Summary
During drought, abscisic acid (ABA) induces closure of stomata via a signaling pathway that involves the calcium (Ca2+)‐independent protein kinase OST1, as well as Ca2+‐dependent protein kinases. However, the interconnection between OST1 and Ca2+ signaling in ABA‐induced stomatal closure has not been fully resolved.
ABA‐induced Ca2+ signals were monitored in intact Arabidopsis leaves, which express the ratiometric Ca2+ reporter R‐GECO1‐mTurquoise and the Ca2+‐dependent activation of S‐type anion channels was recorded with intracellular double‐barreled microelectrodes.
ABA triggered Ca2+ signals that occurred during the initiation period, as well as in the acceleration phase of stomatal closure. However, a subset of stomata closed in the absence of Ca2+ signals. On average, stomata closed faster if Ca2+ signals were elicited during the ABA response. Loss of OST1 prevented ABA‐induced stomatal closure and repressed Ca2+ signals, whereas elevation of the cytosolic Ca2+ concentration caused a rapid activation of SLAC1 and SLAH3 anion channels.
Our data show that the majority of Ca2+ signals are evoked during the acceleration phase of stomatal closure, which is initiated by OST1. These Ca2+ signals are likely to activate Ca2+‐dependent protein kinases, which enhance the activity of S‐type anion channels and boost stomatal closure.
Keywords: abscisic acid (ABA), Ca2+‐indicator, cytosolic Ca2+ signals, OST1 protein kinase, R‐GECO1‐mTurquoise, SLAC1 and SLAH3 anion channels, stomata
Introduction
Land plants control gas exchange with the surrounding atmosphere by modulating the aperture of stomatal pores in the leaf surface (Shimazaki et al., 2007; Kim et al., 2010; Kollist et al., 2014). In the light, stomata open and enable CO2 uptake for photosynthesis, whereas they close during drought to protect plants from desiccation. Several lines of evidence show that the stress hormone abscisic acid (ABA) plays a central role in drought‐induced stomatal closure (Roelfsema et al., 2012; Munemasa et al., 2015). An in‐depth understanding of the molecular mechanisms that underlie ABA‐dependent stomatal closure, therefore, can open new strategies of breeding plants with improved drought tolerance.
Stomata rapidly close after stimulation with extracellular ABA (Guzel Deger et al., 2015), which was taken as an indication that this stress hormone is perceived by a cell surface receptor (Joshi‐Saha et al., 2011). However, recent findings point to a rapid uptake of ABA into guard cells (Boursiac et al., 2013; Merilo et al., 2015), which is followed by the perception through cytosolic PYrabactin Resistant/PYrabactin resistant‐Like/Regulatory Component of ABA Receptors (PYR/PYL/RCAR; Levchenko et al., 2008; Ma et al., 2009; Park et al., 2009). Loss of multiple of these PYR/PYL/RCAR receptors causes stomata to become ABA insensitive (Gonzalez‐Guzman et al., 2012; Merilo et al., 2013), which indicates that these proteins are essential for guard cell ABA perception.
A short signaling pathway leads to activation of the SLow Anion Channel 1 (SLAC1) in guard cells (Roelfsema et al., 2012; Munemasa et al., 2015; Hedrich & Geiger, 2017), in which the protein kinase Open STomata 1 (OST1) is a central player (Mustilli et al., 2002). In the absence of ABA, a group of class 2C protein phosphatases (PP2Cs; including ABA Insensitive 1 and 2) inhibits OST1 (Umezawa et al., 2009; Vlad et al., 2009). Binding of ABA to its PYR1/PYL/RCAR receptors causes them to deactivate the PP2Cs and thus release OST1 from inhibition (Ma et al., 2009; Park et al., 2009). Once OST1 gets activated, it phosphorylates and stimulates SLAC1, which leads to the extrusion of anions and causes a depolarization of the guard cell plasma membrane (Pei et al., 1997; Roelfsema et al., 2004; Geiger et al., 2009; Lee et al., 2009). As a result, depolarization‐dependent potassium (K+) channels are activated, anions and K+ are released by guard cells; this reduces their osmotic content and causes stomatal closure in less than 20 min (Kollist et al., 2014; Guzel Deger et al., 2015; Hedrich & Geiger, 2017).
In addition to the calcium (Ca2+)‐independent OST1 pathway, guard cells are also likely to exhibit a Ca2+‐dependent signaling chain that activates SLAC1, as well as the homologous channel SLAH3 (Geiger et al., 2011; Brandt et al., 2015; Guzel Deger et al., 2015). Such a Ca2+‐dependent pathway was already postulated in the pioneering work of De Silva et al. (1985). Later experiments with Ca2+‐sensitive dyes revealed that ABA can indeed trigger a transient elevation of the cytosolic free Ca2+ concentration in Commelina communis guard cells (McAinsh et al., 1990; Gilroy et al., 1991). The ABA‐dependent rise of the cytosolic Ca2+ level was postulated to activate plasma membrane anion channels, based on experiments in Vicia faba, Arabidopsis and tobacco (Nicotiana tabacum) guard cells (Schroeder & Hagiwara, 1989; Allen et al., 1999; Chen et al., 2010; Stange et al., 2010). However, the hypothesis was challenged by the contrasting finding that ABA is able to activate S‐type anion channels in the absence of cytosolic Ca2+ signals (Levchenko et al., 2005; Marten et al., 2007). One may thus propose that the guard cell ABA signaling pathway is based on a core Ca2+‐insensitive (OST1‐dependent) chain (Cutler et al., 2010), which is modulated by Ca2+‐dependent processes. However, the interconnection between these two branches and their individual roles in stomatal closure have not been resolved.
Most of the aforementioned guard cell studies that address Ca2+ signaling have been carried out with stomata in epidermal peels or epidermal fragments. These isolated tissues offer the advantage that fluorescence signals of guard cells are not disturbed by autofluorescence of mesophyll cells. Moreover, stimuli such as ABA can be easily applied to guard cells in epidermal peels, from the side that faces the leaf interior, whereas the side covered by the cuticle offers a strong barrier for many solutes. However, stomatal movements in epidermal peels are reduced in amplitude and response time, in comparison with the stomatal responses in intact leaves (Willmer & Mansfield, 1969; Roelfsema & Hedrich, 2002). It is thus desirable to work with guard cells in intact leaves, but this approach requires a new generation of reporters that enable cytosolic Ca2+ measurements in intact tissues.
Newly developed genetically encoded Ca2+‐ reporters, such as GCaMP6 and R‐GECO1, display much higher Ca2+‐dependent changes in fluorescence intensity, compared with Yellow Cameleon 3.6 (Zhao et al., 2011; Chen et al., 2013; Waadt et al., 2017). Recently, R‐GECO1 has been fused to mTurquoise to generate a highly sensitive Ca2+ sensor with an internal reference (Waadt et al., 2017). We therefore used intact Arabidopsis leaves that express R‐GECO1‐mTurquoise (RG‐mT) to study Ca2+ signals in stomata that were stimulated with ABA via microcapillaries in contact with the guard cell wall.
Materials and Methods
Plant material and growth conditions
All Arabidopsis thaliana lines were in the Col‐0 background, the ost1‐3, slac1‐3, slah3‐1 single mutants, the slac1‐3/slah3‐1 double mutants, and plants expressing RG‐mT have been described previously (Yoshida et al., 2002; Merilo et al., 2013; Guzel Deger et al., 2015; Waadt et al., 2017). The ost1‐3 mutant was transformed with the RG‐mT construct, as described for wild‐type by Waadt et al. (2017), using the floral dip method and the Agrobacterium tumefaciens strain GV3101 (Zhang et al., 2006). Seeds were sown on sterilized soil, and plants were grown in a growth cabinet with 60% relative humidity, a cycle of 12 h : 12 h, light : dark, temperatures of 21°C (light) and 18°C (dark), and a photon flux density of 100 μmol m−2 s−1. After 12 d, the seedlings were transferred to pots (diameter 6 cm) and grown for another 2–3 wk in the same conditions.
Measurements were carried out on stomata, either in isolated epidermal peels or in intact leaves that were excised with a sharp razor blade from 4‐ to 5‐wk‐old plants and gently fixed in a petri dish (diameter 35 mm) with the adaxial side attached to double‐sided adhesive tape. The leaves were immersed in the following solution: 10 mM potassium chloride (KCl), 1 mM calcium chloride (CaCl2) and 10 mM potassium citrate, pH 5 and illuminated with white light (100 μmol m−2 s−1) for at least 2 h before the start of the experiment.
Epidermal strips were gently peeled with a pair of tweezers from the abaxial side of RG‐mT‐expressing leaves. The strips were fixed on cover slips (diameter 18 mm) with medical adhesive (Medical Adhesive B; Aromando, Düsseldorf, Germany) and placed in the following bath solution: 10 mM KCl, 1 mM CaCl2, 10 mM Mes–bis‐tris propane, pH 6.0.
Microelectrode techniques
Stomata in the abaxial epidermis of intact leaves, or epidermal strips, were visualized with a water immersion objective (W Plan‐Apochromat, 63×/1.0; Carl Zeiss, Jena, Germany) mounted to an upright microscope (Axioskop 2FS; Zeiss). ABA was applied with single‐barreled microelectrodes that were pulled from borosilicate glass capillaries (inner diameter, 0.58 mm; outer diameter, 1.0 mm; Hilgenberg, Malsfeld, Germany; http://www.hilgenberg-gmbh.com) on a horizontal laser puller (P2000; Sutter Instruments Co., Novato, CA, USA). The electrode tips were filled with ABA at the standard concentration of 50 μM, or concentrations ranging from 0.5 to 100 μM to obtain a dose–response curve, and the electrodes then further filled with 300 mM KCl. Control experiments were conducted with 50 μM benzoic acid. The electrodes were connected via silver/silver chloride (Ag/AgCl) half‐cells to a headstage of a custom‐made amplifier (input impedance > 1011 Ω, Ulliclamp01). A glass capillary that was filled with 300 mM KCl and sealed with 300 mM KCl in 2% agarose served as a reference electrode. The microelectrodes were mounted to a piezo‐driven micro‐manipulator (MM3A; Kleindiek Nanotechnik, Reutlingen, Germany) and slowly moved towards the guard cell wall. The tip potential was monitored during manipulation of the microelectrode, and when the electrode came into contact with the guard cell wall it suddenly changed to values more negative than −15 mV. After establishment of a connection between the guard cell wall and microelectrode, ABA, or benzoic acid as control, was ejected from the electrode with a current of −0.8 nA for a period of 20–30 s. Directly after termination of current ejection, the microelectrode was removed from the guard cell wall.
The plasma membrane conductance of guard cells was studied in voltage clamp experiments with double‐barreled microelectrodes. These microelectrodes were fabricated from two borosilicate capillaries (inner diameter, 0.58 mm; outer diameter, 1.0 mm; Hilgenberg), which were aligned, heated, twisted 360°, and pre‐pulled on a vertical puller (L/M‐3P‐A; Heka, Lambrecht/Pfalz, Germany). Subsequently, the joint capillaries were pulled on a horizontal laser puller (P2000; Sutter Instruments Co.). The double‐barreled electrodes were backfilled with 300 mM KCl and had a tip resistance that ranged from 180 to 280 MΩ. Both barrels of the microelectrode were connected by Ag/AgCl half cells to the Ulliclamp01 amplifier, which enables voltage clamp experiments with an internal differential amplifier. Voltage pulses were applied with winwcp software (Dempster, 1997; University of Strathclyde, https://www.strath.ac.uk) and recorded at 1 kHz, using USB‐6002 interfaces (National Instruments, Austin, TX, USA; http://www.ni.com). A dual low‐pass Bessel filter (LPF 202A; Warner Instruments Corp., Hamden, CT, USA) was used to low‐pass filter the electrical signals at 0.5 kHz.
Quantitative fluorescence microscopy
Fluorescence signals of Ca2+‐imaging experiments were obtained from regions of interest in the central part of guard cells that included the nucleus. The measurements were carried out with a charge‐multiplying charge‐coupled device camera (QuantEM; Photometrics; http://www.photometrics.com) that was mounted to a CARV, Crestoptics, Rome, Italy confocal spinning disc unit. Within the CARV unit, three filter wheels were used, while the spinning disc was moved out of the light path. The R‐GECO1 and mTurquoise subunits in RG‐mT were excited with light of an LED illumination system (pE‐4000; CoolLED, Andover, UK) at 435 nm and 580 nm, respectively. The emission signals were passed through dichroic mirrors with cut‐off wavelengths of 450 nm (T450 LPXR; Chroma Technology Corp., Bellows Falls, VT, USA) and 590 nm (FF593 BrightLine; Semrock, http://www.semrock.com) and band filters at 475/28 nm (BrightLine HC; Semrock, Semrock Inc., IDEX Corp.; Lake Forest, IL, USA) and 628/40 nm (BrightLine; Semrock).
Changes in stomatal aperture were monitored during the Ca2+‐imaging experiments, with light provided by a halogen bulb in the microscope lamp, filtered through a far‐red light bandpass filter (713/30 nm). All images were analyzed offline with the image‐j/fiji software package (Schindelin et al., 2012). The statistical and mathematical analysis of the data was carried out with prism 6 and 7 (GraphPad Software, San Diego, CA, USA; https://www.graphpad.com) and origin pro 8 (Originlab Corp., Northampton, MA, USA).
Results
ABA ejection evokes movement of single guard cells
In a previous study, rapid stomatal closure was induced by nanoinfusion of ABA‐containing solution, which was pressure‐injected through open stomata into intact leaves (Guzel Deger et al., 2015). Based on this approach, we found that 20 μM ABA induces closure of stomata within 20 min. However, nanoinfusion alters the optical properties of the leaf surface, which is a disadvantage when it is used in combination with quantitative fluorescence microscopy. We therefore introduced a ‘current‐ejection technique’ to stimulate single guard cells with ABA in intact Arabidopsis leaves (Fig. 1a). Single‐barreled electrodes were slowly moved towards the abaxial epidermis of an intact leaf until the electrode tip came into contact with the guard cell wall (Fig. 1a). In this configuration, electrically charged molecules, such as ABA−, can be ejected from the glass microcapillary into the guard cell wall by application of current pulses.
Figure 1.
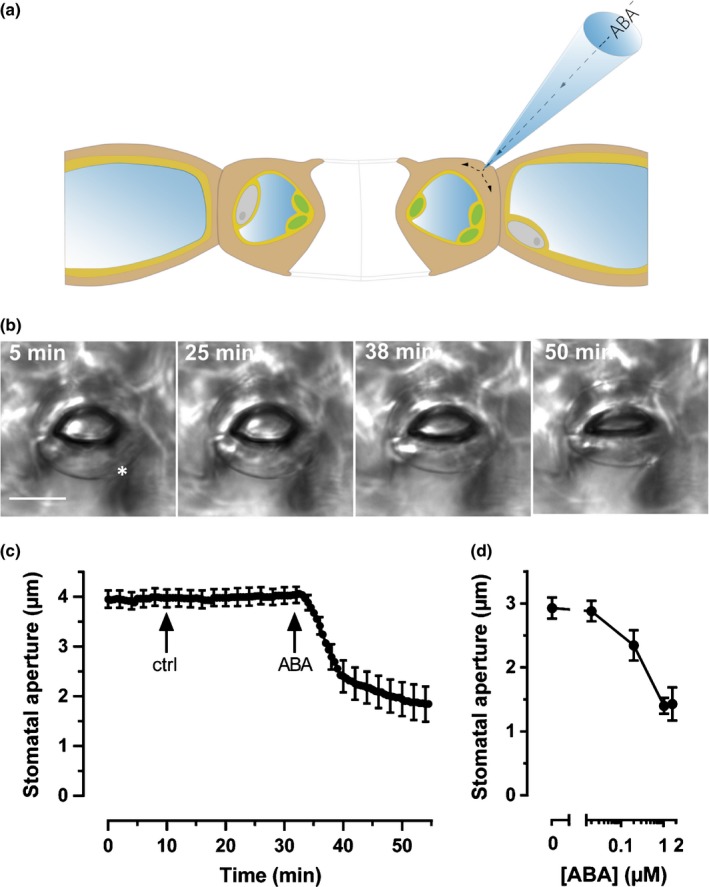
Current ejection of abscisic acid (ABA) induces rapid stomatal closure. (a) Cartoon of the current‐ejection technique. The tip of an electrode was filled with 50 μM ABA (or 50 μM benzoic acid as control) and brought in contact with the wall of a guard cell, in an intact Arabidopsis thaliana leaf. ABA was ejected via the microelectrode, by application of a −0.8 nA current. Stomatal movements were monitored with a charge‐coupled device camera mounted to an upright microscope. (b) Images of a stoma in an intact leaf, acquired before and after current ejection of benzoic acid, as a control (25 min), and ABA (38 and 50 min). The time points marked in the images correspond to the time axis shown in (c). The asterisk marks the position at which the microelectrode was in contact with the guard cell wall. Bar, 10 μm. Note that stomatal movement was mainly due to a change in shape of the guard cell close to the microelectrode, whereas the other guard cell remained bent. See also Supporting Information Videos S1. (c) Time‐dependent changes of the average stomatal aperture, after subsequent application of benzoic acid and ABA, as indicated by arrows. Note that current ejection of benzoic acid did not affect the stomatal aperture, whereas ABA triggered rapid stomatal closure. Average data are shown, ± SE, n = 9. (d) Dose–response curve of ABA‐induced stomatal closure. The dose of ABA was varied by modulating the hormone concentration in the current‐ejection electrode and estimated as explained in Methods S1.
Stimulation with ABA, by a current of −0.8 nA for 20–30 s, caused a rapid reduction of the stomatal aperture (Supporting Information Videos S1). After a lag time of only 1.44 min (Fig. 1b,c; SE = 0.29 min, n = 9), the stomata closed with a maximal velocity of 0.28 μm min−1 (SE = 0.05 μm min−1, n = 9). By contrast, application of benzoic acid as control (−0.8 nA, 20–30 s) did not affect the aperture of stomata (Fig. 1b,c). ABA had a strong impact on the guard cell that was closely located to the tip of the current‐ejection electrode (asterisk in Fig. 1b, left panel), whereas the guard cell on the other side of the pore remained curved (Fig. 1b).
The asymmetric response of guard cells in a stomatal complex suggests that the current‐ejection method only provides ABA to a restricted area of the guard cell wall. This was studied by current ejection of the fluorescent dye Lucifer Yellow CH (LY) using the same conditions as already described for ABA (Fig. S1b,c; Methods S1; Videos S2). Indeed, current ejection of LY resulted in a localized fluorescence signal that decreased exponentially from the tip of the electrode (Fig. S1b,c). Based on the LY experiments, it was estimated that the current‐ejection procedure transferred a short dose of ABA with a local concentration of 1.1 μM (Fig. S1d). The dose of ABA that was applied to guard cells could be modulated by changing the ABA concentration in the current‐ejection electrodes. This revealed that a local ABA concentration of 0.2 μM triggered stomatal closure, with only half of the average magnitude, compared with 1.1 or 1.6 μM ABA (Fig. 1d), whereas guard cells did not respond to 0.02 μM ABA (Fig. 1d).
ABA‐induced Ca2+ signals are associated with initiation and acceleration of stomatal closure
Several genetically encoded fluorescent Ca2+ reporters have become available in recent years, of which RG‐mT was chosen, since it exhibits a strong Ca2+‐dependent change in fluorescence emission ratio and it does not dramatically affect plant growth (Zhao et al., 2011; Waadt et al., 2017). The emission ratio of RG‐mT was calibrated to the cytosolic Ca2+ concentration, using the fluorescent Ca2+‐reporter dye FURA2 (see Fig. S2; Methods S1; Videos S3), and far‐red light was used to monitor closure of the stomatal pore (Fig. 2a–c; Videos S4–S6). Current ejection of ABA caused closure of the stomatal pore in all experiments (n = 41); however, the impact of ABA on the cytosolic Ca2+ level split into three guard cell populations (Fig. 2a–c). In a first group of 22 out of 41 measured cells, ABA triggered a transient rise in the cytosolic free Ca2+ level during the phase in which closure of the stomata accelerated (Fig. 2a; Videos S4). In a second population of guard cells, the Ca2+ signal preceded stomatal closure (seven out of 41 cells; Fig. 2b; Videos S5). Finally, Ca2+ signals were lacking in guard cells during ABA‐induced stomatal closure in the remaining third population of 12 out of 41 cells (Fig. 2c; Videos S6). The occurrence of Ca2+ signals correlated with the speed of stomatal closure (Fig. 2d). Stomata in which Ca2+ signals occurred during stomatal closure reached the half‐maximal response in a significantly shorter period than stomata in which Ca2+ signals were absent (one‐way ANOVA, P = 0.002). On average, stomata with a transient Ca2+ rise displayed a half‐maximal closure within 289 s (SE = 19 s, n = 22), whereas this value was reached later (410 s, SE = 27 s, n = 12) in the absence of Ca2+ signals.
Figure 2.

Abscisic acid (ABA)‐induced Ca2+ signals occur during the initiation or acceleration phase of stomatal closure and speed up stomatal closure. (a–c) Left panels: bright‐field images (upper images) showing the aperture of an Arabidopsis thaliana stoma and pseudo‐color images (lower images) showing the cytosolic Ca2+ concentration in guard cells of the same stoma. The images were obtained at three time points, as indicated in the bright‐field images. The asterisks in the pseudo‐color images indicate the position of contact between the current‐ejection electrode and the guard cell wall. The calibration bar below the images links the color code to [Ca2+]cyt. Bars, 10 μm. See also Supporting Information Videos S4–S6. Right panels: time‐dependent changes in stomatal aperture (closed circles) and the cytosolic free Ca2+ concentration (open squares) in guard cells that were stimulated by current ejection of ABA, as indicated by the arrow. Data are shown for representative cells, which either show (a) an increase of the cytosolic free Ca2+ concentration during stomatal closure (22 out of 41 cells), (b) before stomatal closure (7 out of 41 cells), or (c) no change in the cytosolic Ca2+ level (12 out of 41 cells). (d) ABA‐induced changes in stomatal aperture, averaged for stomata in which a Ca2+ signal occurred before stomatal closure (open triangles, n = 7), during stomatal closure (closed circles, n = 22), or without Ca2+ signals (open circles, n = 12). Arrow indicates the time point of ABA application; error bars represent ± SE. Data points at which the average stomatal aperture significantly differed between the groups of stomata are indicated by lower‐case letters. (e) Frequency distribution of the time period between stimulation with ABA and occurrence of a peak in the cytosolic Ca2+ level. The time period between current ejection of ABA and the maximal cytosolic Ca2+ concentration was calculated for the same cells as displayed in (a–c) and binned in intervals of 100 s. The frequency distribution was fitted with the sum of single (dotted line), double (solid line), and triple (striped line) Gaussian functions. Based on Akaike's information criterion (AICc), a model with two Gaussian functions (solid line) was 55 times more likely as one with a single Gaussian function (dotted line) and 1037 times more likely as one with three Gaussian functions (striped line). The arrow indicates the time point of current ejection of ABA.
By contrast to the experiments with ABA, current ejection of benzoic acid did not cause stomatal closure in any of the 24 experiments. Only in two out of 24 guard cells were transient changes in the cytosolic free Ca2+ concentration observed, and these Ca2+ signals had a smaller amplitude than those elicited by ABA (Fig. S3).
Our data thus indicate that ABA‐induced Ca2+ signals can be clustered into two groups (Fig. 2a,b). In a small group, the Ca2+ signals precede stomatal closure (Fig. 2b), whereas the Ca2+ level rises during stomatal closure in the majority of guard cells (Fig. 2a). The occurrence of these groups was tested by fitting the frequency distribution of cells with Ca2+ signals; the number of cells were plotted against the time interval between stimulation with ABA and occurrence of the peak in the cytosolic Ca2+‐level (Fig. 2e). According to the corrected Akaike information criterion (Burnham et al., 2011), a model based on the sum of two Gaussian functions (solid line in Fig. 2e, R 2 = 0.99) was 55 times more likely than the model with one Gaussian function (dotted line in Fig. 2e, R 2 = 0.75), whereas a model based on the sums of three Gaussian functions was very unlikely (1037 less likely as the sum of two Gaussian functions; striped line in Fig. 2e, R 2 = 0.99). This analysis thus strongly supports that ABA‐induced Ca2+ signals in guard cells occur in two time windows; some are elicited early (before the stomata start to close), whereas others are evoked later (during stomatal closure).
Elevated cytosolic Ca2+ levels rapidly activate S‐type anion channels
Elevated cytosolic Ca2+ levels were shown to activate plasma membrane anion channels in guard cells of several species (Allen et al., 1999; Chen et al., 2010; Stange et al., 2010), but it is unknown how fast this response occurs in Arabidopsis. We therefore studied this response in real time, with guard cells expressing RG‐mT. Guard cells were impaled with double‐barreled electrodes, and cytosolic Ca2+ concentration changes were evoked with voltage pulses (Grabov & Blatt, 1998; Voss et al., 2018) from −100 mV, stepwise for 10 s, to more negative membrane potentials (Fig. 3a,b). The cytosolic Ca2+ level of the cell shown in Fig. 3(a,b) hardly changed in response to a 10 s pulse of −180 mV, but a transient rise in the Ca2+ concentration was triggered by pulses to −200 and −220 mV (Videos S7). During the hyperpolarizing pulses, inward currents are facilitated by K+ channels, which are voltage activated and deactivate at −100 mV in c. 0.5 s (Roelfsema & Prins, 1997). After termination of the voltage pulses in which Ca2+ signals were elicited, an additional conductance was recorded (arrows in Fig. 3a), which transiently reached maximum conductance, at 13.8 s (SE = 0.9 s, n = 27) after the cytosolic Ca2+ peak (Fig. 3a). It is likely that this slow current is facilitated by Ca2+‐activated S‐type anion channels, as was previously shown for tobacco guard cells (Chen et al., 2010; Stange et al., 2010).
Figure 3.
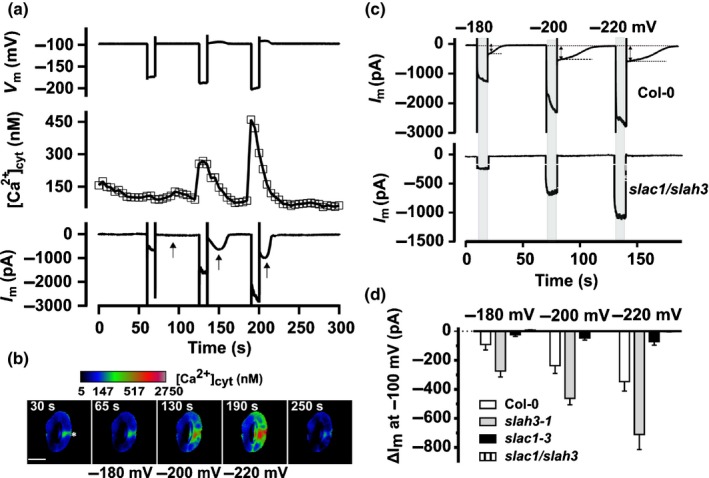
Cytosolic Ca2+ signals rapidly activate SLAC1 and SLAH3 anion channels in Arabidopsis thaliana guard cells. (a) Arabidopsis guard cells were stimulated with 10 s pulses from a potential of −100 mV, to −180, −200, and −220 mV (upper trace). The voltage pulses to −200 and −220 mV evoked a transient increase of the cytosolic free Ca2+ concentration (middle trace). The Ca2+ signals caused activation of inward currents (lower trace) after returning the voltage to −100 mV, as indicated by the arrows below the current trace. (b) Pseudo‐color images that represent the cytosolic free Ca2+ concentration of the same guard cell as in (a) determined from the R‐GECO1‐mTurquoise signal. The images were acquired before, during, and after the stimulation of the cell with hyperpolarizing voltage pulses. The asterisk marks the position at which the right guard cell was impaled with a double‐barreled electrode. Bar, 10 μm. The calibration bar above the images links the color code to [Ca2+]cyt. See also Supporting Information Videos S7. (c) Guard cells were stimulated with voltage pulses from a holding potential of −100 mV, for 10 s to −180, −200, and −220 mV as indicated above the current traces. In wild‐type guard cells these voltage pulses caused activation of anion channels (upper trace) that facilitate inward currents after returning the voltage to −100 mV, as indicated by the dotted lines. These anion currents were absent in guard cells of the slac1‐3/slah3‐1 double mutant (lower trace). (d) Average currents facilitated by S‐type anion channels, as measured in (c), evoked by voltage pulses of −180, −200, and −220 mV in Col‐0 wild‐type (white bars), slah3‐1 (gray bars; see also Fig. S4), slac1‐3 (black bars; see also Fig. S4), and slac1/slah3 (striped bars, note that bars approximate 0 pA). Errors bars represent + SE (Col‐0, n = 9; slah3‐1, n = 8; slac1‐3, n = 10; slac1/slah3, n = 13).
In Arabidopsis, guard cell S‐type anion channels are encoded by SLAC1 and SLAH3 (Negi et al., 2008; Vahisalu et al., 2008; Guzel Deger et al., 2015), and the voltage responses of the slac1 and slah3 loss‐of‐function mutants were therefore compared with wild‐type (Fig. 3c,d). In wild‐type, 10 s pulses from −100 mV, stepwise to −180, −200, and −220 mV, induced inward currents that slowly deactivated after returning to −100 mV (Fig. 3c), just as in guard cells expressing RG‐mT (Fig. 3a). In the slah3‐1 single mutant, the hyperpolarizing pulses elicited currents that had a similar magnitude as in wild‐type (Figs 3d, S4). However, these currents were only detected in six out of 10 slac1‐3 guard cells, where, on average, they had a reduced magnitude (Figs 3d, S4). Finally, the loss of both SLAC1 and SLAH3 caused a complete lack of Ca2+‐activated currents (Fig. 3c,d). These data thus strongly suggest that both SLAC1 and SLAH3 contribute to the Ca2+‐activated conductance in Arabidopsis guard cells.
Loss of OST1 prevents ABA‐induced stomatal closure and alters Ca2+ signals
The protein kinase OST1 plays a central role in ABA‐induced stomatal closure (Mustilli et al., 2002; Merilo et al., 2013; Guzel Deger et al., 2015). However, it is unclear how loss of OST1 affects Ca2+ signals. Guard cells of ost1‐3, expressing RG‐mT, were therefore stimulated by current‐ejection of ABA. In the majority of guard cells, ABA neither induced stomatal closure nor provoked a change in cytosolic free Ca2+ level (Fig. 4a, 26 out of 31 cells; Videos S8). Despite the lack of stomatal closure, transient changes of the cytosolic free Ca2+ concentration were observed in five out of 31 guard cells (Fig. 4b; Videos S9). ABA thus triggered Ca2+ signals in approximately one out of six ost1‐3 guard cells, whereas it evoked Ca2+ signals in three out of four guard cells of wild‐type (Fig. 2). For comparison, current ejection of benzoic acid as control evoked only a Ca2+ signal in one out of 21 ost1‐3 guard cells (Fig. S5).
Figure 4.
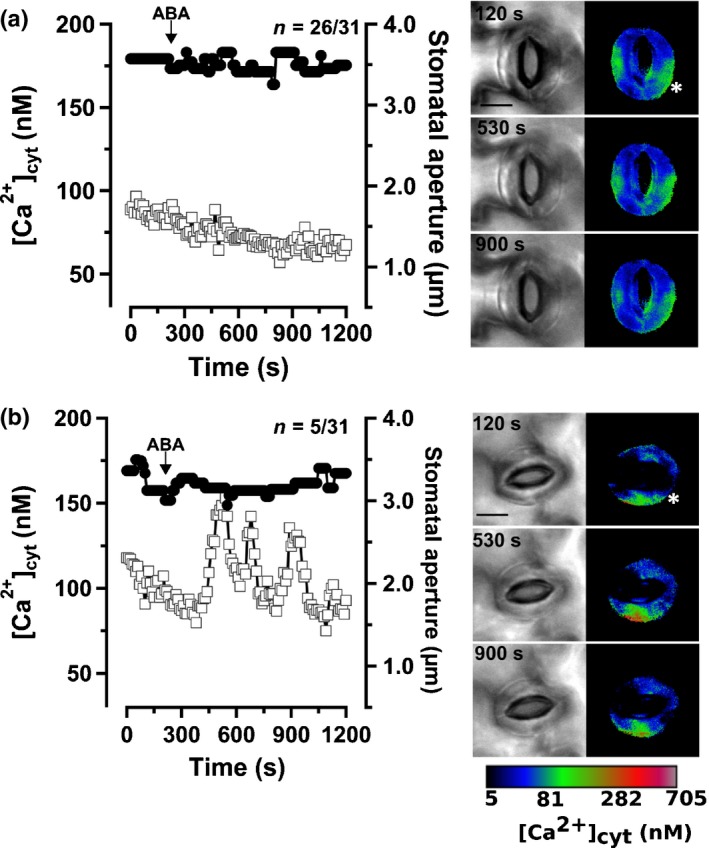
Abscisic acid (ABA) evokes Ca2+ signals only in five out of 31 Arabidopsis thaliana ost1‐3 guard cells. (a, b) Left panels: stomatal aperture (closed circles) and the cytosolic free Ca2+ concentration (open squares) plotted against time of guard cells stimulated by current ejection of ABA, as indicated by the arrow. None of the stomata closed in response to ABA. (a) In 26 out of 31 cells, the guard cells did not display a change in the cytosolic free Ca2+ concentration. (b) ABA triggered repetitive rises in the cytosolic free Ca2+ level in five out of 31 cells; nevertheless, the stomata remained open. (a, b) Right panels: bright‐field (left image) and pseudo‐color (right image) images, showing the stomatal aperture and cytosolic free Ca2+ concentration in the same stomata as in the graphs on the left. The images were obtained at three time points, as indicated in the bright‐field images. The asterisks in the pseudo‐color images indicate the position of contact between the current‐ejection electrode and the guard cell wall. The calibration bar below the images links the color code to [Ca2+]cyt. Bars, 10 μm. See also Supporting Information Videos S8 & S9.
Cytosolic Ca2+ signals trigger rapid activation of anion channels in ost1‐3
OST1 is important for a variety of stomatal responses (Melotto et al., 2006; Xue et al., 2011; Merilo et al., 2013), but it is unclear to what extent it is necessary for Ca2+‐dependent responses in guard cells. Guard cells of ost1‐3, expressing RG‐mT, were therefore stimulated with 10 s hyperpolarization pulses (Fig. 5a,b). Just as in wild‐type, these pulses evoked a transient elevation of the cytosolic Ca2+ level (Videos S10) and activated S‐type anion channels, with a similar voltage dependence as in wild‐type (Fig. 5c). The cytosolic Ca2+ concentration changes were plotted against the currents carried by S‐type anion channels at −100 mV in Fig. 5(d). For wild‐type, a Hill equation was fitted to the data, which revealed that a 90 nM increase of the cytosolic Ca2+ concentration led to a half‐maximal response (Fig. 5d). The Hill equation did not converge to the data of ost1‐3, but the number of cells in which large changes of the cytosolic Ca2+ level occurred was higher in the mutant (Fig. 5d). Combined with the finding that the voltage pulses triggered S‐type anion channel currents with a similar magnitude in ost1‐3 and wild‐type (Fig. 5c), this suggests that ost1‐3 guard cells have a slightly lower Ca2+ responsiveness, as wild‐type.
Figure 5.
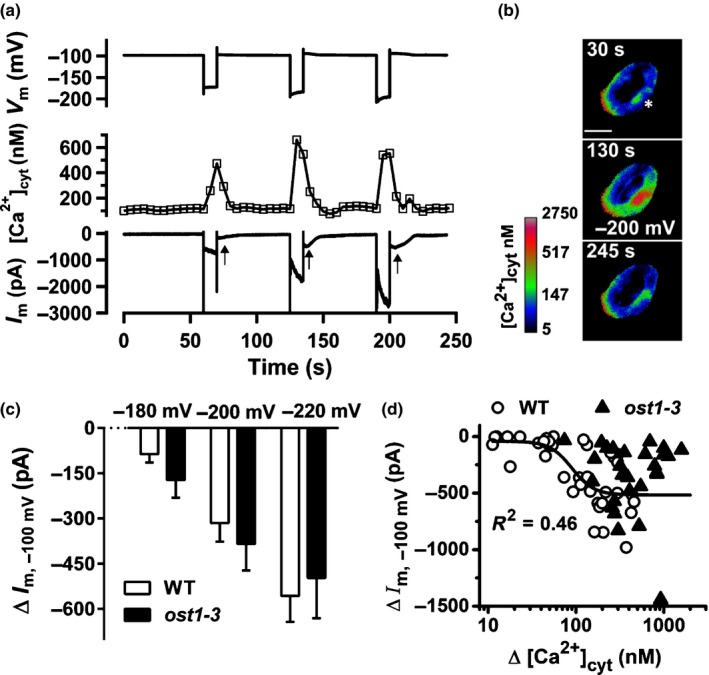
Ca2+‐dependent activation of S‐type anion channels in ost1‐3. (a) An Arabidopsis thaliana ost1‐3 guard cell was stimulated with 10 s voltage pulses from a potential of −100 mV, to −180, −200 and −220 mV (upper trace). The voltage pulses evoked a transient increase of the cytosolic free Ca2+ concentration (middle traces), which caused activation of S‐type anion channels (lower trace) that facilitate inward currents after returning the voltage to −100 mV (arrows below the current trace). (b) Pseudo‐color images that represent the cytosolic free Ca2+ concentration of the same guard cell as in (a), determined from the R‐GECO1‐mTurquoise signal. The images were acquired before, during, and after application of the −200 mV voltage pulse. The asterisk marks the position at which the right guard cell was impaled with a double‐barreled electrode. Bar, 10 μm. The calibration bar next to the images links the color code to [Ca2+]cyt. See also Supporting Information Videos S10. (c) Average change in S‐type anion channel current, recorded at −100 mV and induced by hyperpolarizing pulses to −180, −200, and −220 mV, in wild type (WT, white bars) and ost1‐3 (black bars). Data are from experiments shown in Fig. 3(a) (WT, n = 13) and Fig. 5(a) (ost1‐3, n = 9). Error bars represent + SE. (d) Currents carried by S‐type anion channels, plotted against the peak in the cytosolic Ca2+ concentration, induced by voltage pulses. Data were obtained from 37 voltage pulses applied to 13 WT (open circles) guard cells and 27 voltage pulses in nine guard cells of ost1‐3 (closed triangles). The WT data were fitted with a Hill function, which revealed a half‐maximal response at a change of the cytosolic Ca2+ concentration of 90 nM (SE = 26 nM) and a maximal anion channel current of −516 pA (SE = 74 pA). The Hill function did not converge with the data of ost1‐3.
Discussion
ABA evoked stomatal closure in Arabidopsis in the absence of Ca2+ signals in one out of four stomata, whereas a transient rise in the Ca2+ level was detected in three out of four experiments. These data are in line with early experiments with C. communis, in which ABA‐dependent Ca2+ signals were detected in eight out of 10 stomata (McAinsh et al., 1990) or in 14 out of 38 stomata (Gilroy et al., 1991). This suggests that ABA‐induced Ca2+ signals are common in guard cells, but not absolutely required for stomatal closure.
Ca2+ signals occur in two phases of the guard cell ABA response
The cytosolic Ca2+ signals arose in two phases after stimulation of Arabidopsis guard cells with ABA (Fig. 2). In the majority of cells, the cytosolic Ca2+ concentration increased transiently during the stage in which the stomata were closing. It is feasible that these Ca2+ signals are provoked by the sudden changes in osmotic content of guard cells, which arise at the start of stomatal closure. Such a mechanism is supported by the finding that fast changes in the osmotic content of tobacco guard cells provoke Ca2+ release from intracellular stores (Voss et al., 2016). This class of ABA‐induced Ca2+ signals will not occur in the ost1‐3 mutant, as its stomata do not close in response to ABA, and thus osmotic changes in the cytosol are not evoked by the hormone. As a result, ABA‐induced Ca2+ signals are impaired in ost1‐3 and only five out of 31 ost1‐3 stomata showed changes in the cytosolic Ca2+ level; all of which did not exceed 100 nM (Fig. 4).
ABA can also induce Ca2+ signals that precede closure of the stomatal pore (Fig. 2b), which suggests that the hormone also stimulates Ca2+ channels by a mechanism that does not depend on changes in osmotic pressure. This early response may explain why ABA can also trigger repetitive rises in the Ca2+ concentration of Arabidopsis guard cells in isolated epidermal tissue (Allen et al., 1999, 2001; Klüsener et al., 2002; Islam et al., 2010). Note that in isolated epidermal tissues the ABA‐induced stomatal closure response is less pronounced than in intact leaves (Islam et al., 2010), and osmotically induced Ca2+ signals are therefore less likely to occur. This suggests that ABA evokes these early Ca2+ signals through a mechanism that is not dependent on OST1, but instead through stimulation of nonselective cation channels in the guard cell plasma membrane (Hamilton et al., 2000; Pei et al., 2000; Siegel et al., 2009).
Role of Ca2+ signals in ABA‐induced stomatal closure
ABA‐induced stomatal closure is likely to involve a Ca2+‐independent and ‐dependent signaling mechanisms. The initial Ca2+‐independent step releases the protein kinase OST1 from inhibition (Cutler et al., 2010). In guard cells, OST1 will activate SLAC1, which leads to the release of anions from guard cells and provokes stomatal closure (Geiger et al., 2009; Lee et al., 2009). The Ca2+ signals that can occur before or during stomatal closure probably enhance the activity of SLAC1 and also activate SLAH3, since these two anion channels are also activated by hyperpolarization‐induced Ca2+ signals (Fig. 3). Owing to a further stimulation of the S‐type anion channels in guard cells, Ca2+ signals seem to speed up stomatal closure (Fig. 2d).
The Ca2+‐dependent response is likely to be provoked by Ca2+‐dependent protein kinases (CPKs; Harper et al., 1991; Geiger et al., 2010, 2011; Brandt et al., 2015) and calcineurin B‐like (CBL)‐interacting protein kinases (CIPKs) that bind to CBL proteins (Maierhofer et al., 2014; Kudla et al., 2018). Studies with CPK loss‐of‐function mutants support the function of these protein kinases in ABA‐induced stomatal closure. In cpk8, cpk10, the cpk3/6 double, and the cpk5/6/11/23 quadruple mutants, ABA‐induced stomatal closure was impaired in intact leaves that were floated on solution (Mori et al., 2006; Brandt et al., 2015; Zou et al., 2015). However, experiments with the cpk23 and cpk4/5/6/11 mutants put this general role of CPKs in question. ABA could still induce stomatal closure in the cpk4/5/6/11 mutant (Guzel Deger et al., 2015), and loss of CPK23 even caused plants to become more tolerant to drought (Ma & Wu, 2007). Future studies will thus have to disclose which targets are addressed by individual CPKs and CIPKs and how these interactions contribute to the regulation of stomatal movements.
Cytosolic Ca2+ signals regulate not only plasma membrane ion channels but also the vacuolar two‐pore K+ channels, which are important for stomatal closure (Gobert et al., 2007; Latz et al., 2013; Wang et al., 2015). As suggested by Wheeler & Brownlee (2008), the Ca2+ signals may thus serve as a unifying signal that can coordinate transport processes between the plasma membrane and intracellular membranes. Such a coordinated response is likely to be important for rapid stomatal closure, in which osmolytes are first released from the vacuole into the cytosol and finally extruded across the plasma membrane into the apoplast (Wheeler & Brownlee, 2008; Kollist et al., 2014).
Future directions
In addition to the drought hormone ABA, stomata also respond to a variety of other signals, such as CO2, microbe‐associated molecular patterns, and blue light. Previously, the associated Ca2+ signals were studied in isolated epidermal tissues (Young et al., 2006; Harada & Shimazaki, 2009; Thor & Peiter, 2014), but new genetically encoded Ca2+ sensors now enable experiments with intact leaves. Studies with these new sensors can reveal if guard cell Ca2+ responses are stimulus specific, or if similar Ca2+ signals are recorded, irrespective of the stimulus that induces stomatal closure.
The newly developed sensors will also be of great advantage to study the nature of Ca2+ channels that give rise to Ca2+ signals in guard cells. ABA‐induced activation of Ca2+‐permeable plasma membrane channels in guard cells was reported almost 20 yr ago (Hamilton et al., 2000; Pei et al., 2000), but the genes encoding these channels still need to be uncovered. The osmotically acitavated calcium channels, which are expressed in guard cells, have been associated with osmotically induced Ca2+ signals (Yuan et al., 2014). These channels are thus good candidates for those that generate Ca2+ signals during acceleration phase of ABA‐induced stomatal closure.
Author contributions
RW, RH, and MRGR initiated and designed the study, SH and MN performed the experiments, SH, MN, and MRGR conducted the data analysis and prepared the figures, and SH, HK, RW, RH, and MRGR wrote the manuscript.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Quantification of ABA concentrations evoked by current‐ejection.
Fig. S2 Calibration of RG‐mT with FURA2 in Arabidopsis guard cells.
Fig. S3 Current‐ejection of benzoic acid only induces Ca2+ signals in 2 out of 24 guard cells.
Fig. S4 Inward currents triggered by hyperpolarization of wild type, slah3‐1 and slac1‐3 guard cells.
Fig. S5 Current‐ejection of benzoic acid only induces Ca2+ signals in 1 out of 21 ost1‐3 guard cells.
Methods S1 Procedures to estimate the ABA concentration in the guard cell wall that was imposed by current‐ejection and calibration of R‐GECO1‐mTurquoise with FURA2.
Videos S1 Stomatal closure induced by current‐ejection of ABA.
Videos S2 Current‐ejection of Lucifer Yellow CH (LY) into the wall of an Arabidopsis guard cell.
Videos S3 Calibration of R‐GECO1‐mTuquiose (RG‐mT) with FURA2.
Videos S4 ABA‐induced rise in the cytosolic Ca2+ concentration of a guard cell, during stomatal closure.
Videos S5 ABA‐induced rise in the cytosolic Ca2+ concentration of a guard cell, before stomatal closure.
Videos S6 ABA‐induced stomatal closure in the absence of a cytosolic Ca2+ signal in the guard cells.
Videos S7 Voltage‐induced Ca2+ signals in an Arabidopsis guard cell.
Videos S8 ost1‐3 stoma exposed to ABA, which did neither evoke stomatal closure, nor Ca2+‐signals.
Videos S9 ABA‐induced Ca2+‐signals in an ost1‐3 stoma that were not linked to stomatal closure.
Videos S10 Voltage‐induced Ca2+ signals in an ost1‐3 guard cell.
Acknowledgements
We thank Ingo Dreyer, University of Talca, Chile, for assistance with the analysis of the current‐ejection experiments. This work was supported by grants of the Germany Science Foundation (DFG) ‘Pathogate’: Stomatal control of pathogenic microbe infestation (HE 1640/34‐1; RO 2381/6‐1) to RH and MRGR, the DFG (WA 3768/1‐1) to RW, the China Scholarship Council (CSC) grant (201506350031) to SH and by the Estonian Research Council (grant IUT2‐21) and the European Regional Development Fund (Centre of Excellence in Molecular Cell Engineering) to HK.
References
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffman T, Tang YY, Grill E, Schroeder JI. 2001. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. 1999. Arabidopsis abi1‐1 and abi2‐1 phosphatase mutations reduce abscisic acid‐induced cytoplasmic calcium rises in guard cells. Plant Cell 11: 1785–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Leran S, Corratge‐Faillie C, Gojon A, Krouk G, Lacombe B. 2013. ABA transport and transporters. Trends in Plant Science 18: 325–333. [DOI] [PubMed] [Google Scholar]
- Brandt B, Munemasa S, Wang C, Nguyen D, Yong TM, Yang PG, Poretsky E, Belknap TF, Waadt R, Aleman F et al 2015. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 4: e03599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65: 23–35. [Google Scholar]
- Chen ZH, Hills A, Lim CK, Blatt MR. 2010. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. The Plant Journal 61: 816–825. [DOI] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V et al 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. 2010. Abscisic acid: emergence of a core signaling network. Annual Review of Plant Biology 61: 651–679. [DOI] [PubMed] [Google Scholar]
- De Silva DLR, Hetherington AM, Mansfield TA. 1985. Synergism between calcium ion and abscisic acid in preventing stomatal opening. New Phytologist 100: 473–482. [Google Scholar]
- Dempster J. 1997. A new version of the Strathclyde electrophysiology software package running within the Microsoft windows environment. Journal of Physiology 504P: P57. [Google Scholar]
- Geiger D, Maierhofer T, Al‐Rasheid KAS, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E et al 2011. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Science Signaling 4: ra32. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al‐Rasheid KAS, Grill E et al 2010. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proceedings of the National Academy of Sciences, USA 107: 8023–8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al‐Rasheid KAS et al 2009. Activity of guard cell anion channel SLAC1 is controlled by drought‐stress signaling kinase–phosphatase pair. Proceedings of the National Academy of Sciences, USA 106: 21425–21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Fricker MD, Read ND, Trewayas AJ. 1991. Role of calcium in signal transduction of Commelina guard cells. Plant Cell 3: 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJM. 2007. The two‐pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proceedings of the National Academy of Sciences, USA 104: 10726–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Guzman M, Pizzio GA, Antoni R, Vera‐Sirera F, Merilo E, Bassel GW, Fernandez MA, Holdsworth MJ, Perez‐Amador MA, Kollist H et al 2012. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24: 2483–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. 1998. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proceedings of the National Academy of Sciences, USA 95: 4778–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzel Deger A, Scherzer S, Nuhkat M, Kedzierska J, Kollist H, Brosche M, Unyayar S, Boudsocq M, Hedrich R, Roelfsema MRG. 2015. Guard cell SLAC1‐type anion channels mediate flagellin‐induced stomatal closure. New Phytologist 208: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DWA, Hills A, Kohler B, Blatt MR. 2000. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proceedings of the National Academy of Sciences, USA 97: 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Shimazaki K. 2009. Measurement of changes in cytosolic Ca2+ in Arabidopsis guard cells and mesophyll cells in response to blue light. Plant and Cell Physiology 50: 360–373. [DOI] [PubMed] [Google Scholar]
- Harper JF, Sussman MR, Schaller GE, Putnamevans C, Charbonneau H, Harmon AC. 1991. A calcium‐dependent protein‐kinase with a regulatory domain similar to calmodulin. Science 252: 951–954. [DOI] [PubMed] [Google Scholar]
- Hedrich R, Geiger D. 2017. Biology of SLAC1‐type anion channels – from nutrient uptake to stomatal closure. New Phytologist 216: 46–61. [DOI] [PubMed] [Google Scholar]
- Islam MM, Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. 2010. Roles of AtTPC1, vacuolar Two Pore Channel 1, in Arabidopsis stomatal closure. Plant and Cell Physiology 51: 302–311. [DOI] [PubMed] [Google Scholar]
- Joshi‐Saha A, Valon C, Leung J. 2011. A brand new START: abscisic acid perception and transduction in the guard cell. Science Signaling 4: re4. [DOI] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu HH, Nishimura N, Schroeder JI. 2010. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology 61: 561–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüsener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI. 2002. Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiology 130: 2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MRG. 2014. Closing gaps: linking elements that control stomatal movement. New Phytologist 203: 44–62. [DOI] [PubMed] [Google Scholar]
- Kudla J, Becker D, Grill E, Hedrich R, Hippler M, Kummer U, Parniske M, Romeis T, Schumacher K. 2018. Advances and current challenges in calcium signaling. New Phytologist 218: 414–431. [DOI] [PubMed] [Google Scholar]
- Latz A, Mehlmer N, Zapf S, Mueller TD, Wurzinger B, Pfister B, Csaszar E, Hedrich R, Teige M, Becker D. 2013. Salt stress triggers phosphorylation of the Arabidopsis vacuolar K channel TPK1 by calcium‐dependent protein kinases (CDPKs). Molecular Plant 6: 1274–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan WZ, Buchanan BB, Luan S. 2009. A protein kinase–phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proceedings of the National Academy of Sciences, USA 106: 21419–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko V, Guinot DR, Klein M, Roelfsema MRG, Hedrich R, Dietrich P. 2008. Stringent control of cytoplasmic Ca2+ in guard cells of intact plants compared to their counterparts in epidermal strips or guard cell protoplasts. Protoplasma 233: 61–72. [DOI] [PubMed] [Google Scholar]
- Levchenko V, Konrad KR, Dietrich P, Roelfsema MRG, Hedrich R. 2005. Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals. Proceedings of the National Academy of Sciences, USA 102: 4203–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. 2009. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068. [DOI] [PubMed] [Google Scholar]
- Ma SY, Wu WH. 2007. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Molecular Biology 65: 511–518. [DOI] [PubMed] [Google Scholar]
- Maierhofer T, Diekmann M, Offenborn JN, Lind C, Bauer H, Hashimoto K, Al‐Rasheid KAS, Luan S, Kudla J, Geiger D et al 2014. Site‐ and kinase‐specific phosphorylation‐mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Science Signaling 7: ra86. [DOI] [PubMed] [Google Scholar]
- Marten H, Konrad KR, Dietrich P, Roelfsema MRG, Hedrich R. 2007. Ca2+‐dependent and ‐independent abscisic acid activation of plasma membrane anion channels in guard cells of Nicotiana tabacum . Plant Physiology 143: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. 1990. Abscisic acid‐induced elevation of guard‐cell cytosolic Ca2+ precedes stomatal closure. Nature 343: 186–188. [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. 2006. Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980. [DOI] [PubMed] [Google Scholar]
- Merilo E, Jalakas P, Kollist H, Brosche M. 2015. The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2, and exogenous ABA. Molecular Plant 8: 657–659. [DOI] [PubMed] [Google Scholar]
- Merilo E, Laanemets K, Hu H, Xue S, Jakobsen L, Tulva I, Gonzales‐Guzman M, Rodriguez PL, Schroeder JI, Brosche M et al 2013. PYR/RCAR receptors contribute to ozone‐, reduced air humidity‐, darkness‐ and CO2‐induced stomatal regulation. Plant Physiology 162: 1652–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang YZ, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR et al 2006. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S‐type anion‐ and Ca2+‐permeable channels and stomatal closure. PLoS Biology 4: 1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI. 2015. Mechanisms of abscisic acid‐mediated control of stomatal aperture. Current Opinion in Plant Biology 28: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. 2002. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai‐Yamada M, Uchimiya H, Hashimoto M, Iba K. 2008. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486. [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TFF et al 2009. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. 1997. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild‐type and abi1 and abi2 mutants. Plant Cell 9: 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. 2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734. [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R. 2002. Studying guard cells in the intact plant: modulation of stomatal movement by apoplastic factors. New Phytologist 153: 425–431. [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R, Geiger D. 2012. Anion channels: master switches of stress responses. Trends in Plant Science 17: 221–229. [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Levchenko V, Hedrich R. 2004. ABA depolarizes guard cells in intact plants, through a transient activation of R‐ and S‐type anion channels. The Plant Journal 37: 578–588. [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Prins HBA. 1997. Ion channels in guard cells of Arabidopsis thaliana (L.) Heynh. Planta 202: 18–27. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B et al 2012. fiji: an open‐source platform for biological‐image analysis. Nature Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. 1989. Cytosolic calcium regulates ion channels in the plasma‐membrane of Vicia faba guard‐cells. Nature 338: 427–430. [Google Scholar]
- Shimazaki KI, Doi M, Assmann SM, Kinoshita T. 2007. Light regulation of stomatal movement. Annual Review of Plant Biology 58: 219‐247. [DOI] [PubMed] [Google Scholar]
- Siegel RS, Xue SW, Murata Y, Yang YZ, Nishimura N, Wang A, Schroeder JI. 2009. Calcium elevation‐dependent and attenuated resting calcium‐dependent abscisic acid induction of stomatal closure and abscisic acid‐induced enhancement of calcium sensitivities of S‐type anion and inward‐rectifying K+ channels in Arabidopsis guard cells. The Plant Journal 59: 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange A, Hedrich R, Roelfsema MRG. 2010. Ca2+‐dependent activation of guard cell anion channels, triggered by hyperpolarization, is promoted by prolonged depolarization. The Plant Journal 62: 265–276. [DOI] [PubMed] [Google Scholar]
- Thor K, Peiter E. 2014. Cytosolic calcium signals elicited by the pathogen‐associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytologist 204: 873–881. [DOI] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi‐Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K. 2009. Type 2C protein phosphatases directly regulate abscisic acid‐activated protein kinases in Arabidopsis . Proceedings of the National Academy of Sciences, USA 106: 17588–17593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmaki A, Brosche M, Moldau H, Desikan R et al 2008. SLAC1 is required for plant guard cell S‐type anion channel function in stomatal signalling. Nature 452: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Lauriere C, Merlot S. 2009. Protein phosphatases 2C regulate the activation of the Snf1‐related kinase OST1 by abscisic acid in Arabidopsis . Plant Cell 21: 3170–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss LJ, Hedrich R, Roelfsema MRG. 2016. Current injection provokes rapid expansion of the guard cell cytosolic volume and triggers Ca2+ signals. Molecular Plant 9: 471–480. [DOI] [PubMed] [Google Scholar]
- Voss LJ, McAdam SAM, Knoblauch M, Rathje JM, Brodribb TJ, Hedrich R, Roelfsema MRG. 2018. Guard cells in fern stomata are connected by plasmodesmata, but control cytosolic Ca2+ levels autonomously. New Phytologist 219: 206–215. [DOI] [PubMed] [Google Scholar]
- Waadt R, Krebs M, Kudla J, Schumacher K. 2017. Multiparameter imaging of calcium and abscisic acid and high‐resolution quantitative calcium measurements using R‐GECO1‐mTurquoise in Arabidopsis . New Phytologist 216: 303–320. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dindas J, Rienmuller F, Krebs M, Waadt R, Schumacher K, Wu W‐H, Hedrich R, Roelfsema MRG. 2015. Cytosolic Ca2+ signals enhance the vacuolar ion conductivity of bulging Arabidopsis root hair cells. Molecular Plant 8: 1665–1674. [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Brownlee C. 2008. Ca2+ signalling in plants and green algae – changing channels. Trends in Plant Science 13: 506–514. [DOI] [PubMed] [Google Scholar]
- Willmer CM, Mansfield TA. 1969. A critical examination of use of detached epidermis in studies of stomatal physiology. New Phytologist 68: 363–375. [Google Scholar]
- Xue SW, Hu HH, Ries A, Merilo E, Kollist H, Schroeder JI. 2011. Central functions of bicarbonate in S‐type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO Journal 30: 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K. 2002. ABA‐activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis . Plant and Cell Physiology 43: 1473–1483. [DOI] [PubMed] [Google Scholar]
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. 2006. CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+‐independent phase, and CO2 insensitivity of the gca2 mutant. Proceedings of the National Academy of Sciences, USA 103: 7506–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Yang HM, Xue Y, Kong DD, Ye R, Li CJ, Zhang JY, Theprungsirikul L, Shrift T, Krichilsky B et al 2014. OSCA1 mediates osmotic‐stress‐evoked Ca2+ increases vital for osmosensing in Arabidopsis . Nature 514: 367–371. [DOI] [PubMed] [Google Scholar]
- Zhang XR, Henriques R, Lin SS, Niu QW, Chua NH. 2006. Agrobacterium‐mediated transformation of Arabidopsis thaliana using the floral dip method. Nature Protocols 1: 641–646. [DOI] [PubMed] [Google Scholar]
- Zhao YX, Araki S, Jiahui WH, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T et al 2011. An expanded palette of genetically encoded Ca2+ indicators. Science 333: 1888–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JJ, Li XD, Ratnasekera D, Wang C, Liu WX, Song LF, Zhang WZ, Wu WH. 2015. Arabidopsis CALCIUM‐DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid‐mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 27: 1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Quantification of ABA concentrations evoked by current‐ejection.
Fig. S2 Calibration of RG‐mT with FURA2 in Arabidopsis guard cells.
Fig. S3 Current‐ejection of benzoic acid only induces Ca2+ signals in 2 out of 24 guard cells.
Fig. S4 Inward currents triggered by hyperpolarization of wild type, slah3‐1 and slac1‐3 guard cells.
Fig. S5 Current‐ejection of benzoic acid only induces Ca2+ signals in 1 out of 21 ost1‐3 guard cells.
Methods S1 Procedures to estimate the ABA concentration in the guard cell wall that was imposed by current‐ejection and calibration of R‐GECO1‐mTurquoise with FURA2.
Videos S1 Stomatal closure induced by current‐ejection of ABA.
Videos S2 Current‐ejection of Lucifer Yellow CH (LY) into the wall of an Arabidopsis guard cell.
Videos S3 Calibration of R‐GECO1‐mTuquiose (RG‐mT) with FURA2.
Videos S4 ABA‐induced rise in the cytosolic Ca2+ concentration of a guard cell, during stomatal closure.
Videos S5 ABA‐induced rise in the cytosolic Ca2+ concentration of a guard cell, before stomatal closure.
Videos S6 ABA‐induced stomatal closure in the absence of a cytosolic Ca2+ signal in the guard cells.
Videos S7 Voltage‐induced Ca2+ signals in an Arabidopsis guard cell.
Videos S8 ost1‐3 stoma exposed to ABA, which did neither evoke stomatal closure, nor Ca2+‐signals.
Videos S9 ABA‐induced Ca2+‐signals in an ost1‐3 stoma that were not linked to stomatal closure.
Videos S10 Voltage‐induced Ca2+ signals in an ost1‐3 guard cell.


