Abstract
Background
Insulin is stored within large dense-core granules in pancreatic beta (β)-cells and is released by Ca2+-triggered exocytosis with increasing blood glucose levels. Polarized and targeted secretion of insulin from β-cells in pancreatic islets into the vasculature has been proposed; however, the mechanisms related to cellular and molecular localization remain largely unknown. Within nerve terminals, the Ca2+-dependent release of a polarized transmitter is limited to the active zone, a highly specialized area of the presynaptic membrane. Several active zone-specific proteins have been characterized; among them, the CAST/ELKS protein family members have the ability to form large protein complexes with other active zone proteins to control the structure and function of the active zone for tight regulation of neurotransmitter release. Notably, ELKS but not CAST is also expressed in β-cells, implying that ELKS may be involved in polarized insulin secretion from β-cells.
Scope of review
This review provides an overview of the current findings regarding the role(s) of ELKS and other active zone proteins in β-cells and focuses on the molecular mechanism underlying ELKS regulation within polarized insulin secretion from islets.
Major conclusions
ELKS localizes at the vascular-facing plasma membrane of β-cells in mouse pancreatic islets. ELKS forms a potent insulin secretion complex with L-type voltage-dependent Ca2+ channels on the vascular-facing plasma membrane of β-cells, enabling polarized Ca2+ influx and first-phase insulin secretion from islets. This model provides novel insights into the functional polarity observed during insulin secretion from β-cells within islets at the molecular level. This active zone-like region formed by ELKS at the vascular side of the plasma membrane is essential for coordinating physiological insulin secretion and may be disrupted in diabetes.
Keywords: Pancreatic β-cells, ELKS, Active zone protein, Voltage-dependent Ca2+ channel, Insulin exocytosis, Ca2+ influx
Abbreviations: VDCC, voltage-dependent Ca2+ channel; KO, knockout; TIRFM, total internal reflection fluorescence microscopy; VDA, voltage-dependent activation; VDI, voltage-dependent inactivation; ECM, extracellular matrix
1. Introduction
The hormone insulin is stored in large dense-core granules within pancreatic β-cells and is released by exocytosis in response to elevated extracellular glucose concentrations. Triggered insulin exocytosis appears to involve increases in intracellular ATP concentrations, closure of ATP-sensitive K+ channels, membrane depolarization, and Ca2+ influx through voltage-dependent Ca2+ channels (VDCCs) [1]. However, exocytotic “hot spots” and polarized insulin granule exocytosis in β-cells remain elusive.
Regarding rodent islets, β-cells are generally structured in rosettes of cells surrounding the central pancreatic venous vasculature [2], [3], [4]. There is increasing evidence suggesting that the β-cell plasma membrane encompasses functionally separated compartments, indicating β-cell polarity [5]. In support of this, E-cadherin and GLUT2 have been shown to be located on the lateral regions of the plasma membrane [6], [7]. In the vascular apogee (i.e., the side of the plasma membrane opposite the vasculature), PAR-3 and ZO-1 tend to be positioned where adjacent β-cells project their primary cilia [7].
Interestingly, electron microscopy indicates that insulin granules are enriched along the vascular-facing plasma membrane following cellular stimulation [2], supporting the concept of polarized secretion, i.e., insulin is released from the vascular-facing plasma membrane toward the bloodstream in a Ca2+-dependent manner. The location of insulin exocytosis is under debate since a two-photon imaging study showed that most events are occurring at the “abvascular” compartment of β-cells within intact islets (i.e., cell–cell interfaces away from blood vessels) [8]. However, when two-photon imaging with 3D-measurements were recently employed, it was clear that insulin exocytosis mostly takes place at the vascular-facing plasma membrane of β-cells [9]. Despite these findings, there is still a lack of information on the molecular mechanism of the polarity of the Ca2+ influx and insulin secretion from pancreatic islets.
In neurons, the release of a transmitter, which has a specific polarity, takes place at specialized active zones on the presynaptic membrane. Active zones are defined as slightly electron-dense regions beneath the presynaptic plasma membrane [10]. The cytomatrix at the active zone contains the molecular machinery necessary for docking and fusion of synaptic vesicles [11]. This unique and vital structure contains active zone-specific protein families, including RIM1, Munc13-1, Piccolo (i.e., Aczonin), Bassoon, CAST (i.e., ERC2), and ELKS (i.e., ERC1) [12], [13]. Additionally, VDCCs, cytoskeletal proteins, and SNAREs are associated with the cytomatrix at the active zone [11]. In particular, the CAST/ELKS protein family has been reported to directly bind to Bassoon, Piccolo, and RIM1 and indirectly to Munc13-1 through RIM1, suggesting that CAST/ELKS family proteins may act as a platform for a dynamic multicomplex at the active zone [14], [15]. Moreover, growing evidence suggests the structure and function of the active zones are controlled by CAST and ELKS to tightly regulate polarized neurotransmitter release [15], [16]. Interestingly, ELKS but not CAST is also expressed in pancreatic β-cells and localizes at the vascular-facing plasma membrane of β-cells within islets [17]. We further found that ELKS is one of the key players regulating polarized Ca2+ influx and insulin secretion from islet β-cells [18].
In this review, we provide a brief summary of the recent findings related to the molecular structures and biochemical properties of the CAST/ELKS protein family and discuss the potential role(s) of ELKS in polarized insulin secretion from β-cells.
2. Molecular structures and biochemical properties of the CAST/ELKS protein family in the presynaptic active zone
CAST was first purified from the rat brain [19] and was independently identified by the yeast two-hybrid system as ERC2 [20]. CAST has coiled-coil regions throughout its entire protein structure and contains a unique three amino acid sequence (IWA) at its C-terminus (Figure 1A). CAST has been shown to directly interact with the PDZ domain of RIM1 via this IWA motif [19], [20]. Other active zone proteins, such as Bassoon, Piccolo, and Munc13-1, bind directly to the middle region of CAST [14], [21] (Figure 1A). Additionally, CAST indirectly binds to Munc13-1 through RIM1 [14]. ELKS is a member of the CAST protein family, and it has been reported that its encoding gene is translocated to a different chromosome in thyroid carcinoma [22]. ELKS is named after its high content of the amino acids E, L, K, and S [22]. In 2002, three groups independently identified ELKS within different systems and renamed it CAST2, Rab6IP2, and ERC1 [19], [20], [23], [24]. However, as this protein was first identified as ELKS [22], we used this terminology within this review to avoid confusion. CAST and ELKS show relatively high homology (∼70% amino acid identity), their molecular weight is ∼120 kDa, and they appear to form oligomers with each other [25]. As with CAST, ELKS has been reported to directly bind to Bassoon, Piccolo, and RIM1 and indirectly to Munc13-1 through RIM1 [25]. In addition, the brain-specific variant of Munc13-2, bMunc13-2, interacts with the C-terminal region of ELKS but not with CAST [26] (Figure 1A).
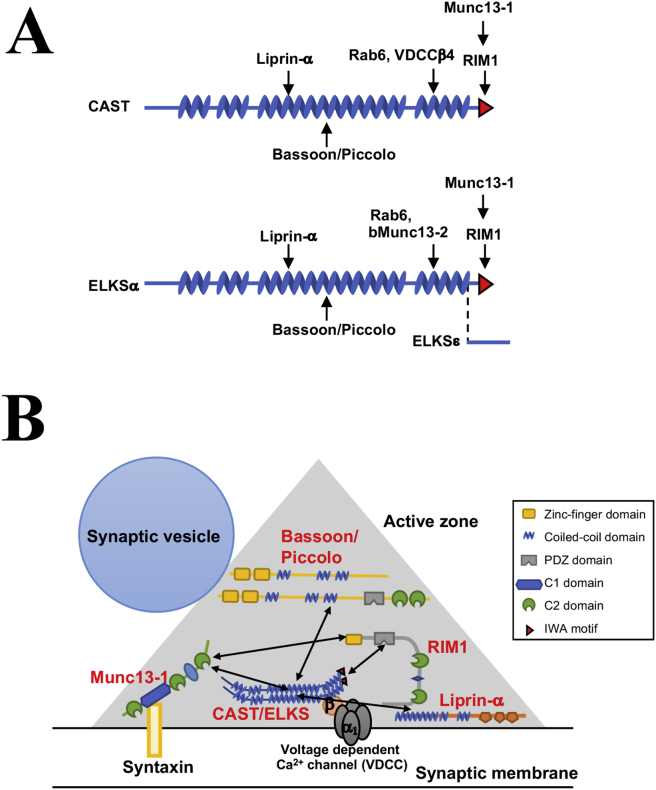
Figure 1.
Molecular structure of CAST and ELKS in presynaptic active zones. (A) Molecular structure of CAST and ELKS. CAST and ELKS are predicted to contain four coiled-coil structures, illustrated as zig-zag objects. The red triangle is the PDZ-binding IWA motif. Arrows indicate binding sites of interacting molecules. ELKSε is the C-terminal spliced isoform. (B) Schema of the interactions of presynaptic active zone proteins and ELKS/CAST-binding proteins.
The physiological significance of the CAST/ELKS-mediated active zone protein interactions remains unknown, but it is well recognized that these CAST/ELKS-dependent protein–protein interactions among active zone proteins may be the molecular basis for the integrity of presynaptic active zones (Figure 1B). Additionally, disruption of CAST-RIM1 binding and/or CAST-Bassoon binding have been shown to significantly impair synaptic transmission in cultured neurons [14]. In addition to these active zone proteins, CAST/ELKS share many synaptic proteins as binding partners, such as Liprin-α, Rab6, and VDCCs [23], [27], [28]. VDCCs such as the N-, P/Q-, R-, and L-types are essential for neurotransmitter release at presynaptic active zones [29], [30], [31], [32]. VDCCs are heteromultimeric protein complexes composed of the pore-forming α1-subunit and the auxiliary α2/δ-, β-, and γ-subunits [33], [34]. In particular, there is evidence of physical and functional interactions between CAST/ELKS family members and VDCCs. CAST and ELKS directly interact with the VDCC-β subunit (β4) [28], [35], and in a BHK cell co-expression system CAST slightly shifts the I-V relationship to the hyperpolarizing direction by about 5 mV without affecting current density [28]. Consistent with these observations, Kaeser et al. recently reported that ELKS enhances presynaptic Ca2+ influx to boost the release probability at inhibitory hippocampal nerve terminals with a Ca2+-imaging method [36]. More recently, electrophysiological analyses of the CAST/ELKS and VDCC relationship have been performed in vivo. For example, the presynaptic Ca2+ influx was strongly reduced within rod photoreceptors of CAST-knockout (KO) and CAST/ELKS-double-KO mice as analyzed by whole-cell voltage-clamp recordings [37]. Moreover, in the calyx of Held synapses in CAST/ELKS-double-KO mice, both VDCC currents and numbers were significantly decreased as was the readily releasable pool size. Intriguingly, loss of CAST/ELKS increased the probability of synaptic vesicle release. It has been suggested that CAST/ELKS complexes regulate the probability of synaptic vesicle release through post-priming, a late step controlling synaptic vesicle release. Several other reviews and articles describe the CAST/ELKS family with additional detail [13], [15], [16].
Therefore, CAST and ELKS tightly regulate the release of polarized neurotransmitters by controlling the structure and function of the active zones; however, other than the brain, the mechanisms by which active zone proteins contribute to cellular functions—such as hormone secretion from endocrine cells—remain poorly understood.
3. ELKS localizes at the vascular-facing plasma membrane of β-cells
β-cells appear to lack active zones, which are ultrastructurally characterized as electron-dense regions of cytoskeletal filaments underneath the plasma membrane. However, active zone proteins, such as ELKS, RIM2, Munc13-1, Piccolo, and Bassoon, and synaptic scaffold proteins, such as Liprin-α, were also found in pancreatic β-cells [9], [17], [38], [39], [40], [41]. Some of these proteins may be involved in the regulation of insulin secretion. Treatment of islets with antisense oligodeoxynucleotides against Piccolo reduced insulin secretion evoked by glucose and a cAMP analog [38]. RIM2 has been demonstrated to regulate the docking and priming of insulin granule exocytosis in a study using RIM2-KO mice [41]. Munc13-1 acts as a priming factor in insulin exocytosis [40]. Thus, identifying the roles of active zone proteins in pancreatic β-cells remains a focus within insulin exocytosis research; however, the functional relationship of these proteins with polarized insulin secretion has not been elucidated.
Because CAST and ELKS have been implicated in the Ca2+-dependent exocytosis of neurotransmitters as described above, we hypothesized that CAST and ELKS are potential candidates for localization at insulin exocytotic “hot spots” in β-cells [17]. Islets in rats express ELKS but not CAST proteins, whereas the brain expresses both CAST and ELKS proteins (Figure 2A). In rodents, islets express two major ELKS splice variants: ELKSα (brain isoform, ∼120 kDa) and ELKSε (ubiquitous isoform, ∼140 kDa) [18]. These variants have a distinct C-terminus. ELKSα has the IWA amino acid motif (Figure 1A).
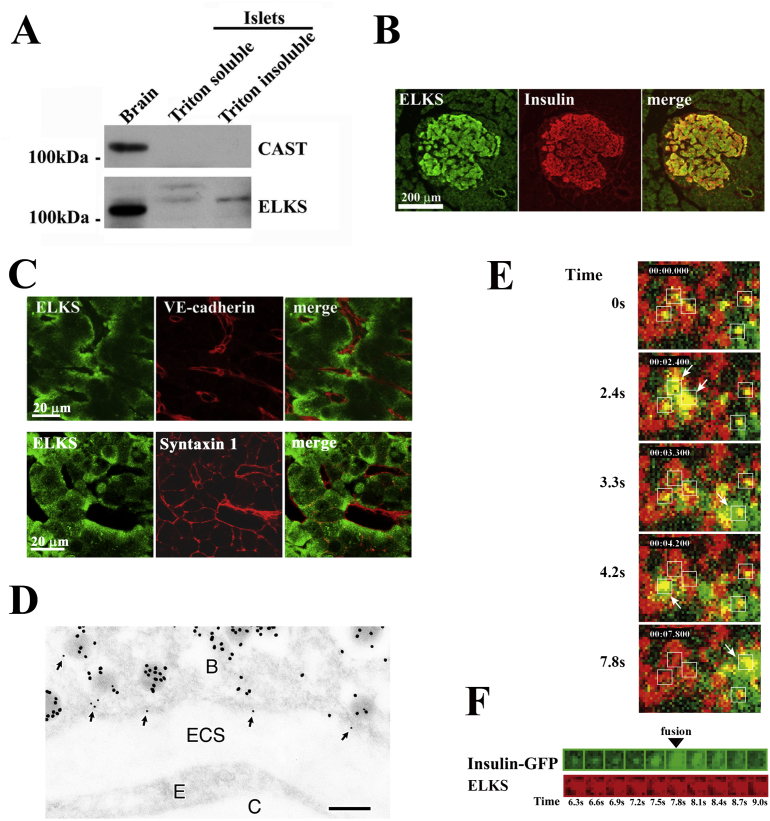
Figure 2.
ELKS is expressed in pancreatic islet β-cells. (A) Immunoblot analysis of pancreatic islet lysates using anti-ELKS and anti-CAST antibodies. Rat brain homogenate was used as a positive control. (B) Pancreatic sections were stained for ELKS and insulin. (C) Localization of ELKS, VE-cadherin (an endothelial cell marker), and Syntaxin 1 in islets. Islets were double-stained using anti-ELKS pAb and anti-VE-cadherin mAb or anti-Syntaxin 1 mAb. (D) Ultrastructural localization of ELKS in β-cells. Note that immunoreactivity of ELKS (small gold particles) was frequently detected close to insulin-containing granules (large gold particles) with docking at the plasma membrane facing a blood capillary (arrows). B: β-cell, ECS: extracellular space, E: endothelial cell, C: blood capillary. Bar, 0.2 μm. (E) ELKS clusters are sites for insulin granule docking and fusion, with docking and fusion of insulin granules occurring at these clusters. TIRF image of GFP-tagged insulin granules and Cy3-labeled ELKS clusters in MIN6 cells and dual-image analysis of GFP-tagged insulin granule motion at ELKS clusters following 50 mM KCl stimulation. The box (1 × 1 μm) indicates the granule to be fused. Timestamp (min:sec:msec) was overlaid. Time 0 indicates the addition of KCl. (F) Sequential images (1 × 1 μm, 300-ms intervals) of a single insulin granule (green) at an ELKS cluster (red) upon stimulation with 50 mM KCl. Adapted from Ohara-Imaizumi et al. (2005) [17].
Double staining for insulin and ELKS showed immunoreactivity of ELKS in insulin-positive β-cells in pancreas sections, indicating that ELKS is most abundant in β-cells (Figure 2B). Higher magnification confocal imaging of islets showed that ELKS was localized at the plasmalemmal region of β-cells, especially those facing blood capillaries labeled with VE-cadherin, a marker of endothelial cells (Figure 2C). However, this pattern differed from the immunostaining pattern of the exocytotic SNARE protein, Syntaxin 1, as it was seen on the entire plasma membrane [42]. In neurons, the t-SNAREs, Syntaxin 1, and SNAP-25, are similarly present on the entire axonal plasma membrane, and are not specifically localized to terminal nerve active zones [43].
Furthermore, immunogold electron microscopy confirmed that ELKS (labeled with small gold particles) localized to the plasma membrane facing the vasculature and was frequently detected in close proximity to insulin (large gold particles)-containing granules docked on the plasma membrane (Figure 2D). Thus, ELKS localizes to the docking sites of insulin granules at the β-cell plasma membrane, and in particular, accumulates near the vasculature in islets, implying that it plays a role in insulin granule exocytosis.
4. ELKS defines the fusion site of insulin granules
In single β-cells cultured from dispersed islets, such localization of ELKS was not observed at the plasma membrane. ELKS immunostaining was primarily observed throughout the plasma membrane in single β-cells and MIN6 β-cells, which predominantly express ELKS but not CAST [17]. We further examined ELKS distribution in the plasma membrane of MIN6 cells using total internal reflection fluorescence microscopy (TIRFM) imaging, of which the evanescent field illumination reaches a <100-nm-thick layer immediately adjacent to the cover glass and illuminates only the plasma membrane under our TIRF conditions [44], [45]. We found that ELKS immunofluorescence was unevenly but locally distributed at separate clusters within the plasma membrane. Interestingly, double immunostaining for ELKS and insulin suggested that the former may interact with insulin granule docking sites. However, we previously showed that Syntaxin 1 clusters are essential for the docking and fusion of insulin granules [46], [47]. We further explored the interactions between insulin granule docking sites, ELKS, and Syntaxin 1 clusters by triple immunostaining analyzed using TIRFM. Most insulin granules colocalized with either ELKS or Syntaxin 1 clusters, and ∼50% of granules docked with ELKS clusters colocalized with Syntaxin 1. In summary, insulin granules preferentially dock with ELKS clusters colocalized with Syntaxin 1 clusters. These results suggest that ELKS may be involved in physiological interactions with insulin granules at docking sites.
Does insulin exocytosis occur at the sites of ELKS clusters? Using live MIN6 cells and TIRF images, we analyzed the interaction(s) between docking and fusion of GFP-tagged insulin granules and ELKS clusters labeled by a TAT-conjugated, Cy3-labeled ELKS antibody. Stimulation of MIN6 cells with high KCl showed that the fusion of insulin granules frequently occurred at ELKS clusters (Figure 2E,F), with ∼60% of all fusion events occurring at these sites. Thus, fusion of insulin granules occurred selectively at ELKS cluster sites in the plasma membrane of MIN6 cells. The link between ELKS and insulin granule fusion sites was also observed in insulin-secreting INS-1 cells [48]. Of note, attenuation of ELKS expression by RNA interference decreased the number of ELKS clusters within the plasma membrane, thereby suppressing glucose-evoked insulin secretion from MIN6 cells [17]. These results indicate that ELKS may regulate insulin granule exocytosis and is a possible candidate for defining the fusion site of insulin exocytosis.
Taken together with the fact that ELKS localizes at the vascular-facing plasma membrane within islets, these data strongly suggest that ELKS forms an active zone-like region at these locations on the β-cell plasma membrane. To confirm this, we performed experiments using β-cell-specific ELKS-KO (ELKS βKO) mice and in situ Ca2+ imaging.
5. ELKS potentiates opening of L-type Ca2+ channels and first-phase insulin secretion
We generated ELKS βKO mice by crossing ELKS floxed (ELKSflox/flox) mice [49] with RIP-Cre mice expressing Cre recombinase in pancreatic β-cells [50] in order to examine the potential role(s) of ELKS in pancreatic β-cells. In islets isolated from ELKS βKO mice, the expression levels of ELKS isoforms were markedly decreased, but no changes were detected in terms of the expression of glucokinase, GLUT2, SNARE proteins required for insulin exocytosis, and active zone proteins (Bassoon, RIM2, and Munc13-1). We confirmed reduction of ELKS expression in insulin-positive β-cells in ELKS βKO mice islets via immunostaining of pancreatic sections (Figure 3A).
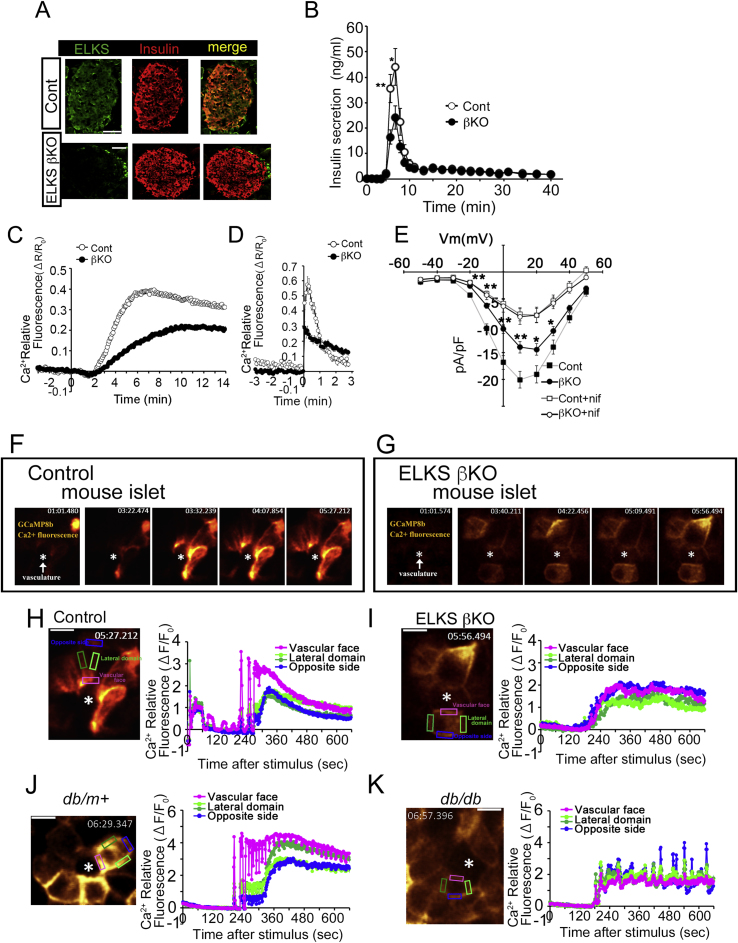
Figure 3.
ELKS controls Ca2+influx via L-type Ca2+channels and first-phase insulin secretion. (A) Immunohistochemical staining for insulin and ELKS in pancreatic islets from control and ELKS βKO mice. Scale bars = 50 μm. (B) Insulin secretion in perfused pancreas in response to glucose (16.7 mM). (C, D) Fura-2 Ca2+ imaging in β-cells from control and ELKS βKO mice stimulated with glucose (22 mM) (C) and high K+ (40 mM) (D). (E) ELKS deficiency reduces the L-type voltage-dependent Ca2+ current. The current (pA/pF)–voltage (mV) relationships recorded in control and ELKS-KO β-cells with or without of nifedipine are shown (nif: 1 μM). (F, G) G-CaMP8b Ca2+ imaging of the β-cell plasma membrane in control (F) and ELKS-KO (G) islets stimulated with glucose (22 mM). The asterisk indicates the vasculature labeled with tomato lectin. (H, I) Typical time course of the fluorescence intensity of G-CaMP8b in the three regions of the β-cell plasma membrane (the vascular-facing plasma membrane, lateral region, and side opposite the vasculature) during glucose stimulation in control (H) and ELKS βKO (I) islets. (J, K) Typical time course of the fluorescence intensity of G-CaMP8b in the three regions of the β-cell plasma membrane (the vascular-facing plasma membrane, lateral region, and side opposite the vasculature) during glucose stimulation in islets from db/m+ mice (J) and db/db mice (K). Scale bars = 10 μm. Time stamps (min:sec:msec) are shown for each image. Results are presented as the means ± SEM. *P < 0.05; **P < 0.01. Adapted from Ohara-Imaizumi et al. (2019) [18].
The oral glucose tolerance test showed impaired glucose tolerance in ELKS βKO mice. Within ELKS βKO islets, insulin secretion evoked by glucose was substantially reduced compared with control islets. Insulin secretion measurements from pancreatic β-cells demonstrated that glucose induces insulin secretion in a characteristic biphasic pattern, with a transient first-phase followed by the second phase sustained component [51], [52], [53]. In the perfused pancreata of ELKS βKO mice, there was selective reduction of the first-phase insulin secretion (Figure 3B). Moreover, insulin secretion induced by high K+ depolarization, which evokes first-phase secretion [54], was also decreased in βKO mice. Our results suggest that ELKS plays a role in glucose-stimulated first-phase insulin secretion. On the other hand, TIRF or electron microscopy did not reveal any change in the number of morphologically docked insulin granules between control and ELKS-KO β-cells. Thus, insulin granule docking was not impaired in ELKS-KO β-cells.
Rapid, marked elevation in Ca2+ influx through the opening of L-type VDCCs is required for first-phase secretion [55], [56]. In cultured primary ELKS-KO β-cells, the first glucose-evoked [Ca2+]i rise was decreased compared with that of control β-cells (Figure 3C). [Ca2+]i rise induced by high K+ was also lower in ELKS-KO β-cells (Figure 3D). Nifedipine, an L-type VDCC inhibitor, diminished the glucose- and high K+-evoked increase in [Ca2+]i in control and ELKS-KO β-cells. This suggests that the deletion of ELKS impairs the increase in [Ca2+]i via L-type VDCCs. In addition, the reduced glucose- and high K+-induced [Ca2+]i increase in ELKS-KO β-cells was similarly restored by both ELKSα and ELKSε gene transfer, suggesting that these isoforms are important for this [Ca2+]i rise in β-cells.
How does ELKS control the entry of Ca2+ into β-cells via L-type VDCCs? ELKS and CAST have been shown to bind to VDCC-β subunits in mouse brain lysate [28], [35], so ELKS may regulate the function of VDCCs by binding to the VDCC-β subunits in pancreatic β-cells. VDCC-β2 and β3 subunits are primarily expressed in mouse islets [57], [58]. Indeed, we uncovered endogenous binding of ELKS to VDCC-β2 and β3 subunits within MIN6 β-cells [18]. Moreover, we revealed the direct interaction of the ELKS N-terminus with the GK domain of VDCC-β2/3 subunits. These findings suggest that the interaction of ELKS and VDCC-β2/3 subunits regulates the glucose-stimulated increase in [Ca2+]i, which is followed by insulin secretion from pancreatic β-cells.
The GK domain of VDCC-β subunits regulates channel opening by interacting with the VDCC-α1 subunit, which forms a pore for the influx of extracellular Ca2+ [59]. In support of this, electrophysiological whole-cell voltage-clamp experiments showed the reduced current density of L-type VDCCs in ELKS-KO β-cells (Figure 3E). Nifedipine reduced Ca2+ currents in control and ELKS-KO β-cells to approximately the same levels, indicating the Ca2+ current is regulated by ELKS through L-type VDCCs. In addition, we confirmed the functional effect of ELKS on L-type VDCCs under whole-cell recording using human embryonic kidney (HEK293) cells [60] transfected with L-type VDCCs (CaV1.2/β2a/α2δ), in the presence or absence of ELKS overexpression [18]. The Ba2+ current density within cells co-expressing L-type VDCCs and ELKS was significantly higher than that in control cells expressing L-type VDCCs without ELKS. Results from the exogenous expression experiments support those of the experiments with the ELKS-KO β-cells showing that ELKS potentiates opening of L-type VDCCs.
As described above, ELKS clusters were identified at insulin granule docking sites colocalized with Syntaxin 1A clusters in the plasma membrane of MIN6 cells [17]. Insulin granules are tethered to an assembly of SNARE proteins near L-type VDCCs [61]. Thus, together with previous findings, our results indicate that ELKS binding to the GK domain of VDCC-β2/3 subunits opens L-type VDCCs. This opening of the L-type VDCCs facilitates the influx of Ca2+ and insulin exocytosis during first-phase secretion of insulin from pancreatic β-cells (Figure 4A).
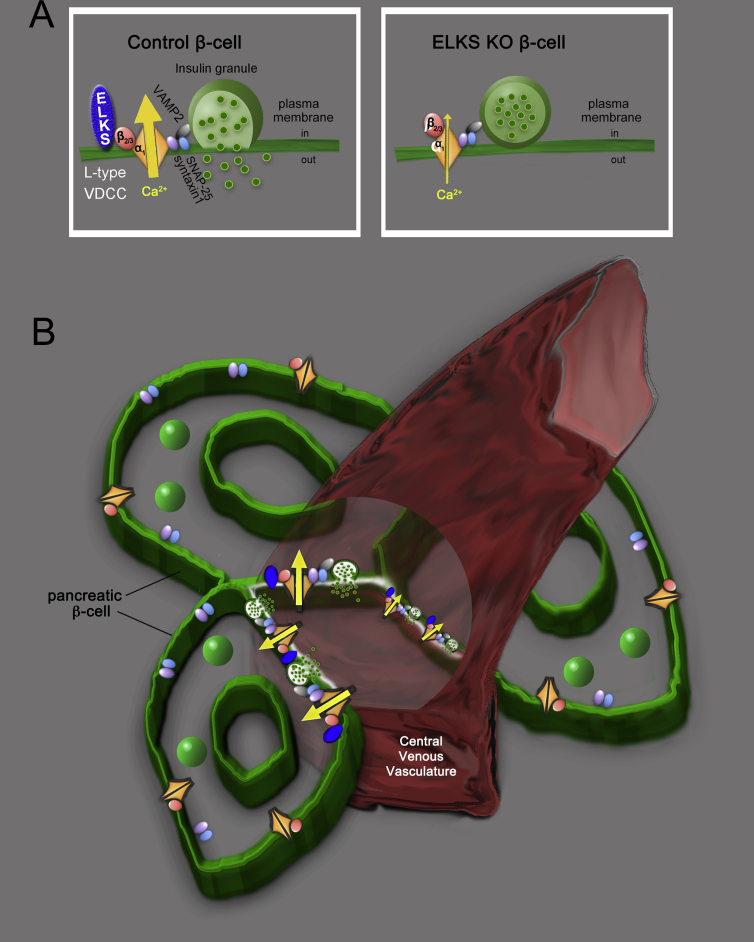
Figure 4.
Schema for regulation of ELKS during insulin secretion from islet β-cells. (A) ELKS has a role in regulating the opening of L-type VDCCs through direct binding to VDCC-β2/3. (B) In pancreatic islets, ELKS localizes at the vascular-facing plasma membrane of β-cells where it forms a complex with L-type VDCCs for insulin exocytosis. This complex controls the polarity of the initial increase in Ca2+ and first-phase insulin secretion into the central venous vasculature. Adapted from Ohara-Imaizumi et al. (2019) [18].
We also investigated the molecular mechanism underlying ELKS regulation of VDCC activity. It has been reported that the β subunit associates with the pore-forming VDCC-α1 subunit and contributes to plasma membrane trafficking and voltage-dependent gating of VDCCs [59]. However, surface expression of VDCC-α1 (CaV 1.2) was not affected by ELKS KD in MIN6 β-cells, suggesting that ELKS does not regulate VDCC trafficking to the plasma membrane. In addition, electrophysiological analysis showed that: i) ELKS deletion did not affect steady-state voltage-dependent activation (VDA) or inactivation (VDI) in ELKS-KO β-cells, and ii) the VDI was identical in HEK293 cells transfected with L-type VDCCs present with or without ELKS. These results suggest that ELKS does not affect voltage-dependent gating of L-type VDCCs. Therefore, ELKS may positively regulate L-type VDCCs by a yet unknown mechanism that differs from that of other molecules that have been shown to interact with β subunits, such as RGK (Rem/Gem/Kir) family proteins [62] and RIM1 [63], [64], [65]. Further research is needed to clarify the molecular mechanism underlying the control of ELKS-mediated facilitation of L-type VDCCs in β-cells.
6. ELKS is important for the influx of Ca2+ and insulin secretion at the vascular-facing plasma membrane of β-cells
ELKS has been reported to be preferentially enriched at the vascular side of the β-cell plasma membrane, whereas VDCC-α1 (CaV1.2) immunostaining was observed on the entire β-cell plasma membrane, as were exocytotic t-SNARE proteins within mouse islets [18]. These data suggest that ELKS may control L-type VDCCs on the vascular-facing plasma membrane in islet β-cells. If so, Ca2+ signals via VDCCs should be detectable initially at the vascular-facing plasma membrane during the first phase of insulin secretion. To test this hypothesis, we investigated Ca2+ signals at the vascular side of the β-cell plasma membrane in situ in islets using a newly synthesized Ca2+ probe employed as an improved high affinity version of GCaMP [66], [67], and DyLight 594-labeled tomato lectin, a marker for vasculature [68]. In control islets, Ca2+ imaging during glucose stimulation showed that Ca2+ signals initially increased at the vascular-facing plasma membrane of the β-cells, and then spread across the entire plasma membrane (Figure 3F). Analysis of this increase in Ca2+ in each plasmalemmal region of β-cells showed that the Ca2+ signal increased first at the vascular face, with the Ca2+ signal gradually increasing initially in the lateral regions and at the side of the plasma membrane opposite the vasculature (Figure 3H). Conversely, ELKS-KO β-cells exhibited a reduced initial increase in Ca2+ at the vascular-facing plasma membrane (Figure 3G). Ca2+ fluorescence intensity increased similarly in all regions (Figure 3I); these increases in Ca2+ were hampered by L-type VDCC blockers within control and ELKS βKO islets. These findings suggest that the plasma membrane of β-cells is functionally heterogeneous in Ca2+ rise patterns, and implies that the ELKS-regulated Ca2+ rise at the vascular-facing plasma membrane is important for first-phase insulin secretion. We therefore propose that ELKS and L-type VDCCs form an insulin secretion complex at the ELKS-localized vascular side of the β-cell plasma membrane for initial polarized Ca2+ influx and first-phase insulin secretion from pancreatic islets (Figure 4B). Use of another Ca2+ probe in mouse islets, R-CaMP1h, revealed a Ca2+ microdomain in the vascular-facing plasma membrane and in the lateral regions; however, which Ca2+ microdomain first appeared remains unclear [69]. The differences from our results may be caused by use of R-CaMP1h in the cytosol. R-CaMP1h has low affinity for Ca2+ (Kd: ∼1.3 μM) [70], whereas the membrane-bound probe, G-CaMP8b, has higher Ca2+ affinity (Kd: ∼44 nM) [18]. In the future, imaging of the increase in Ca2+ and insulin granule exocytosis should be performed simultaneously in islet β-cells. In this case, two-photon imaging of islets transplanted into the anterior chamber of the eye may be a useful tool enabling visualization of in situ β-cell function in a setting where vascular supplies are rewired [71], [72], [73].
7. ELKS and diabetes
Oral glucose tolerance tests have demonstrated impaired glucose tolerance in ELKS βKO mice. Hence, ELKS is required to maintain blood glucose homeostasis, which it accomplishes through its facilitation effect on the Ca2+ increase and first-phase secretion of insulin from β-cells. It is intriguing that db/db mice, a murine model of spontaneous type 2 diabetes, demonstrated reduced ELKS expression within islets. These db/db mice displayed higher fasting blood glucose levels and lower glucose insulin secretion from islets compared with nondiabetic db/m+ mice. Within this model the glucose-induced [Ca2+]i rise showed a slower and decreased peak response versus that of islets from db/m+ mice, which was consistent with previous reports [74], [75]. These findings led us to suspect the possible involvement of decreased expression of ELKS in diabetes. To assess this idea, we performed in situ G-CaMP8b Ca2+ imaging of β-cells within islets from db/db mice and compared these data with that of β-cells from db/m+ mice. The Ca2+ signal initially increased in the vascular-facing region within db/m+ mouse islet β-cells (Figure 3J), similar to that observed in the control mouse islet β-cells. On the other hand, db/db mouse islet β-cells showed an initially impaired Ca2+ rise at the vascular-facing plasma membrane, and Ca2+ levels were elevated equally throughout the plasma membrane regions, similar to that of the ELKS-KO mouse islet β-cells (Figure 3K). Therefore, we speculated that the downregulated ELKS expression may play a role in impaired insulin secretion within type 2 diabetes via a reduced polarized Ca2+ rise. Nevertheless, further studies are needed to understand the relationship between downregulated ELKS expression and diabetes.
8. What is the molecular mechanism behind ELKS delivery to the vascular-facing plasma membrane of β-cells?
The findings above raise new questions. What are the molecular cues for ELKS resulting in its localization to the vascular-facing plasma membrane in pancreatic β-cells? How does ELKS organize the secretory hotspots?
For heterogeneous localization of ELKS, there is likely a polarity cue enabling ELKS to localize to the vascular side of the plasma membrane. One possible mechanism may involve communication between β-cells and endothelial cells [76]. Endothelial cells form capillaries and secrete extracellular matrix (ECM) proteins to generate a basement membrane interacting with adjacent β-cells [77], [78]. Very recently, Gan et al. [79] reported that contact between the ECM and vascular side of the plasma membrane in β-cells triggers spatially confined activation of β1-integrin-dependent focal adhesion in β-cells, which orientates the β-cells and directs targeting of insulin granule fusion to the contact sites. Interestingly, activation of β1-integrin recruits LL5β, a phosphatidylinositol-3,4,5-triphosphate (PIP3)-binding protein, to the rim of focal adhesion [80], and LL5β has been reported to interact with ELKS in Ins-1 β-cells [48]. In addition, the ECM protein laminin interacts extracellularly with VDCCs, which can intracellularly interact further with active zone proteins, including ELKS, Bassoon, and Piccolo, to organize active zones in neuromuscular junctions [81], [82]. Therefore, it is possible that focal adhesion activated by local interaction between β1-integrin in β-cells and ECM proteins secreted from endothelial cells localizes ELKS to the vascular side of the plasma membrane via recruitment of LL5β and organizes secretory hotspots. However, this should be further examined in β-cells.
9. Other active zone proteins
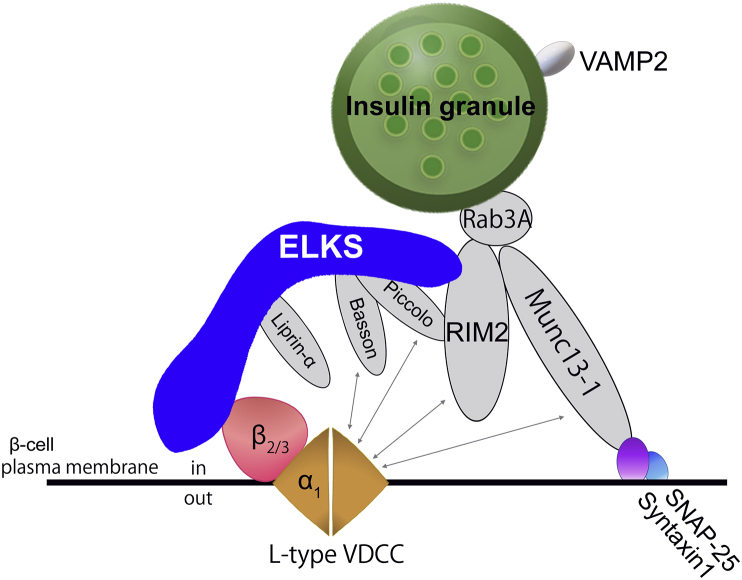
ELKS has been reported to bind other active zone proteins, implying that these proteins are also present at the ELKS-localized vascular side of the β-cell plasma membrane. Immunostaining research has recently shown that RIM2, Piccolo, and Liprin-α were also present at the vascular-facing plasma membrane of β-cells, although RIM2 and Piccolo are also apparently diffusely located in the β-cell cytosol [9]. We also observed that Bassoon was localized at the vascular-facing plasma membrane of β-cells (unpublished data). Therefore, it is necessary to define the relationship between ELKS and other active zone proteins at the vascular-facing plasma membrane of β-cells during polarized insulin secretion within islets. We summarize the current findings below concerning interactions among ELKS and other active zone protein members within the insulin secretory system (Figure 5).
Figure 5.
Interaction model of ELKS and other active zone proteins in pancreatic β-cells. ELKS acts as a platform for a dynamic multicomplex of active zone proteins in order to control insulin secretion.
9.1. RIM2
RIM2 was first identified as a binding partner for Epac2, a cAMP sensor in MIN6 cells [83]. A study using RIM2-null mice revealed that RIM2 is necessary for insulin granule docking to the plasma membrane and for the subsequent priming step, a process required for insulin granules to acquire competence for exocytosis in response to a Ca2+ rise during insulin secretion from β-cells [41]. RIM2 is a multidomain protein interacting with several proteins through its functional domains. While RIM1 interacts with VDCC-β in neurons [65], RIM2 interacts with VDCC-α1 (CaV1.2) and suppresses the VDI of VDCC currents [41], [84]. RIM2 has also been reported to interact with the C-terminal IWA region of ELKS. Because ELKS binds directly to VDCC-β through the N-terminal region, RIM2 is not likely involved in the ELKS control of VDCCs. Similar to ELKSα (with the IWA region), ELKSε (lacking IWA region) rescued the impairment of the [Ca2+]i increase in ELKS-KO β-cells [18]. However, the relationship between ELKS and RIM2 in VDCC regulation remains unclear.
9.2. Munc13-1
Munc13-1 is a mammalian homolog of the Unc13 synaptic protein in Caenorhabditis elegans [85]. As revealed by experiments with C. elegans [86] and mammalian neurons [87], Munc13-1 is required for the exocytosis priming step of insulin granules [39], [88]. Given that Munc13-1 is dispensable for the docking process in neurons and chromaffin cells [87], [89], the docking process of insulin granules is likely to be independent of Munc13-1. Munc13-1 contains a C1-domain (homologous to the phorbol ester- and diacylglycerol-binding region of protein kinase C), three C2-domains (Ca2+-binding and protein-protein interaction domains), a calmodulin binding sequence and a Syntaxin-binding domain essential for the priming process [90]. Munc13-1 interacts with the VDCC-α1 (CaV2.2) through the C2-domain C2B to regulate its gating properties in nerve terminals [91]. Whether Munc13-1 can physically interact with the α-subunit of VDCCs in β-cells remains uncertain. However, competitive inhibition experiments using the C2B domain of Munc13-1 revealed that functional interaction between Munc13-1 and CaV1.2 is likely important for arrangement of L-type VDCCs near the docked insulin granules in order to facilitate granule exocytosis [92]. ELKS has been reported to indirectly bind to Munc13-1 through RIM1 [12], [16]; hence, it is important to unravel the functional relationship between these three proteins within Ca2+ influx and insulin secretion. Notably, it has been reported that Munc13-1 levels in pancreatic islets were reduced in type 2 diabetic patients as well as type 2 diabetic model rats [40], [93].
9.3. Bassoon/Piccolo
Bassoon and Piccolo are large scaffolding proteins present in synaptic active zones. These proteins are structurally related multidomain proteins with 10 highly conserved regions [94]. Bassoon interacts with ELKS, and its binding is crucial for the docking of insulin granules to the plasma membrane with subsequent insulin secretion from MIN6 cells [17]. Piccolo is involved in insulin secretion evoked by the cAMP analog, 8-Br-cAMP, but not by glucose alone in MIN6 cells [38]. Both Bassoon and Piccolo interact with VDCC-α1 directly or indirectly, and Bassoon affects VDCC activity [84], [95]. Both Bassoon and Piccolo interact with a multitude of proteins, including ELKS, RIM2, and Munc13-1.
9.4. Liprin-α
The scaffold protein, Liprin-α, localizes to the vascular-facing plasma membrane in β-cells [9], but so far there are no reports revealing the role of Liprin-α in insulin secretion. Because Liprin-α is required for neurotransmitter release in synapses [96], [97], and it interacts with ELKS, RIM, and CASK [98], it is also potentially involved in insulin secretion from β-cells.
10. Conclusions
Our analyses indicate that ELKS has a role in opening L-type VDCCs at the vascular-facing plasma membrane of β-cells, which leads to an initial polarized Ca2+ influx and first-phase insulin secretion from islets. Our model further clarifies active zone protein control of the polarity of the Ca2+ influx and insulin secretion from β-cells. ELKS maintains blood glucose homeostasis through its facilitation effect on the Ca2+ rise and first-phase secretion of insulin from β-cells. ELKS expression is reduced in diabetic mouse islets with impaired polarized increase of Ca2+ at the vascular-facing plasma membrane of β-cells and insulin secretion. Considering these findings together, ELKS may be a therapeutic target for improvement of glycemic control in type 2 diabetes, which includes abnormalities in first-phase insulin secretion. Further experiments are required to investigate these possibilities in human islets, which differ from mouse islets in structure and have β-cells interspersed with other endocrine cells [99], [100].
Funding
This article is part of a supplement entitled ‘Biomarkers of Beta-Cell Health and Dysfunction: Towards Personalised Diabetes Care. Proceedings of the 20th Servier-IGIS Symposium’. The Symposium and the publication of the Supplement have been made possible by an unrestricted educational grant from Institut la Conférence Hippocrate – Servier Group.
Acknowledgments
This work was supported by grants [No. 17K08547 (M.O.-I.), No. 17K09845 (K.A.), and No. 15H04272 (T.O.)] from the Ministry of Education, Culture, Sports, Science and Technology Japan (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI, Japan, funding from the Japan Diabetes Foundation, Japan (M.O.-I.), Kyorin University School of Medicine Joint research program, Japan (M.O.-I.), and the Gunma University Joint research program, Japan [No. 15010 (K.A.)], and a grant (No. JPMJCR1751) from JST CREST, Japan (T.O.).
Conflict of interest
None declared.
References
- 1.Ashcroft F.M., Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell. 2012;148(6):1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner-Weir S. Morphological evidence for pancreatic polarity of β-cell within islets of langerhans. Diabetes. 1988;37(5):616–621. doi: 10.2337/diab.37.5.616. [DOI] [PubMed] [Google Scholar]
- 3.Weir G.C., Bonner-Weir S. Islets of Langerhans: the puzzle of intraislet interactions and their relevance to diabetes. Journal of Clinical Investigation. 1990;85(4):983–987. doi: 10.1172/JCI114574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granot Z., Swisa A., Magenheim J., Stolovich-Rain M., Fujimoto W., Manduchi E. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metabolism. 2009;10(4):296–308. doi: 10.1016/j.cmet.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roscioni S.S., Migliorini A., Gegg M., Lickert H. Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nature Reviews Endocrinology. 2016;12(12):695–709. doi: 10.1038/nrendo.2016.147. [DOI] [PubMed] [Google Scholar]
- 6.Orci L., Thorens B., Ravazzola M., Lodish H.F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989;245(4915):295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- 7.Gan W.J., Zavortink M., Ludick C., Templin R., Webb R., Webb R. Cell polarity defines three distinct domains in pancreatic β-cells. Journal of Cell Science. 2017;130(1):143–151. doi: 10.1242/jcs.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi N., Kishimoto T., Nemoto T., Kadowaki T., Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297(5585):1349–1352. doi: 10.1126/science.1073806. [DOI] [PubMed] [Google Scholar]
- 9.Low J.T., Zavortink M., Mitchell J.M., Gan W.J., Do O.H., Schwiening C.J. Insulin secretion from beta cells in intact mouse islets is targeted towards the vasculature. Diabetologia. 2014;57(8):1655–1663. doi: 10.1007/s00125-014-3252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landis D., Hall A.K., Weinstein L.A., Reese T.S. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988;1(3):201–209. doi: 10.1016/0896-6273(88)90140-7. [DOI] [PubMed] [Google Scholar]
- 11.Garner C.C., Kindler S., Gundelfinger E.D. Molecular determinants of presynaptic active zones. Current Opinion in Neurobiology. 2000;10(3):321–327. doi: 10.1016/s0959-4388(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 12.Hida Y., Ohtsuka T. CAST and ELKS proteins: structural and functional determinants of the presynaptic active zone. Journal of Biochemistry. 2010;148(2):131–137. doi: 10.1093/jb/mvq065. [DOI] [PubMed] [Google Scholar]
- 13.Südhof T.C. The presynaptic active zone. Neuron. 2012;75(1):11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takao-Rikitsu E., Mochida S., Inoue E., Deguchi-Tawarada M., Inoue M., Ohtsuka T. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. The Journal of Cell Biology. 2004;164(2):301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamada S., Ohtsuka T. CAST: its molecular structure and phosphorylation-dependent regulation of presynaptic plasticity. Neuroscience Research. 2018;127:25–32. doi: 10.1016/j.neures.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Held R.G., Kaeser P.S. ELKS active zone proteins as multitasking scaffolds for secretion. Open Biology. 2018;8(2):170258. doi: 10.1098/rsob.170258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohara-Imaizumi M., Ohtsuka T., Matsushima S., Akimoto Y., Nishiwaki C., Nakamichi Y. ELKS, a protein structurally related to the active zone-associated protein CAST, is expressed in pancreatic beta cells and functions in insulin exocytosis: interaction of ELKS with exocytotic machinery analyzed by total internal reflection fluorescence microscopy. Molecular Biology of the Cell. 2005;16(7):3289–3300. doi: 10.1091/mbc.E04-09-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohara-Imaizumi M., Aoyagi K., Yamauchi H., Yoshida M., Mori M.X., Hida Y. ELKS/Voltage-dependent Ca2+ channel-β subunit module regulates polarized Ca2+ influx in pancreatic β cells. Cell Reports. 2019;26(5):1213–1226. doi: 10.1016/j.celrep.2018.12.106. [DOI] [PubMed] [Google Scholar]
- 19.Ohtsuka T., Takao-Rikitsu E., Inoue E., Inoue M., Takeuchi M., Matsubara K. Cast: a novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. The Journal of Cell Biology. 2002;158(3):577–590. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Liu X., Biederer T., Südhof T.C. A family of RIM-binding proteins regulated by alternative splicing: implications for the genesis of synaptic active zones. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(22):14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Hu B., Zieba A., Neumann N.G., Kasper-Sonnenberg M., Honsbein A. A protein interaction node at the neurotransmitter release site: domains of Aczonin/Piccolo, Bassoon, CAST, and rim converge on the N-terminal domain of Munc13-1. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(40):12584–12596. doi: 10.1523/JNEUROSCI.1255-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakata T., Kitamura Y., Shimizu K., Tanaka S., Fujimori M., Yokoyama S. Fusion of a novel gene, ELKS, to RET due to translocation t(10;12)(q11;p13) in a papillary thyroid carcinoma. Genes, Chromosomes & Cancer. 1999;25(2):97–103. doi: 10.1002/(sici)1098-2264(199906)25:2<97::aid-gcc4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Monier S., Jollivet F., Janoueix-Lerosey I., Johannes L., Goud B. Characterization of novel Rab6-interacting proteins involved in endosome-to-TGN transport. Traffic. 2002;3(4):289–297. doi: 10.1034/j.1600-0854.2002.030406.x. [DOI] [PubMed] [Google Scholar]
- 24.Janoueix-Lerosey I., Jollivet F., Camonis J., Marche P.N., Goud B. Two-hybrid system screen with the small GTP-binding protein Rab6. Identification of a novel mouse GDP dissociation inhibitor isoform and two other potential partners of Rab6. Journal of Biological Chemistry. 1995;270(24):14801–14808. doi: 10.1074/jbc.270.24.14801. [DOI] [PubMed] [Google Scholar]
- 25.Deguchi-Tawarada M., Inoue E., Takao-Rikitsu E., Inoue M., Ohtsuka T., Takai Y. CAST2: identification and characterization of a protein structurally related to the presynaptic cytomatrix protein CAST. Genes to Cells: Devoted to Molecular & Cellular Mechanisms. 2004;9(1):15–23. doi: 10.1111/j.1356-9597.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- 26.Kawabe H., Mitkovski M., Kaeser P.S., Hirrlinger J., Opazo F., Nestvogel D. ELKS1 localizes the synaptic vesicle priming protein bMunc13-2 to a specific subset of active zones. The Journal of Cell Biology. 2017;216(4):1143–1161. doi: 10.1083/jcb.201606086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko J., Na M., Kim S., Lee J.-R., Kim E. Interaction of the ERC family of RIM-binding proteins with the liprin-alpha family of multidomain proteins. Journal of Biological Chemistry. 2003;278(43):42377–42385. doi: 10.1074/jbc.M307561200. [DOI] [PubMed] [Google Scholar]
- 28.Kiyonaka S., Nakajima H., Takada Y., Hida Y., Yoshioka T., Hagiwara A. Physical and functional interaction of the active zone protein CAST/ERC2 and the β-subunit of the voltage-dependent Ca2+ channel. Journal of Biochemistry. 2012;152(2):149–159. doi: 10.1093/jb/mvs054. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T., Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366(6451):156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler D.B., Randall A., Tsien R.W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264(5155):107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 31.Catterall W.A. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24(5–6):307–323. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- 32.Wu L.G., Westenbroek R.E., Borst J.G., Catterall W.A., Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. Journal of Neuroscience. 1999;19(2):726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ertel E.A., Campbell K.P., Harpold M.M., Hofmann F., Mori Y., Perez-Reyes E. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25(3):533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 34.Catterall W.A., Perez Reyes E., Snutch T.P., Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacological Reviews. 2005;57(4):411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 35.Billings S.E., Clarke G.L., Nishimune H. ELKS1 and Ca2+ channel subunit β4 interact and colocalize at cerebellar synapses. NeuroReport. 2012;23(1):49–54. doi: 10.1097/WNR.0b013e32834e7deb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C., Bickford L.S., Held R.G., Nyitrai H., Sudhof T.C., Kaeser P.S. The active zone protein family ELKS supports Ca2+ influx at nerve terminals of inhibitory hippocampal neurons. Journal of Neuroscience. 2014;34(37):12289–12303. doi: 10.1523/JNEUROSCI.0999-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagiwara A., Kitahara Y., Grabner C.P., Vogl C., Abe M., Kitta R. Cytomatrix proteins CAST and ELKS regulate retinal photoreceptor development and maintenance. The Journal of Cell Biology. 2018;217(11):3993–4006. doi: 10.1083/jcb.201704076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimoto K., Shibasaki T., Yokoi N., Kashima Y., Matsumoto M., Sasaki T. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2. Piccolo complex in cAMP-dependent exocytosis. Journal of Biological Chemistry. 2002;277(52):50497–50502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- 39.Kang L., He Z., Xu P., Fan J., Betz A., Brose N. Munc13-1 is required for the sustained release of insulin from pancreatic β cells. Cell Metabolism. 2006;3(6):463–468. doi: 10.1016/j.cmet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Sheu L., Pasyk E.A., Ji J., Huang X., Gao X., Varoqueaux F. Regulation of insulin exocytosis by munc13-1. Journal of Biological Chemistry. 2003;278(30):27556–27563. doi: 10.1074/jbc.M303203200. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda T., Shibasaki T., Minami K., Takahashi H., Mizoguchi A., Uriu Y. Rim2α determines docking and priming states in insulin granule exocytosis. Cell Metabolism. 2010;12(2):117–129. doi: 10.1016/j.cmet.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Nagamatsu S., Fujiwara T., Nakamichi Y., Watanabe T., Katahira H., Sawa H. Expression and functional role of syntaxin 1/HPC-1 in pancreatic beta cells. Syntaxin 1A, but not 1B, plays a negative role in regulatory insulin release pathway. Journal of Biological Chemistry. 1996;271(2):1160–1165. doi: 10.1074/jbc.271.2.1160. [DOI] [PubMed] [Google Scholar]
- 43.Garcia E.P., McPherson P.S., Chilcote T.J., Takei K., De Camilli P. rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. The Journal of Cell Biology. 1995;129(1):105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohara-Imaizumi M., Nakamichi Y., Tanaka T., Ishida H., Nagamatsu S. Imaging exocytosis of single insulin secretory granules with evanescent wave microscopy: distinct behavior of granule motion in biphasic insulin release. Journal of Biological Chemistry. 2002;277(6):3805–3808. doi: 10.1074/jbc.C100712200. [DOI] [PubMed] [Google Scholar]
- 45.Ohara-Imaizumi M., Nishiwaki C., Kikuta T., Nagai S., Nakamichi Y., Nagamatsu S. TIRF imaging of docking and fusion of single insulin granule motion in primary rat pancreatic β-cells: different behaviour of granule motion between normal and Goto-Kakizaki diabetic rat beta-cells. Biochemical Journal. 2004;381(1):13–18. doi: 10.1042/BJ20040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohara-Imaizumi M., Nishiwaki C., Kikuta T., Kumakura K., Nakamichi Y., Nagamatsu S. Site of docking and fusion of insulin secretory granules in live MIN6 β cells analyzed by TAT-conjugated anti-syntaxin 1 antibody and total internal reflection fluorescence microscopy. Journal of Biological Chemistry. 2004;279(9):8403–8408. doi: 10.1074/jbc.M308954200. [DOI] [PubMed] [Google Scholar]
- 47.Ohara-Imaizumi M., Fujiwara T., Nakamichi Y., Okamura T., Akimoto Y., Kawai J. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. The Journal of Cell Biology. 2007;177(4):695–705. doi: 10.1083/jcb.200608132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan T., Liu L., Zhang Y., Wei L., Zhao S., Zheng X. Diacylglycerol guides the hopping of clathrin- coated pits along microtubules for exo-endocytosis coupling. Developmental Cell. 2015;35(1):120–130. doi: 10.1016/j.devcel.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Dong W., Radulovic T., Goral R.O., Thomas C., Montesinos M.S., Guerrero-Given D. CAST/ELKS proteins control voltage-gated Ca2+ channel density and synaptic release probability at a mammalian central synapse. Cell Reports. 2018;24(2):284–293. doi: 10.1016/j.celrep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aoyagi K., Ohara-Imaizumi M., Nishiwaki C., Nakamichi Y., Nagamatsu S. Insulin/phosphoinositide 3-kinase pathway accelerates the glucose-induced first-phase insulin secretion through TrpV2 recruitment in pancreatic β-cells. Biochemical Journal. 2010;432(2):375–386. doi: 10.1042/BJ20100864. [DOI] [PubMed] [Google Scholar]
- 51.Cerasi E., Luft R. The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Acta Endocrinologica. 1967;55(2):278–304. doi: 10.1530/acta.0.0550278. [DOI] [PubMed] [Google Scholar]
- 52.Curry D.L., Bennett L.L., Grodsky G.M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- 53.Straub S.G., Sharp G.W.G. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes/Metabolism Research and Reviews. 2002;18(6):451–463. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 54.Ohara-Imaizumi M., Aoyagi K., Nakamichi Y., Nishiwaki C., Sakurai T., Nagamatsu S. Pattern of rise in subplasma membrane Ca2+ concentration determines type of fusing insulin granules in pancreatic β cells. Biochemical and Biophysical Research Communications. 2009;385(3):291–295. doi: 10.1016/j.bbrc.2009.04.155. [DOI] [PubMed] [Google Scholar]
- 55.Rorsman P., Braun M., Zhang Q. Regulation of calcium in pancreatic α- and β-cells in health and disease. Cell Calcium. 2012;51(3-4):300–308. doi: 10.1016/j.ceca.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulla V., Renström E., Feil R., Feil S., Franklin I., Gjinovci A. Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. The European Molecular Biology Organization Journal. 2003;22(15):3844–3854. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berggren P.-O., Yang S.-N., Murakami M., Efanov A.M., Uhles S., Köhler M. Removal of Ca2+ channel beta3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell. 2004;119(2):273–284. doi: 10.1016/j.cell.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 58.Lee K., Kim J., Köhler M., Yu J., Shi Y., Yang S.-N. Blocking Ca2+ channel β3 subunit reverses diabetes. Cell Reports. 2018;24(4):922–934. doi: 10.1016/j.celrep.2018.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buraei Z., Yang J. Structure and function of the β subunit of voltage-gated Ca2+ channels. BBA - Biomembranes. 2013;1828(7):1530–1540. doi: 10.1016/j.bbamem.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mori M.X., Erickson M.G., Yue D.T. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 2004;304(5669):432–435. doi: 10.1126/science.1093490. [DOI] [PubMed] [Google Scholar]
- 61.Barg S., Eliasson L., Renström E., Rorsman P. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes. 2002;51(Suppl 1):S74–S82. doi: 10.2337/diabetes.51.2007.s74. [DOI] [PubMed] [Google Scholar]
- 62.Yang T., Colecraft H.M. Regulation of voltage-dependent calcium channels by RGK proteins. BBA - Biomembranes. 2013;1828(7):1644–1654. doi: 10.1016/j.bbamem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaeser P.S., Deng L., Wang Y., Dulubova I., Liu X., Rizo J. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144(2):282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirano M., Takada Y., Wong C.F., Yamaguchi K., Kotani H., Kurokawa T. C-terminal splice variants of P/Q-type Ca2+ channel CaV2.1 α1 subunits are differentially regulated by Rab3-interacting molecule proteins. Journal of Biological Chemistry. 2017;292(22):9365–9381. doi: 10.1074/jbc.M117.778829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiyonaka S., Wakamori M., Miki T., Uriu Y., Nonaka M., Bito H. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nature Neuroscience. 2007;10(6):691–701. doi: 10.1038/nn1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakai J., Ohkura M. Probing calcium ions with biosensors. Biotechnology & Genetic Engineering Reviews. 2003;20(1):3–22. doi: 10.1080/02648725.2003.10648035. [DOI] [PubMed] [Google Scholar]
- 67.Ohkura M., Sasaki T., Sadakari J., Gengyo-Ando K., Kagawa-Nagamura Y., Kobayashi C. Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. Public Library of Science one. 2012;7(12):e51286. doi: 10.1371/journal.pone.0051286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brissova M., Fowler M., Wiebe P., Shostak A., Shiota M., Radhika A. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53(5):1318–1325. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 69.Geron E., Boura-Halfon S., Schejter E.D., Shilo B.-Z. The edges of pancreatic islet β cells constitute adhesive and signaling microdomains. Cell Reports. 2015;10(3):317–325. doi: 10.1016/j.celrep.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 70.Akerboom J., Carreras Calderón N., Tian L., Wabnig S., Prigge M., Tolö J. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Frontiers in Molecular Neuroscience. 2013;6:2. doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Speier S., Nyqvist D., Cabrera O., Yu J., Molano R.D., Pileggi A. Noninvasive in vivo imaging of pancreatic islet cell biology. Nature Medicine. 2008;14(5):574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Speier S., Nyqvist D., Köhler M., Caicedo A., Leibiger I.B., Berggren P.-O. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nature Protocols. 2008;3(8):1278–1286. doi: 10.1038/nprot.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leibiger I.B., Caicedo A., Berggren P.O. Non-invasive in vivo imaging of pancreatic β-cell function and survival - a perspective. Acta Physiologica (Oxford, England) 2012;204(2):178–185. doi: 10.1111/j.1748-1716.2011.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gustavsson N., Larsson-Nyrén G., Lindström P. Pancreatic β cells from db/db mice show cell-specific [Ca2+]i and NADH responses to glucose but not to α-ketoisocaproic acid. Pancreas. 2005;31(3):242–250. doi: 10.1097/01.mpa.0000175891.58918.c8. [DOI] [PubMed] [Google Scholar]
- 75.Do O.H., Low J.T., Gaisano H.Y., Thorn P. The secretory deficit in islets from db/db mice is mainly due to a loss of responding beta cells. Diabetologia. 2014;57(7):1400–1409. doi: 10.1007/s00125-014-3226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peiris H., Bonder C.S., Coates P.T.H., Keating D.J., Jessup C.F. The β-cell/EC Axis: how do islet cells talk to each other? Diabetes. 2014;63(1):3–11. doi: 10.2337/db13-0617. [DOI] [PubMed] [Google Scholar]
- 77.Nikolova G., Jabs N., Konstantinova I., Domogatskaya A., Tryggvason K., Sorokin L. The vascular basement membrane: a niche for insulin gene expression and β cell proliferation. Developmental Cell. 2006;10(3):397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 78.Otonkoski T., Banerjee M., Korsgren O., Thornell L.E., Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for β-cell growth and differentiation. Diabetes, Obesity and Metabolism. 2008;10(Suppl. 4):119–127. doi: 10.1111/j.1463-1326.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 79.Gan W.J., Do O.H., Cottle L., Ma W., Kosobrodova E., Cooper-White J. Local integrin activation in pancreatic beta cells targets insulin secretion to the vasculature. Cell Reports. 2018;24(11):2819–2826. doi: 10.1016/j.celrep.2018.08.035. e3. [DOI] [PubMed] [Google Scholar]
- 80.Noordstra I., Akhmanova A. Linking cortical microtubule attachment and exocytosis. F1000Research. 2017;6:469. doi: 10.12688/f1000research.10729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nishimune H. Molecular mechanism of active zone organization at vertebrate neuromuscular junctions. Molecular Neurobiology. 2012;45(1):1–16. doi: 10.1007/s12035-011-8216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishimune H. Active zones of mammalian neuromuscular junctions: formation, density, and aging. Annals of the New York Academy of Sciences. 2012;1274(1):24–32. doi: 10.1111/j.1749-6632.2012.06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ozaki N., Shibasaki T., Kashima Y., Miki T., Takahashi K., Ueno H. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nature Cell Biology. 2000;2(11):805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 84.Shibasaki T., Sunaga Y., Fujimoto K., Kashima Y., Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. Journal of Biological Chemistry. 2004;279(9):7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 85.Brose N., Hofmann K., Hata Y., Sudhof T.C. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. Journal of Biological Chemistry. 1995;270(42):25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- 86.Richmond J.E., Weimer R.M., Jorgensen E.M. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature. 2001;412(6844):338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Augustin I., Rosenmund C., Sudhof T.C., Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400(6743):457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- 88.Kwan E.P., Xie L., Sheu L., Nolan C.J., Prentki M., Betz A. Munc13-1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes. 2006;55(5):1421–1429. doi: 10.2337/db05-1263. [DOI] [PubMed] [Google Scholar]
- 89.Ashery U., Varoqueaux F., Voets T., Betz A., Thakur P., Koch H. Munc13-1 acts as a priming factor for large dense-core vesicles in bovine chromaffin cells. The European Molecular Biology Organization Journal. 2000;19(14):3586–3596. doi: 10.1093/emboj/19.14.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stevens D.R., Wu Z.-X., Matti U., Junge H.J., Schirra C., Becherer U. Identification of the minimal protein domain required for priming activity of Munc13-1. Current Biology. 2005;15(24):2243–2248. doi: 10.1016/j.cub.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 91.Calloway N., Gouzer G., Xue M., Ryan T.A. The active-zone protein Munc13 controls the use-dependence of presynaptic voltage-gated calcium channels. eLife. 2015;4:e07728. doi: 10.7554/eLife.07728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gandasi N.R., Yin P., Riz M., Chibalina M.V., Cortese G., Lund P.-E. Ca2+ channel clustering with insulin-containing granules is disturbed in type 2 diabetes. Journal of Clinical Investigation. 2017;127(6):2353–2364. doi: 10.1172/JCI88491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ostenson C.-G., Gaisano H., Sheu L., Tibell A., Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006;55(2):435–440. doi: 10.2337/diabetes.55.02.06.db04-1575. [DOI] [PubMed] [Google Scholar]
- 94.Gundelfinger E.D., Reissner C., Garner C.C. Role of Bassoon and Piccolo in assembly and molecular organization of the active zone. Frontiers in Synaptic Neuroscience. 2016;7:19. doi: 10.3389/fnsyn.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davydova D., Marini C., King C., Klueva J., Bischof F., Romorini S. Bassoon specifically controls presynaptic P/Q-type Ca2+ channels via RIM-binding protein. Neuron. 2014;82(1):181–194. doi: 10.1016/j.neuron.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 96.Wong M.Y., Liu C., Wang S.S.H., Roquas A.C.F., Fowler S.C., Kaeser P.S. Liprin-α3 controls vesicle docking and exocytosis at the active zone of hippocampal synapses. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(9):2234–2239. doi: 10.1073/pnas.1719012115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spangler S.A., Schmitz S.K., Kevenaar J.T., de Graaff E., de Wit H., Demmers J. Liprin-α2 promotes the presynaptic recruitment and turnover of RIM1/CASK to facilitate synaptic transmission. The Journal of Cell Biology. 2013;201(6):915–928. doi: 10.1083/jcb.201301011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spangler S.A., Hoogenraad C.C. Liprin-alpha proteins: scaffold molecules for synapse maturation. Biochemical Society Transactions. 2007;35(5):1278–1282. doi: 10.1042/BST0351278. [DOI] [PubMed] [Google Scholar]
- 99.Cabrera O., Berman D.M., Kenyon N.S., Ricordi C., Berggren P.-O., Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bosco D., Armanet M., Morel P., Niclauss N., Sgroi A., Muller Y.D. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59(5):1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]