ABSTRACT
Background
The effects of dietary composition on weight loss are incompletely understood. In addition to energy intake, fiber intake, energy density, macronutrient composition, and demographic characteristics have all been suggested to contribute to weight loss.
Objective
The primary aim of this analysis was to assess the role of dietary fiber as a predictor of weight loss in participants who consumed calorie-restricted diets (−750 kcal/d from estimated energy needs) for 6 mo, using data from the POUNDS Lost (Preventing Overweight Using Novel Dietary Strategies) Study—a randomized trial that examined the effects of calorie-restricted diets varying in macronutrient composition on weight loss in adults.
Methods
Data were randomly partitioned to a training data set (70%) in which the effects of fiber and other weight-loss predictors were identified using adjusted Least Absolute Shrinkage and Selection Operator and model averaging. The retained predictors were then fit on the testing data set to assess predictive performance.
Results
Three hundred and forty-five participants (53.9% female) provided dietary records at baseline and 6 mo. Mean ± SD age and BMI for the full sample was 52.5 ± 8.7 y and 32.6 ± 3.9 kg/m2, respectively. Mean ± SD (99% CI) weight change at 6 mo for the full sample was −7.27 ± 5.6 kg (−8.05, −6.48 kg). The final, best fit model (R2 = 0.41) included fiber, energy density, fat, age, adherence, baseline weight, race, and changes from baseline in carbohydrate, fiber, PUFA, and MUFA intake, but the most influential predictor was fiber intake (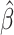 = −0.37; P < 0.0001). In addition, fiber was strongly associated with adherence to the macronutrient prescriptions (P < 0.0001). Interactions between race and adherence, age, baseline weight, carbohydrate, energy density, and MUFAs were also retained in the final model.
= −0.37; P < 0.0001). In addition, fiber was strongly associated with adherence to the macronutrient prescriptions (P < 0.0001). Interactions between race and adherence, age, baseline weight, carbohydrate, energy density, and MUFAs were also retained in the final model.
Conclusion
Dietary fiber intake, independently of macronutrient and caloric intake, promotes weight loss and dietary adherence in adults with overweight or obesity consuming a calorie-restricted diet. This trial was registered at clinicaltrials.gov as NCT00072995.
Keywords: fiber, weight loss, energy density, dietary adherence, LASSO, obesity
Introduction
Approximately 37.9% of men and 41.1% of women in the Unted States were classified as obese [BMI (in kg/m2) ≥30.0] in 2015–2016 and unadjusted obesity rates have steadily increased even since 2007–2008 (1). Half of US adults surveyed between 2013 and 2016 indicated they have made efforts to lose weight within the past year (2). These attempts are often unsuccessful, with only 20% of adults losing ≥10% of their initial body weight (3). Major shortcomings of weight-loss strategies include poor adherence and physiological compensatory mechanisms that can inhibit weight loss and promote weight regain (4–6).
Evidence-based dietary strategies to induce weight loss should focus primarily on achieving an energy deficit (7). As reported previously, calorie-restricted diets varying in macronutrient composition within the Acceptable Macronutrient Distribution Range (AMDR) do not appear to elicit different changes in weight loss (8). However, for extreme macronutrient restriction below the AMDR, there is some evidence that macronutrient composition may influence modest changes in energy stores as well as measures of energy expenditure independently of caloric restriction (6, 9–11). This extreme restriction can lead to decreased consumption of entire food groups and compromise diet quality.
Dietary modifications including increased dietary fiber, decreased energy density, and higher protein intake have been explored as weight-loss facilitators to aid in achieving an energy deficit (12–14). The objective of this study was to evaluate the impact of changes in fiber intake and associated factors as predictors of weight loss for participants with obesity following a calorie-restricted diet for 6 mo. A secondary objective was to identify predictors of dietary adherence. We hypothesized that higher fiber intake would predict weight loss and dietary adherence.
Methods
Design
The POUNDS Lost (Preventing Overweight Using Novel Dietary Strategies) study was a 2-y randomized clinical trial which analyzed the effects of energy-restricted diets with 4 different macronutrient compositions on weight loss in adults who were overweight or obese. The details of the study have been reported previously (8). In short, 811 free-living adults with overweight or obesity were randomly assigned to 1 of 4 energy-restricted diets for 2 y. Major inclusion criteria included being 30–70 y of age and having a BMI between 25 and 40. Major exclusion criteria included diabetes mellitus, unstable cardiovascular disease, use of weight-altering medications, and amotivation for participation. The study was conducted at the Harvard School of Public Health and Brigham and Women's Hospital in Boston; and the Pennington Biomedical Research Center of the Louisiana State University system in Baton Rouge.
The compositions of the 4 diets were 1) 20% fat, 15% protein (low-fat, average-protein); 2) 20% fat, 25% protein (low-fat, high-protein); 3) 40% fat, 15% protein (high-fat, average-protein); and 4) 40% fat, 25% protein (high-fat, high-protein). All diets were designed to include ≥20 g of dietary fiber and <8% of energy from saturated fat. The diets were designed to meet the macronutrient goals and provide essential nutrients from a variety of foods and food groups. The fiber goals were met through the inclusion of grains, fruits, vegetables, nuts, and seeds. See Sacks et al. (8) and the accompanying supplementary appendix for more detail regarding the diets and their compositions. Each participant's diet reflected a 750-kcal/d reduction of energy expenditure as measured by hooded calorimetry at baseline.
Participants met with a study dietitian at screening, after random assignment to their respective diet group, and were scheduled to meet with a dietitian every 8 wk. Participants were instructed via individual counseling with a dietitian and group sessions to achieve their respective dietary goals. The weekly physical activity goal for each participant was 90 min moderate exercise. All study procedures were conducted in accordance with the Institutional Review Boards of the aforementioned institutions and all participants signed individual consent forms.
Measurements
Measurements were collected at baseline, 6 mo, and 24 mo, but only the first 2 sets were used in this substudy. Anthropometric measurements were collected using standardized protocols. Basal metabolic rate was measured using indirect calorimetry which was then multiplied by an activity factor reflective of each participant's reported activity level to estimate energy requirement. Dietary intake data were collected on a random sample of ∼50% of the subjects from a 5-d food record at baseline and from 24-h dietary recalls over 3 nonconsecutive days via phone interviews at the 6-mo follow-up.
Total fiber intake was estimated from the food records and dietary recalls. Energy density was calculated by dividing daily total energy intake by the total weight in grams of the foods and beverages consumed. All foods and beverages (excluding water) were included in the calculation. Previous research by Ledikwe et al. (15) suggests that this method for calculating energy density may have a lower ratio of intraindividual to interindividual variance than other calculation methods that exclude all or certain beverages. This lower CV ratio is useful for improving the power to detect associations between variables (16). Previous reports identified behavioral and dietary components of adherence (17); however, we were interested in adherence to all of the macronutrient goals. Therefore, dietary adherence was defined before analysis as consuming within ±5% of the macronutrient goals for fat and protein for each diet; and was monitored at baseline and 6-mo follow-up.
Statistics
Dietary recall information from baseline and 6-mo follow-up was completed by 345 individuals who were randomly selected. All variables were analyzed for distribution properties. Group differences were explored using independent-sample t tests and Cohen's d. Multiple comparisons were adjusted using the Bonferroni correction where applicable. A P value <0.01 was considered statistically significant.
The model selection process involved randomly partitioning the full data set into 2 subsets: a training data set to identify variables of interest to include in the model, and a testing data set to estimate the prediction capabilities of the model identified by the training data set (18). The data were randomly partitioned into training (70%) and testing (30%) data sets for model selection. Traditional linear regression models utilize ordinary least squares (OLS) which minimizes the residual sum of squares and provides unbiased nonzero estimators with the lowest possible variance. When many variables are included in the model, some of which share correlations with each other, the variance of the OLS parameter estimates increases. Moreover, traditional model selection procedures (e.g., forward, backward, and stepwise) can yield biased parameter estimates, spurious P values, inflated R2 values, and can worsen the problem of collinearity (19). More robust methods for parameter estimation should be employed when a large number of independent variables are available (20). The adaptive Least Absolute Shrinkage and Selection Operator (LASSO) (21, 22) approach for parameter estimation and model selection is useful for data sets containing a large number of variables that share some degree of correlation.
Predictors of weight loss were identified on the training data set using the adaptive LASSO and model Akaike's Information Criterion Corrected (AICC) as the selection criterion such that the shrinkage parameter (λ) in the model with the smallest AICC was chosen (18). Variables included in the model selection process were race; education; income; diet adherence; diet type; and change in energy, energy density, total fat, protein, carbohydrate, fiber, SFA, MUFA, and PUFA intakes from baseline to 6 mo. Multiplicative interaction effects between race and dietary variables were explored.
To minimize the error associated with a single random partition of the data, the randomization and modeling processes were repeated 10,000 times to derive parameter estimates retained in ≥20% of the generated models. To obtain a more parsimonious model, these predictors were then refit on an additional 10,000 random partitions of the data and the retention threshold was increased to 50%. The final predictors were then fit on the original testing data set using OLS to derive the parameter estimates, partial correlation coefficients, and R2.
Logistic regression modeling was used to explore the relation between dietary fiber intake and dietary adherence while controlling for age, gender, and race. Dietary fiber intake was treated both as a continuous variable (change in intake from baseline) and as a percentage of those who met the Adequate Intake (AI) for fiber at the 6-mo follow-up. Fiber intake was chosen as the independent variable because it was not a criterion for dietary adherence. The data analyses for this study were conducted using SAS version 9.4 (SAS Institute Inc.).
Results
Baseline demographics for the total sample (n = 345) are reported in Table 1. The majority of participants were female (53.9%) and white (87.3%). Of this sample, 105 (30.4%) adhered to their macronutrient prescriptions. Adherents reported significantly greater (P < 0.0001) mean fiber intake (25.2 g/d compared with 21.1 g/d; d = 0.722) and weight loss (−9.3 kg compared with −6.4 kg; d = 0.513) than nonadherents. Weight loss by diet type was as follows: −7.4 ± 5.3 kg for the low-fat, average-protein group; −7.5 ± 5.6 kg for the low-fat, high-protein group; −7.3 ± 6.5 kg for the high-fat, average-protein group; and −6.9 ± 5.1 kg for the high-fat, high-protein group. Weight change was not significantly different between diet groups (P = 0.9084).
TABLE 1.
Baseline demographic characteristics of adults with overweight or obesity who provided complete dietary recalls1
| Variable | Baseline estimate |
|---|---|
| Age, y | 52.5 ± 8.7 |
| BMI, kg/m2 | 32.6 ± 3.9 |
| Race | |
| White | 301 (87.3) |
| Nonwhite | 44 (12.8) |
| Gender | |
| Female | 186 (53.9) |
| Male | 159 (46.1) |
| Annual income | |
| < $50,000 | 77 (22.8) |
| $50,000–$100,000 | 142 (42.0) |
| >$100,000 | 119 (35.2) |
| Diet | |
| Low-fat, high-protein2 | 87 (25.2) |
| Low-fat, average-protein3 | 86 (24.9) |
| High-fat, average-protein4 | 89 (25.8) |
| High-fat, high-protein5 | 83 (24.1) |
Values are reported as mean ± SDs or as n (%), n = 345.
Low-fat, high-protein: 20% fat, 25% protein.
Low-fat, average-protein: 20% fat, 15% protein.
High-fat, average-protein: 40% fat, 15% protein.
High-fat, high-protein: 40% fat, 25% protein.
Participants’ weight and dietary intake variables at baseline and 6-mo follow-up for the full sample (n = 345) are shown in Table 2. As expected, there were large changes in all variables from baseline to the 6-mo follow-up. The target intake for fiber (20 g/d) was met by 48.7% of the full sample. Total fat and saturated fat changes from baseline exhibited the greatest change among all of the dietary variables. There were no significant differences in demographics or dietary variables between the initial, the randomly partitioned training, and the randomly partitioned testing data sets.
TABLE 2.
Dietary intake at baseline and 6-mo follow-up for adults with overweight or obesity who provided complete dietary recalls1
| Variable | Baseline | 6-mo follow-up | Change from baseline2 | Effect size3 |
|---|---|---|---|---|
| Weight, kg | 94.3 ± 16.0 | 87.0 ± 16.0 | −7.3 ± 5.6 | 0.454 |
| Energy, kcal/d | 2031 ± 561 | 1614 ± 510 | −417 ± 536 | 0.742 |
| Energy density, kcal/g food | 1.03 ± 0.3 | 0.82 ± 0.2 | −0.21 ± 0.3 | 0.840 |
| Protein, g/d | 89.4 ± 25.8 | 79.1 ± 26.7 | −10.3 ± 28.5 | 0.399 |
| Fat, g/d | 85.0 ± 28.7 | 55.0 ± 25.1 | −30.0 ± 30.4 | 1.046 |
| Carbohydrate, g/d | 225 ± 71.4 | 203 ± 74.7 | −21.6 ± 77.5 | 0.303 |
| Fiber, g/d | 17.5 ± 6.4 | 21.2 ± 8.5 | 3.7 ± 8.3 | 0.575 |
| SFAs, g/d | 27.7 ± 10.5 | 15.2 ± 7.9 | −12.5 ± 11.1 | 1.194 |
| MUFAs, g/d | 33.2 ± 11.7 | 21.8 ± 11.2 | −11.3 ± 12.8 | 0.967 |
| PUFAs, g/d | 17.5 ± 6.4 | 13.2 ± 6.5 | −4.3 ± 7.6 | 0.672 |
Values are means ± SDs, n = 345.
All changes from baseline were statistically significant (P < 0.01).
Effect size represents the standardized difference between means and was estimated using Cohen's d.
Fiber intake at baseline and at the 6-mo follow-up, and changes from baseline across each diet prescription are depicted in Table 3. Each diet group reported increases from baseline. The low-fat, average-protein diet group exhibited the greatest change from baseline (+7.0 ± 9.4 g/d), which was statistically different from the 2 high-fat diets.
TABLE 3.
Fiber intake at baseline and 6-mo follow-up and changes from baseline for each diet prescription, for adults with overweight or obesity who provided complete dietary recalls1
| Diet type | n | Baseline | 6-mo follow-up | Change from baseline2 | P value3 | Effect size4 |
|---|---|---|---|---|---|---|
| Low-fat, high-protein5 | 87 | 16.6 ± 5.8 | 20.1 ± 7.6 | 3.4 ± 7.9a,b | 0.0001 | 0.586 |
| Low-fat, average-protein6 | 86 | 17.7 ± 6.9 | 24.8 ± 9.4 | 7.0 ± 9.4a | <0.0001 | 1.014 |
| High-fat, average-protein7 | 89 | 16.8 ± 5.9 | 19.0 ± 8.2 | 2.1 ± 8.1b | 0.0141 | 0.356 |
| High-fat, high-protein8 | 83 | 18.6 ± 6.7 | 20.7 ± 7.2 | 2.1 ± 6.5b | 0.0036 | 0.3134 |
Values are means ± SDs, n = 345.
ANOVA identified a significant difference in change in fiber intake from baseline across diet groups (P < 0.001). Labeled means in a column without a common letter indicate pairwise differences in fiber change from baseline between diet groups after Bonferroni adjustment (P < 0.01).
The P values correspond to the difference in means from baseline to 6-mo follow-up.
Effect size represents the standardized difference between means from baseline to 6-mo follow-up and was estimated using Cohen's d.
Low-fat, high-protein: 20% fat, 25% protein.
Low-fat, average-protein: 20% fat, 15% protein.
High-fat, average-protein: 40% fat, 15% protein.
High-fat, high-protein: 40% fat, 25% protein.
The parameter estimates were derived from the initial training data set (full model). The LASSO procedure does not allow a hierarchy to be retained; therefore, main effects are not retained for some of their corresponding interaction effects. The final parameter estimates were then averaged over all simulated models. The main effects retained were fiber, fat, PUFAs, and race. Six interactions were also retained: race × adherence, race × age, race × baseline weight, race × carbohydrate, race × energy density, and race × MUFAs.
The variables identified by the adaptive LASSO were then fitted on the original testing data set. The main effects omitted by the LASSO were also included to allow for ease of interpretation. Table 4 displays the OLS solution for the final model including each regressor's respective partial R2 and 99% CIs. Of all the predictors, dietary fiber exerted the most influence on the model (partial R2 = 0.17) and was inversely associated with weight change (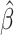 : −0.37; 99% CI: −0.60, −0.14) such that an average increase in dietary fiber of 3.7 g/d (group mean fiber intake change) was associated with a 1.4-kg greater weight loss over 6 mo. Exploration of interactive effects between parameters indicated homogeneity of the association of fiber with race.
: −0.37; 99% CI: −0.60, −0.14) such that an average increase in dietary fiber of 3.7 g/d (group mean fiber intake change) was associated with a 1.4-kg greater weight loss over 6 mo. Exploration of interactive effects between parameters indicated homogeneity of the association of fiber with race.
TABLE 4.
Final model including the regression coefficients identified by the LASSO and added main effects on the full sample of adults with overweight or obesity who provided complete dietary recalls1
| Variable | Estimate | SE | P > |t| | Partial R2 | 99% CI for parameter estimates | |
|---|---|---|---|---|---|---|
| Intercept | −1.37 | 16.1 | 0.93 | — | −43.8 | 41.1 |
| Adherence2 | 1.18 | 3.63 | 0.74 | 0.001 | −8.36 | 10.7 |
| Age, y | 0.24 | 0.18 | 0.19 | 0.020 | −0.24 | 0.73 |
| Baseline weight, kg | −0.14 | 0.15 | 0.36 | 0.010 | −0.55 | 0.27 |
| Carbohydrate,3 g/d | 0.03 | 0.03 | 0.45 | 0.007 | −0.06 | 0.11 |
| Energy density,3 kcal/g food | −9.6 | 10.8 | 0.38 | 0.009 | −37.9 | 18.8 |
| Fat,3 g/d | −0.01 | 0.08 | 0.88 | 0.000 | −0.21 | 0.19 |
| Fiber,3 g/d | −0.37 | 0.09 | < 0.0001 | 0.170 | −0.60 | −0.14 |
| PUFAs,3 g/d | 0.05 | 0.12 | 0.70 | 0.002 | −0.27 | 0.37 |
| MUFAs,3 g/d | 0.26 | 0.21 | 0.22 | 0.017 | −0.30 | 0.83 |
| Race4 | 13.0 | 16.9 | 0.44 | 0.007 | −31.4 | 57.3 |
| Race4 × adherence2 | −3.62 | 3.82 | 0.35 | 0.010 | −13.8 | 6.43 |
| Race4 × age | −0.44 | 0.19 | 0.03 | 0.056 | −0.95 | 0.07 |
| Race4 × baseline weight | 0.07 | 0.16 | 0.66 | 0.002 | −0.35 | 0.49 |
| Race4 × carbohydrate3 | −0.01 | 0.03 | 0.85 | 0.000 | −0.10 | 0.08 |
| Race4 × energy density3 | 13.0 | 10.9 | 0.24 | 0.016 | −15.8 | 41.8 |
| Race4 × MUFAs3 | −0.39 | 0.17 | 0.03 | 0.055 | −0.84 | 0.07 |
| R 2 | 0.410 | |||||
| Adjusted R2 | 0.301 | |||||
The parameter estimates shown were chosen from the model selection process and calculated using the ordinary least squares solution for the full data set (n = 345).
Adherence is a binary variable (1 = achieved macronutrient goals; 0 = otherwise).
Variables are represented as change in grams from baseline.
Race is a binary variable (1 = white; 0 = nonwhite).
Interactions between race and dietary adherence, age, baseline weight, carbohydrate, energy density, and MUFAs were retained in the final model. Although these interactions were not considered statistically significant when modeled on the testing data set, it is important to note they were retained throughout the model selection process. Among the interactions, race × age and race × MUFAs were the most influential (determined by partial correlations) in the training data set and suggest that age and MUFA intake are positively associated with weight loss for white but not nonwhite participants. When averaged values for the predictors were inserted into the final model (Table 4) and controlled for dietary adherence, the final model predicted a greater weight loss for white participants than for nonwhite participants (−8.9 kg compared with −3.5 kg, respectively).
Weight loss increased across each quartile of change in fiber intake from baseline (P < 0.0001). Figure 1 depicts the relation between weight loss and quartiles of fiber intake. Bonferroni pairwise comparisons indicated significant differences between quartile 4 and quartiles 1, 2, and 3 (P < 0.01). Results from the logistic regression models indicated that change in dietary fiber intake was strongly associated with dietary adherence (OR: 1.06; 99% CI: 1.02, 1.10; P = 0.0001). Further, those who met the AI for fiber were more likely to adhere to their prescribed diets (OR: 2.94; 99% CI: 1.56, 5.55; P < 0.0001). There were no significant interactions between fiber intake and race, diet type, or sex.
FIGURE 1.
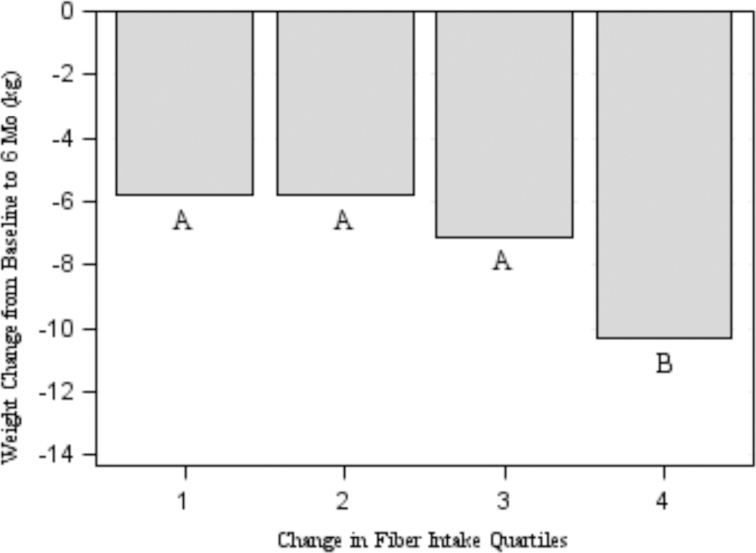
Change in body weight by quartiles of change in fiber intake from baseline to 6 mo in the full sample of adults with overweight/obesity who provided complete dietary recalls for both time points (n = 345). Values for change in fiber intake quartiles are as follows: quartile 1 (n = 86) is −25.01 to −1.78 g/d; quartile 2 (n = 86) is −1.69 to 2.34 g/d; quartile 3 (n = 87) is 2.41–8.33 g/d; and quartile 4 (n = 86) is 8.33–29.39 g/d. Mean ± SD body-weight changes from baseline to 6 mo were −5.8 ± 5.0, −5.8 ± 4.9, −7.1 ± 4.9, and −10.3 ± 6.3 kg for quartiles 1, 2, 3, and 4, respectively. Letters (A–B) indicate pairwise differences between quartiles using Bonferroni's correction for multiple comparisons (α = 0.01).
Discussion
The results of this study are consistent with the hypothesis that dietary fiber predicts weight loss and adherence to the dietary program. The change in fiber intake from baseline to 6 mo was consistently retained as a predictor of weight loss throughout the model selection process and was the strongest predictor of weight loss. The association between increased fiber intake and weight loss is clearly shown in Figure 1. The association of change in fiber intake with weight loss did not vary across macronutrient intake or over levels of dietary adherence or baseline demographics. Change in fiber intake was also strongly associated with adherence to macronutrient prescriptions.
The low-fat, average-protein diet group reported the greatest increase in fiber intake, likely associated with the carbohydrate prescription for the diet (65% energy from carbohydrates). Interestingly, weight change did not vary significantly by diet. This finding, along with the generated model, suggests fiber is important but not sufficient to induce weight loss.
Fiber has been widely studied for its role in promoting satiety and satiation (13, 23, 24). However, these effects appear to vary between different types of fibers; for example, viscous fibers were found to reduce appetite and energy intake more frequently than less viscous fibers (24). These results are echoed by recent studies that reported oatmeal, which is high in β-glucan, improves satiety and suppresses energy intake in subsequent meals (25, 26).
Fiber also appears to affect the metabolizable energy of mixed diets by blunting the digestibility of protein, total fat, and certain SFAs, thereby increasing fecal energy excretion (27–30). These reductions in metabolizable energy are modest but may be more pronounced when sustained over time and coupled with spontaneous reductions in food intake (29). Although fiber has been shown to inhibit absorption of fats in particular, the strength of the association between fiber intake and weight loss did not vary across levels of fat intake in this study. Moreover, it is unclear whether the increased dietary fiber intake promoted changes in the operational taxonomic units (OTUs) within the gut microbiome. These changes in the OTUs could affect energy harvest of the macronutrients, thus increasing fecal energy excretion (31).
Dietary energy density may also influence energy intake and consequently weight status in adults (32). The rationale goes that a diet high in energy content relative to the weight of food in grams is easily consumed in excess, thereby facilitating excessive energy intake and promoting weight gain. Studies that have explored the relation between energy density and weight status have been relatively consistent in their findings (14, 32, 33). Whereas previous studies controlled for race, this study observed a difference in response to changes in energy density by race. It is unclear whether certain racial groups genuinely respond differently to lower energy-dense diets or if baseline energy density was suppressed by certain foods or drinks commonly consumed in these groups.
Previous analysis of the POUNDS Lost trial identified demographic attributes related to weight loss at 6 mo (34). The authors reported that older adults and adults with a BMI >30 at baseline demonstrated greater weight loss at the 6-mo follow-up. They also found no significant differences in percentage weight loss between men and women and between racial groups. Further, subjects who met their fat or protein prescriptions within ±5% reported greater weight loss at 6 mo than those who failed to meet their respective fat or protein goals. Similar to these previous analyses, the present study retained dietary adherence to macronutrient prescriptions as a predictor of weight loss in the final model.
Although race and several interactions were retained after the model selection process, individually, these parameters appeared to have contributed relatively little to the overall model's performance. Although these parameters were not as profound as fiber intake, together, they explain a notable amount of variation in weight change that may be the result of significant disparities in microbiome, environment, and lifestyle between racial groups. The final model predicted a 5.4-kg difference in weight loss between whites and nonwhites when controlling for all other predictors. Previous studies have reported such disparities and emphasized the importance of exploring these differences (35–37). It should be noted, however, that the number of participants in the nonwhite group was relatively small and these results warrant further examination in future studies.
There are several strengths to this study. The relatively large sample size for a single weight-loss study allowed for robust model-selection procedures, which in turn increases our confidence in the accuracy, reliability, and generalizability of the findings. This study also had adequate representation from men and women: 46% and 54%, respectively. In addition, the study was conducted in 2 regions of the United States (New England and Louisiana) which have very different dietary preferences, thus making the findings robust and generalizable.
There were some limitations of this study. The sample is predominantly white and may not generalize well to other racial groups, although the data did allow us to detect racial differences in response. These analyses did not distinguish between the different types of dietary fiber consumed. Lastly, change in energy intake was not retained in the final model as a predictor of weight change. Although unexpected, this could be explained by underreporting of energy intake by participants providing 24-h recalls (38) and has been reported in previous analyses of these data (39). Previous research has also suggested that BMI is positively associated with energy underreporting in adults (38).
In summary, this study identified dietary fiber as a predictor of weight loss. In addition, several other dietary and demographic factors were also predictors of weight loss. Fiber intake was consistently retained throughout the model selection process and was the strongest predictor when fit on the training data set. Despite observed differences in some predictors across racial categories, fiber intake was associated with weight loss and dietary adherence across racial categories.
Acknowledgments
The authors’ responsibilities were as follows—DCM, GAB, CMC, and DHR: designed the research; DCM: analyzed the data and had primary responsibility for the final content; GAB, FMS, and CMC: designed and conducted the original clinical trial; RAB: provided statistical consultation and interpretation; and all authors: read and approved the final manuscript.
Notes
Supported by National Heart, Lung, and Blood Institute grant HL073286 (to FMS) and General Clinical Research Center, NIH grant RR-02635.
Author disclosures: DCM, RAB, FMS, and CMC, no conflicts of interest. GAB is a member of the Herbalife Nutrition Editorial Board and the Medifast Advisory Board. DHR reports financial relationships within the last year with Sanofi, Novo Nordisk, Orexigen, Eisai, Amgen, IFA Celtic, Real Appeal, Scientific Intake, Gila Therapeutics, and Epitomee.
Data described in the article, code book, and analytic code will not be made available because of medical data privacy laws. The protocol and supplementary material have been made publicly available. Any questions regarding the data should be directed to GAB at george.bray@pbrc.edu.
Abbreviations used: AI, Adequate Intake; AICC, Akaike's Information Criterion Corrected; AMDR, Acceptable Macronutrient Distribution Range; LASSO, Least Absolute Shrinkage and Selection Operator; OLS, ordinary least squares; OTU, operational taxonomic unit; POUNDS Lost, Preventing Overweight Using Novel Dietary Strategies.
Contributor Information
Derek C Miketinas, Department of Nutrition and Food Sciences, Texas Woman's University, Houston, TX, USA; Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA, USA.
George A Bray, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA, USA.
Robbie A Beyl, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA, USA.
Donna H Ryan, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA, USA.
Frank M Sacks, Harvard TH Chan School of Public Health, Boston, MA, USA.
Catherine M Champagne, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA, USA.
References
- 1. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319:1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin CB, Herrick KA, Sarafrazi N, Ogden CL. Attempts to lose weight among adults in the United States, 2013–2016. NCHS Data Brief. 2018;(313):1–8. [PubMed] [Google Scholar]
- 3. Nicklas JM, Huskey KW, Davis RB, Wee CC. Successful weight loss among obese US adults. Am J Prev Med. 2012;42:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–604. [DOI] [PubMed] [Google Scholar]
- 5. Blomain ES, Dirhan DA, Valentino MA, Kim GW, Waldman SA. Mechanisms of weight regain following weight loss. ISRN Obes. 2013:210524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall KD, Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology. 2017;152:1718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RFet al.. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. [DOI] [PubMed] [Google Scholar]
- 8. Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo Net al.. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergouignan A, Gozansky WS, Barry DW, Leitner W, MacLean PS, Hill JO, Draznin B, Melanson EL. Increasing dietary fat elicits similar changes in fat oxidation and markers of muscle oxidative capacity in lean and obese humans. PLoS One. 2012;7:e30164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall KD, Bemis T, Brychta R, Chen KY, Courville A, Crayner EJ, Goodwin S, Guo J, Howard L, Knuth NDet al.. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab. 2015;22:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BTet al.. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016;104:324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stelmach-Mardas M, Rodacki T, Dobrowolska-Iwanek J, Brzozowska A, Walkowiak J, Wojtanowska-Krosniak A, Zagrodzki P, Bechthold A, Mardas M, Boeing H. Link between food energy density and body weight changes in obese adults. Nutrients. 2016;8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poutanen KS, Dussort P, Erkner A, Fiszman S, Karnik K, Kristensen M, Marsaux CF, Miquel-Kergoat S, Pentikäinen SP, Putz Pet al.. A review of the characteristics of dietary fibers relevant to appetite and energy intake outcomes in human intervention trials. Am J Clin Nutr. 2017;106:747–54. [DOI] [PubMed] [Google Scholar]
- 14. Vernarelli JA, Mitchell DC, Rolls BJ, Hartman TJ. Dietary energy density and obesity: how consumption patterns differ by body weight status. Eur J Nutr. 2018;57:351–61. [DOI] [PubMed] [Google Scholar]
- 15. Ledikwe JH, Blanck HM, Khan LK, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Dietary energy density determined by eight calculation methods in a nationally representative United States population. J Nutr. 2005;135:273–8. [DOI] [PubMed] [Google Scholar]
- 16. Sempos CT, Johnson NE, Smith EL, Gilligan C. Effects of intraindividual and interindividual variation in repeated dietary records. Am J Epidemiol. 1985;121:120–30. [DOI] [PubMed] [Google Scholar]
- 17. Williamson DA, Anton SD, Han H, Champagne CM, Allen R, LeBlanc E, Ryan DH, McManus K, Laranjo N, Carey VJet al.. Adherence is a multi-dimensional construct in the POUNDS LOST trial. J Behav Med. 2010;33:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedman J, Hastie T, Tibshirani R. The elements of statistical learning. New York (NY): Springer; 2001. [Google Scholar]
- 19. Flom PL, Cassell DL. Stopping stepwise: why stepwise and similar selection methods are bad, and what you should use. In: NorthEast SAS Users Group Inc 20th Annual Conference, 11–14 November, 2007, Baltimore (MD). [Google Scholar]
- 20. Ivanescu AE, Li P, George B, Brown AW, Keith SW, Raju D, Allison DB. The importance of prediction model validation and assessment in obesity and nutrition research. Int J Obes (Lond). 2016;40:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tibshirani R. Regression shrinkage and selection via the LASSO. J R Stat Soc Series B Stat Methodol. 1996;58:267–88. [Google Scholar]
- 22. Zou H. The adaptive LASSO and its oracle properties. J Am Stat Assoc. 2006;101:1418–29. [Google Scholar]
- 23. Astrup A, Vrist E, Quaade F. Dietary fibre added to very low calorie diet reduces hunger and alleviates constipation. Int J Obes. 1990;14:105–12. [PubMed] [Google Scholar]
- 24. Wanders AJ, van den Borne JJ, de Graaf C, Hulshof T, Jonathan MC, Kristensen M, Mars M, Schols HA, Feskens EJ. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes Rev. 2011;12:724–39. [DOI] [PubMed] [Google Scholar]
- 25. Rebello CJ, Johnson WD, Martin CK, Xie W, O'Shea M, Kurilich A, Bordenave N, Andler S, Klinken BJ, Chu YFet al.. Acute effect of oatmeal on subjective measures of appetite and satiety compared to a ready-to-eat breakfast cereal: a randomized crossover trial. J Am Coll Nutr. 2013;32:272–9. [DOI] [PubMed] [Google Scholar]
- 26. Rebello CJ, Johnson WD, Martin CK, Han H, Chu YF, Bordenave N, van Klinken BJ, O'Shea M, Greenway FL. Instant oatmeal increases satiety and reduces energy intake compared to a ready-to-eat oat-based breakfast cereal: a randomized crossover trial. J Am Coll Nutr. 2016;35:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rigaud D, Ryttig KR, Leeds AR, Bard D, Apfelbaum M. Effects of a moderate dietary fibre supplement on hunger rating, energy input and faecal energy output in young, healthy volunteers. A randomized, double-blind, cross-over trial. Int J Obes. 1987;11:73–8. [PubMed] [Google Scholar]
- 28. Baer DJ, Rumpler WV, Miles CW, Fahey GC Jr. Dietary fiber decreases the metabolizable energy content and nutrient digestibility of mixed diets fed to humans. J Nutr. 1997;127:579–86. [DOI] [PubMed] [Google Scholar]
- 29. Zou ML, Moughan PJ, Awati A, Livesey G. Accuracy of the Atwater factors and related food energy conversion factors with low-fat, high-fiber diets when energy intake is reduced spontaneously. Am J Clin Nutr. 2007;86:1649–56. [DOI] [PubMed] [Google Scholar]
- 30. Karl JP, Meydani M, Barnett JB, Vanegas SM, Goldin B, Kane A, Rasmussen H, Saltzman E, Vangay P, Knights Det al.. Substituting whole grains for refined grains in a 6-wk randomized trial favorably affects energy-balance metrics in healthy men and postmenopausal women. Am J Clin Nutr. 2017;105:589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ledikwe JH, Blanck HM, Kettel Khan L, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr. 2006;83:1362–8. [DOI] [PubMed] [Google Scholar]
- 33. Vernarelli JA, Mitchell DC, Rolls BJ, Hartman TJ. Dietary energy density is associated with obesity and other biomarkers of chronic disease in US adults. Eur J Clin Nutr. 2015;54:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bray GA, Ryan DH, Johnson W, Champagne CM, Johnson CM, Rood J, Williamson DA, Sacks FM. Markers of dietary protein intake are associated with successful weight loss in the POUNDS Lost trial. Clin Obes. 2017;7:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Findley K, Williams DR, Grice EA, Bonham VL. Health disparities and the microbiome. Trends Microbiol. 2016;24:847–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mai V, McCrary QM, Sinha R, Glei M. Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutrition. 2009;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Freedman LS, Commins JM, Moler JE, Arab L, Baer DJ, Kipnis V, Midthune D, Moshfegh AJ, Neuhouser ML, Prentice RLet al.. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol. 2014;180:172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vadiveloo M, Sacks FM, Champagne CM, Bray GA, Mattei J. Greater healthful dietary variety is associated with greater 2-year changes in weight and adiposity in the Preventing Overweight Using Novel Dietary Strategies (POUNDS Lost) trial. J Nutr. 2016;146:1552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


