Abstract
Obesity is associated with reduced spontaneous and stimulated growth hormone (GH) secretion and basal insulin-like growth factor I (IGF-1) levels—which in turn is associated with increased prevalence of cardiovascular risk factors. The aim of this study was to investigate: (1) the association of somatotropic axis with cardiometabolic status; (2) the association of somatotropic axis with the Mediterranean diet and nutritional pattern in people with obesity. Cross-sectional observational study was carried out in 200 adult women, aged 36.98 ± 11.10 years with severe obesity (body mass index—BMI of 45.19 ± 6.30 kg/m2). The adherence to the Mediterranean diet and the total calorie intake was assessed. Anthropometric measurements, body composition and biochemical profile were determined along with Growth Hormone (GH)/Insulin like Growth Factor 1 (IGF-1) axis and insulin resistance (homeostatic model assessment for insulin resistance—HoMA-IR). The enrolled subjects were compared after being divided according to GH peak response and according to IGF-1 standard deviation scores (SDS). Derangements of GH peak were detected in 61.5% of studied patients while IGF-1 deficiency was detected in 71% of the population. Both blunted GH peak response and IGF-1 SDS were indicators of derangements of somatotropic axis and were associated with comparable results in terms of cardiometabolic sequelae. Both GH peak and IGF-1 levels were inversely associated with anthropometric and metabolic parameters. The adherence to the Mediterranean diet predicts GH peak response. Fatty liver index (FLI), fat mass (FM) and phase angle (PhA) were predictive factors of GH peak response as well. In conclusion derangements of somatotropic axis is associated with a worse cardiometabolic profile in people with obesity. A high adherence to the Mediterranean diet—and in particular protein intake—was associated with a better GH status.
Keywords: obesity, GH deficiency, IGF-1, insulin resistance, Mediterranean diet
1. Introduction
The GH/IGF-1 axis is finely regulated at multiple steps by neuroendocrine mediators, tissue and soluble receptors and carrier proteins. Growth hormone (GH) secretion—either spontaneous or evoked by provocative stimuli—is markedly disrupted in obesity [1,2]. The alterations of GH/IGF-1 axis in obesity are characterized by the decrease in the half-life of GH along with a reduction in both frequency and amplitude of GH secretory bursts. These phenomena are associated with an increased GH metabolic clearance rate which at the end results in low plasma GH levels [3]. A worse cardiovascular risk profile and body composition with increased cardiometabolic consequences has been detected in obese individuals with a low GH status compared to obese individuals without impairments in the somatotropic axis [1,4,5,6,7]. Moreover, the alterations of GH/IGF-1 axis could encourage the onset of sarcopenic obesity which in turn could represent a further obstacle for physical activity, thus worsening cardiovascular risk and mineral metabolism [8,9]. The low GH status in obesity is considered to be an acquired functional defect; in fact, it has been demonstrated to be reversed after weight loss process. The low GH status seems to be often associated with visceral obesity leading us to hypothesize that fat deposition at abdominal site could be one of the most determining player of GH secretion derangements in obesity. FFA (free fatty acids), which are known to be tightly related to visceral adipose tissue, have been reported to have a role in lowering GH secretion, and the administration of antilipolytic agent (acipimox) restored a normal GH secretion through the inhibition of FFA [10]. Further, dysregulation of GHRH (GH releasing hormone), somatostatin, ghrelin pathways, and hyperinsulinemia contribute to blunt GH secretion in obesity [11]. However, GH/IGF-1 axis function could be regulated by nutritional components as well [12]. Several studies have consistently reported that a high milk intake exerts relevant effects on somatotropic axis by increasing both growth hormone (GH) and insulin-like growth factor-1 (IGF-1) secretion [13,14,15]. Moreover, it has been reported that short-time breastfeeding has been associated with an increased risk of developing childhood obesity [16]. Currently, no studies on the regulation of the somatotropic axis by dietary factors, mainly a Mediterranean diet, were carried out in people with obesity. The aims of this study are two-fold: firstly, to evaluate the association between cardio metabolic status and somatotropic axis in obese individuals; secondly, to investigate the association between the Mediterranean diet and GH secretory status in these subjects.
2. Materials and Methods
2.1. Design and Setting
This is a cross-sectional observational study carried out at the Department of Clinical Medicine and Surgery of the University of Naples Federico II (Italy). The procedures used were in accordance with the guidelines of the Helsinki Declaration on human experimentation. The study was approved by the Ethics Committee of the Federico II University Medical School of Naples (n 5/14). The aim of the study was clearly explained to all the enrolled subjects.
2.2. Population Study
Two hundred adult female individuals (>18 years of age) were enrolled after providing written informed consent. None of the study participants had any known relevant endocrine or non-endocrine diseases. Eligible participants for the study were adult subjects aged 18–75 years with a BMI (body mass index) >35 kg/m2. The participants had normal liver, cardiopulmonary and kidney functions as determined by medical history, physical examination, electrocardiogram, urinalysis blood tests for blood urea nitrogen, creatinine, uric acid, albumin, aspartate aminotransferase, and alanine aminotransferase. Other exclusion criteria were alcohol or drug abuse and history of allergy or intolerance to olive oil. Subjects following a specific dietary regimen for any reason were excluded from the study as well. The study was conducted without support from the pharmaceutical industry.
2.3. Dietary Assessment
A seven day food record was used in order to perform dietary assessment [17,18]. The instructions on how to fill the diary was provided by nutritionists, whom recommended participants to report the previous day’s intake. Participants prospectively filled the diary regarding the rest of the week. The enrolled patients brought back the diary records to the nutritionist who made further enquiries if needed. A commercial software was used to store data [19]. Based on quantities and qualities of food intake, the software calculated not only the daily caloric intake but the amount of macronutrients as well (protein; total, complex, and simple carbohydrates; fibers; total fat, saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA): n-6 PUFA, n-3 PUFA and n-6/n-3 PUFAs ratio; and cholesterol). As previously reported [20,21,22,23,24,25], the adherence to the Mediterranean Diet was assessed by a previously validated 14-items questionnaire) PREDIMED (PREvention with MEDiterranean Diet) questionnaire [26]. The questionnaire was performed by a qualified nutritionist through a face-to-face interview to all the enrolled subjects. Scores of 1 and 0 were assigned for each item. The PREDIMED score was calculated as follows: score 0–5, lowest adherence; score 6–9, average adherence and score ≥10, highest adherence [26], as already reported [27,28,29,30,31].
2.4. Anthropometric Measurements
Subjects dressed in light clothes and no shoes when anthropometric parameters were assessed. The formula of the BMI was the following: weight (kg)/height (m2). A wall-mounted stadiometer was used to assess height. A calibrated scale was used to assess body weight. Waist circumference (WC) was measured to the closest 0.1 cm with a no extensible tape. In all individuals, a sphygmomanometer (Gelman Hawksley Ltd., Sussex, UK) was used to measure systolic (SBP) and diastolic (DBP) blood pressure after the subject rested for a minimum of 10 min.
2.5. Laboratory Test
After fasting overnight, the venous blood samples were collected from the antecubital vein and was stored in vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA). A Roche Modular Analytics System was used to assess all biochemical parameters—including total cholesterol and triglycerides (TG), Alanine Transaminase (ALT), Aspartate Aminotransferase (AST), and γ-Glutamyltransferase (γGT). Low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol were assessed by a direct method (homogeneous enzymatic assay for the direct quantitative determination of LDL and HDL cholesterol). The glucose oxidase method was used to assess fasting plasma glucose. Fasting insulin levels were measured by a solid-phase chemiluminescent enzyme immunoassay using commercially available kits (Immunolite Diagnostic Products Co., Los Angeles, CA, USA). The intra-assay coefficients of variations (CV) was <5.5%. A c-reactive protein (CRP) levels were determined with a nephelometric assay with CardioPhase high-sensitive from Siemens Healthcare Diagnostics (Marburg, Germany). The intra-assay CV for CRP was <4%; low detection limit was >0.1 mg/L. The GH/IGF-1 axis was evaluated by measuring GH peak after GHRH + ARGININE (ARG) and assay of circulating IGF-1 levels. The GHRH (1–29, Geref, Serono, Rome, Italy) + ARG (arginine hydrochloride, Salf, Bergamo, Italy) test was performed according to Ghigo et al. [32]. The GH response after GHRH + ARG was classified as deficient when GH peak was ≤4.2 mg/L and sufficient when GH peak was ≥4.2 mg/L [33]. Serum GH levels were measured by immunoradiometric assay (IRMA) using commercially available kits (HGH-CTK-IRMA, Sorin, Saluggia, Italy). The sensitivity of the assay was 0.02 mg/L. The intra- and inter-assay coefficients of variations (CVs.) were 4.5% and 7.9%, respectively. IGF-1 levels were classified as deficient when the standard deviation scores (SDS) from the mean was <−2 for age and gender and sufficient when the SDS ranged from >−2 to 2.24 [34]. Serum IGF-1 levels were measured by IRMA after ethanol extraction (DSL Inc., Webster, TX, USA); assay sensitivity was 0.8 mg/L. The intra-assay CVs were 3.4%, 3.0% and 1.5% for the low, medium and high points of the standard curve, respectively. The interassay CVs. were 8.2%, 1.5% and 3.7% for the low, medium and high points of the standard curve, respectively. Homeostasis Model Assessment of Insulin Resistance (HoMA-IR) was calculated as previously reported [12].
2.6. Bioelectrical Impedance Analysis
Bioelectrical Impedance Analysis (BIA) body composition was assessed using a BIA phase-sensitive system by experienced observers (an 800-µA current at a signal-frequency of 50 kHz BIA 101 RJL, Akern Bioresearch, Florence, Italy) [35], as previously reported [30,31,36,37]. The exam was performed as suggested by the European Society of Parental and Enteral Nutrition (ESPEN) [38]. Electrodes were placed on the hand and the ipsilateral foot, according to Kushner [39]. The phase angle (PhA) was obtained from conditions under 50 kHz according to the following formula: PhA (°, degrees) = arctangent reactance (Xc)/resistance (R) × (180/π).
2.7. Visceral Adiposity Index
The visceral adiposity index (VAI) has been calculated by the following formula, with triglycerides levels expressed in mmol/L and HDL levels expressed in mmol/L [40]:
| VAI (Female): [WC/36.58 + (1.89 × BMI)] × (TG/0.81) × (1.52/HDL). |
2.8. Fatty Liver Index
The fatty liver index was calculated according to the following formula [41]:
| [100 × exp [0.953 × ln(TG) + 0.139 × BMI + 0.718 × ln(γ-glutamiltransferase) + 0.053 × [WC] − 15.745]/(1 + exp[0.953 × ln(TG) + 0.139 × BMI + 0.718 × ln(γ-glutamiltransferase) + 0.053 × [WC] − 15.745]. |
2.9. Criteria to Define Metabolic Syndrome
According to the National Cholesterol Education Program (NCEP) and Adult Treatment Panel (ATP III) definition, a metabolic syndrome is present if three or more of the following five criteria are met: WC over 88 cm in female, respectively), blood pressure over 130/85 mmHg, fasting TG level over 150 mg/dL, fasting HDL cholesterol level less than 50 mg/dL (in female) and fasting glucose over 100 mg/dL (NCEP-ATP III 2000) [41].
2.10. Statistical Methods
The variable distribution was assessed by Kolmogorov-Smirnov test and results were reported as mean ± SD or as median plus range. Mann–Whitney U test, or Student’s unpaired t-test, was used to assess differences between groups. The significance of differences in frequency distributions was assessed by chi2 (χ2) test. Pearson r or Spearman’s rho correlation coefficients were used to assess correlations between variables. In order to investigate the association among quantitative variables (all food items of the PREDIMED questionnaire and PREDIMED score), bivariate proportional odds ratio (OR) models, 95% interval confidence (IC), and adjusted R2 were performed. In these analyses, we only entered those variables that had a p value of <0.05 in the univariate analysis (partial correlation). To avoid multicollinearity, variables with a variance inflation factor (VIP) >10 were excluded. Values ≤5% were considered statistically significant. Data were stored and analyzed using the MedCalc® package (Version 12.3.0 1993–2012 MedCalc Software bvba-MedCalc Software, Mariakerke, Belgium). The sample size calculations were performed using G POWER software. Cohen’s d (effect size) was determined by calculating the mean difference of IGF-1 levels between obese individuals with and without GH deficiency (GHD) and then dividing the result by the pooled standard deviation [12]. The resulting total minimum sample size—estimated according to a global effect size of 0.7 with type I error of 0.05 and a power of 95%—was 118 subjects. We enrolled 200 subjects to prevent any drop-outs.
3. Results
We report sociodemographic, anthropometric and metabolic characteristics of 200 female subjects with obesity in Table 1. Obesity was present in most of the enrolled subjects. In particular, moderately obesity was detected in 41 subjects (20.5%) while severe obesity was detected in 159 individuals (79.5%). Based on GH peak response and IGF-1 SDS, an altered GH peak and IGF-1 deficiency was found in 123 individuals (61.5%) and 142 individuals (71%), respectively. Insulin-resistance was found in 77.5% (155 subjects), while 44% (88 subjects) had metabolic syndrome. Sociodemographic, anthropometric measurements and metabolic characteristics of 200 obese female participants according to GH status are reported in Table 2. We compared the enrolled subjects according to GH peak response. The same subjects were compared according to IGF-1 SDS as well. As expected, those who were found to have both a blunted GH peak response and IGF-1 deficiency showed worst anthropometric measurements and metabolic profile than obese counterparts with normal GH peak response or with IGF-1 sufficiency.
Table 1.
Socio-demographic, anthropometric measures and metabolic profile of 200 adult females with obesity included in the study.
| Parameters | Mean ± SD |
|---|---|
| Age (years) | 36.98 ± 11.10 |
| BMI (kg/m2) | 45.19 ± 6.30 |
| Grade II obesity | 41, 20.5% |
| Grade III obesity | 159, 79.5% |
| Waist circumference (cm) | 126.74 ± 17.05 |
| SBP (mmHg) | 126.89±15.29 |
| DBP (mmHg) | 80.68 ± 10.84 |
| GH peak response (µg/L) | 3.72 ± 4.29 |
| GH deficit 1 | 123, 61.5% |
| IGF-1 (µg/L) | 151.93 ± 64.93 |
| IGF-1 (SDS) | −2.43 ± 1.85 |
| IGF-1(SDS) deficiency 2 | 142, 71.0% |
| Fasting glucose (mg/dL) | 105.27 ± 36.79 |
| Fasting insulin (µU/mL) | 19.95 ± 11.76 |
| Total cholesterol (mg/dL) | 187.36 ± 47.88 |
| HDL cholesterol (mg/dL) | 50.17 ± 11.94 |
| LDL cholesterol (mg/dL) | 106.54 ± 49.96 |
| Fasting triglycerides (mg/mL) | 151.95 ± 53.10 |
| AST (U/L) | 30.15 ± 16.44 |
| ALT (U/L) | 41.70 ± 30.21 |
| γGT (U/L) | 41.37 ± 25.38 |
| CRP (ng/mL) | 2.33 ± 1.01 |
| HoMA-IR | 6.04 ± 6.55 |
| >Cut-off | 155, 77.5% |
| VAI | 1.86 ± 0.94 |
| >Cut-off | 57, 28.5% |
| FLI | 96.43 ± 6.79 |
| >Cut-off | 197, 98.5% |
| Metabolic syndrome (presence) | 88, 44.0% |
1 GH (growth hormone) peak response after GHRH (GH releasing hormone) + ARG (arginine hydrochloride) was classified as deficient (GH deficit 1) when GH peak was ≤4.2 mg/L. 2 IGF-1 levels were classified as deficient (IGF-1 (insulin-like growth factor I) deficiency 2) when the SDS from the mean was <−2 for age and gender.
Table 2.
Anthropometric measures and metabolic profile of 200 adult females with obesity according to blunted GH peak or IGF-I SDS.
| Parameters | Blunted GH Peak 1 n = 123, 61.5% |
Normal GH Peak 2 n = 77, 35.8% |
p-Value | IGF-1 Deficiency 3 n = 142 (71.0%) |
IGF-1 Sufficiency 4 n = 58 (29.0%) |
p-Value |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | 47.47 ± 6.05 | 41.54 ± 4.84 | <0.001 | 46.61 ± 6.06 | 41.70 ± 5.53 | <0.001 |
| Waist circumference (cm) | 132.11 ± 16.33 | 118.18 ± 14.60 | <0.001 | 129.98 ± 16.09 | 118.83 ± 16.88 | <0.001 |
| SBP (mmHg) | 130.83 ± 15.41 | 120.58 ± 12.87 | <0.001 | 129.19 ± 15.17 | 121.24 ± 14.21 | 0.001 |
| DBP (mmHg) | 83.33 ± 9.32 | 76.45 ± 11.77 | <0.001 | 82.16 ± 10.02 | 77.05 ± 11.94 | 0.002 |
| GH peak response (µg/L) | - | - | - | 2.14 ± 2.78 | 7.59 ± 4.88 | <0.001 |
| IGF-1 (µg/L) | 120.94 ± 44.41 | 201.45 ± 61.91 | <0.001 | - | - | - |
| IGF-1 (SDS) | −3.26 ± 1.12 | −1.11 ± 2.04 | <0.001 | - | - | - |
| Fasting Glucose (mg/dL) | 115.00 ± 40.41 | 89.73 ± 22.94 | <0.001 | 111.67 ± 39.12 | 89.66 ± 24.32 | <0.001 |
| Fasting Insulin (µU/mL) | 24.47 ± 11.59 | 12.73 ± 7.77 | <0.001 | 22.50 ± 11.52 | 13.71 ± 9.93 | <0.001 |
| Total cholesterol (mg/dL) | 195.06 ± 48.20 | 175.08 ± 45.00 | 0.004 | 192.02 ± 47.82 | 175.97 ± 46.49 | 0.031 |
| HDL cholesterol (mg/dL) | 48.09 ± 11.41 | 53.47 ± 12.10 | 0.002 | 48.59 ± 11.57 | 54.00 ± 12.07 | 0.004 |
| LDL cholesterol (mg/dL) | 114.55 ± 50.28 | 93.77 ± 47.00 | 0.004 | 111.39 ± 49.88 | 94.68 ± 48.55 | 0.031 |
| Fasting Triglycerides (mg/dL) | 159.94 ± 53.09 | 139.19 ± 50.89 | 0.007 | 158.30 ± 52.05 | 136.37 ± 52.87 | 0.008 |
| AST (U/L) | 31.17 ± 16.64 | 28.52 ± 16.08 | 0.268 | 30.25 ± 16.36 | 29.91 ± 16.77 | 0.897 |
| ALT (U/L) | 41.54 ± 32.53 | 41.96 ± 26.27 | 0.919 | 41.56 ± 31.25 | 42.03 ± 27.75 | 0.921 |
| γGT (U/L) | 42.26 ± 22.50 | 37.14 ± 19.31 | 0.089 | 42.34 ± 21.61 | 35.28 ± 20.27 | 0.030 |
| CRP (ng/mL) | 2.46 ± 1.02 | 2.12 ± 0.96 | 0.019 | 2.47 ± 1.00 | 1.98 ± 0.95 | 0.002 |
| HoMA-IR | 7.81 ± 7.07 | 3.20 ± 4.36 | <0.001 | 7.08 ± 6.83 | 3.49 ± 5.02 | <0.001 |
| VAI | 2.06 ± 1.01 | 1.55 ± 0.69 | <0.001 | 2.00 ± 0.96 | 1.50 ± 0.75 | <0.001 |
| FLI | 98.23 ± 3.04 | 93.45 ± 9.61 | <0.001 | 97.98 ± 3.18 | 92.64 ± 10.75 | <0.001 |
| Presence of Metabolic Syndrome (n, %) | 75, 61.0% | 13, 16.9% | <0.001 | 75, 52.8% | 13, 22.4% | <0.001 |
1 GH peak response after GHRH + ARG was classified as blunted when GH peak was ≤4.2 mg/L, and 2 sufficient when GH peak was ≥4.2 mg/L. 3 IGF-1 levels were classified as deficient (IGF-1 deficiency) when the SDS from the mean was <−2 for age and gender, and 4 sufficient when the SDS ranged from >−2 to 2.24.
Response frequency of dietary components included in the PREDIMED questionnaire of the subjects at baseline was reported in Table 3. We found that patients with blunted GH peak response consumed less frequently extra virgin olive, fruits, legumes and fish/seafood compared to subjects with normal GH peak response. When the same patients were divided according to IGF-1 SDS, we found that patients with IGF-1 deficiency consumed less fruits and fish/sea food and more soda drink and commercial sweets and confectionery compared to subjects with IGF-1 sufficiency. According to the PREDIMED score a higher percentage of subjects with low adherence to the Mediterranean Diet was found in subjects with blunted GH peak response and when divided according to IGF-1 SDS, low adherence was found in patients with IGF-1 deficiency, compared to their normal counterparts.
Table 3.
Response frequency of dietary components included in the PREDIMED (PREvention with MEDiterranean Diet) questionnaire of 200 adult females with obesity included in the study divided according to blunted GH peak or IGF-1 SDS.
| Questions PREDIMED Questionnaire | Blunted GH Peak 1 n = 123, (61.5%) |
Normal GH Peak 2 n = 77, (35.8%) |
χ2, p-Value | IGF-1 Deficiency 3 n = 142, (71.0%) |
IGF-1 Sufficiency 4 n = 58, (29.0%) |
χ2, p-Value |
|---|---|---|---|---|---|---|
| EVOO as main culinary lipid | 57, 46.3% | 54, 70.1% | 9.71, 0.002 | 75, 52.8% | 36, 62.1% | 1.08, 0.299 |
| EVOO >4 tablespoons | 27, 22.0% | 22, 28.6% | 0.79, 0.373 | 32, 22.5% | 17, 29.3% | 0.069, 0.407 |
| Vegetables ≥2 servings/day | 34, 27.6% | 32, 41.6% | 3.54, 0.059 | 41, 28.9% | 25, 43.1% | 3.16, 0.076 |
| Fruits ≥3 servings/day | 32, 26.0% | 48, 62.3% | 24.53, <0.001 | 46, 32.4% | 34, 58.6% | 10.73, 0.011 |
| Red/processed meats <1/day | 54, 43.9% | 43, 55.8% | 2.25, 0.134 | 65, 45.8% | 32, 55.2% | 1.10, 0.293 |
| Butter, cream, margarine <1/day | 52, 42.3% | 26, 33.8% | 1.11, 0.293 | 56, 39.4% | 22, 37.9% | 0.01, 0.969 |
| Soda drinks <1/day | 39, 31.7% | 30, 39.0% | 0.81, 0.369 | 40, 28.2% | 29, 50.0% | 7.75, 0.001 |
| Wine glasses ≥7/week | 33, 26.8% | 21, 27.3% | 0.01, 0.925 | 37, 26.1% | 17, 29.3% | 0.09, 0.768 |
| Legumes ≥3/week | 56, 45.5% | 47, 61.0% | 3.96, 0.046 | 70, 49.3% | 33, 56.9% | 0.67, 0.412 |
| Fish/seafood ≥3/week | 20, 16.3% | 45, 58.4% | 36.51, <0.001 | 9, 6.3% | 26, 96.6% | 148.69, <0.001 |
| Commercial sweets and confectionery ≤2/week | 50, 40.7% | 39, 50.6% | 1.54, 0.216 | 55, 38.7% | 34, 58.6% | 5.82, 0.016 |
| Tree nuts ≥3/week | 16, 13.0% | 15, 19.5% | 1.06, 0.303 | 20, 14.1% | 11, 19.0% | 0.42, 0.516 |
| Poultry more than red meats | 51, 41.5% | 39, 50.6% | 1.27, 0.261 | 63, 44.4% | 27, 46.6% | 0.02, 0.900 |
| Use of sofrito sauce ≥2/week | 61, 49.6% | 38, 49.4% | 0.01, 0.911 | 69, 48.6% | 30, 51.7% | 0.06, 0.806 |
| PREDIMED categories | ||||||
| Low adherence to the Mediterranean Diet | 95, 77.2% | 32, 41.6% | 24.49, <0.001 | 107, 75.4 | 20, 34.5% | 27.94, <0.001 |
| Average adherence to the Mediterranean Diet | 27, 22.0% | 33, 42.9% | 8.89, 0.001 | 34, 23.9 | 26, 44.8% | 7.59, 0.006 |
| High adherence to the Mediterranean Diet | 1, 0.8% | 12, 15.6% | 14.66, 0.001 | 1, 0.7 | 12, 20.7% | 23.88, <0.001 |
1 GH peak response after GHRH + ARG was classified as blunted when GH peak was ≤4.2 mg/L, and 2 sufficient when GH peak was ≥4.2 mg/L. 3 IGF-1 levels were classified as deficient (IGF-1 deficiency) when the SDS from the mean was <−2 for age and gender, and 4 sufficient when the SDS ranged from >−2 to 2.24.
In Table 4 we reported the total energy and the daily macronutrients intake obtained from the seven-day food records. Although there was no difference in terms of total energy intake, both blunted GH peak response and IGF-1 deficiency were associated with a lower intake of proteins and a higher intake of carbohydrates, compared to their normal counterparts. Further, IGF-1 deficiency was associated with a higher intake of fats compared to subjects with IGF-1 sufficiency. Body composition parameters evaluated by the BIA are shown in Table 4 as well. In particular, both blunted GH peak response and IGF-1 deficiency were associated with lower values of phase angle (PhA) (p < 0.001), intra-cellular water (ICW) (%), and a higher values of extra-cellular water (ECW) compared to their normal counterparts. Additionally, a lower fat free mass (both absolute and percentage) along with a higher fat mass (both absolute and percentage) were detected.
Table 4.
Total energy and macronutrient intake of 200 adult females with obesity included in the study divided according to blunted GH peak or IGF- 1 SDS.
| Parameters | Blunted GH Peak 1 n = 123, 61.5% |
Normal GH Peak 2 n = 77, 35.8% |
p-Value | IGF-1 Deficiency 3 n = 142 (71.0%) |
IGF-1 Sufficiency 4 n = 58 (29.0%) |
p-Value |
|---|---|---|---|---|---|---|
| Dietary assessment | ||||||
| Total energy (kcal) | 2838.97 ± 253.89 | 2783.68 ± 255.47 | 0.136 | 2830.21 ± 250.61 | 2787.00 ± 266.11 | 0.278 |
| Protein (gr of total kcal) | 112.92 ± 14.36 | 127.90 ± 17.98 | <0.001 | 112.32 ± 13.33 | 134.27 ± 16.47 | <0.001 |
| Carbohydrate (gr of total kcal) | 388.99 ± 38.27 | 376.01 ± 36.34 | 0.018 | 388.58 ± 37.19 | 372.78 ± 37.87 | 0.007 |
| Fat (gr of total kcal) | 92.36 ± 10.46 | 85.34 ± 9.84 | <0.001 | 91.84 ± 10.72 | 84.31 ± 8.89 | <0.001 |
| BIA parameters | ||||||
| R (Ω) | 489.47 ± 95.91 | 471.45 ± 80.07 | 0.153 | 488.16 ± 93.22 | 471.56 ± 79.74 | 0.296 |
| Xc (Ω) | 42.20 ± 8.49 | 43.82 ± 7.89 | 0.180 | 42.15 ± 8.13 | 44.73 ± 8.82 | 0.082 |
| PhA (°) | 4.95 ± 0.49 | 5.35 ± 0.70 | <0.001 | 4.97 ± 0.50 | 5.45 ± 0.79 | <0.001 |
| FM (kg) | 68.05 ± 16.24 | 49.98 ± 12.02 | <0.001 | 68.37 ± 16.61 | 45.83 ± 10.15 | <0.001 |
| FM (%) | 53.30 ± 6.71 | 46.73 ± 6.70 | <0.001 | 53.38 ± 6.70 | 45.21 ± 6.49 | <0.001 |
| FFM (kg) | 58.29 ± 8.03 | 55.99 ± 7.37 | 0.041 | 58.41 ± 7.87 | 54.92 ± 7.73 | 0.014 |
| FFM (%) | 46.69 ± 6.71 | 53.28 ± 6.73 | <0.001 | 46.62 ± 6.71 | 54.81 ± 6.54 | <0.001 |
| Skeletal muscle mass (kg) | 29.77 ± 7.12 | 32.52 ± 7.54 | 0.011 | 29.88 ± 7.20 | 33.01 ± 8.08 | 0.020 |
| Skeletal muscle mass (%) | 23.96 ± 6.38 | 31.34 ± 8.97 | <0.001 | 24.01 ± 6.50 | 33.37 ± 9.68 | <0.001 |
| TBW (Lt) | 42.72 ± 5.85 | 40.99 ± 5.39 | 0.035 | 42.75 ± 5.76 | 40.19 ± 5.66 | 0.014 |
| TBW (%) | 34.18 ± 4.90 | 39.00 ± 4.93 | <0.001 | 34.13 ± 4.90 | 40.12 ± 4.79 | <0.001 |
| ICW (Lt) | 20.87 ± 3.35 | 20.87 ± 3.48 | 0.980 | 20.91 ± 3.32 | 20.67 ± 3.71 | 0.708 |
| ICW (%) | 48.77 ± 2.81 | 50.83 ± 3.65 | <0.001 | 48.83 ± 2.87 | 51.33 ± 4.15 | <0.001 |
| ECW (Lt) | 21.86 ± 2.95 | 20.11 ± 2.69 | <0.001 | 21.83 ± 2.93 | 19.52 ± 2.88 | 0.001 |
| ECW (%) | 51.23 ± 2.81 | 49.17 ± 3.65 | <0.001 | 51.17 ± 2.87 | 48.67 ± 4.15 | 0.001 |
| BCMI | 8.34 ± 2.36 | 9.69 ± 2.68 | <0.001 | 8.37 ± 2.39 | 10.00 ± 2.86 | 0.001 |
1 GH peak response after GHRH + ARG was classified as blunted when GH peak was ≤4.2 mg/L, and 2 sufficient when GH peak was ≥4.2 mg/L. 3 IGF-1 levels were classified as deficient (IGF-1 deficiency) when the SDS from the mean was <−2 for age and gender, and 4 sufficient when the SDS ranged from >−2 to 2.24. R (Ω): resistance; Xc (Ω): reactance; PhA: phase angle; FM: fat mass; FFM: fat free mass; TBW: total body water; ICW: intracellular body water; ECW: extracellular body water; BCMI: body cell mass index; BIA: Bioelectrical Impedance Analysis.
Correlation Studies
Correlation analysis were performed to assess the association of GH peak and IGF-1 levels with anthropometric measures and metabolic profile. As expected, both GH peak and IGF-1 levels were inversely associated with anthropometric measurements, SBP/DBP, fasting glucose, fasting insulin, HoMA-IR, total cholesterol, LDL cholesterol, fasting TG, γ-glutamiltransferase (γGT), C reactive protein, VAI, FLI and the percentage of metabolic syndrome. A direct association was found between HDL cholesterol and both GH peak and IGF-1 levels (Table 5). Both GH peak response and IGF-1 levels positively correlated to protein intake while they negatively correlated to carbohydrate and fat intake (Table 6). The correlation analysis between body composition and both GH peak response and IGF-1 levels are showed in Table 6.
Table 5.
Correlation between GH peak response and IGF-1 SDS with Socio-demographic, anthropometric measures and metabolic profile.
| GH peak Response (µg/L) | IGF-1 (SDS) | |||
|---|---|---|---|---|
| Parameters | r | p Value | r | p Value |
| Age (years) | 0.158 | 0.026 | 0.315 | <0.001 |
| BMI (kg/m2) | −0.548 | <0.001 | −0.369 | <0.001 |
| Waist circumference (cm) | −0.484 | <0.001 | −0.331 | <0.001 |
| SBP (mmHg) | −0.354 | <0.001 | −0.261 | <0.001 |
| DBP (mmHg) | −0.315 | <0.001 | −0.303 | <0.001 |
| Fasting glucose (mg/dL) | −0.381 | <0.001 | −0.284 | 0.001 |
| Fasting insulin (µU/mL) | −0.521 | <0.001 | −0.338 | <0.001 |
| Total cholesterol (mg/dL) | −0.239 | 0.001 | −0.228 | 0.001 |
| HDL cholesterol (mg/dL) | 0.292 | <0.001 | 0.273 | <0.001 |
| LDL cholesterol (mg/dL) | −0.265 | <0.001 | −0.246 | <0.001 |
| Fasting triglycerides (mg/dL) | −0.138 | 0.050 | −0.143 | 0.043 |
| AST (U/L) | 0.026 | 0.714 | 0.055 | 0.443 |
| ALT (U/L) | −0.050 | 0.479 | 0.026 | 0.711 |
| γGT (U/L) | −0.134 | 0.050 | −0.191 | 0.007 |
| CRP (ng/mL) | −0.163 | 0.021 | −0.206 | 0.003 |
| HoMA−IR | −0.378 | <0.001 | −0.253 | <0.001 |
| VAI | −0.236 | 0.001 | −0.217 | 0.002 |
| FLI | −0.592 | <0.001 | −0.495 | <0.001 |
| Metabolic syndrome (n, %) | −0.332 | <0.001 | −0.255 | <0.001 |
Table 6.
Correlation between GH peak response and IGF-1 SDS with nutrient intake and BIA parameters.
| GH peak Response (µg/L) | IGF-1 (SDS) | |||
|---|---|---|---|---|
| Parameters | r | p-Value | R | p-Value |
| Dietary assessment | ||||
| Total energy (kcal) | −0.100 | 0.158 | −0.017 | 0.806 |
| Protein (gr of total kcal) | 0.536 | <0.001 | 0.761 | <0.001 |
| Carbohydrate (gr of total kcal) | −0.237 | 0.001 | −0.169 | 0.017 |
| Fat (gr of total kcal) | −0.277 | <0.001 | −0.329 | <0.001 |
| BIA parameters | ||||
| R (Ω) | −0.109 | 0.123 | −0.023 | 0.750 |
| Xc (Ω) | 0.120 | 0.090 | 0.155 | 0.029 |
| PhA (°) | 0.392 | <0.001 | 0.280 | <0.001 |
| FM (kg) | −0.585 | <0.001 | −0.409 | <0.001 |
| FM (%) | −0.528 | <0.001 | −0.328 | <0.001 |
| FFM (kg) | −0.155 | 0.029 | 0.203 | 0.004 |
| FFM (%) | 0.529 | <0.001 | 0.329 | <0.001 |
| Skeletal muscle mass (kg) | 0.251 | <0.001 | 0.111 | 0.120 |
| Skeletal muscle mass (%) | 0.565 | <0.001 | 0.368 | <0.001 |
| TBW (Lt) | −0.161 | 0.024 | −0.200 | 0.005 |
| TBW (%) | 0.529 | <0.001 | 0.330 | <0.001 |
| ICW (Lt) | 0.016 | 0.818 | −0.063 | 0.378 |
| ICW (%) | 0.368 | <0.001 | 0.265 | <0.001 |
| ECW (Lt) | −0.328 | <0.001 | −0.313 | <0.001 |
| ECW (%) | −0.368 | <0.001 | −0.265 | <0.001 |
| BCMI | 0.333 | <0.001 | 0.202 | 0.004 |
In Table 7 the results of bivariate proportional odds ratio model performed to assess the association of GH peak and IGF-1 levels with food items of PREDIMED questionnaire were summarized. On the contrary, the highest odds of soda drinks and commercial sweets and confectionery appeared to have a negative effect on both GH peak and IGF-1 levels. A positive association was found between the consumption of nuts and GH peak response; a positive correlation was found between the consumption of poultry and IGF-1 levels.
Table 7.
Bivariate proportional odds ratio models performed to assess the association of GH peak response and IGF-1 SDS with the dietary components included in the PREDIMED questionnaire.
| GH peak Response (µg/L) n = 200 |
IGF-1 (SDS) n = 200 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | OR | p Value | 95% IC | R2 adj | OR | p Value | 95% IC | R2 adj |
| EVOO as main culinary lipid | 1.12 | 0.003 | 1.04–1.21 | 0.05 | 1.19 | 0.037 | 1.01–1.40 | 0.02 |
| EVOO oil >4 tablespoons | 1.08 | 0.030 | 1.00–1.16 | 0.02 | 1.14 | 0.001 | 0.096–1.35 | 0.01 |
| Vegetables ≥2 servings/day | 1.07 | 0.035 | 1.00–1.15 | 0.02 | 1.31 | 0.002 | 1.10–1.54 | 0.05 |
| Fruits ≥ 3servings/day | 1.22 | <0.001 | 1.12–133 | 0.13 | 1.36 | <0.001 | 1.15–1.62 | 0.07 |
| Red/processed meats <1/day | 1.06 | 0.062 | 0.99–1.14 | 0.02 | 1.07 | 0.394 | 0.09–1.24 | 0.01 |
| Butter, cream, margarine <1/day | 1.01 | 0.753 | 0.95–1.07 | 0.01 | 1.07 | 0.420 | 0.91–1.24 | 0.01 |
| Soda drinks <1/day | 1.09 | 0.014 | 1.02–1.16 | 0.03 | 1.19 | 0.035 | 1.01–1.38 | 0.02 |
| Wine glasses ≥7/week | 0.98 | 0.946 | 0.93–1.07 | 0.01 | 0.99 | 0.863 | 0.83–1.16 | 0.01 |
| Legumes ≥3/week | 1.07 | 0.049 | 1.00–1.15 | 0.02 | 1.16 | 0.066 | 0.99–1.35 | 0.02 |
| Fish/seafood ≥3/week | 1.36 | <0.001 | 1.23–1.51 | 0.24 | 6.98 | <0.001 | 3.83–12.73 | 0.48 |
| Commercial sweets and confectionery ≤2/week | 1.11 | 0.002 | 1.04–1.19 | 0.05 | 1.22 | 0.013 | 1.04–1.44 | 0.03 |
| Tree nuts ≥3/week | 1.08 | 0.049 | 1.00–1.17 | 0.02 | 0.17 | 0.092 | 0.97–1.41 | 0.01 |
| Poultry more than red meats | 1.05 | 0.089 | 0.99–1.13 | 0.01 | 1.17 | 0.042 | 1.00–1.37 | 0.02 |
| Use of sofrito sauce ≥2/week | 1.01 | 0.716 | 0.095–1.08 | 0.01 | 0.09 | 0.087 | 0.085–1.15 | 0.01 |
| PREDIMED categories | ||||||||
| Low adherence to the Mediterranean Diet | 0.81 | <0.001 | 0.75–0.88 | 0.15 | 0.61 | <0.001 | 0.50–0.75 | 0.14 |
| Average adherence to the Mediterranean Diet | 1.06 | 0.046 | 0.99–1.14 | 0.02 | 1.23 | 0.014 | 1.04–1.44 | 0.03 |
| High adherence to the Mediterranean Diet | 1.47 | <0.001 | 1.26–1.69 | 0.19 | 1.96 | <0.001 | 1.45–2.63 | 0.12 |
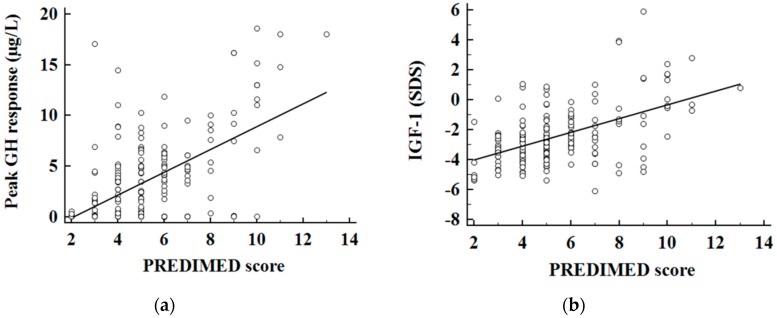
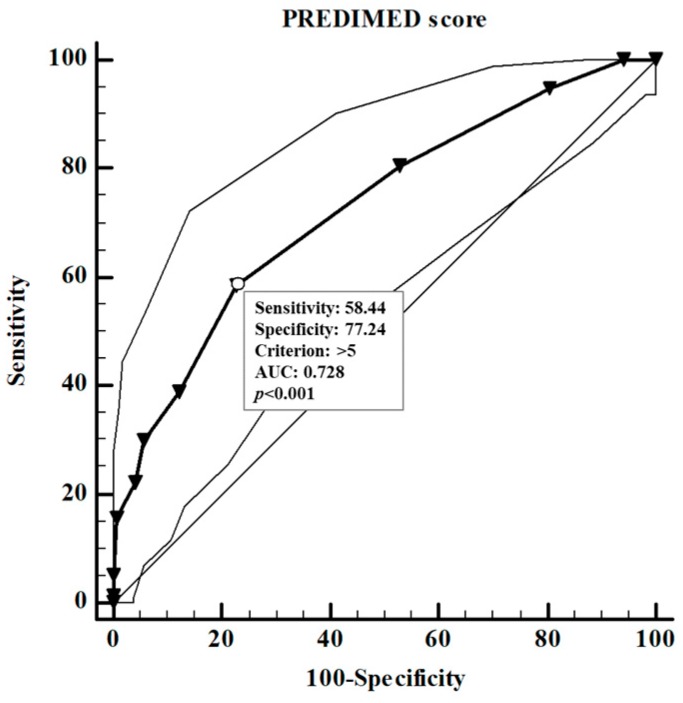
In addition, a positive association was found between the adherence to the Mediterranean Diet and both GH peak response (Figure 1a) and IGF-1 levels (Figure 1b). To evaluate the predictive value of: (a) nutritional parameters; (b) cardiometabolic indices; (c) metabolic syndrome and (d) BIA parameters on GH peak response (µg/L), we performed two multiple linear regression analysis models, which included PREDIMED score and proteins intake (model I) and measures of the body composition parameters (model II). Using model I, both PREDIMED score and proteins intake entered at the first step (p < 0.001) appeared to exert a powerful influence on GH peak response, while the other variables (EVOO, vegetables, fruits, soda drinks, legumes, fish/seafood, commercial sweets and confectionery, tree nuts, carbohydrates and fats) were excluded. Using model II, FLI, FM and PhA were entered at the first step (p < 0.001) and appeared to be the most powerful factors influencing GH peak response while the other variables (HoMA-IR, VAI, metabolic syndrome, and other parameters of the BIA) were excluded. The results of the two models are shown in Table 8. ROC analysis was performed to determine the cut off values of the adherence to the Mediterranean Diet that were predictive of highest GH peak response (Figure 2). A value of PREDIMED score of ≤5.0 (p < 0.001, AUC 0.728) could serve as a threshold for a significant decrease of GH peak response.
Figure 1.
Correlation between PREDIMED (PREvention with MEDiterranean Diet) score and GH peak (a) and IGF-1 SDS (b). Growth hormone (GH); insulin-like growth factor I (IGF-1); standards deviations scores (SDS).
Table 8.
Multiple regression analysis models (stepwise method) with GH Peak response (µg/L) as dependent variable to estimate the predictive value of: nutritional parameters (model 1); fatty liver index and BIA parameters (model 2).
| Parameters | Multiple Regression Analysis | |||
|---|---|---|---|---|
| Model 1 | R2 | β | t | p value |
| PREDIMED score | 0.297 | 0.548 | 9.22 | <0.001 |
| Protein (gr of total kcal) | 0.398 | 0.362 | 5.86 | <0.001 |
| Variables excluded: EVOO, vegetables, fruits, soda drinks, legumes, fish/seafood, commercial sweets and confectionery, tree nuts, carbohydrates and fat. | ||||
| Model 2 | ||||
| FLI | 0.347 | −0.592 | −10.34 | <0.001 |
| FM | 0.453 | −0.382 | −6.22 | <0.001 |
| PhA (°) | 0.479 | 0.181 | 3.30 | <0.001 |
| Variables excluded: HoMA-IR, VAI, metabolic syndrome, and other parameters of the BIA | ||||
Growth hormone (GH); metabolic syndrome; bioelectrical impedance analysis (BIA); phase angle (PhA); fat mass (FM); extra virgin olive oil (EVOO); homeostatic model assessment for insulin resistance (HoMA-IR); visceral adiposity index (VAI); fatty liver index (FLI).
Figure 2.
Identification of a cut-off value of PREDIMED score predicting a blunted GH peak response. A value of PREDIMED score of ≤5.0 (p < 0.001, AUC 0.728) could serve as a threshold for a significantly decrease of GH peak response. A p value in bold type denotes a significant difference (p < 0.05). Growth hormone (GH); area under the curve (AUC).
4. Discussion
The novel findings of this cross-sectional observational study are that both the blunted GH peak response and/or IGF-1 deficiency are associated with a worst body composition and cardiometabolic profile in obesity. Further, nutritional status, in particular the degree of adherence to the Mediterranean Diet and protein intake has been found to be associated with GH peak response in obese patients. Based on ROC curve analysis, a PREDIMED score of ≤5.0 was associated with derangements of GH peak response. Both blunted GH response and IGF-1 deficiency were damage indicators of somatotropic axis and resulted in similar cardiometabolic derangements. In fact, in this study, both blunted GH response and IGF-1 deficiency were associated with an increased fat mass and a decreased free fat mass. These findings were in agreement with previous studies performed in subjects with GHD in which changes in body composition were characterized by reduced lean body mass and increased visceral adiposity [42,43]—a clinical phenotype which has been tightly associated with insulin resistance and glucose derangements. In view of the existing association between visceral fat mass, insulin resistance and non-alcoholic fatty liver disease (NAFLD), we found that derangements of somatotropic axis were associated with increased visceral fat and fat liver in obesity, as demonstrated by higher VAI and FLI. These findings were in line with cross-sectional data reporting an increased visceral fat mass along with increased liver fat content in patients with GHD [44,45,46]. Moreover, several case reports of hypopituitaric patients reported a reduction in liver fat content following GH replacement therapy [47,48,49]. Given the tight association between NAFLD and insulin resistance measured by HoMA-IR [50], we found that derangements of somatotropic axis were associated with increased insulin resistance. As is well known, insulin resistance is an important feature of metabolic syndrome and is associated with low-grade chronic inflammation, endothelial dysfunction and increased cardiovascular mortality [51]. So far, the prevalence of metabolic syndrome is high in GHD patients. Van der Klaauw et al. [52] reported that GHD patients had a more than a two-fold higher prevalence of metabolic syndrome compared to the general population. Attanasio et al. [53] found as well that metabolic syndrome prevalence was increased in GHD patients. In agreement with this, we found that people with obesity with derangements of somatotropic axis had an increased percentage of metabolic syndrome and low-grade chronic inflammation. Moreover, PhA, a parameter obtained from BIA direct measures, such as R and Xc, is widely used as a marker of cellular health [54]. In fact, PhA is currently considered as a predictive marker of mortality and morbidity in several diseases [55]. In a healthy population, PhA could be determined by several factors such as diet, sex, age, BMI, and inflammatory status [56]. PhA provides information on the integrity [54] of a large number of cell membranes and the water distribution in body fluids [55]. Thus, PhA is positively associated with the body cell mass [56,57,58,59] and negatively associated with ECW/ICW ratio [60]. In agreement with other studies in different chronic inflammatory diseases [22,36], in this study we evidenced that a small PhA was detected in obese patients with derangements of somatotropic axis, and we hypothesized that this could be tightly related to the inflammatory status. Of interest, among all BIA measurements, PhA along with FLI and fat mass were predictive factors of GH peak response. Nevertheless, it has been reported that dyslipidemia is one of the most important contributor to the increase of hypopituitarism-related cardiovascular risk [61,62]. Derangements of serum lipid concentrations improved after GH replacement therapy [63]. People with obesity with derangements of somatotropic axis showed a worse lipid profile characterized by increased triglycerides, total and LDL cholesterol along with a reduction of HDL cholesterol. It has been reported as well that hypertension is a common finding in GHD subjects. In line with these data we found that derangements of somatotropic axis were associated with a higher SBP and DBP compared to their healthy counterparts. In addition, we found that derangements of somatotropic axis were associated with a lower adherence to the Mediterranean Diet. In addition, by carefully evaluating their dietary assessment, people with obesity with derangements of somatotropic axis consumed higher amounts of simple carbohydrates, total fats and a lower amount of proteins. The PREDIMED score, and in particular proteins intake, were found to predict GH peak response. In particular, a value of PREDIMED score of ≤5.0 has been identified as the cut-off value associated with a significant decrease of GH peak response. It has already been reported that protein intake could be a powerful stimulus for somatotropic axis [12]. However, our results lead us to hypothesize that the cluster of food components enclosed in the Mediterranean Diet could have an addictive effect to the stimulus of protein intake. Thus, the strong point of our study is that we highlighted the therapeutic role of nutrition on the obesity-related GH/IGF-1 axis derangements. This is of paramount importance because it allows for a tailored nutritional approach in people with obesity developing this hormonal defect. The main limit of the study was the lack of control group of lean subjects. However, in order to minimize this limit, we consider people with obesity without GH/IGF-1 derangement as control.
5. Conclusions
In conclusion, we reported an association between the derangements of somatotropic axis and some cardiovascular risk factors in people with obesity. The nutritional status—in particular the degree of adherence to the Mediterranean Diet and proteins intake—was found to be one of the most predictive factor of GH status in obesity, showing that a novel association exists. Specific cut-off values for the degree of adherence to the Mediterranean Diet could be included as an auxiliary tool in the complex obesity evaluation, contributing to identify those patients who could get additional benefit from careful evaluation of somatotropic axis. Further studies on a large population with obesity and derangements of somatotropic axis and intervention trials are warranted to support the association between the adherence to the Mediterranean Diet and the clinical severity of alterations of somatotropic axis.
Abbreviations
| GH | Growth hormone |
| IGF-I | Insulin-like growth factor I |
| GHD | Growth hormone deficiency |
| BMI | Body mass index |
| BIA | Bioelectrical impedance analysis |
| FLI | Fatty liver index |
| VAI | Visceral adiposity index |
| NAFLD | Non-alcoholic fatty liver disease |
| PhA | Phase angle |
| FM | Fat mass |
| FFM | Free fat mass |
Author Contributions
Conceptualization, G.M. and L.B.; methodology, G.M.; software, L.B.; validation, S.S., A.C. and G.M.; formal analysis, L.B.; investigation, G.M.; resources, C.D.S.; data curation, D.L.; writing—original draft preparation, G.M. and L.B.; writing—review and editing, G.P., D.L. and C.S.; visualization, A.C.; supervision, S.S.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Di Somma C., Pivonello R. Prevalence of the metabolic syndrome in moderately-severely people with obesity with and without growth hormone deficiency. J. Endocrinol. Investig. 2010;33:171–177. doi: 10.1007/BF03346577. [DOI] [PubMed] [Google Scholar]
- 2.Makimura H., Stanley T. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J. Clin. Endocrinol. Metab. 2008;93:4254–4260. doi: 10.1210/jc.2008-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berryman D.E., Glad C.A. The GH/IGF-1 axis in obesity: Pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2013;9:346–356. doi: 10.1038/nrendo.2013.64. [DOI] [PubMed] [Google Scholar]
- 4.Utz A.L., Yamamoto A. Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J. Clin. Endocrinol. Metab. 2008;93:2507–2514. doi: 10.1210/jc.2008-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makimura H., Stanley T. Reduced growth hormone secretion is associated with increased carotid intima-media thickness in obesity. J. Clin. Endocrinol. Metab. 2009;94:5131–5138. doi: 10.1210/jc.2009-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makimura H., Feldpausch M.N. Reduced growth hormone secretion in obesity is associated with smaller LDL and HDL particle size. Clin. Endocrinol. (Oxf.) 2012;76:220–227. doi: 10.1111/j.1365-2265.2011.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savastano S., Di Somma C. Growth hormone status in morbidly people with obesity and correlation with body composition. J. Endocrinol. Investig. 2006;29:536–543. doi: 10.1007/BF03344144. [DOI] [PubMed] [Google Scholar]
- 8.Skrypnik D., Bogdański P. Role of gestational weight gain, gestational diabetes, breastfeeding, and hypertension in mother-to-child obesity transmission. Pol. Arch. Intern. Med. 2019;129:267–275. doi: 10.20452/pamw.4426. [DOI] [PubMed] [Google Scholar]
- 9.Skrypnik D., Bogdański P. Influence of endurance and endurance-strength training on mineral status in women with abdominal obesity: A randomized trial. Medicine (Baltim.) 2019;98:e14909. doi: 10.1097/MD.0000000000014909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordido F., Peino R. Impaired growth hormone secretion in people with obesity is partially reversed by acipimox-mediated plasma free fatty acid depression. J. Clin. Endocrinol. Metab. 1996;81:914–918. doi: 10.1210/jcem.81.3.8772550. [DOI] [PubMed] [Google Scholar]
- 11.Vijayakumar A., Yakar S. The intricate role of growth hormone in metabolism. Front. Endocrinol. (Lausanne) 2011;2:32. doi: 10.3389/fendo.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrea L., Di Somma C. Influence of nutrition on somatotropic axis: Milk consumption in adult individuals with moderate-severe obesity. Clin. Nutr. 2017;36:293–301. doi: 10.1016/j.clnu.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Holmes M.D., Pollak M.N. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol. Biomark. Prev. 2002;11:852–861. [PubMed] [Google Scholar]
- 14.Hoppe C., Udam T.R. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-y-old Danish children. Am. J. Clin. Nutr. 2004;80:447–452. doi: 10.1093/ajcn/80.2.447. [DOI] [PubMed] [Google Scholar]
- 15.Hoppe C., Molgaard C. High intakes of skimmed milk, but not meat, increase serum IGF-I and IGFBP-3 in eight-year-old boys. Eur. J. Clin. Nutr. 2004;58:1211–1216. doi: 10.1038/sj.ejcn.1601948. [DOI] [PubMed] [Google Scholar]
- 16.Szulińska M., Skrypnik D. Effects of Endurance and Endurance-strength Exercise on Renal Function in Abdominally Obese Women with Renal Hyperfiltration: A Prospective Randomized Trial. Biomed. Environ. Sci. 2016;29:706–712. doi: 10.3967/bes2016.095. [DOI] [PubMed] [Google Scholar]
- 17.Welch A.A., McTaggart A. DINER (Data into Nutrients for Epidemiological Research)—A new data-entry program for nutritional analysis in the EPIC-Norfolk cohort and the 7-day diary method. Public Health Nutr. 2001;4:1253–1265. doi: 10.1079/PHN2001196. [DOI] [PubMed] [Google Scholar]
- 18.Escola-Gil J.C., Calpe-Berdiel L. Moderate beer consumption does not change early or mature atherosclerosis in mice. Nutr. J. 2004;3:1. doi: 10.1186/1475-2891-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terapia Alimentare Dietosystem® DS-Medica. [(accessed on 21 July 2019)]; Available online: http://www.dsmedica.info.
- 20.Muscogiuri G., Barrea L. Patient empowerment and the Mediterranean diet as a possible tool to tackle prediabetes associated with overweight or obesity: A pilot study. Hormones (Athens) 2019;18:75–84. doi: 10.1007/s42000-018-0090-9. [DOI] [PubMed] [Google Scholar]
- 21.Barrea L., Annunziata G. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition. 2018;62:7–17. doi: 10.1016/j.nut.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Barrea L., Fabbrocini G. Role of Nutrition and Adherence to the Mediterranean Diet in the Multidisciplinary Approach of Hidradenitis Suppurativa: Evaluation of Nutritional Status and Its Association with Severity of Disease. Nutrients. 2019;11:57. doi: 10.3390/nu11010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrea L., Annunziata G. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients. 2018;10:1971. doi: 10.3390/nu10121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrea L., Muscogiuri G. Coffee consumption, metabolic syndrome and clinical severity of psoriasis: Good or bad stuff? Arch. Toxicol. 2018;92:1831–1845. doi: 10.1007/s00204-018-2193-0. [DOI] [PubMed] [Google Scholar]
- 25.Barrea L., Macchia P.E. Nutrition: A key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J. Transl. Med. 2015;13:303. doi: 10.1186/s12967-015-0658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Gonzalez M.A., Garcia-Arellano A. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE. 2012;7:e43134. doi: 10.1371/journal.pone.0043134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrea L., Muscogiuri G. Association between Mediterranean diet and hand grip strength in older adult women. Clin. Nutr. 2019;38:721–729. doi: 10.1016/j.clnu.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Barrea L., Tarantino G. Adherence to the Mediterranean Diet and Circulating Levels of Sirtuin 4 in Obese Patients: A Novel Association. Oxid. Med. Cell. Longev. 2017;2017:6101254. doi: 10.1155/2017/6101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savanelli M.C., Barrea L. Preliminary results demonstrating the impact of Mediterranean diet on bone health. J. Transl. Med. 2017;15:81. doi: 10.1186/s12967-017-1184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrea L., Muscogiuri G. Mediterranean Diet and Phase Angle in a Sample of Adult Population: Results of a Pilot Study. Nutrients. 2017;9:151. doi: 10.3390/nu9020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrea L., Balato N. Nutrition and psoriasis: Is there any association between the severity of the disease and adherence to the Mediterranean diet? J. Transl. Med. 2015;13:18. doi: 10.1186/s12967-014-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghigo E., Aimaretti G. New approach to the diagnosis of growth hormone deficiency in adults. Eur. J. Endocrinol. 1996;134:352–356. doi: 10.1530/eje.0.1340352. [DOI] [PubMed] [Google Scholar]
- 33.Corneli G., Di Somma C. The cut-off limits of the GH response to GH-releasing hormone-arginine test related to body mass index. Eur. J. Endocrinol. 2005;153:257–264. doi: 10.1530/eje.1.01967. [DOI] [PubMed] [Google Scholar]
- 34.Colao A., Di Somma C. Relationships between serum IGF1 levels, blood pressure, and glucose tolerance: An observational, exploratory study in 404 subjects. Eur. J. Endocrinol. 2008;159:389–397. doi: 10.1530/EJE-08-0201. [DOI] [PubMed] [Google Scholar]
- 35.Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am. J. Clin. Nutr. 1996;64:524S–532S. doi: 10.1093/ajcn/64.3.524S. [DOI] [PubMed] [Google Scholar]
- 36.Barrea L., Macchia P.E. Bioelectrical phase angle and psoriasis: A novel association with psoriasis severity, quality of life and metabolic syndrome. J. Transl. Med. 2016;14:130. doi: 10.1186/s12967-016-0889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrea L., Altieri B. Impact of Nutritional Status on Gastroenteropancreatic Neuroendocrine Tumors (GEP-NET) Aggressiveness. Nutrients. 2018;10:1854. doi: 10.3390/nu10121854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyle U.G., Bosaeus I. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004;23:1430–1453. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Kushner R.F. Bioelectrical impedance analysis: A review of principles and applications. J. Am. Coll. Nutr. 1992;11:199–209. [PubMed] [Google Scholar]
- 40.Amato M.C., Giordano C. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bedogni G., Bellentani S. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savastano S., Di Somma C. The complex relationship between obesity and the somatropic axis: The long and winding road. Growth Horm. IGF Res. 2014;24:221–226. doi: 10.1016/j.ghir.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Attallah H., Friedlander A.L. Visceral obesity, impaired glucose tolerance, metabolic syndrome, and growth hormone therapy. Growth Horm. IGF Res. 2006;16(Suppl. A):S62–S67. doi: 10.1016/j.ghir.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Hong J.W., Kim J.Y. Metabolic parameters and nonalcoholic fatty liver disease in hypopituitary men. Horm. Metab. Res. 2011;43:48–54. doi: 10.1055/s-0030-1265217. [DOI] [PubMed] [Google Scholar]
- 45.Ichikawa T., Hamasaki K. Non-alcoholic steatohepatitis and hepatic steatosis in patients with adult onset growth hormone deficiency. Gut. 2003;52:914. doi: 10.1136/gut.52.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nyenwe E.A., Williamson-Baddorf S. Nonalcoholic Fatty liver disease and metabolic syndrome in hypopituitary patients. Am. J. Med. Sci. 2009;338:190–195. doi: 10.1097/MAJ.0b013e3181a84bde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tai T.S., Lin S.Y. Metabolic effects of growth hormone therapy in an Alstrom syndrome patient. Horm. Res. 2003;60:297–301. doi: 10.1159/000074248. [DOI] [PubMed] [Google Scholar]
- 48.Takano S., Kanzaki S. Effect of growth hormone on fatty liver in panhypopituitarism. Arch. Dis. Child. 1997;76:537–538. doi: 10.1136/adc.76.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi Y., Iida K. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology. 2007;132:938–943. doi: 10.1053/j.gastro.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 50.Isokuortti E., Zhou Y. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: A population-based and inter-laboratory study. Diabetologia. 2017;60:1873–1882. doi: 10.1007/s00125-017-4340-1. [DOI] [PubMed] [Google Scholar]
- 51.Reaven G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 52.Van der Klaauw A.A., Biermasz N.R. The prevalence of the metabolic syndrome is increased in patients with GH deficiency, irrespective of long-term substitution with recombinant human GH. Eur. J. Endocrinol. 2007;156:455–462. doi: 10.1530/EJE-06-0699. [DOI] [PubMed] [Google Scholar]
- 53.Attanasio A.F., Mo D. Prevalence of metabolic syndrome in adult hypopituitary growth hormone (GH)-deficient patients before and after GH replacement. J. Clin. Endocrinol. Metab. 2010;95:74–81. doi: 10.1210/jc.2009-1326. [DOI] [PubMed] [Google Scholar]
- 54.Norman K., Stobaus N. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012;31:854–861. doi: 10.1016/j.clnu.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Stobaus N., Pirlich M. Determinants of bioelectrical phase angle in disease. Br. J. Nutr. 2012;107:1217–1220. doi: 10.1017/S0007114511004028. [DOI] [PubMed] [Google Scholar]
- 56.Kyle U.G., Soundar E.P. Can phase angle determined by bioelectrical impedance analysis assess nutritional risk? A comparison between healthy and hospitalized subjects. Clin. Nutr. 2012;31:875–881. doi: 10.1016/j.clnu.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Siddiqui N.I., Khan S.A. Anthropometric Predictors of Bio-Impedance Analysis (BIA) Phase Angle in Healthy Adults. J. Clin. Diagn. Res. 2016;10:CC01–CC04. doi: 10.7860/JCDR/2016/17229.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbosa-Silva M.C., Barros A.J. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005;82:49–52. doi: 10.1093/ajcn/82.1.49. [DOI] [PubMed] [Google Scholar]
- 59.Piccoli A., Rossi B. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994;46:534–539. doi: 10.1038/ki.1994.305. [DOI] [PubMed] [Google Scholar]
- 60.Chertow G.M., Lowrie E.G. Nutritional assessment with bioelectrical impedance analysis in maintenance hemodialysis patients. J. Am. Soc. Nephrol. 1995;6:75–81. doi: 10.1681/ASN.V6175. [DOI] [PubMed] [Google Scholar]
- 61.Abdu T.A., Neary R. Coronary risk in growth hormone deficient hypopituitary adults: Increased predicted risk is due largely to lipid profile abnormalities. Clin. Endocrinol. (Oxf.) 2001;55:209–216. doi: 10.1046/j.1365-2265.2001.01320.x. [DOI] [PubMed] [Google Scholar]
- 62.Gazzaruso C., Gola M. Cardiovascular risk in adult patients with growth hormone (GH) deficiency and following substitution with GH—An update. J. Clin. Endocrinol. Metab. 2014;99:18–29. doi: 10.1210/jc.2013-2394. [DOI] [PubMed] [Google Scholar]
- 63.Di Somma C., Scarano E. Cardiovascular alterations in adult GH deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2017;31:25–34. doi: 10.1016/j.beem.2017.03.005. [DOI] [PubMed] [Google Scholar]