Abstract
Introduction:
Clinicians intuitively recognize that faster time to hemostasis is important in bleeding trauma patients, but these times are rarely reported.
Methods:
Prospectively collected data from the PROPPR trial were analyzed. Hemostasis was predefined as no intraoperative bleeding requiring intervention in the surgical field or resolution of contrast blush on interventional radiology. Patients who underwent an emergent (within 90 minutes) OR or IR procedure were included. Mixed-effects Poisson regression with robust error variance (controlling for age, injury severity score [ISS], treatment arm, injury mechanism, base excess on admission [missing values estimated by multiple imputation], and time to OR/IR as fixed effects and study site as a random effect) with modified Bonferroni corrections tested the hypothesis that decreased time to hemostasis was associated with decreased mortality and decreased incidence of acute kidney injury (AKI), acute respiratory distress syndrome (ARDS), multiple organ failure (MOF), sepsis, and venous thromboembolism (VTE).
Results:
Of 680 enrolled patients, 468 (69%) underwent an emergent procedure. Patients with decreased time to hemostasis were less severely injured, had less deranged base excess on admission, and lower incidence of blunt trauma (all p<0.05). In 408 patients (87%) in whom hemostasis was achieved, every 15 minute decrease in time to hemostasis was associated with decreased 30-day mortality (RR 0.97, 95% CI 0.94–0.99), AKI (RR 0.97, 95% CI 0.96–0.98), ARDS (RR 0.98, 95% CI 0.97–0.99), MOF (RR 0.94, 95% CI 0.91–0.97), and sepsis (RR 0.98, 95% CI 0.96–0.99), but not VTE (RR 0.99, 95% CI 0.96–1.03).
Conclusion:
Earlier time to hemostasis was independently associated with decreased incidence of 30-day mortality, AKI, ARDS, MOF, and sepsis in bleeding trauma patients. Time to hemostasis should be considered as an endpoint in trauma studies and as a potential quality indicator.
Level of evidence:
Level III (Therapeutic / Care Management)
Keywords: hemorrhage, trauma, massive transfusion
Introduction
Hemorrhage is the leading cause of preventable trauma mortality1,2,3 with median time to exsanguination of 2–3 hours after hospital arrival.4,5 Optimal treatment of the hemorrhaging trauma patient includes transfusion of a balanced ratio of blood products and measures to rapidly acquire mechanical hemorrhage control, i.e. hemostasis.6 Clinicians intuitively recognize that minimization of blood loss by more rapidly obtaining hemostasis reduces physiologic derangement and ischemic organ injury, thereby improving patient outcomes. Several studies have reported that minimizing times to laparotomy,7,8 interventional radiology (IR),9 and plasma and platelet transfusion (hemostatic resuscitation)4,10,11 are associated with improved outcomes in trauma patients, likely by minimizing the time to hemostasis and degree of blood loss. However, time to hemostasis is not routinely reported.12 The Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial found that bleeding trauma patients randomized to high (1:1:1) ratios of plasma and platelets to red blood cells (RBCs) were more likely to have achieved hemostasis and less likely to have died from hemorrhage within the first 24 hours compared to patients randomized to a low (1:1:2) ratio, although there were no differences in 24-hour or 30-day mortality.5 In patients achieving hemostasis, the time from hospital arrival to hemostasis was similar between treatment arms (median 144 vs 131 min, p=0.17). We hypothesized that early achievement of hemostasis was independently associated with reduced mortality and risk of complications.
Methods
Patients and data collection
This was a pre-specified secondary analysis of the PROPPR study, a pragmatic, phase 3, multicenter trial which randomized 680 bleeding trauma patients predicted to require massive transfusion to resuscitation with 1:1:1 versus 1:1:2 plasma to platelets to RBCs during a 17-month period in 2012–2013. This study was approved by the institutional review boards of all 12 participating sites.5
Definitions
Hemostasis was declared by the attending trauma surgeon and defined a priori as no intraoperative bleeding requiring intervention in the surgical field or resolution of contrast blush on IR. Active resuscitation was ended when hemostasis was achieved and the attending surgeon and anesthesiologist agreed that the patient was adequately resuscitated based on improving patient physiology (normalizing vital signs, increasing urine output, or decreasing vasopressor requirement). We included patients who underwent an emergent procedure defined as any operating room (OR) or IR procedure for hemorrhage control within 90 minutes of hospital arrival, consistent with previous studies.13,14 Severe injury was defined as abbreviated injury scale (AIS) ≥3. Definitions of complications were those from the primary paper and were standardized across all institutions.5 Acute kidney injury (AKI) was defined as serum creatinine increase of at ≥0.3 mg/dL or ≥50%, glomerular filtration rate (GFR) decrease of ≥25%, or urine output of <0.5 ml/kg/hr. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin criteria.15 Multiple organ failure (MOF) was defined using the Denver MOF scoring system. Sepsis was defined as systemic inflammatory response syndrome and suspected or known infection. Venous thromboembolism (VTE) was defined as deep venous thrombosis identified by autopsy or imaging, or pulmonary embolism identified on CT angiogram.
Statistical analysis
Stata 14.1 (Stata Corp., College Station, TX) was used for all calculations. Data are summarized as median and interquartile range (IQR) or proportions as appropriate. Patients requiring emergent OR or IR were separated into groups based on time to hemostasis to facilitate univariate and sensitivity analyses. Patients were divided into quartiles based on time to hemostasis with a fifth group created for patients who did not achieve hemostasis. Non-parametric univariate comparisons of continuous data were performed using the Kruskal-Wallis test. Comparisons of categorical data were performed with chi-squared test or Fisher’s exact test for categories with ≤5 expected members. Locally weighted scatterplot smoothing (LOWESS) was used to estimate the incidence of complications versus time to hemostasis.
Mixed-effects Poisson models with robust error variance (which have been previously used to analyze dichotomous outcomes)16 were used to analyze mortality at 24 hours and 30 days, as well as five inflammatory complications (AKI, ARDS, MOF, sepsis, and VTE) to test the hypothesis that decreased time to hemostasis was independently associated with improved outcomes. Out of the 23 predefined complications analyzed in PROPPR, we chose the above complications to analyze in the present study because they were prevalent, clinically important, and thought to be potential consequences of the prolonged ischemia and increased inflammation secondary to delayed hemostasis. Of note, 6-hour mortality was not analyzed because only one patient who achieved hemostasis died within 6 hours of ED arrival. Unadjusted models controlled for study site as a random effect. Adjusted models controlled for study site as a random effect and the following as fixed effects: age, ISS, injury mechanism, treatment arm, admission base excess, and time to OR/IR. These were selected because of their clinical significance and/or statistical significance at the 0.1 level on univariate analysis. Missing admission base excess data were estimated using multiple imputation with predictive mean matching using age, injury mechanism, and ISS as predictors. Modified Bonferroni corrections were performed to control for multiple comparisons. Because these analyses use time to hemostasis as a continuous variable, only patients who achieved hemostasis were included. Sensitivity analyses were then performed which included patients who exsanguinated before hemostasis was achieved. A two-tailed alpha level of 0.05 was used for all statistical tests.
Results
Summary of hemostasis and between-site comparisons
Of 680 patients enrolled, 468 (69%) underwent an emergent OR/IR procedure. These patients had greater incidence of severe abdominal injury (60% vs 27%), decreased incidence of severe head injury (17% vs 34%), and decreased incidence of TBI-related death (6% vs 17%) compared to those not undergoing an emergent OR/IR procedure (all p<0.01).
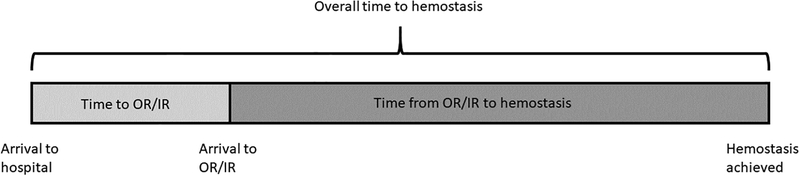
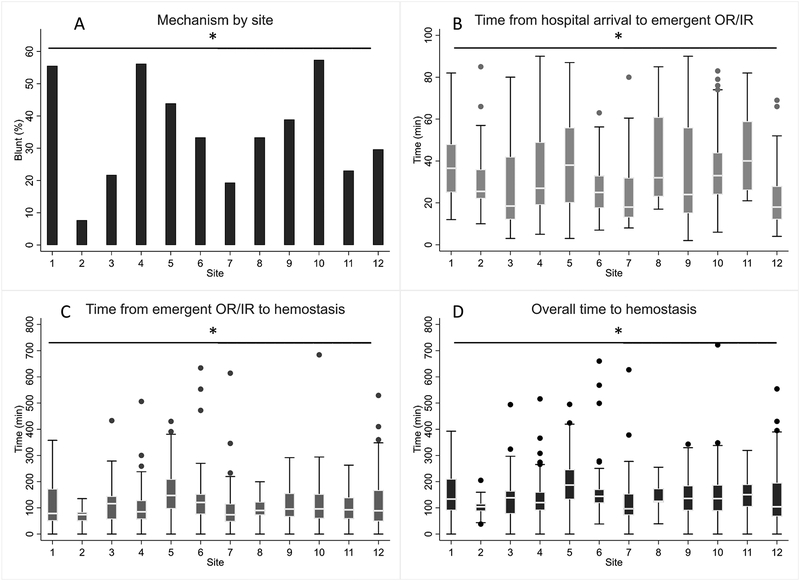
Among patients undergoing emergent OR/IR, hemostasis was achieved in 87% (n=408) with no significant differences between study sites on the chi-squared test (range: 71–91% per site, p=0.43). We examined the time to emergent OR/IR, time from OR/IR to hemostasis, and overall time to hemostasis, which is schematically depicted in Figure 1. Time from OR/IR to hemostasis accounted for the majority of overall time to hemostasis (median 77%, IQR 63–87%). There were significant between-site differences in proportion of blunt trauma (range: 8–57%), use of emergent IR instead of OR (range: 0–20%), as well as time to emergent OR/IR, time from OR/IR to hemostasis, and overall time to hemostasis (all p<0.05). Overall time to hemostasis was significantly higher in blunt versus penetrating trauma (median 139 vs 119 min, p<0.01) and after IR versus OR (median 184 vs 127 min, p<0.01).
Figure 1.
Schematic representation of overall time to hemostasis, which consists of time from hospital arrival to OR/IR and time from OR/IR to hemostasis.
Demographics
Patients who underwent emergent OR/IR (n=468, 69%) were divided into quartiles based on time to hemostasis (group 1, <90 min; group 2, 90 to 129 min; group 3, 130 to 183 min; group 4, >183 min). A fifth group was created for patients who did not achieve hemostasis. There were no differences in age, admission systolic blood pressure or heart rate, or treatment arm between groups (Table 1). Higher group number was associated with increased ISS, more deranged base excess on admission, and higher incidence of blunt trauma. Multiple imputation with predictive mean matching was used to estimate missing admission base excess in 45 patients (10%) using age, ISS, and injury mechanism as predictors. Distribution of base excess before and after imputation was unchanged.
Table 1.
Demographics of patients requiring emergent OR/IR (n=468)
| Variable | Group 1 (n=102) | Group 2 (n=102) | Group 3 (n=104) | Group 4 (n=100) | Group 5 (n=60) | p |
|---|---|---|---|---|---|---|
| Time to hemostasis (min) | <90 | 90 to 129 | 130 to 183 | >183 | No hemostasis | - |
| Age (years) | 32 (24, 47) | 31 (24, 43) | 33 (24, 45) | 32 (24, 48) | 34 (24, 54) | 0.57 |
| ISS | 20 (13, 34) | 25 (14, 38) | 26 (18, 36) | 29 (19, 41) | 34 (25, 45) | <0.01 |
| Base excess (mEq/L) | −7 (−11, −2) | −7 (−12, −4) | −7 (−12, −3) | −9 (−14, −6) | −17 (−19, −12) | <0.01 |
| SBP (mmHg) | 100 (79, 126) | 106 (86, 130) | 108 (86, 123) | 104 (80, 130) | 94 (78, 118) | 0.17 |
| Heart rate (bpm) | 113 (93, 131) | 107 (92, 127) | 120 (97, 133) | 122 (102, 136) | 113 (82, 136) | 0.09 |
| Blunt mechanism (n, %) | 29 (28%) | 36 (35%) | 47 (45%) | 46 (46%) | 28 (47%) | 0.03 |
| 1:1:2 | 49 (48%) | 49 (48%) | 58 (51%) | 51 (51%) | 27 (45%) | |
| Emergent IR (n, %) | 1 (1%) | 1 (1%) | 7 (7%) | 9 (9%) | 1 (2%) | |
| Time from hospital arrival to OR/IR (min) | 20 (15, 28) | 28 (19, 42) | 31 (20, 57) | 34 (21, 55) | 25 (18, 45) | <0.01 |
| Time from hospital arrival to hemostasis (min) | 69 (56, 80) | 111 (101, 120) | 152 (141, 165) | 245 (210, 303) | - | - |
ISS, injury severity score
Outcomes
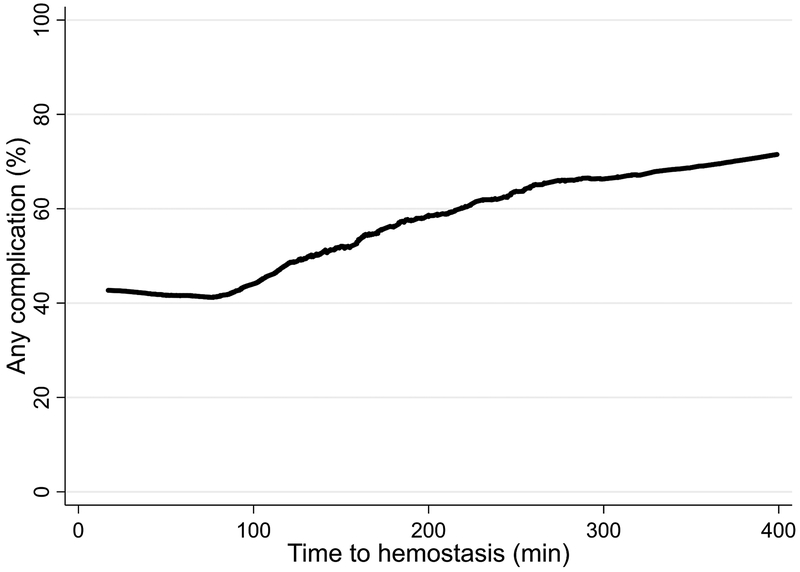
Higher group number was associated with increased transfusion of blood products during active resuscitation, consistent with increased hemorrhage (Table 2). There were no significant differences in mortality or time to death between the first four groups, although cause of death was somewhat different. In contrast, patients in group 5 died quickly (median 2.2 hours) from exsanguination. As group number increased, ICU-free days decreased and incidence of AKI, ARDS, MOF, and sepsis increased. LOWESS regression was used to estimate the rate of any complication (mortality, AKI, ARDS, MOF, sepsis, or VTE) versus time to hemostasis (Figure 3).
Table 2.
Outcomes of patients requiring emergent OR/IR (n=468)
| Variable | Group 1 (n=102) | Group 2 (n=102) | Group 3 (n=104) | Group 4 (n=100) | Group 5 (n=60) | p* |
|---|---|---|---|---|---|---|
| Platelets (units) | 1 (0, 1) | 1 (0, 2) | 1 (1, 2) | 2 (1, 3) | 3 (1, 4) | |
| Mortality at 6 hours (n, %) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 55 (92%) | 1.00 |
| Mortality at 24 hours (n, %) | 1 (1%) | 2 (2%) | 2 (2%) | 1 (%) | 59 (98%) | 1.00 |
| Mortality at 30 days (n, %) | 8 (8%) | 8 (8%) | 8 (8%) | 15 (15%) | 60 (100%) | 0.25 |
| Time to death (days) | 3.6 (1.3, 6.3) | 2.8 (0.8, 11.9) | 7.1 (1.5, 18.5) | 8.8 (2.5, 11.2) | 0.09 (0.05, 0.15) | 0.74 |
| Other | 1 (1%) | 1 (1%) | 0 (0%) | 1 (1%) | 1 (2%) | |
| ICU-free days | 26 (17, 28) | 25 (8, 27) | 22 (11, 26) | 16 (0, 25) | 0 (0, 0) | <0.01 |
| AKI (n, %) | 13 (13%) | 17 (17%) | 29 (28%) | 43 (43%) | 2 (3%) | <0.01 |
| ARDS (n, %) | 10 (10%) | 14 (14%) | 23 (22%) | 33 (33%) | 5 (8%) | <0.01 |
| MOF (n, %) | 0 (0%) | 2 (2%) | 3 (3%) | 14 (14%) | 2 (3%) | <0.01 |
| Sepsis (n, %) | 21 (21%) | 26 (25%) | 43 (41%) | 45 (45%) | 0 (0%) | <0.01 |
| VTE (n, %) | 7 (7%) | 10 (10%) | 16 (15%) | 14 (14%) | 0 (0%) | 0.20 |
| Any complication§ (n, %) | 38 (37%) | 38 (37%) | 61 (59%) | 72 (72%) | 60 (100%) | <0.01 |
for first four groups only
not mutually exclusive
includes mortality, AKI, ARDS, MOF, sepsis, or VTE
RBC, red blood cells; TBI, traumatic brain injury; MOF, multiple organ failure; PE, pulmonary embolism; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; VTE, venous thromboembolism
Figure 3.
Locally weighted scatterplot smoothing (LOWESS) regression of incidence of any complication (mortality, AKI, ARDS, MOF, sepsis, or VTE) versus time to hemostasis.
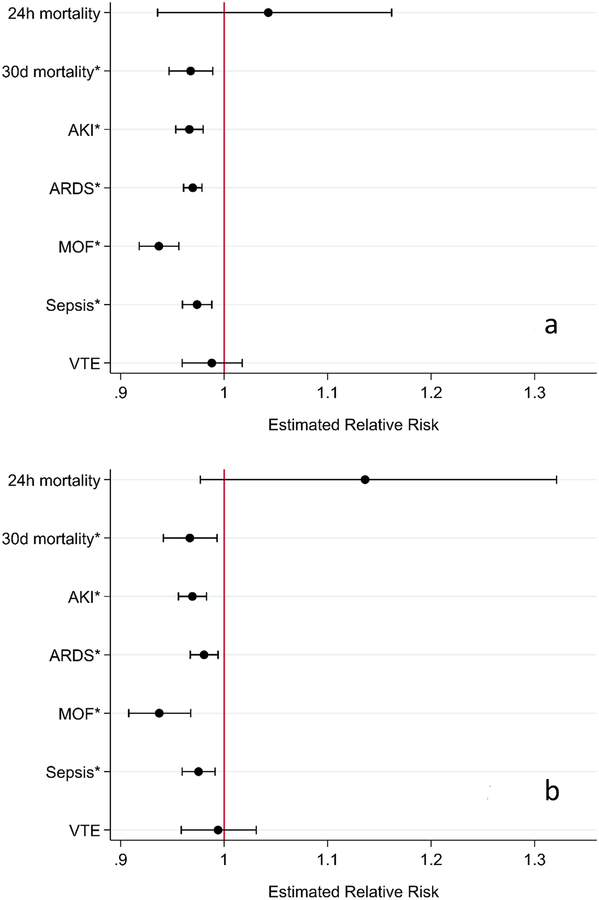
In 408 patients in whom hemostasis was achieved, mixed effects Poisson regression with robust error variance and modified Bonferroni corrections found that every 15 minute decrease in time to hemostasis was associated with decreased 30-day mortality (RR 0.97, 95% CI 0.94–0.99, p<0.001) and decreased incidence of AKI (RR 0.97, 95% CI 0.96–0.98, p<0.001), ARDS (RR 0.98, 95% CI 0.97–0.99, p<0.01), MOF (RR 0.94, 95% CI 0.91–0.97, p<0.001), and sepsis (RR 0.98, 95% CI 0.96–0.99, p<0.001), but not 24-hour mortality (RR 1.14, 95% CI 0.98–1.32, p=0.13) or VTE (RR 0.99, 95% CI 0.96–1.03, p=0.73). Results before and after adjustment for age, ISS, treatment arm, injury mechanism, time to OR/IR, and admission base excess are summarized in Figure 4.
Figure 4A–B.
Mixed effects Poisson regression with robust error variance for patients requiring emergent OR/IR and who achieved hemostasis (n=408). Unadjusted models (A) control for study site as a random effect. Adjusted models (B) control for age, ISS, injury mechanism, time to OR/IR, treatment group, and admission base excess as fixed effects and study site as a random effect. *, statistically significant after modified Bonferroni correction.
Diagnostic studies were performed (Supplement). In terms of global goodness-of-fit, chi-squared tests of the deviance statistic were not significant (p>0.05). Visual inspection of deviance residuals versus predicted values revealed no outliers in three models and 1% outliers in four models (defined as deviance residual < −2 or > +2). Due to the dichotomous outcome, the residuals were not expected to be normally distributed. Instead, we used our models to generate simulated data; visual comparison of actual versus simulated residuals revealed moderate deviations in the sepsis model and no deviations in other models.
Sensitivity Analyses
Patients who did not achieve hemostasis had undefined time to hemostasis and were excluded from the primary analysis, likely leading to survival bias. Therefore, two sensitivity analyses were performed including the 60 patients who exsanguinated before hemostasis was achieved. First, these patients were assigned time to hemostasis of 315 minutes, which was at the 95th percentile for time to hemostasis. After modified Bonferroni correction, decreased time to hemostasis was significantly associated with decreased risk of mortality at 24 hours (RR 0.93, 95% CI 0.91–0.95) and 30 days (RR 0.95, 95% CI 0.93–0.96), as well as decreased risk of AKI (RR 0.97, 95% CI 0.96–0.99) and MOF (RR 0.94, 95% CI 0.91–0.97), but not ARDS (RR 1.00, 95% CI 0.96–1.03), sepsis (RR 0.99, 95% CI 0.97–1.03), or VTE (RR 1.02, 95% CI 0.97–1.07). Another sensitivity analysis was performed in which group assignment (Table 1) was substituted for time to hemostasis. After modified Bonferroni correction, decreasing group number was significantly associated with decreased mortality at 24h and 30 days, as well as decreased risk of MOF, but not AKI, ARDS, sepsis, or VTE.
Discussion
We performed a pre-specified secondary analysis of the PROPPR study and found no significant differences in rate of hemostasis between sites, but there was significant variability in time to hemostasis between sites. We also found significant variability in use of IR between sites and increased time to emergent IR compared to emergent OR, consistent with a previous report.17 Decreased time to hemostasis was independently associated with decreased incidence of 30-day mortality, AKI, ARDS, MOF, and sepsis, but not 24-hour mortality or VTE.
Components of hemostasis acquisition include recognition of significant hemorrhage, transport to the OR/IR suite, and the hemorrhage control procedure itself. Studies have reported improved outcomes with earlier time to operative7,8 or IR intervention9 in bleeding trauma patients. Several scoring methods have also been reported to rapidly identify bleeding patients in the trauma bay,18,19,20 and catheter-based interventions in conjunction with hybrid operating suites may further reduce delays in initiating appropriate therapy.21 However, clinical benefit is ultimately achieved by acquiring earlier definitive hemorrhage control (i.e. hemostasis). Determinants of time to hemostasis after arrival to OR/IR include factors which are non-modifiable (e.g. pattern of injury), modifiable (e.g. surgeons’ skill level and judgment including decision for damage control versus definitive therapy), and potentially modifiable (e.g. availability of hybrid operating capability). Previous studies have used time to OR/IR as a surrogate for time to hemostasis, which does not take into account the actual time required to perform definitive hemorrhage control maneuvers. In the present study, we found that time from OR/IR to hemostasis accounted for most of the overall time to hemostasis (Figure 2).
Figure 2A–D.
Between-site comparisons for patients requiring emergent OR/IR (n=468). Incidence of blunt (versus penetrating) trauma varied significantly between sites (A). Time from hospital arrival to emergent OR/IR (B), time from emergent OR/IR to hemostasis (C), and overall time to hemostasis (D) also varied significantly between sites. Time from emergent OR/IR to hemostasis accounted for median 73% (IQR 63%–87%) of overall time to hemostasis. *, p<0.05.
Hemorrhagic death occurs rapidly with a median time to death of 2–3 hours after hospital arrival.4,5 Oyeniyi et al performed a single-center retrospective study at a level 1 center and found that trauma mortality from hemorrhage significantly decreased after implementation of significant changes in control of bleeding and early resuscitation procedures as part of a hemorrhage control bundle.22 These included earlier transfusion of plasma and platelets, earlier and increased use of temporizing hemorrhage control devices (extremity and junctional tourniquets, pelvic binders, hemostatic dressings, and resuscitative endovascular balloon occlusion of the aorta [REBOA]), and decreased time to OR/IR.6,23,24 We have previously argued that outcomes of clinical studies should be consistent with the disease process being investigated, and have proposed using an outcome of all-cause mortality at 6 hours for future hemorrhage studies.25 Given that purposeful action to effect earlier time to hemostasis will improve patient outcomes after trauma, the results of the current study support the use of time to hemostasis as a clinically significant endpoint for future hemorrhage studies. The overall complication rate was level until about 75 minutes, then steadily increased as time to hemostasis increased (Figure 3). Time to hemostasis may serve as a quality metric for trauma centers, but further studies are needed to identify modifiable and potentially modifiable determinants of time to hemostasis as well as an optimal benchmark.
The limitations of this study are as follows. Importantly, we could not control for several factors impacting time to hemostasis. Some injury patterns, such as retrohepatic caval injuries, result in substantial and difficult-to-control hemorrhage, and such injury patterns are not recapitulated by ISS. Other factors affecting time to hemostasis include the surgeons’ technical skill and judgment, including the decision to perform damage control versus definitive therapy and knowing when to ask for help. Although hemostasis was defined a priori in the PROPPR study as no bleeding requiring intervention in the surgical field or resolution of contrast blush in IR, it is to some degree subjective and consequently subject to bias, possibly accounting for some of the between-study site variability in time to hemostasis. Study sites also had significant differences in rates of blunt versus penetrating injury, which may have also contributed to variation in time to hemostasis. However, we believe that our method of mixed effects regression (using study site as a random effect) accounts for this in our statistical models. Additionally, the ultimate goal of achieving early hemostasis is to reduce blood loss due to hemorrhage, but accurately measuring the volume of blood loss after trauma is prohibitively difficult. We have shown that time to hemostasis more accurately reflects the degree of blood loss compared to time to OR/IR, but time to hemostasis is nevertheless still a surrogate for volume of blood loss. Direct correlation between time to hemostasis and actual volume of blood loss is obfuscated by several factors including rate of hemorrhage. Interestingly, among all 680 patients in the PROPPR trial, the 1:1:1 group had increased proportion of hemostasis (86% versus 78%, p<0.01), but similar time to hemostasis (median 144 vs 131 min, p=0.17), compared to the 1:1:2 group,5 suggesting that higher plasma:RBC and platelet:RBC reduced the rate of hemorrhage instead of allowing clinicians to achieve hemostasis more rapidly. We used time to hemostasis as a continuous variable in our multivariable models, which necessarily excluded 60 patients (15%) who exsanguinated before hemostasis could be achieved, resulting in survivor bias. Sensitivity analyses which included these 60 patients still found significant associations between time to hemostasis versus mortality, MOF, and possibly AKI. In the primary analysis of 408 patients who achieved hemostasis, we demonstrated that increased time to hemostasis was associated with increased incidence of several post-traumatic complications which was further correlated with increased 30-day mortality. Given that it takes time for these complications to manifest, it makes sense that there was no correlation with 24-hour mortality. These limitations are counterbalanced by the strengths of this study, which include our method of mixed-effects analysis, correction for multiple comparisons, and transparency in reporting diagnostics of our statistical tests.
In conclusion, time to hemostasis, but not rate of hemostasis, varied significantly between study sites in the PROPPR trial. Despite previous focus on time from hospital arrival to OR/IR, time from OR/IR to hemostasis accounts for the majority of overall time to hemostasis. Decreased time to hemostasis was independently associated with decreased 30-day mortality as well as incidence of AKI, ARDS, sepsis, and MOF. We find it biologically plausible that delayed hemostasis would result in increased blood loss, greater end organ ischemia, greater organ dysfunction, and increased late mortality. Time to hemostasis should be considered as an endpoint in future hemorrhage studies, and further studies investigating its use as a potential quality metric for trauma centers are warranted.
Supplementary Material
Acknowledgments:
Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) Study Group:
Clinical Coordinating Center, University of Texas Health Science Center at Houston: John B. Holcomb, MD; Charles E. Wade, PhD; Deborah J. del Junco, PhD; Erin E. Fox, PhD; Nena Matijevic, PhD (Laboratory Committee co-Chair); Jeanette M. Podbielski, RN; Angela M. Beeler, BS.
Data Coordinating Center, University of Texas Health Science Center at Houston: Barbara C. Tilley, PhD; Sarah Baraniuk, PhD; Stacia M. DeSantis, PhD; Hongjian Zhu, PhD; Joshua Nixon, MS; Roann Seay, MS; Savitri N. Appana, MS; Hui Yang, MS; Michael O. Gonzalez, MS.
Core Laboratory, University of Texas Health Science Center at Houston: Lisa Baer, MS; Yao-Wei Willa Wang, MD; Brittany S. Hula, MS; Elena Espino, BS; An Nguyen, BS; Nicholas Pawelczyk,BS; Kisha D. Arora-Nutall, BS; Rishika Sharma,MD; Jessica C. Cardenas, PhD; Elaheh Rahbar, PhD; Tyrone Burnett, Jr., BS; David Clark, BS.
Resuscitation Outcomes Consortium, University of Washington: Gerald van Belle, PhD; Susanne May, PhD; Brian Leroux, PhD; David Hoyt, MD; Judy Powell, BSN, RN; Kellie Sheehan, BSN.
Systems Biology Committee, University of California, Berkeley: Alan Hubbard, PhD (co-Chair); Adam P. Arkin, PhD.
Transfusion Committee: John R. Hess, MD, MPH (co-Chair, University of Washington); Jeannie L. Callum, MD (co-Chair, Sunnybrook Health Sciences Centre).
Anesthesiology Committee: Jean-Francois Pittet, MD (Chair, University of Alabama at Birmingham).
Emergency Medicine Committee: Christopher N. Miller, MD (Chair, University of Cincinnati).
PROPPR Clinical Sites (listed in order of number of patients enrolled):
University of Texas Health Science Center at Houston: Bryan A. Cotton, MD, MPH; Laura Vincent, BSN, RN, CCRP; Timothy Welch; Tiffany Poole, DC; Evan G. Pivalizza, MD; Sam D. Gumbert, MD; Yu Bai, MD, PhD; James J. McCarthy, MD; Amy Noland, MD; Rhonda Hobbs, MT(ASCP)SBB.
University of Washington: Eileen M. Bulger, MD; Patricia Klotz, RN; Lindsay Cattin, BA; Keir J. Warner, BS; Angela Wilson, BA; David Boman, BA; Nathan White, MD, MS; Andreas Grabinsky, MD; Jennifer A. Daniel-Johnson, MBBS.
University of California, San Francisco: Mitchell Jay Cohen, MD (Systems Biology and Laboratory Committees co-Chair); Rachael A. Callcut, MD, MSPH; Mary Nelson, RN, MPA; Brittney Redick, BA; Amanda Conroy, BA; Marc P. Steurer, MD, DESA; Preston C. Maxim, MD; Eberhard Fiebig, MD; Joanne Moore; Eireen Mallari, MT.
University of Cincinnati: Peter Muskat, MD; Jay A. Johannigman, MD; Bryce R. H. Robinson, MD; Richard D. Branson, MSc, RRT; Dina Gomaa, BS, RRT; Christopher Barczak, BS, MT(ASCP); Suzanne Bennett, MD; Patricia M. Carey, MD; Helen Hancock, BS, MT(ASCP); Carolina Rodriguez, BA.
University of Southern California: Kenji Inaba, MD; Jay G. Zhu, MD; Monica D. Wong, MS; Michael Menchine, MD, MPH; Kelly Katzberg, MD, FACEP; Sean O. Henderson, MD; Rodney McKeever, MD; Ira A. Shulman, MD; Janice M. Nelson, MD; Christopher W. Tuma, BA, MT(ASCP), SBB; Cheryl Y. Matsushita, BS, MT(ASCP).
Shock, Trauma and Anesthesiology Research—Organized Research Center (STAR-ORC), R Adams Cowley Shock Trauma Center, University of Maryland Medical Center: Thomas M. Scalea, MD; Deborah M. Stein, MD, MPH; Cynthia K. Shaffer, MS, MBA; Christine Wade, BA; Anthony V. Herrera, MS; Seeta Kallam, MBBS; Sarah E. Wade, BS; Samuel M. Galvagno, Jr, DO, PhD; Magali J. Fontaine, MD, PhD; Janice M. Hunt, BS, MT(ASCP) SBB; Rhonda K. Cooke, MD.
University of Tennessee Health Science Center, Memphis: Timothy C. Fabian, MD; Jordan A. Weinberg, MD; Martin A. Croce, MD; Suzanne Wilson, RN; Stephanie Panzer-Baggett, RN; Lynda Waddle-Smith, BSN; Sherri Flax, MD.
Medical College of Wisconsin: Karen J. Brasel, MD, MPH; Pamela Walsh, AS, CCRC; David Milia, MD; Allia Nelson, BS, BA; Olga Kaslow, MD, PhD; Tom P. Aufderheide, MD, MS; Jerome L. Gottschall, MD; Erica Carpenter, MLS(ASCP).
University of Arizona: Terence O’Keeffe, MBChB, MSPH; Laurel L. Rokowski, RN, BSN, MKT; Kurt R. Denninghoff, MD; Daniel T. Redford, MD; Deborah J. Novak, MD; Susan Knoll, MS, MT(ASCP) SBB.
University of Alabama at Birmingham: Jeffrey D. Kerby, MD, PhD; Patrick L. Bosarge, MD; Albert T. Pierce, MD; Carolyn R. Williams, RN, BSN, BSME; Shannon W. Stephens, EMTP; Henry E. Wang, MD, MS; Marisa B. Marques, MD.
Oregon Health and Science University: Martin A. Schreiber, MD; Jennifer M. Watters, MD; Samantha J. Underwood, MS; Tahnee Groat, MPH; Craig Newgard, MD, MPH; Matthias Merkel, MD, PhD; Richard M. Scanlan, MD; Beth Miller, MT(ASCP)SBB.
Sunnybrook Health Sciences Centre: Sandro Rizoli, MD, PhD; Homer Tien, MD; Barto Nascimento, MD, MSc, CTBS; Sandy Trpcic; Skeeta Sobrian-Couroux, RN, CCRP, BHA; Marciano Reis; Adic Pérez, MD; Susan E. Belo, MD, PhD; Lisa Merkley, BA, MLT, CBTS; Connie Colavecchia, BSc, MLT.
Funding: The Pragmatic Randomized Optimal Platelet and Plasma Ratio (PROPPR) trial was sponsored by the U.S. National Heart, Lung, and Blood Institute (U01HL077863) and the U.S. Department of Defense. RC is supported by a T32 fellowship (grant no. 5T32GM008792) from the National Institute of General Medical Sciences.
Conflict of Interest: B.A.C. is a paid consultant to Haemonetics Corp. S.R. reported receiving grant funding from TEM International and CSL Behring. Otherwise, authors report no conflicts of interest.
Footnotes
Presented at the 76th Annual Meeting of the American Association for the Surgery of Trauma, September 13–16, 2017 in Baltimore, MD
References
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93. [DOI] [PubMed] [Google Scholar]
- 2.Harvin JA, Maxim T, Inaba K, Martinez-Aguilar MA, King DR, Choudhry AJ, Zielinski MD, Akinyeye S, Todd SR, Griffin RL, et al. Mortality Following Emergent Trauma Laparotomy: a Multicenter, Retrospective Study: Mortality after Emergent Trauma Laparotomy. J Trauma Acute Care Surg. 2017;83(3):464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotwal RS, Montgomery HR, Kotwal BM, Champion HR, Butler FK Jr, Mabry RL, Cain JS, Blackbourne LH, Mechler KK, Holcomb JB. Eliminating Preventable Death on the Battlefield. Arch Surg. 2011;146(12):1350–8. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. ; PROMMTT Study Group. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. ; PROPPR study group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang R, Holcomb JB. Optimal Fluid Therapy for Traumatic Hemorrhagic Shock. Crit Care Clin. 2017;33(1):15–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke JR, Trooskin SZ, Doshi PJ, Greenwald L, Mode CJ. Time to laparotomy for intra-abdominal bleeding from trauma does affect survival for delays up to 90 minutes. J Trauma. 2002;52(3):420–5. [DOI] [PubMed] [Google Scholar]
- 8.Meizoso JP, Ray JJ, Karcutskie CA 4th, Allen CJ, Zakrison TL, Pust GD, Koru-Sengul T, Ginzburg E, Pizano LR, Schulman CI, et al. Effect of time to operation on mortality for hypotensive patients with gunshot wounds to the torso: The golden 10 minutes. J Trauma Acute Care Surg. 2016;81(4):685–91. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz DA, Medina M, Cotton BA, Rahbar E, Wade CE, Cohen AM, Beeler AM, Burgess AR, Holcomb JB. Are we delivering two standards of care for pelvic trauma? Availability of angioembolization after hours and on weekends increases time to therapeutic intervention. J Trauma Acute Care Surg. 2014;76(1):134–9. [DOI] [PubMed] [Google Scholar]
- 10.Radwan ZA, Bai Y, Matijevic N, del Junco DJ, McCarthy JJ, Wade CE, Holcomb JB, Cotton BA. An emergency department thawed plasma protocol for severely injured patients. JAMA Surg. 2013;148(2):170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, Dubose JJ, Fox EE, Inaba K, Rodriguez CJ, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82(3):605–617. [DOI] [PubMed] [Google Scholar]
- 12.Nascimento B, Rizoli S, Rubenfeld G, Lin Y, Callum J, Tien HC. Design and preliminary results of a pilot randomized controlled trial on a 1:1:1 transfusion strategy: the trauma formula-driven versus laboratory-guided study. J Trauma. 2011;71(5 Suppl 1):S418–26. [DOI] [PubMed] [Google Scholar]
- 13.Clarke JR, Trooskin SZ, Doshi PJ, Greenwald L, Mode CJ. Time to laparotomy for intra-abdominal bleeding from trauma does affect survival for delays up to 90 minutes. J Trauma. 2002;52:420–425. [DOI] [PubMed] [Google Scholar]
- 14.Undurraga Perl VJ, Leroux B, Cook MR, Watson J, Fair K, Martin DT, Kerby JD, Williams C, Inaba K, Wade CE, et al. ; PROPPR Study Group. Damage-control resuscitation and emergency laparotomy: Findings from the PROPPR study. J Trauma Acute Care Surg. 2016;80(4):568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 16.Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174(8):984–92. [DOI] [PubMed] [Google Scholar]
- 17.Chang R, Fox EE, Greene TJ, Eastridge BJ, Gilani R, Chung KK, DeSantis SM, DuBose JJ, Tomasek JS, Fortuna GR Jr, et al. ; for the NCTH Study Group. Multicenter retrospective study of non-compressible torso hemorrhage: anatomic locations of bleeding and comparison of endovascular versus open approach. J Trauma Acute Care Surg. 2017;83(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205(4):541–5. [DOI] [PubMed] [Google Scholar]
- 19.Yücel N, Lefering R, Maegele M, Vorweg M, Tjardes T, Ruchholtz S, Neugebauer EA, Wappler F, Bouillon B, Rixen D; Polytrauma Study Group of the German Trauma Society. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma. 2006. June;60(6):1228–36. [DOI] [PubMed] [Google Scholar]
- 20.Cotton BA, Dossett LA, Haut ER, Shafi S, Nunez TC, Au BK, Zaydfudim V, Johnston M, Arbogast P, Young PP. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010;69 Suppl 1:S33–9. [DOI] [PubMed] [Google Scholar]
- 21.Holcomb JB, Fox EE, Scalea TM, Napolitano LM, Albarado R, Gill B, Dunkin BJ, Kirkpatrick AW, Cotton BA, Inaba K, et al. ; for the Catheter-Based Hemorrhage Control Study Group. Current opinion on catheter-based hemorrhage control in trauma patients J Trauma Acute Care Surg. 2014;76(3):888–93. [DOI] [PubMed] [Google Scholar]
- 22.Oyeniyi BT, Fox EE, Scerbo M, Tomasek JS, Wade CE, Holcomb JB. Trends in 1029 trauma deaths at a level 1 trauma center: Impact of a bleeding control bundle of care. Injury. 2017;48(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrestha B, Holcomb JB, Camp EA, Del Junco DJ, Cotton BA, Albarado R, Gill BS, Kozar RA, Kao LS, McNutt MK, et al. Damage-control resuscitation increases successful nonoperative management rates and survival after severe blunt liver injury. J Trauma Acute Care Surg. 2015;78(2):336–41. [DOI] [PubMed] [Google Scholar]
- 24.Johansson PI, Stensballe J, Oliveri R, Wade CE, Ostrowski SR, Holcomb JB. How I treat patients with massive hemorrhage. Blood. 2014;124(20):3052–8. [DOI] [PubMed] [Google Scholar]
- 25.Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC; PROPPR Study Group. Earlier Endpoints are Required for Hemorrhagic Shock Trials Among Severely Injured Patients. Shock. 2017;47(5):567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.