Abstract
ATP is known to act as an extracellular messenger mediating the propagation of Ca2+ waves in astrocyte networks. ATP mediates Ca2+ waves by activating P2Y purinoceptors, which mobilize intracellular Ca2+ in astrocytes. A number of P2Y purinoceptor subtypes have been discovered, but it is not known which P2Y subtypes participate in transmitting astrocyte Ca2+ waves. Here, we show that ATP analogs that are selective agonists for the P2Y1 subtype of purinoceptor caused release of intracellular Ca2+ in astrocytes from the dorsal spinal cord. The Ca2+ responses were blocked by adenosine-3′-phospho-5′-phosphosulfate, an antagonist known to selectively inhibit P2Y1 but not other P2Y purinoceptor subtypes. Also, we show that P2Y1 mRNA is expressed in dorsal spinal cord astrocytes. Furthermore, expression of P2Y1 in an astrocytoma cell line lacking endogenous purinoceptors was sufficient to permit propagation of intercellular Ca2+ waves. Finally, Ca2+ wave propagation in dorsal spinal cord astrocytes was suppressed by pharmacologically blocking P2Y1 purinoceptors. Together, these results indicate that dorsal spinal astrocytes express functional P2Y1 purinoceptors, which participate in the transmission of Ca2+ waves. Ca2+waves in astrocytes have been implicated as a major signaling pathway coordinating glial and neuronal activity; therefore, P2Y1purinoceptors may represent an important link in cell–cell signaling in the CNS.
Keywords: purinoceptors, astrocytes, fura-2, dorsal spinal cord, Ca2+ waves, ATP
Astrocytes are the predominant cell type in the CNS, outnumbering neurons by a factor of ∼10:1 (Kuffler, 1984). Astrocytes respond to a variety of extracellular stimuli, including ATP and other nucleotides, by raising the intracellular concentration of Ca2+([Ca2+]i). Increased [Ca2+]imodulates various intracellular processes in astrocytes, including differentiation, cytoskeletal reorganization, and the secretion of neuroactive substances (Verkhratsky and Kettenmann, 1996). A rise in [Ca2+]i localized to one part of an astrocyte may propagate throughout the entire cell, and Ca2+ responses may be transmitted from one astrocyte to others, leading to regenerative Ca2+ waves that spread within astrocyte networks (Cornell-Bell et al., 1990; Dani and Smith, 1995; Newman and Zahs, 1997). There is emerging evidence that Ca2+ signaling in astrocytes is a means for information encoding and transmission, which is complementary to and interacts with electrical signaling in neurons (Parpura et al., 1994; Newman and Zahs, 1998; Araque et al., 1999).
Ca2+ signals could conceivably be transmitted from one astrocyte to another via gap junctions (Boitano et al., 1992). However, transmission of Ca2+waves is unaffected by selectively blocking gap junctional coupling in astrocyte cultures (Guan et al., 1997), and Ca2+ waves propagate between astrocytes in the retina, although there is little gap junctional coupling (Feller et al., 1996). Moreover, Ca2+ waves can be transmitted between astrocytes in vitro that are physically separate from one another (Hassinger et al., 1996; Guthrie et al., 1999). Therefore, there must be an alternative mechanism for transmitting Ca2+ signals, such as via a diffusible extracellular messenger. It is known that ATP evokes Ca2+ responses in astrocytes (Salter and Hicks, 1994), and recently, it has been found that ATP is released during Ca2+ wave propagation and that propagation of Ca2+ waves is blocked by broad spectrum antagonists of receptors activated by ATP (Cotrina et al., 1998; Guthrie et al., 1999), i.e., P2Y purinoceptors. Together, these findings indicate that ATP is the diffusible messenger responsible for transmitting Ca2+ waves between astrocytes.
An important unresolved question in previous studies is which subtype of P2Y purinoceptor mediates propagation of Ca2+ waves. P2Y purinoceptors comprise a multigene family in which five bona fide subtypes, P2Y1, P2Y2,P2Y4, P2Y6, and P2Y11, have been identified (Ralevic and Burnstock, 1998). ATP is known to activate a number of these subtypes. Moreover, the antagonists that have been used to inhibit Ca2+ wave propagation do not distinguish between P2Y purinoceptor subtypes (Guthrie et al., 1999; John et al., 1999). Therefore, the P2Y purinoceptor subtype, or subtypes, mediating Ca2+ wave transmission could not be determined. In the present study, we investigated P2Y-mediated Ca2+ signaling and Ca2+ wave propagation in astrocytes using cultures of dorsal spinal cord. These astrocytes express two pharmacologically distinguishable subtypes of P2Y purinoceptors (Ho et al., 1995). We show by pharmacological and molecular methods that one of the receptors is the P2Y1 subtype of purinoceptor and that P2Y1 purinoceptors participate in the transmission of Ca2+waves between astrocytes.
MATERIALS AND METHODS
Primary culture of dorsal spinal cord. Primary dissociated cultures of dorsal spinal cord were prepared from embryonic day 17 (E17) to E18 rats and maintained as described in detail previously (Salter and Hicks, 1994). Briefly, timed pregnant Wistar rats were anesthetized, and embryos were removed surgically. The spinal cord was extracted from each embryo, and the dura was removed. Dorsal horn tissue was isolated according to the open-book technique (Peterson and Crain, 1982). The dorsal half of the cord was then incubated in 0.25% trypsin for 30 min, rinsed, mechanically dissociated by trituration, and then plated onto collagen-coated plastic disks affixed over holes in 35 mm culture dishes. Cells were maintained in DMEM (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS) and 10% horse serum for 1 week. After 1 week, the media was switched to DMEM plus 10% horse serum. Cells were used at 12–15 d in culture.
Generation and maintenance of 1321N1 human astrocytoma cells stably expressing P2Y1. Rat P2Y1(rP2Y1) purinoceptor cDNA (GenBank accession number U22830) was excised from a P2Y1-pGem 11-Z plasmid (from Dr. G. I. Bell, Chicago, IL) and subcloned into the BamHI-XhoI restriction sites of the mammalian expression vector pcDNA3 (Invitrogen, San Diego, CA). rP2Y1-pcDNA3 were grown in bacteria and purified. 1321N1 human astrocytoma cells were obtained from the European Collection of Cell Cultures. It is reported that 1321N1 cells do not express P2 purinoceptors (Boyer et al., 1996), but before using the clone obtained, we confirmed that untransfected cells do not express P2Y1 mRNA and that they do not respond to ATP or other agonists used in the present study. rP2Y1-pcDNA3 was stably transfected into 1321N1 cells using the calcium phosphate method. Transfected cells (1321N1-P2Y1) were grown in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin. After 2 d growth in normal medium, the transfected cells were split into a selection media containing 500 mg/ml G-418 (Life Technologies). Media was exchanged every 2–3 d, and the cells were grown in selection media for 14 d. Isolated colonies of cells demonstrating resistance to G-418 were picked and transferred to 24-well plates in which they were grown in selection medium to near confluence and then transferred to the culture dishes described above. After 24 hr, cells were loaded with fura-2, and calcium responses to applied 2-methylthio-ATP (2-MeSATP) were used as an assay for the determination of cell lines expressing functional P2Y1 purinoceptors. In these lines, expression of P2Y1 mRNA was confirmed using reverse transcription (RT)-PCR as described below. Cell lines found to be expressing P2Y1 were then maintained in normal medium supplemented with 400 mg/ml G-418, and split every 3–4 d. When required for experiments, cells were split and plated onto culture dishes and were used within 2 d.
Single-cell [Ca2+]imeasurements and Ca2+ imaging.The Ca2+-sensitive fluorophore fura-2 (Molecular Probes, Eugene, OR) was used for measuring [Ca2+]iphotometrically in single astrocytes and also for ratiometric imaging. All fluorescence measurements were made from subconfluent areas of the dishes so that individual astrocytes could be readily identified. Single astrocytes were identified using criteria described by Salter and Hicks (1994). Just before recording, cells were incubated at room temperature for 90 min in extracellular recording solution composed of (in mm): NaCl 140, KCl 5.4, CaCl2 1.3, HEPES 25, glucose 33, and tetrodotoxin (TTX) 0.5 μm, pH 7.35, osmolarity 315–320 mOsm, which had been supplemented with bovine serum albumin (BSA) (0.5%) and fura-2 AM (2 μm). Subsequently, the culture dish was thoroughly rinsed with extracellular solution lacking fura-2 AM and BSA and was mounted on an inverted microscope (Diaphot-TMD; Nikon, Mississauga, Canada). To avoid neural–astrocyte signaling, the areas chosen were free from neurons. When required, cultures were bathed in extracellular solution with no added Ca2+ and supplemented with 100 μm EGTA, referred to as Ca2+-free extracellular solution. Cultures were viewed using a 40× CF epifluorescence Fluor objective lens. Recordings were made at room temperature (20–22°C).
For single-cell [Ca2+]imeasurements, recording was done by means of single-photon counting from individual astrocytes (Salter and Hicks, 1994). In brief, light from a compact xenon arc lamp (75 W) was alternately guided through either a 340DF10 nm or a 380DF13 nm wavelength bandpass excitation filter (Omega Optical, Brattleboro, VT) by means of a mirrored chopper rotating at 50 or 60 Hz to the input of an inverted microscope (Diaphot-TMD; Nikon). Emitted light was sent to the side camera port of the microscope at which it entered a dual optical pass adapter (Nikon). Here, the light was directed through a 510DF20 nm bandpass filter by a DM 580 dichroic mirror, after which the light passed through a manually adjustable aperture and was detected by a photomultiplier tube in single-photon counting mode [Photon Technologies Inc. (PTI), London, Ontario, Canada]. The output of the photomultiplier was sampled at a rate of 10 or 20 Hz by an IBM-compatible computer with hardware and software from PTI. All light intensity measurements were analyzed off-line. The free [Ca2+] was calculated using the formula of Grynkiewicz et al. (1985):
where R is the ratio of the fluorescence intensities recorded with the excitation wavelengths of 340 (F1) and 380 (F2) nm.Sf2 andSb2 represent the fura-2 Ca2+-free and Ca2+-bound values, respectively.Rmin is the ratio of fluorescence intensities for F1 andF2 in absence of Ca2+ (i.e., Ca2+-free fura-2), andRmax isF1/F2for Ca2+-saturated fura-2.Kd represents the effective dissociation constant for the Ca2+ fura-2 complex at room temperature. Rmin,Rmax, andKd were determined using an in vitro titration procedure.
Recording of Ca2+ waves was done by ratiometric imaging. Excitation light at 340 and 380 nm was generated by a xenon arc lamp and passed through a high-speed, computer-controlled, variable wavelength monochromator. This light was transmitted to the culture dish via a fiber optic cable. Emitted light was directed through a 510 nm bandpass filter and was detected by an intensified CCD camera. The CCD camera black level was set to >1% to maximize the dynamic range of the instrument. Images were acquired by computer at a rate of ∼2.5 per second and were stored on hard disk. Hardware and software for imaging were from PTI.
Image data were analyzed off-line. The first image in each image set was used as a template for designating each cell as a region of interest within the image. Each 340 nm image was divided, on a pixel-by-pixel basis, by the corresponding 380 nm image producing a ratio image. Averaged values of the ratios within each region of interest were plotted as a function of time. No attempt was made to convert ratio data from images to [Ca2+]i.
Drug application. P2Y agonists were dissolved in extracellular solution or Ca2+-free extracellular solution, as necessary. Agonists were applied to individual astrocytes by pressure ejection from a pipette located ∼20–40 μm from the cell being stimulated. All other drugs were dissolved in extracellular solution and were applied directly to the bath.
Stimulation of Ca2+waves. Ca2+ waves were evoked by mechanical stimulation of an individual astrocyte or 1321N1 cell. A single cell was briefly touched under visual control with the tip (3–5 μm diameter) of a fire-polished glass pipette lowered gradually from a height of ∼3 μm above the cell. The mechanical stimulation was done regularly at 10 min intervals. Adenosine-3′-phospho-5′-phosphosulfate (A3P5PS) or suramin were tested only in cases in which two or more Ca2+ waves were reliably evoked beforehand. Effects of A3P5PS or suramin were analyzed on cells neighboring the one mechanically stimulated, and such neighboring cells were only included in the analysis if they had a Ca2+ response after washing out A3P5PS or suramin that was similar in amplitude to that before A3P5PS.
Reverse transcription-PCR. RNA from E18 embryonic spinal cord, primary cultures of rat dorsal spinal cord, or 1321N1-P2Y1 cells was isolated by a phenol-chloroform method using TRIzol reagent (Life Technologies). Total RNA was used as a template for the synthesis of first strand cDNA using oligo-dT primers to reverse transcribe poly(A+) mRNA (Superscript system; Life Technologies). The product of this reaction was then treated with RNase H (Life Technologies) to degrade RNA hybridized to first-strand cDNA. For all reverse transcription reactions, a negative control was performed in which no reverse transcriptase was added to the reaction tube. The cDNA generated was used as template in a PCR reaction using Vent polymerase (New England Biolabs, Beverly, MA) for 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min, with a final extension time of 6 min. The primers used, 5′ (CATCTCCCCCATTCTCTTCTAC) 3′ (CTTGTGTCTCCGTTCTGCTTG), were complementary to rat P2Y1. Amplified products were subcloned into the pCR Blunt II-TOPO vector (Invitrogen) and sequenced.
Analysis of pharmacological data. For each cell in which concentration–response relationships were investigated, the magnitude of peak Ca2+ response at each concentration of agonist (A) was calculated as a percentage of the maximum peak response of that cell. The mean of the responses of a number of cells was determined at each concentration, and concentration–effect curves were computed by fitting the mean at each agonist concentration with a logistic function: E =Emax/(1 + ([EC50]/[A])n), where Emax is the maximum effect andn is the Hill coefficient. Concentration–inhibition data were collected for A3P5PS, and inhibition curves were constructed by fitting the mean response amplitude at each concentration with the equation E = E0/(1 + ([A3P5PS]/[IC50])n);E0 is the response amplitude in the absence of A3P5PS. Antagonist affinity (Kb) was estimated by using the general form of the Cheng–Prusoff equation (Leff and Dougall, 1993) as described previously (Ho et al., 1995):
Data were compared using Student's t test.p < 0.05 was considered to indicate a statistically significant difference.
Source of reagents. 2-MeSATP, 2-methylthio-ADP (2-MeSADP), and UTP were from Research Biochemicals International (Natick, MA). All other reagents, except where indicated above, were from Sigma-Aldrich, Canada Inc.
RESULTS
Ca2+ responses of dorsal spinal cord astrocytes evoked by 2-MeSATP and 2-MeSADP
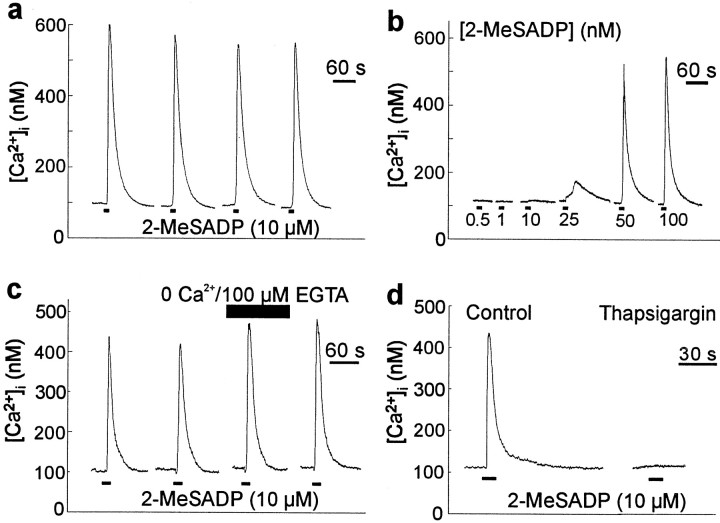
Dorsal spinal astrocytes are known to respond to the P2Y purinoceptor agonists ATP and 2-MeSATP by releasing Ca2+ from an IP3-sensitive intracellular store (Salter and Hicks, 1995). 2-MeSATP was found to be more potent than ATP, suggesting that the Ca2+ responses might be mediated by the P2Y1 purinoceptor subtype (Simon et al., 1995). However, 2-MeSATP is known to stimulate other P2Y purinoceptor subtypes (Ralevic and Burnstock, 1998). Therefore, presently we tested the ADP analog 2-MeSADP, which has been reported to preferentially activate P2Y1 but not the other P2Y purinoceptor subtypes that are sensitive to 2-MeSATP (Communi et al., 1997; Li et al., 1998; Palmer et al., 1998). We found that applying 2-MeSADP (10 μm for 10 sec) evoked transient increases in [Ca2+]i in dorsal spinal astrocytes (n = 25 cells) (Fig.1a). Ca2+ responses evoked by applying 2-MeSADP were reliable and reproducible when the interval between applications was 5 min or greater. The magnitude of the Ca2+ response to 2-MeSADP was graded, varying directly with 2-MeSADP concentration in the range tested (Fig.1b).
Fig. 1.
2-MeSADP causes release of stored Ca2+ in individual spinal cord astrocytes.a, The traces show records of [Ca2+]i from a single dorsal spinal cord astrocyte. 2-MeSADP (10 μm, 10 sec;bottomblack bars) was applied every 5 min to allow maximum recovery between applications. b, In a different cell, 2-MeSADP was applied for 10 sec every 5 min at varying concentrations as indicated below the traces.c, In another cell, 2-MeSADP was applied for 10 sec at 5 min intervals. Five minutes before and during the third agonist application, the cell was bathed in extracellular solution (top black bar) containing 100 μm of the Ca2+ chelator EGTA and no added Ca2+. d, A response to 2-MeSADP (10 μm, 10 sec) is shown on the left. After depletion of intracellular Ca2+ stores by bath application of thapsigargin (1 μm, 10 min), 2-MeSADP (10 μm, 10 sec) was applied to the same cell (right). The gaps in the recordings in this figure and in all others indicate periods when fluorescence signals were not sampled to minimize photobleaching of fura-2.
When 2-MeSADP (10 μm) was applied in extracellular solution with no added Ca2+ and 100 μm EGTA, the Ca2+ responses were similar in magnitude to those evoked in normal extracellular solution (n = 6 cells) (Fig. 1c). Thus, Ca2+ responses to 2-MeSADP did not depend on extracellular Ca2+. To determine whether responses to 2-MeSADP are mediated by release of Ca2+ from intracellular stores, we applied the endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin (1 μm) (Thastrup et al., 1990) in the Ca2+-free extracellular solution. After thapsigargin administration, applying 2-MeSADP (10 μm) did not produce a change in [Ca2+]i(n = 9 cells) (Fig. 1d), which indicates that the 2-MeSADP-evoked responses were mediated by releasing Ca2+ from intracellular stores.
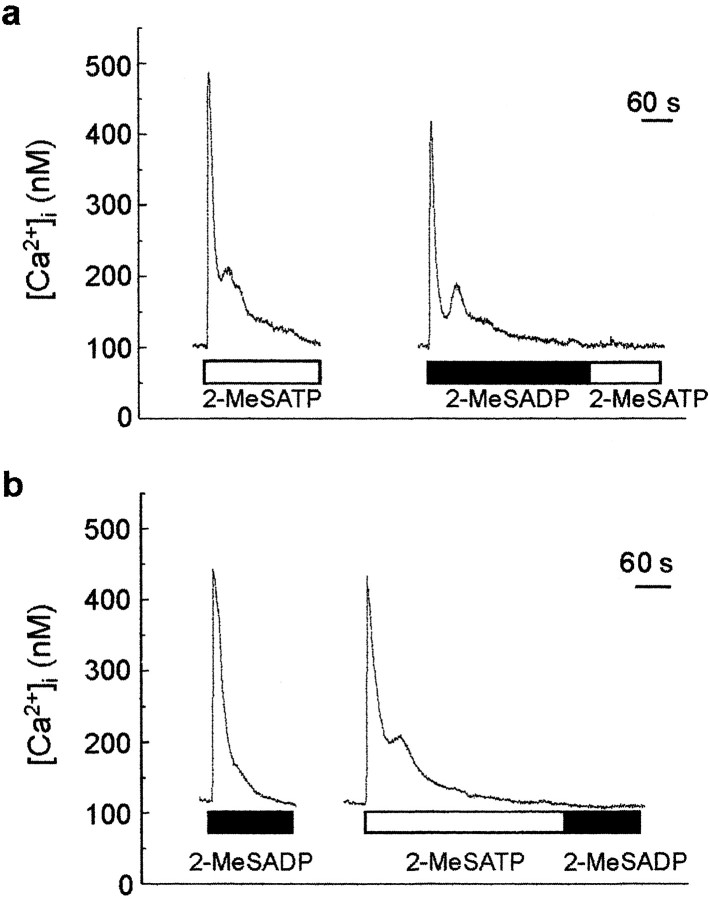
If 2-MeSATP and 2-MeSADP act on a common receptor in astrocytes, then responses to these two agonists would be expected to cross-desensitize. We produced desensitization with prolonged applications of 2-MeSADP (10 μm) or 2-MeSATP (10 μm), which each caused a rapid but transient increase in [Ca2+]i, with [Ca2+]i returning to baseline level by 5 min after the start of the application (Fig.2). Test applications of 2-MeSATP made immediately at the end of a prolonged application of 2-MeSADP produced no change in [Ca2+]i(n = 5 cells) (Fig. 2a). Conversely, a test application of 2-MeSADP in cells in which the response to 2-MeSATP had been desensitized caused no rise in [Ca2+]i(n = 4 cells) (Fig. 2b). Thus, the responses to 2-MeSADP and 2-MeSATP cross-desensitized. In contrast, Ca2+ responses evoked by applying UTP, which is known to cause release of Ca2+from an IP3-sensitive intracellular pool (Idestrup et al., 1998), persisted even when the 2-MeSADP- or 2-MeSATP-evoked responses had been desensitized (data not shown), and hence, the lack of responses to the test applications of 2-MeSADP or 2-MeSATP was not attributable to depletion of intracellular Ca2+ stores. Together, these findings indicate that responses to 2-MeSADP and 2-MeSATP may be mediated via a common type of receptor.
Fig. 2.
Responses to 2-MeSADP and 2-MeSATP cross-desensitize. a, 2-MeSATP (10 μm) was applied continuously until [Ca2+]ireturned to basal level (open bar). After 10 min, 2-MeSADP (1 μm) was applied continuously. After the return of [Ca2+]i to the baseline level, 2-MeSATP was immediately reapplied. b, In another cell, 2-MeSADP (10 μm) was applied continuously. Ten minutes later, 2-MeSATP (10 μm) was applied continuously, and after the return of [Ca2+]i to the baseline level, 2-MeSADP was immediately reapplied.
We next examined the concentration–response relationships for 2-MeSADP and 2-MeSATP. For each cell tested, the minimum concentration at which 2-MeSADP evoked a Ca2+ response was lower than that required for 2-MeSATP (n = 5 cells) (Fig.3). On average, the concentration of 2-MeSADP producing 50% of the maximum response (EC50) was found to be 35 ± 5 nm (n = 9 cells), whereas the EC50 for 2-MeSATP was 200 ± 31 nm (n = 5 cells) (Fig.3b). Therefore, 2-MeSADP was approximately sixfold more potent than 2-MeSATP at evoking Ca2+responses in dorsal spinal astrocytes.
Fig. 3.
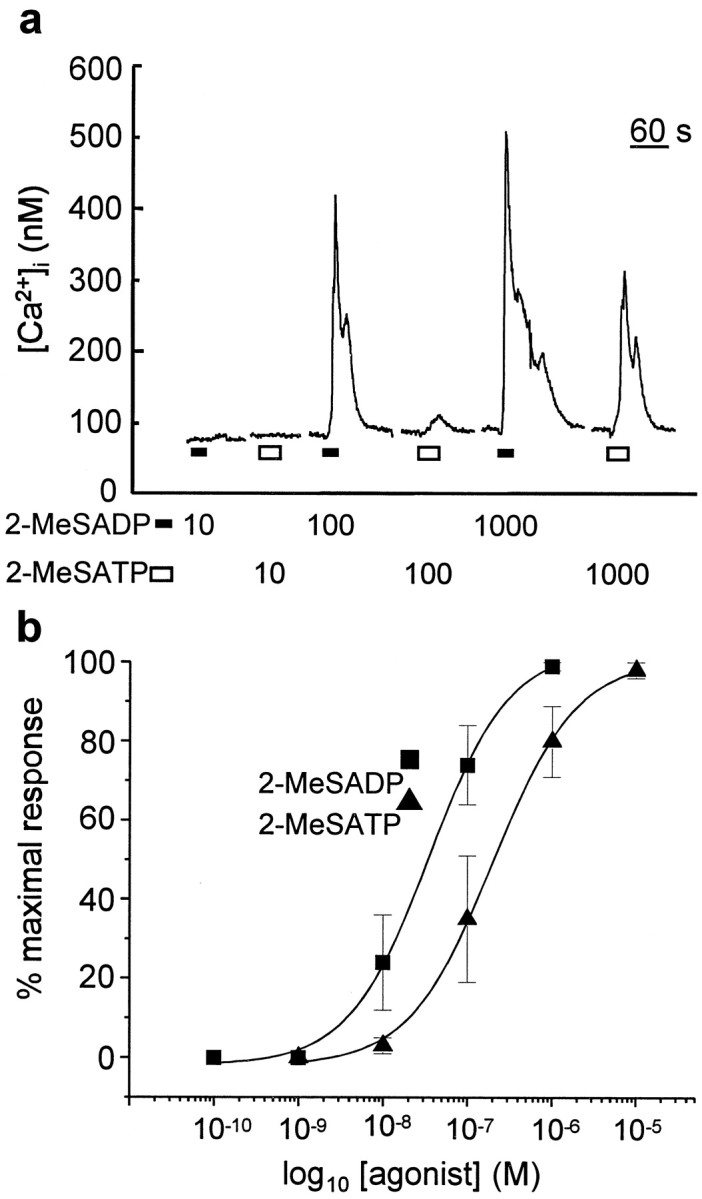
2-MeSADP is more potent than 2-MeSATP.a, Record of [Ca2+]i in a single cell onto which 2-MeSADP (bottom black bars) and 2-MeSATP (bottom white bars) were alternately applied at intervals of 5 min at concentrations indicated below thetrace. b, This graph shows a plot of the concentration–response relationships for 2-MeSADP (squares) and 2-MeSATP (triangles). Each data point is the mean ± SEM of the response to the applied agonist at each concentration for n = 5–9 cells. The curves are the best fits of the means of responses evoked by 2-MeSADP and 2-MeSATP. The equations of the curves are 104/(1 + (3.5e−8/[2-MeSADP])0.9) and 103/(1 + (2e−7/[2-MeSATP])0.9) for 2-MeSADP and 2-MeSATP, respectively.
P2Y1 antagonist differentially blocks Ca2+ responses to 2-MeSATP and 2-MeSADP but not UTP
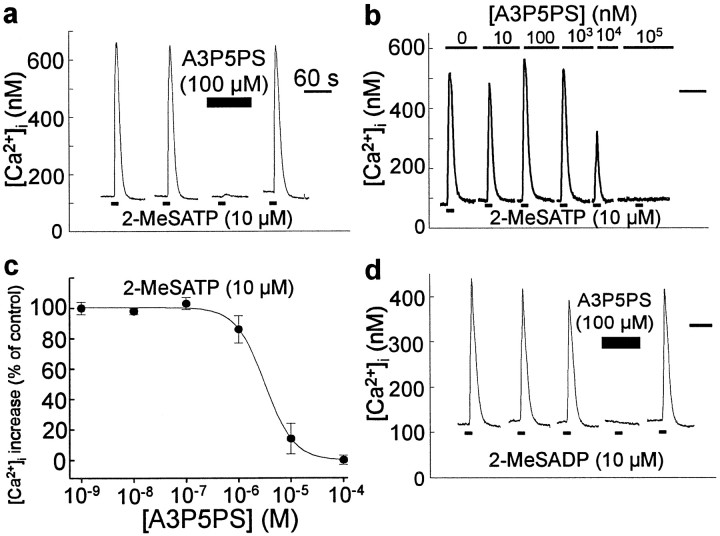
The results above suggest that the purinoceptor subtype may be P2Y1 (Ralevic and Burnstock, 1998). We therefore examined the effect of A3P5PS, which is reported to block responses to activation of heterologously expressed P2Y1purinoceptors but to not affect responses mediated by other P2Y purinoceptors (Boyer et al., 1996). We found that applying A3P5PS (100 μm) inhibited Ca2+ responses evoked by 2-MeSATP (n = 12 cells) (Fig.4a). The Ca2+ responses evoked by 2-MeSATP returned to the control amplitude when A3P5PS was washed out, indicating that the inhibition was reversible. The degree of inhibition of 2-MeSATP-evoked responses was dependent on A3P5PS concentration (Fig.4b,c), and the concentration producing 50% inhibition (IC50) was 2.8 ± 0.4 μm (n = 5 cells). From this, the calculated Kb for A3P5PS on 2-MeSATP-evoked responses was 44 ± 5 nm. In addition, A3P5PS (100 μm) reversibly blocked responses evoked by 2-MeSADP (n = 8 cells) (Fig.4d).
Fig. 4.
Responses to 2-MeSATP and 2-MeSADP are blocked by the P2Y1 receptor antagonist A3P5PS. a, Thetraces show records of [Ca2+]i from a single dorsal spinal cord astrocyte. 2-MeSATP (10 μm, 5 sec) was applied at 5 min intervals. The cell was incubated in bath solution containing A3P5PS (100 μm) 4 min before and during the third agonist application, as indicated by the black bar above thetrace. Normal bath solution was restored after the third agonist application. b, In a different cell, 2-MeSATP (10 μm, 10 sec) was applied at 5 min intervals in the presence of bath solution containing A3P5PS, ranging in concentration from 0 to 100 μm, as indicated above thetraces. c, This graph shows a plot of the concentration-dependence of the inhibition of responses to 2-MeSATP (10 μm) by A3P5PS. Each data point represents the mean ± SEM of the Ca2+ response to 2-MeSATP in the presence of varying concentrations of A3P5PS for five cells. Ca2+ responses are expressed as a percentage of the peak response evoked in the absence of A3P5PS. The curve is the best fit of the means to the logistic equation 96/(1 + ([A3P5PS]/2.8e−6)1.7).d, Record of [Ca2+]i in a different cell onto which 2-MeSADP (10 μm, 10 sec) was applied as indicated by the bottomblack bars. The cell was incubated in bath solution containing A3P5PS (100 μm; top black bar) before and during the fourth agonist application. After this, the antagonist was washed out and replaced with normal extracellular solution. The black bar in the top right corner of a,b, and d indicate 60 sec time scales.
To examine the possibility that A3P5PS might interfere with the Ca2+ release pathway, we tested this compound on Ca2+ responses evoked by UTP, which has been suggested to activate a G-protein-coupled purinoceptor subtype that is distinct from that activated by 2-MeSATP but is also coupled to the release of Ca2+ from IP3-sensitive stores (Idestrup and Salter, 1998). In the presence of A3P5PS (100 μm), responses to UTP (50 μm) were not different from the control level (104 ± 5%, n = 5 cells), indicating that A3P5PS differentially blocks responses to 2-MeSADP and 2-MeSATP but not to UTP (Fig. 5).
Fig. 5.
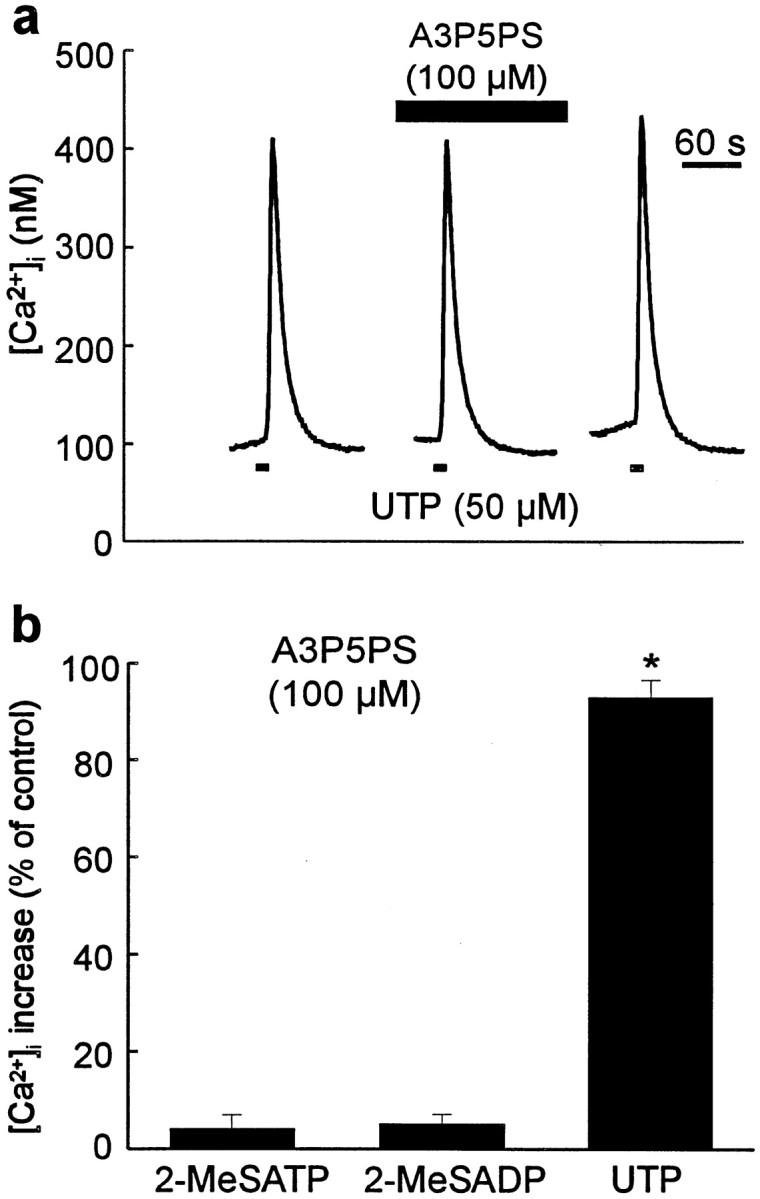
Responses to UTP are unaffected by A3P5PS.a, Record of [Ca2+]i in a cell onto which UTP (50 μm, 5 sec) was applied as indicated by the bottomblack bars, in the presence of a bath solution containing A3P5PS (100 μm; top black bar) before and during the second agonist application. b, The mean peak rise in intracellular Ca2+ evoked by 2-MeSATP (10 μm; n = 12 cells), 2-MeSADP (10 μm; n = 8 cells), and UTP (50 μm; n = 5 cells) in the presence of A3P5PS (100 μm). Ca2+ responses are expressed as a percentage of the peak response evoked in the absence of A3P5PS. *p < 0.05 (Student's ttest).
P2Y1 mRNA is expressed in spinal cord astrocytes and embryonic spinal cord
The results above indicate that the receptor activated by 2-MeSATP and 2-MeSADP has pharmacological properties that match those reported for heterologously expressed P2Y1 purinoceptors (Palmer et al., 1998). To determine whether mRNA encoding P2Y1 is expressed by astrocytes, we did RT-PCR with poly(A+) RNA from the spinal cultures and used primers complementary to rat P2Y1. From this PCR, we generated a 570 bp DNA PCR product (Fig.6a), the sequence of which was found to be identical to that of the corresponding region of rat P2Y1. In contrast, no RT-PCR product was generated from a water-only control or when reverse transcriptase was omitted (Fig. 6a). Therefore, we conclude that P2Y1 mRNA is expressed in dorsal spinal cultures.
Fig. 6.
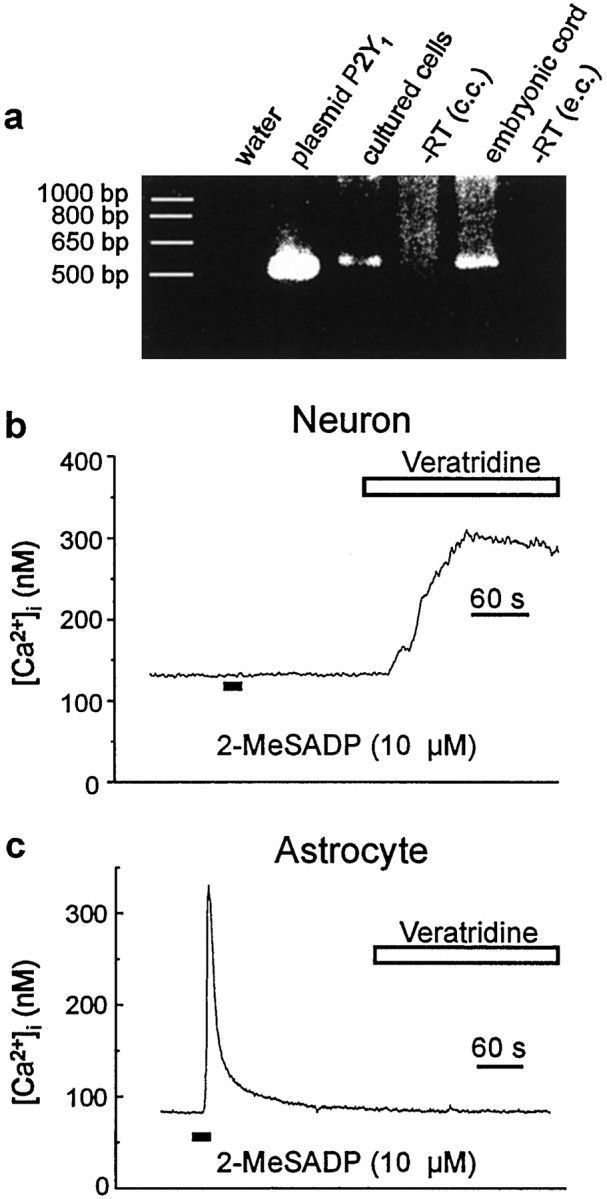
P2Y1 is expressed by dorsal spinal cord astrocytes. a, Result of RT-PCR performed on mRNA from embryonic spinal cord and dorsal spinal cord cultures using primers complementary to the rat P2Y1 purinoceptor. PCR was performed on plasmid P2Y1 as a positive control and on water RT-embryonic cord [−RT (e.c.)] and spinal cord cultures [−RT (c.c.)] as negative controls.b, Record of [Ca2+]i in a neuron. 2-MeSADP (10 μm, 10 sec) was directly applied as indicated by the bottom black bar. The cell was then bathed in extracellular solution containing veratridine (10 μm) and no added TTX as indicated by the white bar. c, Record of [Ca2+]i in an astrocyte onto which 2-MeSADP (10 μm, 10 sec) was directly applied (bottom black bar). After the return of [Ca2+]i to the baseline level, veratridine (10 μm) was bath-applied as indicated by thewhite bar.
These cultures contain both astrocytes and neurons, and therefore it was possible that the P2Y1 mRNA may have come from the neurons. However, with neurons, we found that applying 2-MeSADP (10 μm) caused no change in [Ca2+]i(n = 9 cells) (Fig. 6b). On the other hand, neurons displayed increases in [Ca2+]i in response to the depolarizing agent veratridine. Conversely, astrocytes were unresponsive to veratridine (Fig. 6c). Together, these data indicate that astrocytes are the source of P2Y1 transcript detected in the spinal cord cultures.
We wondered whether P2Y1 might also be expressed in the developing spinal cord at the time point when the cultures were made. With RT-PCR using mRNA from E18 embryonic spinal cord, we generated a 570 bp product, the sequence of which was identical to the corresponding region of rat P2Y1 (Fig.6a). Thus, P2Y1 purinoceptors appear to be expressed in the spinal cord in vivo, as well as in the dorsal spinal cultures.
P2Y1 purinoceptor is sufficient for Ca2+ wave propagation
To determine whether P2Y1 purinoceptors are sufficient to support Ca2+ wave propagation, we stably transfected rat P2Y1 into 1321N1 astrocytoma cells. 1321N1 cells are reported not to endogenously express P2 purinoceptors (Boyer et al., 1996), and we confirmed that these cells did not respond to 2-MeSATP or to UTP (n = 10 cells). With the cell line expressing P2Y1 (1321N1-P2Y1), applying 2-MeSADP (10 μm) or 2-MeSATP (10 μm) evoked Ca2+responses in every cell tested (n = 28 and 7 cells, respectively), and A3P5PS (100 μm) reversibly inhibited the Ca2+ responses by (93 ± 7% inhibition, n = 13 cells tested). In contrast, no 1321N1-P2Y1 cell responded to UTP (50 μm, n = 6 cells tested). Thus, the 1321N1-P2Y1 cells have the pharmacological properties expected for cells expressing P2Y1 but not other P2Y purinoceptors.
To investigate the propagation of Ca2+waves, a single cell was stimulated mechanically by means of a brief touch with a blunt-tipped micropipette. This type of mechanical stimulation reliably caused a transient rise in [Ca2+]i in the cell that was stimulated; the increase in [Ca2+]i peaked within 1 sec after the stimulation, and [Ca2+]i gradually returned to the baseline level over 3–5 min (Fig.7). With the parental 1321N1 cells, the transient rise in [Ca2+]i was restricted to the cell that was stimulated (n = 10 cells). However, with 1321N1-P2Y1 cells, the transient rise in [Ca2+]i in the stimulated cell was followed by Ca2+responses in cells neighboring the one that was touched (Fig.7a). The onset of the Ca2+responses in the neighboring cells was typically 5–10 sec after the beginning of the rise [Ca2+]i in the mechanically stimulated cell. [Ca2+]i peaked rapidly in the neighboring cells and then declined, usually more rapidly than in the cell that was stimulated directly. Thus, with the 1321N1-P2Y1 cells, but not the 1321N1 cells, mechanically stimulating a single cell produced a Ca2+ wave that was transmitted to neighboring cells.
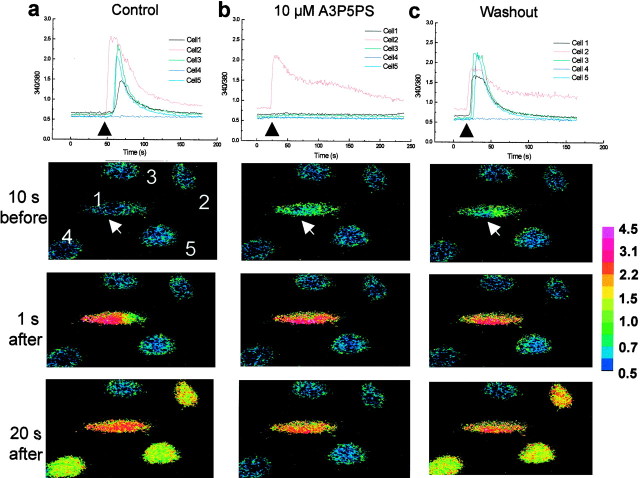
Fig. 7.
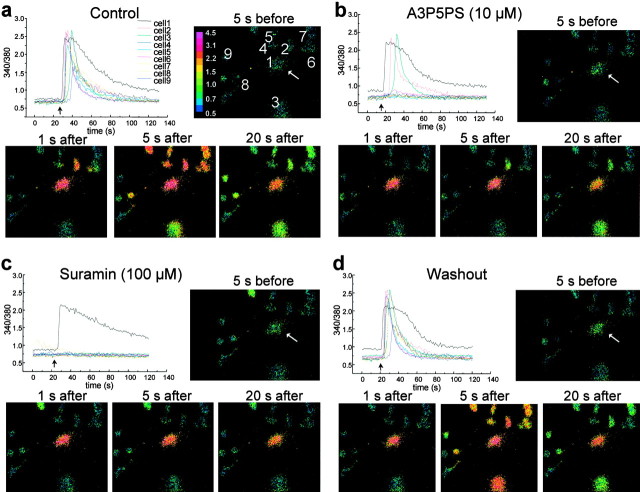
Ca2+ waves in 1321N1-P2Y1 cells are blocked by A3P5PS. In the top graphs, the individual traces show records of the ratio of fluorescence intensities of fura-2 at 340 and 380 nm in five individual 1321N1-P2Y1 cells. Cell 1 was mechanically stimulated at the times indicated by the black arrows. The row of panels below each graph show the corresponding ratiometric images of the experiment represented in the top graph at three different time points: 10 sec before stimulation and 1 and 20 sec after stimulation. The white arrow indicates the cell directly simulated. The color bar on the right indicates the scale of ratio intensity. Cell 1 was stimulated in the absence of antagonist (a), 10 min later in the presence of bath-applied A3P5PS (10 μm) (b), and10 min after washout of A3P5PS (c).
To determine whether blocking P2Y1 purinoceptors could prevent Ca2+ wave propagation between 1321N1-P2Y1 cells, A3P5PS (10 μm) was bath applied after control Ca2+ waves had been evoked. In the example illustrated in Figure 7, application of A3P5PS prevented the propagation of the Ca2+ wave to neighboring cells without preventing the Ca2+ response of the 1321N1-P2Y1 cell that was stimulated mechanically (Fig. 7b). Upon washing out A3P5PS, Ca2+ responses were produced in cells neighboring the one that was stimulated (Fig. 7c), indicating that the effect of A3P5PS was reversible. In eight separate experiments, A3P5PS (10 μm) reversibly blocked the Ca2+ wave transmission to 36 of the 39 neighboring 1321N1-P2Y1 cells examined; in the other three neighboring cells, Ca2+responses were significantly, but not fully, inhibited by A3P5PS (10 μm). Taking these results together, we conclude that P2Y1 purinoceptors are sufficient to support Ca2+ wave propagation.
P2Y1 receptors are required for full Ca2+ wave propagation in a spinal astrocyte network
To determine whether P2Y1 purinoceptors participate in the propagation of Ca2+waves in astrocytes, we tested A3P5PS on mechanically evoked Ca2+ responses in the dorsal spinal cultures. Mechanically stimulating a single dorsal spinal astrocyte evoked a Ca2+ rise in the stimulated cell, which was followed 3–10 sec later by Ca2+responses in neighboring astrocytes (Fig.8a). During bath application of A3P5PS (10–100 μm), the Ca2+ rise in the mechanically stimulated cell persisted, but the propagation of the Ca2+ wave to neighboring astrocytes was suppressed. In the example shown in Figure 8b, A3P5PS blocked propagation of the Ca2+ wave to six of the nine neighboring astrocytes that had been engaged during the control Ca2+ response. Overall, in a total of eight experiments, we followed 57 neighboring astrocytes that were engaged by the Ca2+ wave before A3P5PS application. Of these astrocytes, 18 showed no rise in [Ca2+]i during A3P5PS application, and on average, the amplitude of the peak Ca2+ responses was decreased by 55 ± 5% compared with the control responses. Upon washing out A3P5PS, Ca2+ responses were observed in all cells that had been engaged by the control Ca2+wave (Fig. 8d), indicating that A3P5PS reversibly suppressed the propagation of astrocyte Ca2+waves.
Fig. 8.
Ca2+ waves among dorsal spinal cord astrocytes are suppressed by A3P5PS and suramin. Thetraces show individual records of the ratio of fluorescence intensities of fura-2 at 340 and 380 nm in nine individual astrocytes within a single field. Cell 1 was mechanically stimulated at the times indicated by the black arrow. Thepanels along with each trace are ratiometric images of each experiment taken at four different time points; 5 sec before touch and 1, 5, and 20 sec after stimulation touch. Thewhite arrow indicates the cell stimulated directly by touch. The color bar in the top panelindicates the scale of ratio intensity. a, Stimulation of cell 1 before the addition of antagonists. b, Ten minutes later, stimulation of cell 1 in the presence of A3P5PS (10 μm). c, Ten minutes after the washout of A3P5PS, bath solution containing suramin (100 μm) was applied, and then cell 1 was stimulated. d, Cell 1 was stimulated 10 min after washout of solution containing suramin.
To determine whether the residual propagation of Ca2+ responses was mediated by purinoceptors, we used the broad spectrum P2Y purinoceptor blocker suramin (Ralevic and Burnstock, 1998), which has been shown to block responses of spinal cord astrocytes to ATP, 2-MeSATP, and UTP (Ho et al., 1995). In three experiments, suramin (100–300 μm) was applied after A3P5PS. In 10 of the 13 neighboring astrocytes in these experiments, Ca2+responses were blocked reversibly by A3P5PS (Fig.8c,d); in each of the remaining three cells, the Ca2+ response was significantly and reversibly reduced (average inhibition of 70 ± 16%). Thus, propagation of Ca2+ waves between dorsal spinal astrocytes appears to be mediated primarily by P2Y purinoceptors, with P2Y1 purinoceptors necessary for full propagation of the waves.
DISCUSSION
Our principal conclusions are that dorsal spinal cord astrocytes express P2Y1 purinoceptors coupled to release of intracellular Ca2+ and that these purinoceptors participate in the propagation of Ca2+ waves between dorsal spinal astrocytes. We show that 2-MeSATP and 2-MeSADP caused release of Ca2+ from thapsigargin-sensitive stores, that the responses to 2-MeSATP and 2-MeSADP cross-desensitized with each other but not with responses to UTP, that responses of astrocytes to 2-MeSATP and 2-MeSADP but not to UTP were blocked by A3P5PS, and that P2Y1 mRNA is expressed by the dorsal spinal astrocytes. Furthermore, we found that expression of P2Y1 purinoceptors in a cell line lacking endogenous purinoceptors allowed the propagation of Ca2+ waves. These Ca2+ waves were blocked by A3P5PS, which also suppressed Ca2+ wave propagation between the astrocytes. Thus, P2Y1 purinoceptors are sufficient, without other purinoceptors, to support Ca2+ waves, and P2Y1purinoceptors are required for complete propagation of such waves in dorsal spinal astrocyte networks.
The results of the present study, together with previous work (Salter and Hicks, 1995), indicate that the rank order of potency of agonists at the P2Y1 purinoceptor is 2-MeSADP > 2-MeSATP > ATP when expressed in spinal astrocytes. This rank order of potency for these agonists is identical to that reported for human (Palmer et al., 1998) or rat P2Y1purinoceptors expressed in 1321N1 cells (Tokuyama et al., 1995). 2-MeSATP is also known to activate the P2Y6 and P2Y11 purinoceptor subtypes (Communi et al., 1997; Li et al., 1998). P2Y6 purinoceptors are activated as well by UDP, but we have found that <10% of astrocytes are responsive to this nucleotide and only at concentrations greater than those of 2-MeSADP (M. W. Salter, unpublished data). At P2Y11 purinoceptors, ATP is more potent than 2-MeSATP, and 2-MeSADP is only a weak agonist (Communi et al., 1997). Therefore, the rank order of potency of agonists is not consistent with expression of P2Y6 or P2Y11purinoceptors by dorsal spinal astrocytes.
We find that the Ca2+ responses of dorsal spinal astrocytes to 2-MeSATP and 2-MeSADP are reversibly blocked by A3P5PS. Previously, responses to 2-MeSATP in dorsal spinal astrocytes were shown to be blocked by suramin (Salter and Hicks, 1995). Suramin is known to inhibit heterologously expressed P2Y purinoceptors, such as P2Y1, P2Y2, and P2Y6 (Ralevic and Burnstock, 1998), whereas A3P5PS has been found to block only responses mediated by recombinant P2Y1 purinoceptors but not any of the other purinoceptors tested (P2Y2, P2Y4, or P2Y6) when expressed heterologously in 1321N1 cells (Boyer et al., 1996). Thus, the agonist and antagonist profiles lead to the conclusion that responses to 2-MeSATP and 2-MeSADP are mediated by P2Y1 purinoceptors. Native P2Y1 purinoceptors have been implicated in mediating responses in the rat aorta (Dol-Gleizes et al., 1999) and in platelets (Jin et al., 1998); we report in this study that P2Y1 purinoceptors are functionally expressed in cells from the CNS.
Previously, it has been suggested that dorsal spinal astrocytes express a P2Y purinoceptor subtype in addition to the one that we now identify as P2Y1.The evidence for this second receptor was that responses to 2-MeSATP and UTP do not cross-desensitize (Ho et al., 1995) and that, although 99% of astrocytes are responsive to 2-MeSATP, only 70% of astrocytes respond to UTP. Here, we show that, in contrast to responses to 2-MeSATP and 2MeSADP, responses to UTP were unaffected by A3P5PS. Thus, we demonstrate that there is an antagonist that discriminates between the responses of 2-MeSATP–2-MeSADP and UTP, providing definitive pharmacological evidence for the presence of two distinct P2Y purinoceptor subtypes on dorsal spinal astrocytes. The purinoceptor that was activated by UTP was blocked by pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) (Ho et al., 1995), which distinguished it at the time from the only UTP-activated P2Y purinoceptor that had been cloned, P2Y2. UTP-activated P2Y purinoceptors that have been subsequently identified, P2Y4 and P2Y6, have also been shown to be insensitive to PPADS (Ralevic and Burnstock, 1998). Thus, the identity of the UTP-activated purinoceptor on spinal cord astrocytes remains to be determined.
When we expressed P2Y1 purinoceptors in astrocytoma cells that do not normally express purinoceptors, the cells gained the ability to respond to 2-MeSATP–2-MeSADP and to propagate Ca2+ waves. The lack of Ca2+ wave propagation by the parental 1321N1 cells could not be attributable to the cells being unable to generate Ca2+ signals because these 1321N1 cells have been shown to express receptors other than P2Y purinoceptors, coupled to release of intracellular Ca2+ (Ohuchi et al., 1998). Moreover, 1321N1 cells release nucleotides such as ATP (Lazarowski et al., 1995) and UTP (Lazarowski et al., 1997) in a stimulus-dependent manner. Thus, when the appropriate mediators are released and the remainder of the signaling pathways are present, expressing P2Y1purinoceptors becomes a sufficient condition to permit the cell–cell transmission of Ca2+ waves.
Because the spread of Ca2+ waves in the dorsal spinal cultures was suppressed by the antagonist A3P5PS, native P2Y1 purinoceptors appear to be necessary for the full propagation of Ca2+ waves. As discussed above, essentially all dorsal spinal astrocytes express P2Y1 purinoceptors, whereas only ∼70% of the astrocytes appear to express the other P2Y purinoceptor subtype. Thus, differential expression of the receptors could account for the observation that A3P5PS completely blocked Ca2+ responses in some cells. The residual responses in other cells were likely mediated by the other P2Y purinoceptor subtype because these responses were blocked by suramin.
Blockade of astrocyte Ca2+ waves by suramin has been reported in cultured cortical (Guthrie et al., 1999;John et al., 1999) and brain astrocytes (Cotrina et al., 1998). Moreover, it has been reported that PPADS prevents the spread of Ca2+ waves in cortical astrocytes (Guthrie et al., 1999). Like dorsal spinal astrocytes, cortical astrocytes are responsive to 2-MeSATP and UTP (King et al., 1996) and thus likely express multiple subtypes of P2Y purinoceptors. On the basis of our present results, we expect that blockade of the various receptors would be required to fully suppress Ca2+ wave propagation between cortical astrocytes.
ATP has been shown to be released during Ca2+ wave propagation and has been suggested as the mediator that transmits Ca2+ signals between cortical astrocytes (Guthrie et al., 1999). Although release of ATP could account for the wave propagation between dorsal spinal astrocytes, we cannot eliminate the possibility that another nucleotide mediator might also participate. In addition to release from astrocytes, ATP is well known to be released from presynaptic nerve terminals and to mediate postsynaptic responses in some regions of the CNS (Salter et al., 1993). Astrocyte processes are intimately associated with synapses (Barres, 1991) and are thus strategically localized to sense and respond to synaptically released transmitters. ATP that is released synaptically could therefore evoke Ca2+responses in surrounding astrocytes by activation of P2Y purinoceptors, resulting in the initiation as well as the propagation of Ca2+ waves.
There is emerging evidence that Ca2+ waves in astrocytes lead to alterations in the function of neurons (Newman and Zahs, 1998; Araque et al., 1999). Because the predominant means for transmitting astrocyte Ca2+ waves is via release of ATP, the present results raise the possibility of physiological roles of the astrocyte P2Y1purinoceptors. Given our finding that P2Y1 mRNA is expressed in embryonic spinal cord, one area in which P2Y1 purinoceptors may have a physiological role is in development of the CNS. P2Y1 purinoceptors have been suggested to have a role in the early embryonic development of the chick embryo (Meyer et al., 1999) and in the differentiation of rat striatal astrocytes during development (Abbracchio et al., 1995).
In addition to physiological roles, P2Y1 and other purinoceptors may participate in pathological events. The activation of P2Y purinoceptors on astrocytes is known to stimulate trophic signaling pathways (Neary et al., 1999) and to cause activation of transcription factors, which induce changes in gene expression (Priller et al., 1998). Increased proliferation and differentiation underlie the pathological responses of astrocytes to various noxious stimuli, such as hypoxia, ischemia, and trauma, leading to the formation of glial scars in the CNS (Hatten et al., 1991). ATP released from cells damaged by noxious stimuli could therefore act to induce the reactive response in astrocytes by evoking Ca2+ waves and causing the regenerative release of ATP. P2Y purinoceptor-mediated Ca2+ wave propagation has also been shown to be modulated by neuroimmune mediators, such as interleukin-1β, suggesting that P2Y purinoceptors may play role in the regulation of astrocyte signaling by inflammatory events in the CNS (John et al., 1999).
In conclusion, we have demonstrated that the P2Y1purinoceptor subtype is expressed by dorsal spinal astrocytes and is required for full propagation of Ca2+waves in astrocytes. Given that Ca2+ waves in astrocytes may serve to coordinate astrocyte–astrocyte and astrocyte–neuron signaling, P2Y1 purinoceptors may represent an important link in cell–cell signaling in the CNS.
Footnotes
S.R.F. and C.J.G. contributed equally to this work.
This work was supported by the Medical Research Council (MRC) of Canada (S.R.F. and M.W.S.), the Ontario Neurotrauma Foundation (C.J.G.), and the Nicole Feldman Memorial Fund. C.J.G. is a clinician scientist trainee at the Hospital for Sick Children, and M.W.S. is an MRC scientist. We thank J. L. Hicks and David Wong for preparing and maintaining dorsal horn cultures. We also thank Dr. G. I. Bell for the rat P2Y1 cDNA.
Correspondence should be addressed to Michael W. Salter, Programme in Brain and Behaviour, The Hospital for Sick Children, 555 University Avenue, Toronto, Ontario M5G 1X8, Canada. E-mail:mike.salter@utoronto.ca.
REFERENCES
- 1.Abbracchio MP, Ceruti S, Langfelder R, Cattabeni F, Saffrey MJ, Burnstock G. Effects of ATP analogues and basic fibroblast growth factor on astroglial cell differentiation in primary cultures of rat striatum. Int J Dev Neurosci. 1995;13:685–693. doi: 10.1016/0736-5748(95)00064-x. [DOI] [PubMed] [Google Scholar]
- 2.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Barres BA. New roles for glia. J Neurosci. 1991;11:3685–3694. doi: 10.1523/JNEUROSCI.11-12-03685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 5.Boyer JL, Romero-Avila T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1 receptor. Mol Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- 6.Communi D, Govaerts C, Parmentier M, Boeynaems JM. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- 7.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long- range glial signalling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 8.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signalling. J Neurosci. 1998;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dani JW, Smith SJ. The triggering of astrocytic calcium waves by NMDA-induced neuronal activation. Ciba Found Symp. 1995;188:195–205. doi: 10.1002/9780470514696.ch11. [DOI] [PubMed] [Google Scholar]
- 10.Dol-Gleizes F, Mares AM, Savi P, Herbert JM. Relaxant effect of 2-methyl-thio-adenosine diphosphate on rat thoracic aorta: effect of clopidogrel. Eur J Pharmacol. 1999;367:247–253. doi: 10.1016/s0014-2999(98)00985-6. [DOI] [PubMed] [Google Scholar]
- 11.Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:182–187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 13.Guan X, Cravatt BF, Ehring GR, Hall JE, Boger DL, Lerner RA, Gilula NB. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J Cell Biol. 1997;139:1785–1792. doi: 10.1083/jcb.139.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guthrie PB, Knappenberger J, Segal M, Bennett MVL, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassinger TD, Guthrie PB, Atkinson PB, Bennett MV, Kater SB. An extracellular signalling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci USA. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatten ME, Liem RK, Shelanski ML, Mason CA. Astroglia in CNS injury. Glia. 1991;4:233–243. doi: 10.1002/glia.440040215. [DOI] [PubMed] [Google Scholar]
- 17.Ho C, Hicks J, Salter MW. A novel P2-purinoceptor expressed by a subpopulation of astrocytes from the dorsal spinal cord of the rat. Br J Pharmacol. 1995;116:2909–2918. doi: 10.1111/j.1476-5381.1995.tb15944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Idestrup CP, Salter MW. P2Y and P2U receptors differentially release intracellular Ca2+ via the phospholipase c/inositol1,4,5-triphosphate pathway in astrocytes from the dorsal spinal cord. Neuroscience. 1998;86:913–923. doi: 10.1016/s0306-4522(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 19.Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 20.John GR, Scemes E, Suadicani SO, Liu JS, Charles PC, Lee SC, Spray DC, Brosnan CF. IL-1beta differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels. Proc Natl Acad Sci USA. 1999;96:11613–11618. doi: 10.1073/pnas.96.20.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King BF, Neary JT, Zhu Q, Wang S, Norenberg MD, Burnstock G. P2 purinoceptors in rat cortical astrocytes: expression, calcium- imaging and signalling studies. Neuroscience. 1996;74:1187–1196. doi: 10.1016/0306-4522(96)00209-6. [DOI] [PubMed] [Google Scholar]
- 22.Kuffler SW. Physiology of neuroglia cells.In: From neuron to brain, Ed 2 (Kuffler SW, Nicholls JG, Martin AR, eds), p 324. Sinauer; Sunderland, MA: 1984. [Google Scholar]
- 23.Lazarowski ER, Watt WC, Stutts MJ, Boucher RC, Harden TK. Pharmacological selectivity of the cloned human P2U-purinoceptor: potent activation by diadenosine tetraphosphate. Br J Pharmacol. 1995;116:1619–1627. doi: 10.1111/j.1476-5381.1995.tb16382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarowski ER, Homolya L, Boucher RC, Harden TK. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem. 1997;272:24348–24354. doi: 10.1074/jbc.272.39.24348. [DOI] [PubMed] [Google Scholar]
- 25.Leff P, Dougall IG. Further concerns over Cheng–Prusoff analysis. Trends Pharmacol Sci. 1993;14:110–112. doi: 10.1016/0165-6147(93)90080-4. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Olesky M, Palmer RK, Harden TK, Nicholas RA. Evidence that the p2y3 receptor is the avian homologue of the mammalian P2Y6 receptor. Mol Pharmacol. 1998;54:541–546. doi: 10.1124/mol.54.3.541. [DOI] [PubMed] [Google Scholar]
- 27.Meyer MP, Clarke JD, Patel K, Townsend-Nicholson A, Burnstock G. Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo. Dev Dyn. 1999;214:152–158. doi: 10.1002/(SICI)1097-0177(199902)214:2<152::AID-AJA5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Neary JT, Kang Y, Bu Y, Yu E, Akong K, Peters CM. Mitogenic signalling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J Neurosci. 1999;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohuchi Y, Yanai K, Sakurai E, Fukui H, Yanagisawa T, Watanabe T. Histamine-induced calcium mobilization in single cultured cells expressing histamine H1 receptors: a relationship between its sensitivity and the density of H1 receptors. Int J Mol Med. 1998;1:355–360. doi: 10.3892/ijmm.1.2.355. [DOI] [PubMed] [Google Scholar]
- 32.Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- 33.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 34.Peterson ER, Crain SM. Nerve growth factor attenuates neurotoxic effects of taxol on spinal cord-ganglion explants from fetal mice. Science. 1982;217:377–379. doi: 10.1126/science.6124041. [DOI] [PubMed] [Google Scholar]
- 35.Priller J, Reddington M, Haas CA, Kreutzberg GW. Stimulation of P2Y-purinoceptors on astrocytes results in immediate early gene expression and potentiation of neuropeptide action. Neuroscience. 1998;85:521–525. doi: 10.1016/s0306-4522(97)00653-2. [DOI] [PubMed] [Google Scholar]
- 36.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 37.Salter MW, Hicks JL. ATP-evoked increases intracellular calcium in cultured neurons and glia from the dorsal spinal cord. J Neurosci. 1994;14:1563–1575. doi: 10.1523/JNEUROSCI.14-03-01563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salter MW, Hicks JL. ATP causes release of intracellular Ca2+ via the phospholipase Cb/IP3 pathway in astrocytes from the dorsal spinal cord. J Neurosci. 1995;15:2961–2971. doi: 10.1523/JNEUROSCI.15-04-02961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salter MW, De Koninck Y, Henry JL. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Prog Neurobiol. 1993;41:125–156. doi: 10.1016/0301-0082(93)90006-e. [DOI] [PubMed] [Google Scholar]
- 40.Simon J, Webb TE, King BF, Burnstock G, Barnard EA. Characterisation of a recombinant P2Y purinoceptor. Eur J Pharmacol. 1995;291:281–289. doi: 10.1016/0922-4106(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 41.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokuyama Y, Hara M, Jones EM, Fan Z, Bell GI. Cloning of rat and mouse P2Y purinoceptors. Biochem Biophys Res Commun. 1995;211:211–218. doi: 10.1006/bbrc.1995.1798. [DOI] [PubMed] [Google Scholar]
- 43.Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends Neurosci. 1996;19:346–352. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]