Abstract
The pore-forming α subunits of many ion channels are associated with auxiliary subunits that influence channel expression, targeting, and function. Several different auxiliary (β) subunits for large conductance calcium-dependent potassium channels of the Slowpoke family have been reported, but none of these β subunits is expressed extensively in the nervous system. We describe here the cloning and functional characterization of a novel Slowpoke β4 auxiliary subunit in human and mouse, which exhibits only limited sequence homology with other β subunits. This β4 subunit coimmunoprecipitates with human and mouse Slowpoke. β4 is expressed highly in human and monkey brain in a pattern that overlaps strikingly with Slowpoke α subunit, but in contrast to other Slowpoke β subunits, it is expressed little (if at all) outside the nervous system. Also in contrast to other β subunits, β4 downregulates Slowpoke channel activity by shifting its activation range to more depolarized voltages and slowing its activation kinetics. β4 may be important for the critical roles played by Slowpoke channels in the regulation of neuronal excitability and neurotransmitter release.
Keywords: potassium channel, β subunit, in situhybridization, Slowpoke, maxi K, calcium-dependent potassium channel
Large conductance calcium-dependent potassium (KCa or maxi K) channels are ubiquitous in nerve, muscle, and other cell types (Kaczorowski et al., 1996;Vergara et al., 1998). They play a particularly important role in neuronal signaling, because they respond to both the intracellular calcium concentration and the membrane potential. Neuronal KCa channels are enriched in axons and synaptic terminals (Knaus et al., 1996). They not only contribute to action potential repolarization and the control of firing frequency but also are critical for the regulation of neurotransmitter release (Gho and Ganetzky, 1992; Robitaille and Charlton, 1992; Robitaille et al., 1993; Bielefeldt and Jackson, 1994). These channels are key integrators of neuronal activity, because they provide a link between intracellular biochemical messenger systems and the electrical properties of the plasma membrane.
The pore-forming α subunits of KCa channels are encoded by the Slowpoke genes that have been described in many organisms (Adelman et al., 1992; Butler et al., 1993;Dworetzky et al., 1994; Pallanck and Ganetzky, 1994; Tseng-Crank et al., 1994). Although these α subunits can form functional channels when they are expressed alone in heterologous host cells, they may often be associated with auxiliary subunits in native tissues. For example, KCa channels purified from smooth muscle consist of the α subunit together with a 20–25 kDa membrane protein that has been termed a β subunit (Garcia-Calvo et al., 1994; Hanner et al., 1998). Voltage-dependent sodium, calcium, and potassium channels also copurify with β and other subunits that are important for channel expression, membrane targeting, and modulation (Isom et al., 1994; Rettig et al., 1994; Dunlap et al., 1995; Rhodes et al., 1995; Trimmer, 1998).
Slowpoke channel β subunits have been cloned from several sources. When the first-described β1 subunit (Knaus et al., 1994) is coexpressed with the α subunit, it can modulate Slowpoke channel activity, influence channel modulation by protein kinases, and alter toxin binding to the channel (McManus et al., 1995; Dworetzky et al., 1996; Tseng-Crank et al., 1996). This β1 subunit also binds estradiol and may mediate the activation of KCa current by the hormone in vascular smooth muscle (Valverde et al., 1999). A related protein has been cloned from quail (Oberst et al., 1997), but its functional properties have not yet been investigated. More recently, a protein that confers rapid inactivation on Slowpoke channels has been described. This protein, named β2 by one group (Wallner et al., 1999) and β3 by another (Xia et al., 1999), exhibits ∼45% identity with β1, and the overall membrane topologies of β1 and β2/3 are identical, with two transmembrane regions separated by a large extracellular domain. β1 is expressed prominently in peripheral tissues, including smooth muscle, but only to a very limited extent in brain (Tseng-Crank et al., 1996). β2/3 is expressed in many tissues (Wallner et al., 1999; Xia et al., 1999), and it is likely responsible for the rapid inactivation of KCa current in adrenal chromaffin cells (Solaro and Lingle, 1992; Solaro et al., 1995) and some neurons (Hicks and Marrion, 1998).
The KCa channels formed by coexpressing hSlo and hβ1 are pharmacologically distinct from those present in brain, and a different β subunit has been identified in brain membranes by biochemical approaches (Wanner et al., 1999). We describe here the cloning, expression pattern, and functional properties of a novel Slowpoke auxiliary subunit in human brain. This protein, which we call hβ4, is only distantly related to the known Slowpoke auxiliary subunits. We also have cloned the mouse ortholog mβ4. Under the conditions we have examined, the effects of β4 on Slowpoke channel properties are diametrically opposite to those of β1 and β2/3 in that channel activity is downregulated by β4. Slowpoke β4 may contribute to the modulation of neuronal excitability and neurotransmitter release by Slowpoke family channels.
MATERIALS AND METHODS
Cloning and sequence analysis. hβ4 was identified and cloned using the strategy described in Results (GenBank accession number AF215891). To clone a mouse homolog, a BLAST search of the mouse Expressed Sequence Tag (EST) database was performed using the human sequence as the input. One significant hit was obtained with GenBank accession number AI180680. This EST did not encode a full-length mouse homolog, but the sequence was used to design 3′ and 5′ rapid amplification of cDNA ends (RACE) primers. RACE reactions were performed with these primers using mouse brain cDNA (Clontech, Palo Alto, CA). A full open reading frame could be deduced from the cloned and sequenced RACE products (GenBank accession number AF215892). Two new primers were designed in the 3′ untranslated region after the stop codon and were used to obtain a full-length physical clone from mouse brain cDNA. This was cloned into pcR2.1(TOPO; Invitrogen, San Diego, CA). The hβ4 and mβ4 clones were subcloned and epitope-tagged with a V5-His tag in the mammalian expression vector pcDNA3.1 V5-His (Invitrogen). They also were subcloned into the pIRES2-EGFP vector (Clontech), a bicistronic vector that allows coexpression of hβ4 and green fluorescent protein (GFP) in the same cell. All constructs were sequenced throughout the full open reading frame (ABI Prism system; Brandeis University Sequencing Facility, Waltham, MA). No PCR-induced nucleotide change was observed in any of the RACE products or the cloned mammalian expression constructs. Amino acid alignments and phylogenetic tree calculations were done using DNAStar Inc. (Madison, WI) software. The phylogenetic analysis assumes a biological clock, represented by the distance between sequence pairs.
Tissue and brain region distribution. A PCR fragment from the hβ4 clone was labeled with 32P and used to probe human multiple tissue and brain region Northern blots (Clontech). In situ hybridization was performed with human cRNA probes corresponding to hSlo α subunit, hβ1, hβ2/3, and hβ4, essentially as described previously (Stahl et al., 1999), using 12 μm fresh-frozen sections prepared from cynomolgus monkey tissues and human brain (obtained from the Harvard Brain Tissue Resource Center, Belmont, MA). Human and monkey aorta and adrenal gland sections were used as positive controls for hβ1 and hβ2/3 probes, respectively.
Mammalian cell expression. Human embryonic kidney 293 (HEK 293) cells were maintained in MEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (pen–strep). Chinese hamster ovary (CHO) cells were cultured in Ham's F12 nutrient mixture plus 10% FBS and 1% pen–strep. Cells were seeded on poly-d-lysine-coated coverslips and transfected the next day with appropriate expression plasmids using Lipofectamine Plus (Life Technologies, Gaithersburg, MD) according to the manufacturer's guidelines.
Coimmunoprecipitation and Western blot. These experiments were done as described previously (Zhou et al., 1999). Epitope-tagged hβ4 or mβ4 in pcDNA3.1 was expressed in HEK 293 cells, either alone or together with hSlo or mSlo ([kindly provided by Steve Dworetzky (Bristol-Myers Squibb, Wallingford, CT) and Larry Salkoff (Washington University, St. Louis, MO), respectively]). Forty-eight hours after transfection, the cells were lysed and incubated with antibody directed against mSlo or against the His epitope (Invitrogen). The mSlo antibody recognizes a region in the C-terminal domain of both mSlo and hSlo (H. Wen and I. B. Levitan, unpublished observations). The immune complexes were precipitated by incubation with protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins in the lysates or immunoprecipitates (IPs) were separated on polyacrylamide gels and transferred to a nitrocellulose membrane. After blocking with 5% nonfat milk in TBST (0.1% Tween 20 in Tris-buffered saline), the blots were probed with primary antibodies directed either against mSlo or the V5 epitope. Horseradish peroxidase (HRP)-coupled anti-V5 (Invitrogen) was used as the primary antibody for hβ4/mβ4 blots, so no secondary antibody was required. HRP-coupled donkey anti-rabbit IgG (Amersham Pharmacia Biotech, Arlington Heights, IL) was used as the secondary antibody for hSlo/mSlo blots. Membranes were washed with TBST, and protein was visualized with an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech).
Electrophysiology. HEK 293 and CHO cells were used for recordings 1–3 d after transfection. Cells expressing GFP were identified by their green fluorescence, and all such green cells were found to exhibit current. Macroscopic currents were recorded from inside-out patches within several minutes of detaching the patch or in the whole-cell recording mode after the currents had stabilized. Solutions for inside-out patch recordings consisted of (in mm): 150 KCl, 10 HEPES, 5 Na-EGTA, and 0.5 MgCl2, pH 7.2 on both sides of the patch. Calcium was added to the intracellular side in an appropriate amount to give a final free calcium concentration of 0.3, 1, and 3 μm as calculated with Equal software from Biosoft (Cambridge, UK). Solutions for whole-cell recordings were as follows: bath solution, 145 mm NaCl, 5 mm KCl, 1 mmMgCl2, 1 mmCaCl2, and 10 mm HEPES, pH 7.2; electrode solution, 150 mm KCl, 10 mm HEPES, 5 mm Na-EGTA, 0.5 mm MgCl2, and 10 μm free calcium, pH 7.2. All chemicals were from Sigma (St. Louis, MO) or Fisher Scientific (Houston, TX), except synthetic charybdotoxin, which was purchased from Research Biochemicals (Natick, MA). Experiments were controlled and recorded on-line with pClamp 7 software (Axon Instruments, Foster City, CA). Currents were amplified with an Axopatch 200A amplifier (Axon Instruments). Data were analyzed with pClamp7, filtered off-line at 1 kHz and leak subtracted if necessary. For activation curves, cells were held at −100 mV, and depolarizations in steps of 10 mV were applied for 150 msec. The maximal conductance (Gmax) was calculated from deactivating tail currents. For lower calcium concentrations, Gmax was estimated from recordings done on the same patch at a higher calcium concentration. Conductance data were expressed asG/Gmax and were fitted to the Boltzmann equation. All results are presented as mean ± SEM, and statistical significance was assessed by ANOVA analysis with Bonferroni's multiple comparison post test.
RESULTS
Cloning and sequence analysis of human and mouse Slowpoke β4
To search for novel Slowpoke auxiliary subunits expressed in brain, we used a strategy of identifying brain proteins that are distantly related to the known β subunits. A monkey striatal cDNA library was analyzed by high-throughput single-pass sequencing and automated BLAST searching of ESTs as described previously (Pan et al., 1997). A clone was identified on the basis of limited homology to the N terminus of the quail putative Slowpoke β subunit (GenBank accession number U67865; blastp score, 70; p = 0.00011; no blastn value). It also could be aligned with the N terminus of the human Slowpoke β1 subunit (hβ1). The complete sequence of this clone was determined by primer walking and found to contain an open reading frame encoding a protein of 210 amino acids. A probe comprising the first 213 nucleotides of the open reading frame was used to screen a human fetal brain cDNA library, and several identical clones were obtained and sequenced. The open reading frame of these clones encodes a protein of 210 amino acids (Fig. 1a,top line), identical to the monkey protein. This protein exhibits only 20% amino acid identity with hβ1 [in contrast, hβ1 and hβ2/3 are 43% identical (Wallner et al., 1999; Xia et al., 1999)] (Fig. 1a, bottom line), and so this novel Slowpoke auxiliary subunit is designated hβ4. hβ4 is more closely related to hβ2/3 (29% identity), but it lacks the N-terminal inactivating particle and does not confer inactivation on Slowpoke α subunits (see below).
Fig. 1.
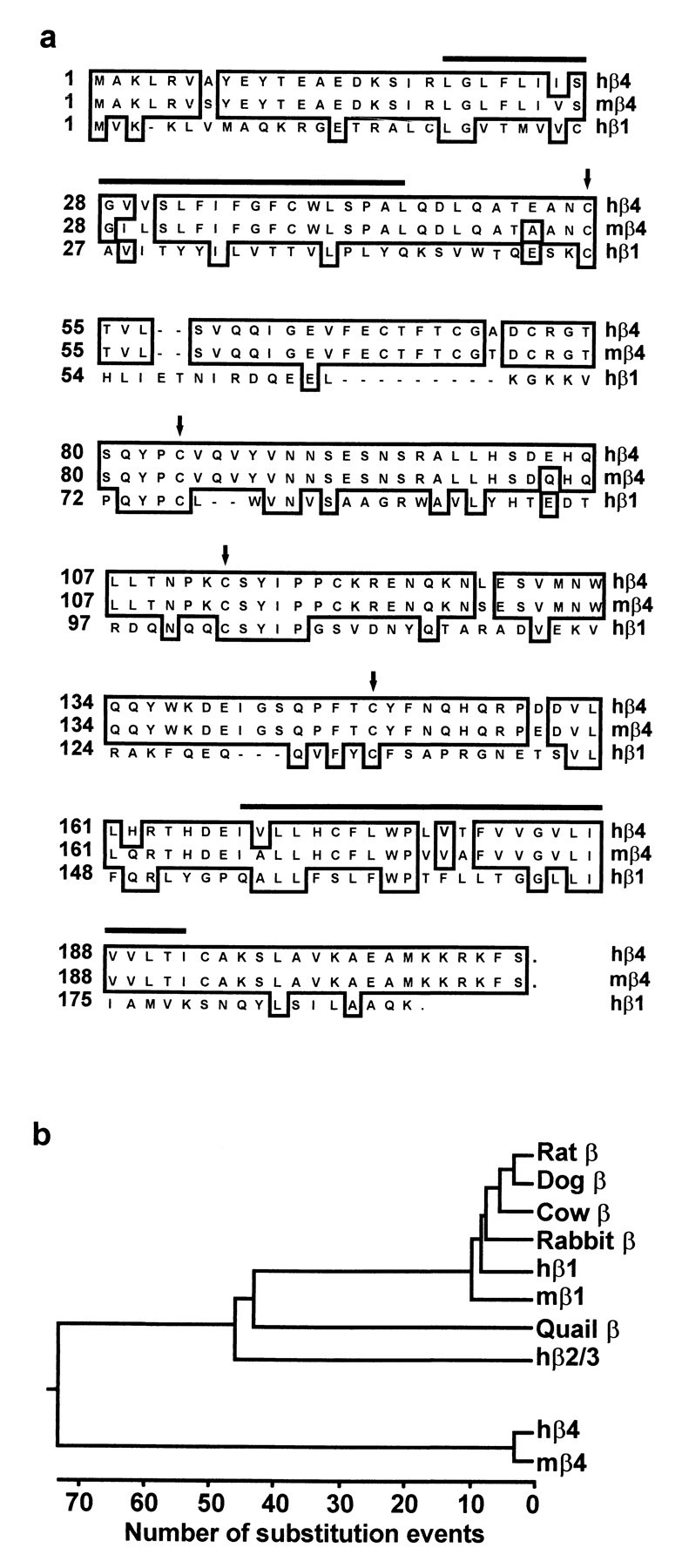
Sequence analysis of Slowpoke β subunits.a, Amino acid sequences of hβ4 (GenBank accession number AF215891), mβ4 (GenBank accession number AF215892), and hβ1 (GenBank accession number U38907) are aligned by the clustal method. Amino acids conserved in the β subunits are boxed. Thehorizontal bars indicate the two predicted membrane-spanning regions in β4, and conserved cysteine residues in the predicted extracellular loops are marked by arrows.b, Phylogenetic tree of Slowpoke β subunits cloned to date. The length of each pair of branches represents the evolutionary distance between sequence pairs, as measured by the number of substitution events. The Slowpoke β4 subunits described in this paper form a gene family distinct from other β subunits, which fall into a separate and evolutionarily conserved family. The GenBank accession numbers for the previously cloned β subunits used in this analysis are as follows: rat β, 1718491; dog β, 1127826; cow β, 508846; rabbit β, 2662318; hβ1, U38907; mβ1, 2347044; quail β, U67865; and hβ2/3, AF099137.
A mouse homolog of hβ4 was identified by BLAST search of the mouse EST database. The mβ4 protein is 94% identical to hβ4, with only a single nonconservative amino acid difference (Fig. 1a). The phylogenetic analysis in Figure 1b emphasizes the distinction between the previously described Slowpoke β subunits and mouse and human β4. Like β1 and β2/3, the β4 subunits contain two predicted membrane-spanning regions (Fig. 1a,horizontal lines), with a large extracellular loop between them containing conserved cysteine residues (Fig. 1a,arrows) and two putative sites for N-linked glycosylation.
Expression pattern of human Slowpoke β4
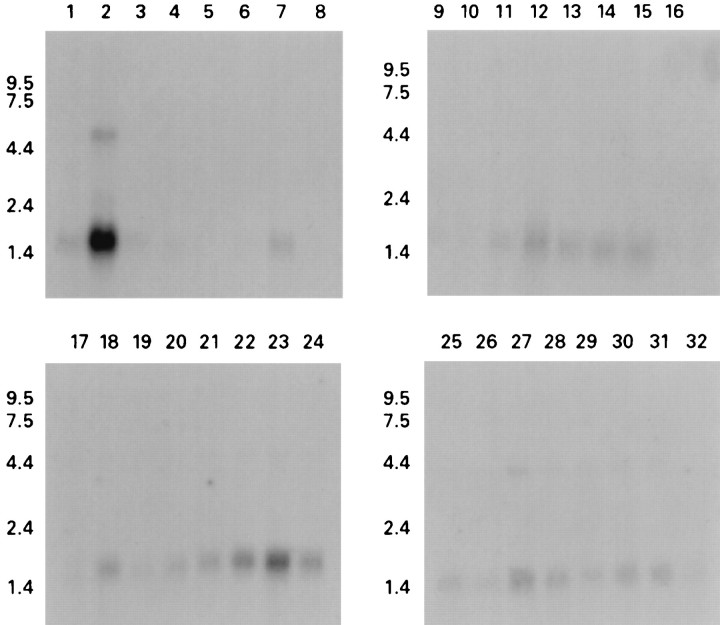
hβ4 expression was first analyzed using multiple tissue Northern blots and brain region Northern blots (Fig.2). hβ4 is expressed predominantly in human brain (Fig. 2, top panels, lane 2), with a major mRNA product at 1.6 kb and a minor one at 5 kb, whereas only limited expression is observed in non-neural tissues (lanes 1, 3–16). Indeed, no signal could be detected in sections of these non-neural tissues by in situhybridization (see below). Within the brain regions analyzed, expression is highest in cortical regions (Fig. 2, bottom panels).
Fig. 2.
Analysis of β4 expression using human multiple tissue (top panels) and brain region (bottom panels) Northern blots. A predominant 1.6 kb band is detected in brain, particularly in cortical regions, and a minor 5 kb band is also seen in brain. Fainter signals can also be detected in peripheral tissues. Lanes: 1, heart; 2, brain;3, placenta; 4, lung; 5, liver; 6, skeletal muscle; 7, kidney,8, pancreas; 9, spleen;10, thymus; 11, prostate;12, testes; 13, ovary; 14, small intestine; 15, colon; 16, peripheral blood leukocyte; 17, cerebellum;18, cerebral cortex; 19, medulla;20, spinal cord; 21, occipital lobe;22, frontal lobe; 23, temporal lobe;24, putamen; 25, amygdala;26, caudate nucleus; 27, corpus callosum;28, hippocampus; 29, whole brain;30, substantia nigra; 31, subthalamic nuclei; 32, thalamus.
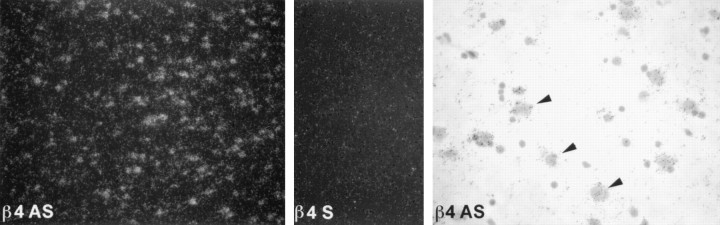
Tissue and regional expression of Slowpoke α and β subunits was analyzed further in human and monkey by in situhybridization. β4 is expressed in all layers of human cortex (Fig.3, left panel), and no signal is detected with a sense (S) probe (Fig. 3,middle panel). At higher magnification (Fig. 3,right panel), hybridization can be seen predominantly over human cortical neurons. Film autoradiography of monkey brain sections (Fig. 4) demonstrates widespread expression of Slowpoke α subunit (Slo) and hβ4 (top panels), particularly in cortex, basal ganglia, infundibulum, and hippocampus. Expression of hβ2/3 is less robust, and virtually no hβ1 mRNA can be detected (Fig. 4, bottom panels). It is also evident from emulsion autoradiography of monkey brain sections that there is a striking overlap in expression of Slowpoke α subunit (Fig. 5, top row) and hβ4 (Fig. 5, middle row) in multiple neuronal populations in cortex (CTX), dentate gyrus, and CA3 regions of hippocampus (HIP) and thalamus (THL). The bottom two rows in Figure 5 confirm that there is more limited expression of hβ2/3 and no expression of hβ1 in these brain regions. β4 expression is also detected in spinal motor neurons, sympathetic neurons of the superior cervical ganglion, and a subpopulation of dorsal root ganglion neurons (data not shown). This extensive brain distribution of β4 may be contrasted with the situation in human aorta in which Slowpoke α subunit (Fig.6, top panel) and hβ1 (Fig. 6, bottom panel) are expressed prominently, but hβ4 mRNA cannot be detected (Fig. 6, middle panel). In addition, despite the faint signal from these tissues on Northern blots, no β4 expression is detected in sections of monkey heart, skeletal muscle, pancreas, liver, testes, lung, or adipose tissue byin situ hybridization (data not shown).
Fig. 3.
Expression of β4 in human cortex. Emulsion autoradiograms of sections from human cortex, labeled with human β4 antisense (β4 AS, left panel) and sense (β4 S,middle panel) probes, were viewed under dark-field illumination (10× magnification). The antisense probe shows β4 expression throughout the cortical layers, whereas the sense probe shows only background. A bright-field image of the same tissue (β4 AS, right panel) shows β4 expression (arrowheads) in cortical neurons (40× magnification).
Fig. 4.
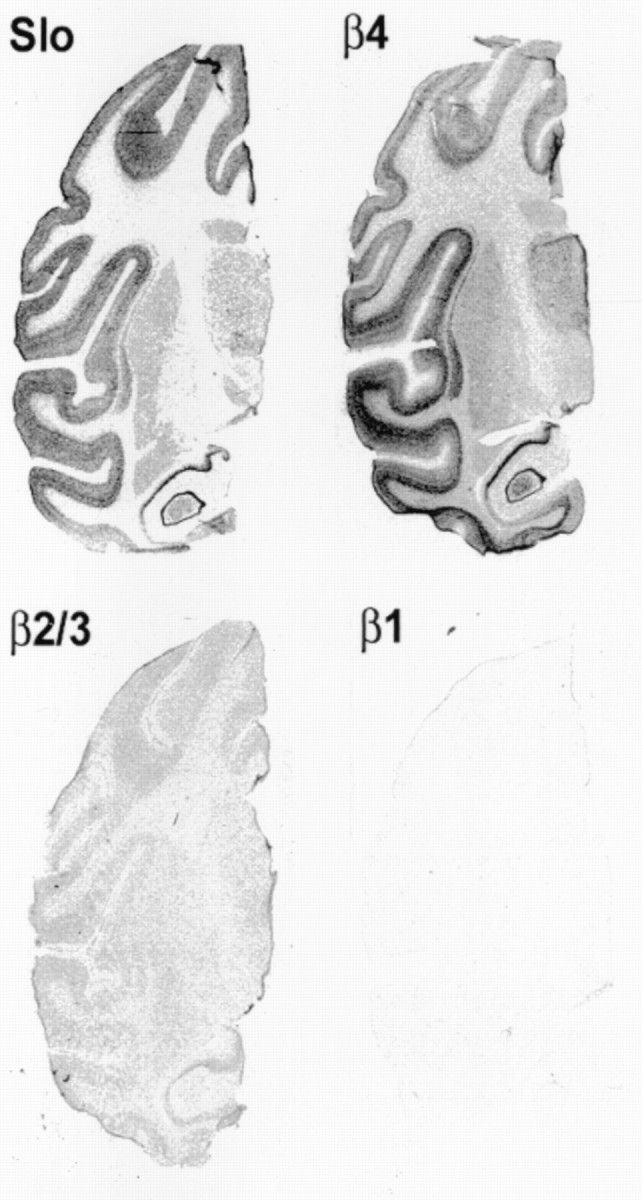
Expression of Slowpoke α and β subunits in monkey brain. Film autoradiograms of monkey brain sections hybridized with antisense probes that detect mRNA encoding Slowpoke α subunit (Slo), β4, β2/3, and β1. Note the overlapping expression of α subunit (Slo) and β4 in multiple brain regions, with considerably lower levels of β2/3 and little or no expression of β1.
Fig. 5.
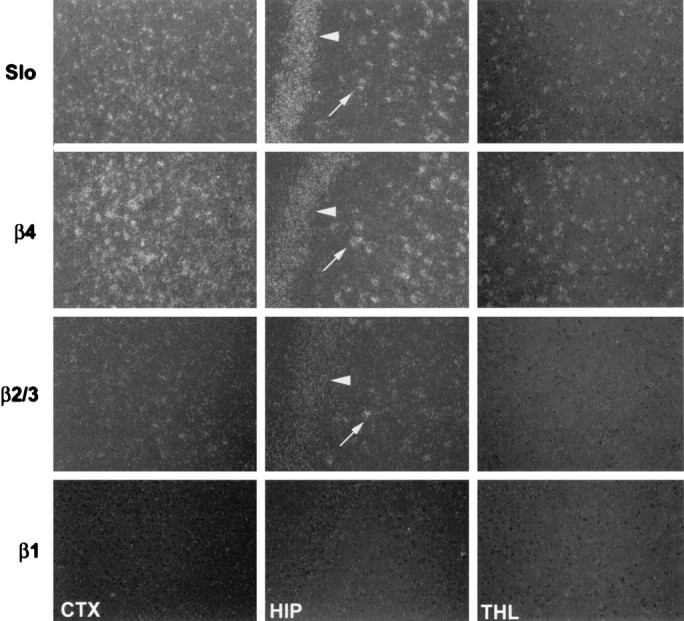
Analysis of Slowpoke α and β subunit expression in different regions of monkey brain. Emulsion autoradiograms of monkey brain sections, labeled with antisense probes to detect Slowpoke α (Slo, top row), β4 (second row), β2/3 (third row), and β1 (bottom row) subunits, were viewed under dark-field illumination. Slo and β4 are expressed in cortex (CTX), in the dentate gyrus (arrow) and CA3 (arrowhead) regions of hippocampus (HIP), and in thalamus (THL). β2/3 is expressed at lower levels and in apparently fewer cells in cortex and hippocampus and is not detected in thalamus. β1 expression is not detectable in any of these areas (10× magnification).
Fig. 6.
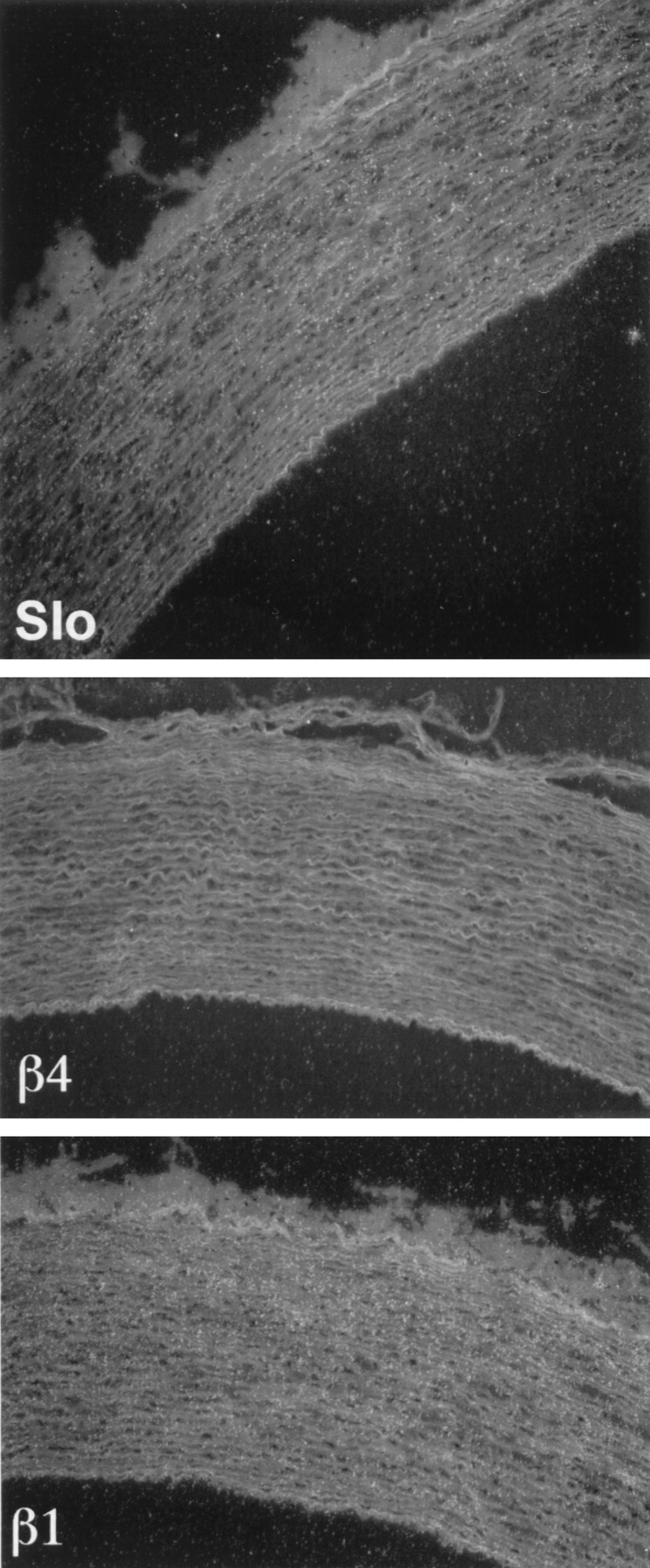
Analysis of Slowpoke α and β subunit expression in monkey aorta. Emulsion autoradiograms of sections of monkey aorta labeled with antisense probes to Slowpoke α (Slo, top panel), β4 (middle panel), and β1 (bottom panel) subunits were viewed under dark-field illumination. Silver grains representing mRNA encoding α and β1 subunits are readily visible over smooth muscle layers in the wall of the monkey aorta, whereas β4 expression is not detectable (10× magnification).
Slowpoke β4 binds to hSlo
To determine whether β4 is indeed a Slowpoke auxiliary subunit, we first asked whether it can coimmunoprecipitate with Slo. When HEK 293 cells are transfected with hSlo together with hβ4 and hSlo is immunoprecipitated with a specific antibody, hβ4 can be detected in the immunoprecipitate (Fig.7a, lane 1). It is interesting that two coimmunoprecipitating protein bands are seen, one with an apparent molecular weight (∼29 kDa) equivalent to that predicted for the epitope-tagged hβ4, and the second several kilodaltons larger (lane 1). Although the higher molecular weight band is barely detectable in the cell lysate (lane 2) and in a hβ4 immunoprecipitate (lane 3), it clearly is enriched relative to the smaller band in the hSlo immunoprecipitate (lane 1). No hβ4 staining is observed in hSlo immunoprecipitates from cells in which either hSlo (lane 4) or hβ4 (lane 5) is transfected alone. A similar result is observed when the interaction between mSlo and mβ4 is analyzed (Fig. 7b). In this case, the higher molecular weight mβ4 band is more evident in the lysate (lane 2) and mβ4 immunoprecipitate (lane 3), but it too preferentially coimmunoprecipitates with mSlo (lane 1). These results suggest that hβ4 and mβ4 may exist in several different post-translationally modified forms, one of which binds preferentially to Slowpoke α subunits. Slowpoke–β4 binding is also observed when the experiment is done by immunoprecipitating epitope-tagged β4 and probing for Slowpoke α subunit with anti-Slowpoke antibodies (data not shown).
Fig. 7.
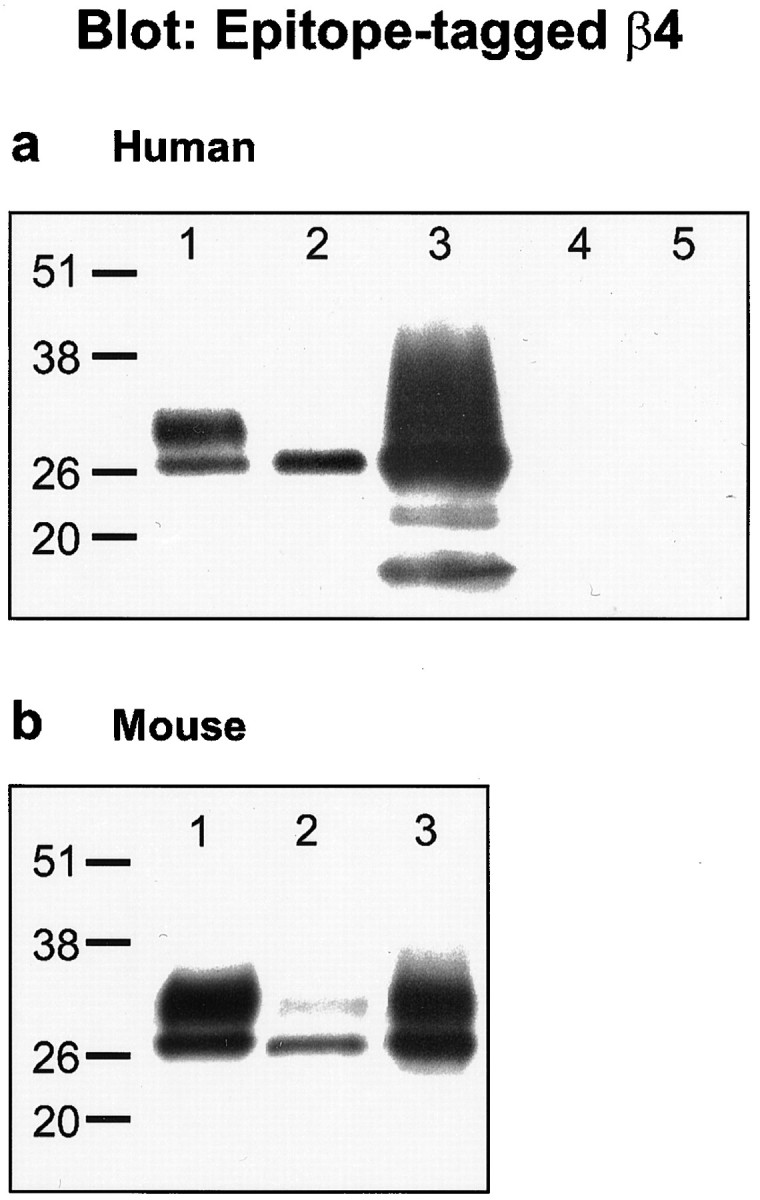
Mammalian Slowpoke α subunits coimmunoprecipitate with β4 subunits. Shown are Western blots of cell lysates or IPs, using an antibody directed against the V5 epitope that detects V5-His tagged β4 subunits. a, Human proteins. Lane 1, hSlo IP from cells transfected with hSlo and hβ4; lane 2, lysate from same cells as in lane1; lane3, anti-His IP from same cells as in lane 1; lane 4, hSlo IP from cells transfected with hSlo alone; lane 5, hSlo IP from cells transfected with hβ4 alone. b, Mouse proteins.Lane 1, mSlo IP from cells transfected with mSlo and mβ4; lane 2, lysate from same cells as in lane 1; lane3, anti-His IP from same cells as inlane 1.
Slowpoke β4 modulates hSlo activation kinetics
hSlo current was measured in inside-out membrane patches from HEK 293 cells transfected with hSlo α subunit. As shown in Figure8a, activation of the current in response to a depolarizing voltage step to +80 mV is much slower in cells cotransfected with hβ4. The time constant (τ) for activation of hSlo current is 7.4 ± 1.2 msec (n = 9) in the absence and 39 ± 7.5 msec (n = 5) in the presence of hβ4 (Fig. 8b). A similar slowing of activation was observed at all other voltages examined between +70 and +120 mV (data not shown). This may be contrasted with hβ1, which does not influence the time course of activation (τ of 7.8 ± 1.4 msec,n = 4) (Fig. 8b). A different pattern is observed when hSlo deactivation is considered. As is evident from inspection of the current traces in Figure 8a, hβ4 has little or no effect on the deactivation kinetics, and this is confirmed by the deactivation τ values in Figure 8c (τ of 3.0 ± 0.9 msec, n = 9 in the absence of hβ4; τ of 5.5 ± 1.9 msec, n = 5 in the presence of hβ4). Again this may be contrasted with hβ1 which, as shown previously (Dworetzky et al., 1996; Tseng-Crank et al., 1996), slows deactivation (τ of 14 ± 1.7 msec, n = 4) (Fig.8c).
Fig. 8.
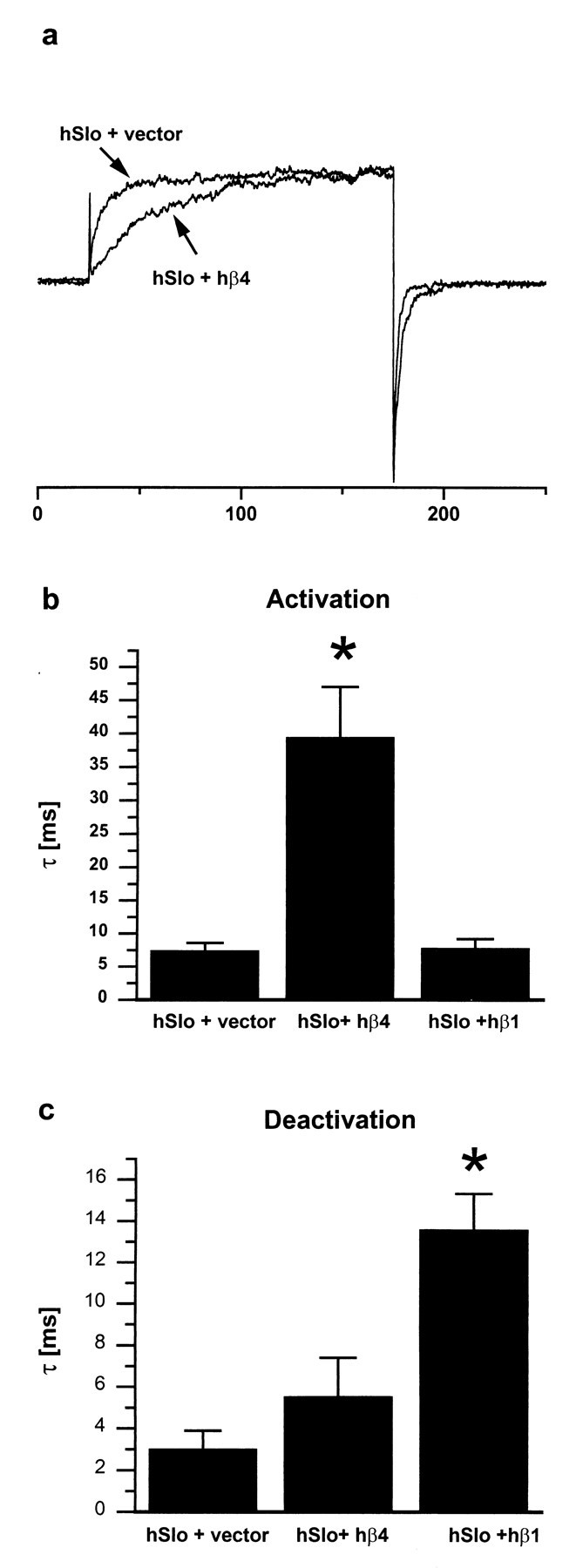
hβ4 decreases Slowpoke activation rate but does not affect its deactivation rate. a, Normalized currents recorded at +80 mV from cells transfected with either hSlo and control vector, or hSlo and hβ4. b and c show time constants (τ) calculated from single exponential fits of current traces. b, Activation kinetics: hβ4 increases the activation time constant significantly (p < 0.001), whereas hβ1 is without effect (p> 0.05). c, Deactivation kinetics: hβ4 does not alter the deactivation time constant (p > 0.05), whereas hβ1 increases it significantly (p< 0.001). Recording conditions: detached inside-out patches, symmetrical K+ solutions, 1 μm free Ca2+ on the intracellular side, holding potential of −100 mV, steps to +80 mV.
Slowpoke β4 modulates the voltage dependence of hSlo activation
hβ4 can also influence the steady-state activation of hSlo. In cells cotransfected with hβ4, the voltage dependence of hSlo activation is shifted some 50 mV to the right compared with cells transfected with hSlo alone (Fig.9a). This requirement for greater depolarization to activate the current is apparent at all calcium concentrations examined in the range from 0.3 to 3 μm (Fig. 9b). In marked contrast, as shown previously by many workers (McManus et al., 1995; Dworetzky et al., 1996; Tseng-Crank et al., 1996; Wallner et al., 1996; Xia et al., 1999), hβ1 shifts the voltage required for half-maximal activation by 20–50 mV in the opposite direction (Fig. 9b). A similar result has been reported for hβ2/3 (Wallner et al., 1999; Xia et al., 1999).
Fig. 9.
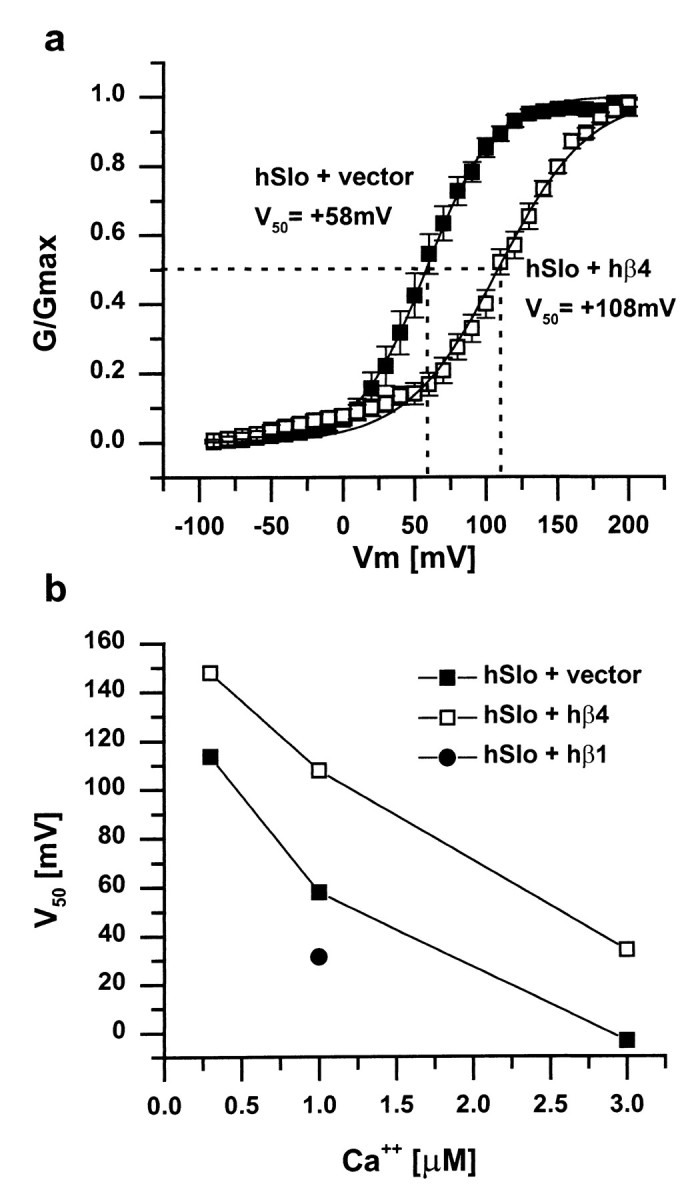
Steady-state activation of hSlo in the presence or absence of hβ1 or hβ4. a, hβ4 shifts the steady-state activation curve to the right, indicating that more depolarized voltages are required to open the channel.V50 (indicated by the dotted lines) is the half maximal activation voltage for each condition. Holding potential of −100 mV, 1 μm free calcium on the intracellular side, data fitted to the Boltzmann equation (n = 11 for hSlo plus vector;n = 10 for hSlo plus hβ4). b,V50 is shifted in the depolarizing direction by hβ4 at all calcium concentrations tested. In contrast, hβ1 shifts the half-maximal activation voltage in the hyperpolarizing direction.
Slowpoke β4 modulates toxin block of hSlo
Because auxiliary subunits often alter the effects of pharmacological agents on Slowpoke channel α subunits, we tested the effects of hβ4 on the block of hSlo current by the scorpion venom toxins charybdotoxin and iberiotoxin, in the whole-cell patch recording configuration. As shown in Figure 10,a and b, in cells transfected with hSlo and control vector, the current is blocked 90% or more by 300 nm of either toxin (filled symbols). In the presence of hβ4, in contrast, no block at all is observed by 300 nm toxin (Fig.10a,b, open symbols), and even as much as 1 μm iberiotoxin is without effect (Fig.10a). hβ1 (Dworetzky et al., 1996; Zhou et al., 1998) and hβ2/3 (Xia et al., 1999) also decrease channel sensitivity to toxins but to a much smaller extent.
Fig. 10.
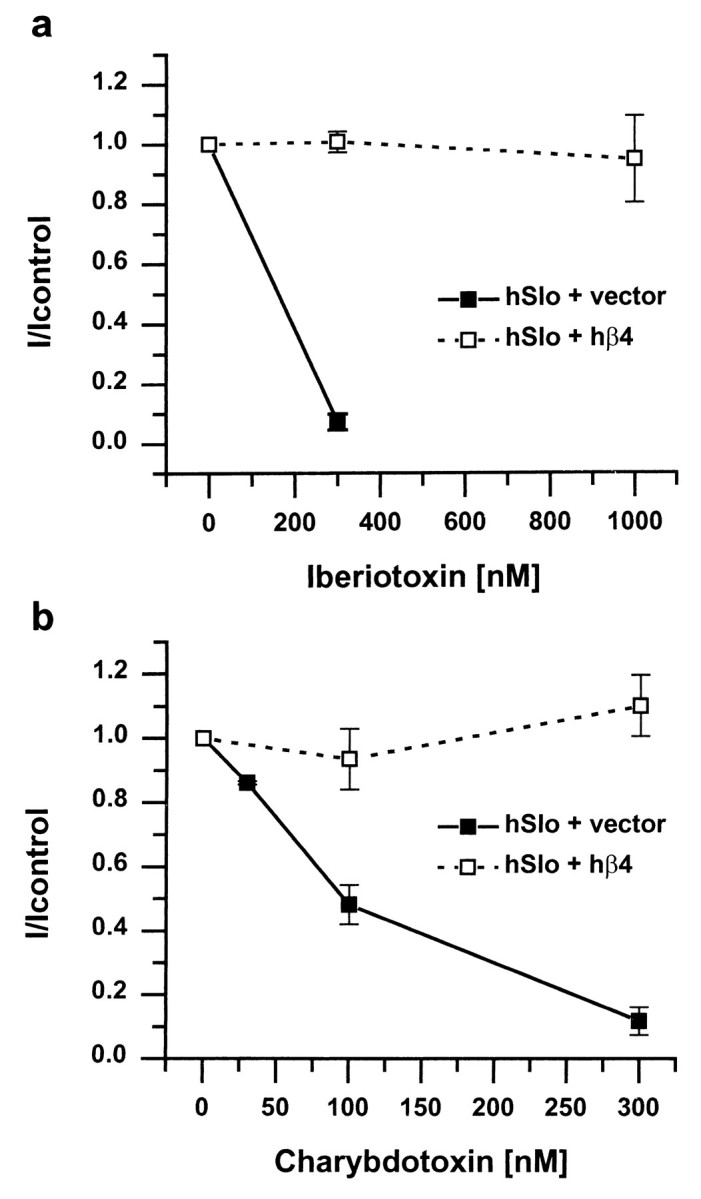
hβ4 protects hSlo from block by iberiotoxin (a) and charybdotoxin (b). Experiments were done in the whole-cell patch-clamp mode, and toxins were applied from the extracellular side. hSlo currents recorded in the presence of different concentrations of toxins were normalized to the control current in the absence of toxin. The filled symbols with solid lines show the block of current by toxins in the absence of hβ4, and the open symbols with dashed lines show that current is not blocked, even by high concentrations of toxin, in the presence of hβ4 (n = 3; holding potential of −85 mV, steps to +105 mV, 10 μm free internal calcium). Note the different concentration axes in a andb.
DISCUSSION
The pattern of neuronal electrical activity can vary widely from one neuron to another. Much of this diversity in electrical activity arises from differences in potassium channel activity in different cells. A large number of genes that encode potassium channels have been identified (Jan and Jan, 1997), and some of these are subject to extensive alternative splicing that can generate even greater diversity (Schwarz et al., 1988; Adelman et al., 1992; Tseng-Crank et al., 1994). Yet another mechanism that is a major contributor to functional diversity is the interaction of the pore-forming α subunits of potassium channels and other channels with a wide variety of auxiliary proteins that can influence channel expression, membrane localization, and gating properties (Isom et al., 1994; Rhodes et al., 1995; Trimmer, 1998).
We describe here human and mouse genes for a novel auxiliary subunit of the Slowpoke family of large conductance KCachannels. This protein, which we call Slowpoke β4, was originally identified as a candidate Slowpoke auxiliary subunit on the basis of its amino acid sequence homology with a known Slowpoke β subunit. Although the predicted membrane topology of β4 is similar to that of β1 and β2/3, the sequence homology in fact is very limited. In addition, β4 exhibits a unique tissue distribution and modulates Slowpoke channel activity very differently from the Slowpoke β subunits that have been described previously. β4 is also distinct from the several Slowpoke-interacting proteins that have been identified recently by yeast two-hybrid screens (Schopperle et al., 1998; Xia et al., 1998; Zhou et al., 1999). Particularly noteworthy is our finding that hβ4 is unique among Slowpoke β subunits in that it is expressed predominantly in the brain and peripheral nervous system. Northern blots show predominant expression in brain, especially cortical regions, and this is readily confirmed by in situhybridization. Although there is a weak signal from several non-neural peripheral tissues on Northern blots, we could not detect expression of β4 in these tissues by in situ hybridization. The most striking result of these localization experiments is that the expression of β4 in brain and peripheral sensory neurons overlaps with that of Slowpoke α subunit, suggesting that β4 is available to interact with and modulate Slowpoke in most neurons. Recent protein purification experiments have identified a Slowpoke-binding protein in rat brain that is immunologically distinct from and smaller than the smooth muscle β1 subunit (Wanner et al., 1999). It will be of interest to determine whether this copurifying protein is related to Slowpoke β4.
The actions of hβ4 on hSlo channel activity are also unique. The two major modulatory effects we have observed, a slowing of activation kinetics and a shift of the voltage dependence of activation to more depolarized voltages, will combine to produce a marked downregulation of channel activity. This may be contrasted with the β1 subunit, which shifts the voltage dependence of the channel to more hyperpolarized voltages and slows deactivation, thereby increasing channel activity. The net effect of hβ2/3 is harder to assess, because it both shifts the voltage dependence to more hyperpolarized voltages and also causes channel inactivation (Wallner et al., 1999;Xia et al., 1999). Nevertheless, it can be predicted with confidence that β1, β2/3, and β4 will have very different effects on the excitability of the cells in which they are expressed. For example, neurons that express hβ4 in their axons and nerve terminals are likely to be more excitable, and release of neurotransmitter is likely to be prolonged compared with those that express β1 or no auxiliary subunit at all. It is also conceivable that auxiliary subunit binding is not constitutive, but itself is regulated by signals that impinge on a neuron, thereby allowing a rapid and dramatic shift in neuronal electrical properties (for an example of this, see Zhou et al., 1999). This idea is especially intriguing in the case of β4 because of our finding that it can exist in cells in at least two distinct forms, one of which binds preferentially to Slowpoke α subunit. It will be important to determine whether such dynamically regulated interactions of auxiliary subunits with ion channels contribute to neuronal plasticity in the mammalian brain and whether these interactions are perturbed in disease states or other pathological situations.
Footnotes
This work was supported in part by a grant to I.B.L from the National Institutes of Health. We thank Dr. F. Benes (Harvard Brain Tissue Resource Center) for providing the human brain samples, Larry Salkoff and Steve Dworetzky for the mSlo and hSlo 1 subunit cDNAs, and Tom Grace at Millennium Pharmaceuticals for assistance with some of the figures.
Correspondence should be addressed to Irwin B. Levitan's present address: Department of Neuroscience, University of Pennsylvania School of Medicine, 215 Stemmler Hall, 3450 Hamilton Walk, Philadelphia, PA 19104-6074. E-mail: levitani@mail.med.upenn.edu.
Dr. Weiger's present address: Department of Neuroscience, University of Pennsylvania School of Medicine, 218 Stemmler Hall, Philadelphia, PA 19104.
Dr. Holmqvist's present address: Millennium Pharmaceuticals, 75 Sidney Street, Cambridge, MA 02139.
REFERENCES
- 1.Adelman JP, Shen K-Z, Kavanaugh MP, Warren RA, Wu Y-N, Lagrutta A, Bond CT, North RA. Calcium- activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- 2.Bielefeldt K, Jackson MB. Phosphorylation and dephosphorylation modulate a Ca2+-activated K+ channel in rat peptidergic nerve terminals. J Physiol (Lond) 1994;475:241–254. doi: 10.1113/jphysiol.1994.sp020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- 4.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 5.Dworetzky SI, Trojnacki JT, Gribkoff VK. Cloning and expression of a human large-conductance calcium-activated potassium channel. Mol Brain Res. 1994;27:189–193. doi: 10.1016/0169-328x(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 6.Dworetzky SI, Boissard CG, Lum-Ragan JT, McKay MC, Post-Munson DJ, Trojnacki JT, Chang C-P, Gribkoff VK. Phenotypic alteration of a human BK (hSlo) channel by hSlob subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J Neurosci. 1996;16:4543–4550. doi: 10.1523/JNEUROSCI.16-15-04543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Calvo M, Knaus H-G, McManus OB, Giangiacomo KM, Kaczorowski GJ, Garcia ML. Purification and reconstitution of the high-conductance, calcium-activated potassium channel from tracheal smooth muscle. J Biol Chem. 1994;269:676–682. [PubMed] [Google Scholar]
- 8.Gho M, Ganetzky B. Analysis of repolarization of presynaptic motor terminals in Drosophila larvae using potassium channel-blocking drugs and mutations. J Exp Biol. 1992;170:93–111. doi: 10.1242/jeb.170.1.93. [DOI] [PubMed] [Google Scholar]
- 9.Hanner M, Vianna-Jorge R, Kamassah A, Schmalhofer WA, Knaus H-G, Kaczorowski GJ, Garcia ML. The β subunit of the high conductance calcium-activated potassium channel. J Biol Chem. 1998;273:16289–16296. doi: 10.1074/jbc.273.26.16289. [DOI] [PubMed] [Google Scholar]
- 10.Hicks GA, Marrion NV. Ca2+-dependent inactivation of large conductance Ca2+-activated K+ (BK) channels in rat hippocampal neurones produced by pore block from an associated particle. J Physiol (Lond) 1998;508:721–734. doi: 10.1111/j.1469-7793.1998.721bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isom LL, De Jongh KS, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12:1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 12.Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol (Lond) 1997;505:267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaczorowski GJ, Knaus HG, Leonard RJ, McManus OB, Garcia ML. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J Bioenerg Biomembr. 1996;28:255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- 14.Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of beta-subunit of high conductance Ca(2+)-activated K+ channel from smooth muscle. J Biol Chem. 1994;269:17274–17278. [PubMed] [Google Scholar]
- 15.Knaus H-G, Schwarzer C, Koch ROA, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G. Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci. 1996;16:955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McManus OB, Helms LMH, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 17.Oberst C, Weiskirchen R, Hartl M, Bister K. Suppression in transformed avian fibroblasts of a gene (CO6) encoding a membrane protein related to mammalian potassium channel regulatory subunits. Oncogene. 1997;14:1109–1116. doi: 10.1038/sj.onc.1200930. [DOI] [PubMed] [Google Scholar]
- 18.Pallanck L, Ganetzky B. Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channel gene, slowpoke. Hum Mol Genet. 1994;3:1239–1243. doi: 10.1093/hmg/3.8.1239. [DOI] [PubMed] [Google Scholar]
- 19.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 20.Rettig J, Heinemann SH, Wunder G, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes KJ, Keilbaugh SA, Barrezueta NX, Lopez KL, Trimmer JS. Association and colocalization of K+ channel α- and β-subunit polypeptides in rat brain. J Neurosci. 1995;15:5360–5371. doi: 10.1523/JNEUROSCI.15-07-05360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 24.Schopperle WM, Holmqvist MH, Zhou Y, Wang J, Wang Z, Griffith LC, Keselman I, Kusinitz F, Dagan D, Levitan IB. Slob, a novel protein that interacts with the slowpoke calcium-dependent potassium channel. Neuron. 1998;20:565–573. doi: 10.1016/s0896-6273(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz TL, Tempel BL, Papazian DM, Jan YN, Jan LY. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988;331:137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- 26.Solaro CR, Lingle CJ. Trypsin-sensitive, rapid inactivation of a calcium-activated potassium channel. Science. 1992;257:1694–1698. doi: 10.1126/science.1529355. [DOI] [PubMed] [Google Scholar]
- 27.Solaro CR, Prakriya M, Ding JP, Lingle CJ. Inactivating and noninactivating Ca2+- and voltage-dependent K+ current in rat adrenal chromaffin cells. J Neurosci. 1995;15:6110–6123. doi: 10.1523/JNEUROSCI.15-09-06110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia L, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- 29.Trimmer JS. Regulation of ion channel expression by cytoplasmic subunits. Curr Opin Neurobiol. 1998;8:370–374. doi: 10.1016/s0959-4388(98)80063-9. [DOI] [PubMed] [Google Scholar]
- 30.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 31.Tseng-Crank J, Godinot N, Johansen TE, Ahring PK, Strobæk D, Mertz R, Foster CD, Olesen S-P, Reinhart PH. Cloning, expression, and distribution of a Ca2+-activated K+ channel β-subunit from human brain. Proc Natl Acad Sci USA. 1996;93:9200–9205. doi: 10.1073/pnas.93.17.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 33.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 34.Wallner M, Meera P, Toro L. Determinant for β-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc Natl Acad Sci USA. 1996;93:14922–14927. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc Natl Acad Sci USA. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wanner SG, Koch RO, Koschak A, Trieb M, Garcia ML, Kaczorowski GJ, Knaus HG. High-conductance calcium-activated potassium channels in rat brain: pharmacology, distribution, and subunit composition. Biochemistry. 1999;38:5392–5400. doi: 10.1021/bi983040c. [DOI] [PubMed] [Google Scholar]
- 37.Xia X-M, Hirschberg B, Smolik S, Forte M, Adelman JP. dSlo interacting protein 1, a novel protein that interacts with large-conductance calcium-activated potassium channels. J Neurosci. 1998;18:2360–2369. doi: 10.1523/JNEUROSCI.18-07-02360.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia X-M, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci. 1999;19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou XB, Schlossmann J, Hofmann F, Ruth P, Korth M. Regulation of stably expressed and native BK channels from human myometrium by cGMP- and cAMP-dependent protein kinase. Pflügers Arch. 1998;436:725–734. doi: 10.1007/s004240050695. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y, Schopperle WM, Murrey H, Jaramillo H, Dagan D, Griffith LC, Levitan IB. A dynamically regulated 14–3-3, Slob, and Slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron. 1999;22:809–818. doi: 10.1016/s0896-6273(00)80739-4. [DOI] [PubMed] [Google Scholar]