Abstract
In spite of the recognition that striatal D2 receptors are critical determinants in a variety of psychomotor disorders, the cellular mechanisms by which these receptors shape neuronal activity have remained a mystery. The studies presented here reveal that D2 receptor stimulation in enkephalin-expressing medium spiny neurons suppresses transmembrane Ca2+ currents through L-type Ca2+ channels, resulting in diminished excitability. This modulation is mediated by Gβγ activation of phospholipase C, mobilization of intracellular Ca2+ stores, and activation of the calcium-dependent phosphatase calcineurin. In addition to providing a unifying mechanism to explain the apparently divergent effects of D2 receptors in striatal medium spiny neurons, this novel signaling linkage provides a foundation for understanding how this pivotal receptor shapes striatal excitability and gene expression.
Keywords: neostriatum, patch clamp, dopamine, neuromodulation, medium spiny neuron, basal ganglia, electrophysiology, single-cell RT-PCR, ion channel, calcium
Disruptions in striatal dopaminergic signaling are thought to underlie a variety of psychomotor disorders including drug abuse, schizophrenia, Tourette's syndrome, and Parkinson's disease (Hornykiewcz, 1973; Meltzer and Stahl, 1976; Sandor, 1993; Nestler and Aghajanian, 1997). In spite of the recognition that alterations in dopaminergic signaling are the basis of these psychomotor disorders, the cellular mechanisms by which dopamine affects striatal function have remained something of a mystery. This is particularly true of D2 receptors. These receptors are expressed at high levels by several groups of neurons in the striatum, including GABAergic medium spiny neurons that project to the globus pallidus and express enkephalin (Gerfen, 1992; Surmeier et al., 1996).
The prevailing model of the striatum (Gerfen, 1992) suggests that D2 receptor stimulation suppresses the activity of enkephalin-expressing striatal medium spiny neurons. This inference is based primarily on two indirect observations. First, dopamine-depleting lesions increase striatal expression of enkephalin, a peptide released by medium spiny neurons expressing D2 receptors (Gerfen, 1992). Second, neuroleptic blockade of D2 receptors increases striatal expression of immediate early genes and glutamic acid decarboxylase (Chesselet et al., 1998). These changes are taken as evidence of D2 receptor-mediated suppression of neural activity and gene transcription. However, there are a number of observations that are difficult to reconcile with this model. For example, D2 receptor stimulation in striatal slices increases the activity of a Ca2+-dependent protein phosphatase (calcineurin) and of Ca2+-dependent mitogen-activated protein (MAP) kinase (Nishi et al., 1997; Yan et al., 1999). D2 receptor stimulation also is necessary for the induction of synaptic plasticity in the striatum (Calabresi et al., 1992). These studies argue that D2 receptor stimulation increases, rather than decreases, activity and intracellular Ca2+ levels in striatal medium spiny neurons.
Direct measurements of neuronal activity have not provided a means of explaining these seemingly contradictory findings. Because the transcriptional and biochemical events at the heart of the signaling discrepancy are Ca2+ dependent, a key question is whether D2 receptors can directly influence intracellular Ca2+ levels. An obvious way this might happen is via the modulation of transmembrane ion channels capable of carrying Ca2+ ions into the cytoplasm. One potential target of this type of modulation is the L-type Ca2+ channel, a channel that has a privileged association with transcriptional regulators in many neurons (Bading et al., 1993; Graef et al., 1999). Although they can be enhanced by several mechanisms (Viard et al., 1999), in medium spiny neurons L-type Ca2+ currents are increased by D1 receptor stimulation of adenylyl cyclase and protein kinase A (Surmeier et al., 1995). Because the best-described effect of striatal D2 receptors is inhibition of adenylyl cyclase (Sibley, 1995), D2receptor activation should, in principle, reduce L-type currents.
The studies described here were intended to test this hypothesis. They show that indeed D2 receptor stimulation suppresses L-type Ca2+ currents in enkephalin-expressing medium spiny neurons. But, the suppression is not mediated by inhibition of adenylyl cyclase. Rather, D2 receptor stimulation mobilizes intracellular Ca2+ stores via Gβγ activation of a phospholipase Cβ1 pathway, leading to a calcineurin-dependent reduction in L-type currents. This novel signaling linkage establishes a mechanism by which D2 receptors can suppress spike activity and Ca2+-dependent gene transcription but activate Ca2+-dependent intracellular enzymes.
MATERIALS AND METHODS
Electrophysiology. Whole-cell recordings from acutely isolated rat striatal neurons were obtained using previously published techniques (Surmeier et al., 1995; Mermelstein et al., 1999). The pipette solution consisted of (in mm): 180N-methyl-d-glucamine (NMG), 40 HEPES, 4 MgCl2, 0–20 BAPTA, 12 phosphocreatine, 2 Na2ATP, 0.2 Na2GTP, and 0.1 leupeptin, pH 7.2–7.3 with H2SO4, 265–270 mOsm/l. The external solution consisted of (in mm): 135 NaCl, 20 CsCl, 1 MgCl2, 10 HEPES, 0.001 TTX, 5 BaCl2, and 10 glucose, pH 7.4 with NaOH, 300–305 mOsm/l. All reagents were obtained from Sigma (St. Louis, MO) except ATP and GTP (Boehringer Mannheim, Indianapolis, IN) and BAPTA, calcineurin autoinhibitory peptide, and leupeptin (Calbiochem, La Jolla, CA). All drugs were prepared according to the manufacturer's specifications and applied with a “sewer pipe” capillary array (Surmeier et al., 1995; Mermelstein et al., 1999). C terminus of β adrenergic receptor kinase 1 (βARK-C) peptide (βARK-Cp) is comprised of residues 548–671 of the rat homolog of βARK. βARK-Cp (4.9 mg/ml) was dialyzed against the recording internal solution. This solution was diluted in the recording internal solution for a final concentration of 1 mg/ml.
Intracellular recordings were performed on rat dorsal neostriatal slices maintained in vitro as reported previously (Hernandez-Lopez et al., 1997). Recording was done in a submerged-type chamber superfused with saline of the same composition (34–36°C). Sharp microelectrodes filled with 3 m K-acetate and 1% biocytin were used. Rectangular current pulses of varying strengths and durations were used to evoke spike activity. Records were obtained with an active bridge electrometer (Neuro Data, Cygnus Technology, Inc., Delaware Water Gap, PA), digitized, and saved on video tapes (40 kHz) for off-line analysis with a personal computer. Neurons were injected with biocytin as described previously. All neurons were medium spiny projection neurons. Experiments were paired, so that records in the presence and absence of bath-applied drugs were compared in the same neuron.
Fluorometry. For combined patch clamp and fluorometry, neurons were loaded with fura-2 pentapotassium salt (100 μm; Molecular Probes, Eugene, OR) through the patch pipette in a chelator-free recording internal solution (see above). Concomitant fluorometry and patch-clamp recording used Ba2+ as the charge carrier to eliminate transmembrane flux as a contributor to the fluorometric signal. For fluorometry without patch recording, neurons were incubated in buffer containing fura-2 AM (5 μm; Molecular Probes) for 25 min at 37°C in the dark. After loading, neurons were rinsed with saline and equilibrated for 20 min at room temperature. Changes in cytoplasmic Ca2+ concentration were determined by measuring the fluorescence ratio (510 nm) after excitation with 340 and 380 nm wavelength light. Emission ratios were corrected for background fluorescence. Measurements were obtained with a Nikon Diaphot equipped with a DeltaScan fluorometry system (Photon Technology International) running proprietary software.
Single-cell reverse transcription-PCR protocol.Protocols similar to those described previously were used (Baranauskas et al., 1999; Mermelstein et al., 1999). The PCR primers were developed from GenBank sequences using OLIGO software (National Biosciences). The primers used for enkephalin and substance P cDNA amplification have been published previously (Surmeier et al., 1996). The primers for phospholipase C β1 (PLCβ1) cDNA (GenBank accession number M20636) were 5′-AAA GGG AAG GTT AGT GAG GAC AG-3′ and 5′-TTC AGG CTA AGG GAT GTT TCT C-3′. The predicted product length was 253 bp. The primers for PLCβ2 cDNA (GenBank accession number AJ011035) were 5′-ATC CAA GCC ATG ACC AAA GTC-3′ and 5′-GTC TCC CAT TTC TGC CTT ATG TG-3′. The predicted product length was 547 bp. The primers for PLCβ3 cDNA (GenBank accession number M99567) were 5′-AGC GCA ACA ACA GCA TCT CAG A-3′ and 5′-CTC TTG CTC CGC CAG TTC AAA G-3′. The predicted product length was 420 bp. The primers for PLCβ4 cDNA (GenBank accession number L15556) were 5′-GGC AAT GAA GCA GTC GAA AGA-3′ and 5′-GGC GTG ATC CTC TGG TGT TCT AT. The predicted product lengths were 209 and 246 bp.
Statistical procedures. Data analysis was performed with SYSTAT (version 5.2; SPSS, Inc., Chicago, IL). Sample statistics are given as means ± SEs. Box plots were used for graphic presentation of the data because of the small sample sizes.
RESULTS
D2 receptor activation reduces Ca2+ currents
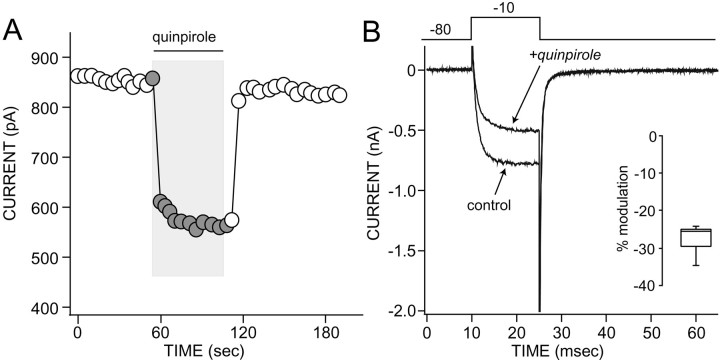
Whole-cell Ba2+ currents through Ca2+ channels were reversibly inhibited by the D2-class receptor agonists (−)-quinpirole (Fig. 1) andR(−)-propylnorapomorphine (NPA) in ∼65% of the acutely isolated medium-sized striatal neurons tested. At saturating agonist concentrations (10 μm), the mean reduction in peak current evoked by a voltage step to −10 mV was 28 ± 2% (n = 5) for quinpirole and 26 ± 3% for NPA (n = 4). Lower agonist concentrations produced smaller, qualitatively similar modulations (0.50–5 μm;n = 6). Previous studies have shown that D2 receptors, like other Gi/o-coupled receptors, inhibit N- and P/Q-type Ca2+ channels but typically do not modulate L-type Ca2+ channels (Yan et al., 1997). However, in medium spiny neurons, the L-type channel antagonist nifedipine significantly reduced the modulation produced by quinpirole, suggesting that L-type channels were a major target of the D2 receptor pathway (Fig.2A,B). The mean modulation in the absence of nifedipine was 29% (n = 8), whereas it was only 10% (n = 6) in the presence of nifedipine (p < 0.05, Kruskal–Wallis).
Fig. 1.
D2-class receptor agonists decrease whole-cell Ba2+ current through Ca2+ channels in acutely isolated striatal neurons.A, Plot of peak Ba2+ currents evoked by a step to −10 mV from a holding potential of −80 mV. Quinpirole (10 μm) reversibly decreased peak currents.B, Representative currents used to constructA. Voltage protocol is shown at the top.Inset, A box plot summary of the percent reduction in peak current produced by quinpirole (n = 5). The central line of the box is the median of the distribution. The edges of the boxare the interquartiles. The lines running from theedge of the box show the extremes of the distribution, excluding outliers.
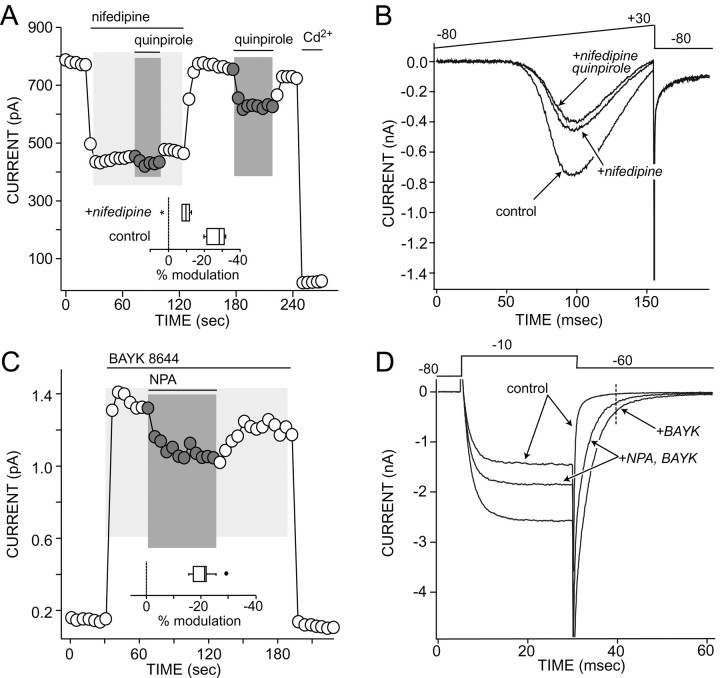
Fig. 2.
D2-class receptor agonists decrease currents through L-type Ca2+ channels.A, Plot of peak Ba2+ current evoked by a voltage ramp (see B). Nifedipine (5 μm) reduced evoked currents and occluded the effects of quinpirole (10 μm); washing nifedipine off restored the quinpirole modulation. Inset, A box plot summary of the modulation in the presence and absence (control) of nifedipine (n = 6). The asterisk is an outlier, defined as a point that is either greater than three halves the interquartile range above the upper interquartile or less than three halves the interquartile range below the lower interquartile (Tukey, 1977). B, Representative currents used to construct A. Voltage protocol is shown at thetop. C, Plot of tail current amplitude evoked by the protocol shown in D and measured at thedashed vertical line in D. BAYK 8644 increased tail amplitudes; NPA reversibly reduced the amplitude.Inset, A box plot summary of the percent reduction in tail current amplitude produced by NPA (n = 13). The filled circle is an outlier.D, Representative current traces used to construct C.
Another way of testing the involvement of L-type Ca2+ channels is via use of the dihydropyridine agonists such as (−)-1,4-dihydro-2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl)-phenyl]-3-pyridine carboxylic acid methyl ester (BAYK) 8644 and 2,5-dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methyl ester (FPL) 64176 (Rampe et al., 1993). These agonists slow the deactivation of L-type Ca2+channels during repolarization of the membrane; this selective slowing provides a convenient way of isolating currents through L-type channels. NPA reversibly reduced the slowly deactivating tail current attributable to L-type Ca2+ channels (see Fig. 2C,D). As shown in Figure 2C,inset box plot, the median reduction in the amplitude of the slow tail current by NPA (10 μm) was just >20% in responsive neurons (n = 13).
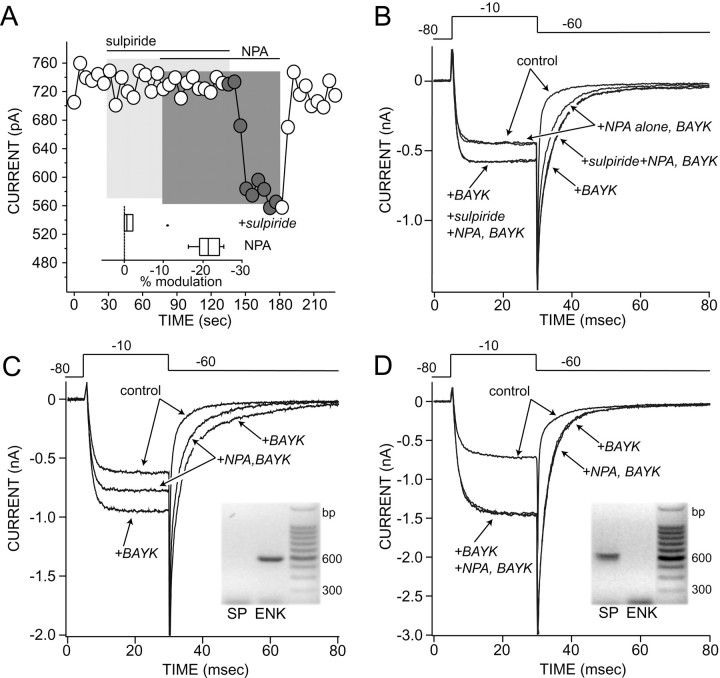
To verify the involvement of D2-class receptors in the response, the ability of (−)-sulpiride to antagonize the response was examined. Sulpiride (5 μm) (Weiss et al., 1985) had no effect of its own on the BAYK-enhanced L-type currents but blocked the effect of NPA (10 μm) on both step and tail currents; the effect of NPA reemerged when sulpiride was washed off the cell (Fig. 3A,B). In six neurons, the median NPA-induced modulation of the slow tail current was 22% in the absence of sulpiride and 2% in its presence (see Fig.3A, inset; p < 0.05, Kruskal–Wallis). The modulation of the current evoked during the depolarizing step also was antagonized by sulpiride (n= 6; median modulation = 4%; p < 0.05, Kruskal–Wallis).
Fig. 3.
The modulation is dependent on D2receptors. A, A plot of Ba2+ current tail amplitudes as a function of time (see Fig. 2D). Sulpiride (5 μm) blocked the effects of NPA (10 μm); washing sulpiride off restored the NPA modulation. Inset, A box plot summary of the modulation in the presence and absence (NPA) of sulpiride (n = 6) is shown. The filled circle is an outlier.B, Representative currents used to constructA are presented. Voltage protocol is shown at thetop. C, NPA modulated Ba2+ currents only in neurons shown by scRT-PCR to express enkephalin (n = 6). Inset, The gel shows the presence of enkephalin (ENK) and absence of substance P (SP) amplicons in this cell.D, Neurons expressing substance P, but not enkephalin, did not respond to NPA (n = 3).Inset, The gel shows the SP amplicon derived from this neuron.
There are three D2-class receptors (D2, D3, or D4) with a high affinity for NPA, quinpirole, and sulpiride. Although the D2 receptor is the predominant striatal isoform, previous studies have identified a substantial subset of medium spiny neurons that express D3 receptors (Surmeier et al., 1996). To determine which of these D2-class receptors was responsible for the modulation, whole-cell recordings were followed by a single-cell reverse transcription (scRT)-PCR analysis. Because the dopamine receptor mRNAs appear to be of relatively low abundance and difficult to detect after whole-cell recording, the scRT-PCR experiments focused on two high-abundance peptide mRNAs that are strongly correlated with receptor expression. D2receptor expression is limited to medium spiny neurons expressing the releasable peptide enkephalin (Gerfen, 1992; Surmeier et al., 1996). On the other hand, D3 receptor expression is limited to a subpopulation of medium spiny neurons expressing substance P in the dorsal striatum (Surmeier et al., 1996). In neurons expressing enkephalin, the modulation of L-type Ca2+channels was robust (Fig. 3C; n = 6; median modulation = 19%), whereas neurons that only expressed substance P failed to exhibit a significant response (Fig.3D; n = 3; median modulation = 0%), clearly implicating D2 receptors in the modulation.
The D2 receptor modulation is not dependent on inhibition of adenylyl cyclase
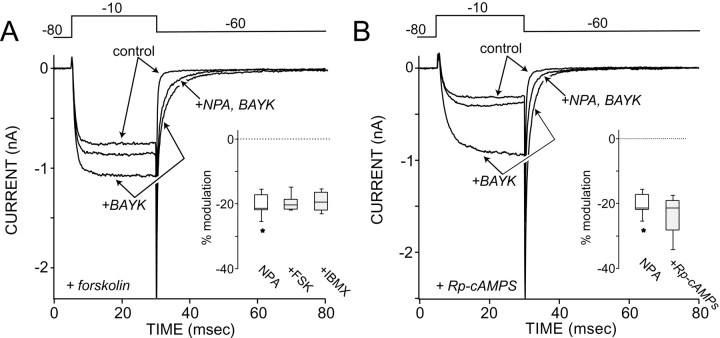
The activation of D2 receptors inhibits adenylyl cyclase activity, reducing cytosolic cAMP levels and protein kinase A (PKA) activity (Sibley, 1995). PKA can enhance L-type Ca2+ channel currents in medium spiny neurons (Surmeier et al., 1995). To test directly whether D2 receptors reduced L-type currents by inhibiting adenylyl cyclase, three experiments were performed. First, adenylyl cyclase was stimulated by incubating neurons in forskolin (1 μm) before NPA exposure. If inhibition of adenylyl cyclase were a key element in the signaling mechanism, forskolin stimulation should increase the absolute magnitude of the NPA modulation (Battaglia et al., 1985). It did not. Although forskolin significantly enhanced tail currents in the absence of BAYK 8644 (control median = 45 pA; n = 13; forskolin median = 74 pA; n = 6; p < 0.05, Kruskal–Wallis), the absolute modulation of BAYK 8644-enhanced tail currents was indistinguishable from that seen in control neurons (Fig.4A; n = 5; median reduction = 20%; p > 0.05, Kruskal–Wallis). The D2 receptor modulation of currents evoked by the test step were unaltered as well (n = 5; median reduction = 20%; p> 0.05, Kruskal–Wallis). A cyclase-dependent mechanism also predicts that blocking the degradation of cAMP should attenuate the D2 modulation. But, the phosphodiesterase inhibitor IBMX (5 μm) did not affect the D2 modulation of slow tail currents (n = 6; median modulation = 19%;p > 0.05, Kruskal–Wallis) or step currents (n = 6; median modulation = 19%;p > 0.05, Kruskal–Wallis). Lastly, blocking the access of cAMP to PKA should blunt the D2modulation. However, as shown in Figure 4B, dialysis with a competitive inhibitor of cAMP, the Rp isomer of cyclic adenosine monophosphothioate (Rp-cAMPS; 10 μm), did not affect the ability of D2 receptors to modulate the slow tail current (n = 4; median modulation = 22%; p > 0.05, Kruskal–Wallis) or step currents (n = 4; median modulation = 20%;p > 0.05, Kruskal–Wallis). These observations, taken together with the fact that D2 receptor activation effectively modulated currents in the absence of receptor-mediated stimulation of adenylyl cyclase, clearly suggest that D2 receptors were working by another mechanism.
Fig. 4.
The D2 receptor modulation is independent of alterations in adenylyl cyclase activity.A, Preincubation of cells in forskolin (1 μm) failed to alter the NPA (5 μm) modulation of BAYK 8644-enhanced tail currents or the modulation of currents evoked by the test step to −10 mV. Voltage protocol is shown at the top. Inset, Box plots of the tail modulation in control (NPA; n = 10), forskolin (FSK; n = 5), and IBMX (n = 6) solutions are shown. Theasterisk is an outlier. These data were not significantly different. B, Cellular dialysis with the cAMP antagonist Rp-cAMPS also failed to alter the NPA modulation of BAYK 8644-enhanced tail currents. Inset, A box plot summary of the tail modulation in control (n = 10) and Rp-cAMPS (n = 4)-dialyzed neurons is shown. These data were not significantly different.
D2 receptors mobilize intracellular Ca2+ via a phospholipase C pathway
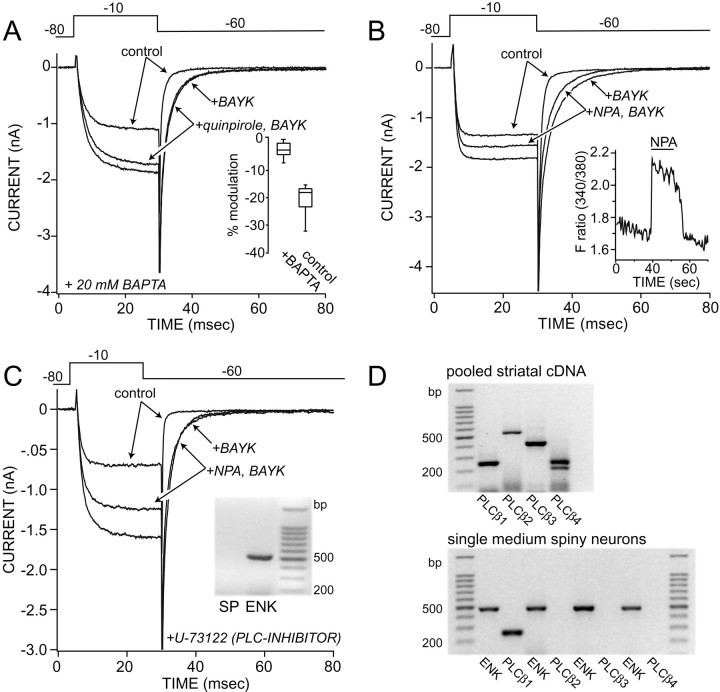
If D2 receptors were not acting via adenylyl cyclase and PKA, then how were they working? A number of studies have shown that L-type Ca2+ currents can be suppressed by elevations of the intracellular Ca2+ concentration (Chad and Eckert, 1986;Armstrong et al., 1991; Lukyanetz et al., 1998). In cells dialyzed with high concentrations of the fast Ca2+chelator BAPTA (20 mm), a concentration sufficient to “clamp” the free Ca2+ concentration at a low nanomolar level, quinpirole had little or no effect on the BAYK-enhanced tail currents (Fig.5A). Although quinpirole failed to modulate the slow tails in these neurons, it continued to reduce the peak current (although to a lesser extent), suggesting that the modulation of non-L-type channels was intact (n = 5; median modulation = 11%). Because BAPTA can have effects unrelated to Ca2+ buffering (Bernheim et al., 1991), cytosolic [Ca2+] was measured directly with fluorometric techniques in voltage-clamped neurons dialyzed with fura-2 (100 μm). These experiments revealed that in neurons in which the slow BAYK tail currents were modulated, NPA also induced a rapid and reversible elevation in cytosolic Ca2+. This elevation occurred in the absence of external Ca2+ and with the cell's membrane held at −80 mV (n = 4) (Fig. 5B), implicating release from intracellular stores. NPA failed to alter intracellular Ca2+ levels in those neurons in which the slow BAYK tail currents were unmodulated (n = 3). To test this linkage further, medium spiny neurons were loaded with fura-2 AM and D2 agonists applied in the presence and absence of extracellular Ca2+. Fluorometric measurements were taken in these neurons without concomitant patch-clamp recording. NPA evoked a calcium transient in 70% of these neurons regardless of whether external Ca2+ was present or not (14/20; data not shown).
Fig. 5.
D2 receptor modulation depends on the release of intracellular Ca2+ via a PLC-dependent mechanism. A, Dialysis with BAPTA (20 mm) blocked the D2 modulation of tail currents but not peak currents (n = 5). Voltage protocol is shown at thetop. Inset, A box plot summarizes the modulations seen with BAPTA internals (n = 5) and matched controls (n = 10). B, NPA (10 μm) reduced BAYK tail currents and increased intracellular Ca2+ levels in the same cells.Inset, The ratio of 510 nm fura-2 emission after excitation at 340 and 380 nm in the same neuron is shown. Measurements were taken while the cell was clamped at −80 mV and in the absence of external Ca2+. C, NPA failed to modulate slow tail currents in enkephalin-expressing neurons dialyzed with the PLC inhibitor U-73122 (n = 9).Inset, The recorded neuron expressed enkephalin but not substance P. D, Top, The gel shows RT-PCR amplicons for PLCβ1–4 in pooled striatal mRNA. Bottom, The gel shows representative amplicons from four ENK-positive medium spiny neurons. Only PLCβ1 mRNA was detected inENK neurons (n = 20).
The best-described mechanism for receptor-mediated mobilization of intracellular Ca2+ stores is via activation of PLC isoforms (Sternweis and Smrcka, 1992). PLC catalyzes the hydrolysis of phosphatidylinositol 4,5-biphosphate, yielding 1,2-diacylglycerol and inositol 1,4,5-triphosphate (IP3). Cytosolic IP3 binds to its cognate receptor, releasing Ca2+from intracellular pools. To determine whether D2receptors relied on a similar mechanism, neurons were dialyzed with the PLC inhibitor U-73122 (10 μm) before D2 receptor stimulation. U-73122 blocked the ability of NPA to reduce the slow, BAYK-enhanced tail currents in enkephalin-expressing neurons (Fig. 5C; n = 9; median modulation = 0%; p < 0.05, Kruskal–Wallis). In contrast, non-L-type currents evoked by the depolarizing voltage step continued to be reduced by NPA (Fig.5C; n = 9; median modulation = 19%; p < 0.05, Kruskal–Wallis). Dialysis with the inactive analog U-73343 (10 μm) failed to alter the D2 modulation of the tail currents (n = 4; median modulation = 21%;p > 0.05, Kruskal–Wallis). PLCβ isoforms are generally thought to mediate receptor-driven responses like the ones observed here (Sternweis and Smrcka, 1992). The involvement of other PLC isoforms, like PLCγ, or tyrosine kinase itself (Diverse-Pierluissi et al., 1997) seems unlikely because of the inability of the tyrosine kinase inhibitor genistein (50 μm) to reduce the D2receptor modulation (n = 3; p > 0.05, Kruskal–Wallis) (Lajiness et al., 1993; Rhee and Bae, 1997). There are four isoforms of PLCβ (1–4) that have been cloned (Exton, 1997). All four isoforms were expressed in pooled striatal tissue (Fig.5D, top); however, when the RT-PCR analysis was limited to neurons expressing enkephalin mRNA, only the PLCβ1 isoform was detected (Fig. 5D, bottom).
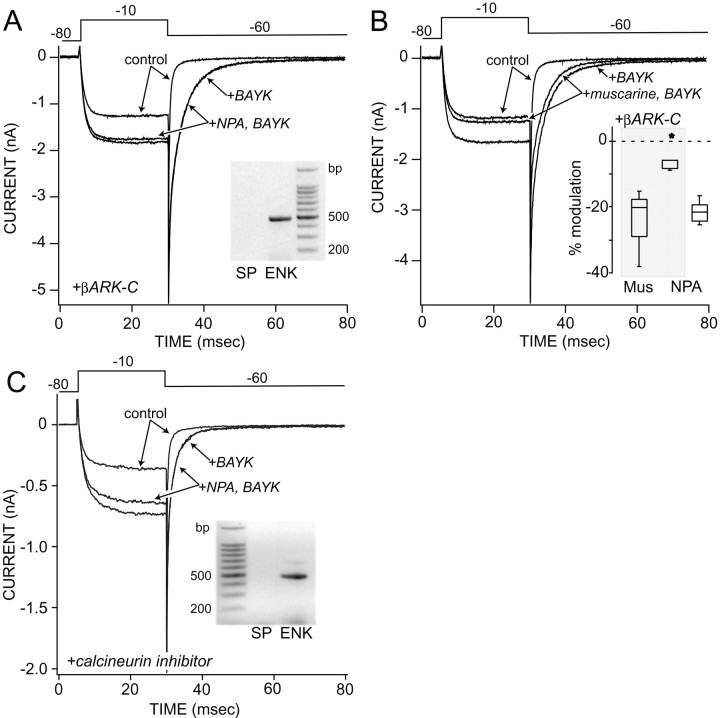
These findings are consistent with the hypothesis that D2 receptors activate PLCβ1. PLCβ1, like the other PLCβ isoforms, is capable of being activated by Gβγ subunits (Exton, 1997; Morris and Scarlata, 1997). To test whether D2 receptors activated PLCβ1 in this way, neurons were dialyzed with an inhibitor of Gβγ signaling (βARK-C peptide; 1 mg/ml) (Koch et al., 1994). βARK-Cp effectively inhibited the NPA modulation of the BAYK-enhanced, L-type tail currents, as well as the peak current, in enkephalin-expressing neurons (Fig.6A). The median modulation of the slow tail currents in the presence of βARK-Cp was 6% (Fig. 6B, inset; n = 5; p < 0.05, Kruskal–Wallis). The NPA modulation of the currents evoked by the depolarizing voltage step was also 6% (n = 5; p < 0.05, Kruskal–Wallis). In the same βARK-Cp-dialyzed neurons, Gqα-linked M1 muscarinic receptors continued to reduce both peak and slow tail currents (Fig. 6B, inset) (Howe and Surmeier, 1995).
Fig. 6.
Inhibitors of Gβγ and calcineurin signaling attenuate the D2 receptor modulation of L-type channels. A, Dialysis with βARK-C peptide (20 μm) blocked the NPA (10 μm) modulation of BAYK (1 μm)-enhanced tail currents as well as peak evoked currents in enkephalin-expressing medium spiny neurons.Inset, The gel from the scRT-PCR profile is shown. The median modulation of the tail currents in enkephalin-expressing neurons (n = 4) was 4% (see B,inset box plot). B, In the same neuron depicted in A, muscarine (Mus; 1 μm) continued to modulate both currents evoked by the step to −10 mV and slow tail currents. Inset, The median muscarinic modulation of the tail currents was 20% in the presence of βARK-C. This was very similar to that seen previously (Howe and Surmeier, 1995) and indistinguishable from the D2modulation (see box plot; the asterisk is an outlier). Previous work has shown that these neurons express high levels of the Gqα-linked M1 receptor.C, Dialysis with the calcineurin autoinhibitory peptide (25 μm) blocked the NPA modulation of the slow tail currents in enkephalin-expressing neurons but not that of the peak currents (n = 4). Inset, The gel shows ENK but not SP amplicons derived from the recorded neuron. Voltage protocol is shown at thetop.
Inhibition of calcineurin blocks the D2 receptor modulation of L-type Ca2+ currents
PLCβ isoforms regulate intracellular Ca2+ levels via the production of IP3. Dialysis with competitive antagonists of IP3 (heparin, 10 mg/ml; n = 6; xestospongin, 1 μm; n = 13) (Simpson et al., 1995; Gafni et al., 1997) antagonized NPA effects on L-type channels in enkephalin-expressing neurons (p < 0.05, Kruskal–Wallis). Lastly, caffeine (10 mm; externally applied), which is known to promote the release from ryanodine-sensitive Ca2+ stores and inhibit IP3-mediated Ca2+release (Simpson et al., 1995), induced an elevation in cytosolic Ca2+ levels and occluded the effects of quinpirole on FPL 64176-enhanced tail currents (n = 10;p < 0.05, Kruskal–Wallis). One potential means by which elevations in cytosolic Ca2+ levels could suppress L-type Ca2+ currents is via the Ca2+-dependent phosphatase calcineurin (Chad and Eckert, 1986). To test this possibility, neurons were dialyzed with a peptide inhibitor of calcineurin (25 μm) (Hashimoto et al., 1990). As shown in Figure 6C, the calcineurin inhibitor significantly reduced the NPA modulation of the BAYK-enhanced tail currents in enkephalin-expressing neurons (n = 4; median modulation = 5%; p < 0.05, Kruskal–Wallis) without blocking the modulation of non-L-type currents (median modulation = 15%; p < 0.05, Kruskal–Wallis). In contrast, inhibition of protein phosphatase 1 and 2A with okadaic acid (1 μm) had no effect on the ability of D2 agonists to suppress the BAYK-enhanced tail current (n = 2; median modulation = 20%;p > 0.05, Kruskal–Wallis).
D2 receptor activation suppresses spike activity evoked from depolarized membrane potentials
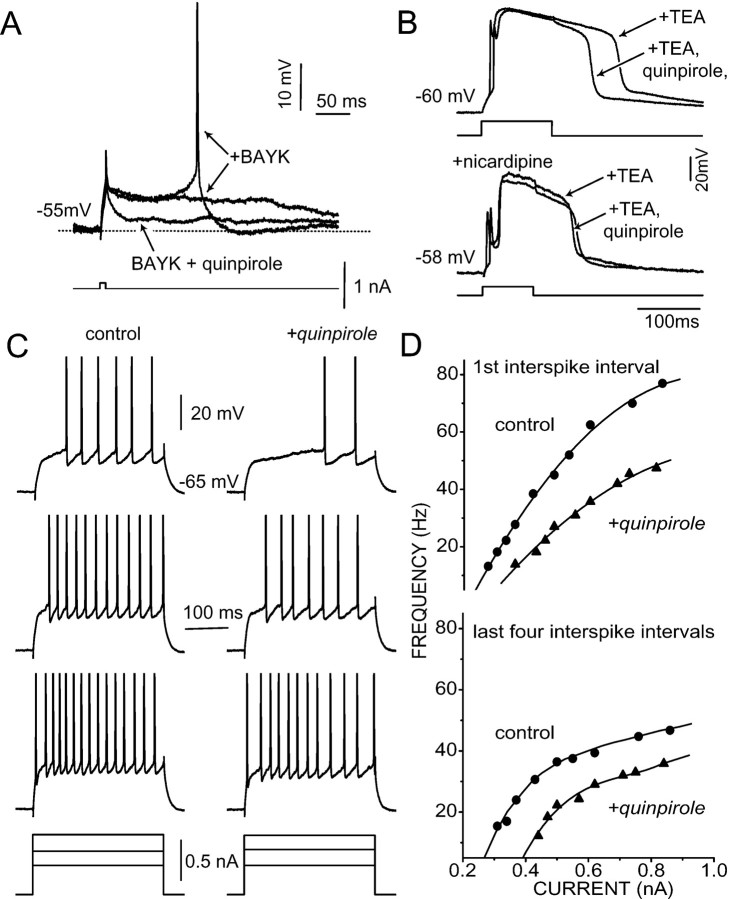
L-type Ca2+ currents are important determinants of evoked spike activity in medium spiny neurons (Hernandez-Lopez et al., 1997). The influence of these currents can be seen by holding medium spiny neurons at a depolarized level, close to that seen in the upstate in vivo. At this potential, a brief current pulse is capable of triggering a prolonged depolarization (hundreds of milliseconds) that occasionally results in spike generation. This type of response was seen in approximately two-thirds of all trials with a given neuron; in the other trials, the membrane potential decayed passively back to the “resting” potential. In the presence of the L-type channel agonist BAYK 8644, this quasibistable response was enhanced in duration and probability (Fig.7A). Spikes become a much more common event in this situation as well. On the other hand, in the presence of the L-type channel antagonist nicardipine (5 μm), the bistable behavior was almost entirely abolished, resulting in passive membrane responses in the vast majority of trials (Hernandez-Lopez et al., 1997). D2receptor stimulation also suppressed the bistable behavior. In the presence of quinpirole (10 μm), the probability of evoking a sustained depolarization dropped significantly (n = 6; p < 0.05, Kruskal–Wallis), even in the presence of BAYK 8644 (Fig. 7A).
Fig. 7.
D2 receptor stimulation suppresses evoked activity in medium spiny neurons recorded in brain slices.A, At depolarized membrane potentials mimicking the upstate in medium spiny neurons, a brief current pulse (see protocolbelow the traces) evokes a sustained depolarization in the presence of BAYK 8644 (2.5 μm). The depolarization was significantly shortened by the addition of quinpirole (10 μm). Similar results were seen in all six cells tested. B, Intracellular recordings from medium spiny neurons in the presence of TEA (20 mm) are shown. Cells were maintained near −60 mV. Top, Quinpirole (10 μm) shortened the duration of the TEA spike (median reduction = 25%; n = 3).Bottom, In the presence of nicardipine (5 μm), the TEA spike was of shorter duration. The addition of quinpirole had little or no effect in the presence of nicardipine (median reduction = 2%; n = 3).C, Activity evoked by intracellular current injection from a depolarized (approximately −65 mV) membrane potential was suppressed by quinpirole. Left, Records evoked by increasing current steps (300 msec in duration) in control conditions are shown. Right, Records taken from the same cell after the addition of quinpirole (10 μm) are shown. Note that in the presence of quinpirole, the discharge frequency decreased for similar current steps. D, Plots of discharge frequency as a function of injected current for the neuron in Care shown. Top, The plot is the frequency (reciprocal of first interspike interval) in the presence and absence of quinpirole (10 μm). Bottom, The average of the last four interspike intervals in the evoked train in the presence and absence of quinpirole is shown. Similar results were obtained in five other responsive neurons.
D2 receptor stimulation also shortened the duration of tetraethylammonium (TEA)-enhanced Ca2+ spikes in medium spiny neurons. When held at −60 mV in the presence of TEA (20 mm), a brief current stimulus evoked an all-or-none Ca2+ spike in medium spiny neurons (Kita et al., 1985; Bargas et al., 1989). In this recording situation, the duration of the Ca2+ spike was 210 ± 35 msec (mean ± SD; n = 40). If nicardipine (5 μm) or nitrendipine (5 μm) were added, the Ca2+ spike was reduced in duration to 150 ± 40 msec (n = 15). As with the L-type channel antagonists, quinpirole (10 μm) reduced the duration of the TEA-induced Ca2+ spike in all three cells tested (mean duration = 160 ± 30 msec) (Fig. 7B, top). In the presence of nicardipine (5 μm), quinpirole failed to exert any further reduction of the Ca2+ spike in three of five neurons (Fig. 7B, bottom).
By providing a sustained depolarizing influence, L-type Ca2+ currents enhance repetitive activity evoked from depolarized membrane potentials in medium spiny neurons (Hernandez-Lopez et al., 1997). D2receptor-mediated suppression of these currents should diminish evoked spiking. Intracellular recordings from medium spiny neurons in tissue slices confirmed this conjecture. Neurons were slightly depolarized (approximately −65 mV) by steady current injection, and then repetitive activity was evoked by current steps. From these “upstate” membrane potentials, quinpirole (10 μm) diminished evoked spiking in 6 of 10 neurons (Fig. 7C). The suppression of repetitive activity was particularly evident with small current injections that come close to mimicking in vivoconditions (Fig. 7D, top). But, the reduction in firing frequency induced by quinpirole was evident via the whole intensity– frequency plot (Fig. 7D). In quinpirole-responsive neurons, the half-maximum frequency was reduced from 45 ± 10 to 33 ± 12 Hz by quinpirole (n= 6; p < 0.05, Kruskal–Wallis). The ability of quinpirole to alter evoked activity was suppressed by blockade of L-type channels with nicardipine (5 μm;n = 3). Taken together, these results clearly argue that D2 receptor modulation of L-type Ca2+ channels results in a suppression of repetitive spiking evoked from depolarized potentials in medium spiny neurons.
DISCUSSION
D2 receptors in striatal medium spiny neurons activate a PLC–IP3–calcineurin cascade
The results presented show that activation of D2 receptors reduces currents through L-type Ca2+ channels, leading to a suppression of evoked spike activity in enkephalin-expressing striatal medium spiny neurons. Even though nearly all striatal effects of D2 receptor activation are ascribed to the inhibition of adenylyl cyclase activity (Sibley, 1995), this signaling linkage was not responsible for the modulation of L-type Ca2+ channels. Manipulation of adenylyl cyclase activity, cAMP metabolism, and dialysis with a competitive inhibitor of cAMP had no effect on the modulation. Rather, the D2 receptor modulation depended on Gβγ protein activation of a PLCβ1-signaling cascade, mobilization of intracellular Ca2+, and activation of calcineurin. This conclusion is based on five observations. First, the D2 receptor suppression of L-type currents was blocked by inhibition of Gβγ signaling. Second, medium spiny neurons expressed readily detectable levels of PLCβ1 mRNA (but not that of other PLCβ isoforms), and the modulation was blocked by inhibitors of PLCβ1. Third, D2receptor stimulation induced the release of Ca2+ from intracellular stores. Fourth, disruption of IP3 signaling or chelation of intracellular Ca2+ blocked the modulation. Lastly, inhibition of the Ca2+-dependent phosphatase calcineurin blocked the modulation. Dephosphorylation by calcineurin has been shown to mediate reductions in L-type Ca2+ currents in a variety of cell types (Chad and Eckert, 1986; Armstrong et al., 1991; Lukyanetz et al., 1998). In an intact preparation, the D2receptor-triggered activation of calcineurin may act cooperatively with a direct Ca2+–calmodulin-mediated inactivation process (Imredy and Yue, 1994; Peterson et al., 1999) to suppress currents through L-type Ca2+channels further.
Although previous biochemical studies of striatal slices have not reported D2 receptor stimulation of PLC (Gupta and Mishra, 1990; Rubinstein and Hitzemann, 1990), striatal cellular heterogeneity complicates the interpretation of these studies. Indiscriminate activation of striatal D2receptors can be expected to have two opposing effects. One is activation of PLC in enkephalinergic medium spiny neurons. The other is diminished acetylcholine release (Drukarch et al., 1990) and a reduction in M1 muscarinic receptor stimulation of PLC in medium spiny neurons (Akins et al., 1990; Bernard et al., 1992). Hence, there may be no net change in striatal PLC activity after global D2 receptor activation. Although they have not provided a clear picture of the signaling mechanism, studies using heterologous expression systems have shown that D2 receptors are capable of stimulating PLC and mobilizing intracellular Ca2+ pools (Vallar et al., 1990; MacKenzie et al., 1994; Yang et al., 1995).
The ability of striatal D2 receptors to mobilize intracellular Ca2+ stores, reduce L-type Ca2+ channel currents, and suppress evoked activity effectively reconciles an apparently divergent set of observations. On one hand, D2 receptor activation is known to increase striatal calcineurin and MAP kinase activity via Ca2+-dependent mechanisms (Nishi et al., 1997; Yan et al., 1999). On the other hand, blockade of D2 receptors or diminished D2 receptor tone is known to increase striatal immediate early gene (IEG) and peptide expression (Chesselet et al., 1998). D2 receptor activation also is necessary for certain forms of striatal use-dependent synaptic plasticity (Calabresi et al., 1992). Our results directly demonstrate calcineurin activation by D2 receptors and provide a mechanism for Ca2+-dependent MAP kinase activation. Calcineurin-mediated suppression of L-type Ca2+ currents will reduce glutamate-induced CRE-binding protein phosphorylation and IEG induction (Rajadhyaksha et al., 1999). By the same token, this D2 receptor-signaling pathway provides a ready alternative to disinhibition of adenylyl cyclase (Ward and Dorsa, 1999) in explaining the ability of D2 receptor antagonists to increase sharply striatal IEG induction after cortical stimulation (Berretta et al., 1999).
D2 receptor activation selectively suppresses activity in enkephalin-expressing medium spiny neurons
In addition to reconciling these more recent observations, our results provide the first direct evidence for one of the oldest conjectures about dopaminergic regulation of striatal activity, namely, that D2 receptor activation selectively suppresses the activity of enkephalin-expressing medium spiny neurons (Albin et al., 1989). This conjecture has served as a cornerstone of basal ganglia models and treatment strategies for Parkinson's disease for over a decade. Yet, the evidence for this conjecture has been indirect or inconclusive (Nicola et al., 2000).
Our results show that D2 receptor stimulation inhibits activity evoked from relatively depolarized membrane potentials mimicking the upstate produced by excitatory cortical or thalamic inputs (Wilson and Kawaguchi, 1996). In vivo, medium spiny neurons move between this depolarized upstate in which they generate spikes and a hyperpolarized “downstate” in which they are quiescent. Although other voltage-dependent channel types are modulated in concert (Surmeier et al., 1992; Surmeier and Kitai, 1993;Waszczak et al., 1998), the D2 receptor suppression of L-type Ca2+ currents is critical to this inhibition of activity. Why? Unlike N- and P/Q-type voltage-dependent Ca2+ channels in medium spiny neurons, L-type channels are active in the subthreshold potential range of the upstate (Bargas et al., 1994; Song and Surmeier, 1996). This property allows them to exert an important influence on the membrane potential near spike threshold, pushing the membrane potential closer or pulling it farther away from spike generation. Medium spiny neurons expressing D1 receptors use this property of L-type Ca2+ currents to enhance evoked activity in the presence of dopamine (Surmeier et al., 1995;Hernandez-Lopez et al., 1997). In contrast, activation of D2 receptors in enkephalin-expressing neurons should reduce both the magnitude and duration of the response to cortical or thalamic excitatory synaptic input, as predicted over a decade ago by Albin et al. (1989). Moreover, by targeting a Ca2+ channel with privileged access to transcriptional regulation (Bading et al., 1993; Graef et al., 1999;Mermelstein et al., 2000), D2 receptors exert a proximal control over gene expression tied to extrinsically driven activity. This proximal coupling may prove to be very important to long-term striatal adaptations triggered by alterations in dopaminergic signaling in Parkinson's disease, prolonged neuroleptic treatment, and drug abuse (Hornykiewcz, 1973; Meltzer and Stahl, 1976; Nestler and Aghajanian, 1997).
Footnotes
This work was supported by National Institutes of Health Grants NS 34696, DA 12958, and TW 01214 to D.J.S. and EY 10291 to H.H. Additional support was provided by Consejo Nacional de Ciencia y Tecnologia Grant 25812N to J.B. and E.G. We thank Sasha Ulrich for assistance with the RT-PCR experiments.
Correspondence should be addressed to Dr. D. James Surmeier, Department of Physiology, Northwestern University Institute for Neuroscience, Northwestern University Medical School, 320 East Superior Street, Chicago, IL 60611. E-mail:j-surmeier@northwestern.edu.
REFERENCES
- 1.Akins PT, Surmeier DJ, Kitai ST. The M1 muscarinic acetylcholine receptor in cultured rat neostriatum regulates phosphoinositide hydrolysis. J Neurochem. 1990;54:266–273. doi: 10.1111/j.1471-4159.1990.tb13310.x. [DOI] [PubMed] [Google Scholar]
- 2.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DL, Rossier MF, Shcherbatko AD, White RE. Enzymatic gating of voltage-activated calcium channels. Ann NY Acad Sci. 1991;635:26–34. doi: 10.1111/j.1749-6632.1991.tb36478.x. [DOI] [PubMed] [Google Scholar]
- 4.Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 5.Baranauskas G, Tkatch T, Surmeier DJ. Delayed rectifier currents in rat globus pallidus neurons are attributable to Kv2.1 and Kv3.1/3.2 K(+) channels. J Neurosci. 1999;19:6394–6404. doi: 10.1523/JNEUROSCI.19-15-06394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bargas J, Galarraga E, Aceves J. An early outward conductance modulates the firing latency and frequency of neostriatal neurons of the rat brain. Exp Brain Res. 1989;75:146–156. doi: 10.1007/BF00248538. [DOI] [PubMed] [Google Scholar]
- 7.Bargas J, Howe A, Eberwine J, Cao Y, Surmeier DJ. Cellular and molecular characterization of Ca2+ currents in acutely isolated, adult rat neostriatal neurons. J Neurosci. 1994;14:6667–6686. doi: 10.1523/JNEUROSCI.14-11-06667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battaglia G, Norman AB, Hess EJ, Creese I. D2 dopamine receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in rat striatum. Neurosci Lett. 1985;59:177–182. doi: 10.1016/0304-3940(85)90196-x. [DOI] [PubMed] [Google Scholar]
- 9.Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernheim L, Beech DJ, Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991;6:859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- 11.Berretta S, Sachs Z, Graybiel AM. Cortically driven Fos induction in the striatum is amplified by local dopamine D2-class receptor blockade. Eur J Neurosci. 1999;11:4309–4319. doi: 10.1046/j.1460-9568.1999.00866.x. [DOI] [PubMed] [Google Scholar]
- 12.Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chad JE, Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol (Lond) 1986;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesselet MF, Delfs JM, Mackenzie L. Dopamine control of gene expression in basal ganglia nuclei: striatal and nonstriatal mechanisms. Adv Pharmacol. 1998;42:674–677. doi: 10.1016/s1054-3589(08)60838-8. [DOI] [PubMed] [Google Scholar]
- 15.Diverse-Pierluissi M, Remmers AE, Neubig RR, Dunlap K. Novel form of crosstalk between G protein and tyrosine kinase pathways. Proc Natl Acad Sci USA. 1997;94:5417–5421. doi: 10.1073/pnas.94.10.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drukarch B, Schepens E, Stoof JC. Muscarinic receptor activation attenuates D2 dopamine receptor mediated inhibition of acetylcholine release in rat striatum: indications for a common signal transduction pathway. Neuroscience. 1990;37:1–9. doi: 10.1016/0306-4522(90)90186-8. [DOI] [PubMed] [Google Scholar]
- 17.Exton JH. Cell signalling through guanine-nucleotide-binding regulatory proteins (G proteins) and phospholipases. Eur J Biochem. 1997;243:10–20. doi: 10.1111/j.1432-1033.1997.t01-1-00010.x. [DOI] [PubMed] [Google Scholar]
- 18.Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 19.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 20.Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- 21.Gupta SK, Mishra RK. The effect of dopamine D1 and D2 receptor agonists on inositol phosphate turnover in rat striatal slices. Biochem Int. 1990;22:887–894. [PubMed] [Google Scholar]
- 22.Hashimoto Y, Perrino BA, Soderling TR. Identification of an autoinhibitory domain in calcineurin. J Biol Chem. 1990;265:1924–1927. [PubMed] [Google Scholar]
- 23.Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornykiewcz O. Dopamine in the basal ganglia. Its role and therapeutic implications. Br Med Bull. 1973;29:172–178. doi: 10.1093/oxfordjournals.bmb.a070990. [DOI] [PubMed] [Google Scholar]
- 25.Howe AR, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J Neurosci. 1995;15:458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imredy JP, Yue DT. Mechanism of Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994;12:1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 27.Kita H, Kita T, Kitai ST. Regenerative potentials in rat neostriatal neurons in an in vitro slice preparation. Exp Brain Res. 1985;60:63–70. doi: 10.1007/BF00237019. [DOI] [PubMed] [Google Scholar]
- 28.Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 29.Lajiness ME, Chio CL, Huff RM. D2 dopamine receptor stimulation of mitogenesis in transfected Chinese hamster ovary cells: relationship to dopamine stimulation of tyrosine phosphorylations. J Pharmacol Exp Ther. 1993;267:1573–1581. [PubMed] [Google Scholar]
- 30.Lukyanetz EA, Piper TP, Sihra TS. Calcineurin involvement in the regulation of high-threshold Ca2+ channels in NG108-15 (rodent neuroblastoma x glioma hybrid) cells. J Physiol (Lond) 1998;510:371–385. doi: 10.1111/j.1469-7793.1998.371bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKenzie RG, VanLeeuwen D, Pugsley TA, Shih YH, Demattos S, Tang L, Todd RD, O'Malley KL. Characterization of the human dopamine D3 receptor expressed in transfected cell lines. Eur J Pharmacol. 1994;266:79–85. doi: 10.1016/0922-4106(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 32.Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- 33.Mermelstein PG, Foehring RC, Tkatch T, Song WJ, Baranauskas G, Surmeier DJ. Properties of Q-type calcium channels in neostriatal and cortical neurons are correlated with beta subunit expression. J Neurosci. 1999;19:7268–7277. doi: 10.1523/JNEUROSCI.19-17-07268.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris AJ, Scarlata S. Regulation of effectors by G-protein alpha- and beta gamma-subunits. Recent insights from studies of the phospholipase C-beta isoenzymes. Biochem Pharmacol. 1997;54:429–435. doi: 10.1016/s0006-2952(97)00032-4. [DOI] [PubMed] [Google Scholar]
- 36.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 37.Nicola SM, Surmeier DJ, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 38.Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 40.Rajadhyaksha A, Barczak A, Macias W, Leveque JC, Lewis SE, Konradi C. L-type Ca(2+) channels are essential for glutamate-mediated CREB phosphorylation and c-fos gene expression in striatal neurons. J Neurosci. 1999;19:6348–6359. doi: 10.1523/JNEUROSCI.19-15-06348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rampe D, Anderson B, Rapien Pryor V, Li T, Dage RC. Comparison of the in vitro and in vivo cardiovascular effects of two structurally distinct Ca++ channel activators, BAY K 8644 and FPL 64176. J Pharmacol Exp Ther. 1993;265:1125–1130. [PubMed] [Google Scholar]
- 42.Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 43.Rubinstein JE, Hitzemann RJ. Further evidence against the coupling of dopamine receptors to phosphoinositide hydrolysis in rat striatum. Biochem Pharmacol. 1990;39:1965–1970. doi: 10.1016/0006-2952(90)90616-s. [DOI] [PubMed] [Google Scholar]
- 44.Sandor P. Gilles de la Tourette syndrome: a neuropsychiatric disorder. J Psychosom Res. 1993;37:211–226. doi: 10.1016/0022-3999(93)90030-j. [DOI] [PubMed] [Google Scholar]
- 45.Sibley DR. Molecular biology of dopamine receptors. In: Ariano MA, Surmeier DJ, editors. Molecular and cellular mechanisms of neostriatal function. Landes; Austin, TX: 1995. pp. 255–272. [Google Scholar]
- 46.Simpson PB, Challiss RA, Nahorski SR. Neuronal Ca2+ stores: activation and function. Trends Neurosci. 1995;18:299–306. doi: 10.1016/0166-2236(95)93919-o. [DOI] [PubMed] [Google Scholar]
- 47.Song W-J, Surmeier DJ. Voltage-dependent facilitation of calcium currents in rat neostriatal neurons. J Neurophysiol. 1996;76:2290–2306. doi: 10.1152/jn.1996.76.4.2290. [DOI] [PubMed] [Google Scholar]
- 48.Sternweis PC, Smrcka AV. Regulation of phospholipase C by G proteins. Trends Biochem Sci. 1992;17:502–506. doi: 10.1016/0968-0004(92)90340-f. [DOI] [PubMed] [Google Scholar]
- 49.Surmeier DJ, Kitai ST. D1 and D2 dopamine receptor modulation of sodium and potassium currents in rat neostriatal neurons. In: Arbuthnott GW, Emson PC, editors. Chemical signaling in the basal ganglia. Elsevier; Amsterdam: 1993. pp. 309–324. [DOI] [PubMed] [Google Scholar]
- 50.Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci USA. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 52.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tukey JW. Exploratory data analysis. Addison-Wesley; Menlo Park, CA: 1977. [Google Scholar]
- 54.Vallar L, Muca C, Magni M, Albert P, Bunzow J, Meldolesi J, Civelli O. Differential coupling of dopaminergic D2 receptors expressed in different cell types. Stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis in LtK-fibroblasts, hyperpolarization, and cytosolic-free Ca2+ concentration decrease in GH4C1 cells. J Biol Chem. 1990;265:10320–10326. [PubMed] [Google Scholar]
- 55.Viard P, Exner T, Maier U, Mironneau J, Nurnberg B, Macrez N. Gbetagamma dimers stimulate vascular L-type Ca2+ channels via phosphoinositide 3-kinase. FASEB J. 1999;13:685–694. doi: 10.1096/fasebj.13.6.685. [DOI] [PubMed] [Google Scholar]
- 56.Ward RP, Dorsa DM. Molecular and behavioral effects mediated by Gs-coupled adenosine A2a, but not serotonin 5-Ht4 or 5-Ht6 receptors following antipsychotic administration. Neuroscience. 1999;89:927–938. doi: 10.1016/s0306-4522(98)00364-9. [DOI] [PubMed] [Google Scholar]
- 57.Waszczak BL, Martin LP, Greif GJ, Freedman JE. Expression of a dopamine D2 receptor-activated K+ channel on identified striatopallidal and striatonigral neurons. Proc Natl Acad Sci USA. 1998;95:11440–11444. doi: 10.1073/pnas.95.19.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss S, Sebben M, Garcia-Sainz JA, Bockaert J. D2-dopamine receptor-mediated inhibition of cyclic AMP formation in striatal neurons in primary culture. Mol Pharmacol. 1985;27:595–599. [PubMed] [Google Scholar]
- 59.Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- 61.Yan Z, Feng J, Fienberg AA, Greengard P. D(2) dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proc Natl Acad Sci USA. 1999;96:11607–11612. doi: 10.1073/pnas.96.20.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang SN, Dasgupta S, Lledo PM, Vincent JD, Fuxe K. Reduction of dopamine D2 receptor transduction by activation of adenosine A2a receptors in stably A2a/D2 (long-form) receptor co-transfected mouse fibroblast cell lines: studies on intracellular calcium levels. Neuroscience. 1995;68:729–736. doi: 10.1016/0306-4522(95)00171-e. [DOI] [PubMed] [Google Scholar]