Abstract
The development of methods to detect damage in macromolecular materials is of paramount importance to understand their mechanical failure and the structure–property relationships of polymers. Mechanofluorophores are useful and sensitive molecular motifs for this purpose. However, to date, tailoring of their optical properties remains challenging and correlating emission intensity to force induced material damage and the respective events on the molecular level is complicated by intrinsic limitations of fluorescence and its detection techniques. Now, this is tackled by developing the first stress‐sensing motif that relies on photon upconversion. By combining the Diels–Alder adduct of a π‐extended anthracene with the porphyrin‐based triplet sensitizer PtOEP in polymers, triplet–triplet annihilation photon upconversion of green to blue light is mechanochemically activated in solution as well as in the solid state.
Keywords: mechanochemistry, pericyclic reactions, photon upconversion, polymers, triplet–triplet annihilation
The optical detection of damage and imminent failure in macromolecular materials would be a convenient method to implement early warning systems but also to understand the origin of their mechanical properties in greater detail to develop strategies for polymer materials with further improved mechanical properties.1, 2, 3 To date, a large variety of mechanochromophores and mechanofluorophores, which alter either their absorption or emission properties upon the application of mechanical stress, have been designed and serve as sensitive molecular‐scale force probes.4, 5, 6, 7, 8 Yet, the continuous development of new mechanophores and their respective detection methods are of paramount importance to correlate the force‐induced molecular changes and their contribution to macroscopic failure of the material with the externally applied force qualitatively and eventually also quantitatively. Popular molecular scaffolds comprise, amongst others, of spiropyran9, 10, 11, 12 and its rhodamine derivatives,13, 14, 15 Diels–Alder adducts with latent anthracenes,16, 17, 18, 19, 20, 21 persistent delocalized radicals,22, 23, 24, 25, 26 and aggregachromic dyes.27, 28, 29
Among these, fluorophores that “turn‐on” upon mechanical force can be considered the most sensitive. They reach their full potential at relatively low concentrations owing to the intrinsically good signal‐to‐noise ratio of fluorescence measurements. This allows to apply only minute amounts of mechanophore, leaving the parent material under investigation, and its mechanical properties, widely unaltered.30 However, at such low mechanophore concentrations not all critical events contributing to damage and failure will be detected. Increasing the fluorophore concentrations would lead to better volume coverage inside of the host material for stress detection, but self‐quenching of more concentrated mechanophores in combination with the strong attenuation of the excitation beam complicate the quantitative relationship between measured fluorescence signal and the number of force‐induced events.31
To overcome this challenge, we propose a new method that intrinsically uses comparably high fluorophore concentrations. We make use of triplet–triplet annihilation photon upconversion (TTA‐UC) as a new stress sensing technique that can be read out using the same technique as for other photoluminescent mechanophores, however, with improved volume coverage inside the host material.32, 33, 34 TTA‐UC relies on the energy transfer between two annihilating fluorophores in the triplet state creating one emissive singlet state fluorophore. This bimolecular photoreaction benefits from high reactant concentrations. Recently, we reported poly‐ (methyl acrylate) (PMA) substituted Diels–Alder adduct 2, which undergoes retro Diels–Alder reaction under application of force, yielding blue fluorescent PMA‐substituted anthracene 1 (Scheme 1 a).20 As 9‐phenylethynylanthracene is a well‐known emitter in TTA‐UC systems,35 we intended to investigate this technique for stress sensing in macromolecular systems. To the best of our knowledge, this stress‐responsive anti‐Stokes shift TTA‐UC system is unprecedented. As the dynamic range of emitter concentrations measurable with regular fluorescence techniques is limited by self‐quenching and the strong attenuation of the excitation beam when reaching the 10−5 m concentration regime, we believe that our approach will allow a more facile correlation of mechanochemical events with the emission signal.
Scheme 1.
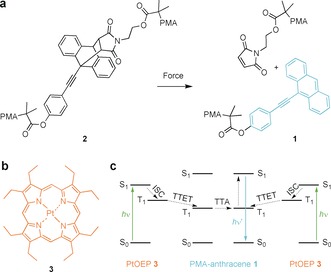
a) Mechanochemical scission of PMA‐substituted anthracene–maleimide mechanophore 2 to yield PMA–anthracene 1. b) Triplet photosensitizer PtOEP 3 employed in this study. c) Reduced Jablonski diagram of the TTA‐UC process employing 1 as annihilator and emitter and 3 as triplet photosensitizer.
TTA‐UC requires a triplet photosensitizer with a slightly higher triplet state energy than that of the emitter. For this purpose, we relied on commercially available and widely investigated platinum octaethylporphyrin (PtOEP) 3 (Scheme 1 b).36, 37 PtOEP shows good intersystem crossing efficiency owing to high spin–orbit coupling induced by the heavy Pt atom with a S0→T1 triplet energy of 1.921 eV.38, 39 Concomitantly, p‐methoxyphenylethynylanthracene (PMPEA) has a slightly lower triplet energy of S0→T1 of 1.621 eV, as calculated by time‐dependent DFT using B3LYP with a PCSEG‐1 basis set. After initial excitation with green light, PtOEP 3 undergoes intersystem crossing (ISC) to its T1 state, which is then transferred to the lower‐lying T1 state of PMA‐anthracene 1 (TTET). Two T1 anthracenes then perform TTA creating one S1 state that decays under the emission of blue light (Scheme 1 c). The absorption as well as emission spectra of all participating species are depicted in Figure 1 highlighting their photophysical compatibility. Latent PMA precursor 2 bearing the Diels–Alder adduct in its center was synthesized according to procedures established by us earlier with Mn=63.9 kDa and ĐM=1.17 through Cu0‐mediated controlled radical polymerization from the respective bifunctional initiator.20
Figure 1.
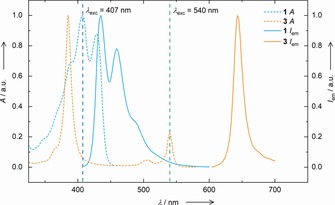
Normalized absorbance (dashed) and emission (solid) spectra of the donor PtOEP 3 (orange) and acceptor reference PMPEA (blue). Also depicted are excitation wavelengths used for TTA‐UC (λ exc=540 nm) and regular fluorescence (λ exc=407 nm).
Initially, we optimized the parameters crucial to the TTA‐UC process employing PMPEA (as a small molecule reference to the polymer‐terminated chromophore 1) and PtOEP 3 in deoxygenated toluene. For this purpose, we conducted TTA‐UC under constant PtOEP 3 concentration with varying PMPEA content (Supporting Information, Figure S1) and vice versa (Supporting Information, Figure S2). Furthermore, excitation intensity‐dependent TTA‐UC was performed (Supporting Information, Figure S3) confirming the non‐linear dependency of the emission intensity from the excitation energy. This non‐linearity is characteristic for the multimolecular TTA process (Supporting Information, Figure S4). After optimization, we determined an effective upconversion quantum yield of Φ TTA‐UC=0.102 % as a relative value compared to the fluorescence emission of Rhodamine B in ethanol.40 Afterwards, we employed PMA‐anthracene 1 instead of small molecule PMPEA adjusting the conditions once more yielding an optimized Φ TTA‐UC=0.0823 % (Supporting Information, Figure S5). The slight decrease in upconversion quantum yield in comparison to the small molecule is intuitive, as the TTA process is diffusion controlled and the attachment of a polymer chain to the emitter reduces its diffusivity in addition to diminishing bimolecular collisions due to statistical screening of the anthracene by the polymer random coil.
Mechanochemical activation of the TTA‐UC process was performed by irradiation of a toluene solution containing polymer 2 and PtOEP 3 with ultrasound (f=20 kHz) exploiting the shear effects induced by collapsing cavitation bubbles of the solvent.41 Samples were taken from this solution at different time intervals and subjected to gel permeation chromatography (GPC) analysis and UV/Vis as well as fluorescence spectroscopy. GPC measurements revealed that over the course of the ultrasonication, the initial signal with a peak molar mass of about 60 kDa had disappeared while a new signal with a peak molar mass approximately half of the initial signal of about 30 kDa had emerged suggesting the force‐induced rupturing of the chains at the central site (Figure 2 a). The selectivity of these cleaving events was confirmed by UV/Vis and fluorescence spectroscopy, generating the typical and expected increase in absorption (Supporting Information, Figure S6) as well as fluorescence (Supporting Information, Figure S7) of anthracene 1. From these absorption measurements, and by employing the molar absorptivity ϵ=17.900 L mol−1 cm−1 of small molecule PMPEA at 407 nm (Supporting Information, Table S1), we then calculated the molar concentrations of PMA‐anthracene 1 as a function of the ultrasonication time corresponding to ca. 68 % conversion of 2 to 1 after 360 min of ultrasonication (Figure 2 b).
Figure 2.
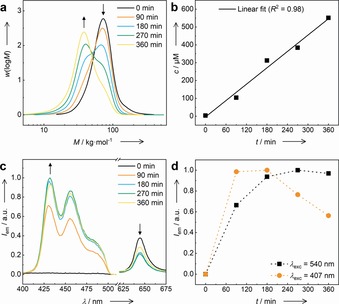
Time snapshots during the mechanochemical generation of PMA‐anthracene 1 by irradiation of polymer 2 with ultrasound in toluene solution (ρ=52 mg mL−1, c=8.14×10−4 m). a) GPC traces. b) Concentration c of free 1 calculated by UV/Vis absorption spectroscopy. c) Normalized TTA upconversion emission spectra (λ exc=540 nm) with intensities increasing for 1 and decreasing for PtOEP 3 (c PtOEP=8.8×10−6 m). d) Normalized emission intensity at λ em=456 nm measured in regular fluorescence (λ exc=407 nm) and TTA‐UC (λ exc=540 nm) mode.
Subsequently, we performed TTA‐UC measurements using green light at λ exc=540 nm at different points in time during the mechanochemical cleaving experiment. As Diels–Alder adduct 2 is optically silent and does not emit owing to its interrupted π‐system, the upconversion process cannot proceed in this structure. Consequently, no emission was measured while performing the TTA‐UC experiment before the start of ultrasonication (Figure 2 c). However, with longer ultrasonication times, and therefore increasing concentration of 1, the upconverted emission intensity increased and the PtOEP emission intensity decreased, highlighting the successful activation of the TTA‐UC process by mechanical force.
Though photon upconversion processes naturally proceed with a considerably lower quantum yield compared to regular fluorescence (Φ TTA‐UC=0.0823 % vs. Φ F=72 %), the advantage of this method becomes obvious when considering the dynamic range of concentrations that yield a proportional signal to the measured emission (Figure 2 d). With increasing ultrasonication time, the proportionality between the concentration of mechanically generated fluorophores and the conventional fluorescence output is not warranted anymore, owing to self‐quenching and the strong attenuation of the excitation beam, when reaching the 10−5 m concentration regime. Conversely, the TTA‐UC intensity, though lower in absolute terms, shows predictable emission behavior scaling with the emitter concentration, as the attenuation of the excitation beam by PtOEP remains constant and low at the employed concentrations. Though the interrelation between emission and fluorophore concentration is complicated on a mechanistic molecular level, regimes of 10−4 m and above become accessible for stress sensing by TTA‐UC under a fixed set of conditions and by generating a calibration curve.
As the detection of forces is generally more relevant in set or arrested materials, we hereafter evaluated our TTA‐UC system in the solid state. We prepared a rubbery poly(hexyl methacrylate) (PHMA) network by free radical polymerization incorporating the anthracene‐maleimide mechanophore as a dimethacrylate crosslinker (1 mol %). This means that each connecting point of the network is occupied by a mechanophore, as we expect the network to cleave at these points, rather than in the more flexible polymer chains between crosslinks. This material was fractured by uniaxial compression with a pellet press leading to irreversible damage to the network structure. The change in fluorescence was readily observed when the samples were irradiated with a UV hand lamp (Figure 3 a,b). To assess the system's TTA‐UC propensity, the triplet sensitizer was incorporated by diffusion by swelling the network in a PtOEP solution (ρ=15 mg mL−1) followed by drying and deoxygenation before fracture yielding an estimated bulk concentration of PtOEP 3 of 0.04 w%. Upon irradiating the fractured sample with a green DPSS laser (50 mW, λ exc=532 nm), the blue UC emission is readily recorded demonstrating that the TTA‐UC mechanism for stress sensing operates properly even in the solid state (Figure 3 c; Supporting Information, Figure S10). Note that swelling the PHMA networks in solvent did not result in mechanophore activation (Supporting Information, Figure S8). In contrast to previous examples where such an effect was observed,42, 43 we attribute the lack of swelling‐activation to the use of rubbery PHMA exhibiting high chain mobility and the higher mechanochemical activation energy of 2 in comparison to dioxetane and spiropyran mechanophores employed in these studies. Furthermore, reference experiments employing blended and thus not covalently anchored mechanophore, as well as mechanophore terminally anchored only on one side confirmed the mechanochemical origin of the observed results (Supporting Information, Figures S9, S11, and S12).
Figure 3.
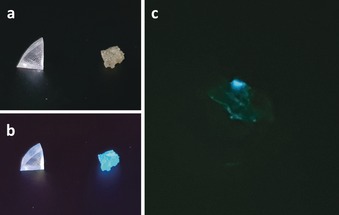
Photographs of the non‐fractured (left) and fractured (right) PHMA network containing mechanophore crosslinker (1 mol %) a) under daylight and b) irradiated with a UV hand lamp (λ exc=365 nm). c) Photograph of a similar fractured PHMA network blended by swelling in a PtOEP solution (15 mg mL−1). The sample was irradiated with a green DPSS laser (50 mW, λ exc=532 nm) and blue UC emission recorded through a 500 nm shortpass filter.
In summary, we presented herein an unprecedented mechanoresponsive TTA‐UC system based on the Diels–Alder adduct of a π‐extended anthracene and maleimide that was blended with PtOEP triplet sensitizer. We utilized this system to detect damage to polymers in solution as well as in the solid state by reading out upconverted blue fluorescence from fractured polymer excited with green light. Hence, we established a unique system capable of anti‐Stokes stress sensing in non‐UV transparent materials. Importantly, by employing this method, the proportionality between fluorophore concentration and output signal is warranted for higher emitter concentrations compared to regular fluorescence. This consequently enhances the dynamic range of available concentrations for stress sensing using mechanofluorophores.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
R.G. and C.B. are grateful for support by a Freigeist‐Fellowship of the Volkswagen Foundation. A.H. acknowledges generous support from the European Union (European Research Council Advanced Grant SUPRABIOTICS No. 694610). Parts of the analytical investigations were performed at the Center for Chemical Polymer Technology CPT, which was supported by the European Commission and the federal state of North Rhine‐Westphalia (Grant no. 300088302). Financial support is acknowledged from the European Commission (EUSMI, 731019).
D. Yildiz, C. Baumann, A. Mikosch, A. J. C. Kuehne, A. Herrmann, R. Göstl, Angew. Chem. Int. Ed. 2019, 58, 12919.
References
- 1. Roberts D. R. T., Holder S. J., J. Mater. Chem. 2011, 21, 8256–8268. [Google Scholar]
- 2. Ciardelli F., Ruggeri G., Pucci A., Chem. Soc. Rev. 2013, 42, 857–870. [DOI] [PubMed] [Google Scholar]
- 3. Jacobs M. J., Blank K., Chem. Sci. 2014, 5, 1680–1697. [Google Scholar]
- 4. Pucci A., Ruggeri G., J. Mater. Chem. 2011, 21, 8282–8291. [Google Scholar]
- 5. Chi Z., Zhang X., Xu B., Zhou X., Ma C., Zhang Y., Liu S., Xu J., Chem. Soc. Rev. 2012, 41, 3878–3896. [DOI] [PubMed] [Google Scholar]
- 6. Ma Z., Wang Z., Teng M., Xu Z., Jia X., ChemPhysChem 2015, 16, 1811–1828. [DOI] [PubMed] [Google Scholar]
- 7. Yuan Y., Yuan W., Chen Y., Sci. China Mater. 2016, 59, 507–520. [Google Scholar]
- 8. Calvino C., Neumann L., Weder C., Schrettl S., J. Polym. Sci. Part A 2017, 55, 640–652. [Google Scholar]
- 9. Davis D. A., Hamilton A., Yang J., Cremar L. D., Van Gough D., Potisek S. L., Ong M. T., Braun P. V., Martínez T. J., White S. R., et al., Nature 2009, 459, 68–72. [DOI] [PubMed] [Google Scholar]
- 10. Li J., Nagamani C., Moore J. S., Acc. Chem. Res. 2015, 48, 2181–2190. [DOI] [PubMed] [Google Scholar]
- 11. Gossweiler G. R., Kouznetsova T. B., Craig S. L., J. Am. Chem. Soc. 2015, 137, 6148–6151. [DOI] [PubMed] [Google Scholar]
- 12. Chen H., Yang F., Chen Q., Zheng J., Adv. Mater. 2017, 29, 1606900. [DOI] [PubMed] [Google Scholar]
- 13. Teng M.-J., Jia X.-R., Chen X.-F., Wei Y., Angew. Chem. Int. Ed. 2012, 51, 6398–6401; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 6504–6507. [Google Scholar]
- 14. Ma Z., Teng M., Wang Z., Yang S., Jia X., Angew. Chem. Int. Ed. 2013, 52, 12268–12272; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 12494–12498. [Google Scholar]
- 15. Wang Z., Ma Z., Wang Y., Xu Z., Luo Y., Wei Y., Jia X., Adv. Mater. 2015, 27, 6469–6474. [DOI] [PubMed] [Google Scholar]
- 16. Song Y.-K., Lee K.-H., Hong W.-S., Cho S.-Y., Yu H.-C., Chung C.-M., J. Mater. Chem. 2012, 22, 1380–1386. [Google Scholar]
- 17. Konda S. S. M., Brantley J. N., Varghese B. T., Wiggins K. M., Bielawski C. W., Makarov D. E., J. Am. Chem. Soc. 2013, 135, 12722–12729. [DOI] [PubMed] [Google Scholar]
- 18. Church D. C., Peterson G. I., Boydston A. J., ACS Macro Lett. 2014, 3, 648–651. [DOI] [PubMed] [Google Scholar]
- 19. Li J., Shiraki T., Hu B., Wright R. A. E., Zhao B., Moore J. S., J. Am. Chem. Soc. 2014, 136, 15925–15928. [DOI] [PubMed] [Google Scholar]
- 20. Göstl R., Sijbesma R. P., Chem. Sci. 2016, 7, 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li H., Göstl R., Delgove M., Sweeck J., Zhang Q., Sijbesma R. P., Heuts J. P. A., ACS Macro Lett. 2016, 5, 995–998. [DOI] [PubMed] [Google Scholar]
- 22. Imato K., Irie A., Kosuge T., Ohishi T., Nishihara M., Takahara A., Otsuka H., Angew. Chem. Int. Ed. 2015, 54, 6168–6172; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 6266–6270. [Google Scholar]
- 23. Imato K., Kanehara T., Ohishi T., Nishihara M., Yajima H., Ito M., Takahara A., Otsuka H., ACS Macro Lett. 2015, 4, 1307–1311. [DOI] [PubMed] [Google Scholar]
- 24. Verstraeten F., Göstl R., Sijbesma R. P., Chem. Commun. 2016, 52, 8608–8611. [DOI] [PubMed] [Google Scholar]
- 25. Ishizuki K., Aoki D., Goseki R., Otsuka H., ACS Macro Lett. 2018, 7, 556–560. [DOI] [PubMed] [Google Scholar]
- 26. Ishizuki K., Oka H., Aoki D., Goseki R., Otsuka H., Chem. Eur. J. 2018, 24, 3170–3173. [DOI] [PubMed] [Google Scholar]
- 27. Löwe C., Weder C., Adv. Mater. 2002, 14, 1625–1629. [Google Scholar]
- 28. Lavrenova A., Balkenende D. W. R., Sagara Y., Schrettl S., Simon Y. C., Weder C., J. Am. Chem. Soc. 2017, 139, 4302–4305. [DOI] [PubMed] [Google Scholar]
- 29. Calvino C., Guha A., Weder C., Schrettl S., Adv. Mater. 2018, 30, 1704603. [DOI] [PubMed] [Google Scholar]
- 30. Göstl R., Clough J. M., Sijbesma R. P., Mechanochemistry in Materials, Royal Society Of Chemistry, London, 2017, pp. 53–75. [Google Scholar]
- 31. Lakowicz J. R., Principles of Fluorescence Spectroscopy, Springer, New York, 2006. [Google Scholar]
- 32. Cheng Y. Y., Khoury T., Clady R. G. C. R., Tayebjee M. J. Y., Ekins-Daukes N. J., Crossley M. J., Schmidt T. W., Phys. Chem. Chem. Phys. 2010, 12, 66–71. [DOI] [PubMed] [Google Scholar]
- 33. Singh-Rachford T. N., Castellano F. N., Coord. Chem. Rev. 2010, 254, 2560–2573. [Google Scholar]
- 34. Zhao J., Ji S., Guo H., RSC Adv. 2011, 1, 937–950. [Google Scholar]
- 35. Huang L., Zhao Y., Zhang H., Huang K., Yang J., Han G., Angew. Chem. Int. Ed. 2017, 56, 14400–14404; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 14592–14596. [Google Scholar]
- 36. Gray V., Dzebo D., Lundin A., Alborzpour J., Abrahamsson M., Albinsson B., Moth-Poulsen K., J. Mater. Chem. C 2015, 3, 11111–11121. [Google Scholar]
- 37. Chidanguro T., Blank D. R., Garrett A., Reese C. M., Schekman J. M., Yu X., Patton D. L., Ayres N., Simon Y. C., Dalton Trans. 2018, 47, 8663–8669. [DOI] [PubMed] [Google Scholar]
- 38. Merkel P. B., Dinnocenzo J. P., J. Lumin. 2009, 129, 303–306. [Google Scholar]
- 39. Tanaka K., Inafuku K., Chujo Y., Chem. Commun. 2010, 46, 4378–4380. [DOI] [PubMed] [Google Scholar]
- 40. Brouwer A. M., Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar]
- 41. Cravotto G., Gaudino E. C., Cintas P., Chem. Soc. Rev. 2013, 42, 7521–7534. [DOI] [PubMed] [Google Scholar]
- 42. Lee C. K., Diesendruck C. E., Lu E., Pickett A. N., May P. A., Moore J. S., Braun P. V., Macromolecules 2014, 47, 2690–2694. [Google Scholar]
- 43. Clough J. M., van der Gucht J., Sijbesma R. P., Macromolecules 2017, 50, 2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


