Abstract
Background
Falls are one of the most common complications after stroke, with a reported incidence ranging between 7% in the first week and 73% in the first year post stroke. This is an updated version of the original Cochrane Review published in 2013.
Objectives
To evaluate the effectiveness of interventions aimed at preventing falls in people after stroke. Our primary objective was to determine the effect of interventions on the rate of falls (number of falls per person‐year) and the number of fallers. Our secondary objectives were to determine the effects of interventions aimed at preventing falls on 1) the number of fall‐related fractures; 2) the number of fall‐related hospital admissions; 3) near‐fall events; 4) economic evaluation; 5) quality of life; and 6) adverse effects of the interventions.
Search methods
We searched the trials registers of the Cochrane Stroke Group (September 2018) and the Cochrane Bone, Joint and Muscle Trauma Group (October 2018); the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 9) in the Cochrane Library; MEDLINE (1950 to September 2018); Embase (1980 to September 2018); CINAHL (1982 to September 2018); PsycINFO (1806 to August 2018); AMED (1985 to December 2017); and PEDro (September 2018). We also searched trials registers and checked reference lists.
Selection criteria
Randomised controlled trials of interventions where the primary or secondary aim was to prevent falls in people after stroke.
Data collection and analysis
Two review authors (SD and WS) independently selected studies for inclusion, assessed trial quality and risk of bias, and extracted data. We resolved disagreements through discussion, and contacted study authors for additional information where required. We used a rate ratio and 95% confidence interval (CI) to compare the rate of falls (e.g. falls per person‐year) between intervention and control groups. For risk of falling we used a risk ratio and 95% CI based on the number of people falling (fallers) in each group. We pooled results where appropriate and applied GRADE to assess the quality of the evidence.
Main results
We included 14 studies (of which six have been published since the first version of this review in 2013), with a total of 1358 participants. We found studies that investigated exercises, predischarge home visits for hospitalised patients, the provision of single lens distance vision glasses instead of multifocal glasses, a servo‐assistive rollator and non‐invasive brain stimulation for preventing falls.
Exercise compared to control for preventing falls in people after stroke The pooled result of eight studies showed that exercise may reduce the rate of falls but we are uncertain about this result (rate ratio 0.72, 95% CI 0.54 to 0.94, 765 participants, low‐quality evidence). Sensitivity analysis for single exercise interventions, omitting studies using multiple/multifactorial interventions, also found that exercise may reduce the rate of falls (rate ratio 0.66, 95% CI 0.50 to 0.87, 626 participants). Sensitivity analysis for the effect in the chronic phase post stroke resulted in little or no difference in rate of falls (rate ratio 0.58, 95% CI 0.31 to 1.12, 205 participants). A sensitivity analysis including only studies with low risk of bias found little or no difference in rate of falls (rate ratio 0.88, 95% CI 0.65 to 1.20, 462 participants). Methodological limitations mean that we have very low confidence in the results of these sensitivity analyses.
For the outcome of number of fallers, we are very uncertain of the effect of exercises compared to the control condition, based on the pooled result of 10 studies (risk ratio 1.03, 95% CI 0.90 to 1.19, 969 participants, very low quality evidence). The same sensitivity analyses as described above gives us very low certainty that there are little or no differences in number of fallers (single interventions: risk ratio 1.09, 95% CI 0.93 to 1.28, 796 participants; chronic phase post stroke: risk ratio 0.94, 95% CI 0.73 to 1.22, 375 participants; low risk of bias studies: risk ratio 0.96, 95% CI 0.77 to 1.21, 462 participants).
Other interventions for preventing falls in people after stroke We are very uncertain whether interventions other than exercise reduce the rate of falls or number of fallers. We identified very low certainty evidence when investigating the effect of predischarge home visits (rate ratio 0.85, 95% CI 0.43 to 1.69; risk ratio 1.48, 95% CI 0.71 to 3.09; 85 participants), provision of single lens distance glasses to regular wearers of multifocal glasses (rate ratio 1.08, 95% CI 0.52 to 2.25; risk ratio 0.74, 95% CI 0.47 to 1.18; 46 participants) and a servo‐assistive rollator (rate ratio 0.44, 95% CI 0.16 to 1.21; risk ratio 0.44, 95% CI 0.16 to 1.22; 42 participants).
Finally, transcranial direct current stimulation (tDCS) was used in one study to examine the effect on falls post stroke. We have low certainty that active tDCS may reduce the number of fallers compared to sham tDCS (risk ratio 0.30, 95% CI 0.14 to 0.63; 60 participants).
Authors' conclusions
At present there exists very little evidence about interventions other than exercises to reduce falling post stroke. Low to very low quality evidence exists that this population benefits from exercises to prevent falls, but not to reduce number of fallers.
Fall research does not in general or consistently follow methodological gold standards, especially with regard to fall definition and time post stroke. More well‐reported, adequately‐powered research should further establish the value of exercises in reducing falling, in particular per phase, post stroke.
Keywords: Female, Humans, Male, Accidental Falls, Accidental Falls/prevention & control, Accidental Falls/statistics & numerical data, Alendronate, Alendronate/administration & dosage, Bone Density Conservation Agents, Bone Density Conservation Agents/administration & dosage, Exercise, Eyeglasses, Randomized Controlled Trials as Topic, Stroke, Stroke/complications, Vitamin D, Vitamin D/administration & dosage, Vitamins, Vitamins/administration & dosage
Plain language summary
Interventions for preventing falls in people after stroke
Review question Which intervention modalities reduce falling post stroke?
Background Falls are commonly reported and occur in up to 73% of people one year post stroke. Not all falls are serious enough to require medical attention but even non‐serious falls may lead to activity restrictions and people developing a fear of falling. They are a factor for predicting future falls, which may restrict the person's activities of daily living and therefore require attention. This review investigated which methods are effective in preventing falls in people after their stroke, either with haemorrhagic or ischaemic aetiology.
Search date 3 September 2018
Study characteristics After searching the literature, we included 14 studies with a total of 1358 participants. We found studies that investigated various interventions for preventing falls: physical exercises; predischarge home visits for hospitalised patients; the provision of single lens distance vision glasses instead of multifocal glasses; a servo‐assistive rollator; and non‐invasive brain stimulation. Included studies conducted their investigations in early to chronic inpatient, outpatient, and community dwelling settings.
Study funding sources None
Key results Exercises appear to reduce the rate of falls, but not the number of people falling post stroke. Among the studies that used exercises as an intervention condition, the majority of studies asked participants to solely perform exercises. One study offered exercises together with additional components, such as educational sessions about falls. Another study offered exercises together with a comprehensive risk assessment and subsequent referrals, such as a review by an optometrist or new shoes, leading to a personalised programme for preventing falls.
Besides exercises, several other interventions aiming to prevent falls post stroke were investigated in the literature. One study administered non‐invasive brain stimulation to people after stroke and the results showed a potential to decrease the number of people falling, but this study needs to be replicated before consideration in clinical practice. There is no evidence at the moment that predischarge home visits, single lens distance vision glasses instead of multifocal glasses or a servo‐assistive rollator reduce the rate of falls or the number of people falling.
None of the included studies reported serious harm related to the intervention conditions.
In summary: there is little evidence that interventions other than exercises are beneficial for preventing falls in people after stroke. The main reason is that there were only a limited number of studies focusing on people after stroke or that included a stroke subgroup in the study. In addition, studies related to falling do not consistently follow known methodological guidelines, particularly in fall definition and time post stroke. More well‐reported, consensual research with an adequate number of participants might further establish the value of exercises in reducing falling post stroke.
Quality of the evidence The quality of the evidence regarding rate of falls and number of fallers ranged from very low to low across the five comparisons, meaning that we have very low to low certainty in these results. The main reasons for downgrading the evidence were the lack of blinding of fall outcome and the majority of comparisons including only one study.
Summary of findings
Summary of findings for the main comparison. Exercise compared to control for preventing falls in people after stroke.
| Exercise compared to control for preventing falls in people after stroke | ||||||
| Patient or population: preventing falls in people after stroke Setting: inpatient and outpatient rehabilitation Intervention: exercise Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with exercise | |||||
| Rate of falls | Study population | Rate ratio 0.72 (0.54 to 0.94) | 765 (8 RCTs) | ⊕⊕⊝⊝ Low 1 2 3 4 5 6 | Exercise may reduce rate of falls but we are very uncertain. Fall rate ratios were calculated if not explicitly stated. | |
| Not applicable | Not applicable | |||||
| Number of fallers | Study population | RR 1.03 (0.90 to 1.19) | 969 (10 RCTs) | ⊕⊝⊝⊝ Very low 1 2 3 4 5 6 7 | ||
| 411 per 1000 | 424 per 1000 (375 to 495) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 All studies had a high/unclear risk of bias regarding blinding of outcome assessment. Furthermore, 6 studies were at additional unclear/high risk on other items of our risk of bias assessment.
2 1 study had > 30% loss to follow‐up. Since major drop‐out was not consistent in all included studies, we decided not to perform an additional downgrading of the evidence for risk of bias.
3 Heterogeneous nature of interventions in both intervention and control conditions.
4 Only 1 study reported very few fall events and number of fallers in both groups, yielding a large 95% confidence interval. Regarding number of fallers, 2 additional studies reported a rather low amount of number of fallers. However, this is to some extent justified since the latter studies also comprised smaller sample sizes. We decided not to the downgrade the evidence level for imprecision of results.
5 In 2 studies, 2 intervention conditions were included. For reasons outlined in the results section of this review, we pooled the effect of the 2 intervention conditions, which might have caused an over‐ or underestimation compared to the separate effects of the interventions. However, since both intervention conditions adopted some kind of exercise treatment, we did not perform an additional downgrade of the evidence.
6 Some heterogeneity was found regarding the included population, being the post stroke phase of the participants. Post stroke phase appeared to range from early subacute to chronic phase post stroke. However, to our knowledge, literature does not consist of convincing evidence to substantiate limitation in applicability of fall‐prevention strategies according to post stroke phase at present. Hence, we concluded not to downgrade for indirectness of evidence.
7 Effect sizes differ widely and inconsistent at both sides of the line of no difference.
Summary of findings 2. Home visits compared to control for preventing falls in people after stroke.
| Home visits compared to control for preventing falls in people after stroke | ||||||
| Patient or population: preventing falls in people after stroke Setting: inpatient rehabilitation Intervention: home visits Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with home visits | |||||
| Rate of falls | Study population | Rate ratio 0.85 (0.43 to 1.69) | 85 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 4 5 6 | Fall rate ratios were calculated if not explicitly stated. | |
| Not applicable | Not applicable | |||||
| Number of fallers | Study population | RR 1.48 (0.71 to 3.09) | 85 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 4 5 6 | ||
| 209 per 1000 | 310 per 1000 (149 to 647) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Substantial amount of unclear and high risk scores regarding study methodology.
2 Falls as a secondary outcome measure and unclear if falls were reported as an adverse event.
3 Allocation of patients to either cohort study or RCT based on empirical decisions.
4 Results based on data of 1 single study.
5 The risk ratio has a strong tendency to be in favour of the control condition.
6 Despite of the fact that only 1 study was found for this comparison, we do not expect the existence of more trials assessing the intervention of interest, since it is a rather exceptional method to reduce falling post stroke.
Summary of findings 3. Single lens distance glasses compared to usual (multifocal) glasses for preventing falls in people after stroke.
| Single lens distance glasses compared to usual (multifocal) glasses for preventing falls in people after stroke | ||||||
| Patient or population: preventing falls in people after stroke Setting: community Intervention: single lens distance glasses Comparison: usual (multifocal) glasses | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual (multifocal) glasses | Risk with single lens distance glasses | |||||
| Rate of falls | Study population | Rate ratio 1.08 (0.52 to 2.25) | 43 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 4 | Fall rate ratios were calculated if not explicitly stated. | |
| Not applicable | Not applicable | |||||
| Number of fallers | Study population | RR 0.74 (0.47 to 1.18) | 43 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 4 | ||
| 739 per 1000 | 547 per 1000 (347 to 872) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Despite of the fact that only 1 study was found for this comparison, we do not expect the existence of more trials assessing the intervention of interest, since it is a rather exceptional method to reduce falling post stroke.
2 Substantial amount of unclear and high risk scores regarding study methodology.
3 Initial target population was elderly regular wearers of multifocal glasses. Hence, conclusions are only applicable on elderly stroke survivors who are regular wearers of multifocal glasses.
4 Results are based on data of 1 single study.
Summary of findings 4. Servo‐assistive rollator compared to control for preventing falls in people after stroke.
| Servo‐assistive rollator compared to control for preventing falls in people after stroke | ||||||
| Patient or population: preventing falls in people after stroke Setting: inpatient rehabilitation Intervention: servo‐assistive rollator Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with servo‐assistive rollator | |||||
| Rate of falls | Study population | Rate ratio 0.56 (0.19 to 1.66) | 42 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 4 | Fall rate ratios were calculated if not explicitly stated. | |
| Not applicable | Not applicable | |||||
| Number of fallers | Study population | RR 0.44 (0.16 to 1.22) | 42 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 4 | ||
| 429 per 1000 | 189 per 1000 (69 to 523) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Despite of the fact that only 1 study was found for this comparison, we do not expect the existence of more trials assessing the intervention of interest, since it is a rather exceptional method to reduce falling post stroke.
2 Substantial amount of unclear and high risk scores regarding study methodology.
3 Results are based on data of 1 single study.
4 A large confidence interval is present in both outcomes due to few events compared to the group sizes.
Summary of findings 5. Other interventions: tDCS compared to sham tDCS for preventing falls in people after stroke.
| Other interventions: tDCS compared to sham tDCS for preventing falls in people after stroke | ||||||
| Patient or population: preventing falls in people after stroke Setting: inpatient rehabilitation Intervention: other interventions: tDCS Comparison: sham tDCS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sham tDCS | Risk with other interventions: tDCS | |||||
| Number of fallers | Study population | RR 0.30 (0.14 to 0.63) | 60 (1 RCT) | ⊕⊕⊝⊝ Low 1 2 3 | ||
| 600 per 1000 | 180 per 1000 (84 to 378) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Despite of the fact that only 1 study was found for this comparison, we do not expect the existence of more trials assessing the intervention of interest, since it is a rather exceptional method to reduce falling post stroke.
2 Results rely solely on data of 1 single study.
3 Effects of 3 different montages of tDCS were pooled and compared to sham tDCS. Stimulation of different brain regions might have different impact on falling post stroke.
Background
Description of the condition
Falls are one of the most common secondary complications after stroke (Davenport 1996; Langhorne 2000). A study including fall events early after stroke showed an incidence of 7% in the first week after stroke onset (Indredavik 2008). Incidence figures from studies collecting data between one and six months post stroke vary from 25% to 37% (Indredavik 2008 and Kerse 2008 respectively). Studies evaluating participants between six and 12 months after stroke report incidences from 40% to 50% (Belgen 2006 and Harris 2005 respectively). One year after stroke, the reported incidence ranges from 55% to 73% (Ashburn 2008 and Sackley 2008 respectively). Not all falls are serious enough to require medical attention, but non‐serious falls are a known predictor for future falls, and can lead to fear of falling and may restrict a person's activities of daily living (Andersson 2008). In summary, serious and non‐serious falls are still among the most common complications after stroke and their increasing incidence poses a challenge for rehabilitation.
Many stroke‐related impairments contribute to deficits of balance and falls, e.g. muscle weakness, sensory loss, reduced attention, and abnormalities of vision and spatial awareness (Weerdesteyn 2008). A fall is a strong predictor of further falls among people with stroke living in the community. However, all people with residual difficulties following a stroke should be considered at increased risk (Ashburn 2008).
Description of the intervention
Few studies have examined fall prevention post stroke, but interventions recommended for the general elderly population who have experienced falls have been reported. Recent evidence outlines the efficacy of different types of interventions to prevent falls in the elderly population (Grossman 2018; Tricco 2017), which mainly comprise multifactorial and exercise interventions. At present, evidence regarding exercise interventions as well as other interventions to tackle falling post stroke is increasing; these interventions presumably work by impacting risk factors for falling post stroke. Various risk factors have already been identified, in particular lack of balance and mobility, and fear of falling (Landers 2016; Maeda 2015; Xu 2018). Rehabilitation might benefit from interventions improving these risk factors. For example, preliminary evidence post stroke shows that various exercise programmes are beneficial for improving balance, fear of falling, and mobility (English 2017; Jung 2015; Van Duijnhoven 2016), and could therefore positively impact on fall occurrence. Additionally, technological advances in assistive devices, such as ankle‐foot orthoses, walking aids (Kuan 1999), and functional electrical stimulation (Burridge 2007), have been suggested to improve mobility. Finally there is increasing interest in the use of rehabilitation technology such as virtual reality and robotics for addressing balance and gait (Laver 2017; Morone 2016), the impairment of which are risk factors for falls.
This review did not focus particularly on the different factors mediating fall occurrence, but investigated the effect of interventions on falling regardless of which risk factors they impacted. Studies mainly included an exercise intervention. Furthermore, literature reports that physical fitness training is a cost‐effective intervention (Collins 2018), with an incremental cost of GBP 2343 per quality‐adjusted life year (QALY) being acceptable according to the National Institute for Health and Care Excellence (NICE) guidelines (NICE 2013). The former statements combined with a fracture risk reducing effect (Eng 2008) outline the multi‐level benefits of exercises, and its potential to decrease falls.
Why it is important to do this review
A summary of the evidence is important for informing evidence‐based practice, and to identify gaps in research. There are existing reviews on the prevention of falls for older people (Cameron 2018; Grossman 2018; Sherrington 2019; Tricco 2017). However, a stroke is a serious condition leading to altered physical, cognitive and psychological impairments specifically related to the problem of falls in this population. In addition, persistent impairments in the later stages after stroke can contribute to an increasing incidence of falls in people after stroke. Since it has been shown that a significant proportion of the total cost of stroke originates from admission to nursing homes and hospitalisation (Demaerschalk 2010), which Lin 2017 found to be increased in fallers compared to non‐fallers, falling results in an increased economic burden. Moreover, from a biopsychosocial point of view, Faes 2010 found that falling is associated with the development of fear of falling resulting in social withdrawal. Interestingly, this fear of falling seems to extend to family caregivers.
Our original Cochrane Review summarised research that investigated the effect of fall prevention interventions in the stroke population and found no significant reduction of falls with exercise interventions, despite a strong fall‐reducing tendency in the chronic phase (Verheyden 2013). The review authors included evidence up to November 2012 and found insufficient evidence that administration of exercise reduces falling in people after stroke. Further studies reporting about the effects of fall prevention after stroke have since been published. Hence, an update of the literature is warranted to provide a coherent understanding of the latest evidence.
Objectives
To evaluate the effectiveness of interventions aimed at preventing falls in people after stroke. Our primary objective was to determine the effect of interventions on the rate of falls (number of falls per person‐year) and the number of fallers. Our secondary objectives were to determine the effects of interventions aimed at preventing falls on 1) the number of fall‐related fractures, 2) the number of fall‐related hospital admissions, 3) near‐fall events, 4) economic evaluation, 5) quality of life, and 6) adverse effects of the interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included controlled trials where participants or clusters were randomly allocated. If cross‐over trials had met our inclusion criteria, we would have included the first phase if the order of assignment was determined randomly.
Types of participants
We included trials with adult participants (over 18 years of age) in the hyperacute, acute, early subacute, late subacute or chronic phase following stroke with a confirmed diagnosis. Diagnosis of stroke comprised ischaemic as well as haemorrhagic events.
We classified people according to the phase post stroke (Bernhardt 2017). The hyperacute phase was within 24 hours post stroke. The acute phase comprised people one to seven days after stroke. The subacute phase was divided into the early subacute phase (one week to three months) and late subacute phase (three to six months). Finally, people in the chronic phase after stroke were those who had suffered a stroke more than six months previously.
We included trials reporting an intervention carried out in a mixed sample of participants, including people after stroke, if data were provided separately (i.e. in a subgroup) for people after stroke.
Types of interventions
We included any intervention where a stated primary or secondary aim was to prevent falls. We classified the interventions according to the taxonomy developed by the Prevention of Falls Network Europe (ProFaNE) (Lamb 2007; Lamb 2011), which proposes the following categories.
Exercises (supervised/unsupervised) including: gait, balance and functional training; strength/resistance exercises; flexibility exercises (e.g. yoga); 3D training (e.g. Tai Chi, Qi Gong); general physical activity; endurance training or others.
Medication (drug target): direct action targeted to specific classes of drugs including: antihypertensives; other cardiovascular agents; vitamin D; calcium; other bone health medication; drugs used in diabetes; anti‐Parkinson drugs; anti‐dementia drugs; antidepressants; antipsychotic/neuroleptic drugs; anxiolytics, hypnotics and sedatives; other central nervous system drugs; urinary antispasmodics; or other specified drugs.
Surgery including: cataract extraction; pacemaker provision; podiatric surgery or intervention; or others.
Management of urinary incontinence (e.g. assisted toileting, bladder retraining).
Fluid or nutrition therapy where the basic objective was to restore the volume and composition of body fluids to normal with respect to water‒electrolyte balance (fluid therapy) or to improve the health status of the individual by adjusting the quantities, qualities, and methods of nutrient intake (nutrition therapy).
Psychological intervention, either individual or in a group, including cognitive (behavioural) intervention, or others.
-
Environment/assistive technology, which includes technical aids for people with disabilities.
Environment (furnishings and adaptations to homes and other premises): direct action including dwelling unit indoors (including entrances); dwelling unit outdoors; public outdoor (e.g. pavement); or relocation.
Environment (aids for personal mobility such as walking aid; wheelchair).
Environment (aids for communication, information and signalling): including optical aids; hearing aids; aids for signalling and indicating; or alarm systems.
Environment (body‐worn aids for personal care and protection) including: body‐worn protective aids; clothes and shoes; or others.
Environmental (social environment) including: staff ratio; staff training; service model change; telephone support; caregiver training; homecare services; or others.
Knowledge interventions including: written material; videos; lectures; or others.
Other interventions/procedures.
We classified interventions into single interventions with one component; multiple interventions with more than one component, but the intervention was the same for all participants; and multifactorial interventions with more than one component and the intervention modified for every participant personally (Lamb 2007).
We compared the intervention for preventing falls with no additional treatment (routine care) or with another type of intervention.
Types of outcome measures
We included only those trials that reported an outcome measure related to the rate of falls or the number of fallers. We included trials where falls were collected either prospectively or retrospectively. We expected to find different definitions of a fall, although a consensus report recommends that a fall should be defined as "an unexpected event in which the participants come to rest on the ground, floor, or lower level." (Lamb 2005).
Primary outcomes
Rate of falls: defined as the ratio between number of fall events in a group and the group sample size (i.e. number of fall events in a group/group sample size). This ratio divided by the length of follow‐up (in years) yielded a standardised measure for fall occurrence (falls per person‐year).
Number of fallers: number of people who fell at least once during the study.
Secondary outcomes
Number of people sustaining fall‐related fractures.
Number of people with fall‐related hospital admissions.
Number of people with near‐fall events (typically defined as an occasion on which a person felt that they were about to fall, but did not actually fall) (Stack 1999).
Economic evaluation.
Quality of life (including psychological aspects such as fear of falling).
Adverse events.
Search methods for identification of studies
See the methods for the Cochrane Stroke Group Specialised register. We searched for trials in all languages and arranged the translation of relevant papers where necessary. We did not include studies published only in abstract form.
Electronic searches
We searched the trials registers of the Cochrane Stroke Group (last searched 3 September 2018) and the Cochrane Bone, Joint and Muscle Trauma Group (last searched October 2018). In addition we searched: the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 9) in the Cochrane Library (Appendix 1); MEDLINE (1950 to 3 September 2018) (Appendix 2); Embase (1980 to 3 September 2018) (Appendix 3); CINAHL (1982 to 3 September 2018) (Appendix 4); PsycINFO (1806 to August 2018) (Appendix 5); AMED (1985 to December 2017) (Appendix 6); and Physiotherapy Evidence Database (PEDro) (www.pedro.org.au) (3 September 2018) (Appendix 7).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist and adapted it for the other databases.
In June 2018, we also searched the following ongoing trials registers (Appendix 8).
ClinicalTrials.gov (www.clinicaltrials.gov);
International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch);
ISRCTN Registry (www.isrctn.com);
Stroke Trials Registry (www.strokecenter.org/trials).
Searching other resources
In an effort to identify further published, ongoing and planned trials we:
checked reference lists of relevant articles;
used Science Citation Index Cited Reference Search for forward tracking of important articles;
contacted original authors and trialists for clarification and further data if trial reports were missing or unclear (Appendix 9).
Data collection and analysis
Selection of studies
For this update, two review authors (SD and WS) independently screened the titles, abstracts and descriptors of the records obtained from the electronic searches and excluded obviously irrelevant studies. We obtained the full text of the remaining studies and independently assessed these for inclusion based on the review eligibility criteria. We resolved disagreements through discussion and with a third review author (GV), and contacted study authors for additional information where required.
Data extraction and management
For this update, two review authors (SD and WS) independently extracted data onto a pre‐tested data extraction sheet. We resolved disagreements through discussion, together with the statistical expert (RP).
Assessment of risk of bias in included studies
Two review authors (SD and WS) independently assessed risk of bias for this update for the following items of each included trial (Higgins 2017): sequence generation (randomisation); allocation concealment; blinding of assessors (for falls); incomplete outcome data; and selective outcome reporting. We included one additional risk of bias item: reliable ascertainment of fall/fallers outcome where 'low risk of bias' means ascertainment of outcome via active registration, e.g. falls diary; 'high risk of bias' if ascertainment relied on participants' recall over a longer period of time (more than one month); and 'unclear risk of bias' if ascertainment relied on participants' recall over a short period of time (one month or less) or if method of ascertainment was not described.
We collected this information on the data extraction sheet and resolved disagreements through discussion.
Measures of treatment effect
Primary outcomes
We used results reported at one year if these were available for trials that monitored falls for longer than one year, and carried out separate analyses pooling information on rate of falls, and risk of falling once or more within a year: treatment effects were measured with the rate ratio and relative risk respectively, following the analyses carried out by Gillespie 2012.
Rate of falls: when the rate ratio and its confidence interval (CI) were presented in the report of a trial, we included them directly in our meta‐analysis. When they were not presented, we calculated rate ratios and their standard errors (SE) based on the number of falls (or mean number of falls) divided by total follow‐up assuming that all participants had the nominal amount of follow‐up, with SEs calculated according to the formula in section 9.4.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Number of fallers: when the risk ratio or the risk difference were reported, we included them directly in our meta‐analysis. Otherwise, we calculated the risk ratio by entering the number of people who fell at least once and the total sample size of the intervention and control condition in Cochrane's meta‐analysis software, Review Manager 5 (Review Manager 2014).
If the required data to calculate either rate of falls or number of fallers were not reported, we contacted the researcher group as outlined in Dealing with missing data in the Methods section.
We used the generic inverse variance method for pooling rate and risk ratios, which we entered according to the information available in the source papers, and we set the software to display results in the original scale. We obtained standard errors of the logarithm of the intervention effect using the method described in section 7.7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions when a properly estimated confidence interval for the intervention effect was presented in the study report (Higgins 2011).
We included unadjusted intervention effects if they were available; otherwise, we considered incorporating adjusted estimates of effect, or calculated estimates of unadjusted effects depending on the validity of obtaining estimates from the information presented in the source report (see Unit of analysis issues). Where necessary, we calculated rate ratio estimates of treatment effect using the method described in section 9.4.8 and calculated risk ratios using the methods described in section 9.2.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The above analysis was based on the one carried out in the Cochrane Review of interventions for preventing falls in older people living in the community (Gillespie 2012), and so we anticipated that the same analysis would be appropriate when restricted to studies in people after stroke.
Secondary outcomes
For our secondary outcomes (number of people sustaining fall‐related fractures, number of people with fall‐related hospital admissions, number of people with near‐fall events, economic evaluation, quality of life, and adverse events), we expected limited and heterogeneous results throughout the included studies. We therefore provide a narrative description of these results.
Unit of analysis issues
We planned to incorporate any cluster‐randomised trials that we found according to the advice in section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and any cross‐over trials according to section 16.4. We used the strategy described in section 7.2.2 of the Cochrane Handbook for Systematic Reviews of Interventions to identify multiple publications of the same trial, and included only the main/first‐reported publication (Higgins 2011).
Dealing with missing data
We contacted study authors to acquire missing data. An email contact template is provided in Appendix 10. We planned a sensitivity analysis in which studies with missing data would have been excluded but we were unable to perform this analysis because of the limited number of included studies.
Assessment of heterogeneity
We assessed heterogeneity visually by means of forest plots and by reporting the I² statistic (Higgins 2003), as described in section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Using this section, we used the following interpretation of the I² statistic.
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
As our search resulted in heterogeneous trials that provided information which could be pooled, we conducted a random‐effects meta‐analysis incorporating random heterogeneity in intervention effect across studies into the standard error of the effect size, so that our findings can be generalised more widely.
Assessment of reporting biases
We discuss possible problems in the Discussion section of our review. We minimised reporting bias by using a comprehensive search strategy, by searching for studies in languages other than English, and by searching the grey literature (see Searching other resources).
Data synthesis
Since the studies we found were of a heterogeneous nature, we performed random‐effects meta‐analyses in all cases. We pooled results from comparable single, multiple and multifactorial interventions as defined in the Types of interventions section above and as presented in the Results section below.
GRADE and 'Summary of findings' table
We created 'Summary of findings' tables for the following outcomes.
Rate of falls
Number of fallers
We used the eight GRADE considerations (study limitations, consistency of the effect, imprecision, indirectness, publication bias, large effect, plausible confounding which would change the effect, and dose response gradient) to assess the body of evidence included in our meta‐analyses (Atkins 2004). We created 'Summary of findings' tables using GRADEpro GDT, and used the Cochrane Handbook for Systematic Reviews of Interventions as a guide to assign qualities of evidence and to justify our decisions regarding downgrading the quality level, added as footnotes below the tables (Higgins 2011 chapter 12.2).
Subgroup analysis and investigation of heterogeneity
We carried out analyses of subgroups of studies (in the (hyper)acute, early/late subacute, and chronic phase after stroke) in an attempt to explain heterogeneity by study characteristics. We were unable to explore the effect of prospective/retrospective data collection or the different forms of data ascertainment, described in the Assessment of risk of bias in included studies section, due to the limited number of included studies.
Sensitivity analysis
In this update, we performed a sensitivity analysis by initially combining low‐bias studies (low risk on all items of the risk of bias assessment, apart from the 'Blinding of outcome assessment' subsection which is consistently at high risk due to the nature of fall assessment), and subsequently adding in the unclear and high‐bias studies to check for noticeable changes in the results.
As we found studies that comprised single, multiple, and multifactorial interventions, we conducted a sensitivity analysis by omitting multiple and multifactorial interventions from the pooled single interventions.
We also performed a sensitivity analysis for phase post stroke, including only studies that ascertained the phase post stroke of their participants. Since stroke recovery is typically more pronounced in the early phases post stroke, we would expect fall‐prevention interventions to be more effective in the earlier phases post stroke compared to the chronic phase (Wagner 2009). We considered the phase post stroke to be ascertained if no ambiguity regarding classification of the included stroke population was present. For example, merely reporting on mean and standard deviation was not sufficient to draw this conclusion.
Results
Description of studies
Results of the search
2013 version
The search strategy of the original review identified 5702 records. Removal of duplicates resulted in 4138 records for initial screening. We obtained a total of 32 full‐text papers for further screening.
2018 version
For the 2018 update of this review, we used the same search strategy to identify studies from 2012 until present. The updated search identified 3618 records. Removal of duplicates resulted in an additional 3272 unique records for initial screening, of which we obtained 26 for full‐text screening.
We present the study flow diagram of the results of our searches in Figure 1.
1.

Study flow diagram.
Notational remark: n is consistently used to denote the sample size in comparisons, whereas N is used to indicate the occurrence of a certain event (e.g. adverse events).
Included studies
After our updated searches, we included in total 14 studies with 1358 participants, of which six were new studies. Details of the included studies can be found in the Characteristics of included studies table, and are summarised below.
All studies were individually randomised controlled trials. We did not retrieve any cluster‐randomised controlled trials or the first phase of any cross‐over trials.
The included studies enrolled between 34 and 170 participants (Holmgren 2010 and Green 2002 respectively), with a median sample size of 91 participants. Age (mean (SD)) of the participants for the experimental and control groups ranged from 57 (11) years (for both groups) in Lau 2012 to 78 (8) years and 79 (8) years respectively in Holmgren 2010. For Lau 2012, the mean age of the participants was under 60 years; seven studies had a mean age between 60 and 69 years (Ada 2013; Andrade 2017; Dean 2012; Mansfield 2018; Marigold 2005; Morone 2016; Taylor‐Piliae 2014); and in the remaining six studies the mean age was 70 years or older.
The studies were carried out in eight different countries: five in Australia (Ada 2013; Batchelor 2012; Dean 2010; Dean 2012; Haran 2010), two in Canada (Mansfield 2018; Marigold 2005), two in the UK (Drummond 2012; Green 2002), and one each in Brazil (Andrade 2017), Italy (Morone 2016), Hong Kong (Lau 2012), Sweden (Holmgren 2010), and the USA (Taylor‐Piliae 2014).
Regarding phases post stroke, both time post stroke and intervention duration were determinants of phase categorization of the included studies. However, we only assigned phases to studies that assured time post stroke of their participants by reporting a range or by using an inclusion criterion regarding time post stroke which assured a correct phase categorization. Four studies recruited people in the chronic phase after stroke (Green 2002; Lau 2012; Mansfield 2018; Marigold 2005). Dean 2012 recruited people in the early and late subacute and chronic phase post stroke, and Holmgren 2010 included people in the late subacute and chronic phase after stroke.
The remaining eight studies were not assigned a phase since there was no specific report on participants' time post stroke, the inclusion criterion spanned multiple phases post stroke, or data comprised solely mean time post stroke with standard deviation.
In 10 studies, people living in the community or receiving outpatient rehabilitation services, or both, were included (Ada 2013; Batchelor 2012; Dean 2012; Green 2002; Haran 2010; Holmgren 2010; Lau 2012; Mansfield 2018; Marigold 2005; Taylor‐Piliae 2014). Four studies carried out their interventions in an institutional or hospital setting (Andrade 2017; Dean 2010; Drummond 2012; Morone 2016).
All studies included both men and women. On average across all studies, 60% of participants consisted of men, ranging from 35% in Haran 2010 to 71% in Lau 2012.
Eight studies evaluated the effect of exercises on falls (Ada 2013; Dean 2010: Dean 2012; Green 2002; Lau 2012; Marigold 2005; Mansfield 2018: Taylor‐Piliae 2014). Ada 2013 compared a combination of treadmill and overground walking with no intervention. Dean 2010 compared treadmill with overground walking. Dean 2012 investigated the WEBB programme, involving task‐related training with progressive balance and strengthening exercises as well as walking and stair climbing in comparison with an exercise class for the upper limb. Green 2002 compared community physiotherapy with no intervention. Lau 2012 examined whole‐body vibration in comparison with the same exercises without vibration. Marigold 2005 compared agility training with stretching and weight‐shifting exercises. Mansfield 2018 investigated perturbation training compared to a traditional balance training programme. Taylor‐Piliae 2014 carried out two comparisons: both Tai Chi and a fitness programme were compared with usual care.
Three studies used interventions that were classified in the ProFaNE taxonomy under environment/assistive technology (Drummond 2012; Haran 2010; Morone 2016). Drummond 2012 considered the effect of predischarge assessment by means of a home visit compared to an assessment conducted in a hospital setting (social environment). Haran 2010 examined the effect of single lens distance vision glasses instead of multifocal glasses (aids for communication, information and signalling). Morone 2016 compared the use of a servo‐assistive rollator with conventional walking‐oriented therapy (aids for personal mobility).
Andrade 2017 used an intervention that was classified in the ProFaNE taxonomy under 'Other interventions/procedures', which considered the effect of active repeated transcranial direct current stimulation (tDCS) compared to sham repeated tDCS. Holmgren 2010 evaluated the effect of a multiple intervention that largely consisted of individualised and home‐based exercises. Finally, Batchelor 2012 examined the effect of a multifactorial intervention that also partly consisted of an individualised home exercise programme but included a comprehensive risk assessment and referral for a wide range of risk factors. For further details of the interventions provided, see Characteristics of included studies.
In two studies, the control group did not receive any intervention (Ada 2013; Green 2002). In two studies, the control group did not receive an additional treatment (usual care only) (Batchelor 2012; Haran 2010). In all but two other studies, the control group received the same amount of therapy but another type of treatment. In the case of Holmgren 2010, the treatment for the control group was not dose‐matched to that of the intervention group; and Taylor‐Piliae 2014 only provided resources, written material, and phone calls for the control condition. For further details of the content of the control group, see the Characteristics of included studies table.
Regarding fall data registration, four studies recorded falls during the intervention period (Batchelor 2012; Dean 2010; Dean 2012; Taylor‐Piliae 2014), three studies recorded falls during a follow‐up period (Lau 2012; Mansfield 2018; Morone 2016), and seven studies recorded falls in both the intervention and follow‐up period (Ada 2013; Andrade 2017; Drummond 2012; Green 2002; Haran 2010; Holmgren 2010; Marigold 2005). The fall registration time ranged from one to 13 months (mean (SD): 8.07 (4.12) months).
Finally, 11 studies were registered in an online database.
Excluded studies
From the 58 full‐text papers that we screened, we excluded 36 studies (see the Characteristics of excluded studies table). We excluded most of the studies (18 out of 36) because falls were collected as a measure of an adverse event: these studies did not therefore have the aim of preventing falls, although they did report them. We excluded four studies for not being randomised controlled trials (Calugi 2016; Gervasoni 2017; Goljar 2016; Mansfield 2017). We excluded Barreca 2004 because the study was not truly randomised; Dai 2013 because they used an inappropriate definition of a fall; Eng 2010 because it was a narrative review; Halvarsson 2011 because the subgroup of people with stroke consisted of only four participants; Johansson 2018 because no stroke subgroup was defined in the full‐text article; and Mayo 1994 because the author was unable to provide us with details and data for the stroke subgroup. For this updated version, we additionally excluded two studies that were included in the 2013 version of this review: Sato 2005a because the publication was retracted; and Sato 2011 because its validity has been questioned. Furthermore, we excluded five studies that awaited classification in the 2013 version: three studies because they were conducted by the same author of the retracted article (Sato 2003; Sato 2005b; Sato 2005c); and two studies because we did not receive additional data needed for classification at present (Cheng 2001; Rosendahl 2008).
Our search did not identify any ongoing trials.
We have insufficient data on number of fallers and unsuitable data (median and interquartile range) to allow calculation of rate of falls from one study (Pedreira 2017); (see the Characteristics of studies awaiting classification table). Since efforts to obtain these data have not yet been fruitful, this study awaits classification.
Risk of bias in included studies
For five out of six items of our 'Risk of bias' assessment, the majority of our included studies scored as having low risk of bias. Only for blinding of outcome assessment (detection bias) did we score the majority of the included studies as having a high risk of bias. Details of 'Risk of bias' assessment for each study are shown in the Characteristics of included studies table. Summary results are shown in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We assessed risk of bias for both random sequence generation and allocation concealment as low in 12 studies (85.7%) and unclear in the remaining two studies (14.3%) (Figure 3).
Blinding
With participant recall or active registration of falls by the participants themselves through a falls calendar or diary, we assessed the risk of bias for blinding as high for all but two included studies (85.7%) (Andrade 2017: low risk, 7.15%; Drummond 2012: unclear risk, 7.15%) (Figure 3).
Incomplete outcome data
We scored the risk of bias for incomplete outcome data addressed as low for all included studies (Figure 3).
Selective reporting
We assessed reporting bias as low in 12 studies (85.7%) and high in the remaining two studies (14.3%) (Figure 3).
Other potential sources of bias
We also assessed whether the falls/fallers outcome was ascertained reliably. For this item, we scored the risk of bias as low for nine studies (64.3%), high for two studies (14.3%) and unclear for the remaining three studies (21.4%) (Figure 3). Of the nine studies that scored low, eight studies used a falls calendar that had to be returned after 10 days, one month, or two months. Six of these studies reminded the participants, if necessary, to fill in these calendars after two weeks (one study) or monthly (five studies). The remaining study used a weekly fall interview. The two studies that scored high used retrospective recall of six months and three months respectively (Dean 2010; Green 2002). We assessed Holmgren 2010, Drummond 2012, and Morone 2016 as being at unclear risk. In Holmgren 2010, it was not apparent whether the falls calendar that they used for the six‐month follow‐up had to be returned monthly, three‐monthly, or after six months, and if there were any follow‐up telephone calls. In Drummond 2012, there was no report on how falls were measured. Finally, Morone 2016 did not report the method of falls self‐registration in the six‐month follow‐up period in which falls were recorded.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Exercises
Our searches identified eight studies that evaluated the effect of exercises on falls (Ada 2013; Dean 2010; Dean 2012; Green 2002; Lau 2012; Mansfield 2018; Marigold 2005; Taylor‐Piliae 2014). As we also identified one multiple interventions trial (Holmgren 2010), and one multifactorial trial (Batchelor 2012), where the intervention largely consisted of an exercise component, we decided to include these two studies under the heading 'Exercises' and combine them with those examining the effect of exercises as a single intervention. Ada 2013 and Taylor‐Piliae 2014 included two treatment groups in their studies. Comparison of two intervention conditions was not the aim of this review (this method is described in section 9.3.9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and in Introduction to Meta‐Analysis 2009)), so we decided to combine the effect sizes of the two intervention conditions in Ada 2013 and Taylor‐Piliae 2014 according to the method described in Introduction to Meta‐Analysis 2009, chapter 25, pages 239‐41. Since Ada 2013 used two 'exact same treatment' groups which differed solely based on intervention time (two and four months), we calculated the mean effect of the intervention groups at two months and compared it with the control group at two months. Both a summary of results and the quality of the evidence regarding the exercises analysis are provided in the Table 1.
Rate of falls
Data
We obtained rate ratio and CI from Batchelor 2012, Dean 2012 and Mansfield 2018. We calculated rate ratio and SE (Measures of treatment effect) in Ada 2013, Dean 2010, Lau 2012, Marigold 2005, and Taylor‐Piliae 2014. We obtained no data on rate of falls from Green 2002 or Holmgren 2010.
Analyses
We pooled the results of eight studies including 765 participants (Ada 2013; Batchelor 2012; Dean 2010; Dean 2012; Lau 2012; Mansfield 2018; Marigold 2005; Taylor‐Piliae 2014), giving a significant reduction in the rate of falls (rate ratio 0.72, 95% CI 0.54 to 0.94, low GRADE evidence) for the experimental group (Analysis 1.1). We were unable to include other trials that included exercises in this analysis due to the lack of stroke‐specific information on falls (Green 2002; Holmgren 2010). The I² statistic (43%) revealed a potential moderate heterogeneity.
1.1. Analysis.
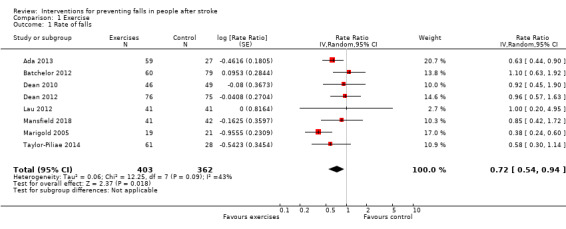
Comparison 1 Exercise, Outcome 1 Rate of falls.
When omitting the multifactorial study by Batchelor 2012, our sensitivity analysis of single interventions resulted in a significant reduction of rate of falls (rate ratio 0.66, 95% CI 0.50 to 0.87, n = 626). Furthermore, we observed a reduction in the I² statistic (34%), reducing the heterogeneity risk to 'might not be important'.
A sensitivity analysis including studies with participants in the chronic phase post stroke found no significant reduction in rate of falls (rate ratio 0.58, 95% CI 0.31 to 1.12, n = 205). The risk for heterogeneity (I² = 52%) remained moderate. We conducted no phase‐related sensitivity analysis for Dean 2010 and Holmgren 2010 since they recruited participants in multiple phases post stroke.
A final sensitivity analysis including solely studies at low risk of bias revealed no significant between‐group difference (rate ratio 0.88, 95% CI 0.65 to 1.20, n = 462), with a reduction in the I² statistic to 0%, indicating absence of heterogeneity (Batchelor 2012; Dean 2012; Mansfield 2018; Taylor‐Piliae 2014). However, after adding in the Ada 2013 study, which is at unclear risk of bias due to an unknown concealment method, the overall finding switches to a significant reduction in rate of falls in favour of the intervention group (rate ratio 0.77, 95% CI 0.61 to 0.98, n = 548). The I² statistic increased to 5%.
Number of fallers
Data
Data for this section were based on the risk difference reported by Dean 2010, the risk ratio reported by Batchelor 2012 and Dean 2012, and the risk ratio calculated (Measures of treatment effect) by the review authors in Ada 2013, Green 2002, Holmgren 2010, Lau 2012, Mansfield 2018, Marigold 2005 and Taylor‐Piliae 2014.
Analyses
We pooled the results of 10 studies with a total of 969 participants (Ada 2013; Batchelor 2012; Dean 2010; Dean 2012; Green 2002; Holmgren 2010; Lau 2012; Mansfield 2018; Marigold 2005; Taylor‐Piliae 2014) (Analysis 1.2). This demonstrated no significant reduction in the number of fallers (risk ratio 1.03, 95% CI 0.90 to 1.19, very low GRADE evidence).
1.2. Analysis.

Comparison 1 Exercise, Outcome 2 Number of fallers.
When omitting the multiple study by Holmgren 2010 and the multifactorial study by Batchelor 2012, our sensitivity analysis of single interventions yielded no significant reduction in the number of fallers (risk ratio 1.09, 95% CI 0.93 to 1.28, n = 796).
A sensitivity analysis including studies with participants in the chronic phase post stroke yielded no significant reduction in number of fallers (risk ratio 0.94, 95% CI 0.73 to 1.22, n = 375). We conducted no phase‐related sensitivity analysis for Dean 2010 and Holmgren 2010 since they recruited participants in multiple phases post stroke.
A final sensitivity analysis including solely studies at low risk of bias did not alter the non‐significant finding related to number of falls (risk ratio 0.96, 95% CI 0.77 to 1.21, n = 462) (Batchelor 2012; Dean 2012; Mansfield 2018; Taylor‐Piliae 2014).
All analyses regarding number of fallers resulted in a risk of heterogeneity that 'might not be important' (I² ranged from 0% in the original analysis to 26% in the sensitivity analysis with low risk of bias studies).
Number of people sustaining fall‐related fractures
Four studies reported on participants sustaining fall‐related fractures. Dean 2012 indicated that one person had a stroke, fractured his shoulder and died in hospital. Lau 2012 reported that none of the falls resulted in any injuries that required medical attention. In Mansfield 2018, no falls resulted in fractures. One person in the agility group in Marigold 2005 sustained a hip fracture, but on a task that was included in both programmes.
Taylor‐Piliae 2014 reported a total of 29 falls that resulted in an injury, of which 8% were evaluated by a health care provider. They did not specify if these injuries involved fractures.
Number of people with fall‐related hospital admissions
No study reported on fall‐related hospital admissions, but three studies reported about the number of falls resulting in a need for medical attention. Lau 2012 indicated that none of the falls resulted in any injuries that required medical attention. Mansfield 2018 and Taylor‐Piliae 2014 reported a total of three and 10 cases, respectively, where participants sought medical attention because of their fall.
Number of people with near‐fall events
We calculated a non‐significant near‐fall rate ratio of 1.11 (95% CI 0.70 to 1.75, n = 89) (see Measures of treatment effect) based on data reported in Taylor‐Piliae 2014.
Economic evaluation
No study that investigated the effect of exercises reported an economic evaluation.
Quality of life
We summarise findings of studies related to quality of life in the table below. Fourteen outcome measures were used across 14 studies, with the vast majority reporting no significant difference between the exercise and control condition. Due to the use of a wide variety of outcome measures, we decided not to pool data within a meta‐analysis, but rather to describe results narratively.
| QOL Measure | Number of studies | Studies | Significant finding? |
| Adelaide Activities Profile | 3 |
Ada 2013 Dean 2010 Dean 2012 |
Dean 2012
|
| SF‐12 / SF‐36 | 3 |
Dean 2012 Holmgren 2010 Taylor‐Piliae 2014 |
Holmgren 2010: favouring intervention at 3 months
|
| Activities‐specific Balance Confidence scale | 3 |
Lau 2012 Mansfield 2018 Marigold 2005 |
No |
| Falls Efficacy Scale (Swedish/International) | 2 |
Batchelor 2012 Holmgren 2010 |
Holmgren 2010: favouring intervention post intervention and at 3 months' follow‐up |
| Frenchay Activities Index | 2 |
Green 2002 Holmgren 2010 |
No |
| Subjective Index of Physical and Social Outcome | 1 | Mansfield 2018 | Mansfield 2018: favouring control at 6, 8, 10 and 12 months' follow‐up |
| EuroQol EQ‐5D‐3L | 1 | Ada 2013 | No |
| Walking Self‐Efficacy Scale | 1 | Ada 2013 | No |
| Hospital Anxiety and Depression Scale | 1 | Green 2002 | No |
| General Health Questionnaire 28 | 1 | Green 2002 | No |
| Physical Activity Scale for Individuals with Physical Disabilities | 1 | Mansfield 2018 | No |
| Nottingham Health Profile | 1 | Marigold 2005 | No |
| Center for Epidemiologic Studies Depression Scale | 1 | Taylor‐Piliae 2014 | No |
| Pittsburgh Sleep Quality Index | 1 | Taylor‐Piliae 2014 | No |
Adverse events
Three trials reported specifically on adverse events. Dean 2012 indicated that no falls or other adverse events occurred during the exercise classes, home programme or assessments. Only one participant withdrew because of the intervention, stating that the exercises had exacerbated an incontinence problem. In Lau 2012, no severe adverse events were reported by the participants, although three indicated mild dizziness during whole‐body vibration therapy, and four had lower‐limb soreness and fatigue (two from the whole‐body vibration group). The study authors reported that all symptoms gradually subsided after the first few sessions of training. Mansfield 2018 reported 48 adverse events: fatigue with training (N = 4), joint pain during or soon after training (N = 25), delayed onset muscle soreness (N = 13), seizure during training (N = 1), and abnormally elevated heart rate and low blood pressure during training (N = 1). Furthermore, four falls occurred related to the study procedure.
Environment/assistive technology
Three studies were classified in the environment/assistive technology section of the ProFaNe taxonomy (Drummond 2012; Haran 2010; Morone 2016). We calculated rate and risk ratios using the methods described in the Measures of treatment effect described in the Methods section.
Social environment
Drummond 2012, who investigated the efficacy of predischarge home visits in the subacute phase post stroke, was categorised in the subcategory of social environment. Both a summary of results and the quality of the evidence regarding the social environment analysis are provided in the Table 2.
Rate of falls
We found no significant reduction in the rate of falls when comparing predischarge assessment by means of a home visit to an assessment conducted in a hospital setting (rate ratio 0.85, 95% CI 0.43 to 1.69, n = 85, very low GRADE evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2 Environment: social environment, Outcome 1 Rate of falls.
Number of fallers
We did not find number of fallers to be significantly different between the two treatment groups (risk ratio 1.48, 95% CI 0.71 to 3.09, n = 85, very low GRADE evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2 Environment: social environment, Outcome 2 Number of fallers.
Economic evaluation
The mean (SD) total cost of a home visit was GBP 183 (GBP 81). The total cost of the control condition was not reported.
Quality of life
Mood as measured by the Stroke Aphasic Depression Questionnaire (10‐item hospital version) differed significantly in favour of the home visits group. We found no significant difference for health‐related quality of life (EQ‐5D).
No information regarding fall‐related fractures, fall‐related hospital admissions, near‐fall events, and adverse events was reported.
Aids for communication, information and signalling
For this section, results relating to vision improvement were based on unpublished data from the stroke subgroup in Haran 2010. Both a summary of results and the quality of the evidence regarding the aids for communication, information and signalling analysis are provided in the Table 3.
Rate of falls
There was no significant reduction in rate of falls when single lens distance vision glasses replaced multifocal glasses for people after stroke (rate ratio 1.08, 95% CI 0.52 to 2.25, n = 43, very low GRADE evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3 Environment: single lens distance glasses versus usual (multifocal) glasses, Outcome 1 Rate of falls.
Number of fallers
There was no significant reduction in the number of fallers when single lens distance vision glasses replaced multifocal glasses for people after stroke (risk ratio 0.74, 95% CI 0.47 to 1.18, n = 43, very low GRADE evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3 Environment: single lens distance glasses versus usual (multifocal) glasses, Outcome 2 Number of fallers.
Number of people sustaining fall‐related fractures
No one with stroke in the intervention group sustained a fracture, compared with one person in the control group.
Number of people with fall‐related hospital admissions
One person with stroke in the intervention group was admitted to hospital once, compared with one person with stroke in the control group admitted to hospital three times.
Quality of life
Data from the SF‐12 physical and mental component score and Falls Efficacy Scale ‒ International version showed no significant difference between groups.
No information regarding near‐fall events, economic evaluation, and adverse events was reported.
Aids for personal mobility
For this section we included Morone 2016, which investigated the effect of a servo‐assistive rollator (i‐walker) on falling post stroke. Both a summary of results and the quality of the evidence regarding the aids for personal mobility analysis are provided in the Table 4.
Rate of falls
No significant reduction was observed regarding the rate of falls between the i‐walker group and the control group (rate ratio 0.56, 95% CI 0.19 to 1.66, n = 42, very low GRADE evidence; Analysis 4.1).
4.1. Analysis.

Comparison 4 Environment: servo‐assistive rollator, Outcome 1 Rate of falls.
Number of fallers
There was no significant reduction in the number of fallers between the i‐walker group and the control group (risk ratio 0.44, 95% CI 0.16 to 1.22, n = 42, very low GRADE evidence; Analysis 4.2).
4.2. Analysis.
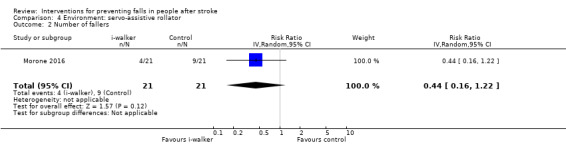
Comparison 4 Environment: servo‐assistive rollator, Outcome 2 Number of fallers.
No information regarding fall‐related fractures, fall‐related hospital admissions, near‐fall events, economic evaluation, quality of life, and adverse events was reported.
Other interventions: transcranial direct current stimulation (tDCS)
We could not classify Andrade 2017, which investigated the effect of different montages of transcranial direct current stimulation on falls post stroke, in any of the specified intervention classes; it was subsequently classified in the 'other interventions' section of the ProFaNe taxonomy. Both a summary of results and the quality of the evidence regarding the tDCS analysis are provided in the Table 5.
Rate of falls
We obtained no data about rate of falls.
Number of fallers
Combining effect sizes of all montages, we found a significant reduction in the number of fallers in favour of the intervention (active tDCS) group (risk ratio 0.30, 95% CI 0.14 to 0.63, n = 60, low GRADE evidence; Analysis 5.1). Furthermore, a significant reduction in the number of fallers was reported for all individual montages of tDCS compared to the sham tDCS condition. Despite a tendency of bilateral stimulation to be more effective in reducing fallers post stroke, we found no significant differences between the three different montages of tDCS. We calculated the risk ratio using the method described in Measures of treatment effect, part of the Methods section.
5.1. Analysis.

Comparison 5 Other interventions: tDCS, Outcome 1 Number of fallers.
Adverse events
No adverse events were reported.
No information regarding fall‐related fractures, fall‐related hospital admissions, near‐fall events, economic evaluation or quality of life was reported.
Discussion
This review focused on the effects of interventions for preventing falls in people after stroke, with secondary outcomes examining the number of people sustaining fall‐related fractures, the number of people with fall‐related hospital admissions, the number of people with near‐fall events, economic evaluation, quality of life (including psychological aspects such as fear of falling), and adverse events.
Summary of main results
Our search strategy resulted in 14 studies being included in this review update (of which six were new studies since publication of the original review) with a total of 1358 participants. Ten studies reported an exercise intervention, three studies an environmental adaptation (providing single lens glasses to users of multifocal glasses, predischarge home visits and a servo‐assistive rollator), one study a multiple intervention, one study a multifactorial intervention and one study transcranial direct current stimulation, which was classified under 'other interventions'. Since both the multiple and multifactorial intervention largely consisted of an exercise component, both studies were included under 'Exercises'.
Exercises
For rate of falls, based on pooled results from eight studies (765 participants) investigating exercises to prevent falling in people after stroke as well as our sensitivity analysis for single interventions (626 participants), we have low confidence that exercise may reduce the rate of falls in favour of the experimental group. Based on our pooled results, exercises resulted in a 28% reduction of the fall rate; and when considering exercises as a single intervention, a 34% reduction of fall rate was observed. Sensitivity analysis for studies conducted in the chronic phase after stroke (205 participants), however, showed little or no difference in the rate of falls. There was also little or no difference in the rate of falls when only studies at low risk of bias were included (462 participants).
For the number of fallers, based on pooled results from 10 studies (969 participants) and sensitivity analyses for single interventions (796 participants), chronic phase after stroke (375 participants), and low risk of bias (462 participants), we have very low confidence in the finding that there may be little or no difference in the number of fallers within the exercises group compared to controls. Overall, our findings suggest that exercises may result in a beneficial reduction in the number of times that fallers fall, but to date there is no clear evidence that exercises change fallers into non‐fallers. The certainty in the evidence (GRADE) for this analysis ranges from very low (number of fallers) to low (rate of falls).
Results for secondary outcome measures were sparse, with the exception of quality of life, but because of the heterogeneity of outcome measures used we decided not to pool these results. Studies assessing the effect of exercises on preventing falls included a total of 14 measures of quality of life. In four of these measures (Adelaide Activities Profile 'service to others' subscale, FES‐I, SF‐36 mental dimension and mental health subscales, and SIPSO), an improvement was reported in favour of the experimental group.
Environmental adaptations
Rate of falls or number of fallers did not appear to reduce when comparing the intervention to the control condition.
Social environment: predischarge home visits compared to predischarge interviews in the hospital (very low GRADE evidence, 85 participants). Mood was found to be increased more in the home visits group.
Vision improvement: provision of single lens distance vision glasses instead of multifocal glasses (very low GRADE evidence, 43 participants).
Aids for personal mobility: use of a servo‐assistive rollator compared to a conventional walking‐oriented therapy (very low GRADE evidence, 42 participants).
All analyses within the 'Environmental adaptations' section relied solely on one study.
Other interventions (transcranial direct current stimulation)
One study (60 participants) investigated the effect of different montages of tDCS compared to a sham tDCS condition. The combined effect size of all montages gives us low certainty that tDCS may reduce the number of fallers (low GRADE evidence).
Overall completeness and applicability of evidence
We were only able to include a limited number of trials with a limited number of participants. In comparison, the review evaluating interventions for preventing falls in older people living in the community included 159 trials with a total of 79,193 participants (Gillespie 2012). Even fewer of the trials presented data required to include them in our analysis of the rate of falls than in the analysis of the number of fallers. Lamb 2005 provided a consensus statement that both outcomes should be provided in trials reporting on interventions evaluating the prevention of falls, and that future trials should include the numbers of both falls and fallers when presenting their results.
Interpretation of results
In contrast to the findings of the original version of this review, exercises may reduce falling post stroke, based on a reduced rate of falls. Our results showed different results in rate of falls and number of fallers. Absence of data on rate of falls from Green 2002 and Holmgren 2010 may have resulted in different findings for the pooled analysis of rate of falls. Of interest are the results of Marigold 2005 and Ada 2013, which are the only trials individually demonstrating a reduction of rate of falls resulting from an intervention programme consisting of agility exercises and treadmill training, respectively. It should be noted that there is a difference for the analysis and subsequent result between Marigold 2005 and our review. In Marigold 2005, the number of falls and the number of fallers were analysed with a Mann‐Whitney U test and a Chi² test respectively, and showed no statistically significant between‐group difference. Based on the information from Marigold 2005, we were able to calculate the parameters of interest for inclusion into our meta‐analysis (see Analysis 1.1 and Analysis 1.2). Surprisingly, for the rate of falls this resulted in a statistically significant between‐group difference in favour of the agility programme (Analysis 1.1). For planning future trials and implementation in clinical practice, this trial seems to give an important message about the content of an intervention to prevent falls. Ada 2013 used two intervention conditions related to exercises, forcing us to pool the two intervention groups into one group to circumvent the problem of study multiplicity in the meta‐analysis. This merge might have caused an under‐ or overestimation of the true effect of the individual exercise programmes. However, in meta‐analyses a general pooling of related control interventions is suggested, therefore this potential bias is intrinsic to the analysis.
Andrade 2017 found tDCS to reduce falling post stroke. However, these results need to be interpreted with caution since the results were obtained from a single study with limited sample size, of which the power analysis was not conducted for the number of fallers. Interestingly, a recent meta‐analysis investigating the effect of tDCS on balance found little or no effects (Li 2018), which seems contrary to the results of Andrade 2017, since balance is an important predictor of falling post stroke (Ashburn 2008; Maeda 2015). Falls remain complex, however, for they are predicted by multiple factors (Ashburn 2008).
Results in Haran 2010 are only to be interpreted for post‐stroke patients who are regular wearers of multifocal glasses.
Regarding environmental adaptations to reduce falling post stroke, Drummond 2012 used in parallel to the randomised controlled trial a cohort study which included patients to whom the clinicians believed a home visit was essential. This has a clinical advantage, but might reduce statistical quality. Furthermore, they described procedural problems in administering the home visits, which were reported to be altered in a definitive trial. Finally, they also found the mean (SD) cost of a home visit to be GBP 183 (GBP 81), which might be an extra limitation for implementation in low‐income countries.
Our hypothesis that fall‐prevention strategies are more effective in earlier phases post stroke remains unconfirmed since we found too few studies with a population in that early rehabilitation post‐stroke phase. Future updates might be potent to answer this query.
In this update, we have used a broad categorisation of interventions to synthesise data, and have not further documented details of interventions. We note that variability is present regarding intervention type within our exercises' meta‐analyses. In our opinion the heterogeneity is substantial, and forms — in combination with the limited number of included studies — the main reason why we did not perform sensitivity analyses on the effect of type of exercise intervention. Exercise, furthermore, is an umbrella term covering a wide range of interventions, which should be described in detail in order to understand key elements such as dose, content and intensity. In a future update of this review, specific analyses of type of exercise intervention could be possible if both future exercise trials are conducted and trials provide adequate detail of exercises investigated. Future sensitivity analyses could investigate whether multiple or multifactorial exercise interventions are more beneficial than single‐type or focused exercise interventions, in order to understand the differential effect of type of exercises provided on falls.
Other remarks
It should be further noted that some trials reported interesting post hoc analyses. Marigold 2005 showed that for their participants with a history of falls, eight out of 15 continued to fall in the intervention group compared with 13 out of 15 in the control group (P = 0.05). Dean 2012 indicated fewer falls in the intervention group for their fast walkers but more falls in the intervention group for their slower walkers. Both studies contribute to the current belief that interventions should be developed for specific subgroups of people with stroke. Again, this information can contribute to the future development of interventions for preventing falls in people after stroke. Andrade 2017 found that the group receiving sham tDCS experienced a higher fall risk compared to the other participants (P < 0.01). This result — combined with their discovery of a bilateral tDCS montage to be associated with significantly higher Berg Balance Scale (BBS) and Falls Efficacy Scale ‒ International (FES‐I) scores compared to anodal and cathodal currents — might be of use when considering a broad scope on fall prevention, including measures for fall risk. It should be noted, however, that only people with high risk of falling were included in the trial. Additionally, no differences between montages were found on the Four Square Step Test (FSST) and Overall Stability Index (OSI), which were both used as outcome measures to quantify the risk of falling.
Holmgren 2010 used an intervention that was classified as multiple, since it comprised exercises, implementing exercises into real‐life situations and educational sessions. The trial found no significant reduction of number of fallers, but a significant increment in quality of life. In addition, the complex aetiology of falls might point towards a more holistic interventional approach, outlining the importance of this idea for planning future trials in this field.
We could neither perform meta‐analyses nor narrative description regarding the benefits of interventions targeted at reducing falling post stroke on our secondary outcomes, due to lack of reporting on these outcomes in the included studies. Future studies are advised to include these outcomes as part of the trial design.
Quality of the evidence
Fall assessment
A major concern arising from the trials included in this review is the definition of falls. Of the 16 trials, only nine provided a definition of a fall, and among these seven different definitions were used, with two trials presenting a definition not referenced to previous literature (Dean 2010; Taylor‐Piliae 2014). Although the content of these different definitions might not be significantly different, uniformity should be sought in future trials evaluating interventions for preventing falls in people after stroke, with a consensual definition of a fall, such as the one developed by the Preventions of Falls Network Europe (Lamb 2005).
Also relating to fall assessment, four of the included studies only collected data on falls during the intervention period. The disadvantage of this approach is that the presence of a latency of physiological changes might cause an overestimation in fall occurrence since benefits from exercises are not yet physically established (Lamb 2005). On the other hand, there may also be a decay of beneficial effects during follow‐up. These limitations strengthen our recommendation to follow the methodological gold standards of fall research presented by Lamb 2005.
Power calculation
For nine out of 14 trials, the primary aim was to prevent falls. For seven of the nine, a power calculation was performed based on establishing a reduction in falls/fallers. Andrade 2017 performed a power calculation to establish this on another primary outcome measure (Four Square Step Test (FSST)) and Taylor‐Piliae 2014 reported about a power calculation to determine their sample size but neither specified time point (a priori or post hoc) nor outcome measure used. The single aim of Holmgren 2010 was to prevent falls, but the power calculation was based on finding an increase in the Berg Balance Scale score, leading to a total of 34 participants recruited. The non‐significant finding for falls and fallers may have resulted from an inadequate sample size for this outcome. Unpublished stroke subgroup results from Haran 2010 should also be interpreted with caution; this analysis is underpowered, since a limited group of the total study sample was selected for our analysis. Future trials need to be of adequate size, with a power calculation based on reasonable estimates of effect size, resulting in trials with many more participants. As an example, Marigold 2005 calculated that, based on their fall data, a sample size of 292 participants per exercise group would be required to detect differences for the number of fallers in a definitive trial. Future trials will probably need to be multicentred and perhaps international.
Risk of bias
The causes of falls in people after stroke are complex, and a trial aimed at preventing falls requires a complex intervention. We assessed the majority of trials included in this review as having a low risk of selection, attrition and reporting biases, as well as a reliable ascertainment of falls/fallers outcome (see Figure 3). Of concern was the level of detection bias (blinding of outcome assessment for falls). In the majority of trials (93.75%), we assessed this as being at high risk of bias, as studies used a self‐reported questionnaire or a falls calendar or diary. These methods rely on active registration by the participant, with telephone calls to the participants if (monthly) fall calendars are not returned. Nevertheless, we assessed this as being at high risk of bias, since the assessors, who were in this case the participants themselves, were probably not blinded to group allocation, and because the accuracy of prospective reporting methods may lead to over‐ or under‐reporting of falls (Lamb 2005). In an attempt to mitigate this risk, Mansfield 2018 used one blinded assessor to interview the participant about the circumstances of the fall, and two blinded assessors to determine if any falls should be excluded from the analysis. This approach could serve as example for future studies and warrants further consideration. Kunkel 2011, comparing retrospective interviews and prospective falls diaries over a 12‐month period in a cohort of 122 people with stroke, found an 83% agreement between the methods in the classification of fallers. Yet frequent repeat fallers reported falls during the retrospective interview but did not record all falls in the diary. Excluding these outliers, a similar number of falls were reported using either method. Our results for detection bias and the findings from Kunkel 2011 indicate that monitoring falls accurately in a chronic, community‐dwelling population remains difficult. Although prospective methods are considered preferable (Hauer 2006), future trials could include both retrospective and prospective methods. Nevertheless, preliminary studies investigating novel assessments of falls, such as portable activity monitors, seem warranted for future research.
To correct for included studies at high/unclear risk of bias (scoring high/unclear risk on one item in addition to the 'blinding of outcome assessment' item), we performed an additional sensitivity analysis in the exercise comparison. Surprisingly, this produced results conflicting with the original significant finding in rate of falls. Subsequent stepwise addition of studies at high/unclear risk of bias yielded results that strongly depended on study selection. Of interest is our observation that by reincorporating Ada 2013 in the analysis, the significant finding returned regardless of the amount of remaining studies at high/unclear risk of bias that were subsequently reincluded. Ada 2013 scored unclear risk of bias at concealment methodology, since details were lacking regarding the adopted concealment method. A conclusive statement with respect to study quality is that more well‐designed, low bias, adequately powered trials are needed to increase quality of the evidence, which would be potent to more robustly outline the true significance of fall‐oriented interventions.
GRADE
This updated version included the GRADE approach according to Atkins 2004. The quality of the evidence ranged from very low in the majority of the comparisons to low in the two comparisons that were found to be significant. The most frequent reasons for downgrading were the lack of blinding, which is difficult to perform on outcome measures related to fall assessment, and the fact that most comparisons only consisted of one study. Future updates of the review might show increased quality of the evidence due to inclusion of more studies per comparison.
Potential biases in the review process
Although we developed comprehensive search strategies for our review, there is still a possibility that we have missed some trials. Nevertheless, as an international group we are familiar with the work of colleagues from around the world active in the domain of falls after stroke, so we were able to include studies reporting on trials that were only recently completed.
Another potential bias of our review might be that we excluded trials that reported falls as an adverse event. It could be hypothesised that, although falls were included in these trials despite the fact that the interventions were not aimed at preventing them, some of them might actually have a positive effect on falls or the number of fallers, or both.
Furthermore, one could disagree with our inclusion of Dean 2010, Dean 2012, Lau 2012, Mansfield 2018 and Marigold 2005 in our pooled analysis, as both arms of these trials received an active exercise component. We believe that our decision was justified as, firstly, there is a general mixture of types of control interventions across our studies, and secondly — and more importantly — we believe that the experimental intervention in these studies was focused on significant aspects of falls prevention. Although both the intervention and the control condition involved a gait‐related approach in Dean 2010, body‐weight‐supported treadmill training was reported to be superior in improving ambulatory kinematics (Mao 2015), which in turn has a predictive capacity to falling (Punt 2017). The control condition in Dean 2012 consisted of upper extremity exercises, which could help prevent falls by improving mobility and balance recovery mechanisms. We feel, however, that the strength‐ and balance‐oriented approach adopted in the intervention condition has a higher probability of reducing falls compared to the control condition. In Lau 2012 the intervention group conducted whole‐body vibration exercises; vibration is thought to improve muscle weakness, the latter being an important predictor for falls (Weerdesteyn 2008). In Mansfield 2018, participants in the intervention condition received perturbation training, adapted to participants' ability and balance impairments, aimed at improving control of balance reactions, which is also related to falls post stroke (Marigold 2006). Similarly in Marigold 2005, the aim of the agility exercises in the experimental group was also clearly more related to falls prevention than the stretching and slow, low‐impact weight‐shifting exercises conducted in the control group. Despite our feeling that inclusion of the previously mentioned studies was justified, we highlight the probability of underestimating the true effect of exercises. Since exercises obviously show a potential to efficiently prevent falling post stroke, offering a similar approach to the control condition might have caused the occurrence of treatment effects in the control conditions, therefore minimising differences between both groups. Taylor‐Piliae 2014 provided usual care to the control condition in addition to written resources, which might have included some exercise component. Details are lacking regarding the content of the usual care condition.
Finally, the categorization of post‐stroke phases in this update version was different from the one used in the original version of this review. In the 2013 version, the categorization per study was made according to the location of the studies' recruited participants. This was a dedicated stroke unit or acute hospital ward (acute stage), a rehabilitation ward or clinic after discharge from an acute ward (subacute stage) or living at home or admitted to institutional care (chronic stage). Despite a profound clinical understanding that efficacy of administered interventions differs according to the location of the participants, experts in the field agree on a phase categorization according to time post stroke (Bernhardt 2017), also based on the biology of stroke recovery. Additionally, we chose to only assign a phase post stroke to studies of which we were absolutely sure of the time post stroke of all recruited patients. Together with our choice to conduct sensitivity analyses by pooling studies according to phase, these decisions were carefully made after intensive discussion with our international review group, including statistical experts in the field of stroke rehabilitation. By following this approach, however, we might have missed studies in our sensitivity analysis that recruited patients in one single phase post stroke but did not clearly indicate this in their report.
Agreements and disagreements with other studies or reviews
Stroke research
Our search strategy identified two systematic reviews on the topic of falls prevention after stroke. First, Batchelor 2010 included 13 studies and found exercises not to be beneficial in reducing either rate of falls or number of fallers post stroke. There is a discrepancy between the studies included in Batchelor 2010 and our review. In Batchelor 2010, the type of interventions included were all those that may affect falls outcome. On that basis, they also included trials in which falls were classified as an adverse event. Their review therefore contains six trials that we have excluded from our review, because we applied the stricter inclusion criterion that interventions had to be aimed at preventing falls. This is an important distinction. Trials evaluating interventions such as very early mobilisation after stroke or early supported discharge include falls as an outcome. The aim of these interventions, however, is not to prevent falls but to improve functional outcome. Because (very) early mobilisation or early supported discharge might be associated with an increase in falls, they are included as an outcome measure. We believe that a stricter approach — i.e. including only trials where the aim was to prevent falls — is justified, as otherwise study hypotheses are mixed and results become difficult to interpret.
Second, Winser 2018 conducted a meta‐analysis regarding the effect of Tai Chi in neurological disorders, including the effect on falling post stroke. They found Tai Chi to reduce falling post stroke, based on one study that was also included in our meta‐analysis (Taylor‐Piliae 2014). Their results agree with our findings of Taylor‐Piliae 2014, although we merged their two intervention conditions and we used a random‐effects model to evaluate the rate ratio, whereas Winser 2018 used a fixed‐effect model to evaluate an odds ratio. Despite the fact that merging both intervention conditions in our review reduced the effect size, our findings support that Tai Chi might be beneficial in preventing falls post stroke. The superiority of one exercise intervention over the other remains unknown at present, since there is a lack of studies investigating the fall‐preventing capacity of different exercise interventions post stroke, and this is probably also an important avenue for future research.
Elderly research
Finally, we feel that a more in‐depth appraisal of fall‐prevention reviews, focusing on elderly people living in the community, is important to the readership of this article. Besides the larger number of studies included in these reviews, there is a first notable contrast with our results in Gillespie 2012: multiple‐component group exercises and individually prescribed multiple‐component home‐based exercises did show a beneficial effect for reducing the rate of falls and the risk of falling in this population. We should be cautious, however, when considering whether these interventions might be suitable for preventing falls in people after stroke. Three trials included in this review (Batchelor 2012, Dean 2012 and Taylor‐Piliae 2014) examined the effect of an intervention containing an exercise programme developed for older people: the Otago Exercise Program (OEP) in Batchelor 2012; the Weight‐bearing Exercise for Better Balance (WEBB) programme in Dean 2012; and the SilverSneakers national fitness programme designed for older people in Taylor‐Piliae 2014, which was merged with the Tai Chi intervention condition for reasons stated in the Results section of this review. None of these trials showed significant between‐group differences for reducing the rate of falls and the number of fallers, again indicating that specific interventions may be required for preventing falls in people after stroke, with strategies aimed at particular deficits that people have after their stroke.
Other reviews concerning fall prevention in elderly people published contradicting results regarding the fall‐preventing capacity of exercises (Cameron 2018; Grossman 2018; Sherrington 2019; Tricco 2017). Sherrington 2019 substantiates the value of Tai Chi, balance‐oriented and functional exercises. Tricco 2017 found exercises, either alone or part of a multifactorial approach, to be associated with lower risk of injurious falls. Grossman 2018 found a small effect of multifactorial interventions, whereas Cameron 2018 express their uncertainty of the effect of exercises on rate of falls or risk of falling. We note, however, that the latter study was conducted in an institutionalised setting, opposed to the other three reviews assessing community‐dwelling older people. In general, literature therefore concludes that exercises have a beneficial fall‐preventing effect in elderly people, which was reflected in the stroke population in this review update. Nevertheless, we stress the importance that the exercise‐related studies included in our review did not find any beneficial effect on number of fallers. The overall inconsistency of significant fall‐reducing effects of the investigated interventions, combined with issues outlined earlier in this discussion, requires us to be cautious and thus express low confidence in our conclusion. If future updates could reach sample sizes comparable to fall research in elderly people, this cautiousness regarding concluding statements might turn into more robust evidence more effectively informing clinical practice.
Authors' conclusions
Implications for practice.
Exercises
Currently, there is low to very low quality evidence that exercises, either as a single intervention or part of a multi‐component intervention, and including ambulation, perturbation/vibration‐based, balance/strength‐oriented or Tai‐Chi training, reduce the rate of falls, but not the number of fallers after stroke. There remains a lack of evidence to draw conclusions of the effects in a specific phase post stroke. Furthermore, there is a general lack of evidence to inform clinicians about potent interventions to prevent fall‐related fractures, fall‐related hospital admissions, near‐fall events, economic factors, quality of life, or adverse events.
Environmental adaptations
There is currently insufficient evidence to reach conclusions about the impact of use of predischarge home visits, provision of single lens distance glasses, or use of a servo‐assistive rollator on the rate of falls or number of fallers. We graded quality of the evidence to be very low in all analyses related to environmental adaptations.
Other interventions (tDCS)
Low‐quality evidence from one study suggests that tDCS may reduce the number of fallers, but there is a need for further evidence before tDCS is introduced into routine clinical practice as an intervention to prevent falls.
General quality of the evidence
Despite the GRADE approach finding quality of the evidence to range from low (exercises (rate of falls) and tDCS) to very low in the remainder of the analyses, our review outlines a strong tendency that clinical practice at this stage will benefit mostly from exercises, based on their low cost, ease of administration and potentially favourable fall‐preventing outcome. Hopefully, future updates of this review will be sufficiently enriched with new literature to provide more conclusive evidence on its value compared to other fall prevention interventions.
Implications for research.
Content
Further studies are needed to evaluate exercises as a single component or part of a multiple or multifactorial programme, with careful consideration of the content of the intervention, taking into account the current knowledge about risk factors for falling after stroke and the possibility that different interventions have to be developed for different subgroups of people after stroke. In addition, larger trials are needed to confirm the potential benefits of (different) exercises regarding falling post stroke.
No studies seemed to include participants in the (hyper)acute phase after their stroke.
Currently, only three domains of potential interventions for preventing falls have been investigated, with the majority of trials investigating exercises. Thus, there remains an important evidence gap, and future trials should consider other types of interventions or inclusion of these types of interventions in multiple or multifactorial approaches, since they also might positively impact on established risk factors for falls, and potentially reduce falls post stroke. Moreover, interpretability of fall research would increase by clarifying the content of interventions using for instance the TIDieR framework (Hoffmann 2014), designed to allow for replication of the intervention as well as translating research into clinical practice.
Methodology
It is important to note that time post stroke was generally reported by means of a mean (SD) which does not allow for accurate phase categorisation, and therefore limits us to draw conclusions regarding phase‐dependent efficacy of fall‐prevention interventions. Combined with the need for more studies in the (hyper)acute phase, we would recommend future trials to restrict inclusion criteria to particular post stroke phases. Moreover, it could focus on the potential of influencing risk factors for falls in people early after stroke, i.e. while still hospitalised, as well as on the assessment of the long‐term effect when people are discharged back into their community.
A general heterogeneity was observed regarding the time point of measuring treatment effect (during intervention vs. follow‐up), which might influence results based on reasons stated in the Quality of the evidence section of this review. This outlines a general need for consensus regarding the optimal timespan of recording falls in a clinical trial, which could be addressed in future research.
Studies investigating fall prevention for people after stroke should be adequately powered, provide a standardised definition of a fall from a consensus statement, use appropriate and accurate methods of fall ascertainment, and apply the current standards for analysis and reporting of data (Lamb 2005), including the CONSORT guidelines.
What's new
| Date | Event | Description |
|---|---|---|
| 3 September 2018 | New search has been performed | Update of the original review (2013) by including the results of an updated search from November 2012 to September 2018. We removed 2 studies from the original review due to publication retraction. We included an additional 6 studies with a total of 532 participants, yielding a new total of 14 included studies with 1358 participants. We applied the GRADE approach including 'Summary of findings' tables in order to summarise key results from our review. |
| 3 September 2018 | New citation required and conclusions have changed | At present very little evidence exists about interventions other than exercises to reduce falling post stroke. Low‐quality to very low quality evidence exists that this population benefits from exercises to prevent falls, but not to reduce number of fallers. |
Acknowledgements
The authors wish to express their sincere gratitude to Sue Forsey and Paula Sands from the University of Southampton School of Medicine library for conducting the searches, and Ann Ashburn for contributing to the original version of this Cochrane Review. We also would like to thank Stephen R Lord for providing stroke subgroup data from the VISIBLE trial (Haran 2010); and Giovanni Morone, Avril Drummond and Louise Ada for the provision of additional data regarding rate of falls (Ada 2013; Drummond 2012; Morone 2016). We additionally express our sincere gratitude to the consumer reviewers Jonathan M Fuchs and Catherine Hofstetter for providing constructive comments to this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Cerebrovascular Disorders] explode all trees #2 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] explode all trees #3 MeSH descriptor: [Brain Ischemia] explode all trees #4 MeSH descriptor: [Carotid Artery Diseases] explode all trees #5 MeSH descriptor: [Intracranial Arterial Diseases] explode all trees #6 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees #7 MeSH descriptor: [Intracranial Hemorrhages] explode all trees #8 MeSH descriptor: [Stroke] explode all trees #9 MeSH descriptor: [Brain Infarction] explode all trees #10 MeSH descriptor: [Vasospasm, Intracranial] explode all trees #11 MeSH descriptor: [Vertebral Artery Dissection] explode all trees #12 (¨stroke¨ or ¨poststroke¨ or ¨post‐stroke¨ or ¨cerebrovasc*¨ or ¨brain vasc*¨ or ¨cerebral vasc*¨ or ¨cva*¨ or ¨apoplex*¨ or ¨SAH¨):ti,ab,kw (Word variations have been searched) #13 (¨brain*¨ or ¨cerebr*¨ or ¨cerebell*¨ or ¨intracran*¨ or ¨intracerebral¨) near/5 (¨isch?emi*¨ or ¨infarct*¨ or ¨thrombo*¨ or ¨emboli*¨ or ¨occlus*¨):ti,ab,kw or (¨brain*¨ or ¨cerebr*¨ or ¨cerebell*¨ or ¨intracerebral¨ or ¨intracranial¨ or ¨subarachnoid¨) near/5 (¨haemorrhage*¨ or ¨hemorrhage*¨ or ¨haematoma*¨ or ¨hematoma*¨ or ¨bleed*¨):ti,ab,kw (Word variations have been searched) #14 MeSH descriptor: [Paresis] explode all trees #15 MeSH descriptor: [Hemiplegia] explode all trees #16 ¨hemipleg*¨ or ¨hemipar*¨ or ¨paresis¨ or ¨paretic:ti,ab,kw (Word variations have been searched) #17 MeSH descriptor: [Gait Disorders, Neurologic] explode all trees #18 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 MeSH descriptor: [Accidental Falls] explode all trees #20 MeSH descriptor: [Accidents] explode all trees #21 MeSH descriptor: [Accident Prevention] explode all trees #22 MeSH descriptor: [Accidents, Home] explode all trees #23 MeSH descriptor: [Accident Proneness] explode all trees #24 (¨fall* or ¨fall‐related¨ or ¨near‐fall¨):ti,ab,kw or (¨slip*¨ or ¨trip*¨):ti,ab,kw or (¨stumble*¨ or ¨tumble*¨):ti,ab,kw or ¨lose¨ near/5 ¨footing¨:ti,ab,kw (Word variations have been searched) #25 #19 or #20 or #21 or #22 or #23 or #24 #26 #18 and #25 #27 MeSH descriptor: [Randomized Controlled Trial] explode all trees #28 MeSH descriptor: [Randomized Controlled Trials as Topic] explode all trees #29 MeSH descriptor: [Random Allocation] explode all trees #30 MeSH descriptor: [Controlled Clinical Trial] explode all trees #31 MeSH descriptor: [Controlled Clinical Trials as Topic] explode all trees #32 MeSH descriptor: [Clinical Trials as Topic] explode all trees #33 MeSH descriptor: [Clinical Trial] explode all trees #34 MeSH descriptor: [Clinical Trials, Phase I as Topic] explode all trees #35 MeSH descriptor: [Clinical Trials, Phase II as Topic] explode all trees #36 MeSH descriptor: [Clinical Trials, Phase III as Topic] explode all trees #37 MeSH descriptor: [Clinical Trials, Phase IV as Topic] explode all trees
#38 MeSH descriptor: [Cross‐Over Studies] explode all trees #39 MeSH descriptor: [Multicenter Studies as Topic] explode all trees #40 MeSH descriptor: [Therapies, Investigational] explode all trees #41 ("randomised controlled trial" or ¨controlled clinical trial¨ or ¨clinical trial¨ or ¨clinical trial phase I¨ or ¨clinical trial phase II¨ or ¨clinical trial phase III¨ or ¨clinical trial phase IV¨):ti,ab,kw or (¨random*¨ or ¨cross‐over¨ or ¨cross over¨ or ¨crossover¨):ti,ab,kw or (¨quasi‐random¨ or ¨quasi random¨ or ¨pseudo‐random¨ or ¨pseudo random¨):ti,ab,kw or ¨coin¨ near/5 (¨flip*¨ or ¨toss¨):ti,ab,kw (Word variations have been searched) #42 #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 #43 #26 and #42 #44 #43 Publication Year from 2012 to 2018
Appendix 2. MEDLINE search strategy
1. cerebrovascular disorders/
2. exp basal ganglia cerebrovascular disease/
3. exp brain ischemia/
4. exp carotid artery diseases/
5. exp intracranial arterial diseases/
6. exp "intracranial embolism and thrombosis"/
7. exp intracranial hemorrhages/
8. stroke/
9. exp brain infarction/
10. vasospasm, intracranial/
11. vertebral artery dissection/
12. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
13. (stroke or post stroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
14. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
15. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
16. hemiplegia/ or exp paresis/
17. (hemipleg$ or hemipar$ or paresis or paretic).tw.
18. exp gait disorders, neurologic/
19. 12 or 13 or 14 or 15 or 16 or 17 or 18
20. accidental falls/ or accidents/ or exp accident prevention/ or accidents, home/ or accident proneness/
21. (fall or falls or faller or fallen or fallers or falling or fall‐related or near‐fall or falls‐efficacy scale).tw.
22. (slip or slips or slipped or slipping or trip or trips or tripped or tripping).tw.
23. (stumble$ or tumble$).tw.
24. (lose adj5 footing).tw.
25. 20 or 21 or 22 or 23 or 24
26. 19 and 25
27. Randomized Controlled Trials as Topic/
28. random allocation/
29. Controlled Clinical Trials as Topic/
30. control groups/
31. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
32. double‐blind method/
33. single‐blind method/
34. Placebos/
35. placebo effect/
36. cross‐over studies/
37. Multicenter Studies as Topic/
38. Therapies, Investigational/
39. Research Design/
40. Program Evaluation/
41. evaluation studies as topic/
42. randomised controlled trial.pt.
43. controlled clinical trial.pt.
44. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
45. multicenter study.pt.
46. (evaluation studies or comparative study).pt.
47. random$.tw.
48. (controlled adj5 (trial$ or stud$)).tw.
49. (clinical$ adj5 trial$).tw.
50. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
51. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
52. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw.
53. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
54. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
55. (coin adj5 (flip or flipped or toss$)).tw.
56. versus.tw.
57. (cross‐over or cross over or crossover).tw.
58. placebo$.tw.
59. sham.tw.
60. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw.
61. controls.tw.
62. (treatment$ adj6 order).tw.
63. 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62
64. 26 and 63
65. limit 64 to humans
66. limit 65 to yr="2012 ‐Current"
Appendix 3. Embase search strategy
1. ‘cerebrovascular disease’/exp
2. ‘basal ganglion hemorrhage’/exp
3. ‘brain ischemia’/exp
4. ‘carotid artery disease’/exp
5. ‘cerebral artery disease’/exp
6. 'intracranial embolism'
7. ‘intracranial thrombosis'
8. ‘brain hemORrhage’/exp
9. ‘brain infarction’/exp
10. ‘brain vasospasm’/exp
11. ‘artery dissection’/exp
12. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11
13. (‘stroke’ OR ‘post stroke’ OR ‘post‐stroke’ OR ‘cerebrovasc$’ OR ‘brain vasc$’ OR ‘cerebral vasc$’ OR ‘cva$’ OR ‘apoplex$’ OR ‘SAH’):ti,ab,kw
14. ((‘brain$’ OR ‘cerebr$’ OR ‘cerebell$’ OR ‘intracran$’ OR ‘intracerebral’) NEAR/5 (‘isch?emi$’ OR ‘infarct$’ OR ‘thrombo$’ OR ‘emboli$’ OR ‘occlus$’)):ti,ab,kw
15. ((‘brain$’ OR ‘cerebr$’ OR ‘cerebell$’ OR ‘intracerebral’ OR ‘intracranial’ OR ‘subarachnoid’) NEAR/5 (‘haemORrhage$’ OR ‘hemORrhage$’ OR ‘haematoma$’ OR ‘hematoma$’ OR ‘bleed$’)):ti,ab,kw
16. ‘hemiplegia’/exp
17. ‘paresis’/exp
18. ((‘brain$’ OR ‘cerebr$’ OR ‘cerebell$’ OR ‘intracerebral’ OR ‘intracranial’ OR ‘subarachnoid’) NEAR/5 (‘haemORrhage$’ OR ‘hemORrhage$’ OR ‘haematoma$’ OR ‘hematoma$’ OR ‘bleed$’)):ti,ab,kw
19. ‘gait disORder’/exp
20. 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19
21. ‘accident’/exp
22. ‘accidents and accident related phenomena’/exp
23. ‘falling’/exp
24. ‘home accident’/exp
25. ‘accident prevention’/exp
26. ‘accident proneness’/exp
27. (‘fall$’ OR ‘fall‐related’ OR ‘near‐fall’ OR ‘falls‐efficacy scale’):ti,ab,kw
28. (‘slip’ OR ‘slips’ OR ‘slipped’ OR ‘slipping’ OR ‘trip’ OR ‘trips’ OR ‘tripped’ OR ‘tripping’):ti,ab,kw
29. (‘stumble$’ OR ‘tumble$’):ti,ab,kw
30. (‘lose’ NEAR/5 ‘footing’):ti,ab,kw
31. 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30
32. ‘controlled clinical trial’/exp
33. ‘randomized controlled trial’/exp
34. 'controlled clinical trial (topic)'/exp
35. ‘randomization’/exp
36. ‘phase 1 clinical trial (topic)’/exp OR ‘phase 2 clinical trial (topic)’/exp OR ‘phase 3 clinical trial (topic)’/exp OR ‘phase 4 clinical trial (topic)’/exp
37. (‘crossover procedure’/exp OR ‘cross‐over’ OR ‘cross over’ OR ‘crossover’):ti,ab,kw
38. ‘multicenter study (topic)’/exp
39. ‘experimental therapy’/exp
40. (‘randomized controlled trial’ OR ‘randomised controlled trial’ OR ‘controlled clinical trial’):ti,ab,kw
41. (‘clinical trial phase I’ OR ‘clinical trial phase ii’ OR ‘clinical trial phase iii’ OR ‘clinical trial phase iv’):ti,ab,kw
42. (‘random allocat$’ OR ‘random$’ OR ‘quasi‐random’ OR ‘quasi random$’ OR ‘pseudo‐random’ OR ‘pseudo random$’):ti,ab,kw
43. 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41
44. 20 AND 31 AND 43
45. Limit 44 to Humans; Publication years: 2012 to 2018
Appendix 4. CINAHL search strategy
S1 (MH "Cerebrovascular Disorders") S2 (MH "Basal Ganglia Cerebrovascular Disease+") S3 (MH "Cerebral Ischemia+") S4 (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis+") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke+") S5 (MH "Carotid Artery Diseases+") S6 (MH "Brain Injuries+") S7 (MH "Cerebral Vasospasm") S8 (MH "Vertebral Artery Dissections") S9 TX (stroke or post stroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH) S10 TX (brain* or cerebr* or cerebell* or intracran* or intracerebral) S11 TX (isch?emi* or infarct* or thrombo* or emboli* or occlus*) S12 S10 AND S11 S13 TX (brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) S14 TX (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*) S15 S13 AND S14 S16 (MH "Hemiplegia") S17 (MH "Paralysis+") S18 TX (hemipleg* or hemipar* or paresis or paretic) S19 (MH "Gait Disorders, Neurologic+") S20 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S12 OR S15 OR S16 OR S17 OR S18 OR S19 S21 (MH "Accidents, Home") OR (MH "Accidental Falls") OR (MH "Accidents") S22 (MH "Safety") S23 TX (fall or falls or faller or fallen or fallers or falling or fall‐related or near‐fall or falls‐efficacy scale) S24 TX (slip or slips or slipped or slipping or trip or trips or tripped or tripping) S25 TX (stumble* or tumble*) S26 TX lose footing S27 S21 OR S22 OR S23 OR S24 OR S25 OR S26 S28 S20 AND S27 S29 (MH "Randomized Controlled Trials") S30 (MH "Random Assignment") S31 (MH "Clinical Trials") S32 (MH "Control Group") S33 (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Intervention Trials") OR (MH "Preventive Trials") OR (MH "Therapeutic Trials") S34 (MH "Placebos") OR (MH "Placebo Effect") S35 (MH "Cross Sectional Studies") OR (MH "Crossover Design") OR (MH "Multicenter Studies") OR (MH "Experimental Studies") OR (MH "Multimethod Studies") S36 (MH "Study Design") OR (MH "Research Methodology") S37 (MH "Program Evaluation") S38 (MH "Evaluation Research") S39 PT randomised controlled trial S40 TX randomised controlled trial S41 PT controlled clinical trial S42 TX controlled clinical trial S43 PT (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv) S44 PT multicenter study S45 TX multicenter study S46 PT (evaluation studies or comparative study) S47 TX (evaluation studies or comparative study) S48 TX random* S49 TX controlled S50 TX (trial* or stud*) S51 S49 AND S50 S52 TX clinical* trial* S53 TX (control or treatment or experiment* or intervention) S54 TX (group* or subject* or patient*) S55 S53 AND S54 S56 TX (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) S57 TX (multicenter or multicentre or therapeutic) S58 TX (trial* or stud*) S59 S57 AND S58 S60 TX (control or experiment* or conservative) S61 TX (treatment or therapy or procedure or manage*) S62 S60 AND S61 S63 TX coin AND TX ( (flip or flipped or toss*) ) S64 TX versus S65 TX (cross‐over or cross over or crossover) S66 TX placebo* S67 TX sham* S68 TX (assign* or alternate or allocat* or counterbalance* or multiple baseline) S69 TX controls S70 TX treatment* AND TX order S71 S52 OR S55 OR S56 OR S59 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 S72 S28 AND S71 S73 S28 AND S71 Limiters ‐ Published Date: 20120101‐20181231; Human
Appendix 5. PsycINFO search strategy
S1 DE “Cerebrovascular Disorders” OR DE “Cerebrovascular Accidents”
S2 DE “Cerebrovascular Disorders” OR DE “Cerebrovascular Accidents”
S3 DE “Basal Ganglia”
S4 DE “Cerebral Ischemia” OR DE “Cerebral Small Vessel Disease”
S5 DE “Carotid Arteries”
S6 DE intracranial arterial disease*
S7 “intracranial embolism and thrombosis”
S8 intracranial embolism
S9 DE “Subarachnoid Hemorrhage” OR DE “Cerebral Hemorrhage”
S10 intracranial vasospasm
S11 DE “Brain Disorders”
S12 DE “Cerebral Arteriosclerosis”
S13 TX (stroke or post stroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH)
S14 TX (brain* or cerebr* or cerebell* or intracran* or intracerebral)
S15 TX (brain* or cerebr* or cerebell* or intracran* or intracerebral) AND TX (isch?emi* or infarct* or thrombo* or emboli* or occlus*)
S16 TX (brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) AND TX (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)
S17 DE “Hemiplegia” OR DE “Hemiparesis”
S18 TX (hemipleg* or hemipar* or paresis or paretic)
S19 TX neurologic gait disorder*
S20 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19
S21 DE “Falls”
S22 DE “Accidents” OR DE “Falls” OR DE “Home Accidents” OR DE “Industrial Accidents” OR DE “Pedestrian Accidents” OR DE “Transportation Accidents” OR DE “Accident Prevention” OR DE “Home Accidents”
S23 DE “Accident Proneness”
S24 TX (fall or falls or faller or fallen or fallers or falling or fall‐related or near‐fall or falls‐efficacy scale)
S25 TX (slip or slips or slipped or slipping or trip or trips or tripped or tripping)
S26 TX (stumble* or tumble*)
S27 TX lose AND TX footing
S28 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27
S29 S20 AND S28
S30 DE “Random Sampling”
S31 DE “Clinical Trials”
S32 DE “Experiment Controls”
S33 DE “Double Bind Interaction”
S34 DE “Placebo”
S35 DE “Experimentation” OR DE “Experimental Methods”
S36 DE “Methodology”
S37 DE “Program Evaluation”
S38 DE “Followup Studies” OR DE “Longitudinal Studies” OR DE “Retrospective Studies”
S39 PT randomised controlled trial
S40 PT randomised controlled trial
S41 TX randomised controlled trial
S42 PT controlled clinical trial
S43 PT controlled clinical trial
S44 TX controlled clinical trial
S45 PT (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv)
S46 PT (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv)
S47 TX (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv)
S48 PT multicenter study
S49 PT multicenter study
S50 TX multicenter study Search modes
S51 TX multicenter study Search modes
S52 PT (evaluation studies or comparative study)
S53 PT (evaluation studies or comparative study)
S54 TX (evaluation studies or comparative study)
S55 TX random*
S56 TX controlled AND TX (trial* or stud*)
S57 TX clinical* trial*
S58 TX (control or treatment or experiment* or intervention) AND TX (group* or subject* or patient*)
S59 TX (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*)
S60 TX (multicenter or multicentre or therapeutic) AND TX (trial* or stud*)
S61 TX (control or experiment* or conservative) AND TX (treatment or therapy or procedure or manage*)
S62 TX coin AND TX (flip or flipped or toss*)
S63 TX versus
S64 TX (cross‐over or cross over or crossover)
S65 TX placebo*
S66 TX sham
S67 TX (assign* or alternate or allocat* or counterbalance* or multiple baseline)
S68 TX controls
S69 TX treatment* AND TX order
S70 S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69
S71 S29 AND S70
S72 Limiters ‐ Publication Year: 2012‐2018 Narrow by Population: ‐ human
Appendix 6. AMED search strategy
S1 cerebrovascular disorders/
S2 exp Basal ganglia/
S3 exp Basal ganglia/
S4 exp Cerebral ischemia/
S5 exp Cerebral ischemia/
S6 Carotid arteries/
S7 intracranial embolism.mp.
S8 brain disease/
S9 exp Cerebral infarction/
S10 exp Cerebral infarction/
S11 vasospasm.mp.
S12 vertebral artery/
S13 cerebrovascular accident/
S14 (stroke or post stroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
S15 ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
S16 ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
S17 ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$or hematoma$ or bleed$)).tw.
S18 ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$or hematoma$ or bleed$)).tw.
S19 hemiplegia/
S20 (hemipleg$ or hemipar$ or paresis or paretic).tw.
S21 gait disorders/
S22 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21
S23 Accident prevention/ or Accidental falls/
S24 accidents/
S25 (fall or falls or faller or fallen or fallers or falling or fall‐related or near‐fall or falls‐efficacy scale).tw.
S26 (slip or slips or slipped or slipping or trip or trips or tripped or tripping).tw.
S27 (stumble$ or tumble$).tw.
S28 (stumble$ or tumble$).tw.
S29 (lose adj5 footing).tw
S30 (lose adj5 footing).tw
S31 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30
S32 S22 AND S31
S33 randomized controlled trials/
S34 random allocation/
S35 controlled clinical trials.mp.
S36 controlled clinical trials.mp.
S37 control groups.mp.
S38 clinical trials/
S39 Double blind method/
S40 single blind method/
S41 Placebos/
S42 placebo effect.mp.
S43 cross‐over studies.mp.
S44 cross‐over studies.mp.
S45 multicenter studies.mp.
S46 therapies investigational.mp.
S47 investigational therapies.mp.
S48 research design/
S49 program evaluation/
S50 evaluation studies.mp.
S51 randomised controlled trial.pt.
S52 randomised controlled trial.pt.
S53 controlled clinical trial.pt.
S54 controlled clinical trial.pt.
S55 (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
S56 multicenter study.pt.
S57 (evaluation studies or comparative study).pt.
S58 random$.tw
S59 random$.tw
S60 (controlled adj5 (trial$ or stud$)).tw.
S61 (controlled adj5 (trial$ or stud$)).tw.
S62 (clinical$ adj5 trial$).tw.
S63 (clinical$ adj5 trial$).tw.
S64 ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
S65 ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
S66 (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
S67 (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
S68 ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw.
S69 ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw.
S70 ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
S71 ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
S72 ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
S73 (coin adj5 (flip or flipped or toss$)).tw.
S74 (coin adj5 (flip or flipped or toss$)).tw.
S75 versus.tw.
S76 (cross‐over or cross over or crossover).tw.
S77 placebo$.tw
S78 placebo$.tw
S79 sham.tw
S80 (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw.
S81 controls.tw.
S82 (treatment$ adj6 order).tw.
S83 (treatment$ adj6 order).tw.
S84 S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81 OR S82 OR S83
S85 S32 AND S84
S86 S32 AND S84
S87 Limiters ‐ Published Date: 20120101‐20171231
Appendix 7. PEDro search strategy
Search combinations:
1: Stroke* AND Fall*
2: Stroke* AND Accident*
3: Stroke* AND Trip*
4: Cerebrovascular AND Accident*
5: CVA AND Accident
6: Brain* AND Fall*
Appendix 8. Clinical trial registers search strategies
Clinicaltrials.gov:
Stroke AND Fall
Filters:
2012 – 01/06/2020
>18 yo
Interventional
Adult (18‐64)
Older Adult (65+)
International clinical trials registry platform search portal:
Condition: cerebrovascular disease OR basal ganglion hemORrhage OR brain ischemia OR carotid artery disease OR intracranial embolism OR intracranial thrombosis OR brain hemORrhage OR brain infarction OR artery dissection OR stroke OR cva* OR apoplex* OR SAH
Intervention: accident OR accidents and accident related phenomena OR falling OR home accident OR accident prevention OR accident proneness OR fall* OR near‐fall OR slip OR slip* OR trip* OR stumble* OR tumble* OR (lose AND footing)
Filters:
Recruitment status: ALL
Phases: ALL
ISRCTN:
Condition: Stroke Keyword: Fall
Stroke trials registry:
Keyword:
Fall
Accident
Filters:
Allocation: randomized
Appendix 9. Author contacting template (ongoing trial)
Dear Prof./Dr. ...,
Our international research group is currently performing an update of a systematic review performed by Verheyden et al, 2013 concerning the effectiveness of interventions to reduce falling post‐stroke (Verheyden GSAF et al. Interventions for preventing falls in people after stroke. (Review) Cochrane Database of Systematic Reviews, 2013). Currently we are screening literature for eligible articles to be included in our update, and your promising ongoing trial appears to meet our inclusion criteria (Study title: "...", Trial registration: ...). More specifically, we would like to check eligibility and whether following data is already available, and if it is by any means possible to obtain these data to incorporate them in the update review. Could you first confirm that your trial adheres to all of the following inclusion criteria:
1. Type of study: controlled trial where participants or clusters were randomly allocated.
2. Type of participants: adult participants (over 18 years of age) in the acute, subacute or chronic phase following stroke with a confirmed diagnosis (diagnosis comprises ischemic as well as hemorrhagic events).
3. Type of intervention: Intervention where a stated primary or secondary aim is to prevent falls.
4. Type of outcome measure: at least 1 outcome measure related to the rate of falls or the number of fallers. Fall collection can be collected either prospectively or retrospectively.
In case you have answered YES to the previous question, would you be able to provide us, by dd/mm/yyyy, the following information:
‐ ...
‐ ...
In the advantageous case of data availability, we kindly request to provide us the data before Friday 31 August 2018 by means of an email forwarded to Stijn Denissen (mail address), Wouter Staring (mail address) and our group supervisor Prof. Dr. Geert Verheyden (mail address) to ensure the progress of the update.
Should you have further questions, please do not hesitate to contact us on the email addresses stated above.
Thank you very much in advance.
Yours sincerely,
Stijn Denissen and Wouter Staring
Appendix 10. Author contacting template (missing data)
Dear Prof./Dr. ..., Our international research group is currently updating a systematic review performed by Verheyden et al, 2013 concerning the effectiveness of interventions to reduce falling post‐stroke (Verheyden GSAF et al. Interventions for preventing falls in people after stroke. (Review) Cochrane Database of Systematic Reviews, 2013). Your study (Study title: "...", Trial registration: ...) has been included in our update version and data has been extracted from the full text article version. In addition to the published results, we would like to check if additional data is available regarding fall rates per treatment group. More specifically, we are interested in following data: ‐ ... ‐ ... In the advantageous case of availability of these additional data, we kindly request to provide us the data before dd/mm/yyyy by means of an email forwarded to Stijn Denissen (mail address), Wouter Staring (mail address) and our group supervisor Prof. Dr. Geert Verheyden (mail address) to ensure the progress of the update. Should you have further questions, please do not hesitate to contact us on the email addresses stated above. Thank you very much in advance. Yours sincerely, Stijn Denissen and Wouter Staring
Data and analyses
Comparison 1. Exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 8 | 765 | Rate Ratio (Random, 95% CI) | 0.72 [0.54, 0.94] |
| 2 Number of fallers | 10 | 969 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.90, 1.19] |
Comparison 2. Environment: social environment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 1 | 85 | Rate ratio (Random, 95% CI) | 0.85 [0.43, 1.69] |
| 2 Number of fallers | 1 | 85 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.71, 3.09] |
Comparison 3. Environment: single lens distance glasses versus usual (multifocal) glasses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 1 | 43 | Rate Ratio (Random, 95% CI) | 1.08 [0.52, 2.25] |
| 2 Number of fallers | 1 | 43 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.47, 1.18] |
Comparison 4. Environment: servo‐assistive rollator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 1 | 42 | Rate ratio (Random, 95% CI) | 0.56 [0.19, 1.66] |
| 2 Number of fallers | 1 | 42 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.16, 1.22] |
Comparison 5. Other interventions: tDCS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of fallers | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.14, 0.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ada 2013.
| Methods | 3‐arm RCT | |
| Participants | 102 community‐dwelling people with stroke | |
| Interventions | The 2 experimental groups received treadmill training without body weight support, structured to increase step length, speed, balance, fitness, and automaticity. Additionally, "overground walking was used each session to reinforce gains achieved during treadmill training." (Page 3) Experimental group 1 and 2 received respectively 4 months and 2 months of the earlier‐mentioned training type The control group did not receive any type of intervention |
|
| Outcomes | Number of fallers and quality of life | |
| Notes | Fall registration during both intervention and follow‐up period. Registration time: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated, independent and concealed randomization was used to assign each participant within the triplet to either ..." (Page 1) |
| Allocation concealment (selection bias) | Unclear risk | "Computer‐generated, independent and concealed randomization was used to assign each participant within the triplet to either ..." (Page 1) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants recorded falls themselves through the use of a falls calendar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Data analysis was performed using intention‐to‐treat analysis." (Page 2) "Missing data were interpolated based on nearest measures taken." (Page 4) |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | Low risk | "At each measurement time, participants were given calendars to cover the period until the next measure and were instructed to record any falls that occurred and bring the calendars to the next measurement session." (Page 3) Pre‐post measurement session interval was 2 months |
Andrade 2017.
| Methods | Sham‐controlled, double‐blinded, parallel RCT | |
| Participants | A total of 60 unilateral, non‐recurring, acute ischaemic stroke patients with a high risk of falling | |
| Interventions | In addition to the same physical rehabilitation programme (1 hour a day, 3 days a week), all participants received an additional 10 sessions (5 consecutive days for 2 weeks) of:
|
|
| Outcomes | Number of fallers and adverse events | |
| Notes | Fall registration during both intervention and follow‐up period. Registration time: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted with randomly permuted blocks, through an online program (www. random.org)." (page 3) |
| Allocation concealment (selection bias) | Low risk | Quote: "... blind allocation in the ratio of 1:1:1:1 was carried out with sequentially numbered and sealed opaque envelopes." (page 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although participants recorded falls themselves through the use of a falls calendar, participants were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results. Quote: "All participants were inserted into the analysis, even if they had attended only one session, following the intention‐to‐treat principle. Sensitivity analysis was applied to choose the treatment of missing data....Missing data were treated by simple imputation." (page 4) |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | Low risk | Quote: "...participants completed information about falls after evaluation, returning calendar pages using prepaid envelopes." (page 4) |
Batchelor 2012.
| Methods | Single blind, multicentre RCT with 12‐month follow‐up | |
| Participants | A total of 156 participants were recruited. Participants were people with stroke at risk of recurrent falls being discharged home from rehabilitation | |
| Interventions | In addition to usual care after discharge (physiotherapy and occupational therapy and follow‐up by the general medical practitioner), the 12‐month study programme consisted of:
|
|
| Outcomes | Rate of falls, number of fallers, number of people sustaining fall‐related fractures, and quality of life | |
| Notes | Fall registration during intervention period. Registration time: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... participants were allocated into either the control group or the intervention group (1:1 allocation ration, simple randomization) using a computer‐generated random allocation sequence ..." (page 2) |
| Allocation concealment (selection bias) | Low risk | Quote: "... participants were allocated into either the control group or the intervention group (1:1 allocation ration, simple randomization) using a computer‐generated random allocation sequence concealed from all researchers in opaque envelopes. Staff independent of the study undertook sequence and concealment." (page 2) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants recorded falls themselves through the use of a falls calendar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | Low risk | Participants returned the falls calendar each month. A researcher blinded to group allocation telephoned participants who did not return their calendar within 2 weeks of the due date |
Dean 2010.
| Methods | Assessor‐blinded RCT | |
| Participants | A total of 126 participants unable to walk within 4 weeks of a stroke who were undergoing inpatient rehabilitation | |
| Interventions | A conventional stroke rehabilitation programme was provided plus up to 30 minutes (5 days a week) of:
Interventions were provided until participants achieved independent walking or were discharged from hospital, resulting in heterogeneity regarding intervention/control condition duration. |
|
| Outcomes | Rate of falls, number of fallers, and quality of life | |
| Notes | Fall registration during intervention period. Registration time: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation sequence was computer‐generated before commencement of the study and centrally located." (page 98) |
| Allocation concealment (selection bias) | Low risk | Quote: "After recruitment, the central office was contacted for allocation so that randomization was secure and concealed." (page 98) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Number of falls was quantified by means of a self‐reported questionnaire |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | High risk | Fall ascertainment method used was retrospective recall (6‐month period) |
Dean 2012.
| Methods | Assessor‐blinded RCT | |
| Participants | A total of 151 community‐dwelling people after stroke | |
| Interventions | Both groups participated in exercise classes of 45 to 60 minutes delivered by a physiotherapist weekly for 40 weeks over a 1‐year period consisting of:
|
|
| Outcomes | Rate of falls, number of fallers, quality of life, and adverse events | |
| Notes | Fall registration during intervention period. Registration time: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation sequence was computer generated before commencement of the study ..." (page 2) |
| Allocation concealment (selection bias) | Low risk | Quote: "... and a set of consecutively numbered, sealed opaque envelopes containing the allocation was centrally generated for each stroke club." (page 2) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants recorded falls themselves through the use of a falls calendar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | Low risk | Participants returned the falls calendar each month. A researcher telephoned participants who did not return their calendar |
Drummond 2012.
| Methods | RCT | |
| Participants | 93 patients transferred from an acute stroke unit with a confirmed diagnosis of stroke. | |
| Interventions | The experimental group received a predischarge home assessment visit with an occupational therapist to identify and address any potential problems in the home environment The control group also received a predischarge home assessment with an occupational therapist, but this was conducted in the hospital. Potential problems were discussed in general terms |
|
| Outcomes | Number of falls, economic evaluation, and quality of life | |
| Notes | Fall registration during both intervention and follow‐up period. Registration time: 1 month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients recruited to the randomized controlled trial were registered using a web‐based randomization program. This was managed by Nottingham Clinical Trials Unit who held a pre‐prepared list in random varying block sizes." (Page 3) |
| Allocation concealment (selection bias) | High risk | No report about concealment was found |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient explanation regarding falls evaluation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analyses were carried out on the basis of intention to treat. For baseline and outcome measures where less than 10% of the total data were missing, mean values were imputed for individual missing items. Where 10% or more data were missing, the entire measure was coded as ‘missing’" (Page 3) |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | Unclear risk | No details except for a blinded researcher who assessed participant outcome |
Green 2002.
| Methods | Single‐masked RCT | |
| Participants | A total of 170 patients with mobility problems more than 1 year after stroke were included in the study | |
| Interventions | Intervention group (n = 85): physiotherapy treatment by an established community physiotherapy service as part of their usual work. Participants treated with a problem‐solving approach at home or in outpatient centre. A standard maximum contact period of 13 weeks with a minimum of 3 contacts per patient was agreed Control group (n = 85): no intervention |
|
| Outcomes | Number of fallers and quality of life | |
| Notes | Fall registration during both intervention and follow‐up period. Registration time: 9 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was achieved by numbered, sealed, opaque envelopes prepared from random number tables ..." (page 200) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was achieved by numbered, sealed, opaque envelopes prepared from random number tables ..." (page 200) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were asked to recall falls themselves |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | High risk | Fall ascertainment method used was retrospective recall (3‐month period) |
Haran 2010.
| Methods | Parallel assessor‐blinded RCT | |
| Participants | A subgroup of 46 people with stroke (n = 606 for the total group) who were regular wearers of multifocal glasses and had an increased risk of falls | |
| Interventions | Intervention group (n = 22): examination by an optometrist with prescription for a pair of single lens distance glasses Control group (n = 24): examination by an optometrist |
|
| Outcomes | Rate of falls, number of fallers, number of fall‐related fractures, and quality of life | |
| Notes | Fall registration during both intervention and follow‐up period. Registration time: 13 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "... by using sequentially numbered opaque envelopes containing group assignment." (page 2) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants recorded falls themselves through the use of a falls calendar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results |
| Selective reporting (reporting bias) | Low risk | All of the studies' pre‐specified outcomes have been reported in the pre‐specified way |
| Reliable ascertainment of falls/fallers outcome | Low risk | Participants returned the falls calendar each month. Follow‐up telephone calls were made as required |
Holmgren 2010.
| Methods | Single‐centre, single‐blinded RCT | |
| Participants | A total of 34 people after stroke with risk of falls were included in the study | |
| Interventions | A 5‐week programme consisting of:
|
|
| Outcomes | Number of fallers, quality of life, and adverse events | |
| Notes | Fall registration during both intervention and follow‐up period. Registration time: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of subjects ... was conducted a minimization software programme ..." (page 117) |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants recorded falls themselves through the use of a falls calendar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results |
| Selective reporting (reporting bias) | High risk | Falls are specified for the total group, but not for the intervention and control group separately |
| Reliable ascertainment of falls/fallers outcome | Unclear risk | Unclear whether the falls calendar that was used throughout the 6‐month follow‐up had to be returned monthly, 3‐monthly or at the end of the 6‐month follow‐up |
Lau 2012.
| Methods | RCT | |
| Participants | A total of 82 people in the chronic phase after stroke participated in the study | |
| Interventions | An 8‐week training programme consisting of:
|
|
| Outcomes | Rate of falls, number of fallers, number of people sustaining fall‐related fractures, number of people with fall‐related hospital admissions, and adverse events | |
| Notes | Fall registration during follow‐up period. Registration time: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "... using sealed opaque envelopes. To ensure concealed allocation, the procedures were performed by an "off‐site" researcher who was not involved in other parts of the study." (page 1410) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants recorded falls themselves through the use of a logbook |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | Low risk | Falls data were collected by means of a monthly interview until 6 months after the end of training |
Mansfield 2018.
| Methods | Multi‐site single‐blind RCT | |
| Participants | A total of 88 community‐dwelling individuals with chronic stroke (> 6 months post stroke) | |
| Interventions | 2 training sessions per week, lasting 1 hour per session, for 6 weeks. These sessions include:
Additionally, participants attended a 1 hour "booster" training 3 and 9 months following the initial training period |
|
| Outcomes | Rate of falls, number of fallers, number of people sustaining fall‐related fractures, number of people with fall‐related hospital admissions, quality of life, and adverse events | |
| Notes | Fall registration during follow‐up period. Registration time: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants will be assigned using blocked stratified randomization with allocation concealment to one of two training group ... To maintain allocation concealment, a variable block size ranging from 4–8 will be used. There will be four strata based on two stratification factors: site (two levels), and frequency of ‘failures’ during baseline reactive balance control assessment (two levels)." (Page 4) |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants will be assigned using blocked stratified randomization with allocation concealment to one of two training groups ..." Quote: "To maintain allocation concealment, a variable block size ranging from 4–8 will be used." (Page 4) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants recorded falls themselves through the use of a falls calendar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results Quote: "Intent‐to‐treat analysis will be used; all individuals with some falls‐monitoring data will be included in the analysis." (Page 8) |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | Low risk | Quote: "Participants will be provided stamped addressed postcards containing a calendar to record falls, which they will complete daily. Participants will be asked to return each postcard to the research team fortnightly. Participants will receive a monthly study newsletter by mail containing health‐related articles of interest, as well as a reminder to complete the postcards. If a participant does not return a postcard within two weeks, the research assistant will call them. In this telephone call, the research assistant will try to ascertain if the participant has experienced a fall in the previous two weeks." (Page 6) |
Marigold 2005.
| Methods | RCT | |
| Participants | A total of 61 community‐dwelling older adults with chronic stroke | |
| Interventions | A 10‐week exercise programme (1‐hour sessions, 3 times a week) consisting of:
|
|
| Outcomes | Rate of falls, number of fallers, number of people sustaining fall‐related fractures, and quality of life | |
| Notes | Fall registration during both intervention and follow‐up period. Registration time: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were ... randomly assigned alphanumeric codes through a random number generator." (page 417) |
| Allocation concealment (selection bias) | Low risk | Quote: "Subsequently, a person independent of the study (i.e. concealed allocation) randomly assigned participants ..." (page 417) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants recorded falls themselves through the use of a falls calendar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results |
| Selective reporting (reporting bias) | High risk | A post hoc primary outcome analysis using a subset of data was performed that was not prespecified |
| Reliable ascertainment of falls/fallers outcome | Low risk | Participants returned the falls calendar each month for a period of 1 year. Follow‐up telephone calls were made if the monthly diary was not returned |
Morone 2016.
| Methods | Prospective RCT | |
| Participants | A total of 44 first‐ever, unilateral stroke patients in the subacute phase (< 90 days post stroke) | |
| Interventions | Both control and intervention groups received 20 sessions (1 session a day, 5 days a week, 4 weeks) of exercises on hand recovery, tone control and improvement of global ability. In addition, 20 therapy sessions (1 session a day, 5 days a week, 4 weeks) were administered involving:
|
|
| Outcomes | Rate of falls and number of fallers | |
| Notes | Fall registration during follow‐up period. Registration time: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The trial was designed as a prospective randomized controlled trial based on CONSORT guidelines. After randomization, which was carried out using a random computer‐generated list, patients were consecutively assigned to one of the two groups" (Page 2) |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed from both patients and physiotherapists; only a non‐clinical experimenter who was not involved in the treatments had access to the randomized lists." (Page 2) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants recorded falls themselves |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | Unclear risk | Falling was only recorded during follow‐up, and specifications lack details about self‐reporting of falling during that period Quote: "(T2) follow‐up, corresponding to the 6 months after T1 during which the number of falls that occurred in the community was self‐reported." (Page 3) |
Taylor‐Piliae 2014.
| Methods | Single‐blind, 3‐group RCT | |
| Participants | A total of 145 community‐dwelling stroke survivors > 3 months post stroke | |
| Interventions | Participants assigned to 1 of the 2 experimental conditions attended a 1‐hour class, 3 times a week, for 12 weeks, involving:
|
|
| Outcomes | Number of fallers, number of people sustaining fall‐related fractures, number of people with fall‐related hospital admissions, number of people with near‐fall events, and quality of life | |
| Notes | Fall registration during intervention period. Registration time: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to TC, SilverSneakers (SS), or UC groups using simple randomization with allocation concealment." (Page 2) Quote: "Subjects drew a slip of paper from a non‐transparent container and were then handed an opaque, sealed envelope match‐ ing the slip of paper, and instructed to open the envelope when they returned home. We selected this method of randomization, e.g. having subjects ‘select’ their group, in order to reduce drop‐outs related to group assignment." (Taylor‐Piliae 2012, page 3) |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were randomly assigned to TC, SilverSneakers (SS), or UC groups using simple randomization with allocation concealment." (Page 2) Quote: "Subjects drew a slip of paper from a non‐transparent container and were then handed an opaque, sealed envelope match‐ ing the slip of paper, and instructed to open the envelope when they returned home. We selected this method of randomization, e.g. having subjects ‘select’ their group, in order to reduce drop‐outs related to group assignment." (Taylor‐Piliae 2012, page 3) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were interviewed weekly regarding the number of falls |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed and unlikely to seriously alter the results Quote: "Sensitivity analyses removing dropouts (nZ131) and comparing differences between groups were similar, except for race/ethnicity, which was no longer significantly different (X² = 4.93, PZ.09). Most dropouts (93%, nZ13) were white/European‐American." (Page 4) "Sensitivity analyses removing dropouts (nZ131, data not reported) were similar to results obtained using intention‐to‐treat analyses." (Page 5) |
| Selective reporting (reporting bias) | Low risk | All of the studies' prespecified outcomes have been reported in the prespecified way |
| Reliable ascertainment of falls/fallers outcome | Low risk | Quote: "...we interviewed participants weekly during the 12‐week intervention on the number of falls and near‐fall events they experienced." (Page 2) |
m/s: metres per second RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barreca 2004 | No true randomisation of participants |
| Bernhardt 2008 | Falls outcome included as adverse event |
| Boysen 2009 | Falls outcome included as adverse event |
| Cadilhac 2011 | Falls outcome included as adverse event |
| Calugi 2016 | Not an RCT |
| Cheng 2001 | No additional data received since publication of the original review |
| Creamer 2018 | Falls outcome included as adverse event |
| Dai 2013 | Inappropriate definition of falls |
| Duncan 2011 | Falls outcome included as adverse event |
| Eng 2010 | Narrative review, not including any new results |
| Gervasoni 2017 | Not an RCT |
| Goljar 2016 | Not an RCT |
| Halvarsson 2011 | Only 4 people with stroke included in the study (3 in the intervention and 1 in the control group) |
| Hesse 2011 | Falls outcome included as adverse event |
| Hill 2015 | Unable to provide details and data for stroke subgroup |
| Johansson 2018 | No stroke population |
| Kluding 2013 | Falls outcome included as adverse event |
| Kong 2009 | Falls outcome included as adverse event |
| Kwok 2005 | Falls outcome included as adverse event |
| Langhorne 2017 | Falls outcome included as adverse event |
| Mansfield 2017 | Not an RCT |
| Mayo 1994 | First author unable to provide details and data for stroke subgroup |
| Plummer‐D'Amato 2012 | Ongoing trial; no data available at present |
| Poletto 2015 | Falls outcome included as adverse event |
| Rosendahl 2008 | No additional data received since publication of the original review |
| Rossi 1990 | Falls outcome included as adverse event |
| Sato 2003 | No additional data received since publication of the original review + same author as retracted studies |
| Sato 2005a | Publication was retracted. Retraction note: "We wish to retract the paper entitled ‘Low‐dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial’ (Cerebrovascular Diseases 2005;20:187–92) by Y Sato, J Iwamoto, T Kanoko and K Satoh as a result of concerns about data integrity and scientific misconduct which have been brought to our attention. For further information see also http://retractionwatch.com/2016/11/09/analysis‐casts‐doubt‐on‐bone‐researchers‐body‐of‐work/. MG Hennerici, Editor‐in‐Chief M Fatar, Managing Editor" |
| Sato 2005b | Publication was retracted. Retraction note: "The following article: Sato Y, Iwamoto J, Kanoko T, Satoh K. Risedronate sodium therapy for prevention of hip fracture in men 65 years or older after stroke. Archives of Internal Medicine 2005;165(15):1743‐8, has been retracted due to acknowledgment of scientific misconduct resulting in concerns about data integrity and inappropriate assignment of authorship. H Bauchner, MD; RF Redberg, MD, MSc" |
| Sato 2005c | No additional data received since publication of the original review + same author as retracted studies |
| Sato 2011 | Questionable validity |
| Schmid 2013 | Falls outcome included as adverse event |
| Stark 2017 | Falls outcome included as adverse event |
| Tao 2015 | Falls outcome included as adverse event |
| Taylor‐Piliae 2011 | Falls outcome included as adverse event |
| Tyson 2018 | Falls outcome included as adverse event |
| Von Koch 2001 | Falls outcome included as adverse event |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Pedreira 2017.
| Methods | RCT |
| Participants | A total of 27 participants with hemiparesis after stroke |
| Interventions | 10 weeks of twice‐weekly physiotherapy visits lasting 1 hour, involving: For the experimental group: Quote: "..trunk mobilizations in the lateral, anterior and posterior directions, and stretching the arms and legs with a duration of 60 seconds, for a total time of 15 minutes, followed by 45 minutes of exercise with Nintendo Wii. The games were performed for 12 minutes each, with a 1‐minute interval between the two games." (Page 2) For the control group: stretching (10 minutes), trunk mobilisation (10 minutes), active (assisted) leg movement (15 minutes), balance training (10 minutes), free gait training (10 minutes) |
| Outcomes | Rate of falls and quality of life |
| Notes |
RCT: randomised controlled trial
Differences between protocol and review
We decided to pool results for one specific type of intervention (i.e. exercises) from single, multiple and multifactorial interventions. However, we additionally reported a sensitivity analysis of single interventions only. Thus, trials were not specifically grouped as single, multiple or multifactorial interventions as specified in our protocol.
Contributions of authors
For the 2013 version
G Verheyden planned the review, worked with A Ashburn and finalised the planning of the review based on comments from V Weerdesteyn, R Pickering, D Hyndman, S Lennon and A Geurts.
G Verheyden and V Weerdesteyn independently screened all search results.
G Verheyden, V Weerdesteyn and S Lennon independently screened full‐text papers, performed data extraction and risk of bias screening. A Ashburn also screened all full‐text papers for eligibility.
R Pickering and G Verheyden performed data analysis.
G Verheyden wrote the draft of the review and revised the draft based on comments from V Weerdesteyn, R Pickering, D Hyndman, S Lennon, A Geurts, and A Ashburn.
For the 2018 version
D Kunkel updated and ran the search strings for AMED and PsycINFO.
S Denissen updated and ran the search strings for all other databases and trial registers.
S Denissen and W Staring independently screened all search results.
S Denissen and W Staring independently screened full‐text papers, performed data extraction and risk of bias screening. G Verheyden provided supervision to the search process.
R Pickering and S Denissen performed data analysis.
S Denissen wrote the draft of the 2018 update version of the review and revised the draft based on comments from W Staring, D Kunkel, V Weerdesteyn, R Pickering, S Lennon, A Geurts and G Verheyden.
Declarations of interest
Stijn Denissen: none known
Wouter Staring: none known
Dorit Kunkel: none known
Ruth M Pickering: none known
Sheila Lennon: none known
Alexander CH Geurts: none known
Vivian Weerdesteyn: none known
Geert SAF Verheyden: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ada 2013 {published data only}
- Ada L, Dean CM, Lindley R. Randomized trial of treadmill training to improve walking in community‐dwelling people after stroke: the AMBULATE trial. International Journal of Stroke 2013;8(6):436‐44. [DOI] [PubMed] [Google Scholar]
Andrade 2017 {published data only}
- Andrade SM, Ferreira JJdA, Rufino TS, Medeiros G, Brito JD, Silva MA, et al. Effects of different montages of transcranial direct current stimulation on the risk of falls and lower limb function after stroke. Neurological Research 2017;39(12):1037‐43. [DOI: 10.1080/01616412.2017.1371473] [DOI] [PubMed] [Google Scholar]
Batchelor 2012 {published data only}
- Batchelor F, Hill K, Mackintosh S, Said C, Fryer C, Whitehead C. Falls prevention after stroke: does adherence to exercise influence falls?. Neurorehabilitation and Neural Repair 2012;26(6):735. [Google Scholar]
- Batchelor F, Hill K, Mackintosh S, Said C, Whitehead C. Does a multifactorial falls prevention programme reduce falls in people with stroke returning home after rehabilitation? A randomised controlled trial. International Journal of Stroke 2010;5:17. [Google Scholar]
- Batchelor FA, Hill KD, Mackintosh SF, Said CM, Whitehead CH. Effects of a multifactorial falls prevention programme for people with stroke returning home after rehabilitation: a randomised controlled trial. Archives of Physical Medicine and Rehabilitation 2012;93(9):1648‐55. [DOI: 10.1016/j.apmr.2012.03.031] [DOI] [PubMed] [Google Scholar]
- Batchelor FA, Hill KD, Mackintosh SF, Said CM, Whitehead CH. The FLASSH study: protocol for a randomised controlled trial evaluating falls prevention after stroke and two sub‐studies. BMC Neurology 2009;9:14. [DOI: 10.1186/1471-2377-9-14] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dean 2010 {published data only}
- Ada L, Dean CM, Morris ME. Supported treadmill training to establish walking in non‐ambulatory patients early after stroke. BMC Neurology 2007;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ada L, Dean CM, Morris ME, Simpson JM, Katrak P. Randomized trial of treadmill walking with body weight support to establish walking in subacute stroke: the MOBILISE trial. Stroke 2010;41(6):1237‐42. [DOI: 10.1161/STROKEAHA.109.569483] [DOI] [PubMed] [Google Scholar]
- Dean CM, Ada L, Bampton J, Morris ME, Katrak PH, Potts S. Treadmill walking with body weight support in subacute non‐ambulatory stroke improves walking capacity more than overground walking: a randomised trial. Journal of Physiotherapy 2010;56(2):97‐103. [DOI] [PubMed] [Google Scholar]
Dean 2012 {published data only}
- Dean CM, Rissel C, Sherrington C, Sharkey M, Cumming R, Lord SR, et al. Weight‐bearing exercise improves mobility in stroke survivors and may prevent falls in faster walkers. Neurorehabilitation and Neural Repair 2012;26(6):730‐1. [DOI] [PubMed] [Google Scholar]
- Dean CM, Rissel C, Sherrington C, Sharkey M, Cumming RG, Lord SR, et al. Exercise to enhance mobility and prevent falls after stroke: the community stroke club randomised trial. Neurorehabilitation and Neural Repair 2012;26(9):1046‐57. [DOI: 10.1177/1545968312441711] [DOI] [PubMed] [Google Scholar]
- Dean CM, Rissel C, Sharkey M, Sherrington C, Cumming RG, Barker RN, et al. Exercise intervention to prevent falls and enhance mobility in community dwellers after stroke: a protocol for a randomised controlled trial. BMC Neurology 2009;9:38. [DOI: 10.1186/1471-2377-9-38] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drummond 2012 {published data only}
- Drummond AE, Whitehead P, Fellows K, Sprigg N, Sampson CJ, Edwards C, et al. Occupational therapy predischarge home visits for patients with a stroke (HOVIS): results of a feasibility randomized controlled trial. Clinical Rehabilitation 2012;27(5):387‐97. [DOI] [PubMed] [Google Scholar]
Green 2002 {published data only}
- Green J, Forster A, Bogle S, Young J. Physiotherapy for patients with mobility problems more than 1 year after stroke: a randomised controlled trial. Lancet 2002;359(9302):199‐203. [DOI] [PubMed] [Google Scholar]
Haran 2010 {unpublished data only}
- Haran MJ, Cameron ID, Ivers IQ, Simpson JM, Lee BB, Tanzer M, et al. Effect on falls of providing single lens distance vision glasses to multifocal glasses wearers: VISIBLE randomised controlled trial. BMJ 2010;340:c2265. [DOI: 10.1136/bmj.c2265] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran MJ, Lord SR, Cameron ID, Ivers RQ, Simpson JM, Lee BB, et al. Preventing falls in older multifocal glasses wearers by providing single‐lens distance glasses: the protocol for the VISIBLE randomised controlled trial. BMC Geriatrics 2009;9:10. [DOI: 10.1186/1471-2318-9-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holmgren 2010 {published data only (unpublished sought but not used)}
- Holmgren E, Lindström B, Gosman‐Hedström G, Nyberg L, Wester P. What is the benefit of a high intensive exercise programme? A randomised controlled trial. Advances in Physiotherapy 2010;12(3):115‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren E, Gosman‐Hedström G, Lindström B, Wester P. What is the benefit of a high‐intensive exercise programme on health‐related quality of life and depression after stroke? A randomised controlled trial. Advances in Physiotherapy 2010;12(3):125‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2012 {published data only}
- Lau RW, Yip SP, Pang MY. Whole‐body vibration has no effect on neuromotor function and falls in chronic stroke. Medicine and Science in Sports and Exercise 2012;44(8):1409‐18. [DOI: 10.1249/MSS.0b013e31824e4f8c] [DOI] [PubMed] [Google Scholar]
Mansfield 2018 {published data only}
- Mansfield A, Aqui A, Centen A, Danells CJ, DePaul VG, Knorr S, et al. Perturbation training to promote safe independent mobility post‐stroke: study protocol for a randomized controlled trial. BMC Neurology 2015;15(1):87. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield A, Aqui A, Centen A, Danells CJ, Depaul VG, Knorr S, et al. Does perturbation‐based balance training prevent falls among individuals with chronic stroke? A randomised controlled trial. BMJ Open 2018;8(8):e021510. [DOI: 10.1186/s12883-015-0347-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marigold 2005 {published data only}
- Marigold DS, Eng JJ, Dawson AS, Inglis JT, Harris JE, Gylfadottir S. Exercise leads to faster postural reflexes, improved balance and mobility, and fewer falls in older persons with chronic stroke. Journal of the American Geriatrics Society 2005;53(3):416‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morone 2016 {published data only}
- Morone G, Annicchiarico R, Iosa M, Federici A, Paolucci S, Cortés U, et al. Overground walking training with the i‐Walker, a robotic servo‐assistive device, enhances balance in patients with subacute stroke: a randomized controlled trial. Journal of Neuroengineering and Rehabilitation 2016;13(1):47. [DOI: 10.1186/s12984-016-0155-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor‐Piliae 2014 {published data only}
- Taylor‐Piliae RE, Hoke TM, Hepworth JT, Latt LD, Najafi B, Coull BM. Effect of Tai Chi on physical function, fall rates and quality of life among older stroke survivors. Archives of Physical Medicine and Rehabilitation 2014;95:816‐24. [DOI: 10.1016/j.apmr.2014.01.001] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barreca 2004 {published data only}
- Barreca S, Sigouin CS, Lambert C, Ansley B. Effects of extra training on the ability of stroke survivors to perform an independent sit‐to‐stand: a randomised controlled trial. Journal of Geriatric Physical Therapy 2004;27(2):59‐64. [Google Scholar]
Bernhardt 2008 {published data only}
- Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke 2008;39(2):390‐6. [DOI: 10.1161/STROKEAHA.107.492363] [DOI] [PubMed] [Google Scholar]
- Sorbello D, Dewey HM, Churilov L, Thrift AG, Collier JM, Donnan G, et al. Very early mobilisation and complications in the first 3 months after stroke: further results from phase II of a very early rehabilitation trial (AVERT). Cerebrovascular Diseases 2009;28(4):378‐83. [DOI: 10.1159/000230712] [DOI] [PubMed] [Google Scholar]
Boysen 2009 {published data only}
- Boysen G, Krarup L, Zeng X, Oskedra A, Kõrv J, Andersen G, et al. ExStroke pilot trial of the effect of repeated instructions to improve physical activity after ischaemic stroke: a multinational randomised controlled clinical trial. BMJ 2009;339:b2810. [DOI: 10.1136/bmj.b2810] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cadilhac 2011 {published data only}
- Cadilhac DA, Hoffmann S, Kilkenny M, Lindley R, Lalor E, Osborne RH, et al. A phase II multicentered single‐blind, randomised, controlled trial of the stroke self‐management programme. Stroke 2011;42(6):1673‐9. [DOI: 10.1161/STROKEAHA.110.601997] [DOI] [PubMed] [Google Scholar]
Calugi 2016 {published data only}
- Calugi S, Taricco M, Rucci P, Fugazzaro S, Stuart M, Dallolio L, et al. Effectiveness of adaptive physical activity combined with therapeutic patient education in stroke survivors at twelve months: a non‐randomized parallel group study. European Journal of Physical and Rehabilitation Medicine 2016;52(1):72‐80. [PubMed] [Google Scholar]
Cheng 2001 {published data only}
- Cheng PT, Wu SH, Liam MY, Wong AM, Tang FT. Symmetrical body‐weight distribution training in stroke patients and its effect on fall prevention. Archives of Physical Medicine and Rehabilitation 2001;82(12):1650‐4. [DOI] [PubMed] [Google Scholar]
Creamer 2018 {published data only}
- Creamer M, Cloud G, Kossmehl P, Yochelson M, Francisco GE, Ward AB, et al. Intrathecal baclofen therapy versus conventional medical management for severe poststroke spasticity: results from a multicentre, randomised, controlled, open‐label trial (SISTERS). Journal of Neurology, Neurosurgery, and Psychiatry 2018;89(6):642‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochelson M, Creamer M, Cloud G, Kossmehl P, Francisco G, Ward A, et al. Intrathecal baclofen therapy decreases pain and improves quality of life compared to conventional medical management in severe post‐stroke spasticity: the SISTERS study. Neurology 2018;90(15 Supplement):5.014. [Google Scholar]
Dai 2013 {published data only}
- Dai C‐Y, Huang Y‐H, Chou L‐W, Wu SC, Wang R‐Y, Lin L‐C. Effects of primary caregiver participation in vestibular rehabilitation for unilateral neglect patients with right hemispheric stroke: a randomized controlled trial. Neuropsychiatric Disease and Treatment 2013;9:477‐84. [DOI: 10.2147/NDT.S42426] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duncan 2011 {published data only}
- Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body‐weight supported treadmill rehabilitation after stroke. New England Journal of Medicine 2011;364(21):2026‐36. [DOI: 10.1056/NEJMoa1010790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eng 2010 {published data only}
- Eng JJ. Fitness and mobility exercise (FAME) programme for stroke. Topics in Geriatric Rehabilitation 2010;26(4):310‐23. [DOI: 10.1097/TGR.0b013e3181fee736] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gervasoni 2017 {published data only}
- Gervasoni E, Parelli R, Uszynski M, Crippa A, Marzegan A, Montesano A, et al. Effects of functional electrical stimulation on reducing falls and improving gait parameters in multiple sclerosis and stroke. American Academy of Physical Medicine and Rehabilitation 2017;9(4):392‐7. [DOI] [PubMed] [Google Scholar]
Goljar 2016 {published data only}
- Goljar N, Globokar D, Puzić N, Kopitar N, Vrabič M, Ivanovski M, et al. Effectiveness of a fall‐risk reduction programme for inpatient rehabilitation after stroke. Disability and Rehabilitation 2016;38(18):1811‐9. [DOI] [PubMed] [Google Scholar]
Halvarsson 2011 {published data only}
- Halvarsson A, Oddsson L, Olsson E, Farén E, Pettersson A, Ståhle A. Effects of new, individually adjusted, progressive balance group training for elderly people with fear of falling and tend to fall: a randomized controlled trial. Clinical Rehabilitation 2011;25(11):1021‐31. [DOI: 10.1177/0269215511411937] [DOI] [PubMed] [Google Scholar]
Hesse 2011 {published data only}
- Hesse S, Welz A, Werner C, Quentin B, Wissel J. Comparison of an intermittent high‐intensity vs continuous low‐intensity physiotherapy service over 12 months in community‐dwelling people with stroke: a randomised trial. Clinical Rehabilitation 2011;25(2):146‐56. [DOI: 10.1177/0269215510382148] [DOI] [PubMed] [Google Scholar]
Hill 2015 {published data only}
- Hill A‐M, McPhail SM, Waldron N, Etherton‐Beer C, Ingram K, Flicker L, et al. Fall rates in hospital rehabilitation units after individualised patient and staff education programmes: a pragmatic, stepped‐wedge, cluster‐randomised controlled trial. Lancet 2015;385(9987):2592–9. [DOI] [PubMed] [Google Scholar]
Johansson 2018 {published data only}
- Johansson E, Jonsson H, Dahlberg R, Patomella A‐H. The efficacy of a multifactorial falls‐prevention programme, implemented in primary health care. British Journal of Occupational Therapy 2018;81(8):474‐81. [Google Scholar]
Kluding 2013 {published data only}
- Dunning K, O'Dell M, Kluding P, Wu SS, Feld J, Ginosian J, et al. The functional ambulation: standard treatment vs. electrical stimulation therapy (fastest) trial for stroke: study design and protocol. Open Access Journal of Clinical Trials 2013;5:39. [Google Scholar]
- Kluding PM, Dunning K, O'Dell MW, Wu SS, Ginosian J, Feld J, et al. Foot drop stimulation versus ankle foot orthosis after stroke: 30‐week outcomes. Stroke 2013;44(6):1660‐9. [DOI] [PubMed] [Google Scholar]
Kong 2009 {published data only}
- Kong KH, Wee SK, Ng CY, Chua K, Chan KF, Venketasubramanian N, et al. A double‐blind, placebo‐controlled, randomised phase II pilot study to investigate the potential efficacy of the traditional Chinese medicine Neuroaid (MLC601) in enhancing recovery after stroke (TIERS). Cerebrovascular Diseases 2009;28(5):514‐21. [DOI: 10.1159/000247001] [DOI] [PubMed] [Google Scholar]
Kwok 2005 {published data only}
- Kwok T, Mok F, Chien WT, Tam E. Does access to bed‐chair pressure sensors reduce physical restraint use in the rehabilitative care setting. Journal of Clinical Nursing 2006;15(5):581‐7. [DOI] [PubMed] [Google Scholar]
Langhorne 2017 {published data only}
- Langhorne P, Wu O, Rodgers H, Ashburn A, Bernhardt J. A Very Early Rehabilitation Trial after stroke (AVERT): a phase III, multicentre, randomised controlled trial. Health Technology Assessment 2017;21(54):1‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansfield 2017 {published data only}
- Mansfield A, Schinkel‐Ivy A, Danells CJ, Aqui A, Aryan R, Biasin L, et al. Does perturbation training prevent falls after discharge from stroke rehabilitation? A prospective cohort study with historical control. Journal of Stroke and Cerebrovascular Diseases 2017;26(10):2174‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayo 1994 {published data only (unpublished sought but not used)}
- Mayo NE, Gloutney L, Levy AR. A randomised trial of identification bracelets to prevent falls among patients in a rehabilitation hospital. Archives of Physical Medicine and Rehabilitation 1994;75(12):1302‐8. [PubMed] [Google Scholar]
Plummer‐D'Amato 2012 {published data only}
- Plummer‐D'Amato P, Kyvelidou A, Sternad D, Najafi B, Villalobos RM, Zurakowski D. Training dual‐task walking in community‐dwelling adults within 1 year of stroke: a protocol for a single‐blind randomized controlled trial. BMC Neurology 2012;12:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poletto 2015 {published data only}
- Poletto SR, Rebello LC, Valença MJ, Rossato D, Almeida AG, Brondani R, et al. Early mobilization in ischemic stroke: a pilot randomized trial of safety and feasibility in a public hospital in Brazil. Cerebrovascular Diseases Extra 2015;5(1):31‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosendahl 2008 {published data only}
- Rosendahl E, Gustafson Y, Nordin E, Lundin‐Olsson L, Nyberg L. A randomized controlled trial of fall prevention by a high‐intensity functional exercise program for older people living in residential care facilities. Aging Clinical and Experimental Research 2008;20(1):67‐75. [DOI] [PubMed] [Google Scholar]
Rossi 1990 {published data only}
- Rossi PW, Kheyfets S, Reding MJ. Fresnel prisms improve visual perception in stroke patients with homonymous hemianopia or unilateral visual neglect. Neurology 1990;40(10):1597‐9. [DOI] [PubMed] [Google Scholar]
Sato 2003 {published data only}
- Sato Y, Metoki N, Iwamoto J, Satoh K. Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in stroke patients. Neurology 2003;61(3):338‐42. [DOI] [PubMed] [Google Scholar]
Sato 2005a {published data only}
- Sato Y, Iwamoto J, Kanoko T, Satoh K. Low‐Dose Vitamin D Prevents Muscular Atrophy and Reduces Falls and Hip Fractures in Women after Stroke: A Randomized Controlled Trial (RETRACTED ARTICLE). Cerebrovascular Diseases 2005;20(3):187‐192. [DOI: 10.1159/000087203] [DOI] [PubMed] [Google Scholar]
Sato 2005b {published data only}
- Sato Y, Iwamoto J, Kanoko T, Satoh K. Risedronate sodium therapy for prevention of hip fracture in men 65 years or older after stroke (RETRACTED ARTICLE). Archives of Internal Medicine 2005;165(15):1743‐8. [DOI] [PubMed] [Google Scholar]
Sato 2005c {published data only}
- Sato Y, Iwamoto J, Kanoko T, Satoh K. Risedronate therapy for prevention of hip fracture after stroke in elderly women. Neurology 2005;64(5):811‐6. [DOI] [PubMed] [Google Scholar]
Sato 2011 {published data only}
- Sato Y, Iwamoto J, Honda Y. An Open‐Label Trial Comparing Alendronate and Alphacalcidol in Reducing Falls and Hip Fractures in Disabled Stroke Patients. Journal of Stroke and Cerebrovascular Diseases 2011;20(1):41‐46. [DOI: 10.1016/j.jstrokecerebrovasdis.2009.10.007] [DOI] [PubMed] [Google Scholar]
Schmid 2013 {published data only}
- Schmid AA, Yaggi HK, Burrus N, McClain V, Austin C, Ferguson J, et al. Circumstances and consequences of falls among people with chronic stroke. Journal of Rehabilitation Research and Development 2013;50(9):1277‐86. [DOI] [PubMed] [Google Scholar]
Stark 2017 {published data only}
- Stark S, Keglovits M, Somerville E, Hu Y‐L, Conte J, Yan Y. Feasibility of a novel intervention to improve participation after stroke. British Journal of Occupational Therapy 2018;81(2):116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tao 2015 {published data only}
- Tao J, Rao T, Lin L, Liu W, Wu Z, Zheng G, et al. Evaluation of Tai Chi Yunshou exercises on community‐based stroke patients with balance dysfunction: a study protocol of a cluster randomized controlled trial. BMC Complementary and Alternative Medicine 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor‐Piliae 2011 {published data only}
- Taylor‐Piliae RE, Coull BM. Community‐based Yang‐style Tai Chi is safe and feasible in chronic stroke: a pilot study. Clinical Rehabilitation 2012;26(2):121‐31. [DOI] [PubMed] [Google Scholar]
Tyson 2018 {published data only}
- Tyson SF, Vail A, Thomas N, Woodward‐Nutt K, Plant S, Tyrrell PJ. Bespoke versus off‐the‐shelf ankle‐foot orthosis for people with stroke: randomized controlled trial. Clinical Rehabilitation 2018;32(3):367‐76. [DOI] [PubMed] [Google Scholar]
Von Koch 2001 {published data only}
- Thorsén AM, Widén Holmqvist LW, Pedro‐Cuesta J, Koch L. A randomised controlled trial of early supported discharge and continued rehabilitation at home after stroke: five‐year follow‐up of patient outcome. Stroke 2005;36(2):297‐303. [DOI] [PubMed] [Google Scholar]
- Thorsén AM, Widén Holmqvist L, Von Koch L. Early supported discharge and continued rehabilitation at home after stroke: 5‐year follow‐up of resource use. Journal of Stroke and Cerebrovascular Diseases 2006;15(4):139‐43. [DOI] [PubMed] [Google Scholar]
- Koch L, Widén Holmqvist L, Kostulas V, Almazan J, Pedro‐Cuesta J. A randomised controlled trial of rehabilitation at home after stroke in southwest Stockholm: outcome at six months. Scandinavian Journal of Rehabilitation Medicine 2000;32(2):80‐6. [DOI] [PubMed] [Google Scholar]
- Koch L, Pedro‐Cuesta J, Kostulas V, Almazan J, Widén Holmqvist L. Randomized controlled trial of rehabilitation at home after stroke: one‐year follow‐up of patient outcome, resource use and cost. Cerebrovascular Diseases 2001;12(2):131‐8. [DOI] [PubMed] [Google Scholar]
- Widén Holmqvist L, Koch L, Kostulas V, Holm M, Widsell G, Tegler H, et al. A randomised controlled trial of rehabilitation at home after stroke in southwest Stockholm. Stroke 1998;29(3):591‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Pedreira 2017 {published data only}
- Pedreira da Fonseca E, Ribeiro Da Silva MN, Pinto EB. Therapeutic effect of virtual reality on post‐stroke patients: randomized clinical trial. Journal of Stroke and Cerebrovascular Diseases 2017;26:94‐100. [DOI: 10.1016/j.jstrokecerebrovasdis.2016.08.035] [DOI] [PubMed] [Google Scholar]
Additional references
Andersson 2008
- Andersson AG, Kamwendo K, Appelros P. Fear of falling in stroke patients: relationship with previous falls and functional characteristics. International Journal of Rehabilitation Research. Internationale Zeitschrift fur Rehabilitationsforschung. Revue Internationale de Recherches de Readaptation 2008;31(3):261‐4. [DOI] [PubMed] [Google Scholar]
Ashburn 2008
- Ashburn A, Hyndman D, Pickering R, Yardley L, Harris S. Predicting people with stroke at risk of falls. Age and Ageing 2008;37(3):270‐6. [DOI: 10.1093/ageing/afn066] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batchelor 2010
- Batchelor F, Hill K, Mackintosh S, Said C. What works in falls prevention after stroke? A systematic review and meta‐analysis. Stroke 2010;41(1):1715‐22. [DOI: 10.1161/STROKEAHA.109.570390] [DOI] [PubMed] [Google Scholar]
Belgen 2006
- Belgen B, Beninato M, Sullivan PE, Narielwalla K. The association of balance capacity and falls self‐efficacy with history of falling in community‐dwelling people with chronic stroke. Archives of Physical Medicine and Rehabilitation 2006;87(4):554‐61. [DOI] [PubMed] [Google Scholar]
Bernhardt 2017
- Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabilitation and Neural Repair 2017;31(9):793‐9. [DOI: 10.1177/1545968317732668] [DOI] [PubMed] [Google Scholar]
Burridge 2007
- Burridge JH, Haugland M, Larsen B, Pickering RM, Svaneborg N, Iversen HK, et al. Phase II trial to evaluate the ActiGait implanted drop‐foot stimulator in established hemiplegia. Journal of Rehabilitation Medicine 2007;39(3):212‐8. [DOI] [PubMed] [Google Scholar]
Cameron 2018
- Cameron ID, Dyer SM, Panagoda CE, Murray GR, Hill KD, Cumming RG, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD005465.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2018
- Collins M, Clifton E, Wijck F, Mead GE. Cost‐effectiveness of physical fitness training for stroke survivors. Journal of the Royal College of Physicians of Edinburgh 2018;48(1):62–8. [DOI] [PubMed] [Google Scholar]
CONSORT
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine 2010;152(11):726‐32. [DOI] [PubMed] [Google Scholar]
Davenport 1996
- Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications after acute stroke. Stroke 1996;27(3):415‐20. [DOI] [PubMed] [Google Scholar]
Demaerschalk 2010
- Demaerschalk BM, Hwang HM, Leung G. US cost burden of ischemic stroke: a systematic literature review. American Journal of Managed Care 2010;16(7):525‐33. [PubMed] [Google Scholar]
Eng 2008
- Eng JJ, Pang MY, Ashe MC. Balance, falls, and bone health: role of exercise in reducing fracture risk after stroke. Journal of Rehabilitation Research and Development 2008;45(2):297‐313. [DOI] [PubMed] [Google Scholar]
English 2017
- English C, Hillier SL, Lynch EA. Circuit class therapy for improving mobility after stroke. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD007513.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faes 2010
- Faes MC, Reelick MF, Joosten‐Weyn Banningh LW, Gier M, Esselink RA, Olde Rikkert MG. Qualitative study on the impact of falling in frail older persons and family caregivers: foundations for an intervention to prevent falls. Aging & Mental Health 2010;14(7):834‐42. [DOI] [PubMed] [Google Scholar]
Gillespie 2012
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD007146.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 29 November 2018. Hamilton (ON): McMaster University (developed by Evidence Prime).
Grossman 2018
- Grossman DC, Curry SJ, Owens DK, Barry MJ, Caughey AB, Davidson KW, et al. Interventions to prevent falls in community‐dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018;319(16):1696‐1705. [DOI: 10.1001/jama.2017.21962] [DOI] [PubMed] [Google Scholar]
Harris 2005
- Harris JE, Eng JJ, Marigold DS, Tokuno CD, Louis CL. Relationship of balance and mobility to fall incidence in people with chronic stroke. Physical Therapy 2005;85(2):150‐8. [PubMed] [Google Scholar]
Hauer 2006
- Hauer K, Lamb SE, Jorstad EC, Todd C, Becker C, the ProFaNE‐group. Systematic review of definitions and methods of measuring falls in randomised controlled fall prevention trials. Age and Ageing 2006;35(1):5‐10. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hoffmann 2014
- Hoffmann T, Glasziou P, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Indredavik 2008
- Indredavik B, Rohweder G, Naalsund E, Lydersen S. Medical complications in a comprehensive stroke unit and an early supported discharge service. Stroke 2008;39(2):414‐20. [DOI] [PubMed] [Google Scholar]
Introduction to Meta‐Analysis 2009
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta‐Analysis. John Wiley & Sons, Ltd, 2009. [DOI: 10.1002/9780470743386.refs; ISBN 9780470057247] [DOI] [Google Scholar]
Jung 2015
- Jung Y, Lee K, Shin S, Lee W. Effects of a multifactorial fall prevention program on balance, gait, and fear of falling in post‐stroke inpatients. Journal of Physical Therapy Science 2015;27(6):1865‐8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerse 2008
- Kerse N, Parag V, Feigin VL, McNaughton H, Hackett ML, Bennett DA, et al. Falls after stroke: results from the Auckland Regional Community Stroke (ARCOS) Study, 2002 to 2003. Stroke 2008;39(6):1890‐3. [DOI: 10.1161/STROKEAHA.107.509885] [DOI] [PubMed] [Google Scholar]
Kuan 1999
- Kuan TS, Tsou JY, Su FC. Hemiplegic gait of stroke patients: the effect of using a cane. Archives of Physical Medicine and Rehabilitation 1999;80(7):777‐84. [DOI] [PubMed] [Google Scholar]
Kunkel 2011
- Kunkel D, Pickering RM, Ashburn AM. Comparison of retrospective interviews and prospective diaries to facilitate fall reports among people with stroke. Age and Ageing 2011;40(2):277‐80. [DOI: 10.1093/ageing/afq177] [DOI] [PubMed] [Google Scholar]
Lamb 2005
- Lamb SE, Jorstad‐Stein EC, Hauer K, Becker C, Prevention of Falls Network Europe and Outcomes Consensus Group. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. Journal of the American Geriatrics Society 2005;53(9):1618–22. [DOI] [PubMed] [Google Scholar]
Lamb 2007
- Lamb SE, Hauer K, Becker C. Manual for the fall prevention classification system. www.profane.eu.org/documents/Falls_Taxonomy.pdf (accessed 30 January 2013).
Lamb 2011
- Lamb SE, Becker C, Gillespie LD, Smith JL, Finnegan S, Potter R, et al. Reporting of complex interventions in clinical trials: development of a taxonomy to classify and describe fall‐prevention interventions. Trials 2011;12:125. [DOI: 10.1186/1745-6215-12-125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landers 2016
- Landers MR, Oscar S, Sasaoka J, Vaughn K. Balance confidence and fear of falling avoidance behavior are most predictive of falling in older adults: prospective analysis. Physical Therapy 2016;96(4):433‐42. [DOI: ] [DOI] [PubMed] [Google Scholar]
Langhorne 2000
- Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke: a multicenter study. Stroke 2000;31(6):1223‐9. [DOI] [PubMed] [Google Scholar]
Laver 2017
- Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD008349.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2018
- Li Y, Fan J, Yang J, He C, Li S. Effects of transcranial direct current stimulation on walking ability after stroke: a systematic review and meta‐analysis. Restorative Neurology and Neuroscience 2018;36(1):59‐71. [DOI: 10.3233/RNN-170770] [DOI] [PubMed] [Google Scholar]
Lin 2017
- Lin MH, Chen SR, Liao MN, Chen YC, Chang WY. Factors and medical costs associated with fall events in hospitalized patients. Journal of Nursing 2017;64(4):44‐52. [DOI] [PubMed] [Google Scholar]
Maeda 2015
- Maeda N, Urabe Y, Murakami M, Itotani K, Kato J. Discriminant analysis for predictor of falls in stroke patients by using the Berg Balance Scale. Singapore Medical Journal 2015;56(5):280‐3. [DOI: 10.11622/smedj.2015033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2015
- Mao Y‐R, Lo WL, Lin Q, Li L, Xiao X, Raghavan P, et al. The effect of body weight support treadmill training on gait recovery, proximal lower limb motor pattern, and balance in patients with subacute stroke. BioMed Research International 2015;2015:175719. [DOI: 10.1155/2015/175719] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marigold 2006
- Marigold DS, Eng JJ. Altered timing of postural reflexes contributes to falling in persons with chronic stroke. Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale 2006;171(4):459‐68. [DOI: 10.1007/s00221-005-0293-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- NICE. Stroke Rehabilitation 2013:CG162.
Punt 2017
- Punt M, Bruijn SM, Wittink H, Port IG, Dieën JH. Do clinical assessments, steady‐state or daily‐life gait characteristics predict falls in ambulatory chronic stroke survivors?. Journal of Rehabilitation Medicine 2017;49(5):402‐9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sackley 2008
- Sackley C, Brittle N, Patel S, Ellins J, Scott M, Wright C, et al. The prevalence of joint contractures, pressure sores, painful shoulder, other pain, falls, and depression in the year after a severely disabling stroke. Stroke 2008;39(12):3329‐34. [DOI: 10.1161/STROKEAHA.108.518563] [DOI] [PubMed] [Google Scholar]
Sherrington 2019
- Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, et al. Exercise for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2019, Issue 1. [DOI: 10.1002/14651858.CD012424.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stack 1999
- Stack E, Ashburn A. Fall events described by people with Parkinson’s disease: implications for clinical interviewing and the research agenda. Physiotherapy Research International 1999;4(3):190‐200. [DOI] [PubMed] [Google Scholar]
Tricco 2017
- Tricco AC, Thomas SM, Veroniki AA, Hamid JS, Cogo E, Strifler L, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta‐analysis. JAMA 2017;318(17):1687‐99. [DOI: 10.1001/jama.2017.15006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Duijnhoven 2016
- Duijnhoven HJ, Heeren A, Peters MAM, Veerbeek JM, Kwakkel G, Geurts ACH, et al. Effects of exercise therapy on balance capacity in chronic stroke: systematic review and meta‐analysis. Stroke 2016;47(10):2603‐10. [DOI] [PubMed] [Google Scholar]
Wagner 2009
- Wagner LM, Phillips VL, Hunsaker AE, Forducey PG. Falls among community‐residing stroke survivors following inpatient rehabilitation: a descriptive analysis of longitudinal data. BMC Geriatrics 2009;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weerdesteyn 2008
- Weerdesteyn V, Niet M, Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. Journal of Rehabilitation Research and Development 2008;45(8):1195‐213. [PubMed] [Google Scholar]
Winser 2018
- Winser SJ, Tsang WWN, Krishnamurthy K, Kannan P. Does Tai Chi improve balance and reduce falls incidence in neurological disorders? A systematic review and meta‐analysis.. Clinical Rehabilitation 2018;32(9):1157‐68. [DOI: ] [DOI] [PubMed] [Google Scholar]
Xu 2018
- Xu T, Clemson L, O'Loughlin K, Lannin NA, Dean C, Koh G. Risk factors for falls in community stroke survivors: a systematic review and meta‐analysis. Archives of Physical Medicine and Rehabilitation 2018;99(3):563‐73. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Verheyden 2013
- Verheyden GSAF, Weerdesteyn V, Pickering RM, Kunkel D, Lennon S, Geurts ACH, et al. Interventions for preventing falls in people after stroke. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD008728.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


