Abstract
The mechanisms responsible for anchoring molecular components of postsynaptic specializations in the mammalian brain are not well understood but are presumed to involve associations with cytoskeletal elements. Here we build on previous studies of neurotransmitter receptors (Allison et al., 1998) to analyze the modes of attachment of scaffolding and signal transducing proteins of both glutamate and GABA postsynaptic sites to either the microtubule or microfilament cytoskeleton. Hippocampal pyramidal neurons in culture were treated with latrunculin A to depolymerize actin, with vincristine to depolymerize microtubules, or with Triton X-100 to extract soluble proteins. The synaptic clustering of PSD-95, a putative NMDA receptor anchoring protein and a core component of the postsynaptic density (PSD), was unaffected by actin depolymerization, microtubule depolymerization, or detergent extraction. The same was largely true for GKAP, a PSD-95-interacting protein. In contrast, the synaptic clustering of Ca2+/calmodulin-dependent protein kinase II (CaMKII)α, another core component of the PSD, was completely dependent on an intact actin cytoskeleton and was partially disrupted by detergent. Drebrin and α-actinin-2, actin-binding proteins concentrated in spines, were also dependent on F-actin for synaptic localization but were unaffected by detergent extraction. Surprisingly, the subcellular distributions of the inhibitory synaptic proteins GABAAR and gephyrin, which has a tubulin-binding motif, were unaffected by depolymerization of microtubules or actin or by detergent extraction. These studies reveal an unsuspected heterogeneity in the modes of attachment of postsynaptic proteins to the cytoskeleton and support the idea that PSD-95 and gephyrin may be core scaffolding components independent of the actin or tubulin cytoskeleton.
Keywords: actin, microtubules, postsynaptic density, GABA receptor, gephyrin, PSD-95, GKAP, CaMKIIα, drebrin, α-actinin-2
The neuronal cytoskeleton is a complex meshwork consisting of microtubules, actin microfilaments, intermediate filaments, and many associated proteins. These systems are responsible for determining neuronal morphology and for transport and anchoring of cellular constituents. Localization of postsynaptic proteins to their sites of function may require interactions with regulatory enzymes, vesicular adapters, molecular motors, scaffolding proteins, and cytoskeletal systems.
More than 90% of excitatory glutamatergic synapses in the mammalian brain occur on dendritic spines, small actin-rich protrusions (Harris and Kater, 1994). Although diverse in size and morphology, spines have a general structure containing longitudinal actin filaments in the neck and a lattice of actin filaments in the head (Landis and Reese, 1983;Cohen et al., 1985; Fifková, 1985). Virtually all excitatory synapses have a pronounced postsynaptic density (PSD) that contains receptors, signal transducing proteins, and cytoskeletal proteins and is typically identified as an electron-dense and almost completely detergent-resistant structure found just beneath the membrane of dendritic spines (Peters et al., 1991; Kennedy, 1997). Key elements of the core PSD include NMDA-type glutamate receptors, Ca2+/calmodulin-dependent protein kinase II (CaMKII), and the PSD-95/SAP90 family of PDZ domain proteins (Kennedy, 1997). CaMKIIα binds to F-actin via its interaction with CaMKIIβ, PSD-95 binds to MAP1A and CRIPT, both of which are microtubule-binding proteins, and the NR1 subunit of the NMDA receptor can bind to low molecular weight neurofilament (Brenman et al., 1998;Ehlers et al., 1998; Niethammer et al., 1998; Shen et al., 1998). Thus individual components of glutamate synapses may be anchored to actin-, tubulin-, or neurofilament-based cytoskeletal systems, or their localization may be independent of these major cytoskeletal elements. In previous studies (Allison et al., 1998), we showed that synaptic clustering of NMDA receptors is largely independent of F-actin, whereas synaptic clustering of AMPA receptor is strongly dependent on F-actin in hippocampal pyramidal cells.
Inhibitory GABAergic synapses occur primarily on cell bodies and on the shafts of dendrites and axon initial segments. The GABAA receptor is thought to be attached to the microtubule cytoskeleton via gephyrin and/or GABARAP (Kirsch and Betz, 1995; Essrich et al., 1998; Wang et al., 1999). Gephyrin binds to microtubules in vitro and is required for synaptic localization of GABAA receptors in hippocampus (Kirsch et al., 1991; Essrich et al., 1998; Kneussel et al., 1999).
Many proteins bind to cytoskeletal components in vitro, but which of these interactions are important for localization of the proteins to the synapse? In this study, we induced depolymerization of actin filaments or microtubules and performed detergent extraction to assess mechanisms of cytoskeletal anchoring of components of glutamate and GABA postsynaptic specializations in hippocampal pyramidal neurons in culture. We show that individual components of spine synapses show a differential dependence on F-actin for localization and, surprisingly, that GABAAR and gephyrin are not dependent on microtubules for synaptic localization. Our results suggest that PSD-95 and gephyrin may be core components of excitatory and inhibitory synaptic scaffolds that are maintained independently of conventional cytoskeletal elements.
MATERIALS AND METHODS
Cell cultures. Rat hippocampal cultures were prepared using previously described methods (Banker and Cowan, 1977; Goslin et al., 1998). Briefly, hippocampi were dissected from 18 d rat embryos and dissociated using trypsin and trituration through a Pasteur pipette. The neurons were plated on coverslips coated with poly-l-lysine in minimal essential medium with 10% horse serum at an approximate density of 2400/cm2. Once the neurons had attached to the substrate, they were transferred to a dish containing a glial monolayer and maintained for up to 4 weeks in serum-free MEM with N2 supplements. Latrunculin A (5 μm) and vincristine (5 μm) were added directly to the culture medium from a concentrated DMSO or methanol stock, respectively. Reversal of the effects of vincristine was accomplished after a 5 hr treatment in vincristine followed by a 24 hr incubation in a fresh glial dish with conditioned MEM plus N2 supplements. Vincristine was obtained from Sigma (St. Louis, MO), and latrunculin A was obtained from Biomol Research Laboratories (Plymouth Meeting, PA). For extraction, the neurons were treated with 1% Triton X-100 and 4% polyethylene glycol (PEG; molecular weight 40,000) in BRB80 buffer (80 mmPIPES, 1 mm MgCl2, 1 mmEGTA) for 5 min, rinsed in BRB80, and fixed as described below.
Immunocytochemistry. Neurons were either fixed at 18–23 d in culture in warm 4% paraformaldehyde, 4% sucrose in PBS for 15 min followed by permeabilization with 0.25% Triton X-100 for 5 min (for immunocytochemistry for GABAAR, drebrin, α-actinin-2, and gephyrin) or simultaneously fixed and permeabilized in methanol for 15 min at −20°C (for immunocytochemistry involving PSD-95, GKAP, and CaMKIIα). The cultures were incubated with 10% bovine serum albumin (BSA) for 30 min at 37°C to block nonspecific staining and incubated with the primary antibodies in 3% BSA. Presynaptic sites were labeled with a rabbit antiserum G95 against synaptophysin (gift of P. DeCamilli, Yale University; 1:8000) or a mouse monoclonal antibody against SV2 (Developmental Studies Hybridoma Bank, Iowa City, IA; 1:50). F-actin was labeled with rhodamine phalloidin (Molecular Probes, Eugene, OR; 1:10,000). All of the following proteins were stained with mouse monoclonal antibodies: α-actinin (clone EA-53, Sigma; 1:20,000), PSD-95 family (clone 6G6–1C9, Affinity BioReagents, Golden, CO; 1:1000; raised against PSD-95/SAP90 but also appears to cross-react with other family members), drebrin (clone M2F6, Medical and Biological Laboratories, Nagoya, Japan; 1:300), CaMKIIα (clone 6G9, Affinity BioReagents; 1:100), GABAAR β2/3 subunit (clone bd17, Boehringer Mannheim, Indianapolis, IN; 1:100), tubulin (clone DM1α, Sigma; 1:1000), and gephyrin (clone R7A, Cedarlane Laboratories, Hornby, Ontario, Canada; 1:1000). Other proteins were stained with rabbit polyclonal antibodies: GKAPs (gift of M. Sheng, Harvard University; 1:300; raised against GKAP1 but recognizes multiple GKAPs), MAP2 (#266, gift of S. Halpain, Scripps Institute, 1:20,000), tubulin (affinity-purified on tubulin immobilized on BrCN-activated Sepharose; 1:300), GluR1 (Upstate Biotechnology, Lake Placid, NY; 1:4000), and NR2A (Upstate Biotechnology; 1:2000). PSD-95 was also stained with a guinea pig polyclonal antibody (gift of M. Sheng; 1:300). Neurons were generally incubated in primary antibodies for 2 hr at 37°C, or overnight at room temperature (NR2A), or ∼40 hr at 4°C (GABAAR), and in appropriate secondary antibodies for 45 min at 37°C. Secondary antibodies were conjugated to fluorescein, Texas Red, or AMCA (Vector Labs, Burlingame, CA; 1:200–1:600). The coverslips were mounted in elvanol with 2% 1,4-diazabicyclo[2,2,2]octane. Fluorescent images of the neurons were obtained using a Zeiss Axioskop microscope with a 63×, 1.4 N.A. lens and a Photometrics series 250 cooled CCD camera. Images were prepared for presentation using OncorImage or Metamorph and Adobe Photoshop software.
DiI labeling. Neurons were labeled with the lipophilic dye DiI as previously described by Goldberg and colleagues (Park et al., 1996; Hasbani et al., 1998). In summary, neurons were fixed for 30 min in 4% formaldehyde, 4% sucrose in PBS, then washed in PBS several times. DiI (0.4 μg/ml freshly made in PBS) was then added for 30 sec followed by another PBS wash. The coverslips were then mounted on hanging well slides in PBS. Labeled cells were photographed, and their position was noted for subsequent staining. The coverslip was removed from the slide, and the neurons were permeabilized (and DiI removed) with 0.25% Triton X-100 for 10 min before they were labeled with rhodamine phalloidin as described above. Individual neurons were relocated and imaged for phalloidin fluorescence.
Quantitation. To quantitate the data from the immunocytochemistry, pyramidal neurons were chosen randomly for image acquisition (10–15 cells each from three to five separate experiments for paired control, extracted, vincristine, and latrunculin A treatments). For each neuron, two dendrites were chosen for analysis from the phase-contrast image, and their length was measured. To count clusters per dendrite length, the digital images were processed using OncorImage imaging software. Before measuring fluorescence intensities, images were background-subtracted by a dark-field image and divided by the image of a uniform fluorescence field to normalize for potential nonuniformity in illumination. Images were subjected to a user-defined intensity threshold to select spines or clusters (with intensity approximately twofold or greater above the parent dendrite), a selection for region of interest, and a count of the number of clusters along each chosen dendrite. The number of synaptic clusters was determined as the number of clusters apposed to punctate synaptophysin or SV2 immunoreactivity. Dendritic protrusion density was determined from randomly chosen DiI-labeled neurons (18–20 cells each from two separate experiments for paired control, vincristine, and latrunculin A treatments). Protrusions were defined by eye to include any spine-like or filopodial-like dendritic protuberance. All image analysis was performed such that the experimenter was blind to the treatment group. The data were compiled in Microsoft Excel, analyzed in Statview, and plotted using CricketGraph.
RESULTS
Both actin filaments and microtubules can be depolymerized separately and reversibly within hippocampal neurons
We have previously established a protocol for reversibly disrupting the F-actin cytoskeleton in mature primary cultures from embryonic rat hippocampus (Allison et al., 1998). Control pyramidal neurons exhibited large concentrations of F-actin, as visualized with rhodamine phalloidin, within their dendritic spines (Fig.1A). Latrunculin A treatment disrupted actin within the neurons without affecting microtubule staining (Fig. 1B). Latrunculin A depolymerized ∼96% of the F-actin within the neuron, when quantified by rhodamine phalloidin staining (Howard and Oresajo, 1985; Knowles and McCulloch, 1992; Zigmond et al., 1998). The loss of F-actin was also shown by Western blot analysis after detergent extraction of living neurons. Although ∼50% of actin was extracted in control cells, ∼94% was extracted in latrunculin A-treated cells (Allison et al., 1998). F-actin indicated by rhodamine phalloidin staining within the spines returned after 24 hr of recovery without latrunculin A (Fig.1C).
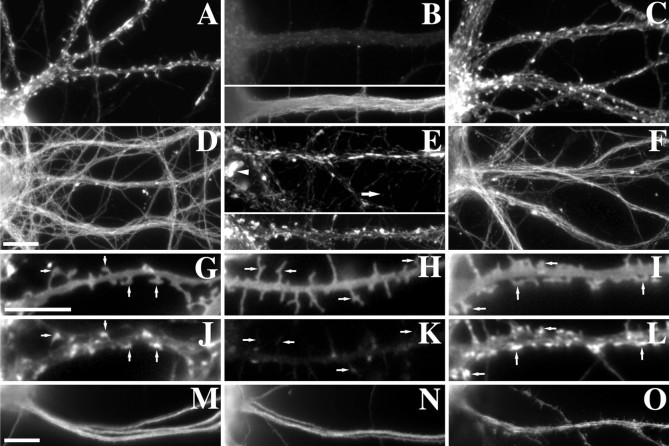
Fig. 1.
Treatment with cytoskeletal depolymerizing drugs reversibly disrupted actin microfilaments or microtubules in cultured hippocampal neurons. A, Rhodamine phalloidin staining of 3 week hippocampal neurons shows a high concentration of F-actin within the dendritic spines of control neurons. B, Treatment with latrunculin A eliminates the F-actin within the neurons, while not affecting microtubule staining (below). C, This effect can be reversed by washing out the drug, allowing the actin filaments to repolymerize. D, Tubulin staining after extraction of control neurons reveals tight bundles of microtubules within the dendrites and axons. E, Treatment with the microtubule depolymerizing agent vincristine eliminates the microtubule bundles, leaving only tubulin paracrystals (arrow indicates smaller axonal paracrystals; arrowhead indicates larger somatodendritic paracrystals), which is characteristic of vincristine treatment. This treatment does not affect the F-actin within spines as seen below. F, Within 24 hr after washing out the vincristine, the microtubule bundles repolymerize.G–L, DiI membrane labeling (G–I) of random subpopulations of neurons reveals the continued presence of dendritic protrusions after cytoskeletal manipulations, regardless of the local concentrations of F-actin (J–L). Control neurons (G, J) and neurons treated with vincristine (I, L) exhibit spines along the shaft of the dendrites with corresponding concentrations of F-actin. Latrunculin A-treated neurons still show protrusions of membrane coming from the dendritic shafts (H) but lacking F-actin (K). M–O, Staining for MAP2 reveals no change after latrunculin A treatment (N) when compared with control (M). Treatment with vincristine does allow MAP2 into the dendritic spines (O) but does not affect polarity. Scale bar, 10 μm.
We have now also established a protocol to disrupt the microtubule cytoskeleton, using the Vinca alkaloid, vincristine. Vincristine disrupts microtubules by binding to tubulin and preventing its polymerization. It also induces tubulin to self-associate into paracrystals (Weber et al., 1975). Many other methods were tried to induce microtubule depolymerization both alone and in combination, including nocodazole, colcemid, vinblastine, and cold treatment, under various conditions and concentrations. These treatments were not effective in the depolymerization of microtubules except at high concentrations, which were then toxic. Vincristine was the only treatment that was successful in depolymerizing microtubules without associated toxicity. Neurons were treated with 5 μmvincristine for 5 hr before extraction and immunostaining for tubulin to reveal microtubules. Control neurons exhibited typical microtubule bundles present within the dendrites and axons (Fig.1D). After treatment with vincristine, the individual microtubules could no longer be seen; they were instead replaced by paracrystals throughout the cell. Both small and relatively large paracrystals could be seen within the cell body and processes of the neurons, but the lack of actual microtubules was evident (Fig.1E) (immunofluorescence intensity for tubulin was reduced by 81.3% between the paracrystals). This treatment did not alter F-actin staining within either the shafts or spines of dendrites (Fig. 1E, bottom). When the drug was removed, the microtubule bundles returned within 24 hr (Fig.1F). Thus these techniques are suitable for assessing the relationship between postsynaptic proteins and the neuronal cytoskeleton.
Because the neuronal cytoskeleton plays such an important role in cell shape and polarity, we looked at the effect that these drug treatments had on the morphology and polarity of the neurons. Staining of a random population of control neurons with the lipophilic dye DiI (Park et al., 1996; Hasbani et al., 1998) reveals a large number of protrusions from the dendrites (45.5 ± 3.8 per 100 μm), representing mostly dendritic spines (Fig. 1G). The spines contain concentrations of F-actin as seen by the corresponding rhodamine phalloidin staining (Fig. 1J). After treatment with latrunculin A, the number of dendritic protrusions does not significantly change (38.9 ± 3.8 protrusions/100 μm,t test, p > 0.1). A similar lack of effect of latrunculin B on the presence of dendritic protrusions has been reported previously (Kim and Lisman, 1999). However, DiI staining revealed an apparent change in the morphology of the protrusions with latrunculin A treatment, corresponding to a large number of elongated filopodia-like protrusions and fewer of the classical mushroom-shaped spines (Fig. 1H). These structures are devoid of detectable F-actin (Fig. 1K). They may be maintained by connection to the presynaptic site via transynaptic proteins. Dendritic profiles of neurons treated with vincristine look virtually identical to those of control cells (Fig.1I,L) (51.02 ± 2.84 protrusions/100 μm, t test, p > 0.1). Microtubule-associated proteins (MAPs), established markers of neuronal polarity, remain in polarized distributions after either treatment. Control cells exhibit normal MAP2 localization within the dendrites (Fig. 1M) (Caceres et al., 1984) and tau in the axons (data not shown; Mandell and Banker, 1996). Neurons treated with latrunculin A have the same staining pattern for MAP2 (Fig.1N) as controls. Although MAP2 is a microtubule-binding protein, the polarity of the MAPs is retained after depolymerization with vincristine (Fig. 1O). The only noticeable difference is the appearance of MAP2 within the dendritic spines after vincristine treatment, presumably because of greater soluble pools of MAP2 within the dendrites. Tau remains axonal after both treatments (data not shown).
Synaptic clusters of PSD-95 and GKAP are maintained independent of actin microfilaments and microtubules
PSD-95/SAP90 and the closely related proteins chapsyn-110/PSD-93 and SAP102, core components of the PSD, are localized to excitatory postsynaptic specializations of hippocampal neurons (Kornau et al., 1995; Müller et al., 1996; Rao et al., 1998). We found previously that the localization of PSD-95 and these closely related cross-reacting family members appears to be largely independent of F-actin (Allison et al., 1998). Here we quantified the effect of latrunculin A on PSD-95 family protein localization and tested the effects of detergent extraction or treatment with the microtubule-depolymerizing agent vincristine. Treatment with either latrunculin A or vincristine resulted in no obvious difference in the pattern of PSD-95 immunoreactivity (Fig.2D,G) and no change in the number of total or synaptic PSD-95-immunoreactive clusters per length of dendrite relative to matched controls (Fig.3). Thus neither microfilaments nor microtubules are required for maintenance of PSD-95 family protein clusters or for maintenance of their synaptic localization. The detergent treatment results in extraction of synaptic vesicle protein markers of presynaptic terminals, and so after detergent extraction we were able to assess only the number of PSD-95 immunoreactive clusters but not synaptic localization. The detergent extraction resulted in a slight increase in the number of PSD-95 clusters (Fig. 3). It may be that the extraction unmasked some clusters that were previously obscured by diffuse dendritic shaft immunoreactivity, or that the detergent partially extracted large clusters of PSD-95, thus apparently breaking up large clusters into multiple smaller clusters. However, the pattern of PSD-95 immunoreactivity was largely unchanged by extraction (Fig. 2J), suggesting that most of the clustered PSD-95 family protein is resistant to detergent extraction.
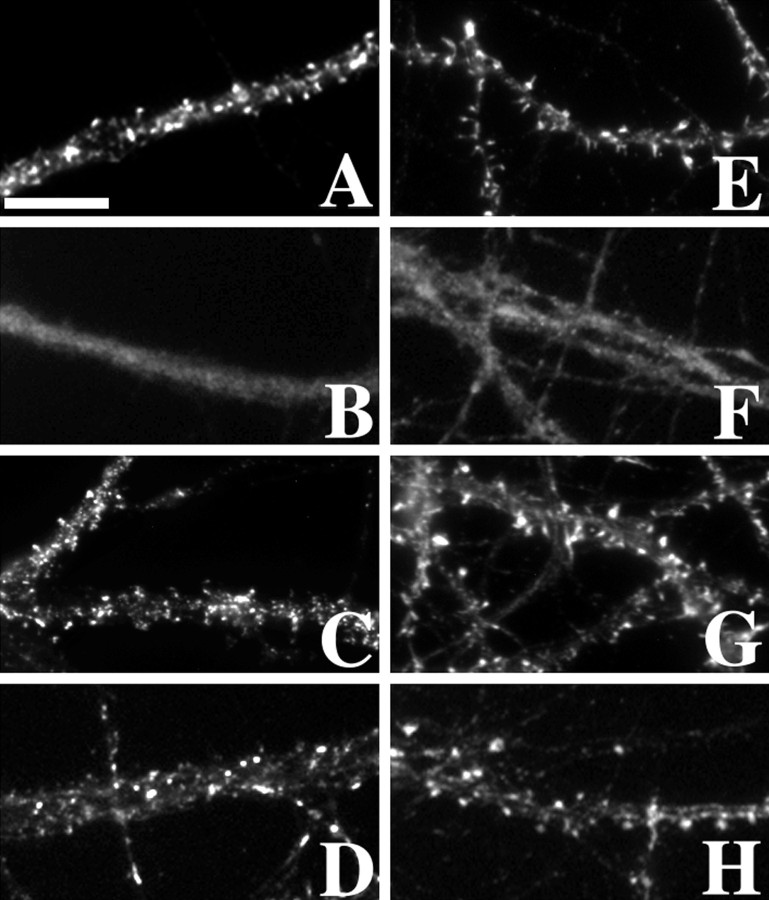
Fig. 2.
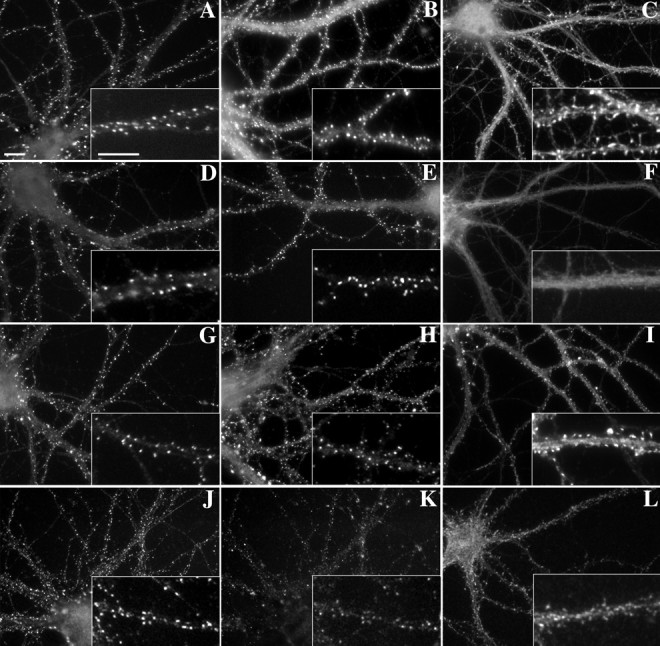
Proteins of the postsynaptic density exhibited different modes of cytoskeletal association.A–C, PSD-95, GKAP, and CaMKIIα, respectively, were found in clusters within dendritic spines and dendrite shafts. Clusters of each protein were primarily synaptic as found by double-labeling for synaptophysin (data not shown).D, E, After actin depolymerization with latrunculin A, both PSD-95 (D) and GKAP (E) clusters remained largely intact.F, Latrunculin A treatment dispersed the CaMKIIα clusters to a diffuse immunoreactivity throughout the dendrites.G–I, Microtubule depolymerization with vincristine had no apparent effect on the distributions of any of these PSD proteins (G, PSD-95; H, GKAP;I, CaMKIIα). J, PSD-95 clusters were resistant to detergent extraction. K, L, GKAP (K) and CaMKIIα (L) clusters, although still present, were reduced in their intensity after detergent extraction, indicating partial extractability. Double staining of PSD-95 and GKAP (J, K) shows the change in relative intensity when compared with controls. In the case of CaMKIIα, it appeared that the staining within the shafts and heads of the spines was extractable, but the staining at the tip within the PSD remained. Scale bars, 10 μm.
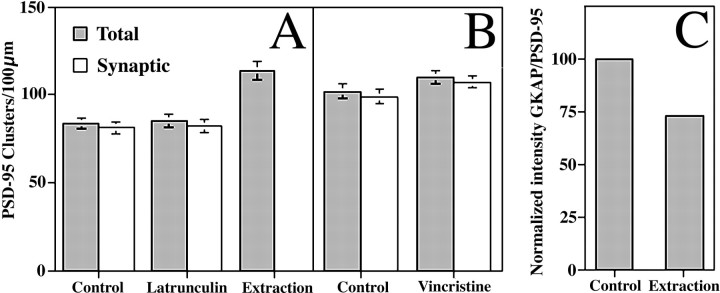
Fig. 3.
PSD-95 clusters were not disrupted by depolymerization of actin filaments or microtubules or by detergent extraction, but GKAP was partially extractable. A, The graph illustrates the number of PSD-95 clusters/100 μm dendrite length for control, latrunculin A-treated, and detergent-extracted neurons. The number of synaptic clusters was determined as the number of PSD-95 clusters apposed to punctate synaptophysin immunoreactivity. None of the changes represents a significant change in the number of clusters (t test, p > 0.1), except for the number of clusters remaining after extraction (ttest, p < 0.0001). B, A second set of experiments was performed to test the effects of vincristine on PSD-95 distribution. The numbers of total or synaptic clusters of PSD-95 were not significantly different between vincristine-treated and matched control groups (t test, p > 0.1). C, This graph indicates the partial detergent extractability of GKAP. For each cluster of GKAP the average immunofluorescence intensity value was divided by the corresponding intensity of PSD-95 immunofluorescence. With the GKAP to PSD-95 ratio normalized to the control neurons, a 27% decrease was seen after extraction. The difference was significant (t test,p < 0.0001).
GKAPs/SAPAPs are a family of four closely related proteins that have been shown to bind to the GK domain of the PSD-95 family and to be major constituents of the PSD in hippocampal neurons (Kim et al., 1997;Takeuchi et al., 1997). Neither latrunculin A nor vincristine had any obvious effect on the localization of GKAPs (Fig.2E,H). Thus, like PSD-95, GKAP clusters were maintained at synapses after disruption of filamentous actin and microtubules. Consistent with the DiI labeling studies described above, PSD-95 and GKAP clusters appeared to be largely maintained on small protrusions off the main dendrite shafts. Detergent extraction on the other hand caused a decrease in the overall intensity of GKAP staining compared with PSD-95. The ratio of GKAP to PSD-95 immunofluorescence was 94% after latrunculin A treatment but dropped to 73% after detergent extraction compared with a normalized control of 100%, indicating that GKAPs are more extractable than some other components of the PSD such as the PSD-95 family.
Clustering of CaMKIIα in spines is dependent on filamentous actin
CaMKII, itself a major PSD protein, can phosphorylate several other PSD proteins and is a key regulator of plasticity at spiny excitatory synapses (for review, see Kennedy, 1998). CaMKIIα has recently been shown to interact with F-actin via the CaMKIIβ subunit and translocate to the PSD on activation of the NMDA receptor (Shen et al., 1998; Shen and Meyer, 1999). As in vivo, CaMKIIα was concentrated within the dendritic spines of hippocampal pyramidal neurons in culture (Fig. 2C). Treatment with latrunculin A caused a dispersal of the clusters as well as a decrease in the overall staining intensity of CaMKIIα (Fig. 2F). Thus, clustering of CaMKIIα at spine synapses is dependent on F-actin. Detergent extraction of the neurons also caused a decrease in the intensity of CaMKIIα immunoreactivity but did not completely disrupt clusters (Fig. 2L). Within spines, CaMKIIα appeared to be extracted predominantly from the body and neck, leaving small puncta of immunoreactivity near the tips of spines, in all likelihood corresponding to the PSD. Depolymerization of microtubules with vincristine had no effect on the distribution of CaMKIIα (Fig.2I).
Actin-binding proteins are dispersed by latrunculin A, but unaffected by vincristine or detergent extraction
α-Actinin-2 and drebrin are two of the major actin-binding proteins concentrated in dendritic spines. Both are thought to be involved in regulating the structure and plasticity of the excitatory synapse, α-actinin-2 through its competitive, calcium-dependent binding to the NMDA receptor (Wyszynski et al., 1997; Zhang et al., 1998; Krupp et al., 1999) and drebrin by regulating binding of F-actin to α-actinin-2 or tropomyosin (Ishikawa et al., 1994). In the hippocampal neurons in culture, concentrations of both α-actinin-2 and drebrin were observed by immunocytochemistry in dendritic spines (Fig.4A,E). Latrunculin A treatment of the neurons led to a complete dispersion of the clusters of both α-actinin-2 and drebrin, as expected for actin-binding proteins (Fig.4B,F). Treatment with vincristine or detergent extraction had no effect on the localizations of α-actinin-2 or drebrin (Fig.4C,D,G,H).
Fig. 4.
Actin-binding proteins of dendritic spines were dispersed by actin depolymerization but unaffected by depolymerization of microtubules or detergent extraction. A,E, The actin-binding proteins α-actinin-2 (A) and drebrin (E) were found to be abundant within the dendritic spines of hippocampal neurons. B, F, After actin depolymerization with latrunculin A, the clusters dissociated, and both α-actinin-2 (B) and drebrin (F) became diffusely localized within the dendrites. C, D, G,H, Neither microtubule depolymerization with vincristine (C, G) nor detergent extraction (D, H) disrupted the clusters of α-actinin-2 (C, D) or drebrin (G, H). Scale bar, 10 μm.
Clusters of GABAAR and gephyrin at inhibitory synapses are maintained independent of microtubules and actin microfilaments
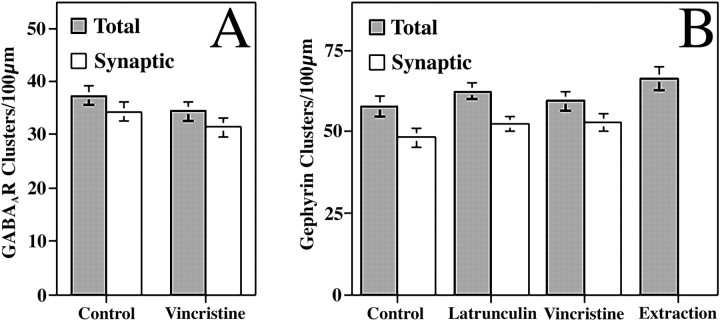
As the main inhibitory neurotransmitter receptor of the brain, the GABAA receptor is found clustered on the shafts of cultured hippocampal neurons opposite GABAergic terminals (Craig et al., 1994). GABAAR immunoreactivity, as visualized with an antibody against the β2/3 subunit, is typically seen as long, thin, primarily synaptic clusters (Fig.5A). Depolymerization of actin with latrunculin A did not affect the distribution pattern of GABAAR (Fig. 5B). Surprisingly, depolymerization of microtubules with vincristine also had no effect on the distribution pattern of GABAAR (Fig.5C). GABAAR clusters remained intact (34.4 ± 1.9 clusters per 100 μm after vincristine treatment compared with 37.4 ± 1.9 in matched controls; p> 0.1) and maintained a synaptic localization (91.3% synaptic with vincristine compared with 91.9% for controls) (see Fig.6 for complete data). GABAAR clusters were also resistant to detergent extraction (Fig. 5D).
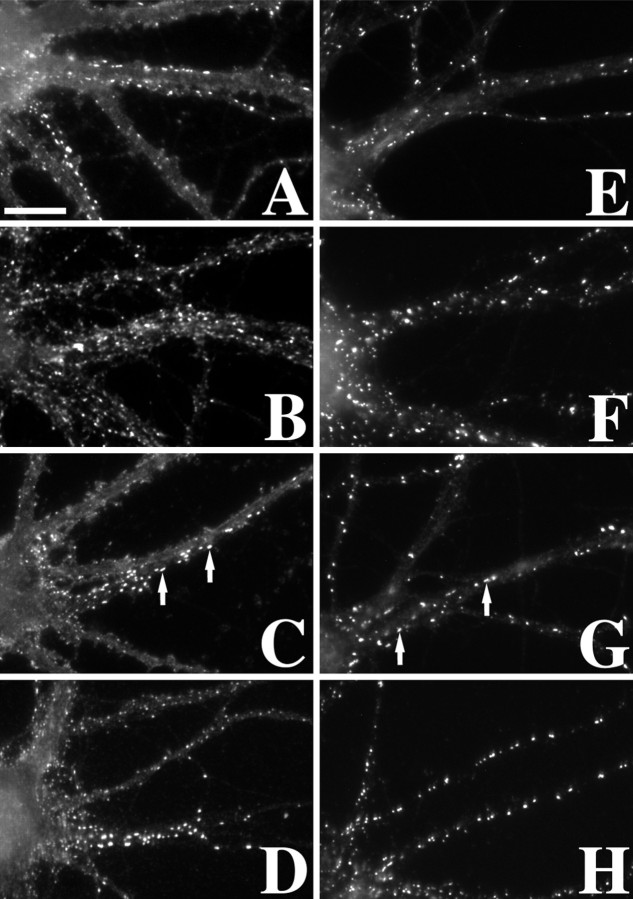
Fig. 5.
Clustering of GABAAR and gephyrin at inhibitory synapses was unaffected by depolymerization of microfilaments or microtubules or by detergent extraction. Immunostaining for the inhibitory neurotransmitter receptor GABAAR β2/3 subunits (A–D) and its putative anchoring protein gephyrin (E–H) was not disrupted by latrunculin A (B, F), vincristine (C, G), or detergent extraction (D, H). Large, elongated, synaptic clusters of both proteins (arrows) still remained, even in the absence of detectable microtubules (as in Fig. 1E), indicating that microtubules are not primarily responsible for anchoring these proteins at inhibitory PSDs. Scale bar, 10 μm.
Fig. 6.
Clustering of inhibitory synaptic proteins was not affected by treatment with vincristine to depolymerize microtubules.A, The total number of clusters and the number of synaptic clusters of GABAAR did not change significantly after vincristine treatment (t test,p > 0.1). B, The number of gephyrin clusters was not affected by treatment with latrunculin A, vincristine, or detergent extraction. Similarly, the number of synaptic clusters of gephyrin did not change after latrunculin A or vincristine treatments. There were no significant differences between groups (ttest, p > 0.1).
Gephyrin, a protein required for synaptic localization of the GABAAR, interacts with tubulin and is thought to either directly or indirectly link the GABAAR to microtubules (Kirsch et al., 1991; Craig et al., 1996; Essrich et al., 1998; Kneussel et al., 1999). Gephyrin immunostaining of mature cultured hippocampal neurons revealed both large synaptic clusters as well as ∼16.4% smaller nonsynaptic clusters on the shafts of dendrites (Fig. 5E). Treatment for 24 hr with latrunculin A had no effect on the distribution pattern of gephyrin (Fig.5F) or on the number of synaptic or nonsynaptic clusters (Fig. 6). Depolymerization of microtubules with vincristine also had no effect on the distribution of gephyrin (Fig.5G). After vincristine treatment, the total number of gephyrin clusters (59.5 ± 2.8 clusters) per dendrite length and number of synaptic clusters (52.9 ± 2.8 synaptic) was not different from controls (57.7 ± 3.2 total clusters and 48.2 ± 2.9 synaptic; p > 0.1 for each). Detergent extraction also had no effect on the distribution of gephyrin (Figs.5H, 6). These results indicate that some mechanism other than F-actin or microtubules is responsible for anchoring gephyrin and GABAAR at inhibitory synapses.
Proteins of the excitatory synapse show differential sensitivities to latrunculin A and detergent extraction
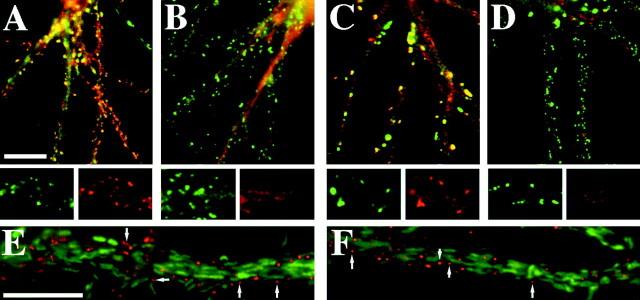
To demonstrate further the selectivity of the treatments, we performed double immunostaining using an antibody to a protein that did change compared with one that did not after the treatment. For the latrunculin A treatment, we compared two core components of the postsynaptic density, NR2A (green) and CaMKIIα (red). Both appeared clustered within the spines of control neurons (Fig. 7A). After treatment with latrunculin A, NR2A clusters remained abundant throughout the neuron, but CaMKIIα no longer appeared concentrated at corresponding locations (Fig. 7B). Before detergent extraction, GluR1 (red) and PSD-95 (green) also both appeared coclustered within dendritic spines (Fig.7C). The extraction removed the GluR1 from the spines but left the PSD-95 immunoreactivity intact (Fig. 7D). These results further demonstrate that the actin depolymerization and detergent extraction disrupted specific interactions, rather than simply inducing nonspecific degradation of the postsynaptic specialization.
Fig. 7.
Treatment with latrunculin A and detergent extraction affected synaptic clustering of different components of the PSD. A, Double immunostaining with CaMKIIα (red) and NR2A (green) showed that both proteins are concentrated within the PSDs of control neurons.B, After treatment with latrunculin A to depolymerize actin, CaMKIIα clusters dispersed (red), whereas the NR2A clusters remained intact (green).C, Similarly, clusters of GluR1 (red) and PSD-95 (green) were seen within control dendritic spines. D, After detergent extraction, GluR1 clusters were extracted (red), but PSD-95 clusters remained (green). E, F, After treatment with vincristine, neither PSD-95 (E, red) nor gephyrin (F, red) colocalizes with tubulin paracrystals (E, F, green). Scale bar, 10 μm.
Tubulin paracrystals are not involved in the stabilization of postsynaptic protein complexes
Treatment with vincristine has been shown to cause the formation of tubulin paracrystals within the cell. These paracrystals are crystalloid structures consisting of tubulin dimers and the pertinent Vinca alkaloid (Bensch et al., 1969; Weber et al., 1975). To show that these paracrystals are not responsible for binding to and stabilizing the synaptic complexes, we have stained for either PSD-95 or gephyrin along with tubulin after vincristine treatment. PSD-95 clusters (Fig.7E, red) do not colocalize with tubulin paracrystals (Fig. 7E, green), nor do gephyrin clusters (Fig. 7F, red). Because the clusters do not colocalize with the paracrystals, nor are they disrupted, we can rule out a role for the paracrystals in stabilization of the synaptic complexes.
DISCUSSION
Several conclusions about the relationship between postsynaptic proteins and their maintenance by the neuronal cytoskeleton can be drawn from the above data. (1) F-actin is responsible for the anchoring of certain spine-specific proteins (CaMKIIα, drebrin, and α-actinin-2). Previously, we have shown that F-actin is also responsible for maintaining the localization of the AMPA receptor on spines (Allison et al., 1998); however, (2) F-actin is not necessary for retaining some of the proteins within the PSD (PSD-95 family, GKAPs, and NMDAR), nor is F-actin required for maintaining the localization of the inhibitory synapse proteins GABAAR and gephyrin. (3) Microtubules are not required to maintain localization of GABAAR and gephyrin at inhibitory synapses or to maintain spine clusters of PSD-95 or any of the other excitatory synapse proteins assayed. (4) With the exception of AMPAR as reported previously, none of the proteins assayed is completely detergent extractable (GKAPs and CaMKIIα are partially extractable). (5) All of this evidence suggests that core postsynaptic specializations (containing PSD-95 family and NMDARs for excitatory synapses, and gephyrin and GABAAR for inhibitory synapses) are rather stable complexes of proteins that once formed, maintain themselves independently of conventional cytoskeletal systems. For summary, see Figure 8.
Fig. 8.
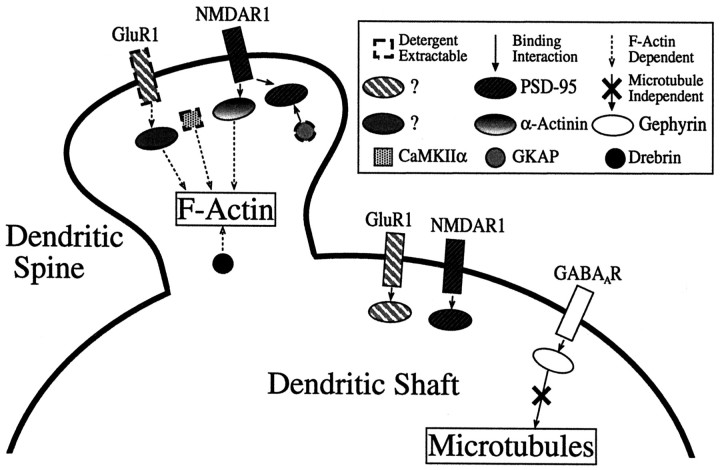
Diagrammatic summary of results. Two different AMPA receptor-binding proteins are postulated to account for the differential detergent extractability and actin dependence of AMPA receptors in spines versus shaft synapses as reported previously (Allison et al., 1998); these may correspond to different forms of GRIP and/or PICK1. The proteins dependent on F-actin for clustering include α-actinin-2, drebrin, CaMKIIα, and AMPAR in spines. GKAP and CaMKIIα are partially detergent extractable, and AMPAR is highly extractable only from spine synapses. All synaptic components were found to be localized independent of microtubules; this is emphasized in the diagram for gephyrin and GABAAR. These results indicate different modes of localization for different components of dendritic spines and suggest that PSD-95 and gephyrin form part of core scaffolds of excitatory and inhibitory synapses maintained independent of association with conventional cytoskeletal systems.
Dependence of synaptic proteins on the actin cytoskeleton
Our data support the idea of two structural and corresponding functional levels of spine PSD organization, a core component and a peripheral actin-associated component (Adam and Matus, 1996). Some proteins are highly dependent on F-actin for their localization (CaMKIIα, drebrin, and α-actinin-2), whereas others appear to be completely independent (PSD-95 and GKAP) (Figs. 2-4, 7). The actin-associated components (CaMKIIα, drebrin, and α-actinin-2) are specifically concentrated only at spiny pyramidal neuron synapses (Jones et al., 1994; Hayashi et al., 1996; Rao et al., 1998;Sík et al., 1998), whereas the actin-independent components (NMDAR, PSD-95, and GKAP) are also concentrated at nonspiny excitatory synapses of interneurons. The actin-associated components of spines appear to have a primary function in activation-induced signal transduction and alterations of the actin cytoskeleton in response to signals initiated through core components of the PSD. The disruption of these actin-dependent proteins via latrunculin A left a membranous protrusion at the presumptive spine site, possibly reflecting attachment of the actin-independent complex via transynaptic proteins to the presynaptic terminal, and further supporting the role for the actin-dependent proteins in ancillary activities. All known components of the inhibitory postsynaptic specializations on dendrite shafts could be considered core components functionally and are independent of F-actin for localization (GABAAR and gephyrin) (Figs. 5, 6).
Actin-associated components of spine synapses include spectrin, myosin V, α-adducin, neurabin, neurabinII/spinophilin, cortactin, and 4.1N (neuronal homolog of erythrocyte protein 4.1) as well as CaMKIIα, drebrin, and α-actinin-2 (Carlin et al., 1983; Morales and Fifková, 1989; Espreafico et al., 1992; Seidel et al., 1995;Allen et al., 1997; Nakanishi et al., 1997; Satoh et al., 1998;Naisbitt et al., 1999; Walensky et al., 1999). Most of these proteins either bind directly to actin, like α-actinin-2 and drebrin, or bind through one additional linkage, like CaMKIIα, which multimerizes with CaMKIIβ, which binds actin (Shen et al., 1998). Thus the localization of these proteins to spines is also likely to depend on F-actin. Many of these actin-associated proteins have additional interactions within the PSD. For example, α-actinin-2 and spectrin both bind to the NMDA receptor, whereas CaMKII binds to myosin V (Wyszynski et al., 1997;Wechsler and Teichberg, 1998; Costa et al., 1999), but as we found for α-actinin-2 and CaMKIIα, these additional linkages may not be sufficient to maintain synaptic localization in the absence of F-actin.
The major function of the actin-associated components of the PSD appears to be in signal transduction and modification of the microfilament arrays in response to synaptic activation, events thought to mediate long-term synaptic plasticity. For example, entry of calcium reduces α-actinin-binding to NMDAR by competitive binding of Ca2+/calmodulin, thus mediating NMDAR inactivation (Zhang et al., 1998; Krupp et al., 1999). A large number of mechanisms appear to act in concert to determine spine morphology, including stabilization of actin filaments by α-actinin-2, spectrin, α-adducin, neurabins, cortactin, and 4.1N, spine elongation by drebrin (Hayashi and Shirao, 1999), cleavage of spectrin by calpain (Seubert et al., 1988), inhibition of α-adducin function by PKC phosphorylation (Matsuoka et al., 1998), and enhanced synaptic localization of cortactin by glutamate stimulation (Naisbitt et al., 1999). Rapid regulation of spine morphology can occur (Halpain et al., 1998; Kaech et al., 1999), and pharmacological blockade (Kirov and Harris, 1999) or synaptic stimulation (Engert and Bonhoeffer, 1999) can induce new spine formation.
Relationship between the microtubule cytoskeleton and synaptic proteins
Tubulin is an abundant protein throughout the neuron that is directly involved in the transport of many intrinsic components to and from the processes. But what, if anything, is tubulin doing in the PSD? Although microtubules are not seen within dendritic spines, tubulin is a major component of biochemically isolated PSD fractions (Blomberg et al., 1977; Matus and Taff-Jones, 1978; Walsh and Kuruc, 1992; Lai et al., 1998). The metabotropic glutamate receptor mGluR1α can interact directly with tubulin (Ciruela et al., 1999), and the NMDAR may bind tubulin either directly or indirectly through PSD-95 and MAP1A or CRIPT (Pedrotti et al., 1994; Brenman et al., 1998; Niethammer et al., 1998;van Rossum et al., 1999). Thus it has been suggested that tubulin, in some form, may have an important function in the organization of excitatory PSDs. However, we found that vincristine, which disrupts microtubules (Fig. 1), does not disrupt maintenance of spines or excitatory PSD components (Figs. 2-4). Thus microtubules are not responsible for synaptic anchoring of NMDA receptors or PSD-95 or for maintenance of the actin cytoskeleton of spines. In addition, because vincristine sequesters tubulin dimers from the cytoplasm into paracrystals, cytoplasmic tubulin is also unlikely to play a major role in synapse organization. Strictly speaking, however, we cannot exclude the involvement of some other form of tubulin (different from microtubules and free tubulin dimer) in the organization of synaptic protein clusters, although we are unaware of any such forms resistant to vincristine and extraction. An alternative role of tubulin near the synapse may be to serve as a substrate for local cytoskeletal modification of the dendrite in response to synaptic activity. Calcium entry through NMDAR modulates MAP-2 phosphorylation (Quinlan and Halpain, 1996), which could locally regulate microtubule stability (van Rossum and Hanisch, 1999).
Microtubules are thought to play a central role in the organization of inhibitory postsynaptic specializations. This idea is based largely on binding properties of components of GABAergic and glycinergic synapses: the GABAAR was initially copurified with tubulin (Item and Sieghart, 1994), gephyrin binds tubulin as well as the glycine receptor β subunit and is required for GABAAR clustering (Kirsch et al., 1991, 1995;Essrich et al., 1998; Kneussel et al., 1999), GABARAP binds the GABAAR γ2 subunit and contains a putative tubulin-binding motif (Wang et al., 1999), and the GABACR binds MAP-1B, which binds tubulin (Hanley et al., 1999). However, microtubules do not directly approach the plasma membrane. Moreover, our results show that microtubules are not required for clustering or for synaptic localization of either GABAAR or gephyrin (Figs. 5, 6). Our results are different from the disruption of clusters of gephyrin in spinal neurons by the microtubule depolymerizing agent demecolcine as reported byKirsch and Betz (1995). There could be a number of reasons for this difference: cell type, differential association of gephyrin with GABAAR versus glycine receptor, pharmacological agents used, the possibility of additional irreversible effects of demecolcine, stages of development, and possible differences in half-life of the synaptic proteins.
Other possible mechanisms for anchoring postsynaptic proteins
This study leads us to the conclusion that simple models for the anchoring of postsynaptic proteins, such as anchoring of excitatory components to F-actin and inhibitory components to microtubules, do not suffice. Of the synaptic components studied here, none were dependent on either F-actin or microtubules for their localization, with the exception of proteins specific to spines (i.e., CaMKII, drebrin, and α-actinin-2). Both F-actin and microtubules are likely necessary for the initial formation or transport of the synaptic structure but not for maintenance of most components of the PSD. An interesting exception is the AMPAR, which is partially dependent on F-actin for maintenance of spine clusters (Allison et al., 1998). In view of recent models suggesting rapid and continuous endocytosis and exocytosis of AMPA receptors at the synapse (e.g., Noel et al., 1999), AMPA receptors may constitute a special case in which actin filaments may be more involved in recycling than in direct anchoring, thus yielding the partial dependence of localization on F-actin.
If neither actin nor microtubules are responsible for anchoring core components of both excitatory and inhibitory synapses, including NMDAR, PSD-95, GABAAR, and gephyrin, then how do they maintain their localization? These proteins may comprise part of postsynaptic densities that are more or less self-anchored or self-maintained. PSDs can be isolated biochemically because they are highly cross-linked, detergent-resistant structures (Peters et al., 1991; Adam and Matus, 1996; Kennedy, 1997). These core interactions need not be static, as evidenced by the activity regulation of NMDAR distribution (Rao and Craig, 1997), but they are likely more stable that those of the actin-associated components. Once a synapse has formed, our data suggest that actin and microtubules are not required for localization of core synaptic proteins but may be more involved in mediating activity-dependent changes in morphology and signaling.
Footnotes
This work was supported by the Markey and Pew Charitable Trusts and National Institutes of Health (NIH) Grant NS33184 to A.M.C., and National Science Foundation Grant MCB 95-31231 and NIH Grant GM52111-01 to V.I.G.. We thank Anna S. Serpinskaya and Huaiyang Wu for excellent technical assistance.
Correspondence should be addressed to Ann Marie Craig, Department of Anatomy and Neurobiology, Washington University School of Medicine, 660 S. Euclid Avenue, Campus Box 8108, St. Louis, MO 63110. E-mail:acraig@pcg.wustl.edu.
REFERENCES
- 1.Adam G, Matus A. Role of actin in the organisation of brain postsynaptic densities. Brain Res Mol Brain Res. 1996;43:246–250. doi: 10.1016/s0169-328x(96)00177-5. [DOI] [PubMed] [Google Scholar]
- 2.Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci USA. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–442. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- 5.Bensch KG, Marantz R, Wisniewski H, Shelanski M. Induction in vitro of microtubular crystals by vinca alkaloids. Science. 1969;165:495–496. doi: 10.1126/science.165.3892.495. [DOI] [PubMed] [Google Scholar]
- 6.Blomberg F, Cohen RS, Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. II. Characterization and arrangement of some of the major proteins within the structure. J Cell Biol. 1977;74:204–225. doi: 10.1083/jcb.74.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenman JE, Topinka JR, Cooper EC, McGee AW, Rosen J, Milroy T, Ralston HJ, Bredt DS. Localization of postsynaptic density-93 to dendritic microtubules and interaction with microtubule-associated protein 1A. J Neurosci. 1998;18:8805–8813. doi: 10.1523/JNEUROSCI.18-21-08805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 1984;315:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- 9.Carlin RK, Bartelt DC, Siekevitz P. Identification of fodrin as a major calmodulin-binding protein in postsynaptic density preparations. J Cell Biol. 1983;96:443–448. doi: 10.1083/jcb.96.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciruela F, Robbins MJ, Willis AC, McIlhinney RA. Interactions of the C terminus of metabotropic glutamate receptor type 1α with rat brain proteins: evidence for a direct interaction with tubulin. J Neurochem. 1999;72:346–354. [PubMed] [Google Scholar]
- 11.Cohen RS, Chung SK, Pfaff DW. Immunocytochemical localization of actin in dendritic spines of the cerebral cortex using colloidal gold as a probe. Cell Mol Neurobiol. 1985;5:271–284. doi: 10.1007/BF00711012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa MC, Mani F, Santoro W, Jr, Espreafico EM, Larson RE. Brain myosin-V, a calmodulin-carrying myosin, binds to calmodulin- dependent protein kinase II and activates its kinase activity. J Biol Chem. 1999;274:15811–15819. doi: 10.1074/jbc.274.22.15811. [DOI] [PubMed] [Google Scholar]
- 13.Craig AM, Blackstone CD, Huganir RL, Banker G. Selective clustering of glutamate and γ-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proc Natl Acad Sci USA. 1994;91:12373–12377. doi: 10.1073/pnas.91.26.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig AM, Banker G, Chang W, McGrath ME, Serpinskaya AS. Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J Neurosci. 1996;16:3166–3177. doi: 10.1523/JNEUROSCI.16-10-03166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehlers MD, Fung ET, O'Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci. 1998;18:720–730. doi: 10.1523/JNEUROSCI.18-02-00720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 17.Espreafico EM, Cheney RE, Matteoli M, Nascimento AA, De Camilli PV, Larson RE, Mooseker MS. Primary structure and cellular localization of chicken brain myosin-V (p190), an unconventional myosin with calmodulin light chains. J Cell Biol. 1992;119:1541–1557. doi: 10.1083/jcb.119.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essrich C, Lorez M, Benson JA, Fritschy JM, Lüscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 19.Fifková E. Actin in the nervous system. Brain Res. 1985;356:187–215. [PubMed] [Google Scholar]
- 20.Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low density culture. In: Banker G, Goslin K, editors. Culturing nerve cells. MIT; Cambridge, MA: 1998. pp. 339–370. [Google Scholar]
- 21.Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanley JG, Koulen P, Bedford F, Gordon-Weeks PR, Moss SJ. The protein MAP-1B links GABAC receptors to the cytoskeleton at retinal synapses. Nature. 1999;397:66–69. doi: 10.1038/16258. [DOI] [PubMed] [Google Scholar]
- 23.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 24.Hasbani MJ, Hyrc KL, Faddis BT, Romano C, Goldberg MP. Distinct roles for sodium, chloride, and calcium in excitotoxic dendritic injury and recovery. Exp Neurol. 1998;154:241–258. doi: 10.1006/exnr.1998.6929. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi K, Shirao T. Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J Neurosci. 1999;19:3918–3925. doi: 10.1523/JNEUROSCI.19-10-03918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi K, Ishikawa R, Ye LH, He XL, Takata K, Kohama K, Shirao T. Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J Neurosci. 1996;16:7161–7170. doi: 10.1523/JNEUROSCI.16-22-07161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard TH, Oresajo CO. A method for quantifying F-actin in chemotactic peptide activated neutrophils: study of the effect of tBOC peptide. Cell Motil. 1985;5:545–557. doi: 10.1002/cm.970050609. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa R, Hayashi K, Shirao T, Xue Y, Takagi T, Sasaki Y, Kohama K. Drebrin, a development-associated brain protein from rat embryo, causes the dissociation of tropomyosin from actin filaments. J Biol Chem. 1994;269:29928–29933. [PubMed] [Google Scholar]
- 29.Item C, Sieghart W. Binding of γ-aminobutyric acidA receptors to tubulin. J Neurochem. 1994;63:1119–1125. doi: 10.1046/j.1471-4159.1994.63031119.x. [DOI] [PubMed] [Google Scholar]
- 30.Jones EG, Huntley GW, Benson DL. α calcium/calmodulin-dependent protein kinase II selectively expressed in a subpopulation of excitatory neurons in monkey sensory-motor cortex: comparison with GAD-67 expression. J Neurosci. 1994;14:611–629. doi: 10.1523/JNEUROSCI.14-02-00611.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaech S, Brinkhaus H, Matus A. Volatile anesthetics block actin-based motility in dendritic spines. Proc Natl Acad Sci USA. 1999;96:10433–10437. doi: 10.1073/pnas.96.18.10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20:264–268. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy MB. Signal transduction molecules at the glutamatergic postsynaptic membrane. Brain Res Brain Res Rev. 1998;26:243–257. doi: 10.1016/s0165-0173(97)00043-x. [DOI] [PubMed] [Google Scholar]
- 34.Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim E, Naisbitt S, Hsueh YP, Rao A, Rothschild A, Craig AM, Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirov SA, Harris KM. Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nat Neurosci. 1999;2:878–883. doi: 10.1038/13178. [DOI] [PubMed] [Google Scholar]
- 37.Kirsch J, Betz H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. J Neurosci. 1995;15:4148–4156. doi: 10.1523/JNEUROSCI.15-06-04148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirsch J, Langosch D, Prior P, Littauer UZ, Schmitt B, Betz H. The 93-kDa glycine receptor-associated protein binds to tubulin. J Biol Chem. 1991;266:22242–22245. [PubMed] [Google Scholar]
- 39.Kirsch J, Kuhse J, Betz H. Targeting of glycine receptor subunits to gephyrin-rich domains in transfected human embryonic kidney cells. Mol Cell Neurosci. 1995;6:450–461. doi: 10.1006/mcne.1995.1033. [DOI] [PubMed] [Google Scholar]
- 40.Kneussel M, Brandstätter JH, Laube B, Stahl S, Müller U, Betz H. Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knowles GC, McCulloch CA. Simultaneous localization and quantification of relative G and F actin content: optimization of fluorescence labeling methods. J Histochem Cytochem. 1992;40:1605–1612. doi: 10.1177/40.10.1527379. [DOI] [PubMed] [Google Scholar]
- 42.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 43.Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Interactions of calmodulin and α-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci. 1999;19:1165–1178. doi: 10.1523/JNEUROSCI.19-04-01165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai SL, Ling SC, Kuo LH, Shu YC, Chow WY, Chang YC. Characterization of granular particles isolated from postsynaptic densities. J Neurochem. 1998;71:1694–1701. doi: 10.1046/j.1471-4159.1998.71041694.x. [DOI] [PubMed] [Google Scholar]
- 45.Landis DM, Reese TS. Cytoplasmic organization in cerebellar dendritic spines. J Cell Biol. 1983;97:1169–1178. doi: 10.1083/jcb.97.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandell JW, Banker GA. A spatial gradient of tau protein phosphorylation in nascent axons. J Neurosci. 1996;16:5727–5740. doi: 10.1523/JNEUROSCI.16-18-05727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin- actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol. 1998;142:485–497. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matus AI, Taff-Jones DH. Morphology and molecular composition of isolated postsynaptic junctional structures. Proc R Soc Lond B Biol Sci. 1978;203:135–151. doi: 10.1098/rspb.1978.0097. [DOI] [PubMed] [Google Scholar]
- 49.Morales M, Fifková E. In situ localization of myosin and actin in dendritic spines with the immunogold technique. J Comp Neurol. 1989;279:666–674. doi: 10.1002/cne.902790412. [DOI] [PubMed] [Google Scholar]
- 50.Müller BM, Kistner U, Kindler S, Chung WJ, Kuhlendahl S, Fenster SD, Lau LF, Veh RW, Huganir RL, Gundelfinger ED, Garner CC. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron. 1996;17:255–265. doi: 10.1016/s0896-6273(00)80157-9. [DOI] [PubMed] [Google Scholar]
- 51.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 52.Nakanishi H, Obaishi H, Satoh A, Wada M, Mandai K, Satoh K, Nishioka H, Matsuura Y, Mizoguchi A, Takai Y. Neurabin: a novel neural tissue-specific actin filament-binding protein involved in neurite formation. J Cell Biol. 1997;139:951–961. doi: 10.1083/jcb.139.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niethammer M, Valtschanoff JG, Kapoor TM, Allison DW, Weinberg TM, Craig AM, Sheng M. CRIPT, a novel postsynaptic protein that binds to the third PDZ domain of PSD-95/SAP90. Neuron. 1998;20:693–707. doi: 10.1016/s0896-6273(00)81009-0. [DOI] [PubMed] [Google Scholar]
- 54.Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- 55.Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol Dis. 1996;3:215–227. doi: 10.1006/nbdi.1996.0022. [DOI] [PubMed] [Google Scholar]
- 56.Pedrotti B, Colombo R, Islam K. Microtubule associated protein MAP1A is an actin-binding and crosslinking protein. Cell Motil Cytoskeleton. 1994;29:110–116. doi: 10.1002/cm.970290203. [DOI] [PubMed] [Google Scholar]
- 57.Peters A, Palay SL, Webster H. The fine structure of the nervous system: neurons and their supporting cells. Oxford UP; New York: 1991. Synapses. pp. 138–211. [Google Scholar]
- 58.Quinlan EM, Halpain S. Postsynaptic mechanisms for bidirectional control of MAP2 phosphorylation by glutamate receptors. Neuron. 1996;16:357–368. doi: 10.1016/s0896-6273(00)80053-7. [DOI] [PubMed] [Google Scholar]
- 59.Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- 60.Rao A, Kim E, Sheng M, Craig AM. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci. 1998;18:1217–1229. doi: 10.1523/JNEUROSCI.18-04-01217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, Takai Y. Neurabin-II/spinophilin. An actin filament-binding protein with one PDZ domain localized at cadherin-based cell-cell adhesion sites. J Biol Chem. 1998;273:3470–3475. doi: 10.1074/jbc.273.6.3470. [DOI] [PubMed] [Google Scholar]
- 62.Seidel B, Zuschratter W, Wex H, Garner CC, Gundelfinger ED. Spatial and sub-cellular localization of the membrane cytoskeleton-associated protein α-adducin in the rat brain. Brain Res. 1995;700:13–24. doi: 10.1016/0006-8993(95)00962-p. [DOI] [PubMed] [Google Scholar]
- 63.Seubert P, Larson J, Oliver M, Jung MW, Baudry M, Lynch G. Stimulation of NMDA receptors induces proteolysis of spectrin in hippocampus. Brain Res. 1988;460:189–194. doi: 10.1016/0006-8993(88)91222-x. [DOI] [PubMed] [Google Scholar]
- 64.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 65.Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIβ functions as an F-actin targeting module that localizes CaMKIIα/β heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- 66.Sík A, Hájos N, Gulácsi A, Mody I, Freund TF. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc Natl Acad Sci USA. 1998;95:3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- 68.van Rossum D, Hanisch UK. Cytoskeletal dynamics in dendritic spines: direct modulation by glutamate receptors? Trends Neurosci. 1999;22:290–295. doi: 10.1016/s0166-2236(99)01404-6. [DOI] [PubMed] [Google Scholar]
- 69.van Rossum D, Kuhse J, Betz H. Dynamic interaction between soluble tubulin and C-terminal domains of N- methyl-d-aspartate receptor subunits. J Neurochem. 1999;72:962–973. doi: 10.1046/j.1471-4159.1999.0720962.x. [DOI] [PubMed] [Google Scholar]
- 70.Walensky LD, Blackshaw S, Liao D, Watkins CC, Weier HU, Parra M, Huganir RL, Conboy JG, Mohandas N, Snyder SH. A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1. J Neurosci. 1999;19:6457–6467. doi: 10.1523/JNEUROSCI.19-15-06457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walsh MJ, Kuruc N. The postsynaptic density: constituent and associated proteins characterized by electrophoresis, immunoblotting, and peptide sequencing. J Neurochem. 1992;59:667–678. doi: 10.1111/j.1471-4159.1992.tb09421.x. [DOI] [PubMed] [Google Scholar]
- 72.Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABAA-receptor-associated protein links GABAA receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- 73.Weber K, Bibring T, Osborn M. Specific visualization of tubulin-containing structures in tissue culture cells by immunofluorescence. Cytoplasmic microtubules, vinblastine-induced paracrystals, and mitotic figures. Exp Cell Res. 1975;95:111–120. doi: 10.1016/0014-4827(75)90615-1. [DOI] [PubMed] [Google Scholar]
- 74.Wechsler A, Teichberg VI. Brain spectrin binding to the NMDA receptor is regulated by phosphorylation, calcium and calmodulin. EMBO J. 1998;17:3931–3939. doi: 10.1093/emboj/17.14.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. Competitive binding of α-actinin and calmodulin to the NMDA receptor. Nature. 1997;385:439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- 76.Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL. Calmodulin mediates calcium-dependent inactivation of N-methyl-d-aspartate receptors. Neuron. 1998;21:443–453. doi: 10.1016/s0896-6273(00)80553-x. [DOI] [PubMed] [Google Scholar]
- 77.Zigmond SH, Joyce M, Yang C, Brown K, Huang M, Pring M. Mechanism of Cdc42-induced actin polymerization in neutrophil extracts. J Cell Biol. 1998;142:1001–1012. doi: 10.1083/jcb.142.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]