Abstract
Purpose
In June 2013, following recommendations from the World Health Organization (WHO) and Food and Drug Administration (FDA), the European Medicines Agency agreed updates to the codeine product information regarding use for pain in children younger than 12 years and children undergoing tonsillectomy or adenoidectomy (TA) for obstructive sleep apnoea. This study was conducted to (a) assess effectiveness of these measures on codeine prescribing in the “real‐world” setting and (b) test feasibility of a study using a common protocol by regulators with access to databases.
Methods
The study was performed using BIFAP (Spain), CPRD (UK), and IMS® Disease Analyzer (France and Germany) databases. Prescribers included general practitioners (GPs) (France and UK), GPs and paediatricians together (Spain), and GPs, paediatricians, and ear, nose, and throat (ENT) specialists separately (Germany). Between January 2010 and June 2015, prevalence of codeine prescribing was obtained every 6 months, and a time series analysis (joinpoint) was performed. Codeine prescribing within ±30 days of TA was also identified. Furthermore, doses, durations, and prior prescribing of other analgesics were investigated.
Results
Over the 5‐year period, codeine prescribing decreased in children younger than 12 years (by 84% in France and Spain, 44% in GP practices in Germany, and 33% in the United Kingdom). The temporal pattern was compatible with the regulatory intervention in France and the United Kingdom, whereas a decrease throughout the study period was seen in Germany and Spain. Decreased prescribing associated with TA was suggested in ENT practices in Germany.
Conclusions
Codeine prescribing for children decreased in line with introduced regulatory measures. Multidatabase studies assessing impact of measures by EU regulators are feasible.
Keywords: children, codeine, drug utilization, pharmacoepidemiology, risk minimization measures
KEY POINTS.
Prescribing of codeine for treatment of pain in children below the age of 12 years decreased in all four countries with a temporal pattern that was compatible with an impact of the RMM in the United Kingdom and in France. There was also evidence of decreased prescribing of codeine after the introduced RMM for children undergoing TA in ear, nose, and throat practices in Germany. The findings of reduced exposure to codeine in children at increased risk of toxicity are reassuring, but further follow‐up of prescribing of codeine in children is warranted to determine if codeine prescribing in children continues to decrease or levels off.
It was also shown that collaborative risk minimisation effectiveness studies using a common protocol by regulators with direct access to electronic health record databases are feasible.
1. INTRODUCTION
Codeine is an opium alkaloid, which has been approved for pain relief, as an antitussive agent, and as an antidiarrhoeal agent in individual countries in the European Union (EU). It is available as a single ingredient (plain codeine) and in combination with other substances. Codeine is converted in the body via the enzyme CYP2D6 into morphine, which is responsible for the analgesic effect of codeine.1, 2 The most severe risk of codeine use is respiratory depression, associated with morphine toxicity.1
Concerns over the safe and effective use of codeine for pain relief in children have been expressed for a number of years.3, 4, 5, 6, 7, 8, 9 In March 2011, the World Health Organization (WHO) deleted codeine from its list of essential medicines in children3 and in 2012, published guidelines on the pharmacological treatment of persisting pain in children, where use of codeine was no longer recommended.4 In August 2012, the United States Food and Drug Administration (FDA) communicated that codeine use in certain children after tonsillectomy or adenoidectomy (TA) may lead to rare but life‐threatening adverse events or death.5, 6 Subsequently, in April 2017, the FDA contraindicated use of codeine in all children below the age of 12 years.10
In October 2012, the European Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) started a review of the use of codeine for treatment of pain in children.11, 12 This led, in June 2013, to the PRAC recommending risk minimisation measures (RMM) including restrictions on its use13 and communications about the safety concerns by national authorities.14, 15, 16, 17, 18, 19, 20, 21, 22 Updated guidelines for analgesic use in children have since been published, in line with the regulatory communications.23, 24, 25
The restrictions of use included that codeine should only be used in children 12 years or older for pain that cannot be relieved by other analgesics such as paracetamol or ibuprofen alone. Also, the lowest effective dose should be used for the shortest period of time in these children. Contraindications were introduced for pain relief in children of all ages undergoing TA for obstructive sleep apnoea and in patients who are CYP2D6 ultra‐rapid metabolisers as they have an increased risk of morphine toxicity due to faster than normal conversion of codeine to morphine.
National competent authorities (NCAs) in the Members States, the EMA, and the European Commission collaborate to regulate medicines in the EU. The monitoring of the safety of medicines has been enhanced as a result of the new European pharmacovigilance legislation, which serves to proactively manage the risk of marketed drugs in a sustainable life‐cycle benefit‐risk management approach.26 Within this legislation, the PRAC is responsible both for implementing RMM and for overseeing their effectiveness.27 With this in mind, a key objective of the present study was to proactively assess the impact of the introduced measures on codeine prescribing for pain in “real‐world” clinical practice. This was to be done by focusing on time trends for prevalence of use of codeine for pain in children younger than 12 years and in those undergoing TA. As prescribing practices across the EU may differ between individual Member States, the study was to be conducted in databases representing a number of EU countries to gain insight into possible variation in impact across the EU. The opportunity would also be taken to use the study to evaluate a model for collaboration within a best evidence strategy to support decision making by the EU medicines regulators.28 Regulators with access to electronic health records (EHR) databases were invited to participate in the study. To this end, the Spanish and United Kingdom NCAs (AEMPS, Spanish Agency for Medicines and Medical Devices and MHRA, Medicines and Healthcare products Regulatory Authority, respectively) collaborated with EMA, who had access to data for France and Germany as “a Best Evidence pilot group.” Hence, the study represents a population of around 260 million EU citizens.
Drug utilisation studies of use of codeine for pain in children before and after regulatory action have been previously performed in Norway and the United States.29, 30, 31 Significant drops in use have been identified. Surveys to prescribers in Sweden and the United States have also provided evidence of reduced prescribing of codeine in children.32, 33
Recognised possible therapeutic alternatives to codeine for treatment of pain in children include, eg, paracetamol, nonsteroidal anti‐inflammatory drugs (NSAIDs), coxibs, and opioid analgesics such as dihydrocodeine, tramadol, morphine, tapentadol, or oxycodone.23, 24, 25, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 However, investigating whether a potential decrease in prescribing of codeine was associated with a shift in prescribing to other analgesics was considered outside the scope of the study.
2. METHODS
2.1. Setting
The agreed study time period was between 1 January 2010 and 31 December 2014 (Spain) or 30 June 2015 (UK, France, and Germany) in line with the frequency of updates of the databases.
The study was conducted in the BIFAP database in Spain, the CPRD database in the United Kingdom, and the IMS® Disease Analyzer databases (IMS®) in France and Germany. All of the included databases collect anonymised EHR from general practitioners (GPs). BIFAP45 also includes data from paediatricians, which together with GPs are considered as primary care physicians, and were analysed together. IMS® Germany includes data from a range of specialist physicians. Data from GPs, paediatricians, and ear, nose, and throat (ENT) specialists in IMS® Germany were analysed separately. Practices in BIFAP include around 16% of the Spanish population45 and practices in CPRD include around 7% of the UK population.46 Around 2% and 3% of all GP practices in France and Germany, respectively, are included in IMS®. Patients in BIFAP, CPRD, and IMS® France are broadly representative of the total population in terms of age and gender,46, 47 whereas children are underrepresented among GP patients in IMS® Germany.47, 48 For coding of symptoms and diagnoses, BIFAP uses the International Classification of Primary Care (ICPC‐2) and ICD‐9, CPRD uses Read Codes and IMS® uses ICD 10. More information about the databases included in the study, and the study population, is provided in Appendix S1.
2.2. Study design
Patients included in the study were those younger than 18 years with at least 1 year of follow‐up in the database. One year of follow‐up was not required for children younger than 1 year.
For the purpose of this study, RMM were considered to be introduced on 28 June 2013, which was the date of endorsement of the PRAC recommendations by the Coordination Group for Mutual Recognition and Decentralised procedures for Human Medicinal Products (CMDh).49 National communications took place subsequent to this and changes to the product information were to be implemented in all EU Member States by 1 October 2013.50
Within the time period of the study, all codeine‐containing products in Germany and Spain were prescription‐only medicines. In France, preparations containing less than 30 mg of codeine did not require a prescription by a physician,51 and in the United Kingdom, some low‐dose forms of combinations including codeine were available without prescription.
A common study protocol focused on codeine used for pain was agreed on 25 November 2015 by a group established by the PRAC. In order to capture use of codeine for treatment of pain, the exposures of interest included products containing codeine alone (ATC code R05DA04), codeine in combination with an analgesic or NSAID (ATC classes N02A and N02B), and codeine in combination with an analgesic and an antihistamine (ATC class N02C). Products containing codeine in combination with menthol, sympathomimetics, antitussives, expectorants, antihistamines (without an analgesic or NSAID), herbal cough ingredients, or an antispasmodic agent or antidiarrhoeal were excluded. Oral solutions and solid oral formulations of plain codeine products were evaluated separately. As products containing plain codeine may be indicated for both pain and cough, a review of patient records was performed to determine the indication more accurately and to exclude prescriptions for nonpain indications.
In BIFAP, indications linked to the prescription for any of the codeine‐containing products in the study were reviewed, and diagnoses related to an indication of pain, or a respiratory or digestive condition, were identified. In children where a linked indication could not be allocated to any of the three indication categories, diagnoses within 14 days prior to the prescription were also reviewed in order to allocate the prescription to one of the defined indication categories. Of the total of 61 302 children with a prescription for one of the codeine‐containing products, 1923 (3.1%) were considered to have received codeine for treatment of pain and were included in further analyses. Children without an allocated indication for treatment (6.1%; n = 3767) along with children considered to have received codeine for a nonpain indication (90.7%; n = 55 612), mostly respiratory indications (88.7%; n = 54 381), were excluded from the study.
In CPRD, treatment of pain was considered for codeine in combination with an analgesic or NSAID, codeine in combination with an analgesic and antihistamine, and prescriptions of plain codeine where no record of cough could be identified within 14 days of the prescription. The majority of children with a prescription for one of the codeine‐containing products, 19 591 of 19 969 (98.1%) were considered to have received at least one prescription of codeine for treatment of pain and were included in further analyses.
In IMS®, treatment of pain was considered for codeine in combination with an analgesic or NSAID, codeine in combination with an analgesic and antihistamine, and prescriptions of plain codeine where no record of cough and no diagnosis likely to indicate cough could be identified within 14 days of the prescription. In IMS® France, the majority of children with a prescription for one of the codeine‐containing products, 3207 of 3385 (94.7%) were considered to have received at least one prescription of codeine for the treatment of pain and were included in further analyses. Corresponding proportions for GP practices, paediatric practices, and ENT practices in IMS® Germany were 59.3% (5672/9571), 53.1% (7574/14 252), and 71.8% (333/464) of children, respectively.
2.3. Analysis
The number of children with a codeine prescription was calculated by age group (younger than 12 y and 12‐17 y), gender, and half‐yearly (every 6 mo) time periods. Key results included prevalence of prescribing of codeine for pain and use of codeine within a period of ±30 days of a TA surgical procedure. Information about how TA was identified within the databases is provided in Appendix S1. TA was identified among children with a codeine prescription. In ENT practices in IMS® Germany, TA was also identified among all children. For information about how daily doses and durations were calculated, see also Appendix S1. The proportion of children that had been prescribed another analgesic (ie, not containing codeine) within a period of 90 days up to the time of the codeine prescription was also calculated.
2.3.1. Analysis of changes in prescribing trends
To evaluate whether changes in prescribing associated with the RMM were statistically significant, a post hoc analysis of prescribing trends for children younger than 12 years was performed using joinpoint regression analysis with log‐linear model.52, 53, 54 Joinpoint regression determines changes in prescribing trend, called joinpoints, and a new joinpoint is added only if there is a statistically significant improvement in the fit of the model. Permutation tests using Monte Carlo methods were used to determine the minimum number of joinpoints required to provide an adequate fit to the data. A significance level of 5% was used to assess the need for each extra joinpoint, starting from zero joinpoints. The timepoint for the joinpoint (with 95% confidence intervals) can then be compared with the dates of the interventions.55
3. RESULTS
3.1. Prevalence of use of codeine for treatment of pain
The most commonly prescribed codeine formulation in France, Spain, and the United Kingdom was codeine in combination with an analgesic or NSAID, whereas in Germany, it was a liquid oral formulation of plain codeine (Table 1). Codeine in combination with both an analgesic and an antihistamine was effectively only prescribed in the United Kingdom (Table 1).
Table 1.
Descriptive overview of children 0‐17 y with a prescription for codeine during the study period
| BIFAP | CPRD | IMS® Francea | IMS® Germany GPa | IMS® Germany PAEDa | IMS® Germany ENTa | |
|---|---|---|---|---|---|---|
| No. of children 0‐17 y included in demographics | 1915 | 17689 | 2983 | 4526 | 6558 | 205 |
| Females (%) | 1097 (57.3) | 11448 (58.4) | 1580 (53.0) | 2355 (52.0) | 3334 (50.8) | 118 (57.6) |
| Children 0‐11 y (%) | 415 (21.7) | 1702 (9.6) | 1571 (52.7) | 1718 (38.0) | 4870 (74.3) | 127 (62.0) |
| Children 12‐17 y (%) | 1499 (78.3) | 15987 (90.4) | 1427 (47.8) | 2889 (63.8) | 1829 (27.9) | 79 (38.5) |
| Plain codeine solution (%) | 375 (19.6) | 779 (4.4) | 1391(46.6) | 3081 (68.1) | 5766 (87.9) | 144 (70.2) |
| Plain codeine solid formulation (%) | 25 (1.3) | 3229 (18.3) | 22 (0.7) | 605 (13.4) | 499 (7.6) | 23 (11.2) |
| Combination with analgesic/NSAID (%) | 1514 (79.1) | 12983 (73.4) | 1601 (53.7) | 992 (21.9) | 343 (5.2) | 41 (20.0) |
| Combination with analgesic and antihistamine (%) | 1 (0.1) | 1092 (6.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other analgesics within 90 d before codeine (%) | 595 (31.1) | 1290 (7.3) | 742 (24.9) | 1803 (39.8) | 2863 (43.7) | 57 (27.8) |
Note. Children included in demographics were all children that had codeine use registered in a half‐year and no codeine use registered in the previous half‐year (BIFAP), or all children with at least 90 d of follow‐up prior to the codeine prescription (CPRD, IMS® France, IMS® Germany). In CPRD, the same patient could contribute to more than one type of codeine product. In IMS® (France, Germany), the same patient could contribute to more than one age group and to more than one type of codeine product. For details about other analgesics within 90 d before codeine, see Appendix S1.
Abbreviations: ENT, ear, nose, and throat practice; GP, general practice; IMS®, IMS® Disease Analyzer; NA, not applicable; PAED, paediatric practice.
Own calculations, based on IMS® Disease Analyzer (France, Germany).
A numerical decrease in overall prescribing of codeine between the start and end of the study period could be seen in all countries, although there was no observed decrease in prescribing of plain codeine in solid oral formulation or codeine in combination with both an analgesic and an antihistamine (Table 2). The decrease was more pronounced in the age group below 12 years with little evidence of a decrease in the use of codeine in children 12 to 17 years, except in Spain (Table 2). The prevalence of prescribing of codeine at the start of the study period was less in children below the age of 12 years compared with that of children 12 to 17 years, except for ENT practices in Germany.
Table 2.
Prevalence (per 10 000) of prescribing of codeine in children 0‐17 y at the start and end of the study period
| Time Period | BIFAP | CPRD | IMS® Francea | IMS® Germany GPa | IMS® Germany PAEDa | IMS® Germany ENTa | |
|---|---|---|---|---|---|---|---|
| All children 0‐17 y | Start | 4.4 | 28.4 | 29.2 | 81.8 | 37.0 | 11.4 |
| End (% change) | 1.4 (−68%) | 22.7 (−20%) | 14.6 (−50%) | 64.3 (−21%) | 29.4 (−21%) | 6.1 (−47%) | |
| Children 0‐11 y | Start | 1.9 | 3.8 | 25.0 | 72.9 | 36.0 | 12.2 |
| End (% change) | 0.3 (−84%) | 2.6 (−33%) | 3.9 (−84%) | 40.6 (−44%) | 23.8 (−34%) | 4.8 (−61%) | |
| Children 12‐17 y | Start | 11.5 | 73.4 | 38.8 | 90.7 | 41.6 | 9.3 |
| End (% change) | 4.2 (−63%) | 71.8 (−2%) | 35.0 (−10%) | 84.0 (−7%) | 47.9 (+15%) | 8.3 (−11%) | |
| Female children 0‐17 y | Start | 3.8 | 37.0 | 29.5 | 81.5 | 38.2 | 13.9 |
| End (% change) | 1.1 (−71%) | 30.6 (−17%) | 18.1 (−39%) | 70.9 (−13%) | 31.6 (−17%) | 8.1 (−42%) | |
| Male children 0‐17 y | Start | 5.0 | 20.2 | 29.1 | 82.0 | 35.8 | 9.1 |
| End (% change) | 1.6 (−68%) | 15.1 (−25%) | 11.3 (−61%) | 58.0 (−29%) | 27.2 (−24%) | 4.3 (−53%) | |
| Plain codeine solution 0‐17 y | Start | 1.5 | 1.1 | 15.3 | 54.3 | 32.67 | 7.8 |
| End (% change) | 0.1 (−93%) | 0.6 (−51%) | 3.0 (−80%) | 41.4 (−24%) | 25.85 (−21%) | 4.2 (−46%) | |
| Solid formulation plain codeine 0‐17 y | Start | 0.0 | 4.3 | 0.0 | 7.7 | 2.0 | 1.2 |
| End (% change) | 0.0 (NA) | 4.6 (+6%) | 0.2 (+∞%) | 10.5 (+36%) | 2.8 (+44%) | 1.1 (−6%) | |
| Combination with analgesic/NSAID 0‐17 y | Start | 2.9 | 21.6 | 14.1 | 20.4 | 2.5 | 2.7 |
| End (% change) | 1.3 (−55%) | 16.2 (−25%) | 11.2 (−81%) | 13.5 (−34%) | 0.7 (−71%) | 0.8 (−72%) | |
| Combination with analgesic and antihistamine 0‐17 y | Start | 0.0 | 2.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| End (% change) | 0.0 (NA) | 2.6 (+20%) | 0.0 (NA) | 0.0 (NA) | 0.0 (NA) | 0.0 (NA) |
Note. Start of the study period was defined as 1 January to 30 June 2010. End of the study period was defined as 1 January to 30 June 2015 in CPRD and in IMS (France, Germany), and as 1 July to 31 December 2014 in BIFAP.
Abbreviations: ENT, ear, nose, and throat practice; GP, general practice; IMS®, IMS® Disease Analyzer; NA, not applicable; PAED, paediatric practice.
Own calculations, based on IMS® Disease Analyzer (France, Germany).
Prescribing trends in the age group below 12 years, in which treatment with codeine for pain was no longer indicated following June 2013, are shown in Figure 1. Prescribing trends in all children are shown in Figure 2.
Figure 1.
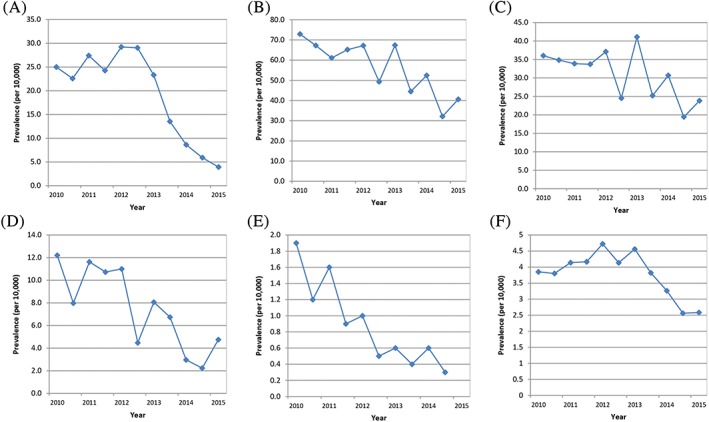
A‐F, Six‐monthly prevalence (per 10 000) of codeine for the treatment of pain in children 0‐11 y in (A) Francea, (B‐D) Germanya, (E) Spain, and (F) the United Kingdom. (A) IMS® France (top left corner). (B) IMS® Germany GP (middle top). (C) IMS® Germany PAED (top right corner). (D) IMS® Germany ENT (bottom left corner). (E) BIFAP (middle bottom). (F) CPRD (bottom right corner) ENT = ear, nose, and throat practice, GP = general practice, IMS® = IMS® Disease Analyzer, PAED = paediatric practice. aOwn calculations, based on IMS® Disease Analyzer (France, Germany) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
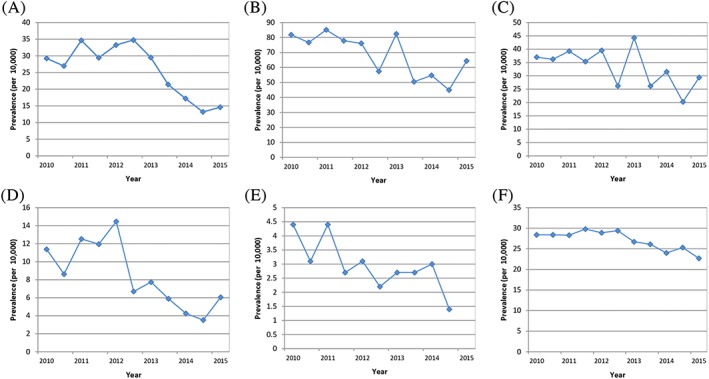
A‐F, Six‐monthly prevalence (per 10 000) of codeine for the treatment of pain in children 0‐17 y in (A) Francea, (B‐D) Germanya, (E) Spain, and (F) the United Kingdom. (A) IMS® France (top left corner). (B) IMS® Germany GP (middle top). (C) IMS® Germany PAED (top right corner). (D) IMS® Germany ENT (bottom left corner). (E) BIFAP (middle bottom). (F) CPRD (bottom right corner) ENT = ear, nose, and throat practice, GP = general practice, IMS® = IMS® Disease Analyzer, PAED = paediatric practice. aOwn calculations, based on IMS® Disease Analyzer (France, Germany) [Colour figure can be viewed at wileyonlinelibrary.com]
In children below 12 years, initial increases in prescribing between 2010 and 2012 were seen in France and the United Kingdom, followed by decreases from 2013 onwards. In contrast, a decrease in prescribing could be seen in Spain already between 2011 and 2012, and this continued into 2013. There were large fluctuations in prescribing in Germany that made it difficult to assess when a decrease in prescribing started.
Joinpoint analysis of prescribing in children below 12 years identified a change in prescribing trend in France and the United Kingdom. No change in prescribing trend was detected in Germany or Spain (Table 3 and Figure 3).
Table 3.
Joinpoint analyses of six‐monthly prescribing trends of codeine for treatment of pain in children 0‐11 y
| No. of Joinpoints | Joinpoint Location (Lower CL, Upper CL) | Slope | Annual Percent Change (Lower CL, Upper CL) | |
|---|---|---|---|---|
| BIFAP | 0 | NA | 1 | −16.6 (−21.7, −11.3) |
| CPRD | 1 | 2013 H1 (2012 H1 − 2013 H2) |
1 2 |
2.7 (−1.1, 6.6) −14.7 (−21.3, −7.6) |
| IMS® Francea | 1 | 2012 H2 (2012 H1 − 2013 H2) |
1 2 |
5.3 (−0.3, 11.2) −32.8 (−39.3, −25.6) |
| IMS® Germany GPa | 0 | NA | 1 | −5.4 (−8.5, −2.2) |
| IMS® Germany PAEDa | 0 | NA | 1 | −3.8 (−7.7, 0.2) |
| IMS® Germany ENTa | 0 | NA | 1 | −10.4 (−16.9, −3.3) |
Note. A log linear joinpoint regression model was selected. Significant annual percent changes are highlighted in bold.
Abbreviations: CL, 95% confidence limit; ENT, ear, nose, and throat practice; GP, general practice; IMS®, IMS® Disease Analyzer; NA, not applicable; PAED, paediatric practice; H1, January to June; H2, July to December.
Own calculations, based on IMS® Disease Analyzer (France, Germany).
Figure 3.
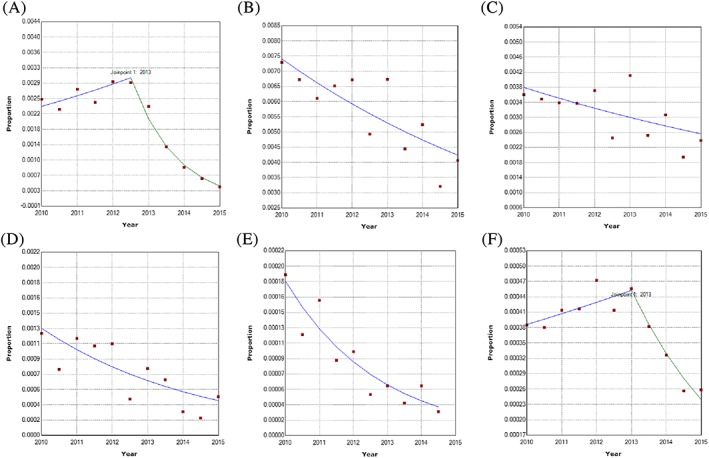
A‐F, Joinpoint analyses of six‐monthly prescribing trends for codeine for treatment of pain in children 0‐11 y in (A) Francea, (B‐D) Germanya, (E) Spain, and (F) the United Kingdom. (A) IMS® France (top left corner). (B) IMS® Germany GP (middle top). (C) IMS® Germany PAED (top right corner). (D) IMS® Germany ENT (bottom left corner). (E) BIFAP (middle bottom). (F) CPRD (bottom right corner). ENT = ear, nose, and throat practice, G = general practice, IMS® = IMS® Disease Analyzer, PAED = paediatric practice, Proportion = proportion of children with a codeine prescription. The dots represent the six‐monthly prevalences. The lines represent the selected joinpoint model. aOwn calculations, based on IMS® Disease Analyzer (France, Germany) [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Use of codeine within 30 days of undergoing TA
In Spain, no child was recorded as having received a prescription for codeine within 30 days of undergoing TA, and in GP practices in Germany, only two children had a prescription for codeine within 30 days of undergoing TA. The proportion of children with a prescription for codeine that had undergone TA within 30 days of the codeine prescription in France, in paediatric and ENT practices in Germany, and in the United Kingdom is shown in Table 4. There seems to be a trend that use of codeine within 30 days of undergoing TA has decreased since 2012, at least in ENT practices in Germany. Only a small proportion (varying from 2% to 7%) of the children that had received codeine within 30 days of undergoing a TA had a diagnosis of sleep apnoea recorded prior to the TA. No child with a diagnosis of sleep apnoea was prescribed codeine within 30 days of the TA after 2013.
Table 4.
Number of children with use of codeine within 30 days of undergoing tonsillectomy or adenoidectomy (TA) (percent of children with a codeine prescription in parenthesis)
| Database (Total No. of Children With a Codeine Prescription) | CPRD (n = 19 591)a | IMS® France (n = 3207)b | IMS® Germany PAED (n = 7574)b | IMS® Germany ENT (n = 333)b |
|---|---|---|---|---|
| All children 0‐17 ya | 429 (2.2%) | 28 (0.9%) | 14 (0.2%) | 35 (10.5%) |
| Children 0‐11 y | 135 (7.5%) | 25 (1.5%) | 14 (0.2%) | 32 (15.6%) |
| Children 12‐17 y | 294 (1.6%) | 3 (0.2%) | 0 (0.0%) | 3 (2.3%) |
| Female children 0‐17 y | 298 (2.3%) | 15 (0.9%) | 8 (0.2%) | 19 (10.2%) |
| Male children 0‐17 y | 131 (1.9%) | 13 (0.9%) | 6 (0.2%) | 16 (11.0%) |
| Oral solution plain codeine | 227 (1.5%) | 21 (1.4%) | 14 (0.2%) | 23 (10.0%) |
| Solid oral formulation plain codeine | 2 (0.1%) | 0 (0.0%) | 0 (0.0%) | 2 (5.0%) |
| Combination with analgesic/NSAID | 132 (16.6%) | 7 (0.4%) | 0 (0.0%) | 10 (14.7%) |
| Combination with analgesic and antihistamine | 73 (1.9%) | NA | NA | NA |
| 1st HY 2010 | 53 (2.1%) | 4 (1.1%) | 3 (0.3%) | 7 (14.9%) |
| 2nd HY 2010 | 59 (2.3%) | 1 (0.3%) | 1 (0.1%) | 5 (12.8%) |
| 1st HY 2011 | 33 (1.3%) | 3 (0.7%) | 2 (0.2%) | 9 (16.7%) |
| 2nd HY 2011 | 61 (2.3%) | 3 (0.8%) | 3 (0.3%) | 6 (11.5%) |
| 1st HY 2012 | 50 (2.0%) | 7 (1.7%) | 3 (0.3%) | 4 (6.6%) |
| 2nd HY 2012 | 52 (2.0%) | 3 (0.7%) | 1 (0.1%) | 2 (7.1%) |
| 1st HY 2013 | 27 (1.2%) | 3 (0.8%) | 1 (0.1%) | 1 (3.2%) |
| 2nd HY 2013 | 40 (1.9%) | 1 (0.4%) | 0 (0.0%) | 1 (4.3%) |
| 1st HY 2014 | 24 (1.3%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) |
| 2nd HY 2014 | 21 (1.1%) | 2 (1.3%) | 0 (0.0%) | 0 (0.0%) |
| 1st HY 2015 | 12 (0.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Note. In BIFAP, no child received codeine within 30 d of undergoing tonsillectomy or adenoidectomy (TA). In IMS® Germany GP, only two children received codeine within 30 d of undergoing TA. Data from BIFAP and IMS® Germany GP are therefore not displayed in the table.
Abbreviations: ENT, ear, nose, and throat practice; GP, general practice; HY, half‐year; IMS®, IMS® Disease Analyzer; NA, not applicable; PAED, paediatric practice.
In CPRD, eight children had a diagnosis of sleep apnoea prior to the TA.
Own calculations, based on IMS® Disease Analyzer (France, Germany). One child in IMS® France, IMS® Germany PAED and IMS® Germany ENT, respectively, had a diagnosis of sleep apnoea prior to the TA.
A total of 8242 TA procedures were identified in ENT practices in Germany. The proportion of children undergoing TA that had received codeine within 30 days decreased over time from 0.4%‐0.6% in 2010‐2011 to 0.1%‐0.3% in 2012‐2013 to 0.0% in 2014‐2015.
Codeine was more often prescribed within the first 7 days of undergoing TA compared with 8 to 30 days after the TA, and up to 30 days before the TA.
3.3. Doses, durations, and use of other analgesics over time
There was no observed decrease in median durations or doses over time and also no evidence of an increase in the proportion of children that had received a prescription for another analgesic within 90 days of the codeine prescription over time; please see Appendix S1 and Tables A1 to A3.
4. DISCUSSION
4.1. Summary of results
The study investigated changes in prescribing of medicines containing codeine for pain in children taking account of RMM introduced in 2013.
A general decrease in prescribing of codeine for treatment of pain over the study period was found in children below the age of 12 years in all of the countries. Changes in prescribing trend that were temporarily compatible with the introduced RMM were identified in France and the United Kingdom, but the wide confidence intervals could not rule out that the rate was declining already before the RMM, eg, at the timepoint when the PRAC review was announced. The decreasing prescribing in children below the age of 12 years in Germany and Spain already before the introduced RMM in Europe may indicate that prescribers were aware of concerns with the use of codeine in children, eg, from earlier WHO or FDA communications or other clinical guidelines that are outside the scope of medicines regulators. Nevertheless, findings suggest an overall more limited impact on prescribing of codeine for pain in children below the age of 12 years during the first 2 years after the RMM in the United Kingdom and in GP and paediatric practices in Germany compared with ENT practices in Germany and practices in France and Spain, based on a total decrease in prescribing from the start to the end of the study period of 33%, 34%, and 44% in the United Kingdom, German GP, and German paediatric practices vs 61%, 84%, and 84% in German ENT practices and French and Spanish practices.
There was a trend that use of codeine within 30 days of undergoing TA decreased since 2012, at least in German ENT practices. Numbers were small, and TA procedures may have been underrecorded in GP and paediatric practices, with some uncertainty around the exact date of the TA procedure.
Children treated for pain with codeine represented only 3.2% of all children (up to 9.3% of children if prescriptions without an identified indication are also allocated to the pain indication) with a prescription for one of the codeine products in Spain compared with 94.7% in France, 51.3% to 71.8% in Germany, and 98.1% in the United Kingdom, which illustrates possible differences in indications for which a drug may be prescribed across countries. On the other hand, treatment of pain was not identified in the same way across all databases, being more stringent in Spain as a result of direct recording of the treatment indication in the BIFAP database. Considering the less strict identification of pain in the other databases, resulting in inclusion of all prescriptions for one of the codeine products except plain codeine prescriptions with an identified diagnosis indicating cough, an apparent lower impact of the RMM might have been expected in France, Germany, and the United Kingdom compared with Spain. However, due to the very small prevalence of use of codeine for pain in children in Spain, there was little power to analyse changes in prescribing over time.
4.2. Feasibility
The study was based on a high‐level common study protocol. Analyses were then customised to the specific databases and health care systems and differed slightly between databases. For example, the indication for treatment was specifically coded and could be obtained directly only in the BIFAP database in Spain, whereas doses and durations were available only in a minority of individual prescriptions in IMS® Germany, making it particularly difficult to estimate the treatment duration in Germany. Considering that the study focused on changes over time rather than absolute values, these slight differences in analyses, while considered acceptable overall, were also acknowledged to make direct comparisons more complex.
4.3. Limitations
In Germany and Spain, prescribing was already decreasing before the introduced RMM, and no changes in prescribing trends associated with the RMM were identified. This might have been due to insufficient power to investigate a step change or steeper decline in an already decreasing prescribing trend. In Spain, power was already low due to a very low baseline level of prescribing. We also included only a limited follow‐up time after the RMM to study changes in prescribing. It is plausible that a longer follow‐up time could have further detected changes in prescribing, linked also to the impact of a second EU‐wide review of codeine for cough and cold in children that started in April 2014 and concluded in March 2015. The outcome of this review was largely in line with the previous recommendations for codeine when used for pain relief.
The purchase of codeine without a prescription (over the counter [OTC] pharmacy sales), which may have taken place in France and the United Kingdom during the study period, could also not be quantified, although the focus of our study was on prescribing by health care professionals. Codeine is now restricted to prescription‐only medicine in France.51 Additionally, hospital use of codeine was not included in the study. This is relevant for underestimation of the results on use of postoperative codeine within 30 days of undergoing TA. Due to differences between databases and health care systems and a stricter definition of use of codeine for treatment of pain in the BIFAP database, the absolute values for prevalence were not comparable. Instead, changes over time were analysed.
Only EU countries with access to EHR databases with information on prescribing could participate in the study. Due to regional differences in prescribing, eg, a predominant prescribing of liquid oral formulation of plain codeine in Germany vs codeine in combination with an analgesic or NSAID in the other countries, findings cannot be extrapolated to countries outside of the study.
4.4. Implications
This study was performed in collaboration between EMA and two NCAs in the context of proactive management of the risks of marketed drugs.27 Existing decreasing trends in prescribing of codeine for treatment of pain in children below the age of 12 years, and in children undergoing TA, at the time of the introduction of RMM are reassuring, even if they may relate to earlier publications or safety communications and cannot be directly attributed to the introduced RMM. Further follow up of the decrease in prescribing may be warranted considering that the RMM aimed not only to decrease but to ultimately eliminate prescribing of codeine for pain in children below the age of 12 years. As codeine has subsequently been contraindicated also for treatment of cough and cold in children below the age of 12 years, a decrease can now be expected for all codeine products and indications. Whether the reduced prescribing of codeine for treatment of pain in children below the age of 12 years was associated with a shift in prescribing to other analgesics or whether prescribing of other analgesics also decreased during the study period was not evaluated. This will instead be addressed in a separate study. The databases included in the study have been found to be broadly representative of their underlying populations,46, 47, 48 comprising a total of around 260 million EU citizens (around 50% of the total EU population), although the exact extent to which prescription estimates from the databases are unbiased in relation to their total underlying population is not known.
A recent systematic review of studies that have measured the impact of regulatory interventions found that only a limited number of regulatory interventions had been evaluated.56 Time series analysis was the most widely used study design (66.0%), including regression‐based approaches to determine statistical significance in 42.5% of all studies with joinpoint regression in only 5.9% of all studies.56
4.5. Conclusion
The current study of the impact of introduced RMM suggested that prescribing of codeine for treatment of pain in children below the age of 12 years decreased over time in all included countries and that in France and the United Kingdom, prescribing changed around the time of the introduced RMM. There was also a more specific suggestion that prescribing in ENT practices in Germany to children undergoing TA decreased after the introduction of the RMM.
ETHICS STATEMENT
The use of data from BIFAP was approved by the AEMPS (Spanish Medicines Agency) as administrator of the database. The use of data from CPRD was approved by the Independent Scientific Advisory Committee for MHRA database research (protocol number: 15_222). The use of data from IMS® Disease Analyzer databases was granted by IMS Health.
CONFLICT OF INTEREST
The authors are employees of the European Medicines Agency or of National Competent Authorities and have no conflicts of interest in relation to this article. Dolores Montero and Julie Williams are members of the PRAC, which developed the risk minimisation measures that were evaluated in the study. No other source of funding was used to assist in the preparation of this article.
DISCLAIMER
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the agencies or organizations with which the authors are affiliated.
Supporting information
Table S1 Median durations in days of codeine for the treatment of pain in children 0‐17 years
Table S2 Median doses in milligram of codeine for the treatment of pain in children 0‐17 years
Table S3 Number of children with prior use of other analgesics within 90 days prior to the codeine prescription (percent of children with a codeine prescription in parenthesis) 1
Hedenmalm K, Blake K, Donegan K, et al. A European multicentre drug utilisation study of the impact of regulatory measures on prescribing of codeine for pain in children. Pharmacoepidemiol Drug Saf. 2019;28:1086–1096. 10.1002/pds.4836
- In June 2013, a series of changes to the marketing authorisation for codeine products in the EU were introduced, including recommendations to not use codeine for treatment of pain in children below the age of 12 years and in children undergoing tonsillectomy or adenoidectomy (TA) due to obstructive sleep apnoea.
- No previously published studies of the impact of introduced risk minimisation measures (RMM) for codeine for treatment of pain in children on the subsequent prescribing of codeine in children in the EU were identified.
- Joinpoint regression analysis is a tool to study drug utilization trends over time and identify time points when significant changes in trend occur.
REFERENCES
- 1. Dean L. In: Pratt V, McLeod H, Dean L, Malheiro A, Rubinstein W, eds. Codeine therapy and CYP2D6 genotype. Bethesda (MD): Medical Genetics Summaries; 2012. [Google Scholar]
- 2. Ingelman‐Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5(1):6‐13. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . Unedited report of the 18th Expert Committee on the Selection and Use of Essential Medicines. Geneva, Switzerland; 2011.
- 4. World Health Organisation . WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses Geneva, Switzerland; 2012. Contract No.: ISBN 978 92 4 154812 0 [PubMed]
- 5. FDA Drug Safety Communication . Codeine use in certain children after tonsillectomy and/or adenoidectomy may lead to rare, but life‐threatening adverse events or death: thasso.com; 2014. [Available from: http://thasso.com/fda‐drug‐safety‐communication‐codeine‐use‐in‐certain‐children‐after‐tonsillectomy‐andor‐adenoidectomy‐may‐lead‐to‐rare‐but‐life‐threatening‐adverse‐events‐or‐death/.
- 6. US Food and Drug Administration . Drug safety communications. Safety review update of codeine use in children; new boxed warning and contraindication on use after tonsillectomy and/or adenoidectomy. US Food and Drug Administration; 2012. [Available from: https://www.fda.gov/downloads/drugs/drugsafety/ucm339116.pdf.
- 7. Fleming ML, Wanat MA. To prescribe codeine or not to prescribe codeine? J Pain Palliat Care Pharmacother. 2014;28(3):251‐254. [DOI] [PubMed] [Google Scholar]
- 8. Friedrichsdorf SJ, Nugent AP, Strobl AQ. Codeine‐associated pediatric deaths despite using recommended dosing guidelines: three case reports. J Opioid Manag. 2013;9(2):151‐155. [DOI] [PubMed] [Google Scholar]
- 9. Racoosin JA, Roberson DW, Pacanowski MA, Nielsen DR. New evidence about an old drug—risk with codeine after adenotonsillectomy. N Engl J Med. 2013;368(23):2155‐2157. [DOI] [PubMed] [Google Scholar]
- 10. FDA . FDA Drug Safety Communication: FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women United States of America, 2017. [Available from: https://www.fda.gov/Drugs/DrugSafety/ucm549679.htm.
- 11. EMA/697484/2012 . Review of codeine‐containing medicines started London, United Kingdom: European Medicines Agency; 2012. [Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Codeine_containing_medicinal_products/Procedure_started/WC500133297.pdf.
- 12. EMEA/H/A‐31/1342 . Assessment report for codeine‐containing medicinal products indicated in the management of pain in children. Committee PRA; 2013. 24 June 2013. Report No.
- 13. EMA/350259/2013 . PRAC recommends restricting the use of codeine when used for pain relief in children London, United Kingdom; 2013. [Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Codeine_containing_medicinal_products/Recommendation_provided_by_Pharmacovigilance_Risk_Assessment_Committee/WC500144445.pdf.
- 14. BfArM . Codeine: genetic polymorphism—paediatric deaths. 2012. [Available from: https://www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/EN/RI/2012/RI‐codeinhaltige‐am‐kinder.html.
- 15. BfArM . Medicinal products containing codeine for treatment of pain in children: key messages from the PRAC for consideration. 2013. [Available from: https://www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/EN/RI/2013/RI‐codeinhaltige‐am‐kinder.html.
- 16. ANSM . Le Comité pour l'Evaluation des Risques en matière de Pharmacovigilance (PRAC) lance une réévaluation du rapport bénéfice/risque des médicaments contenant de la codéine et des médicaments contenant du diclofénac ‐ Retour d'information sur le PRAC. 2012. [Available from: http://ansm.sante.fr/S‐informer/Travaux‐de‐l‐Agence‐Europeenne‐des‐Medicaments‐EMA‐Comite‐pour‐l‐evaluation‐des‐risques‐en‐matiere‐de‐pharmacovigilance‐PRAC/Le‐Comite‐pour‐l‐Evaluation‐des‐Risques‐en‐matiere‐de‐Pharmacovigilance‐PRAC‐lance‐une‐reevaluation‐du‐rapport‐benefice‐risque‐des‐medicaments‐contenant‐de‐la‐codeine‐et‐des‐medicaments‐contenant‐du‐diclofenac‐Retour‐d‐information‐sur‐le‐PRAC.
- 17. ANSM . Médicaments à base de tétrazépam, d'almitrine, de ranélate de strontium et de codéine (chez l'enfant) ‐ Retour d'information sur le PRAC. 2013. [Available from: http://ansm.sante.fr/S‐informer/Travaux‐de‐l‐Agence‐Europeenne‐des‐Medicaments‐EMA‐Comite‐pour‐l‐evaluation‐des‐risques‐en‐matiere‐de‐pharmacovigilance‐PRAC/Medicaments‐a‐base‐de‐tetrazepam‐d‐almitrine‐de‐ranelate‐de‐strontium‐et‐de‐codeine‐chez‐l‐enfant‐Retour‐d‐information‐sur‐le‐PRAC.
- 18. ANSM . Médicaments contenant du diclofénac, de l'hydroxyéthylamidon, de la codéine (pour l'enfant) et solutions pour nutrition parentérale pour prématurés: Avis et recommandations du PRAC. 2013. [Available from: http://ansm.sante.fr/var/ansm_site/storage/original/application/7fdcee31af258aed8eccb755ad52e443.pdf.
- 19. ANSM . Médicaments à base de tétrazépam, d'almitrine, de ranélate de strontium et de codéine (chez l'enfant): avis et recommandations du PRAC ‐ Communiqué de l'EMA. 2013. [Available from: http://ansm.sante.fr/var/ansm_site/storage/original/application/a8691b2745368aa4073132f117ba1efc.pdf.
- 20. MHRA . Codeine‐containing pain relief in children. Safety review initiated following post‐surgical fatalities in ultra‐rapid metabolisers. 2012. [Available from: https://www.gov.uk/drug‐safety‐update/codeine‐containing‐pain‐relief‐in‐children.
- 21. MHRA . Codeine for analgesia: restricted use in children because of reports of morphine toxicity 2013. Available from: https://www.gov.uk/drug‐safety‐update/codeine‐for‐analgesia‐restricted‐use‐in‐children‐because‐of‐reports‐of‐morphine‐toxicity.
- 22. AEMPS . Codeína: restricciones de uso como analgésico en pediatría. 2013. Available from: https://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2013/NI‐MUH_FV_17‐2013‐codeina.htm.
- 23. Royal College of Anaesthetists . Guidance for the administration of codeine and alternative opioid analgesics in children United Kingdom. 2013. Available from: https://www.rcoa.ac.uk/system/files/CodeineGuidance2013.pdf.
- 24. Kvalitetsregistret för tonsilloperation . Nationella riktlinjer för farmakologisk behandling av smärta och illamående i samband med tonsillotomi och tonsillektomi på barn och ungdomar (< 18 år) Sweden. 2013. Available from: https://registercentrum.blob.core.windows.net/ton/r/Nationella‐riktlinjer‐f‐r‐sma‐rtlindring‐vid‐tonsilloperation‐ByUdVd6c.pdf.
- 25. Constant I, Ayari Khalfallah S, Brunaud A, et al. How to replace codeine after tonsillectomy in children under 12 years of age? Guidelines of the French Oto‐Rhino‐Laryngology—Head and Neck Surgery Society (SFORL). Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(4):233‐238. [DOI] [PubMed] [Google Scholar]
- 26. EU collaboration strengthens safety monitoring of medicines . European Commission publishes three‐year report on implementation of pharmacovigilance legislation [press release]. United Kingdom. 2016.
- 27. Arlett P, Portier G, de Lisa R, et al. Proactively managing the risk of marketed drugs: experience with the EMA Pharmacovigilance Risk Assessment Committee. Nat Rev Drug Discov. 2014;13(5):395‐397. [DOI] [PubMed] [Google Scholar]
- 28. EMA/508487/2014 . Draft Reflection paper on a strategy for best evidence. Framework for EU regulatory network to conduct research. London, United Kingdom: European Medicines Agency; 2015:1‐4. [Google Scholar]
- 29. Chua KP, Shrime MG, Conti RM. Effect of FDA investigation on opioid prescribing to children after tonsillectomy/adenoidectomy. Pediatrics. 2017;140(6):e20171765. [DOI] [PubMed] [Google Scholar]
- 30. Goldman JL, Ziegler C, Burckardt EM. Otolaryngology practice patterns in pediatric tonsillectomy: the impact of the codeine boxed warning. Laryngoscope. 2018;128(1):264‐268. [DOI] [PubMed] [Google Scholar]
- 31. Fredheim OMS, Skurtveit S. Provision of analgesics to children before and after the new recommendations on codeine. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke. 2017;137(12‐13):881‐884. [DOI] [PubMed] [Google Scholar]
- 32. Alm F, Jaensson M, Lundeberg S, Ericsson E. Adherence to Swedish guidelines for pain treatment in relation to pediatric tonsil surgery: a survey of the multidisciplinary team. Int J Pediatr Otorhinolaryngol. 2017;101:123‐131. [DOI] [PubMed] [Google Scholar]
- 33. He T, Lardieri AB, Morgan JA. Pharmacist and pediatrician knowledge of codeine use in children. J Pediatr Pharmacol Therapeut. 2018;23(4):293‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chidambaran V, Sadhasivam S, Mahmoud M. Codeine and opioid metabolism: implications and alternatives for pediatric pain management. Curr Opin Anaesthesiol. 2017;30(3):349‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan KW, Martin S, Bell G. Paediatric codeine use after adenotonsillectomy. Anaesthesia. 2014;69(10):1179‐1180. [DOI] [PubMed] [Google Scholar]
- 36. Yellon RF, Kenna MA, Cladis FP, McGhee W, Davis PJ. What is the best non‐codeine postadenotonsillectomy pain management for children? Laryngoscope. 2014;124(8):1737‐1738. [DOI] [PubMed] [Google Scholar]
- 37. Palanisamy A, Bailey CR. Codeine in mothers and children: where are we now? Anaesthesia. 2014;69(7):655‐660. [DOI] [PubMed] [Google Scholar]
- 38. Woolf AD, Greco C. Why can't we retire codeine? Pediatrics. 2014;133(5):e1354‐e1355. [DOI] [PubMed] [Google Scholar]
- 39. Magos TA, Syed MI, Montague ML. Re: More codeine fatalities after tonsillectomy in North American children: time to revise prescribing practice! Clin Otolaryngol. 2014;39(1):69. [DOI] [PubMed] [Google Scholar]
- 40. Benini F, Barbi E. Doing without codeine: why and what are the alternatives? Ital J Pediatr. 2014;40(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anderson BJ. Is it farewell to codeine? Arch Dis Child. 2013;98(12):986‐988. [DOI] [PubMed] [Google Scholar]
- 42. Tremlett MR. Wither codeine? Paediatr Anaesth. 2013;23(8):677‐683. [DOI] [PubMed] [Google Scholar]
- 43. Wong C, Lau E, Palozzi L, Campbell F. Pain management in children: part 2—a transition from codeine to morphine for moderate to severe pain in children. Canadian Pharm J/Revue des Pharmaciens du Canada. 2012;145(6):276‐9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong C, Lau E, Palozzi L, Campbell F. Pain management in children: part 1—pain assessment tools and a brief review of nonpharmacological and pharmacological treatment options. Canadian Pharm J/Revue des Pharmaciens du Canada. 2012;145(5):222‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salvador Rosa A, Moreno Perez JC, Sonego D, Garcia Rodriguez LA, de Abajo Iglesias FJ. The BIFAP project: database for pharmaco‐epidemiological research in primary care. Aten Primaria. 2002;30(10):655‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Information provided by IQVIA/IMS Health. 2016.
- 48. Becher H, Kostev K, Schroder‐Bernhardi D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47(10):617‐626. [DOI] [PubMed] [Google Scholar]
- 49. European Medicines Agency . Restrictions on use of codeine for pain relief in children—CMDh endorses PRAC recommendation London, United Kingdom, 2013. [Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/06/news_detail_001829.jsp&mid=WC0b01ac058004d5c1.
- 50. Annex IV. Timetable for the implementation of the agreement Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Codeine_containing_medicinal_products/Position_provided_by_CMDh/WC500147069.pdf.
- 51. Cracknell C. Codeine now restricted to prescription‐only. The Connexion. 2017. 13 July 2017;Sect. French News.
- 52. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335‐351. [DOI] [PubMed] [Google Scholar]
- 53. National Cancer Institute . Joinpoint Regression Program, version 4.5.0.1 ‐ June 2017. 2017.
- 54. Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62(3):847‐854. [DOI] [PubMed] [Google Scholar]
- 55. Bedson J, Belcher J, Martino OI, et al. The effectiveness of national guidance in changing analgesic prescribing in primary care from 2002 to 2009: an observational database study. Eur J Pain (London, England). 2013;17(3):434‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goedecke T, Morales DR, Pacurariu A, Kurz X. Measuring the impact of medicines regulatory interventions—systematic review and methodological considerations. Br J Clin Pharmacol. 2018;84(3):419‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Median durations in days of codeine for the treatment of pain in children 0‐17 years
Table S2 Median doses in milligram of codeine for the treatment of pain in children 0‐17 years
Table S3 Number of children with prior use of other analgesics within 90 days prior to the codeine prescription (percent of children with a codeine prescription in parenthesis) 1


