Abstract
Background:
Stromal laminins α4 and α5 are differentially regulated in transplant tolerance and immunity, respectively, resulting in altered T cell trafficking. We hypothesized laminins directly regulated T cell activation and polarization.
Methods:
Human and mouse CD4 T cells were activated in Th1, Th2, Th17 or Treg environments with/without laminin α4 and/or α5. Laminin α5 receptors were blocked with anti-α6 integrin or anti-α dystroglycan mAbs, and T cell polarization determined. TCR transgenic TEa CD4 cells that recognized donor alloantigen were transferred into C57BL/6 mice that received alloantigen or cardiac allografts. Laminin receptors were blocked, and TEa T cell migration and differentiation were assessed. Laminin expression was measured in several models of immunity and tolerance.
Results:
In diverse models, laminins α4 and α5 were differentially regulated. Immunity was associated with decreased laminin α4:α5 ratio, while tolerance was associated with an increased ratio. Laminin α4 inhibited CD4+ T cell proliferation and Th1, Th2 and Th17 polarization, but favored Treg induction. Laminin α5 favored T cell activation and Th1, Th2, and Th17 polarization; and inhibited Treg. Laminin α5 was recognized by T cell integrin α6 and important for activation and for inhibition of Treg. Laminin α5 was also recognized by T cell α-dystroglycan and required for Th17 differentiation. Anti-α6 integrin or anti-α-dystroglycan prolonged allograft survival.
Conclusions:
Laminins α4 and α5 are, respectively, co-inhibitory and co-stimulatory ligands for human and mouse CD4 T cells. Laminins and their receptors modulate immune responses by acting as one of the molecular switches for immunity or suppression.
Introduction
Laminins are major components of all basement membranes (BM), the extracellular matrix that separates epithelia or endothelia from underlying tissues.
All laminins are composed of three different disulfide-linked polypeptides, termed α, β and γ. 16 isoforms have been characterized with different properties, functions and localization (for review see Ref. 1). Laminins α4 β1 γ1 (411) and α5 β1 γ1 (511) are the two main isoforms that have been described to modulate immune cell functions and leukocyte migration. The BM of the vascular and lymphatic systems that support immune cell trafficking is composed of these two isoforms.2–4 To infiltrate tissues, leukocytes must first cross a blood or lymphatic endothelial barrier and interact with laminins 411 and 511. This interaction is a key rate-limiting step in leukocyte transmigration and tissue infiltration. In laminin α4 deficient mice, the infiltration of T lymphocytes5 or neutrophils6 into inflamed tissue is reduced. These studies demonstrated that in vivo laminin α4 and laminin 411 favored leukocyte transendothelial migration. Laminin 511 also has an important role in the regulation of leukocyte transmigration. Neutrophils and T cells penetrate vascular BM regions that are characterized by low expression of laminin 511.7,8 Together, these studies suggest that T cell transmigration through endothelial BMs occurs when laminin 411 is present and laminin 511 is weakly expressed. We recently confirmed these opposing roles of laminins on CD4+ T cell transendothelial migration.4 These data demonstrated that laminin 411 is more permissive for CD4+ T cell movement on and into the endothelial cell layer, while laminin 511 prevented it.
In lymph nodes (LN) which are fundamental for the initiation of immunity or tolerance,4,9 laminins 411 and 511 are also the main isoforms expressed and modulate leukocyte infiltration. We observed that these two isoforms were expressed predominantly in the LN cortical ridge (CR), a region that is essential for bringing T lymphocytes and antigen-presenting cells (APC) together.4 Blocking the α6 integrin-binding site on T cells to laminin α5 favored the migration of regulatory suppressive T cells (Treg) and plasmacytoid dendritic cells (pDC) into the LN, while blocking laminin α4 reduced migration. Based on these observations, we hypothesized that the stromal laminins 411 and 511 could modulate not only CD4+ T cell migration in the LN but also activation and polarization by acting as co-stimulatory or coinhibitory ligands.
Materials and Methods
Cardiac transplant and mouse treatment.
C57BL/6 mice received heterotopic cardiac allograft transplants from sex-matched donor BALB/c mice as previously described.10 On the day of transplantation, mice were treated with anti-integrin α6 mAb (clone GoH3, 10μg i.v, Biolegend, San Diego, CA) or isotype control (rat IgG2a), anti- α dystroglycan mAb (clone IIH6C4, 10 μg i.v, Merck Millipore, Darstadt, Germany) or isotype control IgM associated or not with anti-CD40L mAb (clone MR1, 0.25 mg i.v., Bio X Cell, West Lebanon, NH). Anti-integrin α6 mAb or anti- α dystroglycan mAb was administered again every 3 days or once a week when associated with anti-CD40L mAb. Graft function was monitored daily by abdominal palpation.
For donor specific transfusion (DST) treatment, C57BL/6 mice were injected i.v. with 1 × 107 BALB/c splenocytes with or without anti-CD40L mAb. DST cells were obtained from BALB/c spleens.
For CFA treatment, C57BL/6 mice were injected subcutaneously in the flank with emulsified complet freund adjuvant (CFA) (Sigma Aldrich) complemented with 400 μg of mycobacterium tuberculosis (BD Biosciences, San Jose, CA), with or without 200 μg of Ea peptide11 (Anaspec Inc, Fremont, CA).
For the tumor model, mice were inoculated in the flank with 5×105 C57BL/6-derived MC38 colon carcinoma cells maintained as described.12
To induce inflammatory colitis, C57BL/6 mice mice were administered 2% (w/v) DSS (molecular weight of 36,000 – 50,000 Da; MP Biomedicals, Santa Ana, CA) in drinking water for 24 hours or were given water only.
T cell adoptive transfer.
At the time of CFA/Ea immunization or 24h after DST treatment, mice were given 5 × 106 purified CD4+ T cells iv with anti-integrin α6 mAb (clone GoH3, 10μg iv, Biolegend, San Diego, CA) or isotype control, or anti- α dystroglycan mAb (clone IIH6C4, 10 μg iv, Merck Millipore, Darstadt, Germany) or isotype control IgM. CD4+ T cell activation, proliferation and polarizarion were analyzed 3 days after DST treatment or 2 days after CFA/Ea immunization.
In vitro CD4 T cell activation and differentiation.
CD4 T cells from C57BL/6 mice were activated in 96-well plates at a density of 1 × 105 in complete RPMI medium. Cells were activated with coated anti-CD3 (1 or 5 μg/mL, clone 2C11, BD Bioscience) with or without coated laminin 411(2 μg/mL, Biolamina, Matawan, NJ) and/or laminin 511 (1 μg/mL, Biolamina). After 3 days of culture, T cell proliferation and CD69, CD25 and CD44 expression were determined by flow cytometry. For T cell polarization, CD4 T cells from C57BL/6 mice were cultured 5 days with coated anti-CD3 mAb (5μg/mL) and soluble anti-CD28 (1μg/mL, clone 37.51, eBioscience, San Diego, CA) with or without coated laminin 411 and /or laminin 511, in pro-Th1, Th2, Treg or Th17 environments. The laminin α5 receptor was blocked with anti- α6 integrin mAb or with anti- α dystroglycan mAb. All the antibodies were preservative and endotoxin free. After 5 days of culture or after CD4 T cell isolation from the spleen or the LNs, CD4 T cells were activated for 4 hours with phorbol 12-myristate13-acetate (PMA) (40 ng/mL, Sigma-Aldrich, Allentown, PA) and ionomycin (1μg/mL, Sigma-Aldrich) in the presence of brefeldin A (dilution 1:1000, BD Biosciences) and cytokine expression was analyzed by flow cytometry.
Statistics.
Data were analyzed with Prism 4.0c software (GraphPad Software Inc.) using Student’s unpaired 2-tailed t tests (for single variable differences), or 1- or 2-way ANOVA (for multiple variable differences) with Tukey’s or Bonferroni post-tests, as appropriate. Graft survival was plotted using Kaplan-Meier’s curves and differences between groups were estimated using the Log-rank (Mantel-Cox) test. P-values of 0.05 or less were considered statistically significant.
Results
Distinct laminin α4 and α5 expression profiles in the LN following the induction of immunity versus tolerance
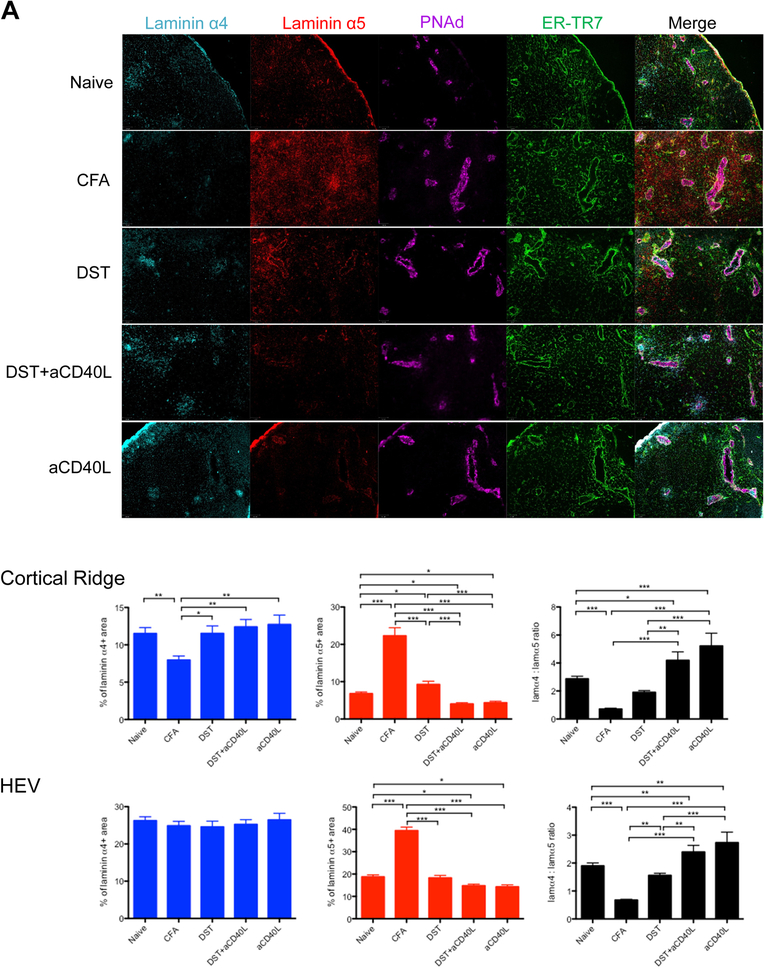
We previously demonstrated that laminin expression changed in response to alloantigen.4 To determine if remodeling was generalizable response, we measured laminins in other models. Differences in expression of the laminin α4 and laminin α5 were evident after induction of inflammation or immunity by subcutaneous injection of CFA or i.v. donor specific transfusion (DST), or tolerance with DST+anti-CD40L mAb or anti-CD40L alone (Figure 1A, Figure S1 and Table S1). The intense inflammatory reaction induced by CFA was associated with decreased laminin α4 in the CR compared to naive LNs. Laminin α5 strongly increased in the CR and HEV. Thus, laminin α4:α5 ratios were significantly lower in the CFA group. Immunization with DST that is associated with a moderate inflammation and allogenic immune response in LNs13 increased the expression of laminin α5 in the CR. The blockade of CD40L alone or the induction of tolerance with DST+anti-CD40L significantly reduced laminin α5 expression in the CR and in the HEV leading to higher laminin α4:α5 ratios compared to the other groups. These results confirmed our previous observation4 and demonstrated that changes in laminin α4 and α5 expression in the LN occurred very rapidly after the induction of immunity or tolerance.
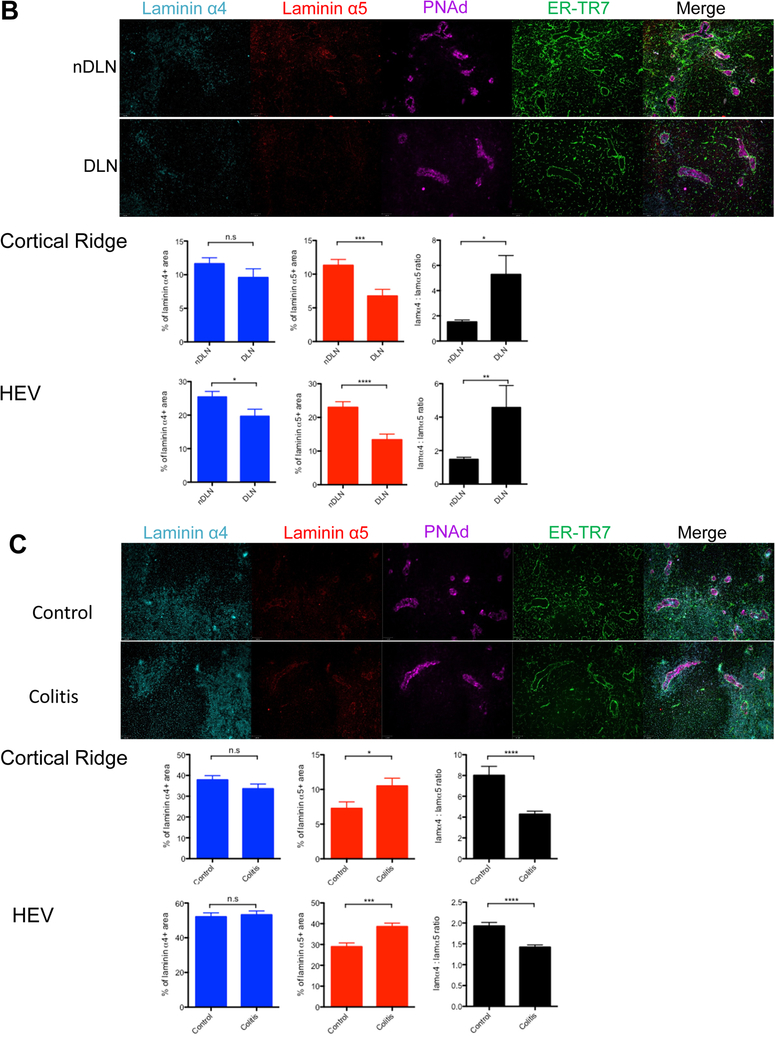
Figure 1.
Laminin α5 and laminin α4 expression differentially regulated in the lymph node following the induction of inflammation, immunity or tolerance. A, Skin LNs from naive, CFA, DST, DST+anti-CD40L and anti-CD40L treated C57BL/6 mice isolated 5 hours after treatment. B, Skin draining and non-draining LNs distal to the s.c. injection site of MC38 tumor cells isolated 48 hours after injection. C, Mesenteric LNs from untreated or DSS treated colitic mice isolated 24 hours after treatment. All LNs sectioned and stained for laminin α4, laminin α5, PNAd and ER-TR7. Representative images, 100X. Percentages of laminin α4 and laminin α5 positive areas, and the ratios of laminin α4: laminin α5 in the cortical ridge (CR) and high endothelial venules (HEV). Data shown as mean ± SEM. n = at least six mice per condition from three independents experiments; 1–4 LNs per mouse, 30–100 HEVs per LN.
*P < 0.05, **P < 0.01, ***P < 0.001.
We also analyzed laminin expression in dLN 48 hours after subcutaneous injection of MC38 tumor cells, an adenocarcinoma known to generate local inhibitory signals.14 In the tumor dLN, the laminin α5 expression in the CR and in the HEV of the DLN was significantly reduced and the laminin α4:α5 ratio strongly increased compared to the non-DLN (Figure 1B).
We next investigated the laminin α4 and α5 expression in the DSS inflammatory colitis model.15 In the mesenteric LN of colitic C57BL/6 mice treated with DSS for 24 hours, laminin α5 expression significantly increased and the laminin α4:α5 ratio decreased compared to the control untreated group (Figure 1C).
Together, these results demonstrated that in multiple distinct models that high laminin α5 expression was associated with immunity and inflammation, while low laminin α5 expression and thus high laminin α4 expression relative to laminin α5 were associated with immune suppression and tolerance. This also suggested that the laminin α4:α5 ratios could dictate the orientation of the immune response and impact T cell activation fate.
Laminin 411 and 511 differentially regulate CD4+ T cell activation
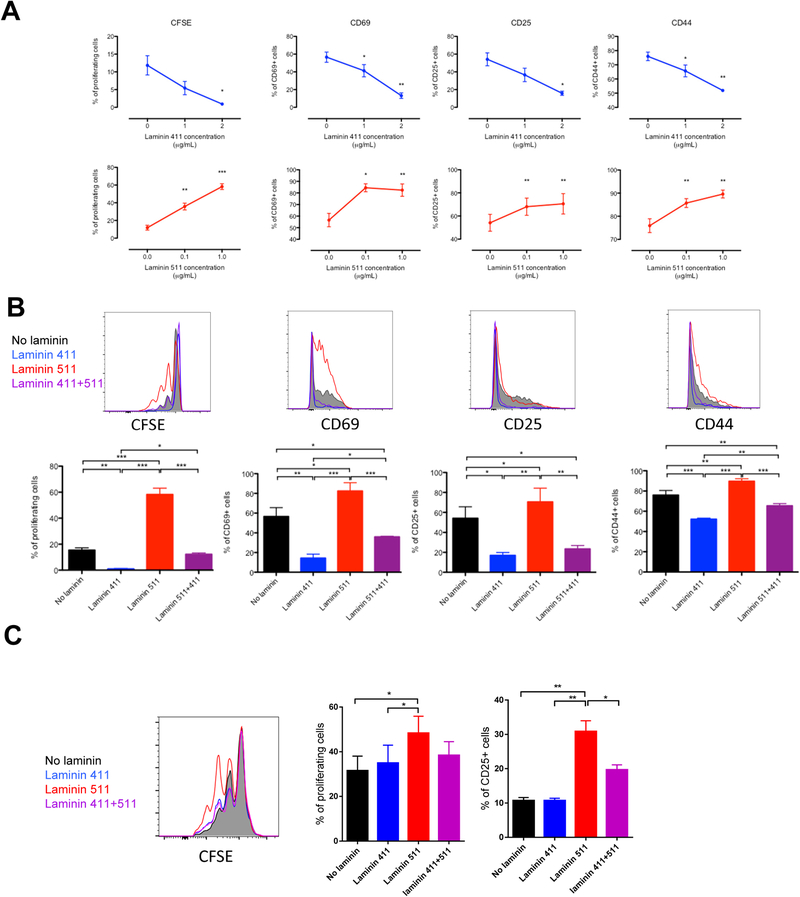
To test the hypothesis that stromal laminin 411 and 511 directly modulated CD4+ T cell activation, we analyzed the proliferation and the expression of activation markers of murine CD4+ T cells activated for 3 days with anti-CD3 at 1μg/mL (Figure 2A) or 5μg/mL (Figure S2A) in presence or not of coated laminin 411 and 511. Coated laminin 411 decreased in a dose dependent manner the proliferation and the expression of CD69, CD25 and CD44. Laminin 511 had the opposite effect and strongly favored T cell activation. Interestingly, the addition of laminin 411 to laminin 511 reduced the stimulatory effect of laminin 511 (Figure 2B and Figure S2B) demonstrating that as observed in vivo, the ratio between these two laminins can dictate the level of T cell activation. We also analyzed the proliferation of human CD4+ T cells activated with anti-CD3/28 beads in presence or not of coated laminins (Figure 2C). As for murine CD4+ T cells, laminin 511 increased human T cell proliferation and activation. Laminin 411 had no direct inhibitory effect on T cell activation but inhibited the immunostimulatory effect of laminin 511, confirming that the laminin 411/511 ratio dictated T cell activation fate also in human. In mice, both laminins slightly increased T cell viability (Figure S2C) excluding the possibility that the inhibitory effect of the laminin 411 on T cell proliferation was due to cell death. Together, these data demonstrated that laminin 411 and 511 are respectively co-inhibitory and co-stimulatory ligands for CD4+ T cells.
Figure 2.
Stromal laminin 411 and 511 differentially modulate CD4+ T cell activation in mice and human. Murine CD4 T lymphocytes evaluated by flow cytometry for proliferation and CD69, CD25, CD44 expression (A, B) after 3 days of culture in plates coated with anti-CD3 (1 μg/mL) and the indicated concentrations of laminin 411 or laminin 511 (A), or with laminin 411 (2 μg/mL) and/or laminin 511 (1 μg/mL) (B). Human CD4+ T lymphocytes (C) evaluated by flow cytometry for proliferation and CD25 expression after 3 days of culture in plates coated with laminin 411 (2 μg/mL) and/or laminin 511 (1 μg/mL) with anti-CD3/CD28 beads (T:Beads ratio = 1:1). Data shown as representative profiles (B, C) and mean ± SEM (A-C) of three independent experiments with samples.
*P < 0.05, **P < 0.01, ***P < 0.001.
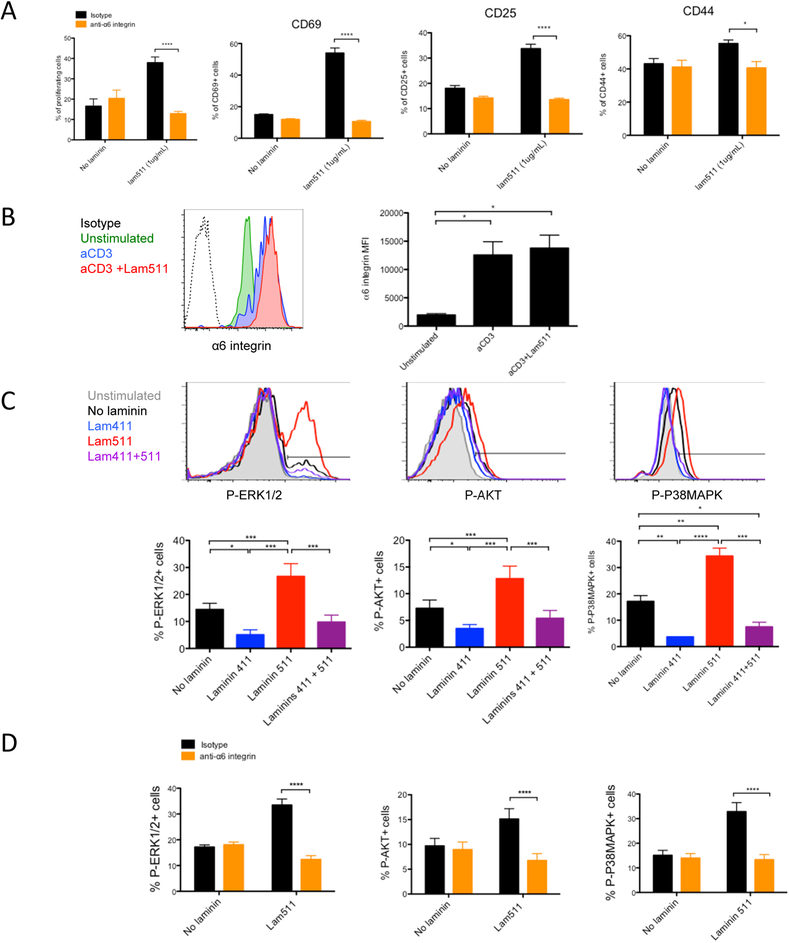
To characterize the laminin receptors, antibodies against potential receptors for laminins were tested (Table 1). Among all the antibodies used, none of them were able to reverse or prevent the laminin 411 inhibitory effect. In contrast, the anti-α6 integrin blocking antibody abrogated the laminin 511 stimulatory effect on CD4+ T cell proliferation and activation (Figure 3A) while it had no effect without laminin 511, demonstrating that α6 integrin is the main T cell receptor for laminin 511, confirming previous observations.16 The α6 integrin receptor is highly expressed on naïve CD4+ T cells and upregulated upon activation (Figure 3B). Integrins favor the activation of Erk, Akt and P38Mapk.17,18 These three pathways are modulated by laminins 411 and 511 (Figure 3C). Laminin 411 decreased the phosphorylation of ERK1/2, AKT and P38MAPK in CD4+ T cells activated with anti-CD3 (Figure 3C). Laminin 511 had the opposite effect and promoted the phosphorylation of these three molecules via the α6 integrin (Figure 3D). Laminin 411 inhibited the stimulatory effect of laminin 511. As these pathways are essential for CD4+ T cell proliferation and activation,19 these results highlight at the molecular level how laminin 411 and 511 regulated T cell activation.
Table 1:
Potential anti-laminin receptor antibodies for in vitro use
| Target Molecule | Source | Clone | Company |
|---|---|---|---|
| α1 integrin | Hamster | Ha31/8 | BD Bioscience |
| α2 integrin | Armenian Hamster | HMα2 | Biolegend |
| α3 integrin | Mouse | ASC-1 | Biolegend |
| α4 integrin | Rat | 9C10 | Biolegend |
| α5 integrin | Armenian Hamster | HMα5–1 | Biolegend |
| α6 integrin | Rat | GoH3 | Biolegend |
| α7 integrin | Mouse | 6A11 | LSBio |
| αM integrin | Rat | M1/70 | Biolegend |
| αV integrin | Rat | RMV-7 | Biolegend |
| β1 integrin | Armenian Hamster | HMβ1–1 | Biolegend |
| β2 integrin | Rat | M18/2 | Biolegend |
| β3 integrin | Armenian Hamster | 2C9.G2 | Biolegend |
| α dystroglycan | Mouse | IIH6C4 | Merck Millipore |
Figure 3.
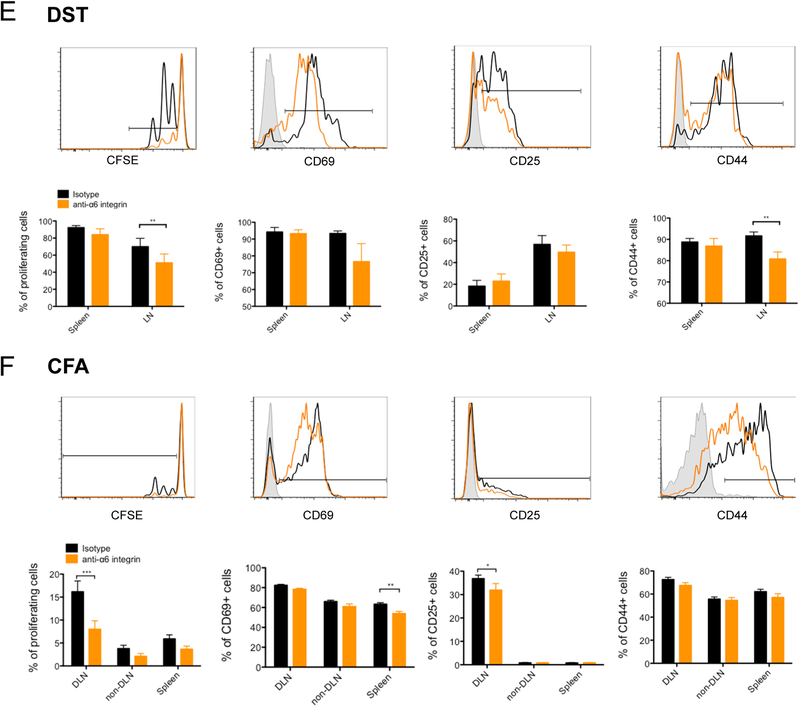
Laminin 511 favors CD4 T cell activation via the receptor α6 integrin. CD4 T lymphocytes evaluated by flow cytometry for proliferation and CD69, CD25, CD44 expression (A), for α6 integrin expression after 3 days of culture (B), or phospho-Erk1/2, phospho-Akt and phospho-P38Mapk (C) after 30 minutes of culture in plates coated with anti-CD3 (1 μg/mL) and with laminin 411 (2 μg/mL) and/or laminin 511 (1 μg/mL) in presence of anti-α6 integrin or isotype control mAb (A, D). Data shown as representative profiles (B, C) and mean ± SEM (A-D) of three independent experiments with duplicate samples. (E) DST or (F) CFA/Ea treated mice received 5 × 106 CFSE-labeled CD45.1 TEa TCR transgenic CD4+ T cells. Mice were euthanized two days post transfer, and donor lymphocytes from spleen and skin LNs assessed for proliferation, CD69, CD25, CD44 and percentage of donor cells (CD4+ CD45.1+) in different organs by flow cytometry. Representative profiles shown together with a quantitative analysis of three independent experiments. Data shown as mean ± SEM of at least six mice per group from three independent experiments.
*P < 0.05, **P < 0.01, ***P < 0.001.
We next determined if the laminin 511 receptor α6 integrin favored CD4+ T cell activation in vivo. C57BL/6 (H-2b) mice were immunized with BALB/c (H-2d) DST or with I-Ed peptide in CFA. These mice also were adoptively transferred with T cell receptor transgenic (TCR Tg) CD4+ T cells from TEa mice that recognize donor I-Ed presented by recipient I-Ab (Ea antigen).11 Mice received anti-α6 integrin blocking mAb or isotype control mAb. In both models, systemic blockade of α6 integrin resulted in decreased proliferation of alloantigen specific CD4 T cells in the LNs (Figure 3E and F) without affecting the proportion of Tea T cells in the LN (Figure S3A) excluding an inhibition of T cell recruitment in the LN. These results confirmed the immunogenic role of this laminin-specific receptor and suggested a co-stimulatory role for laminin 511 in vivo even under strong stimulatory conditions of CFA or fully mismatched donor alloantigen.
Laminin 411 and 511 differentially regulate CD4+ T cell polarization
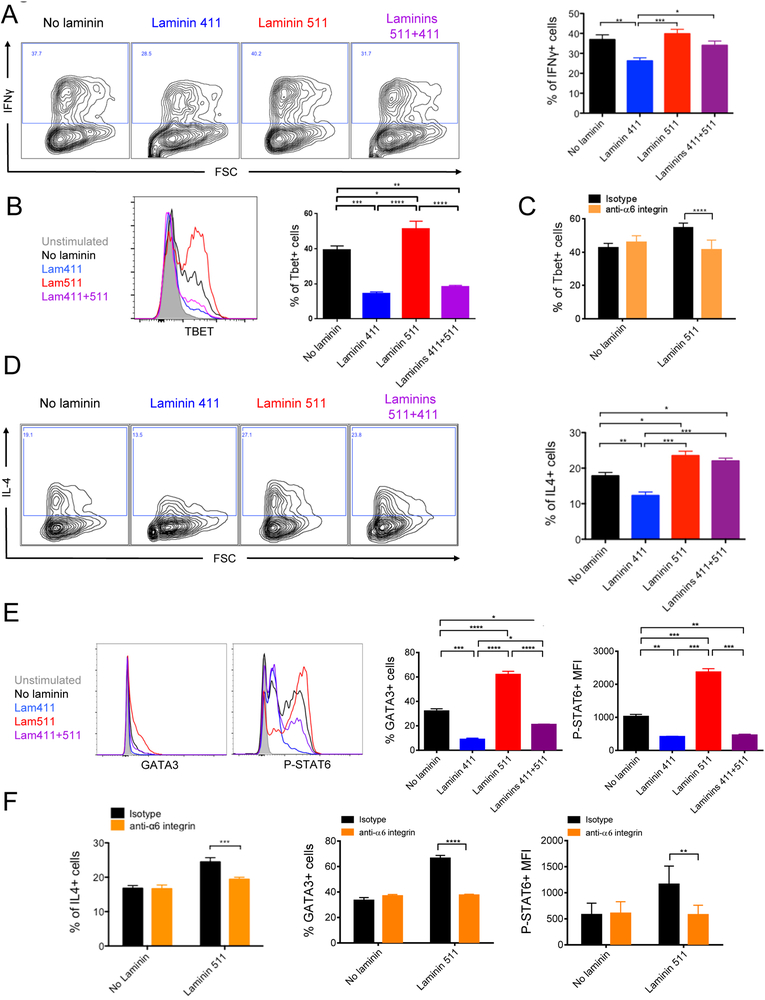
To test the modulation of polarization by laminins, we first tested the impact of laminin 411 and 511 on CD4+ T cells activated in vitro for 5 days with anti-CD3 and anti-CD28 without polarizing cytokines (Figure S2D). In a neutral environment, neither laminin had an impact on Th1, Th2 and Th17 induction. In a pro-Th1 environment (Figure 4A), laminin 511 had no effect on the proportion of IFNγ+ cells but significantly increased Tbet expression (Figure 4B). Addition of laminin 411 significantly decreased IFNγ+ cells, Tbet expression and hence Th1 induction. Addition of laminin 511 to laminin 411 reversed the laminin 411 inhibitory effect on Th1 induction. The laminin 511 effect on Tbet overexpression was mediated by the α6 integrin (Figure 4C).
Figure 4.
Laminin 411 and 511 differentially regulate Th1 and Th2 polarization. CD4 T lymphocytes evaluated by flow cytometry for intracellular expression of IFNγ (A) or IL4 (D) after 5 days or Tbet, Gata3 and phospho-Stat6 after 16 hours culture in pro-Th1 (B,C) or pro-Th2 (E,F) environments with laminin 411 (2 μg/mL) and/or laminin 511 (1 μg/mL) in presence of functional blocking anti-α6 integrin or isotype control mAb (C,F). Data shown as representative profiles and mean ± SEM of at least three independent experiments with duplicate samples.
Addition of laminin 411 to CD4+ cells stimulated in a pro-Th2 environment also inhibited IL-4 and Th2 induction (Figure 4D). Laminin 511 had the opposite effect and increased the proportion of IL-4+ CD4+ T cells (Figure 4D) and promoted the expression of the pro-Th2 transcription factors Gata3 and phospho-Stat6 (Figure 4E). Addition of laminin 411 to 511 completely abrogated the overexpression induced by laminin 511. Each of these laminin 511 driven effects was mediated by the α6 integrin since blocking mAb prevented them (Figure 4F). We next analyzed if the laminin 511 receptor α6 integrin also favored Th1 and Th2 induction in vivo. TEa TCR Tg CD4+ T cells were transferred, with or without anti-α6 integrin mAb, into C57BL/6 mice that received CFA/Ea, and expression of IFNγ and IL4 by TEa cells was analyzed after 3 days (Figure S3B). Systemic blockade of α6 integrin resulted in a slight decreased proportion of IFNγ+ but not IL4+ alloantigen specific CD4+ T cells in the dLN.
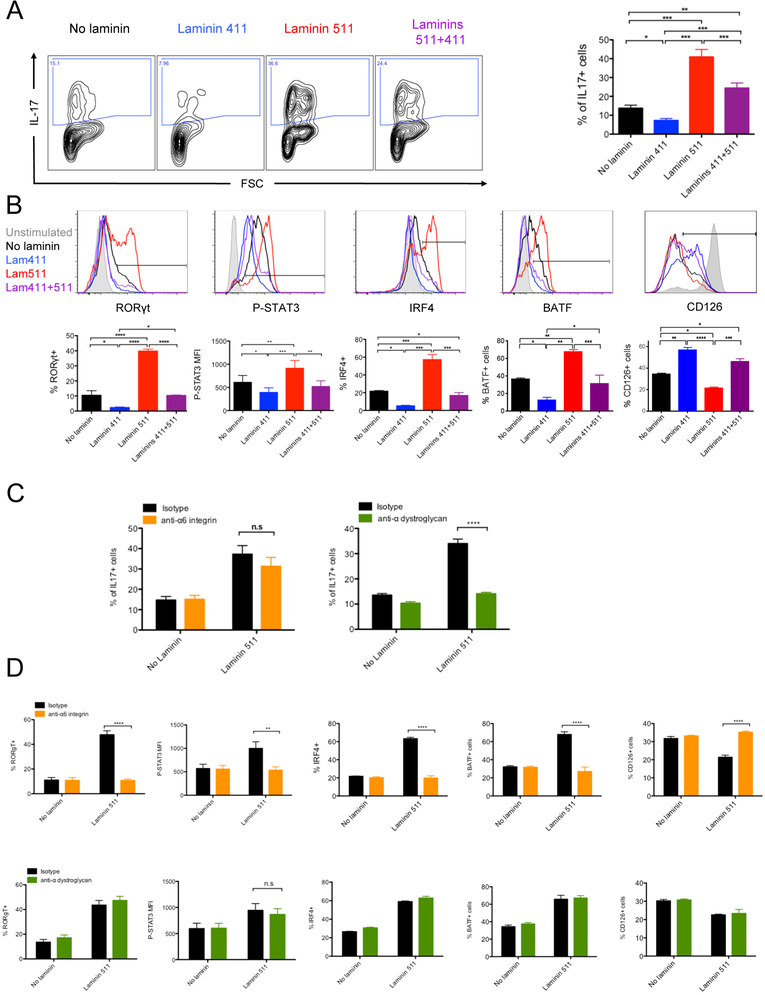
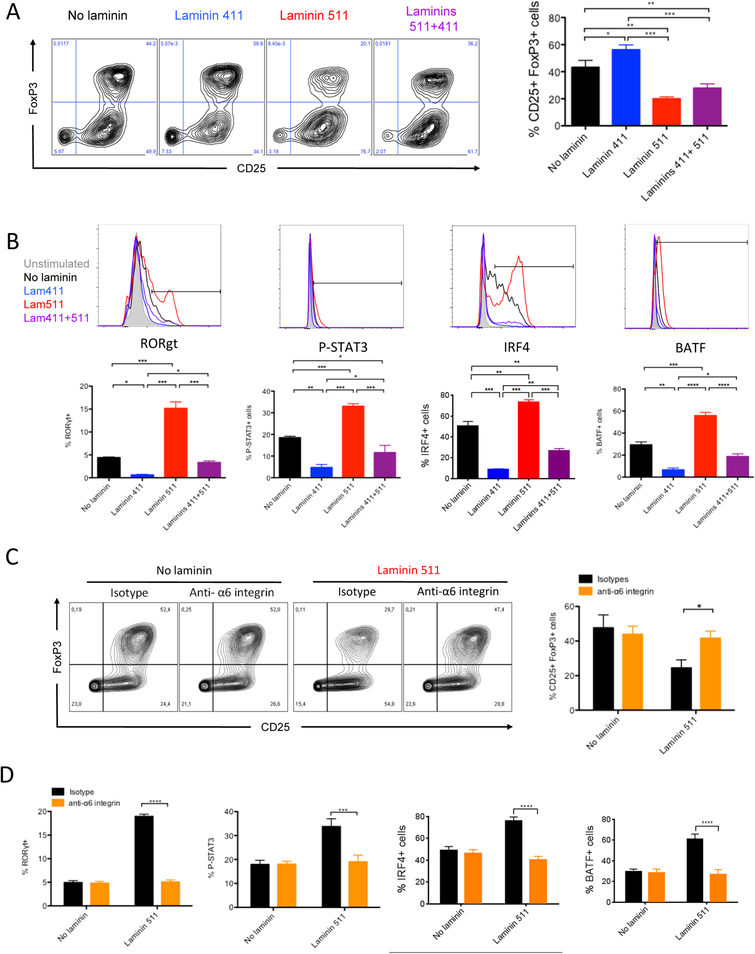
In a pro-Th17 environment, laminin 511 increased Th17 induction by almost 4-fold (Figure 5A). Laminin 511 favored expression of the pro-Th17 factors Rorγt, phospho-Stat3, Irf4, and Batf.20 and reduced the expression of the IL-6 receptor CD126 (Figure 5B). Laminin 411 had the opposite effects and significantly decreased the proportion of IL-17+ cells and the expression of the pro-Th17 transcription factors. Laminin 411 partially reversed the pro-Th17 effect of laminin 511. Addition of the anti-α6 integrin mAb in the culture was unable to inhibit the laminin 511 effect on IL17 production (Figure 5C). Among all the blocking antibodies tested (Table 1), only the blockade of the laminin receptor α-dystroglycan (αDG) abrogated the pro-Th17 effect of laminin 511. Surprisingly, anti-α6 integrin inhibited the effect of laminin 511 on Rorγt, phospho-Stat3, Irf4 and Batf expression while anti-αDG did not (Figure 5D). These results suggested that an unknown pro-Th17 pathway is induced by the laminin 511/αDG axis.
Figure 5.
Laminin 511 promotes Th17 polarization via the α-dystroglycan receptor. CD4 T lymphocytes evaluated by flow cytometry for IL-17 after 5 days of culture (A, C), Rorγt, phopho-Stat3, Irf4, Batf and CD126 (B, D) expression after 16 hours of culture in pro-Th17 environment with laminin 411 (2 μg/mL) and/or laminin 511 (1 μg/mL) in presence of functional blocking anti-α6 integrin mAb, anti-α-dystroglycan or isotype control (C, D). Data shown as representative profiles (A, B) and mean ± AEM (A-D) of at least three independent experiments with duplicate samples. (E) CFA/Ea treated mice received 5 × 106 CFSE-labeled CD45.1 Ea TCR transgenic CD4+ T cells, with or without anti-αDG mAb, euthanized three days post transfer, and donor lymphocytes from spleen and skin LNs assessed as percentage of IL17+ donor cells (CD4+ CD45.1+) in different organs by flow cytometry. Representative profile shown with quantitative analysis of three independent experiments. Data shown as mean ± SEM of eight mice per group from three independent experiments.
*P < 0.05, **P < 0.01, ***P < 0.001.
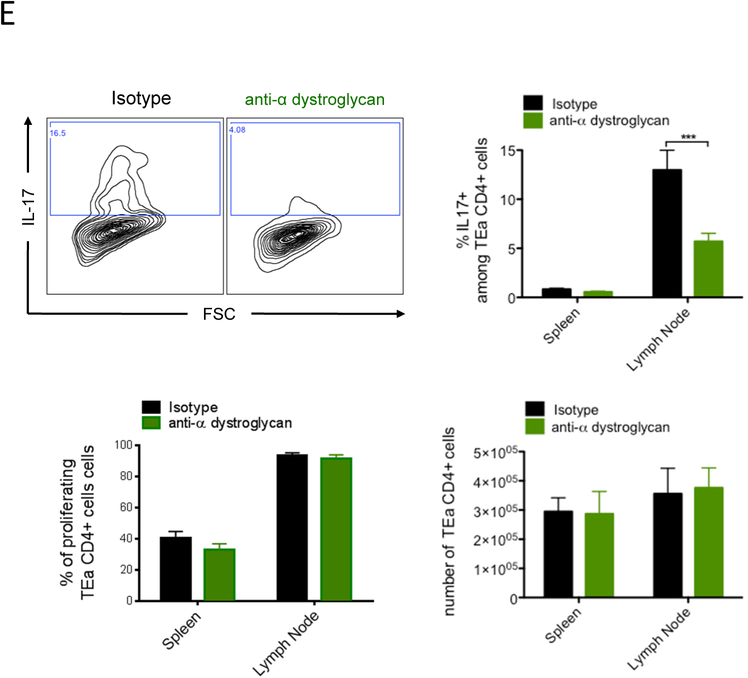
We next analyzed if the laminin 511 receptor αDG also favored Th17 induction in vivo. TEa TCR Tg CD4+ T cells were transferred, with or without anti-αDG mAb, into C57BL/6 mice that received CFA/Ea, and expression of IL-17 by TEa cells was analyzed after 3 days (Figure 5E). Systemic blockade of αDG resulted in a decreased proportion of IL-17+ alloantigen specific CD4+ T cells in the dLN. The percentage of proliferating cells and the absolute number of the TEa CD4+ T cells were unchanged, excluding the possibility that the decreased proportion was due to antibody mediated T cell depletion or a migration defect of the T cells to the LN. Blockade of α6 integrin had no effect on the proportion of IL-17+ cells (Figure S3B). These results demonstrated for the first time a major role for the laminin-specific receptor αDG in Th17 induction in vivo and suggested a co-stimulatory role for laminin 511.
In a pro-Treg environment, laminin 411 significantly increased the proportion of CD25+ FoxP3+ CD4+ T cells (Figure 6A). Laminin 511 had the opposite effect and reduced the proportion of Treg cells by 2-fold. To explore why laminin 511 inhibited Treg induction in presence of TGF β, the expression of pro-Th17 factors was analyzed (Figure 6B). Laminin 511 favored the expression of Rorγt, Irf4 and Batf, and increased Stat3 phosphorylation, demonstrating that in a pro-Treg environment, laminin 511 promoted the induction of Th17 pathways and so decreased Treg polarization. Laminin 411 almost completely inhibited the expression of these pro-Th17 molecules and thus favored Treg induction. The inhibitory effect of laminin 511 on Treg induction was abrogated by anti-α6 integrin mAb (Figure 6C) and not by anti- αDG (data not shown). Induction of pro-Th17 factors by laminin 511 was also inhibited by anti-α6 integrin (Figure 6D).
Figure 6.
Laminin 411 and 511 differentially regulate Treg polarization. CD4 T lymphocytes evaluated by flow cytometry for CD25, FoxP3 expression after 5 days of culture (A, C), Rorγt, phopho-Stat3, Irf4 and Batf (B, D) expression after 16 hours of culture in pro-Treg environment, with laminin 411 (2 μg/mL) and/or laminin 511 (1 μg/mL) in presence of functional blocking anti-α6 integrin or isotype control mAb (C, D). Data shown as representative profiles (A-C) and mean ± SEM (A-D) of at least three independent experiments with duplicate samples.
*P < 0.05, **P < 0.01, ***P < 0.001.
Together, these results demonstrated that laminins 411 and 511 were important regulators for CD4+ T cell polarization and had divergent effects.
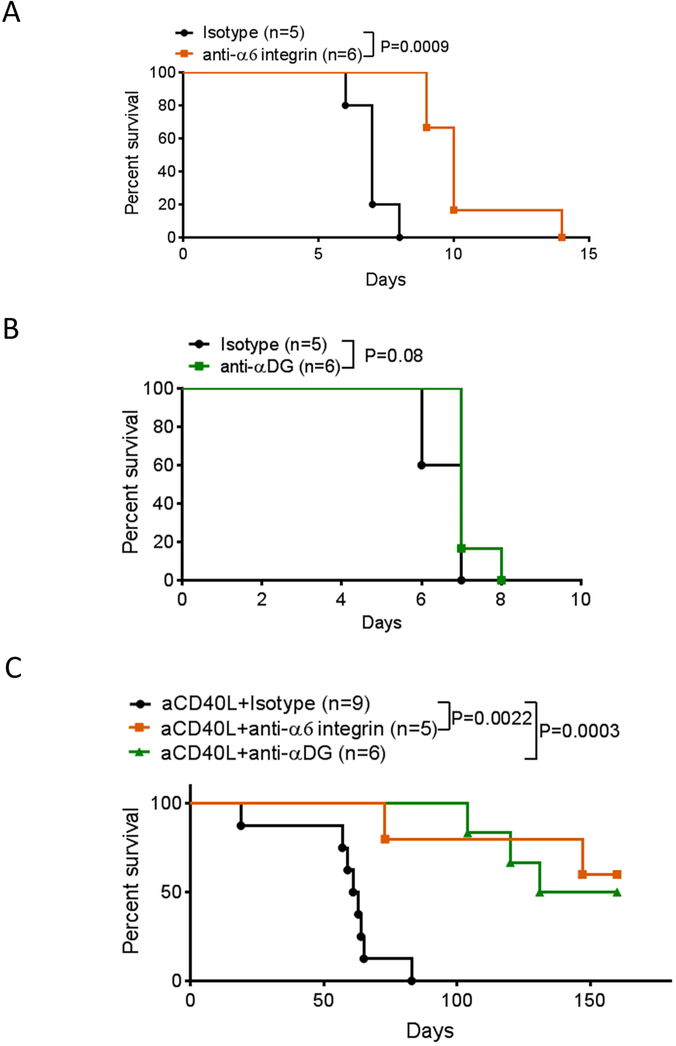
Targeting the laminin 511 receptors α6 integrin and αDG interferes with the induction of alloimmunity and cardiac allograft rejection.
Cardiac allograft rejection is associated with a large decrease in the laminin α4: α5 ratio around post capillary venules, suggesting that the co-stimulatory effect of laminin 511 could also be present in the transplant in addition to the LN (Figure S4). We hypothesized that blocking the main receptors for laminin 511, the α6 integrin and the αDG, would interfere with immunity and favor the generation of immune suppression and tolerance in the LN and in the cardiac transplant. To test this hypothesis, otherwise untreated allograft recipients received anti-α6 integrin mAb or anti-αDG i.v. No side effects or infections have been observed in the treated animals. Anti-α6 integrin resulted in a small but significant prolongation of graft survival (Figure 7A) similar to the protection induced by anti-laminin α5 antibody treatment that we observed previously.4 In comparison to potent, widely used immunosuppressive agents such as CTLA4-Ig21 or rapamycin,22 the protection induced by anti-α6 integrin mAb alone was moderate. Anti-αDG alone had no effect on graft survival (Figure 7B). We further hypothesized that increasing the laminin α4:α5 ratio with anti-CD40L mAb as demonstrated in Figure 1A would improve the efficacy of anti-α6 integrin or anti-αDG treatment for immune protection. Thus, mice were injected once on the day of transplantation with anti-CD40L and treated with or without anti-α6 integrin or anti-αDG (Figure 7C). The addition of anti–α6 integrin or anti–αDG to anti-CD40L treatment resulted in long term protection. These data suggested that increasing the laminin α4:α5 ratio in the LN CR synergized with the inhibition of the laminin 511-α6 integrin or αDG interactions on CD4+ T cells to promote immune suppression and tolerance.
Figure 7.
Blocking laminin 511 receptor α6 integrin and αDG prolongs allogenic cardiac graft survival. C57BL/6 mice received BALB/c heterotopic cardiac allografts on day 0 along with anti-α6 integrin or isotype control mAb alone (10 μg i.v, day 0 and every 3 days) (A), anti- αDG or isotype control mAb alone (B), anti-α6 integrin or anti- αDG (10 μg i.v, day 0 and once a week) with anti-CD40L (250 μg i.v. day 0 only) (C). Data presented as percent survival. n = at least five mice per group.
*P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In this study, we demonstrated that induction of immunity or tolerance modulated very rapidly the expression of laminin α4 and laminin α5 (Figure 1) in the LN. Inflammation and allogenic responses (Figure 1A and C) increased laminin α5 expression rapidly in the CR and HEV of the dLN. Induction of tolerance with anti-CD40L treatment (Figure 1A) or the presence of immunosuppressive environment (Figure 1B) reduced significantly the expression of laminin α5. For laminin α4 expression, only the major inflammation associated with CFA treatment was able to decrease its expression in the CR. It has been clearly demonstrated that the expression of the laminins is differentially controlled by numerous signals such as growth factors, cytokines and proteases.16,23,24 The regulation of laminin expression in the LN cortical ride is a complex process that could involve either laminin polymerization at the cell surface, de novo protein production, laminin degradation and migration of laminin positive cells. These different mechanisms may contribute to the fact that the laminin 411 and 511 composition in the LN is variable and dynamic. However, the rapid overexpression of laminin α5 observed only 5 hours after induction seems to be inconsistent with de novo protein synthesis.
During adaptive immune responses, LNs undergo important modifications in size and structure.25–28 Infection induces transcriptional changes in LN stromal cells resulting in expanded fibroblastic reticular cell networks that persist for months, a process termed “immunological scarring.”25 In our study, we demonstrated that the laminin α4: α5 ratio in the LN was one of the first main modifications associated with LN remodeling and was directly correlated to the state of the immune response. Moreover, this changes in laminin α4 and α5 expression in the LN were persistent for at least 72 hours after induction of immunity or tolerance.4 Based on these observations we hypothesized that laminin isoforms 411 and 511 directly impacted T lymphocyte activation. In vitro studies demonstrated that addition of laminin 411 to anti-CD3 stimulated cultures reduced proliferation and activation (Figure 2A, B). In contrast, addition of laminin 511 increased proliferation and activation by up to 8-fold. Previous studies described the modulation of T cell activation by laminins, but with conflicting results.16,29,30 Discrepancies among reports may be explained by use of poorly characterized laminin preparations isolated from human placenta versus recombinant laminin proteins, variation in laminin concentrations, and use of unpurified lymphocyte populations or T cell lines. Interestingly, the in vivo proliferation analysis in LN of CD4+ T cells in lama4−/− full KO mice showed no difference compared to WT.5 The discrepancy between this study and ours, that showed laminin 411decreased proliferation, may be related to developmental compensatory mechanisms in germline KO mice. Indeed, in these mice, laminin α5 distribution in endothelial basement membranes was altered and associated with substantial modifications of myeloid and T cell migration. These changes could affect T cell activation and proliferation compared to WT and compensate for the loss of the inhibitory effect of laminin 411.
The effect of laminin 511 is mainly mediated by integrin α6 (Figure 3A). The molecular pathways activated by laminin 511, such as ERK1/2, AKT and P38MAPK, were inhibited by anti-α6 integrin antibody. Blocking α6 integrin also reduced CD4 T cell proliferation in different models in vivo (Figure 3E, F), confirming that α6 integrin was a co-stimulatory receptor for CD4 T cells. Blocking α6 integrin prolonged allograft survival and resulted in long-term survival when combined with anti-CD40L mAb (Figure 7). Thus, the laminin 511- α6 integrin axis seems a promising target in transplantation.
The receptor for laminin 411 that mediated T cell inhibition is still unknown despite using multiple different antibodies. This means that the antibodies were unable to block efficiently laminin 411 interaction with its receptor or the receptor is an unknown non-integrin receptor. Laminin 411 was described to bind multiple receptors such as integrins α6- or α7- and α-dystroglycan with poor affinity.31,32This may explain why blocking only one does receptor did not produce any effect. More investigations are needed to characterize laminin 411 receptors that mediated T cell inhibition and which can be an interesting therapeutic targets.
We analyzed the impact of laminin 411 and 511 on CD4 T cell polarization toward the well-described Th1, Th2, Th17, and Treg paths.33 Laminin 511 had little impact on Th1 polarization, slightly increased Th2 CD4 T cell induction, and greatly promoted Th17 induction (Figure 4A, C and5A). The main pathways20 involved in Th17 polarization were upregulated in presence of laminin 511 (Figure 5B). The prototypic Th17 transcription factor Rorγt was also highly upregulated by laminin 511. As for proliferation, the laminin 511 effects on Rorγt expression and phosphorylation of pro-Th17 pathways were mediated by α6 integrin. However, only the blocking of α-dystroglycan34 inhibited IL-17 induced by laminin 511(Figure 5C). The pro-Th17 effect of α-dystroglycan was confirmed in vivo in CFA/antigen immunized mice (Figure 5E). We demonstrated for the first time that α-dystroglycan promotes Th17 generation and could be a promising therapeutic target. Indeed, the blocking of α-dystroglycan prolonged cardiac allograft survival and resulted in long-term survival when combined with anti-CD40L mAb (Figure 7B)
Likely because of activation of pro-Th17 pathways, laminin 511 inhibited induction of FoxP3 regulatory CD4 T cells (Figure 6) via α6-integrin. Interestingly, laminin 411 showed an opposite effect compared to laminin 511. Laminin 411 inhibited Th1, Th2 (Figure 4A and C) and Th17 polarization (Figure 5A), but favored Treg induction (Figure 6A). Both Th17 and Treg cells can develop from naïve CD4+ T cell precursors under the influence of the same cytokine, transforming growth factor β1 (TGFβ1).33 We demonstrated here that laminins 411 and 511 are important factors driving Treg and Th17 plasticity. Th1, Th2 and Th17 have been described to play an important role in allograft rejection. Th1 cells are considered as one of the main actors in acute allograft rejection,35 while Th2 seem to be more involved in chronic rejection.36 The active role of Th17 cells in transplant rejection has been described more recently and is linked to neutrophil recruitment into the graft.37 Due to the central role of CD40L in Th1 activation, anti-CD40L inhibits Th1 induction and acute rejection38 but favors Th2 induction that could be deleterious for transplant outcome as described previously.36 Moreover, it has been demonstrated that in absence of a Th1 alloimmune response, aggressive Th17 lymphocytes come into play and favor allograft rejection in a model of chronic rejection.39 The inhibition of Th2 and Th17 induction associated with anti- α6 integrin and anti- α-dystroglycan that we observed may explain the long term graft protection when these two antibodies are used in association with anti-CD40L treatment (Figure 7C). Thus, targeting the laminin 511 receptors α6 integrin and α-dystroglycan in order to reduce CD4 T cell proliferation and Th1, Th2 and Th17 polarization in favor of Treg cells induction appear as a promising therapeutic strategy for transplantation.
All together, these data demonstrated that laminin 411 and 511 expressed in the LN are co-inhibitory and co-stimulatory ligands, respectively, for CD4 T cells. Laminin 411 and 511 had opposite effects that countered each other. These findings demonstrated that laminins are not only passive LN structural molecules, but also act as one of the molecular switches for tolerance and immunity by directly influencing T cell activation and functions.
Supplementary Material
Acknowledgments:
This work was prepared while Terez Shea-Donohue was employed at University of Maryland School of Medicine. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Financial disclosure: J. S. Bromberg was supported by NIH Grants 1RO1AI114496 and 1RO1AI062765.
Abbreviations
- aDG
alpha dystroglycan
- APC
Antigen Presenting Cell
- BM
Basement Membrane
- CFA
Complete Freund’s Adjuvant
- CR
Cortical Ridge
- DC
Dendritic Cell
- dLN
draining Lymph Node
- DST
Donor-Specific Transfusion
- HEV
High Endothelial Venule
- LN
Lymph Node
- pDC
Plasmacytoid Dendritic Cell
- TCR
T Cell Receptor
- Treg
Regulatory T Cell
- DSS
dextran sodium sulfate
Footnotes
Conflicts of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Simon T, Bromberg JS. Regulation of the Immune System by Laminins. Trends Immunol. 2017;38(11):858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frieser M, Nöckel H, Pausch F, et al. Cloning of the mouse laminin alpha 4 cDNA. Expression in a subset of endothelium. Eur J Biochem. 1997;246(3):727–735. [DOI] [PubMed] [Google Scholar]
- 3.Sorokin LM, Pausch F, Frieser M, et al. Developmental regulation of the laminin alpha5 chain suggests a role in epithelial and endothelial cell maturation. Dev Biol. 1997;189(2):285–300. [DOI] [PubMed] [Google Scholar]
- 4.Warren KJ, Iwami D, Harris DG, et al. Laminins affect T cell trafficking and allograft fate. J Clin Invest. 2014;124(5):2204–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Ivars F, Anderson P, et al. Endothelial basement membrane laminin α5 selectively inhibits T lymphocyte extravasation into the brain. Nat Med. 2009;15(5):519–527. [DOI] [PubMed] [Google Scholar]
- 6.Wondimu Z, Geberhiwot T, Ingerpuu S, et al. An endothelial laminin isoform, laminin 8 (alpha4beta1gamma1), is secreted by blood neutrophils, promotes neutrophil migration and extravasation, and protects neutrophils from apoptosis. Blood. 2004;104(6):1859–1866. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Voisin M-B, Larbi KY, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203(6):1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song J, Zhang X, Buscher K, et al. Endothelial basement membrane laminin 511 contributes to endothelial junctional tightness and thereby inhibits leukocyte transmigration. Cell Rep. 2017;18(5):1256–1269. [DOI] [PubMed] [Google Scholar]
- 9.Sixt M, Kanazawa N, Selg M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22(1):19–29. [DOI] [PubMed] [Google Scholar]
- 10.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–350. [DOI] [PubMed] [Google Scholar]
- 11.Grubin CE, Kovats S, deRoos P, et al. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II–bound self-peptides. Immunity. 1997;7(2):197–208. [DOI] [PubMed] [Google Scholar]
- 12.Sinha P, Parker KH, Horn L, et al. Tumor-induced myeloid-derived suppressor cell function is independent of IFN-γ and IL-4Rα. Eur J Immunol. 2012;42(8):2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quezada SA, Fuller B, Jarvinen LZ, et al. Mechanisms of donor-specific transfusion tolerance: preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood. 2003;102(5):1920–1926. [DOI] [PubMed] [Google Scholar]
- 14.Juneja VR, McGuire KA, Manguso RT, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214(4):895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chassaing B, Aitken JD, Malleshappa M, et al. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:Unit 15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sixt M, Engelhardt B, Pausch F, et al. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood–brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153(5):933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mainiero F, Soriani A, Strippoli R, et al. RAC1/P38 MAPK signaling pathway controls β1 integrin–induced interleukin-8 production in human natural killer cells. Immunity. 2000;12(1):7–16. [DOI] [PubMed] [Google Scholar]
- 18.Moreno-Layseca P, Streuli CH. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014;34:144–153. [DOI] [PubMed] [Google Scholar]
- 19.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nalbant A, Eskier D. Genes associated with T helper 17 cell differentiation and function. Front Biosci (Elite Ed). 2016;8:427–435. [DOI] [PubMed] [Google Scholar]
- 21.Baliga P, Chavin KD, Qin L, et al. CTLA4Ig prolongs allograft survival while suppressing cell-mediated immunity. Transplantation. 1994;58(10):1082–1090. [PubMed] [Google Scholar]
- 22.Nakamura T, Nakao T, Yoshimura N, et al. Rapamycin prolongs cardiac allograft survival in a mouse model by inducing myeloid-derived suppressor cells. Am J Transplant. 2015;15(9):2364–2377. [DOI] [PubMed] [Google Scholar]
- 23.Amin K, Janson C, Sevéus L, et al. Uncoordinated production of Laminin-5 chains in airways epithelium of allergic asthmatics. Respir Res. 2005;6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spenlé C, Lefebvre O, Lacroute J, et al. The laminin response in inflammatory bowel disease: protection or malignancy? PLoS ONE. 2014;9(10):e111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory JL, Walter A, Alexandre YO, et al. Infection programs sustained lymphoid stromal cell responses and shapes lymph node remodeling upon secondary challenge. Cell Rep. 2017;18(2):406–418. [DOI] [PubMed] [Google Scholar]
- 26.Kumar V, Dasoveanu DC, Chyou S, et al. A dendritic-cell-stromal axis maintains immune responses in lymph nodes. Immunity. 2015;42(4):719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burrell BE, Warren KJ, Nakayama Y, et al. Lymph node stromal fiber ER-TR7 modulates CD4+ T cell lymph node trafficking and transplant tolerance. Transplantation. 2015;99(6):1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama Y, Brinkman CC, Bromberg JS. Murine fibroblastic reticular cells from lymph node interact with CD4+ T cells through CD40-CD40L. Transplantation. 2015;99(8):1561–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geberhiwot T, Assefa D, Kortesmaa J, et al. Laminin-8 (alpha4beta1gamma1) is synthesized by lymphoid cells, promotes lymphocyte migration and costimulates T cell proliferation. J Cell Sci. 2001;114(Pt 2):423–433. [DOI] [PubMed] [Google Scholar]
- 30.Gorfu G, Virtanen I, Hukkanen M, et al. Laminin isoforms of lymph nodes and predominant role of alpha5-laminin(s) in adhesion and migration of blood lymphocytes. J Leukoc Biol. 2008;84(3):701–712. [DOI] [PubMed] [Google Scholar]
- 31.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. [DOI] [PubMed] [Google Scholar]
- 32.Nishiuchi R, Takagi J, Hayashi M, et al. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25(3):189–197. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H, Talts JF. Beta1 integrin and alpha-dystroglycan binding sites are localized to different laminin-G-domain-like (LG) modules within the laminin alpha5 chain G domain. Biochem J. 2003;371(Pt 2):289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Elios MM, Josien R, Manghetti M, et al. Predominant Th1 cell infiltration in acute rejection episodes of human kidney grafts. Kidney Int. 1997;51(6):1876–1884. [DOI] [PubMed] [Google Scholar]
- 36.Nocera A, Tagliamacco A, De Palma R, et al. Cytokine mRNA expression in chronically rejected human renal allografts. Clin Transplant. 2004;18(5):564–570. [DOI] [PubMed] [Google Scholar]
- 37.Gorbacheva V, Fan R, Li X, et al. Interleukin-17 promotes early allograft inflammation. Am J Pathol. 2010;177(3):1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hancock WW, Sayegh MH, Zheng XG, et al. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93(24):13967–13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205(13):3133–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.