Abstract
Background and Purpose.
We investigated the prognostic significance of spontaneous intracerebral hemorrhage (ICH) location in presence of severe intraventricular hemorrhage (IVH).
Methods.
We analyzed diagnostic CT scans from 467/500 (excluding primary IVH) subjects from the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR) III trial. We measured ICH engagement with specific anatomic regions, and estimated association of each region with blinded assessment of dichotomized poor stroke outcomes: mortality, modified Rankin Scale 4–6, National Institutes of Health Stroke Scale >4, stroke impact scale <60, Barthel Index <86, and EuroQol Visual Analogue Scale <50 and <70 at days 30 and 180, respectively, using logistic regression models.
Results.
Frequency of anatomic region involvement consisted of thalamus (332 lesions, 71.1% of subjects), caudate (219, 46.9%), posterior-limb internal capsule (PLIC) (188, 40.3%), globus pallidus (GP)/putamen (127, 27.2%), anterior-limb internal capsule (ALIC) (108, 23.1%), and lobar (29, 6.2%). Thalamic location was independently associated with mortality (days 30 and 180) and with poor outcomes on most stroke scales at day 180 on adjusted analysis. PLIC and GP/putamen involvement was associated with increased odds of worse disability at days 30 and 180. ALIC and caudate locations were associated with decreased mortality on days 30 and 180. ALIC lesions were associated with decreased long-term morbidity.
Conclusion.
Acute ICH lesion topography provides important insights into anatomic correlates of mortality and functional outcomes even in severe IVH causing obstructive hydrocephalus. Models accounting for ICH location in addition to volumes may improve outcome prediction and permit stratification of benefit from aggressive acute interventions.
Clinical trial registration.
URL: https://clinicaltrials.gov. Unique identifier: .
Keywords: intracerebral hemorrhage, intraventricular hemorrhage, localization, thalamus, basal ganglia, stroke outcomes
Introduction
Spontaneous intracerebral hemorrhage (ICH) is the most devastating and least treatable type of stroke, causing severe disability among survivors.1 Fewer than half of patients with ICH survive 1 year, and a third survive 5 years.2 Intraventricular hemorrhage (IVH), complicating approximately 45% of acute spontaneous ICH, is often associated with obstructive hydrocephalus and further limits good functional outcome.3–5 The relative impact of ICH location and IVH on functional outcomes in this setting is uncertain and may be important when choosing therapies that benefit one hematoma location but not the other. Spontaneous ICH most commonly involves the basal ganglia and thalamus.6,7 Most studies of ICH typically identify one primarily affected anatomic region, though overlap into other brain regions is common. Hence, there remains significant uncertainty about the relevance of anatomic involvement of ICH on functional outcomes.8,9 The influence of ICH location in the setting of large IVH has not been studied. We therefore aimed to study the association of anatomic involvement of ICH lesions with clinical outcomes in a population with small ICH (<30 mL) and large obstructive IVH. We hypothesized that even small ICH volumes in certain locations may significantly worsen clinical outcomes in severe IVH requiring external ventricular drainage (EVD).
Methods
Study design and patients
We performed a prospective observational cohort study using patients enrolled in the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR) III trial (ClinicalTrials.gov ). CLEAR III was a multicenter, randomized, double-blinded, placebo-controlled trial conducted to determine if pragmatically employed EVD plus intraventricular alteplase improved outcome by removing IVH and controlling intracranial pressure, in comparison to EVD plus saline. The main inclusion criteria were: (1) age 18 to 80 years, (2) spontaneous (hypertensive) ICH with hematoma volume <30 mL, (3) obstruction of the third and/or fourth ventricles, (4) presentation within 24 hours of symptom onset, (5) stability of ICH, IVH, and any EVD tract hemorrhage prior to 72 hours from diagnostic non-contrast computed tomography (CT) scan, and (6) baseline modified Rankin score (mRS) <2. The trial randomized patients to receive up to 12 doses of alteplase or 0.9% saline every eight hours via the EVD until third and fourth ventricles were radiographically open. The methodology and trial results have been published elsewhere.10,11
The study protocol was approved by the appropriate institutional review board at each participating site, and written informed consent was obtained from all participants (or legal representatives or surrogates when applicable). Data are available upon reasonable request and completion of a data use agreement at http://braininjuryoutcomes.com/clear-about.
Measurements
Patient demographics and comorbidities were recorded at time of enrollment. Baseline characteristics included age, gender, stroke comorbidities, and admission severity variables including Glasgow Coma Scale (GCS), National Institutes of Health Stroke Scale (NIHSS), and pre-morbid modified Rankin Scale (mRS).
Neuroimaging was performed on fourth-generation CT scanners at each participating study site. The majority of CT scans consisted of 4–5 mm thick slices through both skull base and cerebrum. A trained researcher and a neuroradiologist from the trial radiology center (VE and DG) analyzed all diagnostic CT scans blinded to treatment and outcomes for ICH location. The ICH locations were defined as lobar, thalamus, globus pallidus (GP)/putamen (basal ganglia), and caudate head, and anterior and posterior limbs of internal capsules (ALIC and PLIC, respectively). Lobar location was selected when structures other than thalamus, basal ganglia, caudate, or internal capsule were involved. When two or more areas were affected, all locations were documented with the location of the largest hematoma component identified as the primary ICH location. Involvement was defined as hyperdensity (>40 Hounsfield units) of any part of the anatomic structure visualized on at least 2 CT scan axial slices. Hematoma and PHE volumes at baseline and 72 hours were calculated independently by three trained researchers (HA, LN, SN), blinded to clinical data and treatment, using computer-assisted multi-slice planimetric and voxel threshold techniques.12 A semi-automated threshold-based approach (range 5–33 Hounsfield units) was applied with adjustment to identify regions of PHE to estimate volumes (cm3) from slice thickness separate to boundaries of blood. Inter-reader reliability was tested with re-analysis after 30% and 60% of the scans were read to assess for drift.12 Thirty-three subjects with primary IVH and no ICH lesion documented on any study CT were excluded from this analysis. All key variables, including primary ICH location, were collected prospectively as part of the trial.
Outcomes
Outcome measures were mortality and blinded assessment of poor functional outcome defined as mRS of 4–6 (primary outcome of the CLEAR III trial) and the following outcomes with thresholds that have discriminatory ability for independent living: NIHSS >4,13 average stroke impact scale (SIS) <60,14 and Barthel Index (BI) <8615 at 30 days and 6 months. The SIS (version 3.0) is a 59-item questionnaire that assesses health-related quality of life in stroke survivors across eight domains: strength, memory and thinking, emotion, communication, activities of daily living, mobility, hand function, and participation.16 CLEAR III participants also completed the EuroQol Visual Analogue Scale (EQ-VAS), which depicts information about the respondents’ subjective health perception, scored on a 20 cm visual analogue scale with endpoints labeled “the best health you can imagine” and “the worst health you can imagine.”17 Outcome measures for poor EQ-VAS in our study were defined as <50 on day 30 and <70 on day 180, based on median values and correlation with other outcome measures.
Statistical Analysis
Binary logistic regression models were used to assess associations between ICH lesion locations and stroke outcomes at days 30 and 180. Multivariable analyses were adjusted for age, ICH volume, 72-hour PHE volume, IVH volume, admission GCS, and treatment group. These variables were chosen based on their clinical relevance, hypothesized, or already recognized influences on functional outcome.18 Each individual anatomic site (caudate, GP/putamen, thalamus, ALIC, PLIC, and lobar) was included in the multivariable model to determine independent relationships between specific sites and outcomes. GP and putamen were combined in logistic regression models due to the small number of GP lesions and their anatomic continuity. Additional multivariable analysis was performed to compare thalamic and non-thalamic ICH location. The calibrations of models were checked using a likelihood ratio and Hosmer-Lemeshow goodness of fit test. We tested for effect modification by ICH volume * site (thalamus versus non-thalamus and by each anatomic site: caudate, GP/putamen, thalamus, ALIC, PLIC, and lobar) by adding anatomical location by ICH volume terms into our regression models. Models were built using forward selection of covariates with p<0.1 in univariable analyses followed by backwards elimination of covariates with p>0.1. Post-hoc descriptive analysis of mortality and primary ICH location by tertile of IVH volume was also performed. Statistical analyses were performed using Stata software (version 14.0, College Station, TX). All analyses were two-tailed, and significance level was determined by p<0.05.
Results
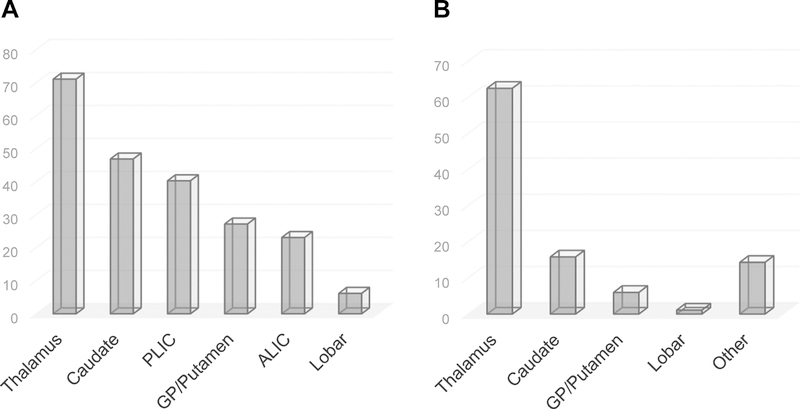
Among the 467 participants of CLEAR III included in this analysis, loss to follow-up for both 30-day and 6-month assessment of the primary outcome was minimal (1.2% and 1.8%, respectively). A total of 1003 ICH lesions in distinct anatomic regions were identified. The most common ICH location of involvement was thalamus (332; 71.1% of subjects), followed by caudate head (219, 46.9%), PLIC (188, 40.3%), GP/putamen (127, 27.2%), ALIC (108, 23.1%), and lobar (29, 6.2%) (Figure 1A). Thalamus followed by caudate had the highest frequency of anatomic locations involved by ICH and the highest frequency of primary ICH locations (Figure 1B). Frequency of involved ICH locations for each primary ICH location is shown in Figure I in the online-only Data Supplement. In primary lesions of ALIC, PLIC, and GP/putamen, the major other involved regions by ICH were thalamus and caudate. In thalamus primary lesions, posterior limb of the internal capsule and caudate were the main associated lesions. In caudate primary lesions, thalamus was the major associated ICH location.
Figure 1.
A. Frequency of all anatomic locations involved by ICH (N=1003). B. Frequency of single primary ICH locations (N=467).
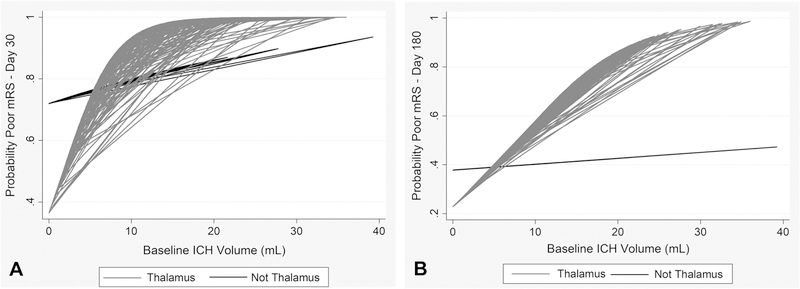
Baseline characteristics and day 180 outcomes of study participants by ICH location and comparison of thalamic and non-thalamic location are shown in Table 1. Mean (±SD) ICH and IVH volumes were 9.9±7.4 mL and 28.7±23.8 mL, respectively; primary thalamic ICH volume was significantly larger and IVH volume smaller compared to non-thalamic primary locations. In the univariable analysis, all stroke functional outcomes were significantly worse in subjects with thalamic versus non-thalamic ICH lesions. After adjusting for confounders, thalamic ICH location (vs. no thalamic ICH) was independently associated with all poor functional outcomes as well as mortality at both day 30 and 180 (Tables 2 and 3, respectively, at bottom). The interaction between ICH volume and thalamic location was significant for poor mRS, NIHSS, SIS (day 30), and BI; this odds ratio indicates an increased odds of a poor outcome for thalamic ICH location vs. not having thalamic ICH for subjects with a median ICH volume. Odds ratios for ICH volume in thalamic and non-thalamic locations are presented in Tables 2 and 3, and the effect of ICH volume on probability of poor mRS by location in Figure 2.
Table 1.
Clinical Factors and Day 180 Outcomes by ICH Location and Thalamic vs. Non-Thalamic ICH Location
| Location | N (%) | Age, Mean (SD) |
Female, n (%) |
Admission GCS, Median (IQR) |
ICH Volume (mL), Mean (SD) |
IVH Volume (mL), Mean (SD) |
Mortality, n (%) |
mRS 4–6, n (%) |
NIHSS >4, n (%) |
SIS <60, n (%) |
BI ≤85, n (%) |
EQ-VAS <70, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thalamus | 332 (71.1) | 58.6 (10.4) | 137 (41.3)* | 10 (6–14) | 11.5 (6.87)*** | 24.13 (18.18)*** | 84 (25.8)* | 123 (50.8)*** | 109 (50.7)*** | 114 (52.3)*** | 150 (63.3)*** | 137 (64.6)** |
| Non-thalamus | 135 (28.9) | 58.5 (13.0) | 67 (49.6) | 10 (7–14) | 5.89 (7.41) | 39.81 (23.04) | 26 (19.5) | 29 (27.1) | 21 (20.8) | 32 (30.8) | 39 (36.8) | 48 (47.5) |
| Caudate | 219 (46.9) | 59.7 (11.5) | 99 (45.2) | 9 (6–13) | 10.73 (7.82) | 36.54 (21.65) | 52 (24.2) | 81 (49.4) | 73 (48.3) | 70 (46.4) | 96 (59.6) | 89 (59.3) |
| PLIC | 188 (40.3) | 59.0 (10.4) | 74 (39.4) | 11 (7–14) | 13.77 (7.22) | 25.33 (20.29) | 52 (27.8) | 82 (60.7) | 76 (61.8) | 76 (60.3) | 99 (75.0) | 85 (70.8) |
| ALIC | 108 (23.1) | 59.2 (11.8) | 58 (53.7) | 8.5 (6–13) | 12.43 (8.28) | 30.96 (19.72) | 21 (19.4) | 39 (44.8) | 31 (40.8) | 39 (49.4) | 43 (51.2) | 42 (54.5) |
| GP/P | 127 (27.2) | 58.8 (11.8) | 62 (48.8) | 9 (6–13) | 14.86 (7.63) | 28.22 (17.84) | 35 (27.8) | 52 (56.5) | 49 (60.5) | 49 (59.8) | 62 (70.5) | 54 (66.7) |
| Lobar | 29 (6.2) | 60.24 (11.15) | 16 (55.2) | 11 (7–15) | 15.84 (8.65) | 34.58 (23.49) | 10 (34.5) | 4 (21.1) | 5 (27.8) | 4 (22.2) | 7 (36.8) | 7 (38.9) |
Comparison of thalamus vs. non-thalamus for each variable (t-test, rank sum test, or chi-square test). BI indicates Barthel Index; EQ-VAS, EuroQol Visual Analogue Scale; GP/P, globus pallidus/putamen; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and SIS, Stroke Impact Scale.
P<0.05.
P<0.01
P<0.001.
Table 2.
Multivariable Models of Association of ICH Lesion Location with Day 30 Outcomes: All Locations (top) and Thalamus vs. Non-Thalamus (bottom)
| Odds Ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Mortality (n=428) | mRS 4–6 (n=434) | NIHSS >4 (n=376) | SIS <60 (n=310) | BI <86 (n=388) | EQ-VAS <50 (n=298) | |
| Thalamus | 4.80 (1.44–15.94)** | 7.05 (2.49–19.95)*** | 3.81 (1.54–9.43)*** | 2.19 (0.73–6.64) | 9.55 (2.66–34.32)*** | 2.06 (0.93–4.57)* |
| IT (ICH*Thalamus) | 1.42 (1.21–1.67)*** | 1.49 (1.20–1.85)*** | ||||
| ICH Volume: Thalamus (per mL) | 1.36 (1.2–1.55)*** | 1.43 (1.21–1.70)*** | ||||
| ICH Volume: Non-Thalamus | 0.96 (0.86–1.07) | 0.96 (0.85–1.09) | ||||
| Caudate | 0.82 (0.37–1.78) | 0.68 (0.27–1.74) | 1.25 (0.64–2.45) | 0.79 (0.33–1.88) | 0.62 (0.23–1.65) | 1.04 (0.54–2.00) |
| PLIC | 0.83 (0.33–2.09) | 1.86 (0.78–4.43) | 1.08 (0.54–2.14) | 2.22 (1.06–4.68)** | 0.85 (0.36–2.04) | 0.82 (0.42–1.61) |
| ALIC | 0.08 (0.01–0.40)*** | 0.52 (0.19–1.41) | 0.53 (0.24–1.17) | 0.81 (0.32–2.09) | 0.63 (0.22–1.83) | 0.52 (0.25–1.08)* |
| GP/Putamen | 3.35 (1.30–9.68)** | 8.99 (1.57–51.35)** | 3.23 (1.33–7.87)*** | 1.82 (0.55–6.02) | 9.21 (1.18–72.07)** | 1.16 (0.54–2.51) |
| IT (ICH*GP/P) | 1.55 (1.14–2.11)*** | 1.57 (1.11–2.22)*** | ||||
| ICH Volume: GP/P (per mL) | 1.48 (1.1–2.01)** | 1.51 (1.1–2.07)** | ||||
| ICH Volume: Non-GP/P | 0.96 (0.86–1.07) | 0.96 (0.85–1.09) | ||||
| Lobar | 0.75 (0.04–14.7) | 2.5 (0.23–27.5) | 0.30 (0.09–1.00)* | 0.51 (0.11–2.35) | 4.82 (0.75–30.87)* | 0.96 (0.26–3.58) |
| Thalamus vs. Non-Thalamus | 4.81 (1.68–13.78)*** | 5.11 (1.91–13.63)*** | 4.84 (2.16–10.87)*** | 2.69 (1.001–7.2)** | 5.64 (1.62–19.62)*** | 2.14 (1.02–4.48)** |
| IT (ICH*Thalamus) | 1.34 (1.13–1.60)*** | 1.18 (1.03–1.35)** | 1.35 (1.07–1.71)** | |||
| ICH Volume: Thalamus (per mL) | 1.43 (1.28–1.61)*** | 1.32 (1.22–1.42)*** | 1.46 (1.26–1.69)*** | |||
| ICH Volume: Non-Thalamus | 1.07 (0.94–1.22) | 1.12 (0.99–1.26) | 1.08 (0.90–1.30) | |||
All models adjusted for age, ICH volume, IVH volume, admission GCS, and treatment group. BI indicates Barthel Index; EQ-VAS, EuroQol Visual Analogue Scale; GP/P, globus pallidus/putamen; IT, interaction term (ICH*Thalamus: ICH volume * thalamus location; ICH*GP/P: ICH volume * globus pallidus/putamen location); mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and SIS, Stroke Impact Scale.
0.05 ≤P <0.10.
0.01 ≤P <0.05.
P<0.01.
Table 3.
Multivariable Models of Association of ICH Lesion Location with Day 180 Outcomes: All Locations (top) and Thalamus vs. Non-Thalamus (bottom)
| Odds Ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Mortality (n=428) | mRS 4–6 (n=431) | NIHSS >4 (n=300) | SIS <60 (n=309) | BI <86 (n=317) | EQ-VAS <70 (n=299) | |
| Thalamus | 2.86 (1.29–6.37)** | 3.92 (1.94–7.93)*** | 4.60 (1.74–12.14)*** | 2.69 (1.22–5.91)** | 3.21 (1.42–7.22)*** | 1.64 (0.82–3.29) |
| IT (ICH*Thalamus) | 1.10 (1.02–1.19)** | 1.17 (1.03–1.34)** | 1.13 (1.04–1.24)*** | 1.12 (1.02–1.23)** | ||
| ICH Volume: Thalamus (per mL) | 1.17 (1.10–1.24)*** | 1.24 (1.15–1.34)*** | 1.16 (1.09–1.23)*** | 1.13 (1.04–1.22)*** | ||
| ICH Volume: Non-Thalamus | 1.06 (1.00–1.13)** | 1.06 (0.95–1.18) | 1.02 (0.96–1.09) | 1.01 (0.93–1.09) | ||
| Caudate | 0.49 (0.26–0.90)** | 0.69 (0.38–1.26) | 1.86 (0.93–3.74)* | 0.60 (0.31–1.16) | 1.19 (0.61–2.31) | 0.97 (0.53–1.79) |
| PLIC | 1.12 (0.60–2.11) | 1.32 (0.73–2.39) | 1.44 (0.71–2.91) | 1.19 (0.65–2.19) | 2.13 (1.07–4.20)** | 1.72 (0.94–3.14)* |
| ALIC | 0.51 (0.23–1.11) | 0.59 (0.32–1.1) | 0.40 (0.17–0.90)** | 1.07 (0.54–2.14) | 0.39 (0.18–0.85)** | 0.56 (0.28–1.12) |
| GP/Putamen | 1.52 (0.78–2.96) | 1.56 (0.83–2.92) | 2.28 (1.02–5.11)** | 2.36 (1.18–4.72)** | 2.54 (1.20–5.38)** | 1.86 (0.95–3.65)* |
| Lobar | 2.72 (1.08–6.82)** | 0.49 (0.19–1.30) | 0.64 (0.13–3.19) | 0.39 (0.09–1.69) | 0.40 (0.10–1.60) | 0.40 (0.13–1.24) |
| Thalamus vs. Non-Thalamus | 2.76 (1.34–5.70)*** | 4.66 (2.39–9.11)*** | 4.36 (1.86–10.22)*** | 2.96 (1.47–5.95)*** | 3.88 (1.92–7.85)** | 2.21 (1.20–4.09)** |
| IT (ICH*Thalamus) | 1.14 (1.05–1.22)*** | 1.19 (1.07–1.32)*** | 1.15 (1.06–1.26)*** | 1.16 (1.06–1.27)*** | ||
| ICH Volume: Thalamus (per mL) | 1.18 (1.12–1.25)*** | 1.20 (1.10–1.31)*** | 1.18 (1.11–1.25)*** | 1.15 (1.06–1.25)*** | ||
| ICH Volume: Non-Thalamus | 1.04 (0.99–1.1) | 1.01 (0.93–1.11) | 1.02 (0.96–1.08) | 0.99 (0.91–1.07) | ||
All models adjusted for age, ICH volume, IVH volume, admission GCS, and treatment group. BI indicates Barthel Index; EQ-VAS, EuroQol Visual Analogue Scale; GP/P, globus pallidus/putamen; IT (ICH*Thalamus), interaction term: ICH volume * thalamus location; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and SIS, Stroke Impact Scale.
0.05 ≤P <0.10.
0.01 ≤P <0.05.
0.001 ≤P <0.01.
Figure 2.
Interaction of ICH volume and thalamic location for outcome prediction. Simple model for thresholding interaction between thalamic location (vs. non-thalamic) and ICH size at (A) day 30 and (B) day 180. A baseline ICH volume threshold below approximately 5 mL appears to have a lower probability of poor mRS compared to non-thalamic ICH in setting of large obstructive IVH. Above this threshold, probability of poor mRS increases by more in thalamus compared to other locations.
The associations between distinct ICH locations and stroke outcomes at day 30 and 180 are shown in Tables 2 and 3, respectively. Following adjustment for age, ICH and IVH volume, 72-hour PHE volume, admission GCS, treatment group and significant interaction terms, thalamic ICH location was significantly associated with increased mortality and poor functional outcome on mRS, NIHSS, and BI at day 30, and with mortality, poor mRS, NIHSS, SIS, and BI at day 180. Caudate ICH was significantly associated with lower mortality at day 180, whereas ALIC location was associated with lower mortality at day 30. At day 180 ALIC location was associated with significantly lower odds of poor NIHSS and poor BI. PLIC location was significantly associated with poor SIS at day 30, and with poor BI at day 180. GP/putamen location was associated with higher mortality and poor outcome on mRS, NIHSS, and BI at day 30, and with higher NIHSS, worse SIS, and BI at day 180. Lobar ICH location was significantly associated with increased mortality, but no significant association with other outcomes. Interaction terms for ICH volume by thalamic and GP/putamen locations were significant in several outcome analyses (day 30 mRS and BI for both, and day 180 mRS, NIHSS, SIS, and BI for thalamus location only).
To better evaluate the effect of increasing IVH volume on mortality for different primary ICH locations, we analyzed subjects with primary thalamic, caudate, and GP/putamen ICH by tertile of IVH volume. Caudate location exhibited a relatively linear relationship between mortality and increasing IVH tertile, while for thalamic and GP/putamen ICH there was evidence of a threshold effect with higher mortality with IVH volume >17.0 mL (Figure 3).
Figure 3.
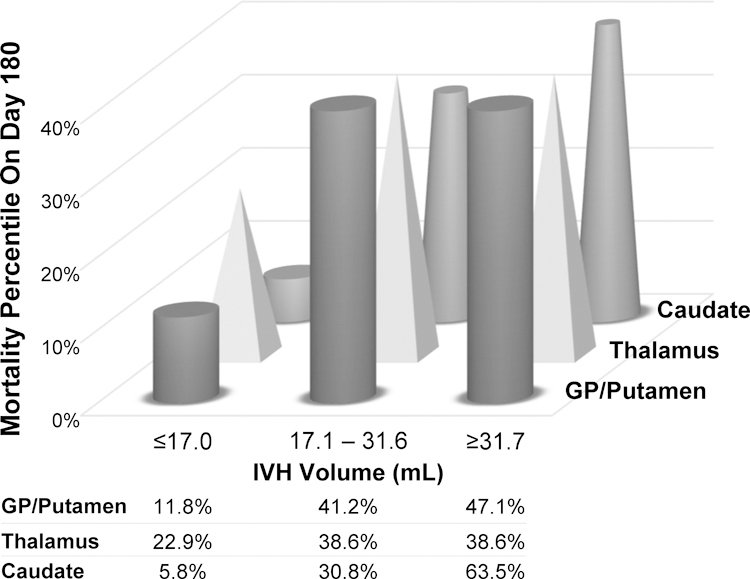
Mortality in major primary ICH location groups by IVH volume tertile (N=388).
Treatment with alteplase was significantly associated with lower day 180 mortality after adjustment for all ICH locations and other confounders (odds ratio [OR], 0.53; 95% CI, 0.32–0.87), but not with other stroke functional outcomes. PHE volume was a significant contributor to day 30 and day 180 mortality in the multivariable analysis of thalamic vs. non-thalamic ICH, but the relationship was inverse to that expected; increase in PHE volume was associated with lower mortality risk. In this analysis and the multivariable analysis of all locations, PHE volume was also independently associated with day 180 poor BI with the expected positive association (OR, 1.07; 95% CI, 1.01–1.13; p=0.02). There were no other significant associations.
Discussion
In this secondary analysis of stroke outcomes from the CLEAR III trial, we observed that despite obstructive IVH requiring external ventricular drainage, anatomic localization of even small parenchymal hemorrhages was a strong determinant of clinical outcomes at both 30 days and even more significantly at 180 days after the acute impact of IVH was over. Thalamic involvement had the most associations with poor outcomes increasing the risk of mortality, and worsened functional outcome including mRS, NIHSS, and BI at 1 and 6 months after hemorrhage onset. Other major regions associated with worsened functional outcome after adjustment for clinical severity were GP/Putamen and PLIC, whereas ALIC and caudate lesions were associated with better stroke outcomes. Lobar location was associated with higher day 180 mortality. The effect of ICH volume on functional outcomes in this setting was modified by thalamus location and in some cases by GP/putamen location, but not by other anatomic regions. Treatment group showed a significant effect for mortality at day 180, but not for other functional outcomes, consistent with results of the main study.19
Thalamic ICH accounts for 8–15% of all ICH.7 In our study of hypertensive IVH, however, thalamic ICH was present in more than 60% of patients, reflecting inclusion criteria of CLEAR III. This high prevalence of thalamic lesions provided a unique opportunity to evaluate the role of thalamic involvement in stroke outcomes. Our results are consistent with other studies, including a large study of 2,066 patients with ICH from the INTERACT2 trial that found thalamic location is more likely to be fatal8 and associated with poor stroke outcomes.7,8,20 The INTERACT2 ICH location study also included small ICH volume (median 10.7 mL) with less IVH (29% overall) and smaller thalamic ICH volume compared to our study (median 7.6 mL). The prevalence of IVH in thalamic ICH ranges from 43% from a stroke registry study from Spain to 65% in the Ethnic/Racial Variations of ICH (ERICH) study database and 71% in a study of 70 thalamic ICH patients from Korea.7,21,22 These studies all report poor outcomes for thalamic ICH. Such epidemiologic data suggest thalamic ICH is a poor prognostic factor both in more general ICH populations without predominant IVH as well as in large obstructive IVH, and that the type of thalamic ICH represented by our cohort is not likely to be significantly different from other ICH populations.
The high mortality risk of thalamic ICH is usually explained due to anatomical proximity of the thalamus to the third ventricle, hence a higher chance of intraventricular extension and brainstem compression9; we found this association despite adjustment for IVH and ICH volume. In fact, IVH volume in our study was significantly smaller in subjects with thalamus as either the primary ICH location (median IVH volume 17.5 mL versus 28.0 mL in subjects with non-thalamic ICH; p<0.001), or any thalamic involvement of ICH. Arboix et al. evaluated patients with thalamic ICH from a stroke registry and reported a mortality rate of 19%.21 Altered consciousness at presentation, presence of IVH, and advanced age were found to be independent predictors of in-hospital mortality. In our study, these factors are less apparent given the similarity in age, GCS, and presence of obstructive IVH in all subjects, and exclusion of subjects with early brainstem herniation syndromes. Arboix et al. compared patients with thalamic ICH to those with internal capsule-basal ganglia hemorrhage and found that history of chronic liver disease, sensory symptoms, nausea and/or vomiting, and ataxia were significantly more frequent in patients with thalamic hemorrhage.21 We did not assess chronic liver disease, but we found no differences in coagulation parameters between thalamic and non-thalamic primary ICH location. Factors not assessed in this analysis that may be important to outcome assessment in thalamic ICH include involvement of specific thalamic nuclei and recovery of consciousness over the longer term.
We found a strong association between GP/putamen ICH location and worse stroke outcomes, including early mortality, in line with previous reports.8,23 On the other hand, caudate ICH location was not associated with significant effect on functional outcomes after adjustment for ICH and IVH volumes, except for lower mortality at day 180. Median IVH volume was highest, however, in subjects with primary caudate lesions, suggesting IVH volume reduction may have significant impact on good outcomes for this location where the ICH is relatively benign.
Anterior and posterior limbs of the internal capsule had opposite impacts on stroke outcomes, which is likely related to the strategic anatomical location of pyramidal tract within the PLIC.8,24,25 Damage to the pyramidal tracts affecting motor function had significant impact on mRS and SIS, known to be biased toward motor disability.26 Lobar ICH lesions, accounted for less than 6% of our cohort, and lobar engagement was associated with higher day 180 mortality but no significant associations with functional outcomes, inconsistent with previous reports of association of lobar ICH with better functional outcomes after adjustment for ICH volume,1,27 with better quality of life,28 and with lower morbidity.29 Higher mortality was not expected, though lobar engagement of ICH was associated with the largest ICH volumes in this cohort, and lobar lesions engaged all other regions fairly equally (Figure I, online-only Data Supplement). For survivors, it has been suggested that underlying cerebral amyloid angiopathy and age-related neurodegenerative changes in lobar hemorrhage may be a protective factor against mass effect and poor outcomes.30 Our analysis did confirm known associations between older age and worse neurologic outcomes, adjusting for all locations.9 Age was significantly associated with every poor outcome indicator analyzed and with mortality at day 180. The 3 lesion sites associated with poor outcomes (thalamus, GP/putamen, PLIC) were all significantly associated with worse BI at 180 days, suggesting any parenchymal hematoma in these areas, regardless of size, can impact activities of daily living. The odds of poor quality of life assessment on the EQ-VAS at thresholds of 50 at day 30 and 70 at day 180 were significantly associated with thalamic ICH versus not thalamus, but not with other ICH locations.
Overall, this study supports the concept that ICH topography provides important information with regards to long-term functional outcomes independent of ICH and IVH volumes. This has been reported for ischemic stroke,25 for a heterogeneous cohort of ICH subjects,8 and, we can now report, for ICH where IVH appears to be the clinically dominant lesion. The significance of ICH location in recovery has also been demonstrated in functional neuroimaging studies including a functional diffusion mapping (fDM) study that showed a strong association between thalamic lesions and worse stroke outcomes (OR, 15.64; 95% CI, 1.40–174.57).31 Differences in fDMs between thalamic and non-thalamic lesions were not assessed.
Major strengths of this study included prospective assessment of multiple stroke outcome scales at early and late time points in a large cohort of ICH/IVH patients with minimum missing data. Lesion topography was performed with a structured atlas-based approach by a team of qualified neuroimaging readers and adjudicated by a neuroradiologist blinded to clinical outcomes. The major limitation of this study is lack of generalizability to ICH subjects with volumes >30 mL, infratentorial ICH, or IVH not requiring an EVD. However, our objective was to evaluate significance of ICH when IVH was the dominant severity factor, and inclusion of only small ICH allowed potentially more discrete segregation of anatomic regions for purpose of understanding relationships with specific outcome measures. The percentage of involvement by anatomic location was not studied, which is a limitation, and the smaller size of some structures (ALIC, PLIC, and caudate) may have limited finding significant interaction effects of location by volume for smaller structures. The use of only CT scans for anatomic localization may limit accuracy of anatomic definition due to gantry angle and variable slice thickness. Magnetic resonance imaging was only available in a minority of subjects. Finally, because the chosen cutoffs for Barthel Index, NIHSS and SIS relate to functional independence they may overestimate the similarities between results. A larger population that meets statistical requirements for proportional distribution will be required to assess the congruence or not of the entire scales of mRS, BI, SIS etc.
Conclusion
Our study findings support prognostication of ICH based on the location of relatively small hemorrhages even in the presence of large obstructive IVH. In this setting, thalamic and lobar ICH engagement were the only sites independently associated with increased risk of death. Deep lesions were associated with greater odds of residual disability predominantly at 6 months, with exception of caudate lesions, while ALIC lesions were associated with better stroke outcomes. Understanding ICH location and contribution to functional outcomes is beneficial to patient selection for aggressive interventions and to guide meaningful endpoints for clinical trials.
Supplementary Material
Acknowledgements
The authors thank Megan Clark, Science Writer at the Division of Brain Injury Outcomes for editorial support and assistance.
Source of funding
CLEAR III was supported by National Institute of Neurological Disorders and Stroke grant U01NS062851, awarded to Dr. Hanley.
Disclosures
Dr. Parry-Jones is supported by an NIHR Clinician Scientist Award (CS-2014-14-005). Drs. Ziai, Awad, and Hanley report grant support from the National Institutes of Health during the conduct of the study. Dr. Awad reports medicolegal consulting. Dr. Hanley reports consulting for BrainScope, Neurotrope, Op2Lysis, Portola Pharmaceuticals and Medtronic, and non-financial research support from Genentech. Dr. Ziai reports consulting for C.R. Bard, Inc. The other authors report no conflicts.
References
- 1.Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: The func score. Stroke. 2008;39:2304–2309. [DOI] [PubMed] [Google Scholar]
- 2.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: Systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660–667. [DOI] [PubMed] [Google Scholar]
- 3.Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: Risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767–774. [DOI] [PubMed] [Google Scholar]
- 4.Hinson HE, Hanley DF, Ziai WC. Management of intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2010;10:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanley DF. Intraventricular hemorrhage: Severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40:1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu YZ, Wang JW, Luo BY. Epidemiological and clinical characteristics of 266 cases of intracerebral hemorrhage in Hangzhou, China. J Zhejiang Univ Sci B. 2013;14:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SH, Park KJ, Kang SH, Jung YG, Park JY, Park DH. Prognostic factors of clinical outcomes in patients with spontaneous thalamic hemorrhage. Med Sci Monit. 2015;21:2638–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delcourt C, Sato S, Zhang S, Sandset EC, Zheng D, Chen X, et al. Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology. 2017;88:1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arboix A, Comes E, Garcia-Eroles L, Massons J, Oliveres M, Balcells M, et al. Site of bleeding and early outcome in primary intracerebral hemorrhage. Acta Neurol Scand. 2002;105:282–288. [DOI] [PubMed] [Google Scholar]
- 10.Ziai WC, Tuhrim S, Lane K, McBee N, Lees K, Dawson J, et al. A multicenter, randomized, double-blinded, placebo-controlled phase III study of clot lysis evaluation of accelerated resolution of intraventricular hemorrhage (CLEAR III). Int J Stroke. 2014;9:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan T, Awad I, Keyl P, Lane K, Hanley D. Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl. 2008;105:217–220. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Arima H, Wu G, Heeley E, Delcourt C, Zhou J, et al. Prognostic significance of perihematomal edema in acute intracerebral hemorrhage: pooled analysis from the intensive blood pressure reduction in acute cerebral hemorrhage trial studies. Stroke. 2015;46:1009–1013. [DOI] [PubMed] [Google Scholar]
- 13.Johnston KC, Wagner DP. Relationship between 3-month National Institutes of Health Stroke Scale score and dependence in ischemic stroke patients. Neuroepidemiology. 2006;27:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canadian Partnership for Stroke Recovery. Stroke Impact Scale (SIS) Evaluation Summary. https://www.strokengine.ca/en/quick/sis_quick/ Accessed Feb 2, 2019.
- 15.Uyttenboogaart M, Stewart RE, Vroomen PC, De Keyser J, Luijckx GJ. Optimizing cutoff scores for the Barthel index and the modified Rankin Scale for defining outcome in acute stroke trials. Stroke. 2005;36:1984–7. [DOI] [PubMed] [Google Scholar]
- 16.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. [DOI] [PubMed] [Google Scholar]
- 17.Fayers PM, Machin D. Scores and Measurements: Validity, Reliability, Sensitivity In: Fayers PM, Machin D, eds. Quality of Life: The Assessment, Analysis and Interpretation of Patient-Reported Outcomes. 2nd ed. Chichester, UK: John Wiley & Sons Ltd; 2007: 77–108. [Google Scholar]
- 18.Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 19.Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: Results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori S, Sadoshima S, Ibayashi S, Fujishima M, Iino K. Impact of thalamic hematoma on six-month mortality and motor and cognitive functional outcome. Stroke. 1995;26:620–626. [DOI] [PubMed] [Google Scholar]
- 21.Arboix A, Rodriguez-Aguilar R, Oliveres M, Comes E, Garcia-Eroles L, Massons J. Thalamic haemorrhage vs internal capsule-basal ganglia haemorrhage: Clinical profile and predictors of in-hospital mortality. BMC Neurology. 2007;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leasure AC, Sheth KN, Comeau M, Aldridge C, Vashkevich A, Rosand J, et al. Characterization of deep, supratentorial intracerebral hemorrhage in the Ethnic/Racial Variations of ICH Study [AAN abstract P6.322]. Neurology. 2018;90(suppl):P6.322. [Google Scholar]
- 23.Miyai I, Suzuki T, Kang J, Volpe BT. Improved functional outcome in patients with hemorrhagic stroke in putamen and thalamus compared with those with stroke restricted to the putamen or thalamus. Stroke. 2000;31:1365–1369. [DOI] [PubMed] [Google Scholar]
- 24.Englander RN, Netsky MG, Adelman LS. Location of human pyramidal tract in the internal capsule: Anatomic evidence. Neurology. 1975;25:823–826. [DOI] [PubMed] [Google Scholar]
- 25.Puig J, Pedraza S, Blasco G, Daunis IEJ, Prados F, Remollo S, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR. 2011;32:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu O, Cloonan L, Mocking SJ, Bouts MJ, Copen WA, Cougo-Pinto PT, et al. Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke. 2015;46:2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zia E, Engstrom G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. 2009;40:3567–3573. [DOI] [PubMed] [Google Scholar]
- 28.Christensen MC, Mayer S, Ferran JM. Quality of life after intracerebral hemorrhage: Results of the factor seven for acute hemorrhagic stroke (FAST) trial. Stroke. 2009;40:1677–1682. [DOI] [PubMed] [Google Scholar]
- 29.Kim KH. Predictors of 30-day mortality and 90-day functional recovery after primary intracerebral hemorrhage: Hospital based multivariate analysis in 585 patients. J Korean Neurosurg Soc. 2009;45:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cordonnier C, Leys D, Dumont F, Deramecourt V, Bordet R, Pasquier F, et al. What are the causes of pre-existing dementia in patients with intracerebral haemorrhages? Brain. 2010;133:3281–3289. [DOI] [PubMed] [Google Scholar]
- 31.Tsai YH, Hsu LM, Weng HH, Lee MH, Yang JT, Lin CP. Functional diffusion map as an imaging predictor of functional outcome in patients with primary intracerebral haemorrhage. Br J Radiol. 2013;86:20110644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.