas LAMB3 is expressed only by keratinocytes. Long-term wound closure in RDEB patients treated with COL7A1-modified CEA may be hindered by absence of dermal fibroblasts expressing COL7A1. Hypothetically, expression of COL7A1 in both fibroblasts and keratinocytes may be required to facilitate early production of anchoring fibrils and stable wound closure. This could be achieved using a bilayer dermal–epidermal skin substitute.
In preclinical studies, correction of a COL7A1 mutation in RDEB patient-derived keratinocytes and fibroblasts was achieved using CRISPR-based genome editing10. Although COL7A1 gene expression levels in edited fibroblasts and keratinocytes were 15.7% and 11.0% of normal levels, respectively, anchoring fibrils were observed in skin substitutes containing corrected cells after transplantation to immunodeficient mice. However, the skin substitutes in that study were initially transplanted under skin flaps in mice, rather than being grafted orthotopically to full-thickness wounds10,11. Although this enabled formation of anchoring fibrils by 1 month after “deflapping,” this type of surgical procedure would not be easily translated to RDEB patients. The current study utilized engineered skin substitutes (ESS), a model that was previously evaluated in clinical trials as an adjunctive treatment for patients with greater than 50% TBSA burns12–14. ESS containing autologous fibroblasts and keratinocytes were shown to provide stable, long-term wound closure in burn patients, with minimal need for regrafting and negligible scarring14. This bilayer, organotypic skin model permits paracrine interactions between fibroblasts and keratinocytes that promote rapid tissue maturation in vitro and long-term graft stability after transplantation, including establishment of a basal stem cell compartment, basement membrane deposition, and formation of anchoring fibrils15–18. The current study was undertaken to determine whether COL7 protein expression is required in one or both layers of ESS for anchoring fibril formation and suppression of blistering.
Materials and Methods
Isolation of Primary Fibroblasts and Keratinocytes
Skin samples used for establishing primary cultures were classified as “discard skin” by the attending surgeons and were de-identified prior to delivery to the lab for cell isolation. Normal skin was from a healthy 24-year-old plastic surgery patient, and RDEB skin was from a 24-year-old RDEB patient. The University of Cincinnati Institutional Review Board determined that collection of de-identified discard skin samples did not constitute human subjects research and was therefore exempt from requirements for informed consent. COL7A1 sequencing was performed by the Laboratory of Genetics and Genomics at Cincinnati Children’s Hospital Medical Center to confirm the clinical diagnosis of RDEB (data not shown).
Primary fibroblasts and keratinocytes were isolated from skin and cultured as detailed elsewhere19–21, with slight modifications. Overnight incubation at 4°C in Dispase (Roche Life Sciences, Indianapolis, IN, USA) was used to separate epidermis and dermis for the normal skin sample; this step was omitted for the RDEB skin sample, as the epidermis and dermis were easily separated without enzymatic digestion. Fibroblasts were cultured in Gibco™ DMEM (Low Glucose; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10 ng/ml epidermal growth factor (EGF; Peprotech, Rocky Hill, NJ, USA), 5 µg/ml human insulin (Sigma-Aldrich; St. Louis, MO, USA), 0.5 mg/ml hydrocortisone (Sigma-Aldrich), 0.1 mM ascorbic acid-2-phosphate (AA2P; Sigma-Aldrich), 4% fetal bovine serum (FBS; Thermo Fisher Scientific), and 1× penicillin-streptomycin-fungizone (PSF; Thermo Fisher Scientific). Keratinocyte medium consisted of modified MCDB153 prepared in-house21 with 0.06 mM calcium chloride and supplemented with 0.2% bovine pituitary extract (Hammond Cell Tech, Windsor, CA, USA), 1 ng/ml EGF, 5 µg/ml human insulin, 0.5 mg/ml hydrocortisone, and 1× PSF. Flasks coated with collagen (Coating Matrix; Thermo Fisher Scientific) were used to establish the primary keratinocyte cultures, but were not used after keratinocytes were passaged; feeder cells were not used. All cells were harvested when they reached 80–90% confluence and were cryopreserved in their respective growth medium containing 10% dimethyl sulfoxide and 20% FBS using a controlled rate freezer. Cells were recovered from liquid nitrogen storage using the same culture medium as for initial culture except that the calcium chloride concentration of the keratinocyte medium was increased to 0.2 mM. Cells were expanded through one additional passage prior to preparation of engineered skin.
Preparation of ESS
ESS were prepared essentially as previously described21. Briefly, fibroblasts (0.5 × 106/cm2) were inoculated onto sterile collagen-glycosaminoglycan scaffolds supported at the air–liquid interface using polyvinyl acetal sponges. Keratinocytes (1.0 × 106/cm2) were inoculated onto the dermal substrates 24 h later and ESS were transferred to cotton pads supported by perforated stainless steel platforms for incubation at the air–liquid interface for 2 weeks (37°C, 5% CO2). Culture medium for ESS consisted of DMEM/F12 (Sigma-Aldrich) supplemented with 1 mM strontium chloride, 0.3% FBS, 1× ITS Supplement (Sigma-Aldrich), 10 µg/ml linoleic acid, 0.1 mM AA2P, 20 pM triiodothyronine, 0.5 µg/ml hydrocortisone, 5 ng/ml keratinocyte growth factor (Peprotech), 1 ng/ml basic fibroblast growth factor (Peprotech), and 1× PSF19. Four groups of grafts were prepared: group 1, RDEB fibroblasts and RDEB keratinocytes; group 2, RDEB fibroblasts and normal keratinocytes; group 3, normal fibroblasts and RDEB keratinocytes; and group 4, normal fibroblasts and normal keratinocytes.
Grafting to Mice and Collection of Tissue Samples
Animal studies were performed with the approval of the University of Cincinnati (UC) Institutional Animal Care and Use Committee (IACUC) and in accordance with UC IACUC guidelines. Immunodeficient mice (NIH-III-nude strain; Charles River Laboratories, Wilmington, MA, USA) were used (n = 32) to enable engraftment of ESS containing human cells. A full-thickness wound (2 cm × 2 cm) was prepared on the flank of each mouse, leaving the panniculus carnosus layer intact. ESS were cut to 2 cm × 2 cm squares and were sutured to the wounds with an overlying piece of N-terface non-adherent dressing (Winfield Laboratories, Richardson, TX, USA). Grafts were dressed with gauze using tie-over stents; gauze was coated with antimicrobial ointment consisting of equal parts Nystatin, Mupirocin, and Neosporin (Johnson & Johnson Consumer Companies, Inc., New Brunswick, NJ, USA). Dressings were covered with Tegaderm Transparent Film Dressing (3M, St. Paul, MN, USA) and mice were wrapped with Coban self-adherent bandages (3M). Dressings were removed at 2 weeks after surgery; mice that were not euthanized until 3 weeks after grafting remained without dressings from week 2 to week 3. Eight mice were grafted for each group. Four mice died during the study period (two each in groups 3 and 4) and were excluded from the analysis. Two mice per group were euthanized at week 1. For weeks 2 and 3, three mice/group were sacrificed for groups 1 and 2, and two mice/group for groups 3 and 4. ESS were excised and multiple biopsies were collected for processing for both histological sections and transmission electron microscopy (TEM). Biopsies fixed in 10% formalin were processed, paraffin-embedded, sectioned, and stained with Tango stain (catalog #852; Anatech Ltd., Battle Creek, MI, USA) by the Shriners Hospitals for Children – Cincinnati Histology Special Shared Facility. For cryosections, biopsies were embedded frozen using OCT Compound (Fisher HealthCare, Pittsburgh, PA, USA), and for TEM, biopsies were fixed in Karnovsky’s fixative (Electron Microscopy Sciences, Hatfield, PA, USA).
Quantitative Analysis of Blister Formation
To analyze blistering, multiple non-overlapping microscopic fields (2–9 per section) were photographed at 10× magnification using a Nikon Microphot-FXA microscope (Nikon, Melville, NY, USA) and Spot Digital Camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA). For each image, the total length of the dermal–epidermal junction and the total length of any blisters present at the dermal–epidermal junction were measured using Nikon Elements software. Percent blistering was calculated using the formula: blister length/total dermal–epidermal junction length × 100. Statistical analysis was performed using SigmaStat version 13.0 (Systat Software, Inc., San Jose, CA, USA). Analyses of group differences at each time point were performed using One Way Analysis of Variance on Ranks, and pairwise comparisons at each time point were performed using the Rank Sum test; these tests were used because the data were not normally distributed. Differences were considered statistically significant at P < 0.05.
Immunohistochemistry
Localization of specific antigens in cryosections was performed by immunohistochemistry using routine procedures. COL7 detection utilized a rabbit polyclonal antibody directed against full-length human collagen VII (catalog #ab93350; Abcam, Cambridge, MA, USA), diluted 1:50 and incubated with sections overnight at 4°C, followed by a chicken anti-Rabbit IgG (H+L) antibody labeled with Alexa Flour 488 (catalog #A21441; Thermo Fisher Scientific), diluted 1:400 and incubated for 1 h at room temperature. A second antibody specific for human COL7 was also used: a mouse monoclonal antibody against human collagen VII (clone LH7.2; catalog #MAB1345; Sigma-Aldrich), which is directed against the N-terminal portion of the protein22, diluted 1:400 and incubated with sections for 30 min at room temperature. The Mouse on Mouse (M.O.M.) Basic Kit (catalog # BMK-2202; Vector Laboratories, Burlingame, CA, USA) was used to block background staining prior to use of a secondary anti-mouse antibody in the in vivo ESS samples, which were excised from mice. This was followed by incubation with a donkey anti-mouse IgG (H+L) antibody labeled with Alexa Fluor 594 (catalog #A21203, Thermo Fisher Scientific), diluted 1:400 and incubated for 30 min at room temperature. Laminin detection was performed using a rat monoclonal antibody (catalog #MBS570086; MyBioSource, San Diego, CA, USA), diluted 1:200 and incubated with sections for 1 h at room temperature, followed by a donkey anti-rat IgG (H+L) antibody labeled with Alexa Fluor 594 (catalog #SA5-10028, Thermo Fisher Scientific), diluted 1:400 and incubated for 1 h at room temperature. Collagen IV was detected in sections using a rabbit polyclonal antibody (catalog #507; Yo Proteins, Rönninge, Sweden), diluted 1:50 and incubated overnight at 4°C, and human E-cadherin was detected using a rabbit monoclonal antibody (catalog #NB110-56937; Novus Biologicals, Centennial, CO, USA), diluted 1:100 and incubated overnight at 4°C. Both were followed by the chicken anti-Rabbit Alexa Fluor 488 antibody. Human keratinocytes in ESS were detected using a mouse monoclonal antibody against human leukocyte antigen (HLA) ABC (clone W6/32 HLK; catalog #CLHLA-01F; Cedarlane Laboratories, Burlington, NC, USA) directly conjugated to fluorescein isothiocyanate; the antibody was diluted 1:50 and sections were incubated overnight at 4°C. This anti-HLA-ABC antibody is directed against a common, non-polymorphic epitope of the HLA-A, B, and C loci, according to information provided by the manufacturer, and was therefore used to detect human cells in ESS after grafting to mice. Vectashield Antifade Mounting Medium with 4′,6-diamidino-2-phenylindole (DAPI; catalog #H-1200, Vector Laboratories) was used to mount coverslips and counterstain nuclei. Sections were viewed and photographed with an Eclipse 90i microscope equipped with a DS-Ri1 Digital Microscope Camera (Nikon Instruments Inc.). Z-stacking was used to improve depth of field of digital images, and all images for a given antibody were collected using identical settings for each tissue section.
TEM
Biopsies of ESS were incubated overnight at 4°C in Karnovsky’s fixative (Electron Microscopy Sciences) and then transferred to sodium cacodylate buffer, 0.2 M, pH 7.4 (Electron Microscopy Sciences). Tissue was post-fixed in 1% osmium tetroxide containing 1.5% potassium ferrocyanide. Following dehydration, tissues were embedded in Spurr resin (Sigma-Aldrich), sectioned using a RMC-MT6000XL ultramicrotome (RMC Inc., Tucson, AZ, USA), and stained with uranyl acetate and lead citrate. Sections were viewed and selected images were digitally photographed using a JEM-1230 transmission electron microscope (JEOL USA Inc., Peabody, MA, USA).
Results
Fibroblasts and keratinocytes isolated from RDEB patient skin and were cultured using the same media formulations and culture conditions optimized in our laboratory for normal fibroblasts and keratinocytes. The RDEB fibroblasts did not exhibit any overt morphological abnormalities. However, unlike normal keratinocytes, the RDEB keratinocytes did not form colonies with tightly associated cells (data not shown)23.
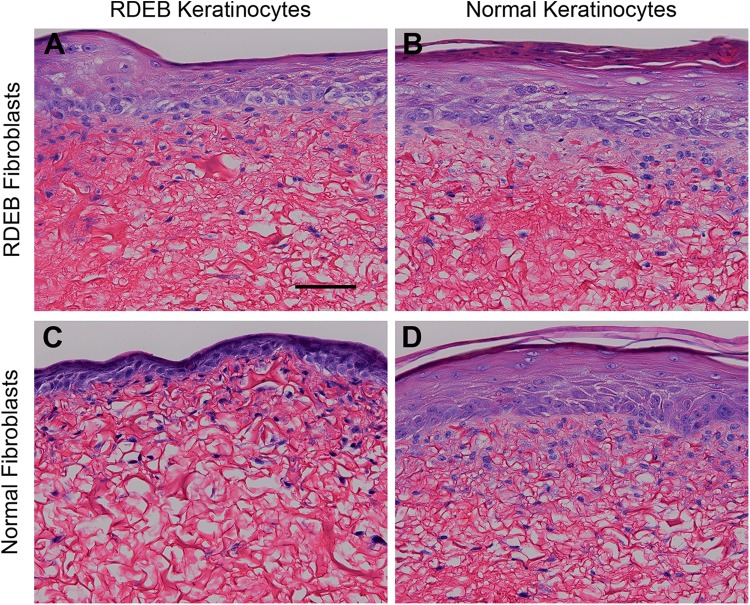
ESS prepared with all RDEB-derived cells (group 1) or combinations of RDEB or normal fibroblasts and keratinocytes (groups 2 and 3) were morphologically similar to ESS prepared with all normal cells (group 4). ESS in all groups displayed a fibroblast-populated scaffold and a stratified epidermal layer with a cornified surface (Fig. 1). Although the epidermis of ESS in group 3 (normal fibroblasts and RDEB keratinocytes) appeared slightly thinner than ESS in other groups, differences in thickness were variable and not statistically significant (data not shown).
Figure 1.
Histological sections of engineered skin substitutes (ESS). Shown are Tango-stained histological sections of ESS prepared with RDEB fibroblasts and RDEB keratinocytes (group 1; A), RDEB fibroblasts and normal keratinocytes (group 2; B), normal fibroblasts and RDEB keratinocytes (group 3; C), and normal fibroblasts and normal keratinocytes (group 4; D). Sections are oriented with the epidermis at the top of each photo. Scale bar in A (100 µm) is for all panels.
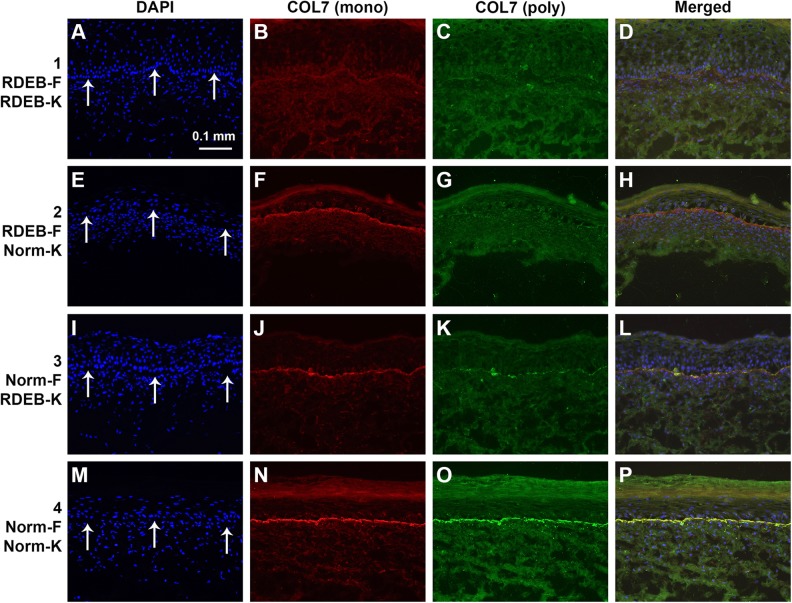
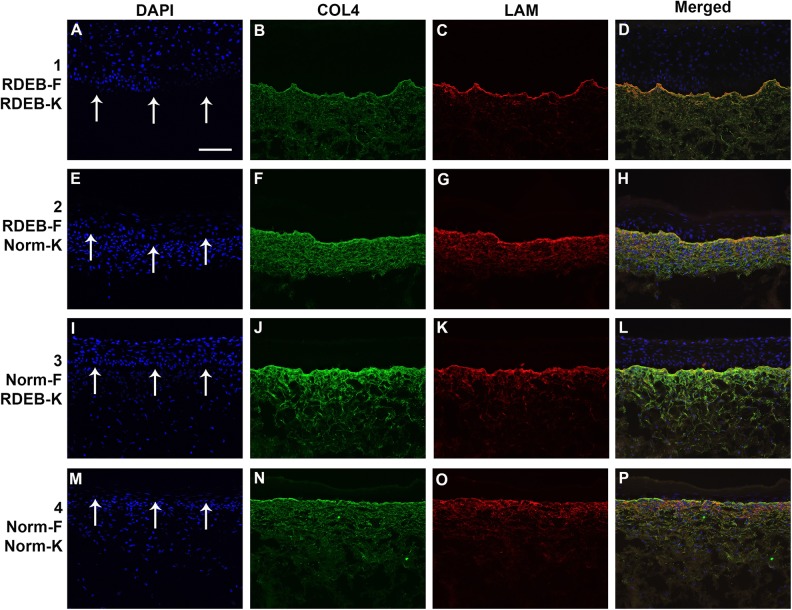
COL7 expression was undetectable by immunohistochemistry in sections of ESS prepared with all RDEB-derived cells (group 1) analyzed at the end of the in vitro culture period (Fig. 2). COL7 was detected at the dermal–epidermal junction of ESS in vitro in groups 2 and 3, prepared with combinations of normal or RDEB fibroblasts and keratinocytes, but the highest COL7 levels were observed in ESS of group 4, prepared with all normal cells (Fig. 2). Basement membrane components collagen IV (COL4) and laminin (LAM) were detected in vitro in ESS of all four groups, with no significant difference in protein levels among groups (Fig. 3).
Figure 2.
Localization of collagen VII (COL7) in sections of engineered skin substitutes (ESS) in vitro. Shown are sections of ESS prepared with RDEB fibroblasts and RDEB keratinocytes (group 1; A-D), RDEB fibroblasts and normal keratinocytes (group 2; E–H), normal fibroblasts and RDEB keratinocytes (group 3; I–L), and normal fibroblasts and normal keratinocytes (group 4; M–P). Note that each row depicts a single section photographed using different fluorescent illumination. Immunohistochemistry was performed to localize COL7 using two different antibodies: a monoclonal antibody (B, F, J, N; red), specific for human COL7, and a polyclonal antibody (C, G, K, O; green) that cross-reacts with mouse COL7. Nuclei were counterstained using 4′,6-diamidino-2-phenylindole (DAPI; A, E, I, M; blue). Arrows in A, E, I, and M indicate location of dermal–epidermal junction. Scale bar in A (100 µm) is same for all panels.
Figure 3.
Deposition of basement membrane in ESS in vitro. Shown are sections of ESS from week 2 in vitro. ESS were prepared with RDEB fibroblasts and keratinocytes (group 1; A–D), RDEB fibroblasts and normal keratinocytes (group 2; E–H), normal fibroblasts and RDEB keratinocytes (group 3; I–L), and normal fibroblasts and keratinocytes (group 4; M–P), as indicated. Note that each row depicts a single section photographed using different fluorescent illumination. Immunohistochemistry was performed to localize collagen IV (COL4; B, F, J, N; green) and laminin (LAM; C, G, K, O; red). Nuclei were counterstained using DAPI (A, E, I, M; blue). Arrows in A, E, I, and M indicate location of dermal–epidermal junction. Scale bar in A (100 µm) is same for all panels.
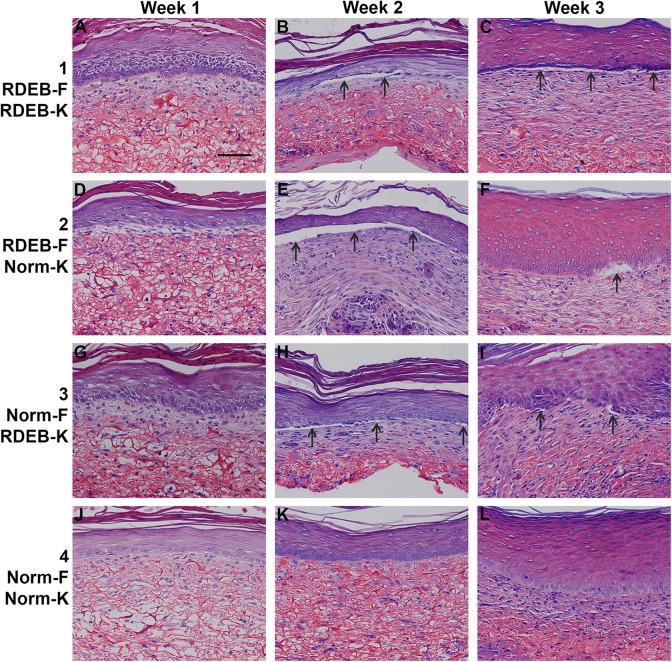
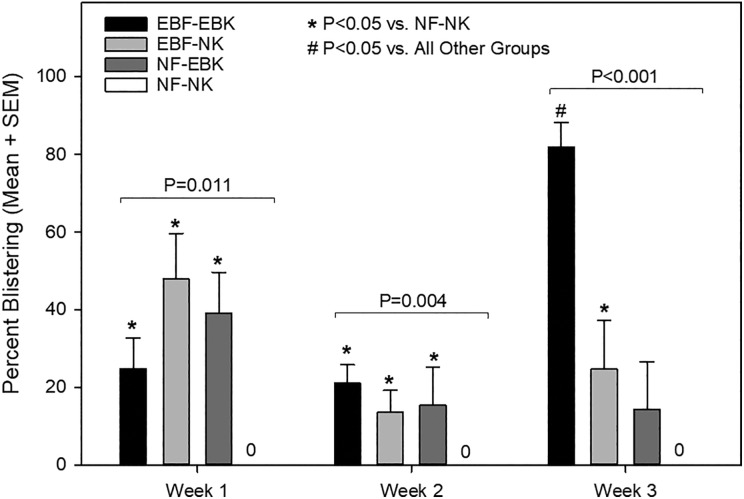
After transplantation to mice, ESS in all four groups displayed evidence of epidermal stratification and fibroblast proliferation (Fig. 4). After grafting, histological evidence of epidermal blistering was observed in groups 1–3, in ESS prepared with RDEB fibroblasts and/or keratinocytes; this was not observed in group 4 ESS prepared with all normal cells (Figs. 4 and 5). At 3 weeks after grafting, the most severe epidermal blistering was observed in ESS prepared with RDEB keratinocytes and RDEB fibroblasts (Figs. 4–5).
Figure 4.
Histological sections of engineered skin substitutes (ESS) in vivo. Shown are Tango-stained histological sections of ESS from week 1 (left; A, D, G, J), week 2 (center; B, E, H, K), and week 3 (right; C, F, I; L) in vivo. ESS were prepared with RDEB fibroblasts and keratinocytes (group 1; A–C), RDEB fibroblasts and normal keratinocytes (group 2; D–F), normal fibroblasts and RDEB keratinocytes (group 3; G–I), and normal fibroblasts and keratinocytes (group 4; J–L), as indicated. Arrows indicate examples of epidermal blistering in ESS prepared with RDEB fibroblasts and/or keratinocytes. Scale bar in A (100 µm) is same for all panels.
Figure 5.
Quantification of blistering in engineered skin substitutes (ESS) in vivo. ESS were prepared with RDEB fibroblasts (EBF) or normal fibroblasts (NF) and RDEB keratinocytes (EBK) or normal keratinocytes (NK) and blistering was evaluated at weeks 1, 2, and 3 after grafting to mice. Blistering values are based on the total length of blisters as a percent of total length of the dermal–epidermal junction in non-overlapping microscopic fields. Values plotted are means + standard error of the mean (SEM). No blistering was observed for grafts prepared with all normal cells (NF–NK); thus, these values are zero for each time point. Group differences at each time point were statistically significant and P-values are indicated. Significant pairwise differences are indicated by symbols (*, P < 0.05 vs. NF–NK; #, P < 0.05 vs. all other groups at the same time point).
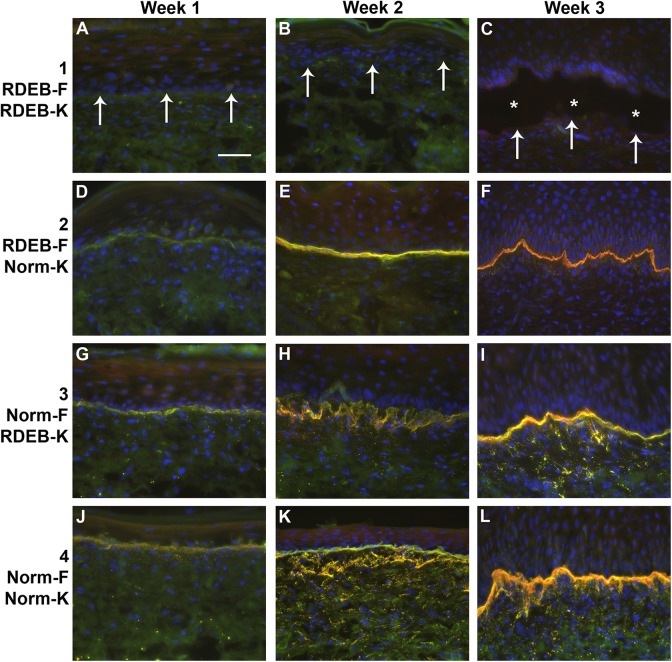
Expression of COL7 was detected in ESS in vivo in grafts prepared with any normal cells (groups 2–4); COL7 was undetectable in ESS prepared using both RDEB fibroblasts and keratinocytes (group 1). Levels of COL7 increased over time, from week 1 through week 3 after grafting, and appeared highest in grafts prepared with normal fibroblasts and keratinocytes (group 4) at 3 weeks after grafting (Fig. 6). Two antibodies were used for detection of human COL7 protein; a mouse monoclonal antibody, specific for human COL7, and a rabbit polyclonal antibody, which showed slight cross-reactivity with mouse COL7 (Fig. 6 and Supplemental Fig. S1). Immunohistochemistry was performed using control sections of human skin from the RDEB patient, normal human skin, and mouse skin, which demonstrated specificity of the monoclonal antibody for human COL7 (Supplemental Fig. S1).
Figure 6.
Localization of collagen VII (COL7) in engineered skin substitutes (ESS) in vivo. Immunohistochemistry was performed to localize COL7 using two different antibodies: a monoclonal antibody (red), specific for human COL7, and a polyclonal antibody (green) that cross-reacts with mouse COL7. Nuclei were counterstained using DAPI (blue). Merged fluorescent images are shown. Shown are sections of ESS from week 1 (left; A, D, G, J), week 2 (center; B, E, H, K), and week 3 (right; C, F, I, L) in vivo. ESS were prepared with RDEB fibroblasts and keratinocytes (group 1; A–C), RDEB fibroblasts and normal keratinocytes (group 2; D–F), normal fibroblasts and RDEB keratinocytes (group 3; G–I), and normal fibroblasts and keratinocytes (group 4; J–L), as indicated. Arrows (A–C) indicate locations of dermal–epidermal junctions, and asterisks (C) indicate epidermal blistering, in ESS prepared with all RDEB cells. Scale bar in A (50 µm) is same for all panels.
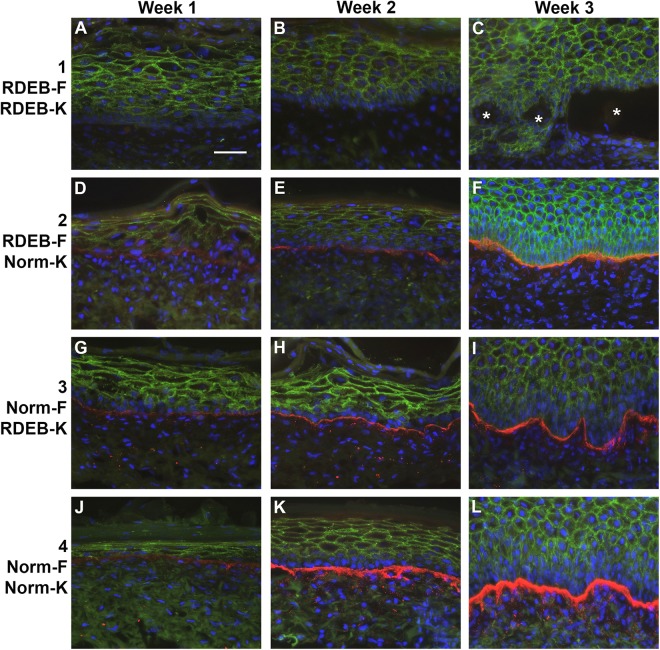
Immunohistochemistry with an antibody against HLA was used to examine engraftment by detecting the presence of human cells in grafted ESS. Positive HLA-ABC immunostaining was virtually undetectable in normal or RDEB ESS at weeks 1 and 2 after grafting. This is consistent with previous studies in our laboratory; HLA-ABC levels are typically low or undetectable at early time points after grafting but increase over time in vivo (data not shown). At 3 weeks after grafting, HLA-ABC-positive keratinocytes were observed in ESS in groups 2 and 4, which were prepared using normal keratinocytes (Supplemental Fig. S2). Positive immunostaining for HLA-ABC was not detected in grafts prepared using RDEB keratinocytes (groups 1 and 3). As a control for the HLA-ABC immunostaining, expression was observed in sections of both native RDEB patient skin and normal human skin (Supplemental Fig. S2). To confirm engraftment of human cells in ESS from all groups, a human-specific antibody against E-cadherin, a protein expressed on the surface of epithelial cells, was used. The specificity of this antibody for human E-cadherin was demonstrated by immunohistochemistry with control sections of human RDEB and normal skin, and mouse skin (Supplemental Fig. S3). Localization of human E-cadherin in ESS in vivo was observed in all groups and all time points, confirming engraftment of human epidermal cells in ESS (Fig. 7).
Figure 7.
Localization of human E-cadherin and collagen VII (COL7) in engineered skin substitutes (ESS) in vivo. Immunohistochemistry was performed to localize human E-cadherin (green), a marker for human epidermal cells, and COL7 (red); nuclei were counterstained using DAPI (blue). Shown are sections of ESS from week 1 (left; A, D, G, J), week 2 (center; B, E, H, K), and week 3 (right; C, F, I, L) in vivo. ESS were prepared with RDEB fibroblasts and keratinocytes (group 1; A–C), RDEB fibroblasts and normal keratinocytes (group 2; D–F), normal fibroblasts and RDEB keratinocytes (group 3; G–I), and normal fibroblasts and keratinocytes (group 4; J–L), as indicated. Asterisks (C) indicate epidermal blistering in ESS prepared with all RDEB cells. Scale bar in A (50 µm) is same for all panels.
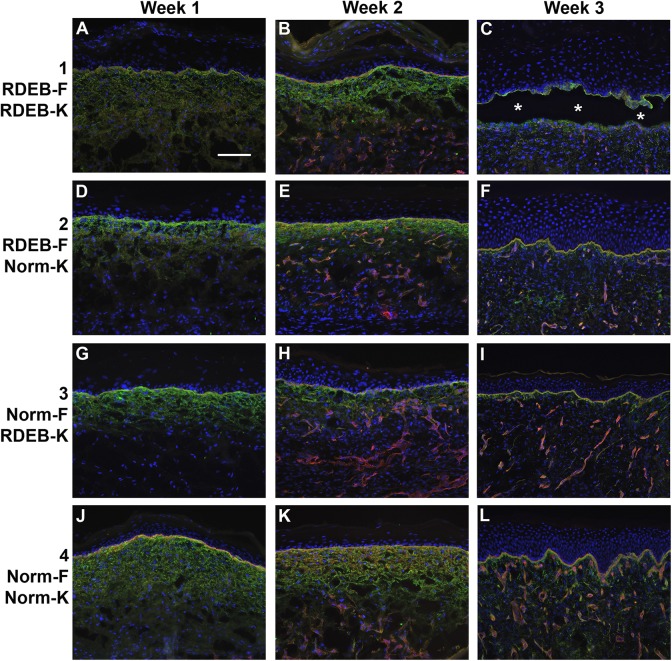
Basement membrane proteins COL4 and LAM were detected in ESS of all four groups at all time points (Fig. 8). Staining was evident in the basement membrane of the dermal–epidermal junction and in blood vessels. There was little noticeable difference in staining intensity among groups 2–4, but ESS in group 1, prepared with RDEB fibroblasts and keratinocytes, displayed reduced COL4 and LAM staining at the dermal–epidermal junction at week 3 after grafting (Fig. 8C). This may be due to epidermal blistering, which disrupted the structure of the dermal–epidermal basement membrane. Vascularization of ESS was evident in sections of ESS from all groups at weeks 2 and 3 after grafting, but appeared to be reduced in ESS in group 1, particularly at 3 weeks after grafting.
Figure 8.
Deposition of basement membrane in ESS in vivo. Immunohistochemistry was performed to localize collagen IV (green) and laminin (red); nuclei were counterstained using DAPI (blue). Shown are sections of ESS from week 1 (left; A, D, G, J), week 2 (center; B, E, H, K), and week 3 (right; C, F, I, L) in vivo. ESS were prepared with RDEB fibroblasts and keratinocytes (group 1; A–C), RDEB fibroblasts and normal keratinocytes (group 2; D–F), normal fibroblasts and RDEB keratinocytes (group 3; G–I), and normal fibroblasts and keratinocytes (group 4; J–L), as indicated. Asterisks (C) indicate epidermal blistering in ESS prepared with all RDEB cells. Scale bar in A (50 µm) is same for all panels.
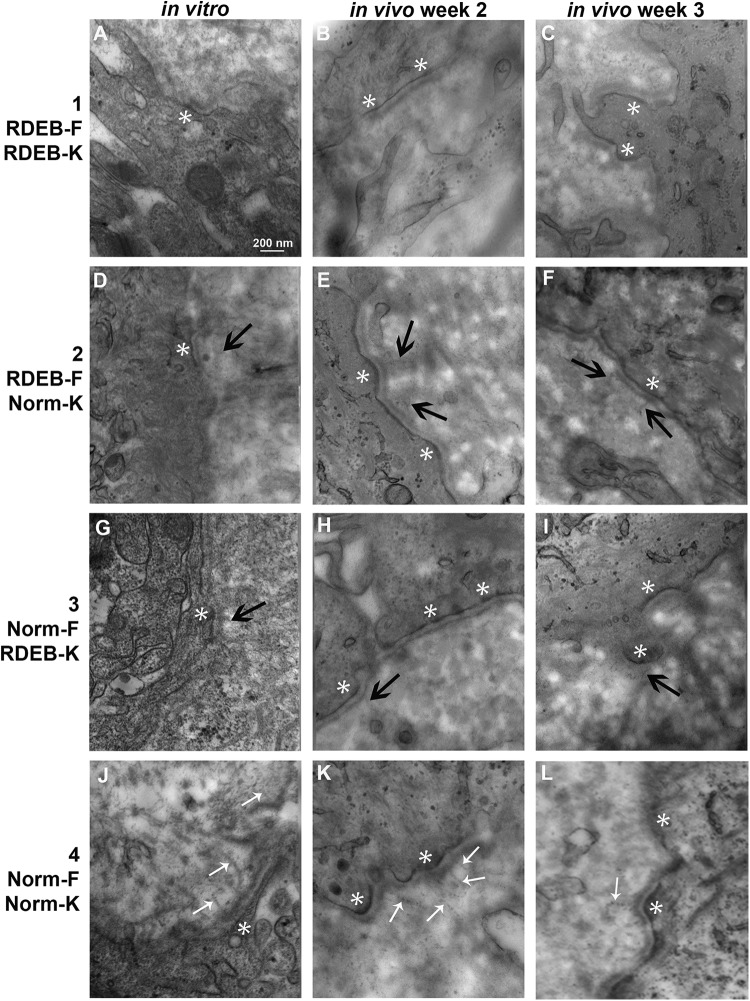
TEM was used to visualize the ultrastructural components of the basement membrane zone of ESS in vitro and in vivo at all time points (Fig. 9). In ESS in vitro, the basement membrane with a lamina densa and lamina lucida was apparent in all samples. Anchoring fibrils appeared morphologically normal, with clear striations, in samples of ESS containing normal fibroblasts and keratinocytes (group 4) in vitro (Fig. 9J). In ESS containing combinations of normal and RDEB fibroblasts and keratinocytes (groups 2 and 3), hints of structures resembling anchoring fibrils were observed, but these appeared diffuse and without the clear striations observed in normal anchoring fibrils (Fig. 9D, G). Anchoring fibrils were not observed in vitro in ESS containing all RDEB cells (group 1; Fig. 9A). At 1 week after grafting, no anchoring fibrils were observed in any of the samples examined, including group 4 ESS containing normal fibroblasts and keratinocytes (data not shown). By 2 and 3 weeks after grafting, morphologically normal anchoring fibrils were observed in group 4 ESS (Fig. 9K–L), but similar to what was observed with ESS in vitro, the structures observed in ESS of groups 2 and 3 appeared diffuse and without clear striations (Fig. 9E,F, H,I). No anchoring fibrils were observed in group 1 ESS at weeks 2 or 3 in vivo (Fig. 9B,C).
Figure 9.
Transmission electron microscopy of dermal–epidermal junction in engineered skin substitutes (ESS). Shown are images of ESS prepared with RDEB fibroblasts and keratinocytes (group 1; A–C), RDEB fibroblasts and normal keratinocytes (group 2; D–F), normal fibroblasts and RDEB keratinocytes (group 3; G–I), and normal fibroblasts and keratinocytes (group 4; J–L), as indicated, collected at the end of the in vitro incubation period (left; A, D, G, J), and at 2 weeks (center; B, E, H, K) and 3 weeks (right; C, F, I, L) after grafting. Anchoring fibrils appeared morphologically normal in samples of ESS prepared with all normal cells (J, K, L; small white arrows); anchoring fibrils in other samples, if present, appeared diffuse and without clear striations (black arrows). Hemidesmosomes were evident in vitro at 2 and 3 weeks in vivo (white asterisks). Original magnification was 80,000×; scale bar in A (200 nm) is the same for all sections.
Discussion
This study was undertaken to determine whether COL7 expression is required in both fibroblasts and keratinocytes in a bilayer skin substitute, or whether expression in only dermal fibroblasts or epidermal keratinocytes would suffice to prevent blistering and enable formation of anchoring fibrils. Previous studies involving this engineered skin model demonstrated deposition of COL7 at the basement membrane in vitro, prior to grafting24. Therefore, we anticipated that anchoring fibrils might form in ESS in vitro. Because COL7 deposition at the basement membrane was observed in ESS prepared with normal cells in only one layer, dermis or epidermis, we expected that ESS in these groups would display anchoring fibril formation in vitro. However, we only observed morphologically normal anchoring fibrils in ESS prepared with both normal fibroblasts and keratinocytes, suggesting that COL7 expression is required in both epidermis and dermis for anchoring fibril formation in vitro. Anchoring fibrils were not observed at week 1 in vivo; this may be due to the extensive proliferation and remodeling that occurs in ESS in the first week after grafting17. Although some fibril-like structures were observed in ESS in groups 2 and 3 after 2 weeks in vivo, these did not resemble normal anchoring fibrils. The current study only examined grafts up to 3 weeks after transplantation; thus, it is possible that formation of morphologically normal anchoring fibrils is delayed rather than inhibited in vivo in the “hybrid” ESS containing combinations of normal and RDEB fibroblasts and keratinocytes. Future studies involving later time points will be required to determine if anchoring fibrils eventually form in vivo in bilayer skin substitutes containing COL7-expressing cells in only the dermis or epidermis.
A recent report cautioned that murine COL7 was able to form anchoring fibrils in human reconstructed skin grafted to mice25. That report used the same monoclonal antibody against human COL7, clone LH7.2, as used in the current study. We confirmed that this antibody is human specific by immunohistochemistry with control sections of RDEB patient skin, normal human skin, and mouse skin. In contrast to the previous report by Bremer et al.25, murine COL7 was not detected in our study in grafts of ESS containing RDEB fibroblasts and keratinocytes at 1 to 3 weeks after grafting. This may be due to differences in the models used in the two studies. In the current study, the grafts formed distinct dermal and epidermal layers prior to grafting, whereas the previous study involved transplantation of fibroblasts and keratinocytes in a chamber and in vivo reconstitution of skin layers25. In addition, their analysis was performed at 10 weeks after grafting. If our model were to be examined at 10 weeks or more after transplantation, it is possible that some deposition of murine collagen VII may be observed.
We were surprised to see that RDEB keratinocytes in ESS were deficient in expression of HLA-ABC at 3 weeks after grafting, despite clear evidence of persistence of human keratinocytes in vivo demonstrated by human E-cadherin localization. Although specific HLA haplotypes were previously reported to be associated with RDEB26, the antibody used for the analysis in the current study is reportedly directed at non-polymorphic epitopes of the HLA-ABC antigens. Positive HLA-ABC immunostaining was observed in the epidermis of the RDEB patient skin sample used for isolation of primary cells, at levels similar to normal human skin. Therefore, we expected HLA-ABC to be expressed similarly in RDEB and normal keratinocytes in ESS in vivo. In numerous previous studies in our laboratory, we have observed that expression of HLA increases over time after grafting, with low to undetectable levels at very early time points in vivo (unpublished data). The absence of detectable HLA staining in ESS containing RDEB keratinocytes at 3 weeks in vivo may reflect a delay in tissue maturation after grafting. A long-term time course, which was beyond the scope of the current study, will be required to resolve this issue.
Restoration of COL7 expression is the goal of cutaneous gene therapy for RDEB. For patients with JEB, in which the mutation affects laminin, a protein expressed only in keratinocytes, CEA appears to be a very effective means of ex vivo gene therapy7. It is less clear whether this approach will be sufficient for stable, long-term wound closure in patients with RDEB because COL7 is normally expressed by both epidermal keratinocytes and dermal fibroblasts. Although a previous clinical trial in patients with RDEB demonstrated wound closure and anchoring fibril formation in grafts containing cultured keratinocytes genetically modified by retroviral transduction to express COL7, the results were variable and lasting benefits were not seen for all patients8. In that study, the authors speculated that the decline in healing over time might have resulted from a reduced number of stem cells in the patient skin biopsies, or that the corrected cells in the grafts were out-competed by cells in the wound bed not expressing COL78,9. A limitation of ex vivo gene therapies that utilize CEA is inclusion of only a single cell type, which may reduce stability of grafted cells. Previous studies from our group determined that a robust dermal fibroblast layer improved tissue development in ESS27. Similar results have been reported by others28–30, emphasizing the importance of paracrine interactions between fibroblasts and keratinocytes for optimal skin morphogenesis. The reduced long-term graft stability observed in RDEB patients treated with COL7A1-expressing CEA8 may have resulted, in part, from the absence of a dermal layer to support epithelial cell proliferation, stratification, and establishment of a stem cell niche. CEA is used as an adjunctive treatment for burn patients, but epidermal blistering and fragility are among the limitations encountered with this therapy31. These limitations are not observed in ESS because it contains both an epidermal and a dermal layer. Previous clinical studies in pediatric burn patients demonstrated the potential for long-term, stable wound closure after grafting of autologous ESS13. Deposition of basement membrane in vitro, prior to grafting24, is believed to suppress blister formation after transplantation to full-thickness wounds. Therefore, we predict that a bilayer skin substitute model, such as ESS, will be superior to CEA for use in cutaneous gene therapy for RDEB.
Blisters were not observed in ESS prepared with normal fibroblasts and keratinocytes at any time point examined, whereas blistering was observed in ESS prepared using RDEB fibroblasts and/or keratinocytes. The most severe blistering was observed in ESS containing all RDEB cells at 3 weeks after grafting. Blistering was relatively minor and was similar in groups 1–3 at 1 and 2 weeks after grafting. At these time points, the grafts were secured by sutures and covered with dressing materials until biopsies were collected for analysis, which likely protected the grafts and inhibited blistering in ESS prepared with RDEB cells. One week after suture and dressing removal, at 3 weeks after grafting, significantly more blistering was observed in ESS prepared using RDEB fibroblasts and RDEB keratinocytes compared with ESS containing mixtures of RDEB and normal cells, suggesting that these grafts were more susceptible to blistering in the absence of wound dressings.
A bilayer skin substitute model containing gene-edited RDEB fibroblasts and keratinocytes was recently investigated in preclinical studies using a mouse xenograft model10. Izmiryan et al. used CRISPR-based gene editing and homology-directed repair to restore COL7 expression in skin substitutes in vivo to approximately 20–26% of normal levels; this was sufficient to enable formation of anchoring fibrils10. That study demonstrated the enormous therapeutic potential of the combined approaches of gene editing and tissue engineering for treatment of RDEB. However, the model used in that study was limited in its clinical utility compared with ESS. The culture of primary keratinocytes used in that study was performed using lethally irradiated murine 3T3-J2 feeder cells, which would result in classification of this skin substitute as a xenograft by the US Food and Drug Administration32. In addition, the skin substitutes were transplanted using a skin flap procedure10, which would be difficult to translate clinically for treatment of RDEB patients. The model of engineered skin utilized in the current study is prepared using primary keratinocytes cultured without use of mouse feeder cells. This culture method was previously optimized for clinical translation, and was tested clinically in over 50 pediatric burn patients with large (>50% TBSA) full-thickness burns13,14. In patients with the largest burns, up to 60% TBSA was healed using autologous ESS13. Based on clinical experiences with burn patients, this model appears well suited as a platform for cutaneous gene therapy for RDEB.
Conclusions
High levels of COL7 were observed at the dermal–epidermal junction of ESS in groups 2–4 by 2 to 3 weeks after grafting. Based on this observation, we expected that ESS containing normal, COL7-expressing cells in only one layer would be sufficient to support anchoring fibril formation, even if the other layer contained native RDEB cells. If this were the case, it could greatly simplify cutaneous gene therapy efforts, requiring genetic modification of only fibroblasts or keratinocytes. However, the results of the current study suggest that the benefits achieved by inclusion of a dermal layer may only be realized if both keratinocytes and fibroblasts express COL7. Further studies examining basement membrane structure at later time points after transplantation in vivo will be required to determine whether anchoring fibril formation is inhibited or merely delayed by expression of COL7 in a single layer of this bilayer skin substitute.
Supplemental Material
Supplementary_Materials for Collagen VII Expression Is Required in Both Keratinocytes and Fibroblasts for Anchoring Fibril Formation in Bilayer Engineered Skin Substitutes by Dorothy M. Supp, Jennifer M. Hahn, Kelly A. Combs, Kevin L. McFarland, Ann Schwentker, Raymond E. Boissy, Steven T. Boyce, Heather M. Powell and Anne W. Lucky in Cell Transplantation
Acknowledgments
The authors gratefully acknowledge the technical assistance of Mary Rolfes at the Shriners Hospitals for Children – Cincinnati Histology Special Shared Facility for processing and sectioning of tissue samples, and the staff of the Laboratory of Genetics and Genomics at Cincinnati Children’s Hospital Medical Center for DNA sequencing. In addition, we thank Mark Kleiner for assistance with preparation of culture media, Christopher Lloyd for preparation of the collagen scaffolds, and Hunter Morgan for critical reading of the manuscript.
Footnotes
Ethical Approval: Ethical approval was obtained by the University of Cincinnati Institutional Review Board, which determined that this research (Study ID# 2013-4582) does not meet the definition of human subjects research as it involves collection of de-identified surgical discard tissue.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the University of Cincinnati Institutional Animal Care and Use Committee approved protocol (#04-04-19-01).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by the Shriners Hospitals for Children (grant #85102-CIN-18 to D.S. and grant #84050-CIN-13 to S.B.).
ORCID iD: Dorothy M. Supp  https://orcid.org/0000-0002-2429-6630
https://orcid.org/0000-0002-2429-6630
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Chung HJ, Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin. 2010;28(1):93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cutlar L, Greiser U, Wang W. Gene therapy: pursuing restoration of dermal adhesion in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2014;23(1):1–6. [DOI] [PubMed] [Google Scholar]
- 3. Guerra L, Odorisio T, Zambruno G, Castiglia D. Stromal microenvironment in type VII collagen-deficient skin: the ground for squamous cell carcinoma development. Matrix Biol. 2017;63:1–10. [DOI] [PubMed] [Google Scholar]
- 4. Webber BR, Tolar J. From marrow to matrix: novel gene and cell therapies for epidermolysis bullosa. Mol Ther. 2014;23(6):987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larcher F, Del RM. Innovative therapeutic strategies for recessive dystrophic epidermolysis bullosa. Actas Dermosifiliogr. 2015;106(5):376–382. [DOI] [PubMed] [Google Scholar]
- 6. Mavillo F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A, Maruggi G, Ferrari G, Provasi E, Bonini C, Capurro S, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nature Med. 2007;12(12):1397–1402. [DOI] [PubMed] [Google Scholar]
- 7. Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, Scaglione D, Reichelt J, Klausegger A, Kneisz D, Romano O, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551(7680):327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siprashvili Z, Nguyen NT, Gorell ES, Loutit K, Khuu P, Furukawa LK, Lorenz HP, Leung TH, Keene DR, Rieger KE, Khavari P, et al. Safety and wound outcomes following genetically corrected autologous epidermal grafts in patients with recessive dystrophic epidermolysis bullosa. JAMA. 2016;316(17):1808–1817. [DOI] [PubMed] [Google Scholar]
- 9. Nystrom A, Bruckner-Tuderman L. Gene therapy for epidermolysis bullosa: sticky business. Mol Ther. 2016;24(12):2035–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Izmiryan A, Ganier C, Bovolenta M, Schmitt A, Mavilio F, Hovnanian A. Ex Vivo COL7A1 correction for recessive dystrophic epidermolysis bullosa using CRISPR/Cas9 and homology-directed repair. Mol Ther Nucleic Acids. 2018;12:554–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Titeux M, Pendaries V, Zanta-Boussif MA, Decha A, Pironon N, Tonasso L, Mejia JE, Brice A, Danos O, Hovnanian A. SIN retroviral vectors expressing COL7A1 under human promoters for ex vivo gene therapy of recessive dystrophic epidermolysis bullosa. Mol Ther. 2010;18(8):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyce ST, Kagan RJ, Yakuboff KP, Meyer NA, Rieman MT, Greenhalgh DG, Warden GD. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann Surg. 2002;235(2):269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyce ST, Simpson PS, Rieman MT, Warner PM, Yakuboff KP, Bailey JK, Nelson JK, Fowler LA, Kagan RJ. Randomized, paired-site comparison of autologous engineered skin substitutes and split-thickness skin graft for closure of extensive, full-thickness burns. J Burn Care Res. 2017;38(2):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boyce ST, Kagan RJ, Greenhalgh DG, Warner P, Yakuboff KP, Palmieri T, Warden GD. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J Trauma. 2006;60(4):821–829. [DOI] [PubMed] [Google Scholar]
- 15. Boyce ST, Rice RK, Lynch KA, Supp AP, Swope VB, Kagan RJ, Supp DM. Assessment of replication rates of human keratinocytes in engineered skin substitutes grafted to athymic mice. Wound Repair Regen. 2012;20(4):544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smiley AK, Klingenberg JM, Aronow BJ, Boyce ST, Kitzmiller WJ, Supp DM. Microarray analysis of gene expression in cultured skin substitutes compared with native human skin. J Invest Dermatol. 2005;125(6):1286–1301. [DOI] [PubMed] [Google Scholar]
- 17. Klingenberg JM, McFarland KL, Friedman A, Boyce ST, Aronow BJ, Supp DM. Engineered human skin substitutes undergo large-scale genomic reprogramming and normal skin-like maturation following transplantation to athymic mice. J Invest Dermatol. 2010;130(2):587–601. [DOI] [PubMed] [Google Scholar]
- 18. Harriger MD, Warden GD, Greenhalgh DG, Kagan RJ, Boyce ST. Pigmentation and microanatomy of skin regenerated from composite grafts of cultured cells and biopolymers applied to full-thickness burn wounds. Transplantation. 1995;59(5):702–707. [DOI] [PubMed] [Google Scholar]
- 19. Boyce ST, Lloyd CM, Kleiner MC, Swope VB, Abdel-Malek Z, Supp DM. Restoration of cutaneous pigmentation by transplantation to mice of isogeneic human melanocytes in dermal-epidermal engineered skin substitutes. Pigment Cell Melanoma Res. 2017;30(6):531–540. [DOI] [PubMed] [Google Scholar]
- 20. Hahn JM, Glaser K, McFarland KL, Aronow BJ, Boyce ST, Supp DM. Keloid-derived keratinocytes exhibit an abnormal gene expression profile consistent with a distinct causal role in keloid pathology. Wound Repair Regen. 2013;21(4):530–544. [DOI] [PubMed] [Google Scholar]
- 21. Boyce ST. Methods for the serum-free culture of keratinocytes and transplantation of collagen-GAG-based skin substitutes. Methods Mol Med. 1999;18:365–389. [DOI] [PubMed] [Google Scholar]
- 22. Lapiere JC, Hu L, Iwasaki T, Chan LS, Peavey C, Woodley DT. Identification of the epitopes on human type VII collagen for monoclonal antibodies LH 7.2 and clone I, 185. J Dermatol Sci. 1994;8(2):145–150. [DOI] [PubMed] [Google Scholar]
- 23. Christiano AM, Greenspan DS, Lee S, Uitto J. Cloning of human type VII collagen. Complete primary sequence of the alpha 1(VII) chain and identification of intragenic polymorphisms. J Biol Chem. 1994;269(32):20256–20262. [PubMed] [Google Scholar]
- 24. Boyce ST, Supp AP, Swope VB, Warden GD. Vitamin C regulates keratinocyte viability, epidermal barrier, and basement membrane formation in vitro, and reduces wound contraction after grafting of cultured skin substitutes. J Invest Dermatol. 2002;118(4):565–572. [DOI] [PubMed] [Google Scholar]
- 25. Bremer J, Kramer D, Eichhorn DS, Gostynski A, Diercks GFH, Jonkman MF, van den Akker PC, Pasmooij AMG. Murine type VII collagen distorts outcome in human skin graft mouse model for dystrophic epidermolysis bullosa [published online ahead of print July 17, 2018]. Exp Dermatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaidya S, Tyring SK, Johnson LB, Fine JD. HLA and epidermolysis bullosa. Association between the HLA complex and recessive dystrophic epidermolysis bullosa. Arch Dermatol. 1991;127(10):1524–1527. [PubMed] [Google Scholar]
- 27. Boyce ST, Swope VB, Supp AP, Warden GD. Fibroblasts in cultured skin substitutes stimulate epidermal barrier and increase cellular viability. J Burn Care Rehabil. 1999;20(1):S197. [DOI] [PubMed] [Google Scholar]
- 28. Wojtowicz AM, Oliveira S, Carlson MW, Zawadzka A, Rousseau CF, Baksh D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014;22(2):246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coulomb B, Friteau L, Baruch J, Guilbaud J, Chretien-Marquet B, Glicenstein J, Lebreton-Decoster C, Bell E, Dubertret L. Advantage of the presence of living dermal fibroblasts within in vitro reconstructed skin for grafting in humans. Plast Reconstruct Surg. 1998;101(7):1891–1903. [DOI] [PubMed] [Google Scholar]
- 30. El Ghalbzouri A, Ponec M. Diffusible factors released by fibroblasts support epidermal morphogenesis and deposition of basement membrane components. Wound Repair Regen. 2004;12(3):359–367. [DOI] [PubMed] [Google Scholar]
- 31. Sood R, Roggy D, Zieger M, Balledux J, Chaudhari S, Koumanis DJ, Mir HS, Cohen A, Knipe C, Gabehart K, Coleman JJ. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: the Indiana University experience. J Burn Care Res. 2010;31(4):559–568. [DOI] [PubMed] [Google Scholar]
- 32. Guidance for Industry. Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans. 2016. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/UCM533036.pdf. (accessed 4 February 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Materials for Collagen VII Expression Is Required in Both Keratinocytes and Fibroblasts for Anchoring Fibril Formation in Bilayer Engineered Skin Substitutes by Dorothy M. Supp, Jennifer M. Hahn, Kelly A. Combs, Kevin L. McFarland, Ann Schwentker, Raymond E. Boissy, Steven T. Boyce, Heather M. Powell and Anne W. Lucky in Cell Transplantation