Abstract
Heme-mediated oxidative modification of low-density lipoprotein (LDL) plays a crucial role in early atherogenesis. It has been shown that hydrogen sulfide (H2S) produced by vascular smooth muscle cells is present in plasma at a concentration of about 50 μmol/L. H2S is a strong reductant which can react with reactive oxygen species like superoxide anion and hydrogen peroxide. The current study investigated the effect of H2S on hemin-mediated oxidation of LDL and oxidized LDL (oxLDL)-induced endothelial reactions. H2S dose dependently delayed the accumulation of lipid peroxidation products—conjugated dienes, lipid hydroperoxides (LOOH), and thiobarbituric acid reactive substances—during hemin-mediated oxidation. Moreover, H2S decreased the LOOH content of both oxidized LDL and lipid extracts derived from soft atherosclerotic plaque, which was accompanied by reduced cytotoxicity. OxLDL-mediated induction of the oxidative stress responsive gene, heme oxygenase-1, was also abolished by H2S. Finally we have shown that H2S can directly protect endothelium against hydrogen peroxide and oxLDL-mediated endothelial cytotoxicity. These results demonstrate novel functions of H2S in preventing hemin-mediated oxidative modification of LDL, and consequent deleterious effects, suggesting a possible antiatherogenic action of H2S.
Keywords: Hydrogen sulfide, Low-density lipoprotein, Lipid hydroperoxide, Hemin, Heme oxygenase-1
Introduction
Oxidative modification of LDL is currently recognized as one of the early events in atherogenesis [1,2]. Oxidized LDL contributes to atherogenesis in the following ways: (i) it is readily ingested by macrophages through the scavenger receptor that is distinct from the LDL receptor, (ii) it is chemotactic for the circulating monocytes, (iii) it increases monocyte adhesion and it inhibits the motility of macrophages already present in the lesion, and (iv) it stimulates release of cytokines and growth factors and it is cytotoxic to endothelial and smooth muscle cells. However, despite extensive evidence implicating oxidized LDL in the development of cardiovascular disease, the exact mechanisms whereby LDL is oxidized in vivo are still being debated.
We previously suggested that heme—which may be derived from oxidation and decomposition of hemoglobin during intravascular hemolysis [3]—mediates the oxidative modification of LDL [4]. In vitro exposure of LDL to hemin promotes extensive oxidative modification of both lipid and apolipoprotein components of LDL, resulting in the formation of an oxidized form of LDL which is cytotoxic to the vasculature. In response to challenge with this material, cells up-regulate the stress responsive genes heme oxygenase-1 (HO-1) and ferritin [5–8]. Up-regulation of HO-1 and ferritin genes in the endothelium occurs in the early phase of progression of atherosclerotic lesions [9], possibly reflecting a cellular response to heme and/or heme-iron-generated lipid peroxidation products. There is growing evidence that induction of HO-1 and ferritin is protective against atherosclerosis. Induction of the HO-1-ferritin system in LDL-receptor knockout mice decreases the formation of atherosclerotic lesions, whereas inhibition of HO-1 enzyme activity by tin protoporphyrin leads to accelerated atherosclerosis in these mice [10].
Hydrogen sulfide (H2S) has been traditionally considered as a toxic gas; however, recently it has been identified as the third endogenous gasotransmitter beside CO and NO [11]. Studies reveal that H2S, which is generated enzymatically by many types of mammalian cells, is detectable in serum and most tissues at a concentration of about 50 μmol/L [12]. In the vasculature, H2S is produced by vascular smooth muscle cells by the pyridoxal-5-phosphate (vitamin B6)-dependent enzyme cystathionine--lyase that uses L-cysteine as a substrate [13]. Hydrogen sulfide exerts a number of physiological actions in the cardiovascular system: (i) it dilates blood vessels mostly, if not exclusively, by a mechanism that involves opening of ATP-sensitive K+ channels of smooth muscle cells [14], (ii) it is cardioprotective against ischemic reperfusion damage and myocardial inflammation [15], and (iii) it preserves both mitochondrial structure and function after the injury [16]. Mounting evidence suggests that H2S has a direct effect on the development of atherosclerosis. Indeed, H2S induces apoptosis [17,18] and suppresses endothelin-induced proliferation of vascular smooth muscle cells [19], which in turn might reduce the growth of atherosclerotic lesions. It influences vascular inflammatory reactions [20], and inhibits vascular calcification, a process involving transformation of vascular smooth muscle cells into an osteoblast-like phenotype [21]. It has been shown that progression of atherosclerosis is significantly slower in patients with Down syndrome, a state of H2S overproduction [22].
It has been previously shown that plasma thiols inhibit hemin-mediated oxidation of LDL [23], and that exogenous administration of an H2S donor reduced lipid peroxidation—including levels of myocardial malondialdehyde, plasma malondialdehyde, and conjugated diene—in a myocardial injury model [23,24]. Therefore we hypothesized that H2S might inhibit hemin-mediated oxidative modification of LDL, thereby contributing to the antiatherogenic effect of H2S. Indeed, our results show that H2S dose dependently prolongs accumulation of oxidation products during hemin-mediated oxidative modification of LDL and decreases the cytotoxic effect of oxidized LDL and of lipid extracted from atheromatous lesions (types IV and Va) [25] on human endothelial cells. Examination of the underlying mechanisms revealed that H2S readily reacts with, and reduces the level of, lipid hydroperoxides (LOOH), which is the main contributor to the cytotoxic effect of both oxidized LDL [26] and lipid derived from atheroma. Moreover, H2S can directly suppress endothelial cell cytotoxicity caused by either hydrogen peroxide or LOOH. We suggest that both protective mechanisms might be important in the antiatherogenic effects of this gas.
Materials and methods
Materials
Medium 199, fetal calf serum, and HankTs balanced salt solution (HBSS) were purchased from Life Technologies, and dispase II was from Boehringer Mannheim (Vienna, Austria). All other reagents utilized were purchased from Sigma unless otherwise specified. NaHS was used to generate H2S in solution.
Cell culture
As in our previous studies [8], human umbilical vein endothelial cells (HUVECs) were removed from human umbilical veins by exposure to dispase and cultured in medium 199 containing 15k fetal calf serum, penicillin (100 U/ml), streptomycin (100 U/ml), and heparin (5 U/ml) supplemented with L-glutamine, sodium pyruvate, and endothelial cell growth factor. Endothelial cells were identified by their morphology and by the presence of von Willebrand factor.
Preparation and oxidation of human low-density lipoprotein
LDL was isolated from plasma derived from EDTA (1 mg/ml)-anticoagulated venous blood of healthy volunteers who had fasted overnight. Density of plasma was adjusted to 1.3 g/ml with KBr, and a two-layer gradient was made in a Quick-Seal polyallomer ultracentrifuge tube (Beckman Instruments) by layering 0.9k NaCl on 10 ml of density adjusted plasma, which was then centrifuged at 302,000 g for 3 h at 4°C (VTi 50.2 rotor, Beckman Instruments) [27]. The LDL samples were kept at 4°C and protected from light, and the protein content was determined by the BCA protein assay (Pierce, Rockford, IL). Oxidized LDL was prepared by incubating LDL (200 μg/ml protein content diluted in saline) with hemin (5 μmol/L) at 37°C overnight, buffered with Hepes (10 mmol/L, pH 7.4).
Tissue sample lipid extraction and oxidation
Specimens of human atherosclerotic lesions (types IV and Va) [25] were obtained from aorta or its primary branches of deceased heart-beating donors for organ transplantation. Removal of tissue samples from these donors was approved by the Scientific and Research Ethic Comity of Scientific Council of Health of the Hungarian Government. Entire vessels and complete lesions were removed during donation, immediately washed with saline, dried, weighed, frozen in liquid nitrogen, and stored at −70 °C until assay. Blood vessel sections were classified by their morphology. Lesions with thickened intima and large lipid core but no sign of disruption were used to extract lipids from by chloroform:methanol (2:1 v/v) according to the method of Bligh and Dyer [28]. Oxidized lipid extract was prepared by incubating lipid extract (1 mg lipid/ml diluted in saline) with hemin (5 μmol/L) at 37°C overnight, buffered with Hepes (10 mmol/L, pH 7.4).
Detection of oxidative modification of LDL and lipid extract
Conjugated diene formation and generation of thiobarbituric acidreactive substances (TBARS) in LDL were measured as in our previous studies [4]. Briefly, for conjugated diene measurement LDL was diluted to 50 μg/ml in saline, and optical density was determined at 234 nm. For TBARS determination 600 μl of thiobarbituric-acid reagent (0.375 w/v% 2-thiobarbituric acid, 2 v/v% HCl, 15 v/v% trichloroacetic acid) was added to 300 μl of LDL (200 μg/ml) and then boiled for 15 min. Following centrifugation (10,000 g, 15 min) the optical density of supernatant was determined at 532 nm. LOOH content of LDL was detected using the Ferrous Oxidation in Xylenol orange (FOX) assay [29]. LOOH content of lipid extracts was determined by an iodometric method. Briefly, 100 μl of lipid suspension was extracted in 110 μl of chloroform and then acetic acid (120 μl) was added to 80 μl of the organic phase. After incubating this mixture with 40 μl KI (1.2 mg/ml in distilled water) for 5 min in the dark, 600 μl of 50 mmol/L cadmium acetate was added to stop the reaction. After a centrifugation (2000 g, 10 min), the optical density of the aqueous phase was measured at 353 nm. Results were calculated by using the molar extinction coefficient for of 2.19 104 M−1 cm−1 and are expressed as nanomole lipid hydroperoxides per milligram tissue.
Hemin determination
Total heme content of the reaction mixtures was measured by a colorimetric assay (QuantiChrom Heme Assay Kit, BioAssay System, Hayward, CA).
Endothelial cell cytotoxicity assay
Confluent HUVEC, grown in 96-well tissue culture plates, were washed twice with HBSS and then exposed to oxidized LDL (200 μg/ ml) or oxidized lipid extracts derived from atheroma (2 mg/ml) alone, or exposed to oxidized LDL or lipid extracts pretreated with H2S (25, 50, 100, or 200 μmol/L) at 37°C for 30 min. After a 4-h incubation, the test solutions were replaced with 100 μl of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (0.5 mg/ml) solution in HBSS, and cells were incubated for an additional 6 h. Then the MTT solution was removed, 100 μl of DMSO was added, and the optical density at 570 nm was measured. In other experiments endothelial cells were pretreated with H2S (50 μmol/L) at 37°C for 4 h and then challenged with hemin (5 μmol/L at 37°C for 1 h), followed by oxidative stress generated by hydrogen peroxide (100 and 200 μmol/L) or oxidized LDL (100 and 200 μg protein/ml). After 4 h of incubation the MTT assay was performed.
Heme oxygenase-1 mRNA analysis
Oxidized LDL (200 μg/ml) was pretreated with H2S (25, 50, 100, or 200 μmol/L) at 37 °C for 30 min and then diluted 4 times with HBSS. Confluent endothelial cells were treated with LDL samples (50 μg/ml) for 1 h, followed by a 4-h incubation in complete media. Total RNA was isolated and HO-1 and 18S rRNA levels were measured by real-time PCR. RNA was reverse-transcribed using Superscript II reverse transcriptase (Invitrogen). Real-time PCR was carried out using the iCycler iQ Real Time PCR System (Bio-Rad Laboratories, Hercules, CA). The 25- l reaction mixtures contained 0.3 nmol/L of forward and reverse primers and 0.13 nmol/L of fluorescent probe for HO-1 (F, 5′-TTCTTGTTCTACGGCTTGCTAC-3′; R, 5′-CTCCAT CCAGATCTCCAGCACT-3′; P, 5′-FAM-CTCAACATCCAGCTCTTTGAGGAGTGCAG-3′) or those for 18S rRNA (F, 5′-TTCACCACCATGGAGAAGGC-3′; R, 5V-GGCATGGACTGTGGTCATGA-3′; P, 5′-FAM-TACCACATCCAAGGAAGGCAGCAGG-5′), 3 mmol/L MgCl2, 0.2 mmol/L dNTPs, and 0.05 U/ml Taq DNA polymerase (Invitrogen, Carlsbad, CA). Results were normalized by 18S rRNA levels and are presented as fold increase compared to vehicle-treated control.
Western blot analysis
HUVECs grown in 6-well plates were washed and exposed to oxidized LDL which was pretreated with H2S as described above. After 1 h exposure, LDL was replaced by complete media, and cells were further incubated for 8 h. Cells were lysed in Tris-HCl (10 mmol/L) containing Triton X-100 (1k), Nonidet P-40 (0.5k), and protease inhibitors (Complete Mini, Roche, Basel, Switzerland). Protein content was determined, and 20 μg of cell lysate was electrophoresed on 12.5k SDS-PAGE, blotted onto nitrocellulose membrane (Hybond-ECL, Amersham Biosciences, Buckinghamshire, UK). After blocking, the membrane was incubated with polyclonal anti-HO-1 antibody (Calbiochem, Darmstadt, Germany). Antigen–antibody complex was visualized with the horseradish peroxidase chemiluminescence system according to the manufacturerTs instructions (Amersham Biosciences, Buckinghamshire, UK). Quantification of HO-1 induction was performed using computer-assisted videodensitometry (AlphaDigiDoc RT, Alpha Innotech Coorporation, San Leandro, CA).
Statistics
Data are shown as mean SD. Statistical analysis was performed by ANOVA test followed by post hoc, Newmann-Keuls test for multiple comparisons. Significance was set at a P < 0.05 level.
Results
Effect of H2S on oxidative modification of LDL induced by hemin
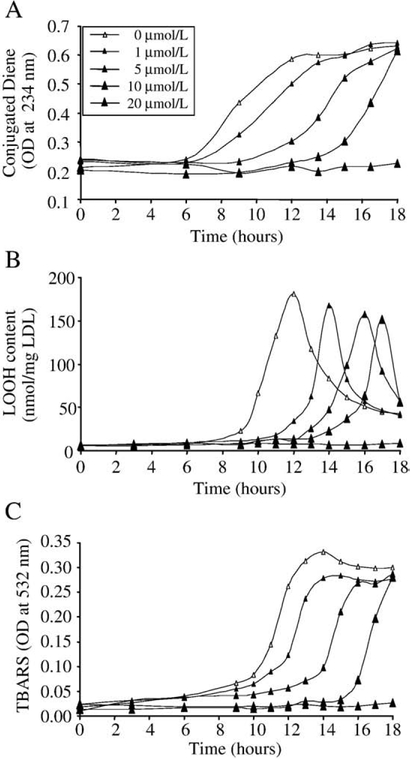
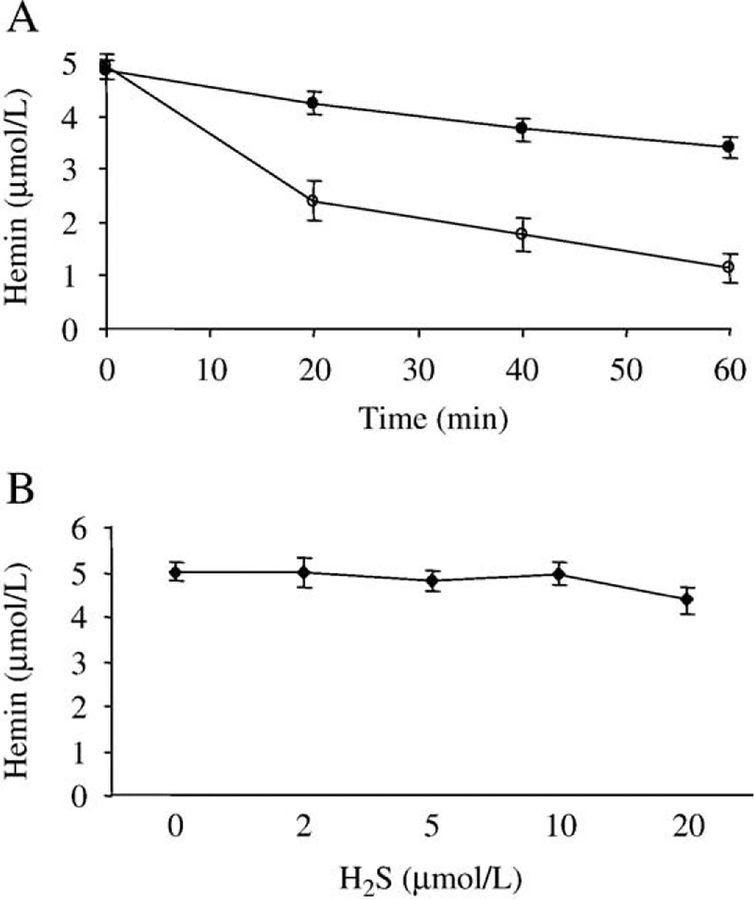
Sodium hydrogen sulfide, which was used to generate H2S, dose dependently prolonged hemin-mediated oxidative modification of LDL. Oxidation of LDL was mediated by the addition of hemin (5 μmol/L) followed by measuring the accumulation of lipid peroxidation products: conjugated dienes (Fig. 1A, empty triangles), lipid hydroperoxides (Fig.1B, empty triangles), and TBARs (Fig.1C, empty triangles). The presence of subphysiologic concentrations of H2S (5–20 μmol/L, black triangles) during hemin-mediated LDL oxidation significantly increased the length of the initiation phase of the lipid peroxidation reaction. Possible explanations of the observed effect are: (i) H2S can scavenge hemin [30], and reduce the availability of free hemin which acts as a catalyst in the oxidation reaction or (ii) H2S acts as a reductant and similarly to its reaction with H2O2 can reduce lipid hydroperoxides which are the ultimate source of lipid alkoxyl radicals, the main radical species that propagate transition metal-induced lipid peroxidation. In our experiments a high concentration (100 μM) of H2S in aqueous solution reduced hemin content by about 50k (4.92 μmol/L starting concentration vs 2.4 μmol/L) within 20 min at 37°C. In contrast, in the presence of LDL hemin content decreased much slower; 87k of the starting hemin was still present after 20 min and 70k after 1 h of incubation (Fig. 2A). Low concentrations (2–10 μmol/L) of H2S did not decrease the hemin content of reaction mixtures containing LDL (200 μg/ml) over a period of 6 h (Fig. 2B).
Fig. 1.
H2S inhibits formation of lipid peroxidation products during hemin-mediated oxidation of LDL in a dose-dependent manner. LDL (200 mg/mL) was incubated with hemin (5 mol/L) alone (empty triangle) or in the presence of H2S at a concentration of 1, 5, 10, or 20 mol/L (closed triangles) at 37°C. Lipid peroxidation was monitored by measuring the formation of conjugated dienes (A), LOOH (B), and TBARs (C) for 18 h. Figure shows a representative of three separate experiments.
Fig. 2.
Time and dose dependency of hemin scavenging by H2S. (A) Hemin (5 mmol/L) was incubated with H2S (100 μmol/L) in the absence (open circles) or in the presence of LDL (200 μg/ml) (closed circles) for the indicated time at 37°C, and then hemin content was determined as described under Materials and methods. (B) Hemin (5 μmol/L) was incubated with H2S (2–10 μmol/L) in the presence of LDL (200 μg/ml) for 6 h at 37°C, and then hemin content was determined. Figure shows mean of three independent experiments. Error bars denote standard deviations.
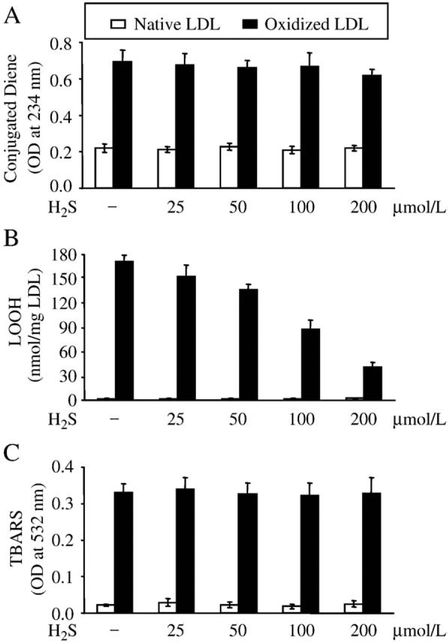
H2S dose dependently decreases LOOH content of oxidized LDL
To examine the possible effect of H2S on lipid peroxidation products, first LDL (200 μg/ml protein) was oxidized with hemin (5 μmol/L, 37°C, overnight), and then the oxidized LDL was treated with H2S for 30 min, and the concentration of lipid peroxidation products was measured. Conjugated diene and TBAR contents of oxidized LDL were unaffected by H2S (Figs. 3A and C). In contrast, H2S dose dependently decreased LOOH content of previously oxidized LDL (Fig. 3B), resulting in a 15, 21, 50, and 77k decrease of LOOH content by 25, 50, 100, and 200 μmol/L of H2S, respectively, compared to untreated oxidized LDL. H2S did not interfere with the assays used to estimate the concentrations of lipid peroxidation products as shown by control measurements using native LDL (Figs. 3A–C, empty bars).
Fig. 3.
H2S dose dependently decreases LOOH content of oxidized LDL. LDL (200 mg/ml) was oxidized with hemin (5 μmol/L) for 12 h at 37°C, and then treated with H2S at the indicated concentrations for 30 min at 37°C. Following treatment levels of conjugated dienes (A), LOOH (B), and TBARs (C) were measured. Data are derived from five separate experiments. Error bars denote standard deviations. Statistical significance is indicated by one (Pb0.05) or two (Pb0.01) asterisks.
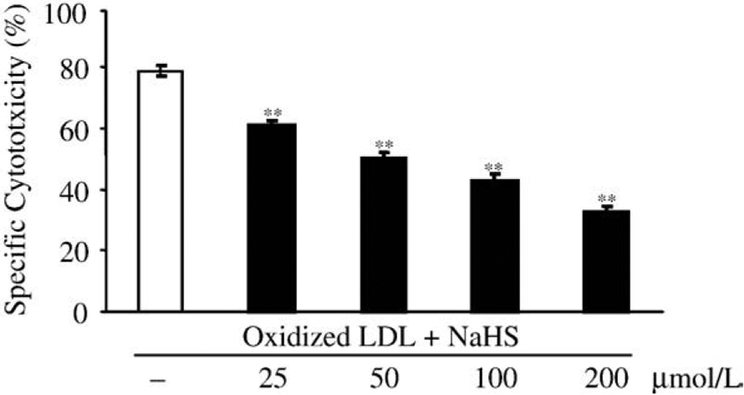
Cytotoxic effect of oxidatively modified LDL is dose dependently decreased by H2S
LDL oxidized with hemin was found to be markedly cytotoxic to endothelial cells. Moreover, we have previously shown that oxidized LDL is cytotoxic to vascular endothelium mainly due to its LOOH content [26,31]. Therefore we asked whether the decreased LOOH content of H2S-treated oxidized LDL might parallel its reduced cytotoxicity. To answer this question, LDL was oxidized with hemin (5 μmol/L) overnight, followed by a 30-min treatment with H2S at the labeled concentrations. As shown in Fig. 4, pretreatment of oxidized LDL with H2S prior to the exposure of HUVECs markedly and dose dependently suppressed endothelial cell cytotoxicity.
Fig. 4.
H2S dose dependently abolishes cytotoxic effects of oxidized LDL on HUVECs. LDL (200 μg/ml) was oxidized with hemin (5 μmol/L) for 12 h at 37°C, and then treated with H2S at the indicated concentrations for 30 min at 37°C. HUVEC grown on a 96-well plate were washed twice with HBSS and then challenged by the LDL samples for 4 h. MTT assay was performed to measure cytotoxicity. Data are derived from three separate experiments performed in triplicates. Error bars denote standard deviations. Statistical significance is indicated by one (Pb0.05) or two (Pb0.01) asterisks.
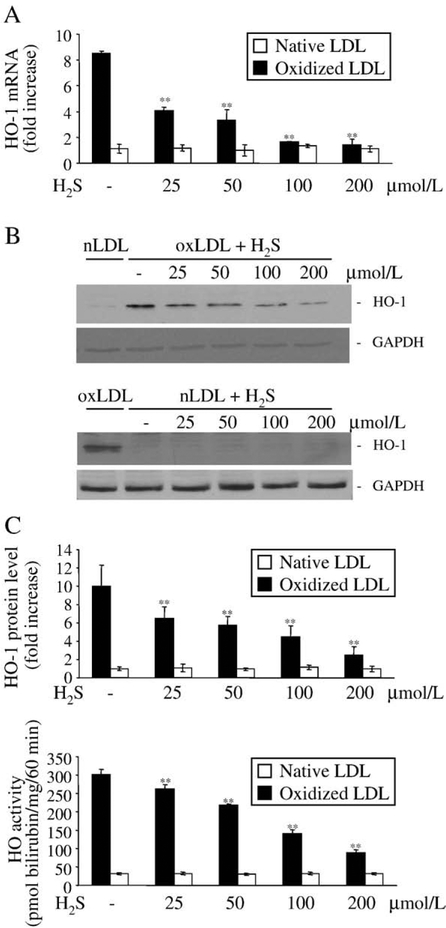
H2S decreases the redox-sensitive heme oxygenase-1 gene induction provoked by oxidized LDL
As a defense mechanism against oxidized LDL-mediated cytotoxicity, endothelial cells up-regulate heme oxygenase-1 when cells are exposed to oxidized LDL at a sublethal dose. Similar to the previous experiment, LDL was first oxidized with heme overnight, and then treated with H2S for 30 min prior to treatment of the cells. As shown on Fig. 5, pretreatment of the oxidized LDL with H2S dose dependently abolished HO-1 induction provoked by oxidized LDL. Treatment of oxidized LDL with 25 μM H2S resulted in a decrease of about 50k in HO-1 mRNA induction (Fig. 5A) provoked by oxidized LDL which was accompanied by an approximately 40k decrease of HO-1 protein induction as shown by Western blot (Fig. 5B). The decreased HO-1 protein expression went along with decreased HO activity of endothelial cells as shown in panel C. In contrast, native LDL in neither the presence nor the absence of H2S provokes the induction of HO-1 (Figs. 5A–C, empty bars).
Fig. 5.
Heme oxygenase-1 induction mediated by oxLDL is diminished by H2S in a dose-responsive manner. Oxidized or native LDL (200 μg/ml) was pretreated with H2S (25, 50, 100, or 200 μmol/L) at 37°C for 30 min, and then diluted 4 times with HBSS. (A) For HO-1 mRNA detection confluent endothelial cells were treated with LDL samples (50 μg/mL) for 1 h, followed by a 4-h incubation in complete media. HO-1 mRNA copy numbers were determined by real-time RT-PCR as described under Materials and methods and were normalized by 18S rRNA copies. Fold increase was calculated using vehicle-treated cells as control (B). To determine HO-1 protein levels HUVECs grown on 6-well plates were treated with LDL samples (50 μg/ml) for 1 h, followed by a 8-h incubation in complete media. Cells were lysed and Western blot was performed as described under Materials and methods. After detection of HO-1 membrane was striped and reprobed for GAPDH to prove equal loading. Quantification of HO-1 induction was performed using computer-assisted videodensitometry. (C) HO-1 activity measured at 8 h after exposure of HUVECs to the same LDL as above. Data for panels A and C are derived from three separate experiments. Error bars denote standard deviations. Statistical significance is indicated by one (P<0.05) or two (P<0.01) asterisks. Western zblot (B) is a representative of three separate experiments.
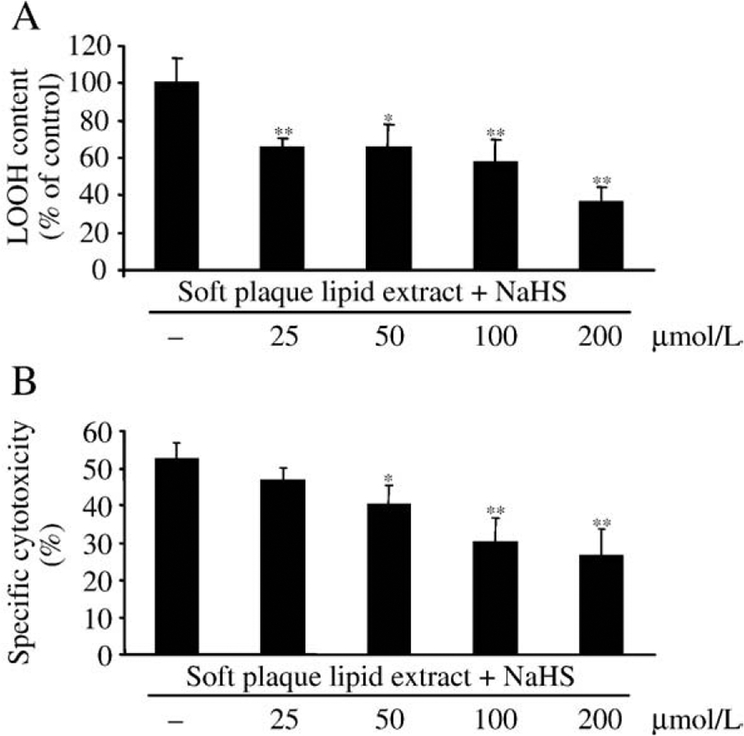
H2S decreases lipid hydroperoxide level and cytotoxicity of lipid extracted from atherosclerotic soft plaque
Hemin exposure of lipid extracted from atherosclerotic lesions leads to the rapid amplification of lipid peroxidation products in the lipid extract, which in turn induces endothelial cytotoxicity because of the high lipid hydroperoxide content. We found that H2S dose dependently decreased the LOOH content of oxidized lipid extract derived from atherosclerotic lesion (Fig. 6A), which was accompanied by decreased endothelial cytotoxicity (Fig. 6B).
Fig. 6.
H2S dose dependently decreases LOOH content and cytotoxicity of oxidized lipid extract derived from soft plaque. Lipid was extracted from human atherosclerotic lesions as described under Materials and methods. Oxidized extracted lipid (1 mg lipid/ml) was oxidized with hemin (5 μmol/L) for 16 h at 37°C and then treated with H2S at the indicated concentrations for 30 min at 37°C. Following H2S treatment LOOH levels of samples were determined (A). To measure the effect of H2S on the cytotoxicity of oxidized lipid extract, HUVECs grown in 96-well plates were treated with samples generated as above for 4 h, and then MTT assay was performed (B). Data are derived from three separate experiments performed in duplicates. Error bars denote standard deviations. Statistical significance is indicated by one (Pb0.05) or two (Pb0.01) asterisks.
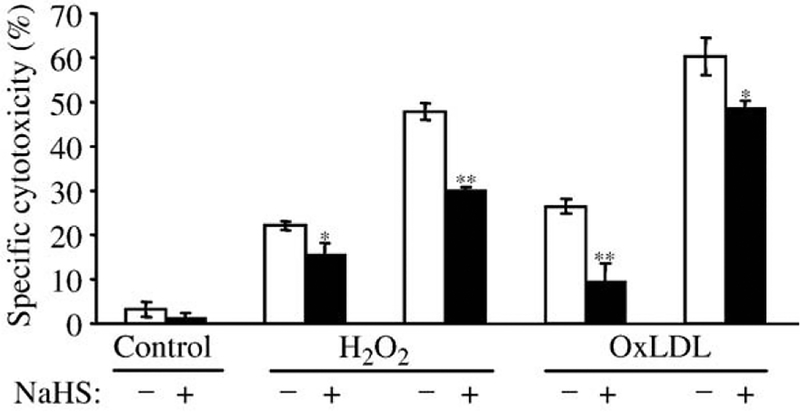
Direct antioxidant effect of H2S against H2O2 or oxidized LDL-mediated endothelial cytotoxicity
We further tested the possibility that H2S might provide protection for endothelial cells against oxidized LDL or hydrogen peroxide-mediated cell cytotoxicity. Endothelial cells were pretreated with H2S (50 μM) for 4 h and then challenged with hemin (5 μmol/L for 1 h), followed by oxidative stress generated by hydrogen peroxide or oxidized LDL. After a 4-h incubation MTT assay was performed. HUVECs pretreated with H2S were more resistant to oxidative damage provoked by both hydrogen peroxide and oxidized LDL, and decreased the cytotoxic effect of H2O2 (100 and 200 μmol/L by 31.8 and 37.5k, respectively, and cytotoxic effect of oxidized LDL (100 and 200 μg protein/ml) by 64.2 and 20.0k, respectively (Fig. 7).
Fig. 7.
H2S protects HUVECs from cytotoxicity mediated by both H2O2 and oxidized LDL. HUVECs grown in 96-well plates were pretreated with H2S (50 μmol/L) at 37°C for 4 h and then challenged with hemin (5 μmol/L at 37°C for 1 h), followed by oxidative stress generated by hydrogen peroxide (100 and 200 μmol/L) or oxidized LDL (100 and 200 μg/ml). After a 4-h incubation MTT assay was performed as described under Materials and methods. Data are derived from five separate experiments performed in triplicates. Error bars denote standard deviations. Statistical significance is indicated by one (Pb0.05) or two (Pb0.01) asterisks.
Discussion
Hydrogen sulfide is increasingly being recognized as an important signaling molecule in the cardiovascular and nervous systems [11]. In the CNS it functions as not only a neuromodulator [32,33], but also a neuroprotectant against oxidative stress [34]. H2S is involved in the regulation of vascular tone [14], myocardial contractility [24], neurotransmission, and insulin secretion [35]. Deficiency of H2S was observed in various animal models of arterial [36] and pulmonary hypertension [37], AlzheimerTs disease [38], gastric mucosal injury [39], and liver cirrhosis [40]. Exogenous H2S ameliorates myocardial dysfunction associated with the ischemia/reperfusion injury [41,42] and reduces the damage of gastric mucosa induced by antiinflammatory drugs [39].
Hemin has been shown to cause endothelial cell damage directly or by promoting the conversion of LDL to cytotoxic oxidized products [4]. In fact there are several types of defenses, which can protect against the deleterious effects of heme. The first line of defense is haptoglobin, an acute phase plasma protein that can bind cell-free hemoglobin dimers and shelter the prosthetic heme groups of hemoglobin [43]. When circulating haptoglobin is depleted, free heme in plasma can be scavenged by hemopexin, a soluble protein that binds free heme with the highest affinity of any other protein described so far [44]. Once the scavenging capacity of hemopexin is exhausted free heme can be scavenged by plasma albumin. Despite the potential for heme binding by plasma proteins, LDL particles successfully compete for heme released from ferrihemoglobin. In fact it has been estimated that approximately 80k of hemin added to whole plasma binds immediately to LDL and high-density lipoprotein [45]. Once heme is inserted into the LDL particle it promotes extensive oxidative modification of LDL, which can be amplified by preformed lipid hydroperoxides within the LDL or other oxidizing agents. This will lead to oxidative scission of the heme group and the release of heme iron, which catalyzes further oxidative breakdown of heme and oxidation of the LDL.
Heme and heme-catalyzed oxidation of LDL are cytotoxic to vascular endothelium. In response, cells up-regulate the last line of defense against the deleterious effects of free heme—the stress responsive genes heme oxygenase-1 and ferritin [4]. Evidence that oxidation of free hemoglobin in plasma could threaten vascular endothelial integrity via oxidative modification of LDL and that toxic species of LDL accumulate in vivo [31] derived from experiments involving LDL isolated from the plasma of the heme oxygenase-1-deficient child reported earlier [46].
Recent studies reveal that H2S is generated by many types of mammalian cells, including vascular smooth muscle cells, and plasma concentration of hydrogen sulfide is around 50–100 μmol/L in humans [12]. H2S is a strong reductant and may easily react with other compounds, especially with reactive oxygen and nitrogen species (ROS and RNS). It has been shown that H2S reacts with superoxide anion [47], hydrogen peroxide [24], peroxynitrite [48], and hypochlorite [49], leading, in all cases, to the protection of proteins and lipids from ROS/RNS-mediated damage.
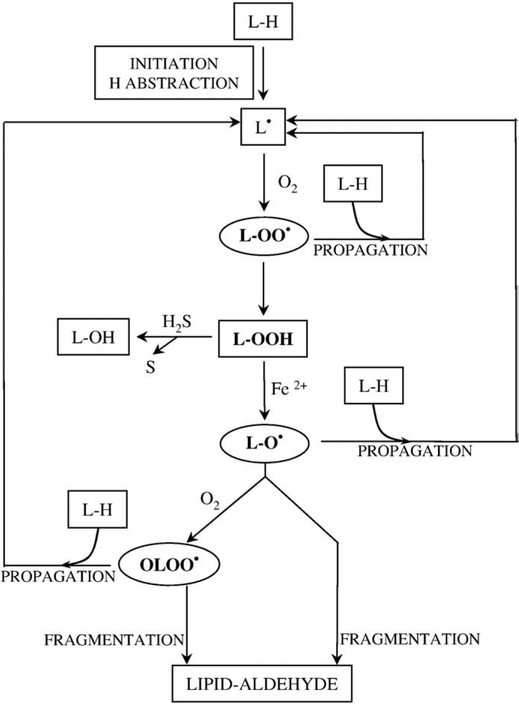
In our study we have examined the role that H2S may play in the hemin-mediated oxidative modification of LDL and consequent endothelial cell reactions. We found that H2S dose dependently delays the accumulation of lipid peroxidation products during hemin-mediated oxidative modification of LDL. Although we have previously shown that hemin at concentrations between 3 and 5 μmol/L does not strictly influence the kinetics of hemin-mediated LDL oxidation [50], to understand the nature of this inhibition we first tested the ability of H2S to reduce the availability of hemin in our system. In fact, high concentrations of H2S in an aqueous solution can readily reduce hemin content. In contrast, low concentrations of H2S (up to 10 μmol/L) in the presence of LDL did not reduce hemin content of the reaction mixture, perhaps because hemin intercalated into the LDL was unavailable for reaction. H2S is a strong reductant, which can readily react with H2O2 resulting in H2O and sulfur. Based on the analogy between chemical reactions of inorganic and organic peroxides, we hypothesized that H2S can slow down heme-mediated lipid peroxidation by reacting with LOOH. Radical species propagate transition metal-induced lipid peroxidation, lipid peroxyl- and lipid alkoxyl radicals, and as previously demonstrated [53] epoxy-allylic peroxyl radicals (OLOO·). As LOOH is the ultimate source of alkoxyl radicals and epoxy-allylic peroxyl radicals, its elimination with H2S would explain the observed effect (Fig. 8). Nevertheless, the possibility that H2S oxidation products may influence LDL oxidation remains as another mechanism that is untested at this time. Treating oxidized LDL with H2S resulted in a dose-dependent decrease of the lipid hydroperoxide content of the oxidized LDL, while levels of other lipid peroxidation products—conjugated dienes and TBARS—remained unaffected. In previous studies we have shown that there is a strong connection between the lipid hydroperoxide content and the toxicity of the LDL oxidized by hemin, and that the enzymatic reduction of LOOH to LOH yields LDL with minimal toxicity [26]. This observation and the fact that the cytotoxic effect of oxidized LDL could be mimicked by an organic hydroperoxide, cumene hydroperoxide, on an equimolar basis prompted us to hypothesize that the reduced lipid hydroperoxide content of H2S-treated oxidized LDL is consonant with its reduced endothelial cytotoxicity.
Fig. 8.
Proposed mechanism of the protective effect of H2S. Lipid peroxidation starts with a H atom abstraction which leads to the formation of a lipid radical (L•). Lipid radical reacts with moleacular oxygen forming lipid peroxyl radical, one of the radical species which can further propagate lipid peroxidation by reacting with an adjacent polyunsaturated fatty acid producing a new lipid radical and lipid hydroperoxide (LOOH). In the presence of transition metals, e.g., iron, LOOH can form alkoxyl radicals (LO•) and epoxy-allylic peroxyl radicals (OLOO•) which can propagate the reaction. As LOOH is the ultimate source of alkoxyl radicals and epoxy-allylic peroxyl radicals its decomposition by H2S to lipid alcohols (L-OH) slows down lipid peroxidation.
The present results agree with these earlier observations and show that the reduction of lipid hydroperoxide content by H2S attenuates cytotoxic effects of oxidized LDL. We have previously shown that oxidized LDL at sublethal doses up-regulates the HO-1 gene. Agarwal et al. recently investigated the mechanism by which oxidized LDL regulates the expression of HO-1. They found that among the components of oxLDL, the most potent inducer of HO-1 is a lipid hydroperoxide—the 13-hydroperoxyoctadecadienoic acid (13-HPODE), which transcriptionally regulates the HO-1 through a 13-HPODE-specific regulatory element in the human HO-1 promoter [51]. In our previous study we have demonstrated that the HO-1 inducing ability of oxidatively modified LDL strongly depends on its total LOOH content; hence we then examined the effect of H2S on the induction of HO-1 provoked by oxidized LDL. Our results demonstrate that pretreatment of the oxidized LDL with H2S dose dependently decreases HO-1 mRNA and protein induction and HO-1 activity of the cells.
Oxidative modification of LDL is a key event in the development of atherosclerosis which disorder is characterized by thickening and hardening of the vessel wall due to accumulation of lipids, fibrous tissue, blood components, and calcium in the subendothelial space. Lipid peroxidation products are present in lipid extracts derived from atherosclerotic lesions, and hemin exposure further amplifies LOOH content and cytotoxicity of lipid extracts. In this study we have shown that H2S dose dependently decreases LOOH content and cytotoxic effects of oxidized lipid extract derived from atherosclerotic lesion. Interestingly, recent studies have shown that H2S protects neurons from glutamate-induced [34] and vascular smooth muscle cells from homocysteine-induced oxidative stress [52]. Our present results indicate that H2S pretreatment of cells inhibited cytotoxicity provoked by both H2O2 and oxidized LDL, indicating that H2S may play a vital role as an antioxidant.
We also investigated the pH dependency of this reaction. Our results indicate that at lower pH (5.0) formation of lipid hydroperoxides, TBARS, and conjugated dienes are all increased (data not shown). On the other hand, the protective effects of H2S are significantly decreased in such an acidic environment. We offer two hypotheses: (i) Since the effects of H2S are likely to be evanescent given that it is a gas with a vapor pressure of 18.75 105 Pa, we hypothesize that this acidic pH facilitates the evaporation of H2S, hence decreasing its availability in the solution. (ii) Considering an in vivo situation a local acidic pH is a common consequence of local inflammation. Vascular smooth muscle cells are able to synthesize up to 100 μM H2S under physiological conditions. Hence one may argue that inflammation, which is accompanied with acidic pH, can decrease the protective effects of such physiological concentrations that may translate to increased susceptibility of LDL to be oxidized.
Our findings indicate a novel role for H2S in inhibiting hemin-induced oxidative modification of LDL through a mechanism involving a reduction of LOOH content. Downstream processes induced by LOOH—like endothelial cytotoxicity or induction of HO-1—are also abolished by H2S. These results may shed new light on the relationship between H2S and atherosclerosis and perhaps may lead to new approaches for combating complications related to atherosclerosis.
Acknowledgments
This work was supported by Hungarian Government Grants OTKA-K61546, ETT-337/2006, RET-06/2004, and MTA-DE-11003. We thank Erika Barna for technical assistance.
Abbreviations:
- HBSS
HankTs balanced salt solution
- HO-1
heme oxygenase-1
- H2S
hydrogen sulfide
- HUVECs
human umbilical vein endothelial cells
- LDL
low-density lipoprotein
- LOOH
lipid hydroperoxides
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- oxLDL
oxidized LDL
- BARS
thiobarbituric acid-reactive substances
References
- [1].Steinberg D; Parthasarathy S; Carew TE; Khoo JC; Witztum IL Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med 320:915–924; 1989. [DOI] [PubMed] [Google Scholar]
- [2].Steinberg D Oxidative modification of LDL and atherogenesis. Circulation 95: 1062–1071; 1997. [DOI] [PubMed] [Google Scholar]
- [3].Bunn HF; Jandl JH Exchange of heme among hemoglobins and between hemoglobin and albumin. J. Biol. Chem 243:465–475; 1968. [PubMed] [Google Scholar]
- [4].Balla G; Jacob HS; Eaton JW; Belcher JD; Vercellotti GM Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial cell injury. Arterioscler. Thromb 11:1700–1711; 1991. [DOI] [PubMed] [Google Scholar]
- [5].Abraham NG; Lavrovsky Y; Schwartzman ML; Stoltz RA; Levere RD; Gerritsen ME; Shibahara S; Kappas A Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc. Natl. Acad. Sci. USA 92:6798–6802; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Agarwal A; Balla J; Balla G; Croatt AJ; Vercellotti GM; Nath KA Renal tubular epithelial cells mimic endothelial cells upon exposure to oxidized LDL. Am. J. Physiol 271:814–823; 1996. [DOI] [PubMed] [Google Scholar]
- [7].Balla G; Jacob HS; Balla J; Rosenberg M; Nath K; Apple F; Eaton WJ; Vercellotti GM Ferritin: A cytoprotective antioxidant strategem of endothelium. J. Biol. Chem 267:18148–18153; 1992. [PubMed] [Google Scholar]
- [8].Balla J; Jacob HS; Balla G; Nath K; Eaton JW; Vercellotti GM Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc. Natl. Acad. Sci. USA 90:9285–9289; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang LJ; Lee TS; Lee FY; Pai RC; Chau LY Expression of heme oxygenase-1 in atherosclerotic lesions. Am. J. Pathol 152:711–720; 1998. [PMC free article] [PubMed] [Google Scholar]
- [10].Ishikawa K; Sugawara D; Wang XP; Suzuki K; Itabe H; Maruyama Y; Lusis AJ Heme oxygenase-1 inhibits atherosclerotic lesion formation in LDL receptor knockout mice. Circ. Res 88:506–512; 2001. [DOI] [PubMed] [Google Scholar]
- [11].Wang R TwoTs company, threeTs a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 16:1792–1798; 2002. [DOI] [PubMed] [Google Scholar]
- [12].Doeller JE; Isbell TS; Benavides G; Koenitzer J; Patel H; Patel RP; Lancaster JR Jr. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal. Biochem 341:40–51; 2005. [DOI] [PubMed] [Google Scholar]
- [13].Erickson PF; Maxwell IH; Su LJ; Baumann M; Glode LM Sequence of cDNA for rat cystathionine -lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem. J 269:335–340; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao W; Zhang J; Lu Y; Wang R The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 20:6008–6016; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sivarajah A; McDonald MC; Thiemermann C The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock 26:154–161; 2006. [DOI] [PubMed] [Google Scholar]
- [16].Elrod JW; Calvert JW; Morrison J; Doeller JE; Kraus DW; Tao L; Jiao X; Scalia R; Kiss L; Szabo C; Kimura H; Chow CW; Lefer DJ Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 104:15560–15565; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang G; Sun X; Wang R Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 18:1782–1784; 2004. [DOI] [PubMed] [Google Scholar]
- [18].Yang G; Wu L; Wang R Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 20:553–555; 2006. [DOI] [PubMed] [Google Scholar]
- [19].Du J; Hui Y; Cheung Y; Bin G; Jiang H; Chen X; Tang C The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels 19:75–80; 2004. [DOI] [PubMed] [Google Scholar]
- [20].Jeong SO; Pae HO; Oh GS; Jeong GS; Lee BS; Lee S; Kim du Y Hydrogen sulphide potentiates interleukin-1-induced nitric oxide production via enhancement of extracellular signal-regulated kinase activation in rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun 345:938–944; 2006. [DOI] [PubMed] [Google Scholar]
- [21].Wu SY; Pan CS; Geng B; Zhao J; Yu F; Pang YZ; Tang CS Hydrogen sulphide ameliorates vascular calcification induced by vitamin D3 plus nicotine in rats. Acta Pharmacol. Sin 27:299–306; 2006. [DOI] [PubMed] [Google Scholar]
- [22].Kamoun P; Belardinelli MC; Chabli A; Lallouchi K; Chadefaux-Vekemans B Endogenous hydrogen sulphide overproduction in Down syndrome. Am. J. Med. Genet 116:310–311; 2003. [DOI] [PubMed] [Google Scholar]
- [23].Lynch SM; Campione AL; Moore MK Plasma thiols inhibit hemin-dependent oxidation of human low-density lipoprotein. Biochim. Biophys. Acta 1485:11–22; 2000. [DOI] [PubMed] [Google Scholar]
- [24].Geng B; Chang L; Pan C; Qi Y; Zhao J; Pang Y; Du J; Tang C Endogenous hydrogen sulphide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun 318:756–763; 2004. [DOI] [PubMed] [Google Scholar]
- [25].Stary HC; Chandler AB; Dinsmore RE; Fuster V; Glagov S; Insull W Jr.; Rosenfeld ME; Schwartz CJ; Wagner WD; Wissler RW A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation 92:1355–1374; 1995. [DOI] [PubMed] [Google Scholar]
- [26].Nagy E; Jeney V; Yachie A; Szab RP; Wagner O; Vercellotti GM; Eaton JW; Balla G; Balla J Oxidation of hemoglobin by lipid hydroperoxide associated with low-density lipoprotein (LDL) and increased cytotoxic effect by LDL oxidation in heme oxygenase-1 (HO-1) deficiency. Cell. Mol. Biol. (Noisy-le-grand) 51:377–385; 2005. [PubMed] [Google Scholar]
- [27].Belcher JD; Balla J; Balla G; Jacobs DR Jr.; Gross M; Jacob HS; Vercellotti GM Vitamin E, LDL, and endothelium. Brief oral vitamin supplementation prevents oxidized LDL-mediated vascular injury in vitro. Arterioscler. Thromb 13:1779–1789; 1993. [DOI] [PubMed] [Google Scholar]
- [28].Bligh EG; Dyer WJ A rapid method of total lipid extraction and purification. Can. J. Biochem. Biophys 37:911–917; 1959. [DOI] [PubMed] [Google Scholar]
- [29].Jiang ZY; Hunt JV; Wolff SP Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem 202:384–389; 1992. [DOI] [PubMed] [Google Scholar]
- [30].Berzofsky JA; Peisach J; Horecker BL Sulfheme proteins. IV. The stoichiometry of sulfur incorporation and the isolation of sulfhemin, the prosthetic group of sulfmyoglobin. J. Biol. Chem 247:3783–3791; 1972. [PubMed] [Google Scholar]
- [31].Jeney V; Balla J; Yachie A; Varga Z; Vercellotti GM; Eaton JW; Balla G Prooxidant and cytotoxic effects of circulating heme. Blood 100:879–887; 2002. [DOI] [PubMed] [Google Scholar]
- [32].Abe K; Kimura H The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci 16:1066–1071; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kimura H Hydrogen sulfide as a neuromodulator. Mol. Neurobiol 1:13–19; 2002. [DOI] [PubMed] [Google Scholar]
- [34].Kimura Y; Kimura H Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 10:1165–1167; 2004. [DOI] [PubMed] [Google Scholar]
- [35].Yang W; Yang G; Jia X; Wu L; Wang R Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol 569:519–531; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yan H; Du J; Tang C The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem. Biophys. Res. Commun 313:22–27; 2004. [DOI] [PubMed] [Google Scholar]
- [37].Chunyu Z; Junbao D; Dingfang B; Hui Y; Xiuying T; Chaoshu T The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem. Biophys. Res. Commun 302:810–816; 2003. [DOI] [PubMed] [Google Scholar]
- [38].Eto K; Asada T; Arima K; Makifuchi T; Kimura H Brain hydrogen sulfide is severely decreased in AlzheimerTs disease. Biochem. Biophys. Res. Commun 293: 1485–1488; 2002. [DOI] [PubMed] [Google Scholar]
- [39].Fiorucci S; Antonelli E; Distrutti E; Rizzo G; Mencarelli A; Orlandi S; Zanardo R Inhibition of hydrogen sulfide generation contributes to gastric injury caused by non-steroidal anti-inflammatory drugs. Gastroenterology 129:1210–1224; 2005. [DOI] [PubMed] [Google Scholar]
- [40].Fiorucci S; Antonelli E; Mencarelli A; Orlandi S; Renga B; Rizzo G; Distrutti E The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 42:539–548; 2005. [DOI] [PubMed] [Google Scholar]
- [41].Bian JS; Yong QC; Pan TT; Feng ZN; Ali MY; Zhou S; Moore PK Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J. Pharmacol. Exp. Ther 316:670–678; 2006. [DOI] [PubMed] [Google Scholar]
- [42].Zhu YZ; Wang ZJ; Ho P; Loke YY; Zhu YC; Huang XW; Huang SH Hydrogen sulfide and its cardioprotective effects in myocardial ischemia in experimental rats. J. Appl. Physiol 102:261–268; 2007. [DOI] [PubMed] [Google Scholar]
- [43].Kristiansen M; Graversen JH; Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature 409:198–201; 2001. [DOI] [PubMed] [Google Scholar]
- [44].Hrkal Z; Vodrzka Z; Kalousek I Transfer of heme from ferrihemoglobin and ferrihemoglobin isolated chains to hemopexin. Eur. J. Biochem 15:73–78; 1974. [DOI] [PubMed] [Google Scholar]
- [45].Miller YI; Shaklai N Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim. Biophys. Acta 1454:153–164; 1999. [DOI] [PubMed] [Google Scholar]
- [46].Yachie A; Niida Y; Wada T; Igarashi N; Kaneda H; Toma T; Ohta K; Kasahara Y; Koizumi S Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest 103:129–135; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mitsuhashi H; Yamashita S; Ikeuchi H; Kuroiwa T; Kaneko Y; Hiromura K; Ueki K Oxidative stress dependent conversion of hydrogen sulfide to sulfite by activated neutrophils. Shock 24:529–534; 2005. [DOI] [PubMed] [Google Scholar]
- [48].Whiteman M; Armstrong JS; Chu SH; Jia-Ling S; Wong BS; Cheung NS; Halliwell B The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite dscavengerT? J. Neurochem 90:765–768; 2004. [DOI] [PubMed] [Google Scholar]
- [49].Whiteman M; Cheung NS; Zhu YZ; Chu SH; Siau JL; Wong BS; Armstrong JS Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem. Biophys. Res. Commun 326:794–798; 2005. [DOI] [PubMed] [Google Scholar]
- [50].Ujhelyi L; Balla J; Muszbek L; Kakuk G; Belcher J; Jacob HS; Vercellotti GM; Balla G A microassay to assess the oxidative resistance of low-density lipoproteins. Clin. Chem 44:1762–1764; 1998. [PubMed] [Google Scholar]
- [51].Hill-Kapturczak N; Voakes C; Garcia J; Visner G; Nick HS; Agarwal A A cis-acting region regulates oxidized lipid-mediated induction of the human heme oxygenase-1 gene in endothelial cells. Arterioscler. Thromb. Vasc. Biol 23:1416–1422; 2003. [DOI] [PubMed] [Google Scholar]
- [52].Yan SK; Chang T; Wang H; Wu L; Wang R; Meng QH Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem. Biophys. Res. Commun 351:485–491; 2006. [DOI] [PubMed] [Google Scholar]
- [53].Thomas A; Dix; Lawrence J., Marnett. Conversion of linoleic acid hydroperoxide to hydroxy,keto,epoxyhydroxy, and trihydroxy fatty acids by hematin. J. Biol. Chem 260:5351–5357; 1985. [PubMed] [Google Scholar]