Abstract
Drosophila transient receptor potential (TRP) is a prototypical member of a novel family of channel proteins underlying phosphoinositide-mediated Ca2+ entry. Although the initial stages of this signaling cascade are well known, downstream events leading to the opening of the TRP channels are still obscure. In the present study we applied patch-clamp whole-cell recordings and measurements of Ca2+ concentration by ion-selective microelectrodes in eyes of normal and mutant Drosophilato isolate the TRP and TRP-like (TRPL)-dependent currents. We report that anoxia rapidly and reversibly depolarizes the photoreceptors and induces Ca2+ influx into these cells in the dark. We further show that openings of the light-sensitive channels, which mediate these effects, can be obtained by mitochondrial uncouplers or by depletion of ATP in photoreceptor cells, whereas the effects of illumination and all forms of metabolic stress were additive. Effects similar to those found in wild-type flies were also found in mutants with strong defects in rhodopsin, Gq-protein, or phospholipase C, thus indicating that the metabolic stress operates at a late stage of the phototransduction cascade. Genetic elimination of both TRP and TRPL channels prevented the effects of anoxia, mitochondrial uncouplers, and depletion of ATP, thus demonstrating that the TRP and TRPL channels are specific targets of metabolic stress. These results shed new light on the properties of the TRP and TRPL channels by showing that a constitutive ATP-dependent process is required to keep these channels closed in the dark, a requirement that would make them sensitive to metabolic stress.
Keywords: TRP and TRPL channels, Drosophila mutants, anoxia, mitochondrial uncouplers, ion-selective microelectrodes, metabolic stress
The Drosophila transient receptor potential (TRP) protein (Minke et al., 1975; Montell and Rubin, 1989) and its homologous protein TRP-like (TRPL) (Phillips et al., 1992) are photoreceptor channel proteins that are activated by the inositol lipid signaling cascade (Devary et al., 1987; Bloomquist et al., 1988). These channels constitute the main route of Ca2+ entry into photoreceptor cells (Hardie and Minke, 1992; Peretz et al., 1994a,b; Hardie, 1996; Niemeyer et al., 1996). A trp mutant was recovered as a spontaneously occurring mutant with a transient receptor potential (Cosens and Manning, 1969; Minke et al., 1975). Subsequently, a trplmutant was isolated (Niemeyer et al., 1996), and in thetrpl;trp double mutant the response to light is abolished, indicating that TRP and TRPL make up all light-activated channels or are required for their activation (Niemeyer et al., 1996; Reuss et al., 1997; Scott et al., 1997).
Cloning and sequencing of the Drosophila TRP protein (Montell and Rubin, 1989) have led to the discovery of a novel family of channel proteins, related to TRP, that is conserved throughout evolution from Caenorhabditis elegans to humans (for review, see Birnbaumer et al., 1996; Friel, 1996; Minke and Selinger, 1996;Montell, 1997; Putney and McKay, 1999; Harteneck et al., 2000). Heterologous expression of Drosophila TRP has demonstrated that TRP is the first molecularly identified channel subunit that can be activated by Ca2+ store depletion (Vaca et al., 1994; Petersen et al., 1995; Gillo et al., 1996; Xu et al., 1997). This makes the Drosophila TRP channel protein a valuable tool for studies of the mechanism underlying phosphoinositide-mediated Ca2+ entry and the role of vertebrate TRP in this process (Berridge, 1995; Birnbaumer et al., 1996; Friel, 1996; Kiselyov et al., 1998; Putney and McKay, 1999). Indeed, the Drosophila TRP and TRPL channels are rather unique among members of the growing family of TRP-related channels, because the physiological function of TRP channels, in vivo, is known only in Drosophila. Therefore, knowledge of the properties of Drosophila TRP and TRPL channels is likely to shed light on the possible function and gating mechanisms of vertebrate TRP homologs.
In the present study we discovered that several processes that induced metabolic stress rapidly activated the TRP and TRPL channels in the dark in vivo. The robust effect of metabolic stress revealed a rare property of channel proteins. Thus, although it is unlikely that depletion of ATP is the physiological mechanism underlying TRP and TRPL activation, this striking phenomenon provides an insight into the physiological properties of TRP and TRPL channels and makes current models of their activation mechanism doubtful.
MATERIALS AND METHODS
Fly stocks. White-eyed Drosophila melanogaster of the Oregon-R strain (WT), Musca domestica, and Calliphora erythrocephala were used for the experiments as indicated. The white-eyed Drosophilamutants included trpP343,norpAP24,ninaEora,Gαq1, and the double mutanttrpl302;trpP343.
Electrophysiology, [K+], and [Ca2+] measurements. Intracellular and electroretinogram (ERG) recordings from intact Drosophilawere performed as described previously (Peretz et al., 1994a). For measurements of concentration changes of Ca2+ and K+, the basic method applied to the large flies (Sandler and Kirschfeld, 1991) and to Drosophila (Peretz et al., 1994a) was performed as described previously in detail. The Drosophilapreparation was identical to that for intracellular and ERG recordings. We used both double-barreled and single-barreled electrodes. When a single-barreled ion-selective electrode was used, it was inserted just below the intact cornea, and the reference electrode was placed in an electrode gel separately on the intact cornea at the center of the eye. No significant difference was found in the results of the two methods, except that the single-barreled method caused less damage to the eye (Peretz et al., vk1994a). For silanization of the ion-selective barrel, we used vapors of N,N, dimethyltrimethylsylililamine (Fluka 41720) at 200°C according to the method of Munoz et al.(1983). The Ca2+sensor mixture was based on the neutral carrier ETH 1001, calcium ionophore I–Cocktail A (Art.-Nr. 21048, Fluka D-7910, Neu-Ulm Germany). It was made syrupy by adding polyvinylchloride (13–16%) and tetrahydrofuran (Aldrich, Milwaukee, WI). The K+ sensor mixture was the potassium ionophore I–Cocktail B (Art.-Nr.60398, Fluka). The dark value of retinal [Ca2+]outin Drosophila used to calculate [Ca2+] was 1.43 mm. This value was determined in previous studies (Peretz et al., 1994a). The reported Ca2+signal in all traces was differential: the potential measured by the ERG electrode was subtracted from that of the Ca2+-sensitive electrode to give the Ca signal, which is a measure of [Ca2+]out.
Light stimulation. For all measurements, orange light (OG 590, Schott edge filter) from a Xenon high-pressure lamp (PTI, LPS 220, operating at 50 W) was delivered to the compound eye either by means of a fiber optic (in intact flies) or as epi illumination via an objective lens (in situ). The maximal luminous intensity at the eye surface was 1.0 log units above the intensity for a half-maximal response of the most common type of fly photoreceptors (type R1–6).
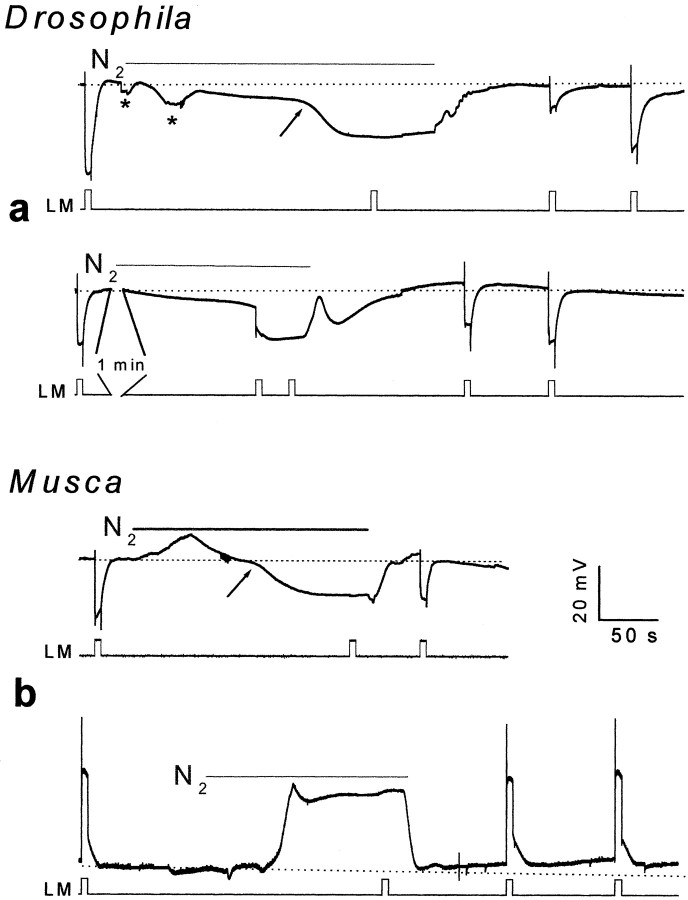
Anoxia. Anoxia was obtained by blowing nitrogen (N2) on the abdomen and thorax of the fly. The onset and offset of N2 application was accompanied by voltage artifact, probably because of N2-induced movement of the eye muscles (see Fig.1, asterisk).
Fig. 1.
Anoxia induced a rapid and reversible depolarization of fly photoreceptors in the dark and abolished light excitation. a, Top panel, Extracellular voltage change recordings of intact Drosophila eye in response to orange light (OG590, attenuated by 1 log unit, in all Figures) followed by application of anoxia (N2, as indicated) and by additional light pulses, which test the recovery from anoxia. The light monitor is indicated below (LM). Note that the second light pulse of the top panel and the third pulse at thebottom panel did not elicit any response. Thearrow indicates the onset of the second phase of the response to anoxia of larger amplitude. Movement artifacts, which probably resulted from movements of the fly eye after anoxia, are indicated by an asterisk (in all Figures).a, Bottom panel, The experiments of thetop traces were repeated in anotherDrosophila fly except that an additional orange light pulse was applied during the initial response to anoxia of small amplitude. A 1 min break is indicated in the bottom traces. b, Top panel, The experiments of a (top traces) were repeated in intact Musca fly. Bottom panel, Intracellular recordings from a single photoreceptor cell in response to the orange light pulses and anoxia. Note that the initial small and slow response to anoxia is absent.
Whole-cell recordings. For whole-cell patch-clamp recordings, dissociated Drosophila ommatidia were prepared from newly emerged flies (<1 hr after eclosion), and whole-cell patch-clamp recordings were performed as described previously (Peretz et al., 1994a). For current measurements data were sampled at 1000 Hz using the Digidata card and analyzed by pClamp 7.0 software (Axon Instruments).
Solution. The bath solution contained (in mm): 120 NaCl, 5 KCl, 10 TES buffer (N-tris-(hydroxymethyl)-methyl-2-amino-ethanesulfonic acid, pH 7.15), 4 MgSO4, 1.5 CaCl2 (except when low Ca2+ medium was used as indicated). For all of the experiments, an internal solution that blocked K+ channels was used. The whole-cell recording pipette contained (in mm): 120 CsCl, 15 tetraethylammonium (TEA) Cl, 2 MgSO4, 10 TES buffer, pH 7.15, 4 MgATP, 0.4 Na2GTP, 1 NAD. In part of the experiments, ATP and NAD were omitted. The external solution was perfused via a perfusion system at a rate of 25 chambers per minute.
RESULTS
Anoxia depolarizes fly photoreceptors in the dark
Previous studies on the physiology of the honey bee retina have demonstrated that insect photoreceptor cells quickly depolarize during application of anoxia in the dark, but the underlying molecular mechanism is not clear (Dimitracos and Tsacopoulos, 1985; Minke and Tsacopoulos, 1986). A study of the effects of anoxia onDrosophila photoreceptors is likely to provide important information on the molecular mechanism underlying anoxia actions because of the power of Drosophila genetics. Detailed measurements of oxygen consumption of honey bee retina reveal that the rate of oxygen consumption (Q) in the dark has a mean level as high as Q = 30 μl of O2/cm3 photoreceptor tissue per minute (Tsacopoulos et al., 1981). Strikingly, the retinalQ of the fly Calliphora that is maintained in the dark is threefold higher (Hamdorf and Kaschef, 1964). Thus, blowing N2 over the fly is expected to dramatically reduce the partial oxygen pressure (pO2) of the retina. Given the high oxygen consumption rate of the tissue, N2 should further lead to a decrease of pO2 to anoxic level (Stavenga and Tinbergen, 1983; Dimitracos and Tsacopoulos, 1985).
The experiments of Figure 1 examined whether the rapid anoxia-induced depolarization, which was described for the honey bee photoreceptors, is also observed in the fly retinal cells in vivo. Application of N2 to intact Drosophila flies during extracellular recordings from the eye resulted in a two-phased corneal negative voltage change in the dark. The initial phase had a slow rise time and small amplitude, whereas the subsequent phase had a much faster rise time and larger amplitude (arrow). The voltage change reached a steady-state level during the anoxia and was accompanied by abolishment of the corneal negative extracellular response to light (the ERG) (Fig.1a). Both the corneal negative voltage changes in the dark and the inactivation of the ERG quickly recovered on removal of anoxia (Fig. 1a). In the honey bee retina, illumination with a single intense flash of light causes a fall of pO2 as large as 40 mmHg (Tsacopoulos and Lehmenkuhler, 1977). Accordingly, it is expected that the effect of illumination will be additive to that of anoxia. Indeed, illumination greatly accelerates the onset of the larger response to anoxia, suggesting that anoxia and light act synergistically (Fig.1a, bottom).
Effects of anoxia similar to those of Figure 1a were also observed during extracellular and intracellular recordings from the larger flies Musca (Fig. 1b) andCalliphora (n = 5). Intracellular recordings from photoreceptors of intact Musca eye showed that anoxia induced a rapid depolarization that was accompanied by a reversible abolishment of the receptor potential in response to light (Fig.1b, bottom). The initial small and slow voltage change in response to anoxia, which was observed in the extracellular recordings (Fig. 1a), was missing in the intracellular recordings, suggesting that this phase did not arise in the photoreceptor cells (see below). Figure 1 thus demonstrates that anoxia depolarizes the photoreceptor cells of the fly. Bridge measurements inMusca and Calliphora photoreceptors during intracellular recordings revealed that the anoxia-dependent depolarization was accompanied by a conductance increase of amplitude similar to that induced by intense light (n = 4). Taken together, the apparent synergism between the response to anoxia and light, the conductance increase, and the abolishment of the response to light suggest that anoxia opens directly or indirectly the light-sensitive channels in the dark (see below).
The large phase of the anoxia-induced depolarization arises from activation of the TRP and TRPL channels
Anoxia is known to cause a reduction in K+ gradient of invertebrate photoreceptor cells caused by inhibition of the Na-K pump (Dimitracos and Tsacopoulos, 1985). In addition, anoxia increases cellular Ca2 (Lo et al., 1980), which may open Ca2+-activated K+ channels. Accumulation of K+ in the extracellular space leads to depolarization of the photoreceptor cells (Coles and Tsacopoulos, 1979;Dimitracos and Tsacopoulos, 1985; Minke and Tsacopoulos, 1986; Sandler and Kirschfeld, 1991). The control experiments of Figure2 were designed to demonstrate that the larger depolarization phase arose from specific activation of the TRP and TRPL channels rather than inhibition of the Na-K pump or other mechanisms causing accumulation of external K+. We simultaneously measured voltage changes and extracellular K+ concentration ([K+]out) using K+-selective microelectrodes. Figure2a shows in WT flies that the ERG response to illumination was accompanied by a reversible increase of [K+]out during light as reported previously (Coles and Tsacopoulos, 1979; Dimitracos and Tsacopoulos, 1985; Minke and Tsacopoulos, 1986; Sandler and Kirschfeld, 1991). Anoxia induced a small accumulation of K+ with relatively short latency, followed by a larger phase of increase in [K+]out(arrow). To determine what part of the K+ signal is attributable to activation of the TRP and TRPL channels, we repeated the measurements of [K+]out in the double mutanttrpl302;trpP343, which lacks both TRP and TRPL channels. In thetrpl302;trpP343mutants, in which the light response was completely abolished (Scott et al., 1997), only the initial slow and smaller signals were observed reaching a steady state, thus indicating that the small but not the large phase of the voltage change resulted from accumulation of K+ in the extracellular space. Because this slow phase was not observed in intracellular recordings, it may reflect depolarization of some other cell type, perhaps the pigment (glia) cells that are very sensitive, much more than the photoreceptor cells, to external [K+] (Coles and Tsacopoulos, 1979) (see Discussion). The larger phase, which depends on the presence of TRP and TRPL channels (Fig. 2b), reflects an efflux of K+ from the photoreceptor cell as expected from K+ acting as a counter ion for Na+ and Ca2+ influx through the light-sensitive channels (Ranganathan et al., 1991; Hardie and Minke, 1992).
Fig. 2.
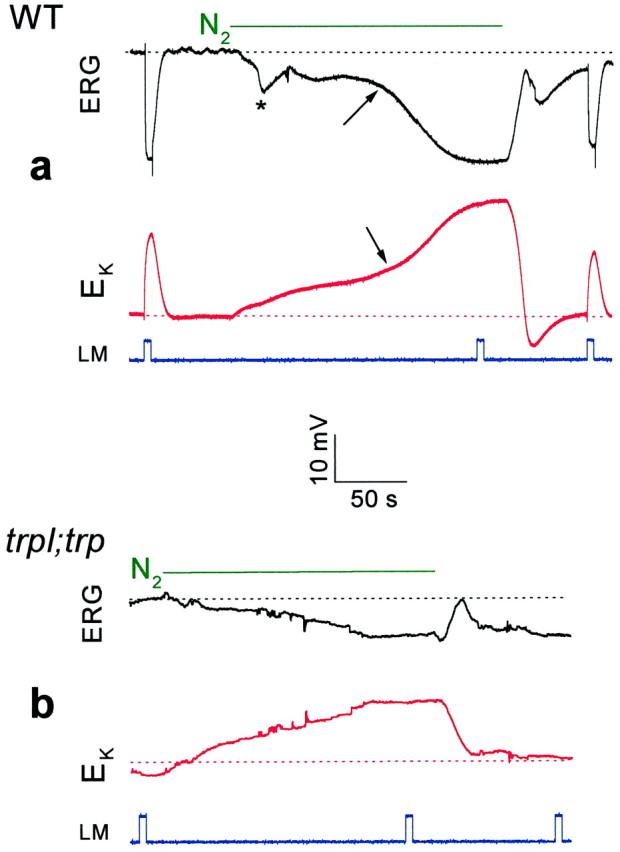
Measurements of [K+]out in WT and thetrpl302;trp343mutant show that only the second and large phase of the response to anoxia arise from activation of TRP and TRPL channels.a, Extracellular voltage change (ERG,top panel, in black) and potentiometric measurements with a K+-selective microelectrode (EK, bottom panel, inred) in response to orange lights and anoxia, in wild-type (WT) Drosophila. On average, the maximal Δ[K+]out during anoxia was 7.26 ± 1.83 mm (n= 4), assuming that [K+]out in the dark is 4 mm (Sandler and Kirschfeld, 1991). The calibration applies for both the ERG and the potentiometric measurements with the K+-selective microelectrode.b, The experiments of a were repeated in the double mutanttrpl302;trp343. Note that there is no response to light, and the second phase of the response to anoxia is absent. On average, the maximal Δ[K+]out was 2.64 ± 0.54 mm (n = 4) during anoxia.
Anoxia reversibly opens the TRP and TRPL channels in the dark at a late stage of the cascade
Measurements of voltage or [K+]out do not distinguish between direct activation of the light-sensitive channels and secondary activation via other channels [mainly voltage-gated K+ channels (Hardie et al., 1991)]. It is well established that Ca2+ influx into the photoreceptors takes place almost exclusively via the TRP and TRPL channels (Hardie and Minke, 1992; Peretz et al., 1994a). We therefore measured Ca2+ influx during anoxia to identify the specific component of the response to anoxia, which arises directly from activation of these channels. We also took advantage ofDrosophila mutants, which are defective in proteins crucial for the phototransduction cascade, to localize the transduction stage that underlies the effects of anoxia. Using Ca2+-selective microelectrodes, we measured in WT flies the well described reduction in extracellular Ca2+ concentration ([Ca2+]out) during illumination (Fig. 3a) (see also Sandler and Kirschfeld, 1991; Peretz et al., 1994a). The reduction of [Ca2+]outduring illumination arises from Ca2+influx into the photoreceptor cells caused by openings of TRP and TRPL channels (Peretz et al., 1994a). Application of anoxia indeed induced, after a delay, a reduction in [Ca2+]out (Fig.3a, bottom). In contrast to the K+ signal, the Ca2+ signal did not show the short-latency small-phase response to anoxia but only the larger phase, which had a rise time and kinetics similar to the larger phase of voltage change recorded from the same eye. This observation indicates that in response to anoxia the large depolarization phase and Ca2+ influx are caused by openings of the light-sensitive channels. Figure 3b shows that illumination of the blind mutant norpAP24, in which light-activated phospholipase C (PLC) is missing (Bloomquist et al., 1988; Pearn et al., 1996), did not elicit any response to light as monitored by either voltage or [Ca2+]out changes as expected (Fig. 3b). Application of anoxia in the dark induced a voltage response, similar to that of WT, with an initial small phase and a subsequent larger phase. The calcium signal, which accompanied the larger phase of the voltage change, was similar in WT and the mutant except for a faster onset in the mutant and an overshoot after anoxia was turned off (Fig. 3). We also examined the effects of anoxia on the ninaEora mutant, which is an opsin Rh1 null mutant (O'Tousa et al., 1989), and on theGαq1 mutant, which has a highly reduced level of light-activated G-protein (Scott et al., 1995). The effects of anoxia on these mutants were similar to those observed in WT flies except that in the Gαq1 mutant the latency of onset of the larger phase of the response to anoxia was approximately two times longer relative to wild type (n = 3, see below). The results thus show that anoxia affects a late stage of the phototransduction cascade downstream to PLC activation.
Fig. 3.
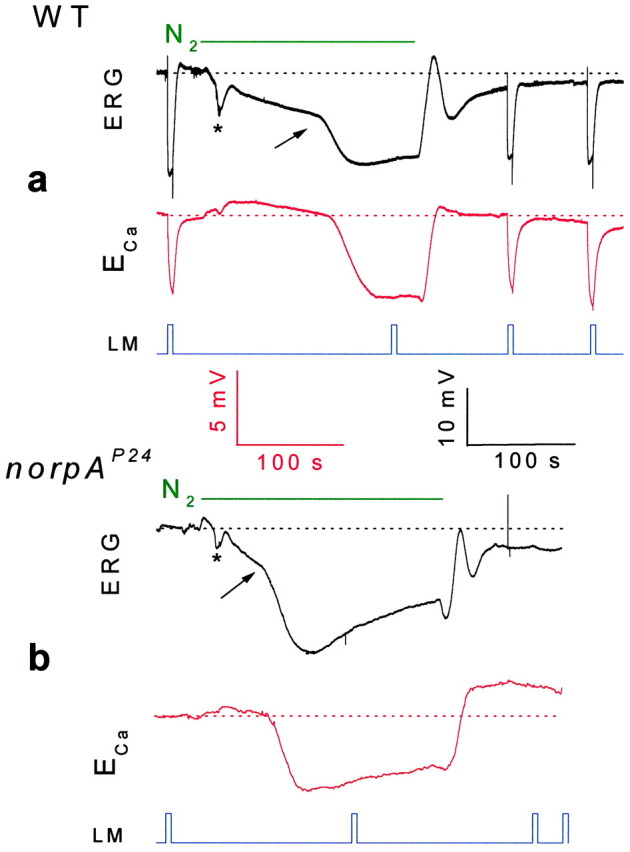
Anoxia activated the TRP and TRPL channels in both WT (a) and the PLC null mutant (norpAP24, b) as monitored by Ca2+ influx. Extracellular voltage change (ERG, top traces in botha and b, in black) and potentiometric measurements with Ca2+-selective microelectrode (ECa, bottom traces in both a and b, inred) in response to orange lights and anoxia in WTDrosophila andnorpAP24 mutant are shown. Note that there is no response to light in thenorpAP24 mutant and the initial slow phase of the electrical response to anoxia is missing in the Ca2+ signals of both WT and the mutant. The calibrations for the ERG records are indicated in black, and the calibrations for the potentiometric measurements with the Ca2+-selective microelectrode are indicated inred (in Figs. 3 and 4).
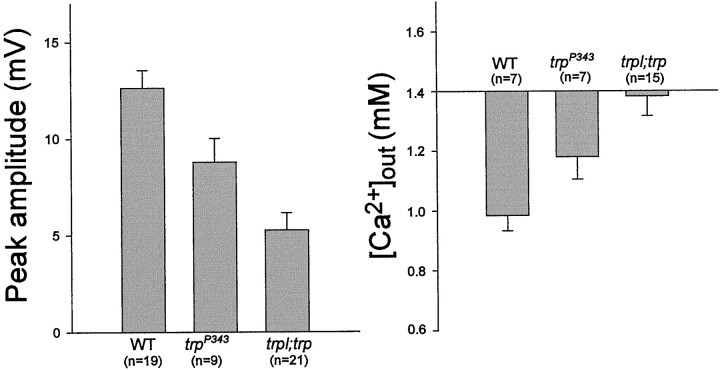
An additional demonstration that anoxia opens both TRP and TRPL channels in the dark was obtained by measuring Ca2+ influx in the trp mutant, in which TRP is missing, and in the trpl;trp double mutant, which lacks both channels (Scott et al., 1997). The ERG response to light of the trpP343 mutant revealed the typical decline toward baseline during illumination, whereas the Ca2+ signal was transient and relatively small, as reported previously (Fig.4a) (Peretz et al., 1994a). Application of anoxia to thetrpP343 mutant activated initially the slow and small phase of the voltage response (Fig.4a). However, in contrast to WT and the other mutants mentioned above, a significantly smaller amplitude of the second faster phase of the voltage and Ca2+ signal were observed in trpP343 flies (Figs. 4a, 5). The small Ca2+ signal in response to light and anoxia in the mutant reflects influx of Ca2+ into the photoreceptors via the TRPL channels (Peretz et al., 1994a).
Fig. 4.
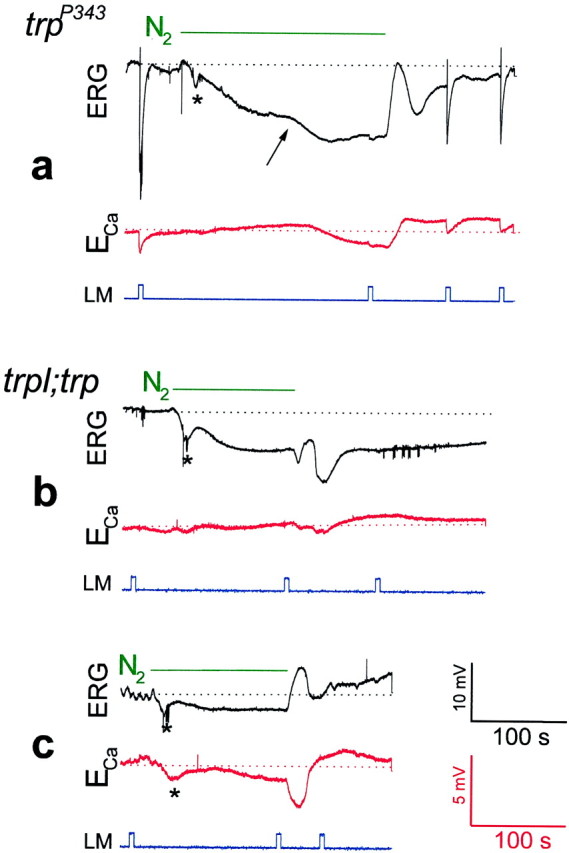
Genetic elimination of TRP (a) resulted in a reduced Ca2+ influx through the remaining TRPL channels in response to anoxia, whereas elimination of both TRP and TRPL (b, c) virtually abolished TRP- and TRPL-dependent signals and Ca2+ influx. The same paradigm of Figure 3 was repeated in Figure 4 except that the null trp mutanttrpP343 (a) and the null double mutanttrpl302;trpP343(in two different flies) (b, c) were used. Note that in the trpP343mutant the second phase of the electrical response to anoxia and the Ca2+ influx were relatively small but were maintained as long as anoxia was applied, in contrast to the transient responses to light. Also note that there is no response to light in the trpl;trp mutant and that the second phase of the electrical response to anoxia was absent. The negative small and slow Ca2+ signal in c revealed variability in sign and appeared in only ∼30% of the mutant flies.
Fig. 5.
Histograms showing maximal voltage changes, which include both the slow and fast phases (left) and Δ[Ca2+]out (right), in response to anoxia in WT, trp, andtrpl;trp mutants. The error bars are SEM.
An interesting characteristic of the response to anoxia of thetrpP343 mutant is the existence of a maintained component in both voltage and Ca2+ signals as long as anoxia was applied, as in WT (Fig. 4a). This maintained response to anoxia in the mutant is in sharp contrast to the transient nature of the response to continuous light.
To firmly establish that the Ca2+ influx in response to anoxia is caused by activation of TRP and TRPL channels, we measured [Ca2+]out in response to anoxia in the double mutanttrpl302;trpP343. In this mutant the response to light is completely abolished (Figs.2b, 4b) (Scott et al., 1997). Application of anoxia induced only the initial small voltage change, with neither the subsequent larger voltage change nor any significant change in [Ca2+]out during the initial 2 min of anoxia (Figs. 4b, 5). In some (∼30%) mutant flies, very small changes in [Ca2+]out were observed (Fig. 4c). These small changes in [Ca2+]o were variable in sign and amplitude and may reflect activation of the Na-Ca exchanger operating in the forward or reverse mode caused by variable reduction in the Na+ or Ca2+ gradients across the cells during anoxia in some vulnerable flies (Hardie, 1995).
The lack of virtually any Ca2+ influx in the trpl;trp double mutant in response to anoxia (Fig. 5) suggests that the mechanism that controls the opening of the TRP and TRPL channels is the target of anoxia.
Inhibition of mitochondria mimicked the effects of anoxiain vivo
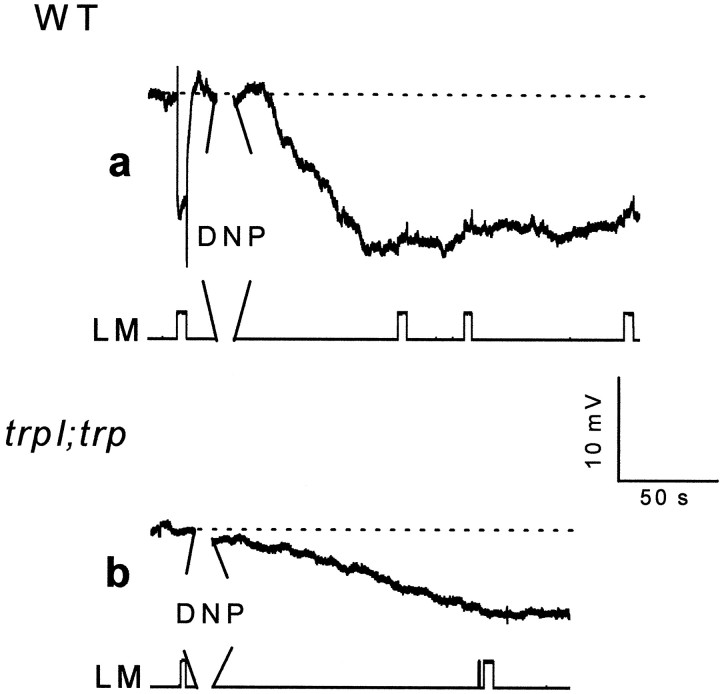
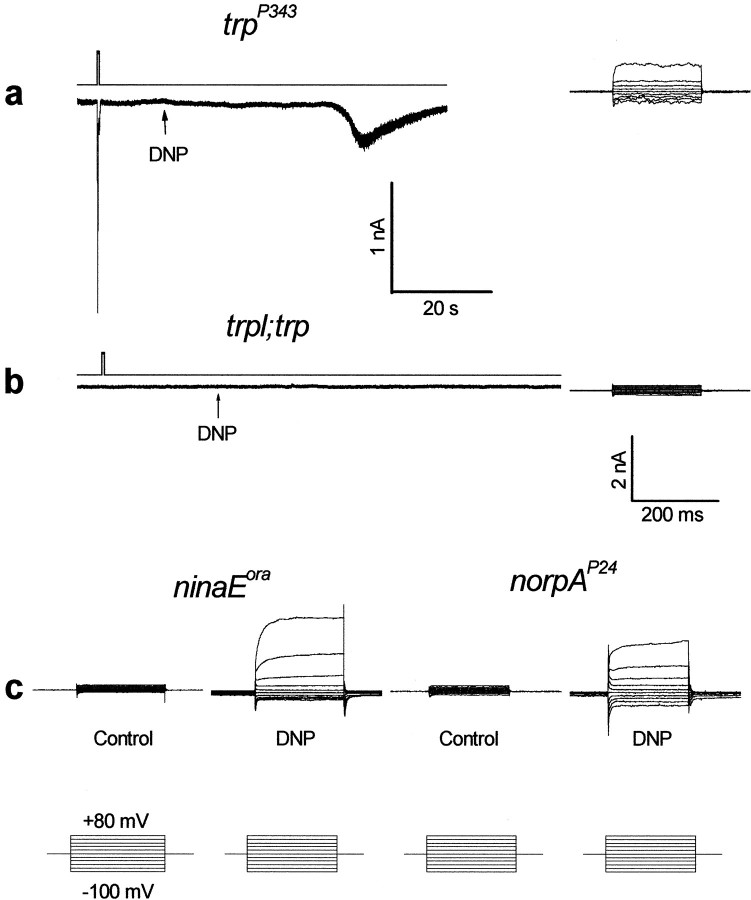
Fly photoreceptor cells are known to have a large number of mitochondria (Boschek, 1971). To investigate whether the effects of anoxia on Drosophila retina are attributable to impaired function of the mitochondria, 2,4-dinitrophenol (DNP) and carbonyl cyanide m chlorophenylhydrazone (CCCP) were applied to the intact eye. DNP and CCCP are known uncouplers of the oxidative chain for ATP production in the mitochondria (McLaughlin and Dilger, 1980). Figure 6a shows that application of DNP to the intact eyes of wild-typeDrosophila induced a negative voltage change and abolished light excitation in a manner similar to the larger phase of the response to anoxia. The effects of DNP were partially reversible ∼20 min after the application (n = 3). Similar measurements performed in the double mutanttrpl302;trpP343(Fig. 6b) showed smaller and slower voltage changes as in the case of anoxia (see below). Similar results were obtained after application of CCCP (n = 3). Measurements of [Ca2+]out could not be obtained because DNP strongly reduced the sensitivity of the Ca2+-selective microelectrodes. The effects of DNP on wild-type flies were accelerated when combined with either anoxia or illumination, thus suggesting that light stimulation and all forms of metabolic stress were additive (see below). These experiments further suggest that mitochondria uncouplers mimicked the effects of anoxia in wild-type flies through effects on the TRP and TRPL channels. Whole-cell recordings in single photoreceptor cells added more conclusive evidence to the in vivo studies.
Fig. 6.
The mitochondria uncoupler 2,4-dinitrophenol (DNP) mimicked the effects of anoxia as monitored by extracellular voltage changes. a, The paradigm of Figure1a was repeated in WT Drosophila, except that an additional pipette containing either 1 or 10 mm DNP in Ringer's solution was inserted into the retina, and DNP was applied by pressure injection (during the 30 sec break in a andb). It is estimated that DNP is diluted ∼10- to 40-fold in the eye. Control injections of Ringer's solution without DNP had no effect. b, The paradigm of awas repeated in the double mutanttrpl302;trpP343.
Mitochondrial uncouplers and depletion of ATP activate the TRP and TRPL channels in the dark in situ
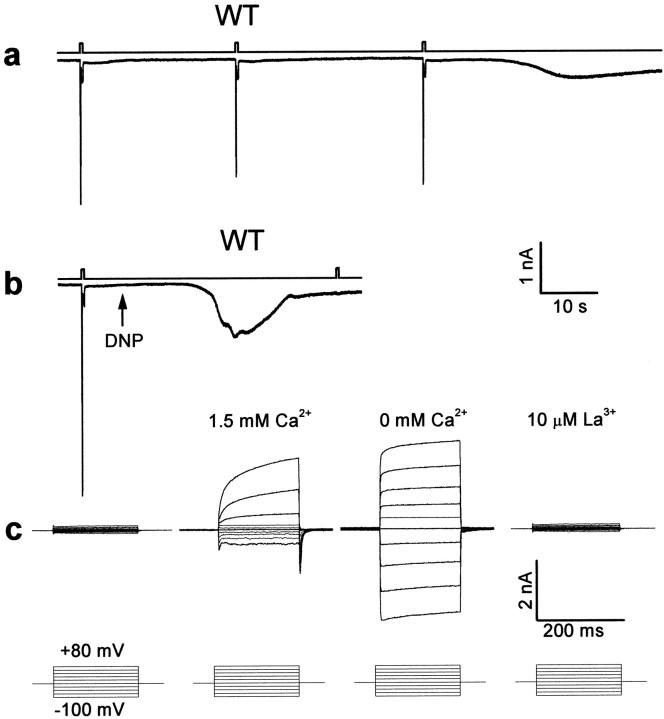
Impairment of mitochondria function is expected to deplete ATP from the photoreceptor cells. To investigate more directly whether depletion of ATP from the photoreceptor cells activates the TRP and TRPL channels, we used whole-cell patch-clamp recordings in isolated ommatidia preparations (Hardie, 1991; Ranganathan et al., 1991; Hardie and Minke, 1992). When ATP and α-nicotinamide adenine dinucleotide (NAD) were omitted from the recording pipette of WT cells, few light pulses of medium intensity induced an inward current in the dark after a delay of 108.5 ± 10.4 sec (n = 8, from the time of establishing the whole-cell recordings). This current was accompanied by elimination of the response to light and by increased noise level (Fig. 7a). Inclusion of ATP and NAD in the pipette prevented the induction of the inward dark current by repeated illumination, for at least 6 min (n = 12). This observation suggests that although light pulses are known to reduce the ATP level in photoreceptor cells (Dimitracos and Tsacopoulos, 1985), the supplement of exogenous ATP and NAD probably prevented depletion of ATP by illumination for at least 6 min. Application of 0.1 mm DNP to the bath (Fig.7b) during recordings with pipettes without ATP and NAD induced the inward current in the dark in WT flies after a delay of only 19.7 ± 3.5 sec (n = 4) (Fig. 7b). When DNP (0.1 mm) was included in the pipette solution (without ATP and NAD), the inward current was induced in <30 sec (n = 4) from the onset of whole-cell recording.
Fig. 7.
Single-cell functional analysis by whole-cell recordings from newly eclosed flies showing that depletion of ATP activates the TRP and TRPL channels of WT cells. Omission of ATP and NAD from the recording pipette (in all traces) induced, after a few light pulses, or after application of DNP, a constitutive activation of the light-sensitive channels. This channel activity was indicated by the appearance of a transient phase, which was followed by a sustained noisy inward current that had all the characteristics of the TRP- or TRPL-dependent current. None of these currents were observed in the presence of ATP and NAD in the pipette or before the dark inward current was induced. This was demonstrated by application of voltage steps in the dark during whole-cell recordings from photoreceptor cells, which revealed only small leak currents (c,left). The establishment of the whole-cell recordings took place ∼10 sec before the beginning of the traces in this Figure.a, Typical light-induced currents of a WT cell in response to three orange lights was followed by the appearance of slow inward current in the dark when the pipette solution had no ATP and NAD. The membrane voltage was held at −50 mV. The top traces in each pair indicate the duration of the orange light stimuli. b, The onset of the dark inward current was accelerated by application of 0.1 mm DNP (arrow, in a different cell). The inward current was induced 19.7 ± 3.5 sec after application of DNP (n = 4) compared with 108.5 ± 19.7 sec (n = 8) without application of DNP under similar recording conditions (a). Note that no additional response to light was obtained after the dark inward current was induced. c, A comparison of families of current traces elicited by a series of voltage steps in the range of −100 to +80 mV in steps of 20 mV (bottom row), from photoreceptors of wild-type flies under the following conditions: in the dark before the inward current was induced, at the peak of the inward current at 1.5 mm external Ca2+, after Ca2+ was removed from the external medium (0 Ca2+), and after 10 μmLa3+ was applied to the external medium (as indicated).
In either trpP343 or in the double mutanttrpl302;trpP343, no dark current was elicited during prolonged recordings (<10 min) using pipettes without ATP and NAD (n = 6). Application of 0.1 mm DNP either to the bath or into the pipette induced a noisy inward current of small amplitude intrp flies (Fig. 8a,left) (n = 5). In the double mutanttrpl302;trpP343, DNP (applied either to the bath or in the pipette) or CCCP without ATP and NAD in the pipette had no effect (Fig. 8b) (n = 8), even after incubation for 16 min.
Fig. 8.
The effects of DNP on Drosophilamutants. a, A relatively slow induction of a small and noisy inward current in the dark was observed intrpP343 after application of 0.1 mm DNP (arrow) during recordings without ATP and NAD in the pipette. Inward currents could not be induced without application of DNP under similar recording conditions. The right traces show a family of current traces elicited by a series of voltage steps as in Figure 7c at 1.5 mmexternal Ca2+. Similar families of current traces of similar amplitudes were observed at 0 Ca2+ medium (n = 3). b, Recording from thetrpl302;trpP343double mutant using pipettes in which ATP and NAD were omitted and 0.1 mm DNP was applied to the external medium as indicated. No dark inward current was observed even 16 min after the beginning of whole-cell recordings. The right traces show a family of current traces elicited by a series of voltage steps as in Figure7c at 1.5 mm external Ca2+. Similar results were obtained from four different cells in which DNP was included in the recording pipette. Note that neither light-induced currents nor inward currents in the dark could be induced in the double mutant. c, The effects of DNP (applied through the pipette) on theninaEora, Gαq1, andnorpAP24 mutants. Thetraces are families of current traces elicited by a series of voltage steps as in Figure 7c at 1.5 mm external Ca2+. The typical TRP-dependent current is observed only in cells in which the recording pipette included 0.1 mm DNP but not in control cells of the same retinae, as indicated. Similar results were obtained from three to six different cells of each mutant. The traces recorded from theGαq1 mutant are not shown. The lower calibration applies to all families of current traces elicited by a series of voltage steps.
The inward current of WT cells, which was induced in the dark after illumination or DNP application in the absence of ATP and NAD, had all the characteristics of the TRP-dependent current (Hardie and Minke, 1994a,b). In WT cells in zero external Ca2+ the current showed inward and outward rectification (Fig. 7c) (0 mmCa2+, n = 7), whereas the amplitude of inward current at negative holding potentials was greatly reduced in the presence of Ca2+ in the medium (Fig. 7c) (1.5 mmCa2+, n = 12). La3+, a potent Ca2+ channel blocker that mimics thetrp phenotype (Hochstrate, 1989; Suss Toby et al., 1991;Hardie and Minke, 1992, 1994a; Niemeyer et al., 1996), completely blocked the TRP-dependent current (Fig. 7c,right) (n = 5), as described previously (Hardie and Minke, 1994a). In the trp mutant, after application of DNP the resulting inward current had all the characteristics of the TRPL-dependent current of trp mutant flies. It had small amplitude, high noise (Fig. 8a), and near absence of inward rectification (Fig. 8a,right) even at 0 Ca2+ level (n = 3) (see also Hardie and Minke, 1994b; Reuss et al., 1997). The effects of DNP were additive with light or absence of ATP and NAD; thus illumination accelerated the onset of the effect of DNP, whereas inclusion of ATP and NAD in the pipette increased the latency of TRP channel activation to 2–4 min (WT, n = 4).
To firmly establish that the effect of DNP under our experimental conditions operates exclusively on the TRP and TRPL channels, we examined the effect of DNP on the isolated ommatidia of the null rhodopsin mutant, ninaEora, on the hypomorph G-protein mutant, Gαq1, and on the null PLC mutant, norpAP24. The experiments depicted in Figure 8c show that inclusion of 0.1 mm DNP in the recording pipette induced, in the above mutants, openings of channels with properties typical for the TRP channels. This was revealed by families of current traces measured at variable membrane voltage (Fig. 8c) (n = 3–5 for each mutant). Control experiments that were performed on ommatidia of these mutants in the dark, when DNP was neither included in the recording pipette nor applied to the bath, did not show these currents during recordings of at least 6 min (Fig. 8c) (n = 3–6 for each mutant). The TRP-dependent current was induced by DNP in <30 sec from the time of establishing the whole-cell recordings in thenorpAP24 andninaEora mutants but much slower (2–3 min) in the Gαq1 mutant (n = 4), which is consistent with the in vivo measurements in this mutant.
In summary, the results show that elimination of ATP and NAD from the recording pipette, combined with conditions that deplete ATP from the mitochondria, opened the TRP and TRPL channels in the dark by affecting a late stage of the transduction cascade.
DISCUSSION
TRP channel proteins are the main candidates for surface membrane, phosphoinositide-mediated Ca2+ entry channels, not only in Drosophila but also in mammalian tissues in which a large number of TRP homologs have been identified (Birnbaumer et al., 1996; Garcia and Schilling, 1997; Montell, 1997;Putney and McKay, 1999). Although several mechanisms have been recently proposed to account for TRP gating (Birnbaumer et al., 1996; Kiselyov et al., 1998; Chyb et al., 1999; Hofmann et al., 1999; Putney and McKay, 1999; Ma et al., 2000), the gating mechanism of TRP channels remains unknown (Berridge et al., 2000).
In the present study we demonstrated that metabolic stress in intactDrosophila eye rapidly and reversibly activated the light-sensitive channels TRP and TRPL. A dependence of membrane potential on oxidative metabolism is a common feature of small nerve cells (Payne, 1981). A rapid depolarization of stimulated anoxic photoreceptors has been reported for various invertebrate species (Baumann and Mauro, 1973; Wong et al., 1976; Payne, 1981; Dimitracos and Tsacopoulos, 1985), and a substantial reduction of K+ gradient has been demonstrated in bee photoreceptors during anoxia (Dimitracos and Tsacopoulos, 1985). Our study shows that accumulation of external K+ was mainly secondary to openings of TRP and TRPL channels. In a study of locust photoreceptors, it has been suggested that anoxia-induced depolarization results from residual random activation of the light-sensitive channels (Payne, 1981). In none of the previous studies has an unequivocal demonstration that the light-sensitive channels are the primary targets of anoxia and their molecular identification been demonstrated. The combination ofDrosophila mutants, in which the light-sensitive channels have been removed genetically, together with measurements of Ca2+ influx, which functionally identify these channels, allowed unequivocal demonstration that anoxia rapidly, reversibly, and specifically activates the light-sensitive channels TRP and TRPL.
The present study demonstrates that activation of TRP and TRPL in the dark results from depletion of ATP. The depletion of ATP probably directly activates the channels and not earlier stages of the transduction cascade because of the following reasons. (1) Anoxia activated the TRP and TRPL channels at a stage downstream to PLC because anoxia induced Ca2+ influx in the PLC null mutant, norpAP24. (2) Activation of earlier stages results in production of unitary events (quantum bumps) (Scott and Zuker, 1998; Chyb et al., 1999), whereas depletion of ATP was shown here to produce a smooth response with channel noise (see also Hardie and Minke, 1994a). (3) Application of anoxia to the null trp mutant in vivo and application of DNP in situ produced maintained openings of the TRPL channels. This observation suggests that the maintained openings originate downstream to the presumably late stage, which underlies the transient response to light of trp mutants (Scott et al., 1997).
The results suggest that mitochondria are the primary target of anoxia leading to a reduction in cellular ATP. Indeed, previous studies on the large fly Calliphora have demonstrated that application of N2 rapidly and reversibly inhibited a specific transient green fluorescence that normally arises from functional mitochondria in the eye (Stavenga and Tinbergen, 1983).
The observation that impaired mitochondria function leads to openings of TRP and TRPL channels has important implications for previous studies. In these studies, activation of various TRP channels has been obtained by several means, including oxidative stress (Balzer et al., 1999) and application of polyunsaturated fatty acids (PUFAs) (Chyb et al., 1999). The latter is of special interest because linoleic acid and several other long-chain fatty acids have been shown to react as efficient uncouplers of mitochondria in various cells (Arslan et al., 1984; Hermesh et al., 1998). Our results, therefore, suggest that the action of PUFAs on TRP and TRPL channels is indirect and results from their action as mitochondria uncouplers.
The mechanism underlying activation of TRP and TRPL channels by depletion of ATP is not clear. However, the need to supply ATP in the dark at a high rate for proper function of the TRP and TRPL channels accounts for the well known, but unexplained, phenomenon of a high rate of oxygen consumption of insect retina in the dark (Tsacopoulos et al., 1981). The results, thus, suggest that a constitutive ATP-dependent process operating at a late stage of the phototransduction cascade is required to keep the TRP and TRPL channels closed in the dark. This makes these channels extremely sensitive to anoxia and may lead to cell death under metabolic stress, not only in Drosophila but also in vertebrate cells, which express TRP channels (Balzer et al., 1999). The present study thus provides a novel target for metabolic stress with possible implications for brain damage, because activation of TRP induces massive Ca2+ influx and TRP homologs are expressed in the brain (Garcia and Schilling, 1997; Putney and McKay, 1999; Harteneck et al., 2000). A recent study has shown that specific mutations in the transmembrane domain of DrosophilaTRP channel, which caused constitutive activation of TRP channels, resulted in a rapid photoreceptor cell death in vivo (Yoon et al., 2000).
The present study opens up a new avenue of research that may lead to elucidation of TRP gating and explain many of the seemingly contradictory reports on pharmacological properties and mechanism of activation of TRP channels (Putney and McKay, 1999; Harteneck et al., 2000).
Footnotes
This research was supported by grants from National Institutes of Health (EY 03529), The Israel Science Foundation (ISF), The Minerva Foundation, the US-Israel Binational Science Foundation (BSF), and the German Israeli Foundation (GIF). We thank Drs. C. S. Zuker for thetrpl and Gαq1 mutants and W. L. Pak for the trpP343mutant andtrpl302;trpP343double mutant. We also thank Drs. R. C. Hardie, Z. Selinger, and Z. Paroush for stimulating discussions and Z. Selinger and M. Treinin for critical reading of this manuscript.
Correspondence should be addressed to Baruch Minke, Department of Physiology, The Hebrew University-Hadassah Medical School, Jerusalem 91120, Israel. E-mail: minke@md2.huji.ac.il.
REFERENCES
- 1.Arslan P, Corps AN, Hesketh TR, Metcalfe JC, Pozzan T. cis-Unsaturated fatty acids uncouple mitochondria and stimulate glycolysis in intact lymphocytes. Biochem J. 1984;217:419–425. doi: 10.1042/bj2170419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzer M, Lintschinger B, Groschner K. Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovasc Res. 1999;42:543–549. doi: 10.1016/s0008-6363(99)00025-5. [DOI] [PubMed] [Google Scholar]
- 3.Baumann F, Mauro A. Effect of hypoxia on the change in membrane conductance evoked by illumination in arthropod photoreceptors. Nat New Biol. 1973;244:146–148. doi: 10.1038/newbio244146b0. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ. Capacitive calcium entry. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge MJ, Lipp P, Bootman MD. Signal transduction: the calcium entry pas de deux. Science. 2000;287:1604–1605. doi: 10.1126/science.287.5458.1604. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaumer L, Zhu X, Jiang MS, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc Natl Acad Sci USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 8.Boschek BC. On the fine structure of the peripheral retina and lamina ganglionaris of the fly, Musca domestica. Z Zellforsch. 1971;118:369–409. doi: 10.1007/BF00331193. [DOI] [PubMed] [Google Scholar]
- 9.Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- 10.Coles JA, Tsacopoulos M. Potassium activity in photoreceptors, glial cells and extracellular space in the drone retina: changes during photostimulation. J Physiol (Lond) 1979;290:525–549. doi: 10.1113/jphysiol.1979.sp012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 12.Devary O, Heichal O, Blumenfeld A, Cassel D, Suss E, Barash S, Rubinstein CT, Minke B, Selinger Z. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc Natl Acad Sci USA. 1987;84:6939–6943. doi: 10.1073/pnas.84.19.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitracos SA, Tsacopoulos M. The recovery from a transient inhibition of the oxidative metabolism of the photoreceptors of the drone (Apis mellifera). J Exp Biol. 1985;119:165–181. [Google Scholar]
- 14.Friel DD. TRP: its role in phototransduction and store-operated Ca2+ entry. Cell. 1996;85:617–619. doi: 10.1016/s0092-8674(00)81021-1. [DOI] [PubMed] [Google Scholar]
- 15.Garcia RL, Schilling WP. Differential expression of mammalian TRP homologues across tissues and cell lines. Biochem Biophys Res Commun. 1997;239:279–283. doi: 10.1006/bbrc.1997.7458. [DOI] [PubMed] [Google Scholar]
- 16.Gillo B, Chorna I, Cohen H, Cook B, Manistersky I, Chorev M, Arnon A, Pollock JA, Selinger Z, Minke B. Coexpression of Drosophila TRP and TRP-like proteins in Xenopus oocytes reconstitutes capacitative Ca2+ entry. Proc Natl Acad Sci USA. 1996;93:14146–14151. doi: 10.1073/pnas.93.24.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamdorf K, Kaschef AH. Der Sauerstoffverbrauch des Facettenauges von Calliphora erythrocephalla in Abhangigkeit von der Temperatur und dem Ionenmilieu. Z Vgl Physiol. 1964;48:251–265. [Google Scholar]
- 18.Hardie RC. Whole-cell recordings of the light induced current in dissociated Drosophila photoreceptors: evidence for feedback by calcium permeating the light-sensitive channels. Proc R Soc Lond B Biol Sci. 1991;245:203–210. [Google Scholar]
- 19.Hardie RC. Photolysis of caged Ca2+ facilitates and inactivates but does not directly excite light-sensitive channels in Drosophila photoreceptors. J Neurosci. 1995;15:889–902. doi: 10.1523/JNEUROSCI.15-01-00889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardie RC. INDO-1 measurements of absolute resting and light-induced Ca2+ concentration in Drosophila photoreceptors. J Neurosci. 1996;16:2924–2933. doi: 10.1523/JNEUROSCI.16-09-02924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 22.Hardie RC, Minke B. Spontaneous activation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994a;103:389–407. doi: 10.1085/jgp.103.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardie RC, Minke B. Calcium-dependent inactivation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994b;103:409–427. doi: 10.1085/jgp.103.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardie RC, Voss D, Pongs O, Laughlin SB. Novel potassium channels encoded by the Shaker locus in Drosophila photoreceptors. Neuron. 1991;6:477–486. doi: 10.1016/0896-6273(91)90255-x. [DOI] [PubMed] [Google Scholar]
- 25.Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 26.Hermesh O, Kalderon B, Bar TJ. Mitochondria uncoupling by a long chain fatty acyl analogue. J Biol Chem. 1998;273:3937–3942. doi: 10.1074/jbc.273.7.3937. [DOI] [PubMed] [Google Scholar]
- 27.Hochstrate P. Lanthanum mimicks the trp photoreceptor mutant of Drosophila in the blowfly Calliphora. J Comp Physiol [A] 1989;166:179–187. doi: 10.1007/BF00193462. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 29.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 30.Lo MV, Wong F, Pak WL. Increase in intracellular free calcium concentration of Limulus photoreceptors caused by a metabolic inhibitor. Vision Res. 1980;20:539–544. doi: 10.1016/0042-6989(80)90129-7. [DOI] [PubMed] [Google Scholar]
- 31.Ma HT, Patterson RL, van-Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin SG, Dilger JP. Transport of protons across membranes by weak acids. Physiol Rev. 1980;60:825–863. doi: 10.1152/physrev.1980.60.3.825. [DOI] [PubMed] [Google Scholar]
- 33.Minke B, Selinger Z. The roles of trp and calcium in regulating photoreceptor function in Drosophila. Curr Opin Neurobiol. 1996;6:459–466. doi: 10.1016/s0959-4388(96)80050-x. [DOI] [PubMed] [Google Scholar]
- 34.Minke B, Tsacopoulos M. Light induced sodium dependent accumulation of calcium and potassium in the extracellular space of bee retina. Vision Res. 1986;26:679–690. doi: 10.1016/0042-6989(86)90082-9. [DOI] [PubMed] [Google Scholar]
- 35.Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- 36.Montell C. New light on TRP and TRPL. Mol Pharmacol. 1997;52:755–763. doi: 10.1124/mol.52.5.755. [DOI] [PubMed] [Google Scholar]
- 37.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 38.Munoz JL, Deyhimi F, Coles JA. Silanization of glass in the making of ion-sensitive microelectrodes. J Neurosci Methods. 1983;8:231–247. doi: 10.1016/0165-0270(83)90037-7. [DOI] [PubMed] [Google Scholar]
- 39.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 40.O'Tousa JE, Leonard DS, Pak WL. Morphological defects in oraJK84 photoreceptors caused by mutation in R1–6 opsin gene of Drosophila. J Neurogenet. 1989;6:41–52. doi: 10.3109/01677068909107099. [DOI] [PubMed] [Google Scholar]
- 41.Payne R. Suppression of noise in a photoreceptor by oxidative metabolism. J Comp Physiol. 1981;142:181–188. [Google Scholar]
- 42.Pearn MT, Randall LL, Shortridge RD, Burg MG, Pak WL. Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutants. J Biol Chem. 1996;271:4937–4945. doi: 10.1074/jbc.271.9.4937. [DOI] [PubMed] [Google Scholar]
- 43.Peretz A, Sandler C, Kirschfeld K, Hardie RC, Minke B. Genetic dissection of light-induced Ca2+ influx into Drosophila photoreceptors. J Gen Physiol. 1994a;104:1057–1077. doi: 10.1085/jgp.104.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peretz A, Suss-Toby E, Rom-Glas A, Arnon A, Payne R, Minke B. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 1994b;12:1257–1267. doi: 10.1016/0896-6273(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 45.Petersen CCH, Berridge MJ, Borgese MF, Bennett DL. Putative capacitative calcium entry channels: expression of Drosophila trp and evidence for the existence of vertebrate homologues. Biochem J. 1995;311:41–44. doi: 10.1042/bj3110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- 47.Putney JW, McKay RR. Capacitative calcium entry channels. Bioessays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 48.Ranganathan R, Harris GL, Stevens CF, Zuker CS. A Drosophila mutant defective in extracellular calcium-dependent photoreceptor deactivation and rapid desensitization. Nature. 1991;354:230–232. doi: 10.1038/354230a0. [DOI] [PubMed] [Google Scholar]
- 49.Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron. 1997;19:1249–1259. doi: 10.1016/s0896-6273(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 50.Sandler C, Kirschfeld K. Light induced extracellular Ca2+ and Na+ concentration changes in the retinal of Calliphora: involvement in the mechanism of light adaptation. J Comp Physiol [A] 1991;169:229–311. [Google Scholar]
- 51.Scott K, Zuker CS. Assembly of the Drosophila phototransduction cascade into a signalling complex shapes elementary responses. Nature. 1998;395:805–808. doi: 10.1038/27448. [DOI] [PubMed] [Google Scholar]
- 52.Scott K, Becker A, Sun Y, Hardy R, Zuker C. Gqa protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron. 1995;15:919–927. doi: 10.1016/0896-6273(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 53.Scott K, Sun Y, Beckingham K, Zuker CS. Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell. 1997;91:375–383. doi: 10.1016/s0092-8674(00)80421-3. [DOI] [PubMed] [Google Scholar]
- 54.Stavenga DG, Tinbergen J. Light dependence of oxidative metabolism in fly compound eyes studied in vivo by microspectrophotometry. Naturwissenschaften. 1983;70:618–620. [Google Scholar]
- 55.Suss-Toby E, Selinger Z, Minke B. Lanthanum reduces the excitation efficiency in fly photoreceptors. J Gen Physiol. 1991;98:849–868. doi: 10.1085/jgp.98.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsacopoulos M, Lehmenkuhler A. A double-barrelled Pt-microelectrode for simultaneous measurement of PO2 and bioelectrical activity in excitable tissues. Experientia. 1977;33:1337–1338. doi: 10.1007/BF01920167. [DOI] [PubMed] [Google Scholar]
- 57.Tsacopoulos M, Poitry S, Borsellino A. Diffusion and consumption of oxygen in the superfused retina of the drone (Apis mellifera) in darkness. J Gen Physiol. 1981;77:601–628. doi: 10.1085/jgp.77.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am J Physiol. 1994;267:C1501–C1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- 59.Wong F, Wu CF, Mauro A, Pak WL. Persistence of prolonged light-induced conductance change in arthropod photoreceptors on recovery from anoxia. Nature. 1976;264:661–664. doi: 10.1038/264661a0. [DOI] [PubMed] [Google Scholar]
- 60.Xu XZS, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- 61.Yoon J, Cohen Ben-Ami H, Hong YS, Park S, Strong LLR, Bowman J, Geng C, Baek K, Minke B, Pak WL. Novel mechanism of massive photoreceptor degeneration caused by mutations in the trp gene of Drosophila. J Neurosci. 2000;20:649–659. doi: 10.1523/JNEUROSCI.20-02-00649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]