Abstract
BRAF mutations have been identified as targetable, oncogenic mutations in many cancers. Given the paucity of treatments for primary brain tumors and the poor prognosis associated with high-grade gliomas, BRAF mutations in glioma are of considerable interest. In this review, we present the spectrum of BRAF mutations and fusion alterations present in each class of primary brain tumor based on publicly available databases and publications. We also summarize clinical experience with RAF and MEK inhibitors in patients with primary brain tumors and describe ongoing clinical trials of RAF inhibitors in glioma. Sensitivity to RAF and MEK inhibitors varies among BRAF mutations and between tumor types as only class I BRAF V600 mutations are sensitive to clinically available RAF inhibitors. While class II and III BRAF mutations are found in primary brain tumors, further research is necessary to determine their sensitivity to third-generation RAF inhibitors and/or MEK inhibitors. We recommend that the neuro-oncologist consider using these drugs primarily in the setting of a clinical trial for patients with BRAF-altered glioma in order to advance our knowledge of their efficacy in this patient population.
Keywords: BRAF, BRAF V600E, MEK, glioma, glioblastoma, astrocytoma, dabrafenib, trametinib, vemurafenib, encorafenib
1. Introduction
The RAF serine/threonine protein kinases have been studied extensively over the past 17 years since their initial discovery as oncogenes. BRAF (v-raf murine viral oncogene homolog B1), in particular, has been implicated as an oncogene in many different cancers [1]. There are three RAF family members (RAF1, also known as CRAF, BRAF, and ARAF). Under physiologic conditions, these are activated when they are bound by active RAS, thereby displacing the RAF autoinhibitory domain from the active site [2]. RAF proteins then homo- or heterodimerize and phosphorylate downstream targets MEK1 and MEK2 (encoded by the genes MAP2K1 and MAP2K2, respectively). Activated MEK kinases in turn phosphorylate and activate ERK1 and ERK2, which translocate to the nucleus and induce transcriptional pathways, leading to cellular proliferation, survival, and dedifferentiation [3].
BRAF functions as an oncogene when its kinase domain remains in the active, open configuration, either due to a point mutation, in-frame deletion, or fusion with another gene that removes the regulatory domain or prevents it from blocking the active site [4]. Of note, in cells with the BRAF V600E mutation, the nonmutated BRAF gene still produces wild-type protein that is free to dimerize and activate downstream ERK signaling accordingly.
The BRAF V600E mutation is present in approximately 60% of melanoma, 40% of non-small cell lung cancer (NSLCL), and 12% of colorectal cancer [1]. RAF inhibitors demonstrated notable early successes as monotherapy in patients with advanced or metastatic melanoma [5,6]. Resistance to RAF inhibitor monotherapy unexpectedly emerges in most patients, however, after an average of 5–6 months. Using a MEK inhibitor in patients following the emergence of resistance to a RAF inhibitor leads to a marginal improvement in survival (1.8 months) [7]. Up-front combination of RAF and MEK inhibitors, however, may delay or prevent the common mechanisms of treatment-emergent resistance that occur with monotherapy and improves progression-free and overall survival in patients with advanced melanoma [8,9,10]. Currently, there are three combinations of RAF and MEK inhibitors approved by the United States Food and Drug Administration (U.S. FDA) for patients with BRAF V600E/K mutations in advanced or metastatic melanoma, NSCLC, or anaplastic thyroid cancer: vemurafenib/cobimetinib (Genentech), dabrafenib/trametinib (Novartis), and encorafenib/binimetinib (Array BioPharma).
The remarkable responses seen in patients with BRAF-mutated cancers have attracted attention in the field of neuro-oncology. Given the dismal prognosis of patients with glioblastoma and the lack of therapeutic options for many primary central nervous system tumors, some neuro-oncologists are using off-label RAF and MEK combination treatment for patients with recurrent high-grade glioma [11,12,13,14]. This has led to anecdotal reports of positive responses, as well as positive clinical trial data, though most neuro-oncologists still have little experience with these drugs [15]. Moreover, next-generation sequencing (NGS) of tumor specimens has enabled identification of other mutations in the BRAF gene in primary brain tumors, but many of these do not respond to FDA-approved RAF inhibitors and may in fact progress more rapidly. Here, we summarize the mutations in BRAF described to date in glioma and provide an overview of their functional implications for tumor biology and treatment with targeted drugs.
2. BRAF Mutation Classes
Over 100 unique mutations in the BRAF gene have been identified in cancer [16]. Through extensive work in melanoma, it is clear that these mutations lead to ERK activation via different functional mechanisms. These mutations have been grouped into three classes based on their dependence on dimerization and on activation by RAS for activity; these properties determine their sensitivity to RAF inhibitors [17]. While V600E is the most common mutation in BRAF, other mutations are being identified in glioma due to increasing use of clinical NGS. Many of these mutations are within the kinase domain, while others are located across the gene and have less well-understood functional consequences.
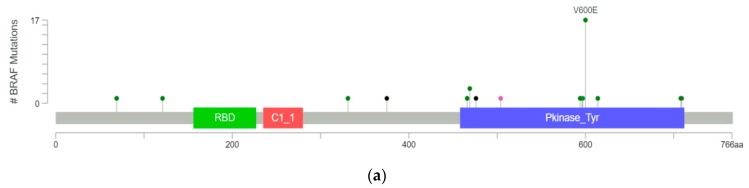
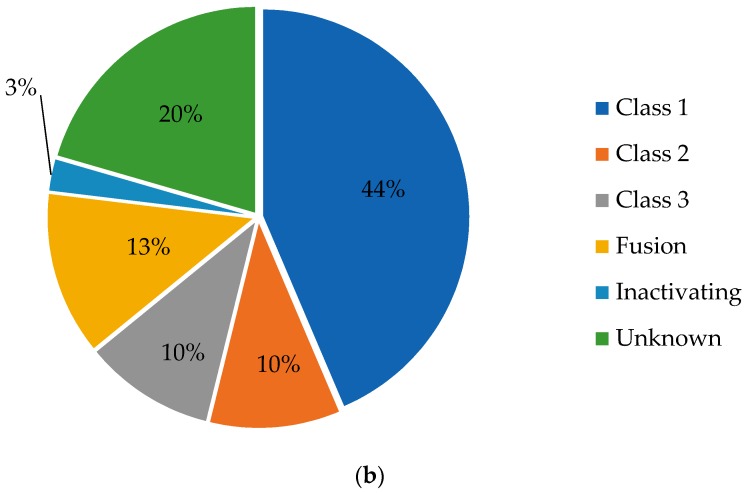
We investigated the complete range of BRAF mutations described to date in publicly available databases. Using cBioPortal.org, multiple patient cohorts were queried for glioma with mutations in BRAF (MSK-IMPACT Clinical Sequencing Cohort and TCGA) [16,18]. Thirty-nine gliomas with BRAF mutations were identified in cBioPortal, of which 17 (44%) had V600E mutations, eight had other known activating mutations (20%), and five had fusions conferring kinase activity (13%). One specimen had a mutation known to inactivate the BRAF kinase function (3%), but the functional significance of the remaining eight mutations are unknown or likely to be insignificant (Figure 1).
Figure 1.
BRAF mutations found in 39 gliomas as identified from MSK-IMPACT and TCGA databases in cBioPortal displayed as a (a) lollipop plot identifying unique mutations (excluding fusions) and (b) a pie chart showing mutation types divided by class.
2.1. Class I Mutations
Similar to other cancers, the most common mutation in the BRAF gene in glioma is the c.1799T>A mutation [1]. This leads to a substitution from valine to glutamic acid at position 600 (V600E). The mutation releases the autoinhibitory domain from the active site through a conformational change, permitting BRAF monomers to adopt their open, active configuration. This allows BRAF V600-mutated monomers to activate downstream MEK1/2 independent of dimerization [2,19]. Valine substitutions to several other amino acids (R, K, D) have been described, though these have not been reported in glioma. V600E mutations strongly activate downstream ERK signaling, leading to suppression of upstream RAS activity through negative feedback from active ERK, resulting in low basal RAS activity in these cells [20]. V600 substitutions are known class I mutations as they are independent of both upstream RAS activation and the need for dimerization [21].
BRAF V600E mutations have been described in a variety of adult and pediatric gliomas, including pleomorphic xanthoastrocytoma (PXA; 60–80%), ganglioglioma (20–70%), pilocytic astrocytoma (PA; 9–10%), low-grade glioma (LGG; 5–15%), pediatric glioblastoma (pGBM; 20%), and adult GBM (3%) [22,23,24]. While the mutation is less common in adult GBM, it is relatively enriched for in the epithelioid subtype of GBM and possibly in low-grade astrocytoma as well [24,25,26]. In patients with papillary craniopharyngioma, 95% of tumors have BRAF V600E mutations [27]. BRAF mutations are exceedingly rare in ependymomas [28]. Despite a relatively low incidence in adults, the potential for targeted therapy makes BRAF V600 mutations in recurrent gliomas significant as prognosis is poor and treatment options are very limited.
2.2. Class II Mutations
A subset of non-V600E mutations in BRAF activate MEK though dimerization but without a requirement for activation by RAS [17]. These class II mutations undergo constitutive, RAS-independent dimerization, leading to increased ERK activation with low RAS activity due to negative feedback [17]. Common class II point mutations, such as K601E/N/T, L597Q/V, and G469A/V/R, have all been identified in glioma, but their relative frequency is unknown. Additionally, BRAF in-frame deletions can function as class II mutations. In-frame deletions removing part of the β3-αC loop have been identified in glioma and other tumors [29,30], leading to a shortened αC helix that is constrained into the active confirmation, preventing autoinhibition and resulting in increased kinase activity [31]. These are also dependent on dimerization for activity in a RAS-independent fashion.
BRAF fusions also function as class II mutations. The most common fusion is KIAA1549–BRAF, which was first identified in low-grade glioma and is composed of the N-terminal dimerization domain of KIAA and the C-terminal kinase domain of BRAF [32]. In this fusion (and the vast majority of BRAF fusions described to date), the BRAF regulatory domain is lost, leading to increased affinity for dimerization and allowing BRAF kinase activity independent of upstream regulation [33]. Fusion mutations are very common in low-grade glioma, where they are found in the majority of pilocytic astrocytoma [32,34,35]. It has been postulated that the cell of origin in pilocytic astrocytoma may have inherent properties that allow BRAF fusions to drive oncogenesis, but the precise mechanism underlying this finding is not known [36]. KIAA1549–BRAF fusions have also been reported in rare patients with GBM, PXA, and ependymoma [37]. In addition to KIAA1549, many other proteins have been reported as fusion partners to BRAF in glioma, all consisting of an N-terminal dimerization domain from another protein and the C-terminal kinase domain of BRAF, thereby removing the regulatory domain of BRAF without impairing its ability to dimerize (Table 1).
Table 1.
List of class I, II, and III mutations identified in BRAF-mutated brain tumors from cBioPortal and other published literature.
| Class I | Class II | Class III |
|---|---|---|
| V600E | G469A | G466E |
| G469R | D594G | |
| L597R | G596D | |
| T599_W604ins [38] | ||
| T599dup [39] | ||
| KIAA1549–BRAF fusion | ||
| BCAS1–BRAF fusion [40] | ||
| CCDC6–BRAF fusion | ||
| CDC42BPB–BRAF fusion [38] | ||
| ERC2–RAF1 fusion [38] | ||
| FAM131B–BRAF fusion [41] | ||
| FXR1–BRAF fusion [42] | ||
| GIT2–BRAF [43] | ||
| KLHL7–BRAF fusion [38] | ||
| RNF130–BRAF fusion [41] | ||
| TEMEM106B–BRAF fusion [40,44] |
2.3. Class III Mutations
Class III mutations function very differently from class I or II mutations. These mutations have impaired, or sometimes absent (i.e., D594G), kinase activity [45,46]. Class III mutations are dependent on RAS and upstream input from receptor tyrosine kinases (RTKs), for their activity [47,48]. Class III mutants bind more tightly to activated RAS than does wild-type BRAF, leading to increased activation of the wild-type binding partner (BRAF, ARAF, or CRAF) upon dimerization [45]. These amplifiers of ERK signaling often occur in conjunction with other mutations that increase upstream RAS activity, such as RAS mutations, NF1 loss, or RTK mutations or amplification [21]. Several class III mutations with impaired kinase function, such as G466E/A/V or G596D/R, have been identified in glioma, as has the kinase dead mutation D594G (Table 1). Of the four class III mutations seen in the cBioPortal dataset, two have co-occurring homozygous deletion of NF1 and one has amplification of EGFR (ERBB1).
3. RAF and MEK Inhibitors
3.1. Type I RAF Inhibitors
Type I RAF inhibitors are ATP-competitive small molecules that selectively bind to and inhibit all RAF monomers, not only BRAF V600E [49]. These drugs are quite effective at inhibiting ERK in cells where ERK signaling is driven by BRAF V600E, and there are three FDA-approved inhibitors in this class for some systemic cancers: vemurafenib, dabrafenib, and encorafenib [50,51,52]. A preclinical RAF inhibitor and sister compound to vemurafenib, PLX4720, successfully inhibits MEK and ERK phosphorylation, as well as downstream AKT phosphorylation, in BRAF V600E-mutated astrocytoma cell lines but not in wild-type tumor cell lines [53]. It also prolongs survival of mice with xenografts from BRAF-mutant brain tumors but not wild-type tumors [53].
Type I RAF inhibitors lead to increased ERK signaling in cells with wild-type RAF and non-V600E mutations by facilitating the formation of RAF dimers, particularly BRAF–CRAF heterodimers [49,54,55]. When bound to RAF, these drugs also activate the catalytic domain of the RAF binding partner [49]. This leads to increased downstream signaling (so-called paradoxical activation) and may even accelerate tumor growth in patients whose tumors are not driven by class I mutations [56]. For this reason, type I RAF inhibitors should only be used in tumors with BRAF V600E mutations (Table 2). Even in this selected group, most tumors develop resistance to RAF inhibitor monotherapy, which has prompted combination therapy with MEK inhibitors (see Section 3.4).
Table 2.
RAF, MEK, and ERK inhibitors in clinical use and their current status during development.
| RAF Inhibitors | |||
| Generation | Drug Name | Manufacturer | FDA Phase |
| 1st | Sorafenib (Nexavar) | Bayer/Onyx Pharmaceuticals | Approved for hepatocellular and renal cell carcinoma |
| 2nd | Vemurafenib (Zelboraf) | Genentech | Approved for BRAF V600E advanced melanoma and Erdheim–Chester Disease |
| 2nd | Dabrafenib (Tafinlar) | Novartis | Approved for BRAF V600E/K melanoma or metastatic non-small cell lung cancer |
| 2nd | Encorafenib (Braftovi) | Array BioPharma | Approved for BRAF V600E/K advanced melanoma |
| 3rd | TAK-580 | Millennium Pharmaceuticals | Phase I/II ongoing |
| 3rd | PLX8394 | Plexxikon | Phase I/IIa ongoing |
| 3rd | BGB283 | BeiGene | Phase 1 ongoing |
| 3rd | LY3009120 | Eli Lilly | Phase I terminated |
| 3rd | BAL3833 (CCT3833) | Basilea | Phase 1 completed |
| MEK Inhibitors | |||
| Drug Name | Manufacturer | FDA Phase | |
| Cobimetinib (Cotellic) | Genentech | Approved for BRAF V600E advanced melanoma | |
| Trametinib (Mekinist) | Novartis | Approved for BRAF V600E/K melanoma or metastatic non-small cell lung cancer | |
| Binimetinib (Mektovi) | Array BioPharma | Approved for BRAF V600E/K advanced melanoma | |
| Selumetinib | AstraZeneca | Breakthrough Therapy Designation; Phase II trials ongoing | |
| RO5126766 | Chugai Pharmaceutical | Phase I ongoing | |
| HL-085 | Shanghai Kechow Pharma | Phase I ongoing | |
| ERK Inhibitors | |||
| Drug Name | Manufacturer | FDA Phase | |
| Ulixertinib | Merck | Phase I/IIa completed | |
| LY3214996 | Eli Lilly & Company | Phase I ongoing | |
| LTT462 | Novartis | Phase Ib ongoing | |
3.2. Paradox Breakers
A third generation of RAF inhibitors, known as “paradox breakers” inhibit BRAF without promoting dimerization, thereby preventing paradoxical upregulation of ERK signaling [57,58,59]. Paradox breakers have the potential to be effective against V600E mutations (class I), splice variants, and upstream RAS mutations and are currently under development [60]. One paradox breaker, PLX8394, is effective against class I and II BRAF mutations and also disrupts dimer formation as a means to inhibit ERK signaling [61]. It is currently under clinical development in phase I clinical trials (NCT02428712), as are other paradox breakers (Table 2) [62]. Another compound inhibits SRC kinases in addition to RAF, thereby preventing paradoxical ERK signaling upregulation [63].
3.3. Dimer Disrupters
Another category of RAF inhibitors, known as “dimer disrupters”, function by disrupting RAF homo- or heterodimerization, thereby preventing activation of downstream MEK. Dimer disrupters are potentially powerful inhibitors as they can interfere with signaling through wild-type RAF as well as mutant RAF. These are effective against class II mutations, both dimer-dependent, activating point mutations as well as fusions in preclinical testing [17,64]. Two—TAK-580 and BGB-283—are currently being evaluated in early-phase clinical trials (NCT03429803; NCT02610361) [57,61].
3.4. MEK Inhibitors
Reactivation of ERK signaling is a common mechanism of resistance to RAF inhibitors. To mitigate this, RAF inhibitors have been combined with MEK inhibitors in patients with BRAF V600E-mutated tumors. MEK inhibitors function as allosteric inhibitors, preventing the conformational change of MEK into its active form upon phosphorylation by RAF. This then prevents MEK from phosphorylating downstream ERK. MEK inhibitor monotherapy leads to nondurable responses in patients with BRAF V600E-mutated melanoma [65]. Treating patients whose BRAF-mutant tumors are already resistant to RAF inhibitors is also ineffective [7]. Combination RAF/MEK therapy, however, delays the onset of resistance by inhibiting multiple targets in the same pathway simultaneously, preventing rebound reactivation and producing deeper inhibition of ERK signaling, and is FDA-approved for several cancer types (Table 2) [8,9,10].
MEK inhibitors can also be effective against other mutations that cause hyperactive ERK signaling. By virtue of the fact that MEK is downstream of RAF, MEK inhibitors have potential efficacy against tumors with RAS mutations, type I or II BRAF mutations, RTK amplification or mutations, NF1 loss, and EGFR overexpression. MEK inhibitor monotherapy, while insufficient at preventing growth in most tumors, shows promising results in low-grade pediatric glioma with the KIAA1549–BRAF fusion or NF1 loss-of-function [66,67].
3.5. ERK Inhibitors
Another potential mechanism to prevent emergent resistance to RAF inhibitors is to target ERK itself. ERK inhibitors have the potential to inhibit ERK signaling driven by class I, II, and III mutations, as well as other genomic events, by virtue of their direct inhibition of this central node in signaling. This strategy avoids the potential for paradoxical activation and may decrease the possibility of emergent resistance when used in combination with a RAF inhibitor. The risk of ERK inhibitors is that they may have a lower therapeutic index and increased toxicity. ERK1/2 inhibitors are currently being evaluated in humans with some promising early results against tumors with class II activating mutations (Table 2) [68].
4. BRAF Mutations in Brain Tumor Subtypes and Sensitivity to Targeted Therapy
4.1. Pilocytic Astrocytoma
MAPK/ERK pathway mutations have been identified in approximately 95% of pilocytic astrocytoma (PA) [42]. The most common mutation in PA is the KIAA1549–BRAF fusion, which occurs in approximately 60–70% of PA [42,69]. Fusions between BRAF and other proteins have been identified less frequently in PA (Table 1). KIAA1549–BRAF fusions in PA are associated with improved prognosis compared to PA without fusions, which may be due to the greater propensity of these tumors to senesce [70,71]. Given the high frequency of fusions in PA, type II RAF inhibitors, specifically the dimer disrupter TAK-580, are being tested in this population (Table 3).
Table 3.
Summary of primary brain tumor types and clinical data supporting RAF-targeted therapy in each tumor type.
| Inhibitor | |||||||
|---|---|---|---|---|---|---|---|
| Brain Tumor Type | Mutation | Incidence | Type I RAF | Type II RAF | RAF Dimer | MEK | RAF + MEK |
| Pilocytic astrocytoma | KIAA1549–BRAF | 60–70% [42,69] | Not active [33] | TAK-580 (NCT03429803) | Selumetinib [66] Binimetinib [67] (NCT02285439) | ||
| V600E | 10% [22,42,72] | Dabrafenib/Trametinib case series [73] | |||||
| Pediatric low-grade astrocytoma | V600E | 20–35% [72,74] | Dabrafenib [75] Vemurafenib (NCT01748149, NCT03220035) | PLX8394 (NCT02428712) | TAK-580 (NCT03429803) | Trametinib [76] (NCT02124772) | Dabrafenib/Trametinib (NCT02684058; NCT02124772) |
| KIAA1549–BRAF | Preclinical activity [33] | ||||||
| Pediatric high-grade astrocytoma | V600E | 10–20% [23,77] | Vemurafenib (NCT01748149, NCT03220035) | Dabrafenib/Trametinib (NCT02684058) | |||
| Adult low-grade astrocytoma | V600E | 5–15% [24] | [15] | Dabrafenib/Trametinib (NCT02034110) | |||
| Adult high-grade astrocytoma | V600E | 3% [22,24,78] | Vemurafenib [15] | Dabrafenib/Trametinib [79] (NCT02034110) Encorafenib/Binimetinib (NCT03973918) |
|||
| Pleomorphic xanthoastrocytoma | V600E | 70% [22] | Vemurafenib [15] | Dabrafenib/Trametinib [79] (NCT02034110) Encorafenib/Binimetinib (NCT03973918) |
|||
| Ganglioglioma | V600E | 50% [72,80] | Vemurafenib case reports [15,81,82,83,84,85,86,87] Dabrafenib case [81] |
Case reports [82,88,89,90] | |||
| Papillary craniopharyngioma | V600E | 95% [27] | Dabrafenib/Trametinib (NCT03224767) | ||||
The class I BRAF V600E mutation has been identified in approximately 10% of PA and is mutually exclusive with BRAF fusions [22,42,72]. BRAF V600E-mutated PA appears to be associated with an inferior prognosis compared to PA with wild-type BRAF, which may be due to the fact that class I mutations are such strong drivers of ERK signaling and correlate with the relative absence of BRAF fusions in high-grade glioma [72]. A small case series has shown that combined RAF and MEK inhibitor therapy can effect profound responses in patients with BRAF V600E-mutated PA [73].
Targeted therapy with MEK inhibitors alone can also be effective in pilocytic astrocytoma, in contrast to melanoma. A phase II study of selumetinib in pediatric patients with pilocytic astrocytoma containing either KIAA1549–BRAF or BRAF V600E showed a sustained response rate of 36% (9/25). Another 11 patients (44%) had prolonged stable disease for a median of 36.4 months [66]. Interestingly, both types of BRAF mutation were responsive to selumetinib, though the response rate may be higher in tumors with BRAF fusions rather than those with BRAF V600E mutations. A clinical trial evaluating safety and efficacy of the MEK1/2 inhibitor, binimetinib, is underway in children with glioma and other tumors containing KIAA1549–BRAF fusions or other activating mutations with some early signals of efficacy (Table 3) [67].
4.2. Pediatric Astrocytoma
While pediatric low-grade gliomas (pLGG) have relatively few mutations overall, 82% have a mutation in the ERK signaling pathway. BRAF V600E mutations are found in 20–35% of non-PXA, non-ganglioglioma pLGG and confer a worse prognosis and relative insensitivity to chemotherapy [72,74]. Ten-year survival in one retrospective study was 27% for BRAF V600E-mutated pLGG compared to 60% for wild type [72]. Pediatric oligodendroglioma, which occur only rarely, have also been identified to harbor KIAA1549–BRAF fusions, suggesting they are different from their adult counterparts [91].
Pediatric glioblastoma have BRAF mutations in approximately 10–20% of cases [23,77]. In contrast to low-grade glioma, BRAF V600E in pGBM appears to confer a more indolent clinical course compared to pGBM with wild-type BRAF [77]. One study investigating the genetics of secondary GBM found 39% of secondary GBM contained BRAF V600E mutations, while none contained BRAF fusions or IDH1 mutations, suggesting BRAF V600E either permits or promotes malignant transformation [92]. That study also found a better overall survival in secondary GBM with BRAF V600E mutation than without [92]. This may suggest a “cap” to BRAF V600E’s oncogenicity, making it a driver mutation that is relatively aggressive compared to other LGG but relatively mild compared to other driver mutations in pGBM.
The response of pediatric glioma to targeted therapy appears to be quite good. A retrospective institutional study found 6/6 progressive pLGG responded to targeted therapy, with no tumors progressing while on treatment (median follow-up 18.5 months) [72]. Another institutional experience found 1/7 pLGG did not respond to treatment with vemurafenib [93]. The interim analysis of a clinical trial administering dabrafenib to children with BRAF V600-mutated relapsed or refractory LGG found a RR of 41%, with another 41% of patients maintaining stable disease for six months or longer [75]. Studies in children evaluating the effect of RAF inhibitors alone or in combination with MEK inhibitors are currently ongoing (Table 3). Given the large proportion of pLGG with ERK pathway activation but without class I mutations, ongoing studies of novel RAF inhibitors in glioma are of great interest (Table 3). MEK inhibitor monotherapy may also be effective in patients with LGG, as hinted at in a phase I study [76].
4.3. Adult Astrocytoma
BRAF V600E mutations are relatively common in young adults with the types of tumors commonly seen in pediatric patients, such as PXA and LGG. BRAF V600E-mutated LGG in young adults are associated with a better clinical course than BRAF wild-type LGG [94]. The size and number of cases in the meta-analysis describing this trend was small and IDH status was not accounted for, but it may indicate a ceiling for the level of oncogenicity BRAF has as a driver mutation, similar to the trend seen in pGBM.
Glioblastoma in adults have BRAF V600E mutations only infrequently (~3%) [22,24,78]. The effect of BRAF V600E mutations on the clinical course of GBM in adults is unclear and has not been sufficiently studied to date, primarily given the rarity of these tumors. BRAF mutations in GBM are more common in young adults (<45 years) than older adults [95]. In young adults (aged 18–35), BRAF V600E mutations are associated with improved overall survival compared with wild type [96]. In adults aged 35–70 years, a small series showed improved progression-free survival compared to case-matched controls, suggesting a trend similar to that seen in young adults [97].
The sensitivity of adult glioma to RAF inhibitors is not as well-defined as in pediatrics, but preliminary evidence suggests it may differ from that in pediatric patients. In a basket study of vemurafenib in adults with BRAF V600E-mutated gliomas, the response rate of adults with PXA was high (43%; 3/7) and similar to pediatric patients, but the response rate in GBM and anaplastic astrocytoma was much lower at 9% (1/11) [15]. In a study of combined dabrafenib/trametinib in adults with high-grade glioma, interim analysis shows a response rate of 22% in grade III and 29% in grade IV glioma [79]. A trial investigating the efficacy of a different RAF/MEK inhibitor pair (encorafenib/binimetinib) is currently underway (Table 3). All studies to date have been performed in recurrent glioma, and given the relatively low efficacy of RAF-targeted therapy in this population, it should not take the place of first-line standard of care therapy except when given in the context of a clinical trial. In both glioma studies, as well as in individual case reports, there are examples of gliomas that initially responded to combination therapy but then progressed [13,14,98]. It is unclear whether resistance occurs via emergent mechanisms, intratumoral heterogeneity, or incomplete drug penetration of the blood–brain barrier.
4.4. PXA
Pleomorphic xanthoastrocytoma (PXA) are rare but have a high rate of BRAF V600E mutations (~70%) and a low rate of fusions [22]. BRAF V600E mutations are associated with improved overall and progression-free survival in both grade II and grade III PXA [99,100]. There are many reports documenting cases of PXA responding positively to targeted therapy: three with a complete response, eight with a partial response, two with stable disease, and one with progressive disease [11,12,13,14,98,101,102,103,104]. A basket trial including seven patients with PXAs showed that 42% responded to vemurafenib and another 42% had stable disease for more than six months (Table 3) [15]. While the reports to date are primarily anecdotal and suffer from reporting bias, they suggest PXA are quite sensitive to targeted therapy with RAF and MEK inhibitors.
4.5. Ganglioglioma
BRAF V600E mutations are common in ganglioglioma, occurring in approximately 50% [72,80]. Patients with ganglioglioma with BRAF V600E mutations appear to have worse progression-free survival compared with nonmutated GG in children and young adults [105]. In pediatric patients with grade I gangliogliomas, several cases have been identified with both H3 K27M and BRAF V600E mutations [80]. Some of these patients were observed to have a relatively indolent disease course despite the H3 K27M mutation (40%), suggesting these tumors may not behave as aggressively as diffuse midline glioma, K3 K27M mutant [106].
Positive responses to targeted therapy with RAF inhibitor monotherapy or combination therapy have been observed in adults with anaplastic ganglioglioma (Table 3). Children with anaplastic ganglioglioma refractory to other treatments have also experienced sustained responses to BRAF-targeted therapy [84,85,86,89,90]. In one case, the tumor responded for three months, then treatment was discontinued for medical reasons. The tumor grew back rapidly and vemurafenib was reinstituted, and the tumor responded again [87]. These findings suggest RAF inhibitors may be effective for high-grade ganglioglioma, though a larger, randomized study is unlikely to ever be completed given the rarity of this tumor type.
4.6. Craniopharyngioma
Following the discovery that BRAF V600E mutations are quite common in papillary craniopharyngiomas, Brastianos et al. described the case of a patient with a remarkable response to combination therapy with dabrafenib and trametinib [27,107]. Subsequently, there have been several case reports of responses to vemurafenib [108] or dabrafenib monotherapy [109] or to combination therapy [110,111]. Progression after initial response to monotherapy with RAF inhibitors has been observed [108]. There is currently a phase II clinical trial evaluating efficacy of dabrafenib plus trametinib in patients with papillary craniopharyngioma (Table 3). This is an intriguing study given the paucity of chemotherapies available for patients with craniopharyngioma.
5. Resistance to RAF-Targeted Therapy in Brain Tumors
Despite the success of RAF/MEK inhibitor combination therapy in pediatric glioma, some tumors are nonresponders or develop resistance over time to targeted therapy [66]. There are emerging reports of nonresponse and acquired resistance to RAF inhibition in adult glioma [13,14,15,98].
Mechanisms of resistance to RAF inhibitor monotherapy often involve maintenance of ERK addiction through upregulation of other pathway activators, either upstream or downstream of mutant BRAF. Increased activation or expression of surface RTKs, such as EGFR, via loss of feedback inhibition by ERK can lead to activation of RAF signaling [112,113]. Emergent mutations in NRAS or loss of function mutations in NF1 can increase activation and dimerization of RAF kinases [114,115]. BRAF itself can be aberrantly spliced, leading to expression of a protein that is able to dimerize independently of RAS, thereby avoiding inhibition [116]. These and other resistance mechanisms have been described and reviewed in detail for melanoma, colorectal cancer, and others [117,118].
It is unclear whether gliomas develop resistance via mechanisms that have been described in other cancers or whether resistance emerges in unique, lineage-specific patterns. The mechanism of acquired resistance to dabrafenib in one pediatric glioma was identified as a novel in cis mutation in BRAF (BRAF V600E L514V), which, upon biochemical characterization, was found to enhance dimerization, thereby decreasing sensitivity to dabrafenib [64]. It is not yet clear whether similar mechanisms will be seen in the majority of gliomas that develop resistance, and further research elucidating resistance mechanisms is ongoing.
6. Conclusions
BRAF mutations, particularly the V600E mutation and KIAA1549–BRAF fusions, are present in a significant subset of primary brain tumors. Given the paucity of treatment options available for patients with high-grade and recurrent glioma, there is significant interest in the potential of RAF/MEK inhibitors. Based on our current understanding of the mechanism by which different BRAF mutations activate ERK signaling, sensitivity to type I RAF inhibitors is mutation-dependent and limited to BRAF V600E mutant tumors. While clinical trials are still ongoing, there is preliminary evidence for a range of positive response rates in different types of glioma with BRAF V600E mutations. Even in recurrent adult GBM, where the response rate is much lower than PXA and likely between 10% and 25%, this represents a significant improvement over currently available standard therapies for these patients. We strongly recommend limiting the use of RAF/MEK inhibitors to the setting of a clinical trial, but combination therapy may represent a viable treatment option for patients with BRAF V600-mutated recurrent glioma who are not eligible for trial.
For class II or III mutations, including BRAF-fusions, preclinical data and data in other cancers clearly support the inefficacy of type I RAF inhibitors. In tumors with these mutations, novel RAF inhibitors that prevent paradoxical activation, those that disrupt BRAF dimerization, or small molecule inhibitors targeting MEK or ERK may have potential. These strategies are not yet clinically validated. In patients with these mutations, RAF-targeted therapy should only be considered in the setting of a clinical trial.
As we begin to understand the therapeutic potential of RAF inhibition in glioma, it must be recognized that tumor heterogeneity will affect responses in ways not yet fully understood. Whether BRAF mutations are present in all cells in a glioma or only a subset of tumor cells remains controversial and certainly has the potential to affect tumor response to targeted therapy. In addition, the effects of co-occurring mutations on tumorigenicity and sensitivity to targeted therapy remain to be more fully explored in brain tumors.
In summary, knowledge of the specific BRAF mutation and its biochemical effects on ERK signaling are critical for determining whether a patient could benefit from RAF-targeted therapy. While radiation with or without chemotherapy remains the mainstay of treatment for many primary brain tumors, ongoing research will clarify the role of RAF-targeted agents in patients with these tumors.
Author Contributions
This article was conceptualized and drafted by K.C.S. Review and editing by S.A.G. and C.A.P. Funding by K.C.S. and C.A.P.
Funding
This research was funded by the Musella Foundation.
Conflicts of Interest
K.C.S. and S.A.G. declare no conflict of interest. C.A.P. is a paid consultant for Genentech.
References
- 1.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF Gene in Human Cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Cutler R.E., Jr., Stephens R.M., Saracino M.R., Morrison D.K. Autoregulation of the Raf-1 serine/threonine Kinase. Proc. Natl. Acad. Sci. USA. 1998;95:9214–9219. doi: 10.1073/pnas.95.16.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daum G., Eisenmann-Tappe I., Fries H.W., Troppmair J., Rapp U.R. The ins and outs of Raf kinases. Trends Biochem. Sci. 1994;19:474–480. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 4.Pratilas C.A., Solit D.B. Therapeutic Strategies for Targeting BRAF in Human Cancer. Rev. Recent. Clin. Trials. 2007;2:121–134. doi: 10.2174/157488707780599393. [DOI] [PubMed] [Google Scholar]
- 5.Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauschild A., Grob J.J., Demidov L.V., Jouary T., Gutzmer R., Millward M., Rutkowski P., Blank C.U., Miller W.H., Jr., Kaempgen E., et al. Dabrafenib in BRAF-Mutated Metastatic Melanoma: A Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 7.Kim K.B., Kefford R., Pavlick A.C., Infante J.R., Ribas A., Sosman J.A., Fecher L.A., Millward M., McArthur G.A., Hwu P., et al. Phase II Study of the MEK1/MEK2 Inhibitor Trametinib in Patients with Metastatic BRAF-Mutant Cutaneous Melanoma Previously Treated with or without a BRAF Inhibitor. J. Clin. Oncol. 2013;31:482–489. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long G.V., Stroyakovskiy D., Gogas H., Levchenko E., de Braud F., Larkin J., Garbe C., Jouary T., Hauschild A., Grob J.J., et al. Combined BRAF and MEK Inhibition Versus BRAF Inhibition Alone in Melanoma. N. Engl. J. Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 9.Dummer R., Ascierto P.A., Gogas H.J., Arance A., Mandala M., Liszkay G., Garbe C., Schadendorf D., Krajsova I., Gutzmer R., et al. Overall Survival in Patients with BRAF-Mutant Melanoma Receiving Encorafenib Plus Binimetinib Versus Vemurafenib Or Encorafenib (COLUMBUS): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2018;19:1315–1327. doi: 10.1016/S1470-2045(18)30497-2. [DOI] [PubMed] [Google Scholar]
- 10.Larkin J., Ascierto P.A., Dreno B., Atkinson V., Liszkay G., Maio M., Mandala M., Demidov L., Stroyakovskiy D., Thomas L., et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 11.Brown N.F., Carter T., Kitchen N., Mulholland P. Dabrafenib and Trametinib in BRAFV600E Mutated Glioma. CNS Oncol. 2017;6:291–296. doi: 10.2217/cns-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migliorini D., Aguiar D., Vargas M.I., Lobrinus A., Dietrich P.Y. BRAF/MEK Double Blockade in Refractory Anaplastic Pleomorphic Xanthoastrocytoma. Neurology. 2017;88:1291–1293. doi: 10.1212/WNL.0000000000003767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreck K.C., Guajardo A., Lin D.D.M., Eberhart C.G., Grossman S.A. Concurrent BRAF/MEK Inhibitors in BRAF V600-Mutant High-Grade Primary Brain Tumors. J. Natl. Compr. Cancer Netw. 2018;16:343–347. doi: 10.6004/jnccn.2017.7052. [DOI] [PubMed] [Google Scholar]
- 14.Johanns T.M., Ferguson C.J., Grierson P.M., Dahiya S., Ansstas G. Rapid Clinical and Radiographic Response with Combined Dabrafenib and Trametinib in Adults with BRAF-Mutated High-Grade Glioma. J. Natl. Compr. Cancer Netw. 2018;16:4–10. doi: 10.6004/jnccn.2017.7032. [DOI] [PubMed] [Google Scholar]
- 15.Kaley T., Touat M., Subbiah V., Hollebecque A., Rodon J., Lockhart A.C., Keedy V., Bielle F., Hofheinz R.D., Joly F., et al. BRAF Inhibition in BRAF(V600)-Mutant Gliomas: Results from the VE-BASKET Study. J. Clin. Oncol. 2018;36:3477. doi: 10.1200/JCO.2018.78.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer. Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao Z., Torres N.M., Tao A., Gao Y., Luo L., Li Q., de Stanchina E., Abdel-Wahab O., Solit D.B., Poulikakos P.I., et al. BRAF Mutants Evade ERK-Dependent Feedback by Different Mechanisms that Determine their Sensitivity to Pharmacologic Inhibition. Cancer. Cell. 2015;28:370–383. doi: 10.1016/j.ccell.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles using the cBioPortal. Sci. Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pratilas C.A., Taylor B.S., Ye Q., Viale A., Sander C., Solit D.B., Rosen N. (V600E)BRAF is Associated with Disabled Feedback Inhibition of RAF-MEK Signaling and Elevated Transcriptional Output of the Pathway. Proc. Natl. Acad. Sci. USA. 2009;106:4519–4524. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lito P., Pratilas C.A., Joseph E.W., Tadi M., Halilovic E., Zubrowski M., Huang A., Wong W.L., Callahan M.K., Merghoub T., et al. Relief of Profound Feedback Inhibition of Mitogenic Signaling by RAF Inhibitors Attenuates their Activity in BRAFV600E Melanomas. Cancer. Cell. 2012;22:668–682. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao Z., Yaeger R., Rodrik-Outmezguine V.S., Tao A., Torres N.M., Chang M.T., Drosten M., Zhao H., Cecchi F., Hembrough T., et al. Tumours with Class 3 BRAF Mutants are Sensitive to the Inhibition of Activated RAS. Nature. 2017;548:234–238. doi: 10.1038/nature23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindler G., Capper D., Meyer J., Janzarik W., Omran H., Herold-Mende C., Schmieder K., Wesseling P., Mawrin C., Hasselblatt M., et al. Analysis of BRAF V600E Mutation in 1,320 Nervous System Tumors Reveals High Mutation Frequencies in Pleomorphic Xanthoastrocytoma, Ganglioglioma and Extra-Cerebellar Pilocytic Astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 23.Dahiya S., Emnett R.J., Haydon D.H., Leonard J.R., Phillips J.J., Perry A., Gutmann D.H. BRAF-V600E Mutation in Pediatric and Adult Glioblastoma. Neuro Oncol. 2014;16:318–319. doi: 10.1093/neuonc/not146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behling F., Barrantes-Freer A., Skardelly M., Nieser M., Christians A., Stockhammer F., Rohde V., Tatagiba M., Hartmann C., Stadelmann C., et al. Frequency of BRAF V600E Mutations in 969 Central Nervous System Neoplasms. Diagn. Pathol. 2016;11:55. doi: 10.1186/s13000-016-0506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinschmidt-DeMasters B.K., Aisner D.L., Foreman N.K. BRAF VE1 Immunoreactivity Patterns in Epithelioid Glioblastomas Positive for BRAF V600E Mutation. Am. J. Surg. Pathol. 2015;39:528–540. doi: 10.1097/PAS.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatae R., Hata N., Suzuki S.O., Yoshimoto K., Kuga D., Murata H., Akagi Y., Sangatsuda Y., Iwaki T., Mizoguchi M., et al. A Comprehensive Analysis Identifies BRAF Hotspot Mutations Associated with Gliomas with Peculiar Epithelial Morphology. Neuropathology. 2017;37:191–199. doi: 10.1111/neup.12347. [DOI] [PubMed] [Google Scholar]
- 27.Brastianos P.K., Taylor-Weiner A., Manley P.E., Jones R.T., Dias-Santagata D., Thorner A.R., Lawrence M.S., Rodriguez F.J., Bernardo L.A., Schubert L., et al. Exome Sequencing Identifies BRAF Mutations in Papillary Craniopharyngiomas. Nat. Genet. 2014;46:161–165. doi: 10.1038/ng.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pajtler K.W., Witt H., Sill M., Jones D.T., Hovestadt V., Kratochwil F., Wani K., Tatevossian R., Punchihewa C., Johann P., et al. Molecular Classification of Ependymal Tumors Across all CNS Compartments, Histopathological Grades, and Age Groups. Cancer. Cell. 2015;27:728–743. doi: 10.1016/j.ccell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratt D., Camelo-Piragua S., McFadden K., Leung D., Mody R., Chinnaiyan A., Koschmann C., Venneti S. BRAF activating mutations involving the β3-αC loop in V600E-negative anaplastic pleomorphic xanthoastrocytoma. Acta Neuropathol. Commun. 2018;6:24. doi: 10.1186/s40478-018-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S.H., Zhang Y., Van Horn R.D., Yin T., Buchanan S., Yadav V., Mochalkin I., Wong S.S., Yue Y.G., Huber L., et al. Oncogenic BRAF Deletions that Function as Homodimers and are Sensitive to Inhibition by RAF Dimer Inhibitor LY3009120. Cancer. Discov. 2016;6:300–315. doi: 10.1158/2159-8290.CD-15-0896. [DOI] [PubMed] [Google Scholar]
- 31.Foster S.A., Whalen D.M., Ozen A., Wongchenko M.J., Yin J., Yen I., Schaefer G., Mayfield J.D., Chmielecki J., Stephens P.J., et al. Activation Mechanism of Oncogenic Deletion Mutations in BRAF, EGFR, and HER2. Cancer. Cell. 2016;29:477–493. doi: 10.1016/j.ccell.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Bar E.E., Lin A., Tihan T., Burger P.C., Eberhart C.G. Frequent Gains at Chromosome 7q34 Involving BRAF in Pilocytic Astrocytoma. J. Neuropathol. Exp. Neurol. 2008;67:878–887. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 33.Sievert A.J., Lang S.S., Boucher K.L., Madsen P.J., Slaunwhite E., Choudhari N., Kellet M., Storm P.B., Resnick A.C. Paradoxical Activation and RAF Inhibitor Resistance of BRAF Protein Kinase Fusions Characterizing Pediatric Astrocytomas. Proc. Natl. Acad. Sci. USA. 2013;110:5957–5962. doi: 10.1073/pnas.1219232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sievert A.J., Jackson E.M., Gai X., Hakonarson H., Judkins A.R., Resnick A.C., Sutton L.N., Storm P.B., Shaikh T.H., Biegel J.A. Duplication of 7q34 in Pediatric Low-Grade Astrocytomas Detected by High-Density Single-Nucleotide Polymorphism-Based Genotype Arrays Results in a Novel BRAF Fusion Gene. Brain Pathol. 2009;19:449–458. doi: 10.1111/j.1750-3639.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones D.T., Kocialkowski S., Liu L., Pearson D.M., Backlund L.M., Ichimura K., Collins V.P. Tandem Duplication Producing a Novel Oncogenic BRAF Fusion Gene Defines the Majority of Pilocytic Astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones D.T., Gronych J., Lichter P., Witt O., Pfister S.M. MAPK Pathway Activation in Pilocytic Astrocytoma. Cell Mol. Life Sci. 2012;69:1799–1811. doi: 10.1007/s00018-011-0898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonelli M., Badiali M., Moi L., Buttarelli F.R., Baldi C., Massimino M., Sanson M., Giangaspero F. KIAA1549:BRAF Fusion Gene in Pediatric Brain Tumors of various Histogenesis. Pediatr. Blood Cancer. 2015;62:724–727. doi: 10.1002/pbc.25272. [DOI] [PubMed] [Google Scholar]
- 38.Pekmezci M., Villanueva-Meyer J.E., Goode B., Van Ziffle J., Onodera C., Grenert J.P., Bastian B.C., Chamyan G., Maher O.M., Khatib Z., et al. The Genetic Landscape of Ganglioglioma. Acta Neuropathol. Commun. 2018;6:47. doi: 10.1186/s40478-018-0551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller K.E., Kelly B., Fitch J., Ross N., Avenarius M.R., Varga E., Koboldt D.C., Boue D.R., Magrini V., Coven S.L., et al. Genome Sequencing Identifies Somatic BRAF Duplication c.1794_1796dupTAC;p.Thr599dup in Pediatric Patient with Low-Grade Ganglioglioma. Cold Spring Harb Mol. Case Stud. 2018;4 doi: 10.1101/mcs.a002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chmielecki J., Bailey M., He J., Elvin J., Vergilio J.A., Ramkissoon S., Suh J., Frampton G.M., Sun J.X., Morley S., et al. Genomic Profiling of a Large Set of Diverse Pediatric Cancers Identifies Known and Novel Mutations Across Tumor Spectra. Cancer Res. 2017;77:509–519. doi: 10.1158/0008-5472.CAN-16-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryall S., Arnoldo A., Krishnatry R., Mistry M., Khor K., Sheth J., Ling C., Leung S., Zapotocky M., Guerreiro Stucklin A., et al. Multiplex Detection of Pediatric Low-Grade Glioma Signature Fusion Transcripts and Duplications using the NanoString nCounter System. J. Neuropathol. Exp. Neurol. 2017;76:562–570. doi: 10.1093/jnen/nlx042. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J., Wu G., Miller C.P., Tatevossian R.G., Dalton J.D., Tang B., Orisme W., Punchihewa C., Parker M., Qaddoumi I., et al. Whole-Genome Sequencing Identifies Genetic Alterations in Pediatric Low-Grade Gliomas. Nat. Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helgager J., Lidov H.G., Mahadevan N.R., Kieran M.W., Ligon K.L., Alexandrescu S. A Novel GIT2-BRAF Fusion in Pilocytic Astrocytoma. Diagn. Pathol. 2017;12:82. doi: 10.1186/s13000-017-0669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsiao S.J., Karajannis M.A., Diolaiti D., Mansukhani M.M., Bender J.G., Kung A.L., Garvin J.H., Jr. A Novel, Potentially Targetable TMEM106B-BRAF Fusion in Pleomorphic Xanthoastrocytoma. Cold Spring Harb Mol. Case Stud. 2017;3:a001396. doi: 10.1101/mcs.a001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wan P.T., Garnett M.J., Roe S.M., Lee S., Niculescu-Duvaz D., Good V.M., Jones C.M., Marshall C.J., Springer C.J., Barford D., et al. Mechanism of Activation of the RAF-ERK Signaling Pathway by Oncogenic Mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/S0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 46.Ikenoue T., Hikiba Y., Kanai F., Tanaka Y., Imamura J., Imamura T., Ohta M., Ijichi H., Tateishi K., Kawakami T., et al. Functional Analysis of Mutations within the Kinase Activation Segment of B-Raf in Human Colorectal Tumors. Cancer Res. 2003;63:8132–8137. [PubMed] [Google Scholar]
- 47.Summers M.G., Smith C.G., Maughan T.S., Kaplan R., Escott-Price V., Cheadle J.P. BRAF and NRAS Locus-Specific Variants have Different Outcomes on Survival to Colorectal Cancer. Clin. Cancer Res. 2017;23:2742–2749. doi: 10.1158/1078-0432.CCR-16-1541. [DOI] [PubMed] [Google Scholar]
- 48.Zheng G., Tseng L.H., Chen G., Haley L., Illei P., Gocke C.D., Eshleman J.R., Lin M.T. Clinical Detection and Categorization of Uncommon and Concomitant Mutations Involving BRAF. BMC Cancer. 2015;15:779. doi: 10.1186/s12885-015-1811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poulikakos P.I., Zhang C., Bollag G., Shokat K.M., Rosen N. RAF Inhibitors Transactivate RAF Dimers and ERK Signalling in Cells with Wild-Type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zelboraf [Package Insert] Genentech USA, Inc.; South San Francisco, CA, USA: 2017. [Google Scholar]
- 51.Tafinlar [Package Insert] Novartis Pharmaceuticals Corporation; East Hanover, NJ, USA: 2018. [Google Scholar]
- 52.Braftovi [Package Insert] Array BioPharma Inc.; Boulder, CO, USA: 2019. [Google Scholar]
- 53.Nicolaides T.P., Li H., Solomon D.A., Hariono S., Hashizume R., Barkovich K., Baker S.J., Paugh B.S., Jones C., Forshew T., et al. Targeted Therapy for BRAFV600E Malignant Astrocytoma. Clin. Cancer Res. 2011;17:7595–7604. doi: 10.1158/1078-0432.CCR-11-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heidorn S.J., Milagre C., Whittaker S., Nourry A., Niculescu-Duvas I., Dhomen N., Hussain J., Reis-Filho J.S., Springer C.J., Pritchard C., et al. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatzivassiliou G., Song K., Yen I., Brandhuber B.J., Anderson D.J., Alvarado R., Ludlam M.J., Stokoe D., Gloor S.L., Vigers G., et al. RAF Inhibitors Prime Wild-Type RAF to Activate the MAPK Pathway and Enhance Growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 56.Karajannis M.A., Legault G., Fisher M.J., Milla S.S., Cohen K.J., Wisoff J.H., Harter D.H., Goldberg J.D., Hochman T., Merkelson A., et al. Phase II Study of Sorafenib in Children with Recurrent Or Progressive Low-Grade Astrocytomas. Neuro Oncol. 2014;16:1408–1416. doi: 10.1093/neuonc/nou059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang C., Spevak W., Zhang Y., Burton E.A., Ma Y., Habets G., Zhang J., Lin J., Ewing T., Matusow B., et al. RAF Inhibitors that Evade Paradoxical MAPK Pathway Activation. Nature. 2015;526:583–586. doi: 10.1038/nature14982. [DOI] [PubMed] [Google Scholar]
- 58.Jin T., Lavoie H., Sahmi M., David M., Hilt C., Hammell A., Therrien M. RAF Inhibitors Promote RAS-RAF Interaction by Allosterically Disrupting RAF Autoinhibition. Nat. Commun. 2017;8:1211. doi: 10.1038/s41467-017-01274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okimoto R.A., Lin L., Olivas V., Chan E., Markegard E., Rymar A., Neel D., Chen X., Hemmati G., Bollag G., et al. Preclinical Efficacy of a RAF Inhibitor that Evades Paradoxical MAPK Pathway Activation in Protein Kinase BRAF-Mutant Lung Cancer. Proc. Natl. Acad. Sci. USA. 2016;113:13456–13461. doi: 10.1073/pnas.1610456113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basile K.J., Le K., Hartsough E.J., Aplin A.E. Inhibition of Mutant BRAF Splice Variant Signaling by Next-Generation, Selective RAF Inhibitors. Pigment Cell. Melanoma Res. 2014;27:479–484. doi: 10.1111/pcmr.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao Z., Gao Y., Su W., Yaeger R., Tao J., Na N., Zhang Y., Zhang C., Rymar A., Tao A., et al. RAF Inhibitor PLX8394 Selectively Disrupts BRAF Dimers and RAS-Independent BRAF-Mutant-Driven Signaling. Nat. Med. 2019;25:284–291. doi: 10.1038/s41591-018-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong D.S., Hollebecque A., Gordon M.S., Flaherty K.T., Shapiro G., Rodon J., Millward M., Ramdas N., Zhang W., Gao L., et al. A First-in-Human Dose Phase 1 Study of LY3009120 in Advanced Cancer Patients. JCO. 2017;35:2507. doi: 10.1200/JCO.2017.35.15_suppl.2507. [DOI] [Google Scholar]
- 63.Girotti M.R., Lopes F., Preece N., Niculescu-Duvaz D., Zambon A., Davies L., Whittaker S., Saturno G., Viros A., Pedersen M., et al. Paradox-Breaking RAF Inhibitors that also Target SRC are Effective in Drug-Resistant BRAF Mutant Melanoma. Cancer. Cell. 2015;27:85–96. doi: 10.1016/j.ccell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J., Yao Z., Jonsson P., Allen A.N., Qin A.C.R., Uddin S., Dunkel I.J., Petriccione M., Manova K., Haque S., et al. A Secondary Mutation in BRAF Confers Resistance to RAF Inhibition in a BRAF V600E-Mutant Brain Tumor. Cancer. Discov. 2018;8:1130–1141. doi: 10.1158/2159-8290.CD-17-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flaherty K.T., Robert C., Hersey P., Nathan P., Garbe C., Milhem M., Demidov L.V., Hassel J.C., Rutkowski P., Mohr P., et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N. Engl. J. Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 66.Fangusaro J., Onar-Thomas A., Young Poussaint T., Wu S., Ligon A.H., Lindeman N., Banerjee A., Packer R.J., Kilburn L.B., Goldman S., et al. Selumetinib in Paediatric Patients with BRAF-Aberrant Or Neurofibromatosis Type 1-Associated Recurrent, Refractory, Or Progressive Low-Grade Glioma: A Multicentre, Phase 2 Trial. Lancet Oncol. 2019;20:1011–1022. doi: 10.1016/S1470-2045(19)30277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robison N., Pauly J., Malvar J., Gruber-Filbin M., de Mola R.L., Dorris K., Bendel A., Bowers D., Bornhorst M., Gauvain K., et al. LGG-44. A phase I dose escalation trial of the MEK1/2 inhibitor MEK162 (binimetinib) in children with low-grade gliomas and other Ras/Raf pathway-activated tumors. Neuro-Oncology. 2018;20:i114. doi: 10.1093/neuonc/noy059.385. [DOI] [Google Scholar]
- 68.Sullivan R.J., Infante J.R., Janku F., Wong D.J.L., Sosman J.A., Keedy V., Patel M.R., Shapiro G.I., Mier J.W., Tolcher A.W., et al. First-in-Class ERK1/2 Inhibitor Ulixertinib (BVD-523) in Patients with MAPK Mutant Advanced Solid Tumors: Results of a Phase I Dose-Escalation and Expansion Study. Cancer. Discov. 2018;8:184–195. doi: 10.1158/2159-8290.CD-17-1119. [DOI] [PubMed] [Google Scholar]
- 69.Johnson A., Severson E., Gay L., Vergilio J.A., Elvin J., Suh J., Daniel S., Covert M., Frampton G.M., Hsu S., et al. Comprehensive Genomic Profiling of 282 Pediatric Low- and High-Grade Gliomas Reveals Genomic Drivers, Tumor Mutational Burden, and Hypermutation Signatures. Oncologist. 2017;22:1478–1490. doi: 10.1634/theoncologist.2017-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Becker A.P., Scapulatempo-Neto C., Carloni A.C., Paulino A., Sheren J., Aisner D.L., Musselwhite E., Clara C., Machado H.R., Oliveira R.S., et al. KIAA1549: BRAF Gene Fusion and FGFR1 Hotspot Mutations are Prognostic Factors in Pilocytic Astrocytomas. J. Neuropathol. Exp. Neurol. 2015;74:743–754. doi: 10.1097/NEN.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hawkins C., Walker E., Mohamed N., Zhang C., Jacob K., Shirinian M., Alon N., Kahn D., Fried I., Scheinemann K., et al. BRAF-KIAA1549 Fusion Predicts Better Clinical Outcome in Pediatric Low-Grade Astrocytoma. Clin. Cancer Res. 2011;17:4790–4798. doi: 10.1158/1078-0432.CCR-11-0034. [DOI] [PubMed] [Google Scholar]
- 72.Lassaletta A., Zapotocky M., Mistry M., Ramaswamy V., Honnorat M., Krishnatry R., Guerreiro Stucklin A., Zhukova N., Arnoldo A., Ryall S., et al. Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. J. Clin. Oncol. 2017;35:2934. doi: 10.1200/JCO.2016.71.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Drobysheva A., Klesse L.J., Bowers D.C., Rajaram V., Rakheja D., Timmons C.F., Wang J., Koral K., Gargan L., Ramos E., et al. Targeted MAPK Pathway Inhibitors in Patients with Disseminated Pilocytic Astrocytomas. J. Natl. Compr. Cancer Netw. 2017;15:978–982. doi: 10.6004/jnccn.2017.0139. [DOI] [PubMed] [Google Scholar]
- 74.Ho C.Y., Mobley B.C., Gordish-Dressman H., VandenBussche C.J., Mason G.E., Bornhorst M., Esbenshade A.J., Tehrani M., Orr B.A., LaFrance D.R., et al. A Clinicopathologic Study of Diencephalic Pediatric Low-Grade Gliomas with BRAF V600 Mutation. Acta Neuropathol. 2015;130:575–585. doi: 10.1007/s00401-015-1467-3. [DOI] [PubMed] [Google Scholar]
- 75.Kieran M.W., Bouffet E., Tabori U., Broniscer A., Cohen K., Hansford J., Geoerger B., Hingorani P., Dunkel I., Russo M., et al. The First Study of Dabrafenib in Pediatric Patients with BRAF V600-Mutant Relapsed or Refractory Low-Grade Gliomas. Ann. Oncol. 2016;27:vi552–vi587. doi: 10.1093/annonc/mdw435.09. [DOI] [PubMed] [Google Scholar]
- 76.Banerjee A., Jakacki R.I., Onar-Thomas A., Wu S., Nicolaides T., Young Poussaint T., Fangusaro J., Phillips J., Perry A., Turner D., et al. A Phase I Trial of the MEK Inhibitor Selumetinib (AZD6244) in Pediatric Patients with Recurrent or Refractory Low-Grade Glioma: A Pediatric Brain Tumor Consortium (PBTC) Study. Neuro Oncol. 2017;19:1135–1144. doi: 10.1093/neuonc/now282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korshunov A., Ryzhova M., Hovestadt V., Bender S., Sturm D., Capper D., Meyer J., Schrimpf D., Kool M., Northcott P.A., et al. Integrated Analysis of Pediatric Glioblastoma Reveals a Subset of Biologically Favorable Tumors with Associated Molecular Prognostic Markers. Acta Neuropathol. 2015;129:669–678. doi: 10.1007/s00401-015-1405-4. [DOI] [PubMed] [Google Scholar]
- 78.Ballester L.Y., Fuller G.N., Powell S.Z., Sulman E.P., Patel K.P., Luthra R., Routbort M.J. Retrospective Analysis of Molecular and Immunohistochemical Characterization of 381 Primary Brain Tumors. J. Neuropathol. Exp. Neurol. 2017;76:179–188. doi: 10.1093/jnen/nlw119. [DOI] [PubMed] [Google Scholar]
- 79.Wen P., Alexander S., Yung-Jue B., van den Bent M.J., Gazzah A., Dietrich S., de Vos F., van Linde M.E., Lai A., Chi A., et al. Efficacy and Safety of Dabrafenib + Trametinib in Patients with recurrent/refractory BRAF V60E-Mutated High-Grade Glioma (HGG) Neuro Oncol. 2018;20:vi238. [Google Scholar]
- 80.Pages M., Beccaria K., Boddaert N., Saffroy R., Besnard A., Castel D., Fina F., Barets D., Barret E., Lacroix L., et al. Co-Occurrence of Histone H3 K27M and BRAF V600E Mutations in Paediatric Midline Grade I Ganglioglioma. Brain Pathol. 2018;28:103–111. doi: 10.1111/bpa.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meletath S.K., Pavlick D., Brennan T., Hamilton R., Chmielecki J., Elvin J.A., Palma N., Ross J.S., Miller V.A., Stephens P.J., et al. Personalized Treatment for a Patient with a BRAF V600E Mutation using Dabrafenib and a Tumor Treatment Fields Device in a High-Grade Glioma Arising from Ganglioglioma. J. Natl. Compr. Cancer Netw. 2016;14:1345–1350. doi: 10.6004/jnccn.2016.0145. [DOI] [PubMed] [Google Scholar]
- 82.Touat M., Gratieux J., Condette Auliac S., Sejean K., Aldea S., Savatovsky J., Perkins G., Blons H., Ligon K.L., Idbaih A., et al. Vemurafenib and Cobimetinib Overcome Resistance to Vemurafenib in BRAF-Mutant Ganglioglioma. Neurology. 2018;91:523–525. doi: 10.1212/WNL.0000000000006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garnier L., Ducray F., Verlut C., Mihai M.I., Cattin F., Petit A., Curtit E. Prolonged Response Induced by Single Agent Vemurafenib in a BRAF V600E Spinal Ganglioglioma: A Case Report and Review of the Literature. Front. Oncol. 2019;9:177. doi: 10.3389/fonc.2019.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bautista F., Paci A., Minard-Colin V., Dufour C., Grill J., Lacroix L., Varlet P., Valteau-Couanet D., Geoerger B. Vemurafenib in Pediatric Patients with BRAFV600E Mutated High-Grade Gliomas. Pediatr. Blood Cancer. 2014;61:1101–1103. doi: 10.1002/pbc.24891. [DOI] [PubMed] [Google Scholar]
- 85.del Bufalo F., Carai A., Figa-Talamanca L., Pettorini B., Mallucci C., Giangaspero F., Antonelli M., Badiali M., Moi L., Bianco G., et al. Response of Recurrent BRAFV600E Mutated Ganglioglioma to Vemurafenib as Single Agent. J. Transl. Med. 2014;12:356. doi: 10.1186/s12967-014-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rush S., Foreman N., Liu A. Brainstem Ganglioglioma Successfully Treated with Vemurafenib. J. Clin. Oncol. 2013;31:e159–e160. doi: 10.1200/JCO.2012.44.1568. [DOI] [PubMed] [Google Scholar]
- 87.Aguilera D., Janss A., Mazewski C., Castellino R.C., Schniederjan M., Hayes L., Brahma B., Fogelgren L., MacDonald T.J. Successful Retreatment of a Child with a Refractory Brainstem Ganglioglioma with Vemurafenib. Pediatr. Blood Cancer. 2016;63:541–543. doi: 10.1002/pbc.25787. [DOI] [PubMed] [Google Scholar]
- 88.Beland B., Tsang R.Y., Sutherland G. Unprecedented Response to Combination BRAF and MEK Inhibitors in Adult Anaplastic Ganglioglioma. J. Neurooncol. 2018;137:667–669. doi: 10.1007/s11060-018-2760-5. [DOI] [PubMed] [Google Scholar]
- 89.Toll S.A., Tran H.N., Cotter J., Judkins A.R., Tamrazi B., Biegel J.A., Dhall G., Robison N.J., Waters K., Patel P., et al. Sustained Response of Three Pediatric BRAF(V600E) Mutated High-Grade Gliomas to Combined BRAF and MEK Inhibitor Therapy. Oncotarget. 2019;10:551–557. doi: 10.18632/oncotarget.26560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marks A.M., Bindra R.S., DiLuna M.L., Huttner A., Jairam V., Kahle K.T., Kieran M.W. Response to the BRAF/MEK Inhibitors dabrafenib/trametinib in an Adolescent with a BRAF V600E Mutated Anaplastic Ganglioglioma Intolerant to Vemurafenib. Pediatr. Blood Cancer. 2018;65:e26969. doi: 10.1002/pbc.26969. [DOI] [PubMed] [Google Scholar]
- 91.Kumar A., Pathak P., Purkait S., Faruq M., Jha P., Mallick S., Suri V., Sharma M.C., Suri A., Sarkar C. Oncogenic KIAA1549-BRAF Fusion with Activation of the MAPK/ERK Pathway in Pediatric Oligodendrogliomas. Cancer Genet. 2015;208:91–95. doi: 10.1016/j.cancergen.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 92.Mistry M., Zhukova N., Merico D., Rakopoulos P., Krishnatry R., Shago M., Stavropoulos J., Alon N., Pole J.D., Ray P.N., et al. BRAF Mutation and CDKN2A Deletion Define a Clinically Distinct Subgroup of Childhood Secondary High-Grade Glioma. J. Clin. Oncol. 2015;33:1015–1022. doi: 10.1200/JCO.2014.58.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Del Bufalo F., Ceglie G., Cacchione A., Alessi I., Colafati G.S., Carai A., Diomedi-Camassei F., De Billy E., Agolini E., Mastronuzzi A., et al. BRAF V600E Inhibitor (Vemurafenib) for BRAF V600E Mutated Low Grade Gliomas. Front. Oncol. 2018;8:526. doi: 10.3389/fonc.2018.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vuong H.G., Altibi A.M.A., Duong U.N.P., Ngo H.T.T., Pham T.Q., Fung K.M., Hassell L. BRAF Mutation is Associated with an Improved Survival in Glioma-a Systematic Review and Meta-Analysis. Mol. Neurobiol. 2017;55:3718–3724. doi: 10.1007/s12035-017-0599-y. [DOI] [PubMed] [Google Scholar]
- 95.Ferguson S.D., Xiu J., Weathers S.P., Zhou S., Kesari S., Weiss S.E., Verhaak R.G., Hohl R.J., Barger G.R., Reddy S.K., et al. GBM-Associated Mutations and Altered Protein Expression are More Common in Young Patients. Oncotarget. 2016;7:69466–69478. doi: 10.18632/oncotarget.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang R.Q., Shi Z., Chen H., Chung N.Y., Yin Z., Li K.K., Chan D.T., Poon W.S., Wu J., Zhou L., et al. Biomarker-Based Prognostic Stratification of Young Adult Glioblastoma. Oncotarget. 2016;7:5030–5041. doi: 10.18632/oncotarget.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schreck K., Vera E., Aboud O., Acquaye A., Boris L., Briceno N., Brown M., Chung H., Crandon S., Garren N., et al. PATH-28. The natural history of braf v600e-mutated glioblastomas in adults. Neuro-Oncology. 2018;20:vi164. doi: 10.1093/neuonc/noy148.684. [DOI] [Google Scholar]
- 98.Chamberlain M.C. Salvage Therapy with BRAF Inhibitors for Recurrent Pleomorphic Xanthoastrocytoma: A Retrospective Case Series. J. Neurooncol. 2013;114:237–240. doi: 10.1007/s11060-013-1176-5. [DOI] [PubMed] [Google Scholar]
- 99.Ida C.M., Rodriguez F.J., Burger P.C., Caron A.A., Jenkins S.M., Spears G.M., Aranguren D.L., Lachance D.H., Giannini C. Pleomorphic Xanthoastrocytoma: Natural History and Long-Term Follow-Up. Brain Pathol. 2015;25:575–586. doi: 10.1111/bpa.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tabouret E., Bequet C., Denicolai E., Barrie M., Nanni I., Metellus P., Dufour H., Chinot O., Figarella-Branger D. BRAF Mutation and Anaplasia may be Predictive Factors of Progression-Free Survival in Adult Pleomorphic Xanthoastrocytoma. Eur. J. Surg. Oncol. 2015;41:1685–1690. doi: 10.1016/j.ejso.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Usubalieva A., Pierson C.R., Kavran C.A., Huntoon K., Kryvenko O.N., Mayer T.G., Zhao W., Rock J., Ammirati M., Puduvalli V.K., et al. Primary Meningeal Pleomorphic Xanthoastrocytoma with Anaplastic Features: A Report of 2 Cases, One with BRAF(V600E) Mutation and Clinical Response to the BRAF Inhibitor Dabrafenib. J. Neuropathol. Exp. Neurol. 2015;74:960–969. doi: 10.1097/NEN.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee E.Q., Ruland S., LeBoeuf N.R., Wen P.Y., Santagata S. Successful Treatment of a Progressive BRAF V600E-Mutated Anaplastic Pleomorphic Xanthoastrocytoma with Vemurafenib Monotherapy. J. Clin. Oncol. 2016;34:e87–e89. doi: 10.1200/JCO.2013.51.1766. [DOI] [PubMed] [Google Scholar]
- 103.Leaver K.M., Zhang N., Ziskin J.L., Vogel H., Recht L., Thomas R.P. Response of Metastatic Glioma to Vemurafenib. Neuro-Oncol. Pract. 2016;3:268–271. doi: 10.1093/nop/npv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burger M.C., Ronellenfitsch M.W., Lorenz N.I., Wagner M., Voss M., Capper D., Tzaridis T., Herrlinger U., Steinbach J.P., Stoffels G., et al. Dabrafenib in Patients with Recurrent, BRAF V600E Mutated Malignant Glioma and Leptomeningeal Disease. Oncol. Rep. 2017;38:3291–3296. doi: 10.1093/neuonc/nox168.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dahiya S., Haydon D.H., Alvarado D., Gurnett C.A., Gutmann D.H., Leonard J.R. BRAF(V600E) Mutation is a Negative Prognosticator in Pediatric Ganglioglioma. Acta Neuropathol. 2013;125:901–910. doi: 10.1007/s00401-013-1120-y. [DOI] [PubMed] [Google Scholar]
- 106.Louis D.N., Giannini C., Capper D., Paulus W., Figarella-Branger D., Lopes M.B., Batchelor T.T., Cairncross J.G., van den Bent M., Wick W., et al. CIMPACT-NOW Update 2: Diagnostic Clarifications for Diffuse Midline Glioma, H3 K27M-Mutant and Diffuse astrocytoma/anaplastic Astrocytoma, IDH-Mutant. Acta Neuropathol. 2018;135:639–642. doi: 10.1007/s00401-018-1826-y. [DOI] [PubMed] [Google Scholar]
- 107.Brastianos P.K., Shankar G.M., Gill C.M., Taylor-Weiner A., Nayyar N., Panka D.J., Sullivan R.J., Frederick D.T., Abedalthagafi M., Jones P.S., et al. Dramatic Response of BRAF V600E Mutant Papillary Craniopharyngioma to Targeted Therapy. J. Natl. Cancer Inst. 2015;108 doi: 10.1093/jnci/djv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aylwin S.J., Bodi I., Beaney R. Pronounced Response of Papillary Craniopharyngioma to Treatment with Vemurafenib, a BRAF Inhibitor. Pituitary. 2016;19:544–546. doi: 10.1007/s11102-015-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Himes B.T., Ruff M.W., Van Gompel J.J., Park S.S., Galanis E., Kaufmann T.J., Uhm J.H. Recurrent Papillary Craniopharyngioma with BRAF V600E Mutation Treated with Dabrafenib: Case Report. J. Neurosurg. 2018;130:1299–1303. doi: 10.3171/2017.11.JNS172373. [DOI] [PubMed] [Google Scholar]
- 110.Rostami E., Witt Nystrom P., Libard S., Wikstrom J., Casar-Borota O., Gudjonsson O. Recurrent Papillary Craniopharyngioma with BRAFV600E Mutation Treated with Neoadjuvant-Targeted Therapy. Acta Neurochir. 2017;159:2217–2221. doi: 10.1007/s00701-017-3311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roque A., Odia Y. BRAF-V600E Mutant Papillary Craniopharyngioma Dramatically Responds to Combination BRAF and MEK Inhibitors. CNS Oncol. 2017;6:95–99. doi: 10.2217/cns-2016-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Montero-Conde C., Ruiz-Llorente S., Dominguez J.M., Knauf J.A., Viale A., Sherman E.J., Ryder M., Ghossein R.A., Rosen N., Fagin J.A. Relief of Feedback Inhibition of HER3 Transcription by RAF and MEK Inhibitors Attenuates their Antitumor Effects in BRAF-Mutant Thyroid Carcinomas. Cancer. Discov. 2013;3:520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prahallad A., Sun C., Huang S., Di Nicolantonio F., Salazar R., Zecchin D., Beijersbergen R.L., Bardelli A., Bernards R. Unresponsiveness of Colon Cancer to BRAF(V600E) Inhibition through Feedback Activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 114.Nazarian R., Shi H., Wang Q., Kong X., Koya R.C., Lee H., Chen Z., Lee M.K., Attar N., Sazegar H., et al. Melanomas Acquire Resistance to B-RAF(V600E) Inhibition by RTK or N-RAS Upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nissan M.H., Pratilas C.A., Jones A.M., Ramirez R., Won H., Liu C., Tiwari S., Kong L., Hanrahan A.J., Yao Z., et al. Loss of NF1 in Cutaneous Melanoma is Associated with RAS Activation and MEK Dependence. Cancer Res. 2014;74:2340–2350. doi: 10.1158/0008-5472.CAN-13-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poulikakos P.I., Persaud Y., Janakiraman M., Kong X., Ng C., Moriceau G., Shi H., Atefi M., Titz B., Gabay M.T., et al. RAF Inhibitor Resistance is Mediated by Dimerization of Aberrantly Spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Samatar A.A., Poulikakos P.I. Targeting RAS-ERK Signalling in Cancer: Promises and Challenges. Nat. Rev. Drug Discov. 2014;13:928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 118.Lim S.Y., Menzies A.M., Rizos H. Mechanisms and Strategies to Overcome Resistance to Molecularly Targeted Therapy for Melanoma. Cancer. 2017;123:2118–2129. doi: 10.1002/cncr.30435. [DOI] [PubMed] [Google Scholar]