Abstract
We studied coactivation-based cortical plasticity at a psychophysical level in humans. For induction of plasticity, we used a protocol of simultaneous pairing of tactile stimulation to follow as closely as possible the idea of Hebbian learning. We reported previously that a few hours of tactile coactivation resulted in selective and reversible reorganization of receptive fields and cortical maps of the hindpaw representation of the somatosensory cortex of adult rats (Godde et al., 1996). In the present study, simultaneous spatial two-point discrimination was tested on the tip of the right index finger in human subjects as a marker of plastic changes. After 2 hr of coactivation we found a significant improvement in discrimination performance that was reversible within 8 hr. Reduction of the duration of the coactivation protocol revealed that 30 min was not sufficient to drive plastic changes. Repeated application of coactivation over 3 consecutive days resulted in a delayed recovery indicating stabilization of the improvement over time. Perceptual changes were highly selective because no transfer of improved performance to fingers that were not stimulated was found. The results demonstrate the potential role of sensory input statistics (i.e., their probability of occurrence and spatiotemporal relationships) in the induction of cortical plasticity without involving cognitive factors such as attention or reinforcement.
Keywords: coactivation, associative pairing, somatosensory, tactile, perceptual learning, humans, cortical reorganization, plasticity, Hebbian learning, attention
Training and learning induce powerful reorganizational changes, which are referred to as use- or experience-dependent plasticity. In owl monkeys, Recanzone et al. (1992b) demonstrated a direct relation between cortical plastic changes and improvement of psychophysically assessed performance. The recent development of noninvasive-imaging techniques made it possible to study in humans the impact of modified use. These studies indicated that parallel to improvement of behavioral performance, extensive use resulted in substantial changes of cortical representations (Cohen et al., 1993; Pascual-Leone and Torres, 1993; Elbert et al., 1995; Pantev et al., 1998; Sterr et al., 1998). Although these studies confirmed the relevance of cortical plasticity for everyday life, they did not determine the crucial stimulus parameters associated with altered use that lead to the observed reorganization. From a number of animal studies, the importance of temporally correlated inputs and thus the characteristics of the input statistics had been hypothesized to play a key role (Clark et al., 1988; Fregnac et al., 1988; Allard et al., 1991; Ahissar et al., 1992; Diamond et al., 1993; Wang et al., 1995; Cruikshank and Weinberger, 1996b). In fact, since Hebb (1949), and even since James (1890), the aspect of simultaneity has become a metaphor in neural plasticity, although the exact role of Hebbian mechanisms in use-dependent plasticity remains controversial (Cruikshank and Weinberger, 1996a; Edeline, 1996; Ahissar et al., 1998).
To study the effects of variation of input statistics, we introduced a paradigm of coactivation in which temporally coherent inputs were generated by the simultaneous pairing of tactile stimuli (Godde et al., 1996). In this initial study we demonstrated that the simultaneous coactivation protocol was able to induce within a few hours reversible reorganization in adult rat somatosensory cortex (SI). Changes were characterized by a selective enlargement of the cortical territory and of the receptive fields representing the stimulated skin fields. A control protocol of the identical stimulus pattern applied to only a single skin site evoked no changes, indicating that coactivation was essential for induction. More generally, our protocol offers the advantage to study systematically the impact of input probabilities by variation of the degree of simultaneity or consistently anticorrelated inputs that is currently under investigation.
The selective and local changes within the cortical map implied that early sensory cortical processing was affected. Only those areas that underwent a specific alteration in stimulation without engaging cognitive factors became reorganized. It is evident, however, that plastic changes are further subject to modification via attention, meaning, and reward (Ahissar and Hochstein, 1993; Ito et al., 1998;Buchner et al., 1999).
To address the question of how much relevance plastic reorganization induced by pure variation of the input statistics (i.e., the temporal and spatial probability distributions of sensory inputs) has on a perceptual level, we tested the impact of a coactivation protocol in humans. Assuming that in humans the tactile coactivation protocol induces equivalent reorganizational processes as described for rat somatosensory cortex, we expected that discrimination performance should be subject to modification. The results showed that a few hours of coactivation induced a fast and reversible discrimination improvement as indicated by a lowering of the spatial two-point discrimination threshold.
MATERIALS AND METHODS
We studied 21 healthy, right-handed subjects (14 male and 7 female) between 22 and 35 years of age in different experimental groups as described below. Because a number of subjects participated more than once, we were able to analyze the data separately with respect to their status as naive or non-naive subjects. Generally, experiments in which non-naive subjects participated were separated by at least 6 weeks. Simultaneous spatial two-point discrimination performance was tested in a two-alternative forced-choice tactile discrimination task. Seven pairs of needles (diameter, 200 μm) with separation distances of 0.7, 1.0, 1.3, 1.6, 1.9, 2.2, and 2.5 mm were used. In addition, zero distance was tested with a single needle. The needles were mounted on a rotatable disk that allowed us to switch rapidly between distances. To accomplish a rather uniform and standardized type of stimulation, we installed the disk in front of a plate that was movable up and down. The arm and fingers of the subjects were fixated on the plate, and the subjects were then asked to move the arm down. The down movement was arrested by a stopper at a fixed position above the needles. The test finger was held in a hollow containing a small hole through which the finger came to touch the needles at approximately the same indentations in each trial. Each distance of the needles was tested 10 times in randomized order, resulting in 80 single trials per session. The subject had to decide immediately whether he or she had the sensation of one or two tips. Generally, the index finger of the right hand (right-IF) was tested. The middle finger of the right hand (right-MF) or the index finger of the left hand (left-IF) served as a control.
The subject's responses (“0” for one tip and “1” for two tips) were summed for each distance separately. A sum of 10 indicates that in each of the 10 trials the subject indicated that he or she had perceived two tips. These values were plotted against tip distance as a psychometric function and were fitted by means of a logistic maximum likelihood estimation [adapted from Harvey (1986)]. The threshold was taken from the fitted curve at that distance for which a level of 50% correct responses was reached.
Experimental testing of the right-IF was performed on 7 consecutive days that were denoted day −4 to day 2, with day −4 the first day of the test period, day 0 the first day at which the tactile coactivation protocol was applied, and day 2 the second day after coactivation. The 5 d (day −4 to day 0) before coactivation were used as the training period to allow the subjects to reach a constant level of performance. On the fifth day of the training period (day 0), the discrimination performance of the control finger (right-MF or left-IF) was additionally tested.
After the discrimination thresholds of the test and the control fingers were measured on day 0, the coactivation protocol was applied to the test finger (right-IF). The discrimination performance of both the test and the control fingers were retested immediately after termination of the coactivation protocol. Assessment of discrimination performance of the test finger was repeated for 2 consecutive days (day 1 and day 2).
The timing of the coactivation protocol was the same as that in our previous neurophysiological study. To prevent habituation during the long-lasting stimulation over several hours, we presented the applied stimuli at eight different interstimulus intervals (ISIs) between 100 and 3000 msec in pseudorandomized order, resulting in a mean stimulation frequency of 1 Hz (Godde et al., 1996). The duration of each pulse was 10 msec. This protocol was used for a group of 11 subjects. The remaining subjects were tested with a slightly modified protocol, in which ISIs were randomized between 8 and 1761 msec, resulting in a mean frequency of 1.7 Hz. Because the outcome of the experiments was unaffected by the slight differences in average frequency, we pooled the data for further analysis.
Pulses were recorded on tape and were played back via portable tape recorders (Walkman), allowing unrestrained mobility of the subjects during the coactivation period. In fact, all subjects resumed their normal day's work. To apply coactivation, a small solenoid with a diameter of 8 mm was mounted to the tip of the right index finger and was used to transmit the tactile stimuli of the coactivation protocol to the skin. The solenoid allowed simultaneous stimulation of the selected skin portions leading to coactivation of all partially overlapping and nonoverlapping receptive fields within this area. Coactivation stimuli were applied at suprathreshold intensities. Subjects were instructed not to attend the stimulation. Stimulation duration was 6, 2, or 0.5 hr. A control group was sham-stimulated with the stimulator attached to the test finger but without application of the coactivation stimuli. Cumulative effects of coactivation were tested by repeated application of the coactivation protocol on 3 consecutive days. All data were statistically analyzed using ANOVA or one-tailed Student's t test.
RESULTS
A total of 21 right-handed subjects was tested in a two-alternative forced-choice discrimination paradigm to measure simultaneous spatial two-point discrimination thresholds on the tip of the right-IF. A coactivation protocol of associative pairing of tactile stimulation was applied to induce plastic changes of discrimination performance.
Learning curves: naive and non-naive subjects
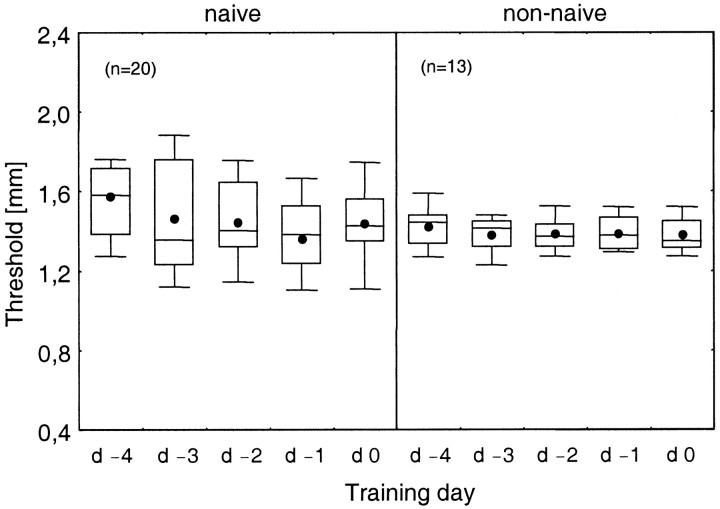
To obtain a stable performance of discrimination and to separate coactivation-induced changes from effects related to simple-task learning, we tested subjects on 5 consecutive days (day −4 to day 0) before the coactivation protocol was applied. The resulting learning curves were computed separately for naive (n = 20) and non-naive (n = 13) subjects. Naive subjects showed a significant improvement of their discrimination performance as indicated by a decrease of their mean discrimination thresholds from 1.57 mm measured on day −4 to 1.44 mm assessed on day 0 [Fig.1; ANOVA,F(3,57) = 4.28; p = 0.0036]. Contrasting the performance on day −4 with that on the other days revealed a significantly lower performance at the first training session than at the following sessions [F(1,19) = 6.621; p = 0.019].
Fig. 1.
Learning curves of naive (left) and non-naive (right) subjects. Thresholds for spatial two-point discrimination as a function of the day of training before induction of changes by a coactivation protocol [day −4 (d −4) to day 0 (d0)] are shown. In this and subsequent figures dots represent the mean thresholds; horizontallineswithinboxes represent the medians.Boxes show the top and bottom quartiles, and theoutliercaps are placed on the top and bottom deciles.
At day −4 non-naive subjects started with a discrimination performance that was significantly lower than the level of naive subjects on their first day of testing (1.42 mm; t31 = 2.01; p = 0.027). In general, non-naive subjects showed a more constant discrimination behavior and a smaller variance throughout the initial training period than did naive subjects. However, differences were not significant [F(1,31) = 1.28; p = 0.266], and naive subjects improved rapidly over the next days of testing. As a consequence, on day 0, naive and non-naive subjects were on approximately the same level of performance (1.42 and 1.38 mm, respectively; t31 = 0.90;p = 0.186).
Changes of the two-point discrimination threshold by a coactivation protocol
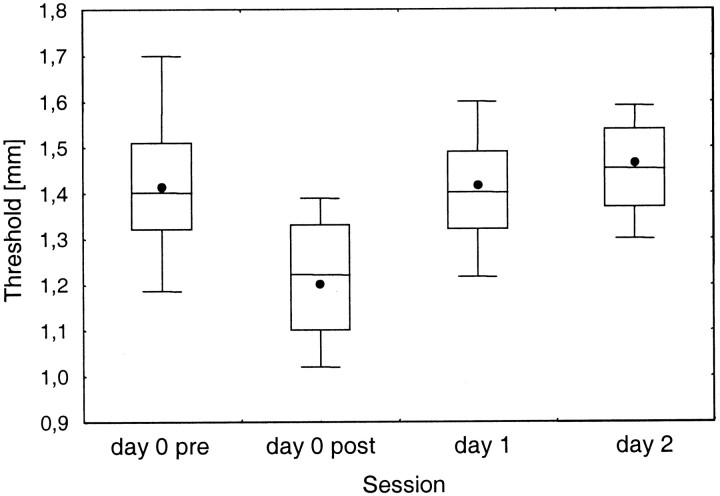
The coactivation protocol was applied at the fifth day of the initial training period (denoted day 0) when the subjects had reached a stable level of performance. In 21 (16 naive, 5 non-naive) subjects, discrimination thresholds were tested before coactivation of 2 or 6 hr duration (day 0 pre), immediately after coactivation (day 0 post), and on 2 consecutive days (day 1, day 2). A multifactorial repeated measures ANOVA revealed a significant coactivation effect on discrimination thresholds [F(3,54) = 6.24; p < 0.001] but no interaction with the status of the subjects as naive or non-naive [F(3,54) = 0.046; p = 0.987] or with the duration of the coactivation [F(3,54) = 0.098; p = 0.961]. Therefore, the results from all subjects independent of their status were pooled for further analysis. Figure2 summarizes the thresholds obtained for the different test sessions. On day 0, the mean discrimination thresholds were reduced from 1.42 mm before coactivation (day 0 pre) to 1.20 mm after coactivation (day 0 post). Significance was tested by apost hoc Scheffé's test (p < 0.001). On the first day after coactivation (day 1), thresholds returned to control values (1.42 mm;p = 0.99). Continuation of testing on the second day after coactivation (day 2) revealed the maintenance of a stable discrimination performance as indicated by the threshold of 1.47 mm (p = 0.97).
Fig. 2.
Effects of coactivation on discrimination thresholds (n = 21). Thresholds were measured at the end of the training period (day 0) before and after application of the coactivation protocol (day 0 pre, day 0 post, respectively) and on the 2 following days (day 1, day 2).
Minimal duration of coactivation
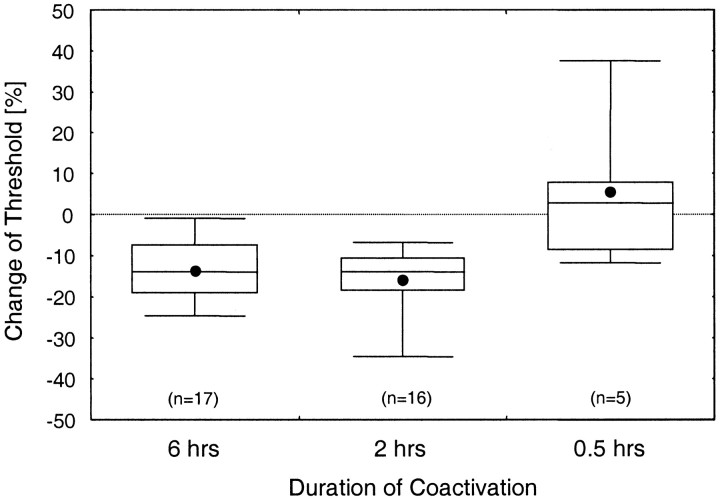
To find the minimal duration necessary to evoke changes of discrimination performance, we tested the efficiency of the coactivation protocol by comparing the results of stimulation of 6 hr (n = 17) and 2 hr (n = 16) with that of only 0.5 hr (n = 5).
As shown in Figure 3, we found a significant difference in threshold changes between the groups [ANOVA,F(2,35) = 7.26; p = 0.0023]. After 6 hr of coactivation, average discrimination thresholds were reduced by 14% from 1.45 to 1.24 mm (t16 = 4.69; p = 0.0002). Two hours of coactivation resulted in a reduction of 16% from 1.38 to 1.16 mm (t15 = 7.89;p < 0.0001). In contrast, when the coactivation protocol was applied for only 30 min, discrimination thresholds remained unaffected, indicating that a critical lower boundary for induction of coactivation-induced changes was reached (1.38 vs 1.44 mm;t4 = −0.55; p = 0.6103). A post hoc Scheffé's test revealed that the coactivation effects were different for durations of 6 and 2 hr compared with 0.5 hr (p = 0.0078 and 0.0028, respectively).
Fig. 3.
Effects of different durations of the coactivation protocol. Shown are relative changes of discrimination thresholds (comparing day 0 pre with day 0 post) after 6, 2, and 0.5 hr of coactivation.
Time course of recovery of coactivation effects
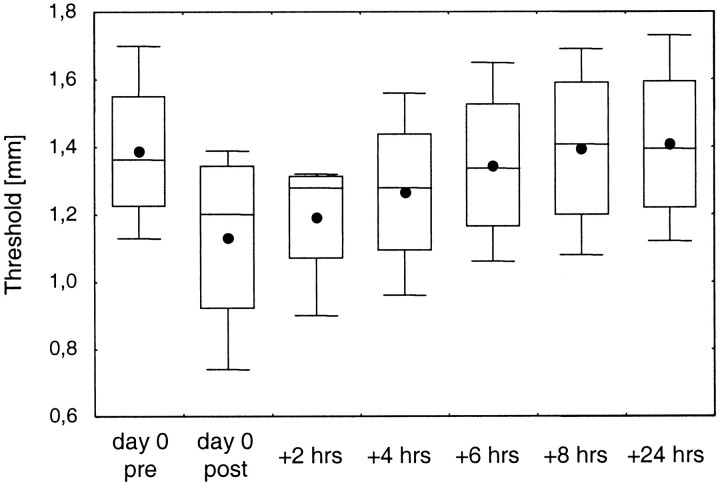
To investigate the time course of the recovery, we measured discrimination thresholds in four subjects 2, 4, 6, and 8 hr after termination of the application of the coactivation protocol for 2 hr. We found that the thresholds recovered continuously over time (Fig.4). A one-way repeated measures ANOVA with thresholds as the repeated measure showed high significance of this recovery effect [F(6,18) = 11.99; p < 0.0001]. A post hocScheffé's test showed that the thresholds 2 hr after termination of coactivation were still significantly lower than that before coactivation (1.20 vs 1.39 mm; p = 0.011). Four hours after termination of coactivation the differences were still evident (mean threshold = 1.27 mm) but did not reach the significance level (p = 0.1886). Full recovery was reached 8 hr after termination of coactivation (threshold = 1.40 mm;p > 0.9999)
Fig. 4.
Recovery of the coactivation effect on discrimination thresholds (n = 4). Thresholds are shown for the before and after conditions (day 0 pre, day 0 post, respectively) and for measurements 2, 4, 6, 8, and 24 hr after termination of the coactivation protocol.
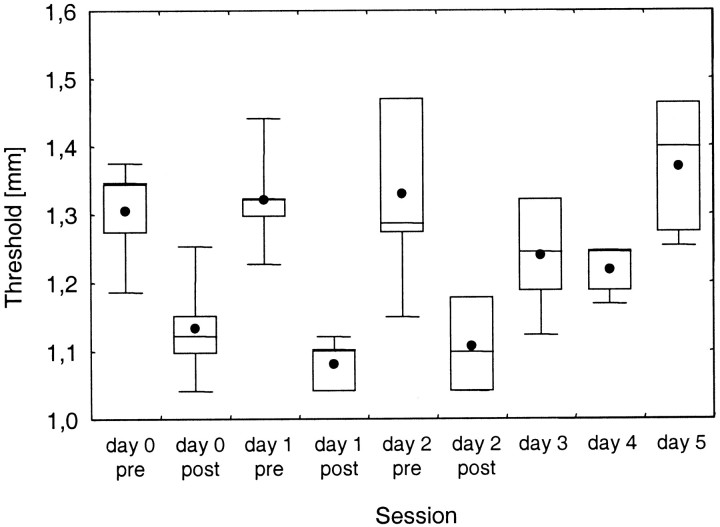
Cumulative effects of coactivation
In five subjects the coactivation protocol was applied for 2 hr on 3 consecutive days to study possible cumulative effects of repeated coactivation. On each of the 3 d (day 0, day 1, and day 2), discrimination thresholds were measured immediately before and after coactivation. After each of the three successive coactivation protocols, thresholds were similarly affected (Fig.5). Mean discrimination thresholds were reduced from 1.30 to 1.13 mm (day 0), from 1.32 to 1.08 mm (day 1), and from 1.33 to 1.11 mm (day 2), confirming the general effects of coactivation shown in Figure 2. A one-way repeated measures ANOVA with before and after coactivation as the repeated measures and the day of coactivation as the factor reveals significance for the coactivation effect [F(1,12) = 165.3;p < 0.0001] but not for different days of performance [F(2,12) = 0.08;p = 0.922] and no interaction [F(2,12) = 1,54; p = 0.2543].
Fig. 5.
Cumulative effects of repeated coactivation (n = 5). Discrimination thresholds before and after (pre, post, respectively) coactivation applied on 3 consecutive days (day 0, day 1, day 2) and on 3 d after the last coactivation application (day 3, day 4, day 5) are shown.
In contrast to the robustness of the coactivation-induced improvement of the discrimination thresholds, a marked effect of the repeated coactivation became apparent after the third day of coactivation, consisting of a significant delay of recovery. Although after the first and second coactivation average thresholds returned to the precontrol level within 24 hr, this was not the case after the third application. After the second coactivation, the mean threshold decreased from 1.33 mm before coactivation (day 2 pre) to 1.11 mm after coactivation (day 2 post) (t4 = 6.43; p = 0.0015). When subjects were tested on the following day (day 3), the mean threshold reached an intermediate level of 1.24 mm (t4 = 3.37; p = 0.014). This improved level of performance was maintained throughout the next 24 hr (day 4), in which the same thresholds could be determined (1.22 mm; t4 = 2.26;p = 0.0.043). Only 72 hr after the coactivation protocol (day 5) did the thresholds return to normal preconditions (1.37 mm; t4 = −1.46;p = 0.109). These results indicate that repeated coactivation affects the time course of recovery, thereby stabilizing the coactivation-induced discrimination performance.
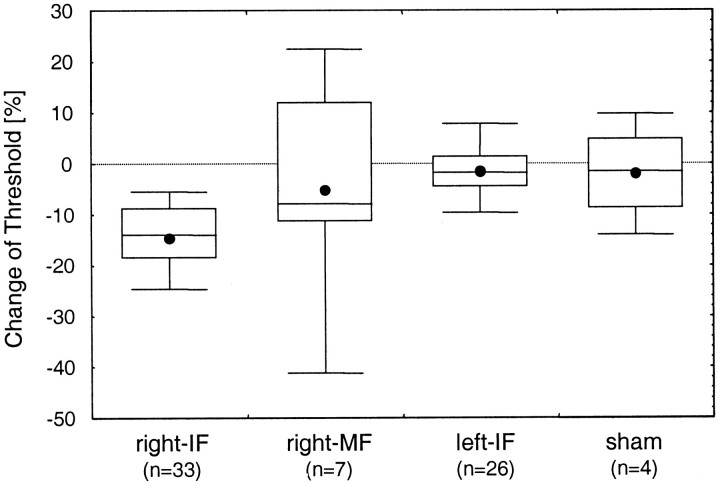
Controls and sham stimulation
To eliminate unspecific effects of the coactivation protocol, we performed a number of control tests. First, the corresponding index finger of the left hand (left-IF; n = 26) and the middle finger of the tested right hand (right-MF; n = 7) served as control fingers. Second, in a series of sham experiments, the entire procedure was followed as described with the exception that no coactivation was applied through the stimulator (n = 4; duration of sham stimulation, 6 hr). Figure6 summarizes the results for the test and control fingers as well as for the sham experiments. The average threshold of the control finger was 1.39 mm for the right-MF and 1.50 mm for the left-IF. After application of the coactivation protocol, the thresholds were 1.34 mm (+5%) for the right-MF and 1.47 mm (+2%) for the left-IF, revealing no significant changes in performance [t6 = 0.50 andp = 0.634 (right-MF);t25 = 1.33 and p = 0.195 (left-IF)]. The mean threshold of the sham-stimulated right-IF was 1.43 mm before sham stimulation and 1.40 mm (+2%) after sham stimulation (t3 = 0.44;p = 0.691). Taken together, none of the control tests performed revealed indications for changes of discrimination performance.
Fig. 6.
Controls. Relative changes of discrimination thresholds (comparing day 0 pre with day 0 post) were measured on the tip of the right-IF, the right-MF, and the left-IF after coactivation was applied to the right index finger. In addition, the result of a sham stimulation protocol (sham) applied to the right index finger is shown.
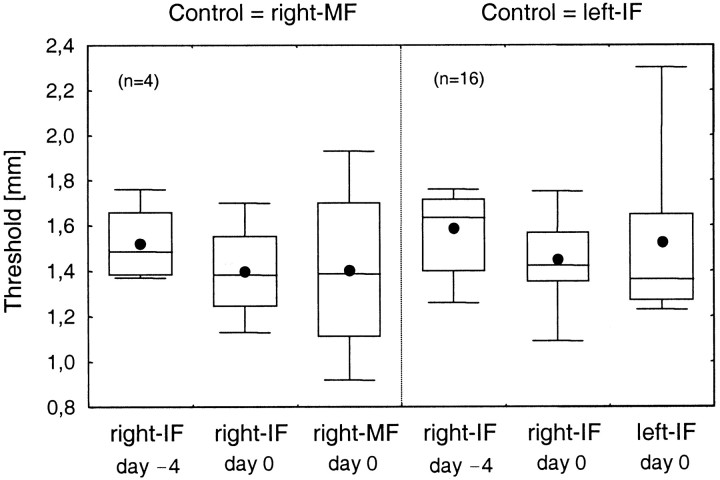
Transfer of discrimination performance during initial training
The control experiments had demonstrated that the coactivation effect was not transferable from the test finger to another finger, either to a neighboring finger of the same hand or to the corresponding finger of the other hand. The transfer of trained abilities is considered an important marker of that level of a sensory pathway from periphery to higher cortical areas at which changes are most likely to occur (Karni and Sagi, 1991; Recanzone et al., 1992a; Schoups et al., 1995; Fahle, 1997). We therefore addressed the question whether the rapid improvement of discrimination performance during the initial training period was subject to transfer to other fingers. For naive subjects we compared the discrimination thresholds of the control fingers (right-MF or left-IF) assessed at the end of the training period (day 0) with the thresholds of the right-IF tested at the first day (day −4) as well as at the end of the training period (day 0).
As shown in Figure 7, in four subjects tested with the right-MF as the control finger, on day 0 the mean threshold of the right-MF (1.41 mm) was comparable with the threshold of the right-IF (1.40 mm; t3 = 0.08;p = 0.94) although on day 0 the right-MF was tested for the first time. This superior performance of the right-MF was substantiated when comparing thresholds obtained for the right-IF (1.52 mm) when tested for the first time (day −4) with the threshold of the right-MF under the same conditions (1.41 mm) indicative for transfer of a training effect. In 16 subjects tested with the left-IF as the control finger, a similar behavior was found. The mean threshold of the left-IF (1.52 mm) was in between the thresholds assessed for the right-IF on day 0 (1.45 mm) and on day −4 (1.59 mm). However, none of the differences were significant [t3 = 0.65 and p = 0.56 (control, right-MF); t15 = 1.07 and p = 0.30; t15 = 0.57 and p = 0.58 (control, left-IF)]. Yet, the results indicate a trend that the initial learning can be transferred to another finger, possibly preferentially easier to a finger of the same hand than to the corresponding finger of the other hand.
Fig. 7.
Transfer of improvement of discrimination performance during the initial training period. Discrimination thresholds were measured for the test finger (right-IF) on the first day of testing (day −4) and at the end of the initial training period (day 0) and for the control fingers on day 0 (corresponding to the first day of testing of the control fingers). Left,Subjects with the right-MF as the control finger. Right,Subjects with the left-IF as the control finger.
DISCUSSION
Coactivation-induced plasticity
We used spatial discrimination performance as a probe to study reorganizational effects of the variation of input statistics on human perception. Plastic changes were induced by an simultaneous, Hebbian-like pairing of natural (i.e., tactile) stimulation resulting in temporally coherent coactivation. We found that 2 hr of coactivation could drive a 14% improvement of the spatial discrimination performance of human subjects. This change was fully reversible within 4–8 hr. To establish a lower limit of the efficiency of the coactivation protocol, we found that 30 min were not sufficient to evoke threshold changes. Repeated application of coactivation on 3 consecutive days resulted in a delayed recovery indicating stabilization of the improvement over time. Perceptual changes were highly selective because no transfer of changes to the middle finger of the same hand or to the index finger of the left hand was found. The data imply that human spatial discrimination performance is subject to improvement by a purely Hebbian coactivation protocol and that spatially highly specific plastic processes can be induced without involving attention or reinforcement. The short timescale of the coactivation-induced reorganization and the aspect of reversibility support the assumption of fast modulations of synaptic efficiency in dynamically maintained networks. In this experiment we used simultaneity in the sense of strict coincidence. Further experiments are needed to study possible effects of temporal delays and temporal pattern on coactivation-induced plasticity.
Relation of psychophysical changes to cortical reorganization
In our previous electrophysiological experiments performed in the hindpaw representation of rat somatosensory cortex (Godde et al., 1996), nonoverlapping or only partially overlapping receptive fields on the hindpaw were used for coactivation. After a few hours, receptive fields showed normal, low-threshold cutaneous characteristics but were increased in size by integration of the stimulated skin sites. The size of the cortical area representing the stimulated skin fields increased severalfold. As a consequence, the topography of the hindpaw was dominated by the representation of the stimulation sites indicative for integration of inputs. This result is in accordance with the observation of Wang et al. (1995), who showed that synchronously applied stimuli resulted in the integration of inputs in the cortical maps, whereas stimuli applied asynchronously were segregated. On the basis of studies using magnetoencephalography, Liepert et al. (1999)reported that 45 min of synchronous movements of the thumb and foot resulted in a reduction of the distance between the corresponding current sources in primary motor cortex, whereas asynchronous movements evoked no significant changes (Liepert et al., 1999).
If we assume that the coactivation protocol results in comparable changes in both man and rat, the enhancement of the discrimination performance might at first appear surprising in view of the reported receptive field enlargement. However, it is a frequent finding that there is a discrepancy between perceptual thresholds and single-neuron properties. Hyperacuity, for example, cannot be explained on the basis of concepts of receptive field sizes of single cells (Westheimer, 1979). Coactivation-induced plasticity included an enlargement of receptive fields accompanied by an increase of receptive field overlap and an enlargement of the representational maps, thus increasing the number of neurons activated by the stimulation. In addition, temporal aspects of neuron responses were changed in terms of response duration (Godde et al., 1996) and paired-pulse behavior (H. R. Dinse, unpublished observations). It is well established that repetitively applied stimuli alter the cortical response behavior (Lee and Whitsel, 1992; Tommerdahl et al., 1998; Buonomano, 1999). It seems reasonable that all changes taken in concert enable cortical networks to perform a faster and more elaborate decoding and processing of information (Dinse et al., 1997).
From a theoretical point of view, the “coarse coding” principle (Hinton et al., 1986; Baldi and Heiligenberg, 1988; Eurich and Schwegler, 1997) was used to explain high-resolution performance by a population of neurons with broad-tuning characteristics; with sufficient overlap, each desired resolution can be achieved. Computer simulation using our electrophysiological data predicted a reduction in discrimination threshold by 15–20% on the pads and by 10–15% on the digits (Eurich et al., 1997). Population-coding approaches allow optimal reconstruction of a desired parameter (Georgopoulos et al., 1986; Salinas and Abbott, 1994). Jancke et al. (1999) showed that a population of neurons recorded from cat visual area 17 represented the actual position of a stimulus with deviations severalfold smaller than the average receptive field size.
In our psychophysical experiments, we did not test for localization abilities. Evidence of a trade-off between localization and discrimination was provided by Sterr et al. (1998) who reported that in three-finger Braille readers stimuli on the reading fingers were more often mislocalized than that on control fingers. This finding suggests that spatial discrimination performance might benefit from enlarged receptive fields on the cost of localization performance.
Learning curves, transfer, and generalization
The degree of transfer of learning-induced changes is considered an important marker of that level of the sensory pathway where changes are most likely to occur (Karni and Sagi, 1991; Recanzone et al., 1992a). In perceptual learning, no general rules seem to apply, but transfer appears to be highly task- and modality-specific. In the visual system perceptual learning can be highly specific for stimulus location, orientation, or color (Schoups et al., 1995; Crist et al., 1997; Fahle, 1997). In contrast, learning of a tactile hyperacuity task has been shown to be completely transferable to the same finger of the opposite hand (Sathian and Zangaladze, 1998).
In our study the coactivation effect was restricted to the stimulated index finger with no effects on the middle finger of the same hand or the index finger of the opposite hand. The differences in transfer seen in the tactile hyperacuity task and in our coactivation protocol might indicate different mechanisms being involved in perceptual learning and in improvement of performance after passive stimulation. We observed an initial learning period that consisted only of the first two training sessions. It is assumed that this initial improvement reflects mainly the learning of the task in terms of cognitive aspects to find an optimal strategy (cf. Recanzone et al., 1992a). In contrast to the effects induced by the coactivation protocol, there was a trend for a partial transfer of this initial improvement to another finger, possibly preferentially easier to a finger of the same hand than to the same finger of the contralateral hand. The lack of initial learning in the group of non-naive subjects who started with lower thresholds than naive subjects further supports this view.
Possible changes in the hand are unlikely to result from the soft stimuli of the coactivation protocol, and unspecific effects of the test stimuli have been eliminated by the sham and control experiments. However, subcortical nuclei have been shown to contain significant plastic capacities (Florence and Kaas, 1995; Faggin et al., 1997; Jones and Pons, 1998; Melzer and Smith, 1998; Nicolelis et al., 1998;Xu and Wall, 1999). The considerable spatial selectivity of the coactivation effects provides a fairly direct argument that the underlying neural changes are most probably occurring within early representations that must contain well ordered topographic maps to allow for this selectivity. Recent studies have stressed a crucial cortical role in mediating plastic changes (Darian-Smith and Gilbert, 1995; Wang et al., 1995; Florence et al., 1998; Kaas, 1999; Krupa et al., 1999). In our view, a cortical involvement is directly supported by the evidence from our electrophysiological experiments performed in the SI.
Reversibility and stability of coactivation-induced changes
To examine possible long-term effects, we applied the coactivation protocol on 3 consecutive days. Repeated coactivation had no effect on the magnitude of the threshold changes but affected the time course of recovery. After the third day, thresholds did not return to normal but remained at an intermediate level for 2 consecutive days, indicating that prolonged coactivation acts to stabilize the obtained perceptual changes. Conceivably, the short period of maintained changes is most likely caused by the short period of induction and must not necessarily reflect characteristics of the coactivation. This view is supported by psychophysical experiments addressing the long-term retention of perceptual learning of a tactile hyperacuity task (Sathian and Zangaladze, 1998). When subjects were tested some months later, the long-term retention of learning was limited, and further practice was required to stabilize performance.
Input statistics versus attention
Attention plays an important role in learning processes and cortical plasticity (Recanzone et al., 1992b; Ahissar and Hochstein, 1993; Weinberger, 1995; Goldstone, 1998; Buchner et al., 1999). However, recent experiments indicated that attentional mechanisms themselves were subject to practice (Ito et al., 1998). A similar conclusion was reached by Sireteanu and Rettenbach (1995) who showed that training transforms serial search tasks to parallel tasks. Perceptual learning of this type is often characterized by a high specificity to stimulus parameters such as location or orientation, suggesting the involvement of early stages of cortical processing (Karni and Sagi, 1991; Crist et al., 1997; Fahle, 1997). It is suggested that specific high-level attentional mechanisms act to control changes at early visual-processing levels via top-down modulations (Ahissar and Hochstein, 1993). In animal experiments, pairing of sensory stimulation with electrical stimulation of the nucleus basalis was shown to result in rapid and selective reorganization (Rasmusson and Dykes, 1988; Edeline et al., 1994; Bakin and Weinberger, 1996; Bjordahl et al., 1998; Kilgard and Merzenich, 1998). In addition, lesion of the cholinergic system that provides modulatory input from the basal forebrain to the neocortex has been shown to prevent plastic reorganization (Baskerville et al., 1997;Sachdev et al., 1998), implying that cholinergic inputs may represent one example of top-down modulatory inputs.
As discussed above, the coactivation protocol was introduced as a tool to study in vivo consequences of pure input statistics. In learning, the term association is often used to refer to a linkage between stimulation and reward. We used this term to indicate an association between the stimuli that are used for coactivation. The electrophysiological experiments were performed in anesthetized animals (Godde et al., 1996) eliminating the involvement of attentional mechanisms. In the human psychophysical experiments, subjects were instructed not to attend the stimulation. In fact, during the several hours of coactivation all subjects continued their normal business work. The engagement in normal day work had not been possible without the simultaneous attentive engagement in other perceptual and motor tasks. We therefore conclude that the changes of thresholds observed in these experiments are most likely caused by the tactile coactivation patterns. Consequently imposing such pattern seems sufficient to drive perceptual changes within a few hours. Further experiments are under way to study the implications of asynchronous stimulation.
Footnotes
We acknowledge support of the Institute for Neuroinformatics (Bochum, Germany) where the experiments were performed. B.G. was supported by the Volkswagen-Stiftung Grant AZ I/73035 (junior research group “cortical reorganization and learning”). We thank Dr. Jancke for helpful discussion of this manuscript.
Correspondence should be addressed to Dr. Ben Godde, University of Tübingen, Institute of Medical Psychology, Gartenstrasse 29, 72074 Tübingen, Germany. E-mail:benjamin.godde@uni-tuebingen.de.
REFERENCES
- 1.Ahissar M, Hochstein S. Attentional control of early perceptual learning. Proc Natl Acad Sci USA. 1993;90:5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahissar E, Vaadia E, Ahissar M, Bergman H, Arieli A, Abeles M. Dependence of cortical plasticity on correlated activity of single neurons and on behavioral context. Science. 1992;257:1412–1415. doi: 10.1126/science.1529342. [DOI] [PubMed] [Google Scholar]
- 3.Ahissar E, Abeles M, Ahissar M, Haidarliu S, Vaadia E. Hebbian-like functional plasticity in the auditory cortex of the behaving monkey. Neuropharmacology. 1998;37:633–655. doi: 10.1016/s0028-3908(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 4.Allard TT, Clark SA, Jenkins WM, Merzenich MM. Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J Neurophysiol. 1991;66:1048–1058. doi: 10.1152/jn.1991.66.3.1048. [DOI] [PubMed] [Google Scholar]
- 5.Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldi P, Heiligenberg W. How sensory maps could enhance resolution through ordered arrangements of broadly tuned receivers. Biol Cybern. 1988;59:313–318. doi: 10.1007/BF00332921. [DOI] [PubMed] [Google Scholar]
- 7.Baskerville KA, Schweitzer JB, Herron P. Effects of cholinergic depletion on experience-dependent plasticity in the cortex of the rat. Neuroscience. 1997;80:1159–1169. doi: 10.1016/s0306-4522(97)00064-x. [DOI] [PubMed] [Google Scholar]
- 8.Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behav Neurosci. 1998;112:467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- 9.Buchner H, Reinartz U, Waberski TD, Gobbele R, Noppeney U, Scherg M. Sustained attention modulates the immediate effect of de-afferentiation on the cortical representation of the digits: source localization of somatosensory evoked potentials in humans. Neurosci Lett. 1999;260:57–60. doi: 10.1016/s0304-3940(98)00948-3. [DOI] [PubMed] [Google Scholar]
- 10.Buonomano DV. Distinct functional types of associative long-term potentiation in neocortical and hippocampal pyramidal neurons. J Neurosci. 1999;19:6748–6754. doi: 10.1523/JNEUROSCI.19-16-06748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark SA, Allard TT, Jenkins WM, Merzenich MM. Receptive fields in the body-surface map in adult cortex defined by temporally correlated input. Nature. 1988;332:444–445. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LG, Brasil N, Pascual-Leone A, Hallett M. Plasticity of cortical motor output organization following deafferentation, cerebral lesions, and skill acquisition. Adv Neurol. 1993;63:187–200. [PubMed] [Google Scholar]
- 13.Crist RE, Kapadia MK, Westheimer G, Gilbert CD. Perceptual learning of spatial localization: specificity for orientation, position, and context. J Neurophysiol. 1997;78:2889–2894. doi: 10.1152/jn.1997.78.6.2889. [DOI] [PubMed] [Google Scholar]
- 14.Cruikshank SJ, Weinberger NM. Evidence for the Hebbian hypothesis in experience-dependent physiological plasticity of neocortex: a critical review. Brain Res Rev. 1996a;22:191–228. doi: 10.1016/s0165-0173(96)00015-x. [DOI] [PubMed] [Google Scholar]
- 15.Cruikshank SJ, Weinberger NM. Receptive-field plasticity in the adult auditory cortex induced by Hebbian covariance. J Neurosci. 1996b;16:861–875. doi: 10.1523/JNEUROSCI.16-02-00861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci USA. 1993;90:2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinse HR, Godde B, Hilger T, Haupt SS, Spengler F, Zepka R. Short-term functional plasticity of cortical and thalamic sensory representations and its implication for information processing. Adv Neurol. 1997;73:159–178. [PubMed] [Google Scholar]
- 19.Edeline JM. Does Hebbian synaptic plasticity explain learning-induced sensory plasticity in adult mammals? J Physiol (Paris) 1996;90:271–276. doi: 10.1016/s0928-4257(97)81437-4. [DOI] [PubMed] [Google Scholar]
- 20.Edeline JM, Hars B, Maho C, Hennevin E. Transient and prolonged facilitation of tone-evoked responses induced by basal forebrain stimulations in the rat auditory cortex. Exp Brain Res. 1994;97:373–386. doi: 10.1007/BF00241531. [DOI] [PubMed] [Google Scholar]
- 21.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 22.Eurich CW, Schwegler H. Coarse coding: calculation of the resolution achieved by a population of large receptive field neurons. Biol Cybern. 1997;76:357–363. doi: 10.1007/s004220050349. [DOI] [PubMed] [Google Scholar]
- 23.Eurich CW, Dinse HR, Dicke U, Godde B, Schwegler H. Coarse coding accounts for improvement of spatial discrimination after plastic reorganization in rats and humans. In: Gerstner W, Germond A, Hasler M, Nicaud JD, editors. Artificial neural networks, Proceedings of ICANN 1997. Springer; New York: 1997. pp. 55–60. [Google Scholar]
- 24.Faggin BM, Nguyen KT, Nicolelis MA. Immediate and simultaneous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proc Natl Acad Sci USA. 1997;94:9428–9433. doi: 10.1073/pnas.94.17.9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahle M. Specificity of learning curvature, orientation, and vernier discriminations. Vision Res. 1997;37:1885–1895. doi: 10.1016/s0042-6989(96)00308-2. [DOI] [PubMed] [Google Scholar]
- 26.Florence SL, Kaas JH. Large-scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. J Neurosci. 1995;15:8083–8095. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 28.Fregnac Y, Shulz D, Thorpe S, Bienenstock EL. A cellular analogue of visual cortical plasticity. Nature. 1988;333:367–370. doi: 10.1038/333367a0. [DOI] [PubMed] [Google Scholar]
- 29.Georgopoulos AP, Schwarz A, Kettner RE. Neuron population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- 30.Godde B, Spengler F, Dinse HR. Associative pairing of tactile stimulation induces somatosensory cortical reorganization in rats and humans. NeuroReport. 1996;8:281–285. doi: 10.1097/00001756-199612200-00056. [DOI] [PubMed] [Google Scholar]
- 31.Goldstone RL. Perceptual learning. Annu Rev Psychol. 1998;49:585–612. doi: 10.1146/annurev.psych.49.1.585. [DOI] [PubMed] [Google Scholar]
- 32.Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput. 1986;18:623–632. [Google Scholar]
- 33.Hebb DO. The organization of behavior. Wiley; New York: 1949. [Google Scholar]
- 34.Hinton GE, McClelland JL, Rumelhart DE. Distributed representations. In: Feldman JA, Hayes PJ, Rumelhart DE, editors. Parallel distributed processing. Exploration in the microstructure of cognition, Vol I, Foundations. MIT; Cambridge, MA: 1986. pp. 77–109. [Google Scholar]
- 35.Ito M, Westheimer G, Gilbert CD. Attention and perceptual learning modulate contextual influences on visual perception. Neuron. 1998;20:1191–1197. doi: 10.1016/s0896-6273(00)80499-7. [DOI] [PubMed] [Google Scholar]
- 36.James W. Psychology: brief course. Harvard UP; Cambridge, MA: 1890. [Google Scholar]
- 37.Jancke J, Erlhagen W, Dinse HR, Akhavan AC, Giese M, Steinhage A, Sch÷ner G. Parametric population representation of retinal location: neuronal interaction dynamics in cat primary visual cortex. J Neurosci. 1999;19:9016–9028. doi: 10.1523/JNEUROSCI.19-20-09016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones EG, Pons TP. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science. 1998;282:1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- 39.Kaas JH. Is most of neural plasticity in the thalamus cortical? Proc Natl Acad Sci USA. 1999;96:7622–7623. doi: 10.1073/pnas.96.14.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci USA. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 42.Krupa DJ, Ghazanfar AA, Nicolelis MA. Immediate thalamic sensory plasticity depends on corticothalamic feedback. Proc Natl Acad Sci USA. 1999;96:8200–8205. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee CJ, Whitsel BL. Mechanisms underlying somatosensory cortical dynamics. I. In vivo studies. Cereb Cortex. 1992;2:81–106. doi: 10.1093/cercor/2.2.81. [DOI] [PubMed] [Google Scholar]
- 44.Liepert J, Terborg C, Weiller C. Motor plasticity induced by synchronized thumb and foot movements. Exp Brain Res. 1999;125:435–439. doi: 10.1007/s002210050700. [DOI] [PubMed] [Google Scholar]
- 45.Melzer P, Smith CB. Plasticity of cerebral metabolic whisker maps in adult mice after whisker follicle removal. II. Modifications in the subcortical somatosensory system. Neuroscience. 1998;83:43–61. doi: 10.1016/s0306-4522(97)00333-3. [DOI] [PubMed] [Google Scholar]
- 46.Nicolelis MA, Katz D, Krupa DJ. Potential circuit mechanisms underlying concurrent thalamic and cortical plasticity. Rev Neurosci. 1998;9:213–224. doi: 10.1515/revneuro.1998.9.3.213. [DOI] [PubMed] [Google Scholar]
- 47.Pantev C, Oostenveld R, Engelien A, Ross B, Roberts LE, Hoke M. Increased auditory cortical representation in musicians. Nature. 1998;392:811–814. doi: 10.1038/33918. [DOI] [PubMed] [Google Scholar]
- 48.Pascual-Leone A, Torres F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- 49.Rasmusson DD, Dykes RW. Long-term enhancement of evoked potentials in cat somatosensory cortex produced by co-activation of the basal forebrain and cutaneous receptors. Exp Brain Res. 1988;70:276–286. doi: 10.1007/BF00248353. [DOI] [PubMed] [Google Scholar]
- 50.Recanzone GH, Jenkins WM, Hradek GT, Merzenich MM. Progressive improvement in discriminative abilities in adult owl monkeys performing a tactile frequency discrimination task. J Neurophysiol. 1992a;67:1015–1030. doi: 10.1152/jn.1992.67.5.1015. [DOI] [PubMed] [Google Scholar]
- 51.Recanzone GH, Merzenich MM, Jenkins WM, Grajski K, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b of owl monkeys trained in a frequency discrimination task. J Neurophysiol. 1992b;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 52.Sachdev RN, Lu SM, Wiley RG, Ebner FF. Role of the basal forebrain cholinergic projection in somatosensory cortical plasticity. J Neurophysiol. 1998;79:3216–3228. doi: 10.1152/jn.1998.79.6.3216. [DOI] [PubMed] [Google Scholar]
- 53.Salinas E, Abbott LF. Vector reconstructing from firing. J Comput Neurosci. 1994;1:89–107. doi: 10.1007/BF00962720. [DOI] [PubMed] [Google Scholar]
- 54.Sathian K, Zangaladze A. Perceptual learning in tactile hyperacuity: complete intermanual transfer but limited retention. Exp Brain Res. 1998;118:131–134. doi: 10.1007/s002210050263. [DOI] [PubMed] [Google Scholar]
- 55.Schoups AA, Vogels R, Orban GA. Human perceptual learning in identifying the oblique orientation: retinotopy, orientation specificity and monocularity. J Physiol (Lond) 1995;483:797–810. doi: 10.1113/jphysiol.1995.sp020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sireteanu R, Rettenbach R. Perceptual learning in visual search: fast, enduring, but non-specific. Vision Res. 1995;35:2037–2043. doi: 10.1016/0042-6989(94)00295-w. [DOI] [PubMed] [Google Scholar]
- 57.Sterr A, Müller MM, Elbert T, Rockstroh B, Pantev C, Taub E. Perceptual correlates of changes in cortical representation of fingers in blind multifinger Braille readers. J Neurosci. 1998;18:4417–4423. doi: 10.1523/JNEUROSCI.18-11-04417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tommerdahl M, Delemos KA, Favorov OV, Metz CB, Vierck CJ, Jr, Whitsel BL. Response of anterior parietal cortex to different modes of same-site skin stimulation. J Neurophysiol. 1998;80:3272–3283. doi: 10.1152/jn.1998.80.6.3272. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- 60.Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westheimer G. Cooperative neural processes involved in stereoscopic acuity. Exp Brain Res. 1979;36:585–597. doi: 10.1007/BF00238525. [DOI] [PubMed] [Google Scholar]
- 62.Xu J, Wall JT. Evidence for brainstem and supra-brainstem contributions to rapid cortical plasticity in adult monkeys. J Neurosci. 1999;19:7578–7590. doi: 10.1523/JNEUROSCI.19-17-07578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]