Abstract
The pathogenic mechanism linking presenilin-1 (PS-1) gene mutations to familial Alzheimer's disease (FAD) is uncertain, but has been proposed to include increased neuronal sensitivity to degeneration and enhanced amyloidogenic processing of the β-amyloid precursor protein (APP). We investigated this issue by using gene targeting with the Cre-lox system to introduce an FAD-linked P264L mutation into the endogenous mouse PS-1 gene, an approach that maintains normal regulatory controls over expression. Primary cortical neurons derived from PS-1 homozygous mutant knock-in mice exhibit basal neurodegeneration similar to their PS-1 wild-type counterparts. Staurosporine and Aβ1–42 induce apoptosis, and neither the dose dependence nor maximal extent of cell death is altered by the PS-1 knock-in mutation. Similarly, glutamate-induced neuronal necrosis is unaffected by the PS-1P264L mutation. The lack of effect of the PS-1P264L mutation is confirmed by measures of basal- and toxin-induced caspase and calpain activation, biochemical indices of apoptotic and necrotic signaling, respectively. To analyze the influence of the PS-1P264L knock-in mutation on APP processing and the development of AD-type neuropathology, we created mouse lines carrying mutations in both PS-1 and APP. In contrast to the lack of effect on neuronal vulnerability, cortical neurons cultured from PS-1P264L homozygous mutant mice secrete Aβ42 at an increased rate, whereas secretion of Aβ40 is reduced. Moreover, the PS-1 knock-in mutation selectively increases Aβ42 levels in the mouse brain and accelerates the onset of amyloid deposition and its attendant reactive gliosis, even as a single mutant allele. We conclude that expression of an FAD-linked mutant PS-1 at normal levels does not generally increase cortical neuronal sensitivity to degeneration. Instead, enhanced amyloidogenic processing of APP likely is critical to the pathogenesis of PS-1-linked FAD.
Keywords: presenilin; amyloid; plaque, neuronal necrosis; neuronal apoptosis; plaque; amyloid precursor protein; Aβ; familial Alzheimer's Disease; gene targeting
Mutations in the β-amyloid precursor protein (APP), presenilin-1 (PS-1), and presenilin-2 (PS-2) genes are a leading cause of early onset familial Alzheimer's disease (FAD) and cosegregate with FAD in an autosomal dominant manner (Price et al., 1998; Selkoe, 1998). All forms of AD are characterized by loss of neurons and synapses in specific brain regions, and deposition of protein aggregates as Aβ-containing amyloid plaques in the brain parenchyma, leptomeninges, and cerebrovasculature, and as tau-containing intraneuronal neurofibrillary tangles. At least two leading hypotheses have emerged for the pathogenic mechanisms of the mutations. According to the “amyloid cascade” hypothesis, APP and PS mutations promote formation from APP of the highly insoluble Aβ42 variant, whose progressive aggregation triggers the amyloid and synaptic abnormalities and neuronal loss. It is supported by findings that all of the FAD-linked mutations examined so far increase selectively the production or concentration of Aβ42 (Citron et al., 1992; Cai et al., 1993; Suzuki et al., 1994; Borchelt et al., 1996; 1997; Duff et al., 1996; Scheuner et al., 1996; Oyama et al., 1998). Transgenic mouse lines overexpressing FAD-linked mutant forms of APP develop amyloid deposits, neuritic dystrophy, and reactive gliosis preferentially in brain regions vulnerable in AD (Games et al., 1995; Hsiao et al., 1996; Sturchler-Pierrat et al., 1997; Frautschy et al., 1998), which are accelerated by the co-overexpression of mutant PS-1 (Borchelt et al., 1997; Holcomb et al., 1998). Aβ aggregates demonstrate neurotoxic and neurotropic properties that could contribute to neuronal and neuropil alterations in the AD brain and disrupt neural circuit function (Yankner, 1996; Phinney et al., 1999). Nevertheless, the amyloid cascade hypothesis has been challenged on several fronts and remains unproven. Moreover, in transgenic mice the mutant APP and PS-1 are expressed at abnormal levels and without endogenous regulatory controls over gene splicing and expression, which adds to the complexity of evaluating the pathogenic significance of transgene-induced abnormalities.
A second postulate for FAD-linked mutations is the “endangerment” hypothesis, which proposes that APP and PS mutations enhance neuronal sensitivity to degeneration. It is based on findings that expression of mutant APP or PS in cell culture either is directly cytotoxic or enhances susceptibility to apoptotic and necrotic insults (Wolozin et al., 1996; Yamatsuji et al., 1996). In these studies, however, APP and presenilin mutants are overexpressed, raising the possibility that the endangering effects may be dependent on nonphysiological expression levels (Czech et al., 1998; Nishimura et al., 1998; Uetsuki et al., 1999). Gene targeting has been used as an alternative means of introducing an FAD-linked mutation into PS-1 without resorting to overexpression and reportedly increases the sensitivity of primary hippocampal neurons to several insults (Guo et al., 1999a,b; Katayama et al., 1999). On the other hand, endangerment is not observed in all studies in which mutant PS-1 is expressed in primary neurons (Bursztajn et al., 1998).
To create faithful mouse genetic models of FAD and investigate further the pathogenic mechanism of the mutations, we have used gene targeting to modify the endogenous APP and PS-1 loci. Here, we have studied a mouse line carrying a PS-1 P264L targeted mutation and evaluated the influences of this FAD-linked mutation, when expressed at normal levels under endogenous control mechanisms, on cortical neuronal vulnerability to degeneration, amyloidogenic APP processing, and the development of AD-type amyloid neuropathology.
MATERIALS AND METHODS
Mutant mouse lines. The PS-1 P264L knock-in mouse line was derived using a two-step mutagenesis strategy similar to that used previously for the mouse APP gene (Reaume et al., 1996) and is described in detail elsewhere (Dorfman et al., 1998; D. G Flood, A. G. Reaume, K. S. Dorfman, Y.-G. Lin, D. M. Lang, S. P. Trusko, M. J. Savage, R. Siman, and R. W. Scott, unpublished observations). Briefly, a PS-1 targeting vector was constructed consisting of a mouse genomic fragment spanning exon 8 and including portions of the surrounding introns and bearing base changes in the coding region at codons 264 and 265. The former introduces a proline-to-leucine substitution at codon 264, whereas the latter is a silent substitution that creates a novel AflII restriction site. The primary structure of the mutagenized portion of exon 8 was confirmed by nucleotide sequencing. The targeting vector was introduced via electroporation into the R1 line of ES cells (129 mouse strain), and homologous recombinants were identified by a positive–negative drug selection scheme. DNA samples were screened by Southern hybridization using PS-1 probes flanking the 5′ arm of homology, the 3′ arm of homology, and an internal probe. Next, a chimeric founder mouse that exhibited germline transmission of the mutant PS-1 allele was produced by embryo aggregation of one of the targeted ES cell clones. A cytomegalovirus/cre expression construct was injected into the pronucleus of zygotes produced from this founder. These embryos were transferred to pseudopregnant recipient females and allowed to develop to term. The pups born from this procedure were scored for the loss of the neomycin cassette by PCR. Of the six pups born, one exhibited loss of the neomycin cassette. The integrity of the PS-1 locus in this presumptiveneo− mouse was confirmed by comprehensive restriction enzyme mapping. From this founder, heterozygous PS-1 P264L/wt and homozygous PS-1 P264L/P264L lines were established in the CD-1 outbred background.
Heterozygous and homozygous mutant PS-1 mice were cross-bred with either a mouse line overexpressing an APP695swetransgene carrying the FAD-linked Swedish double mutation (Tg2576;Hsiao et al., 1996) or a mouse line carrying a targeted APPswe double knock-in mutation and a “humanized” Aβ domain (APPswe KI; Reaume et al., 1996). PCR was used for genotyping analyses. For all of the experiments reported here comparing mice wild-type for APPand PS-1 or carrying mutations in APP orPS-1 alone, or in both, siblings of the various genotypes were used to control for variations in genetic background.
Northern analysis. PS-1 expression in mouse brain was measured in the presence or absence of the P264L targeted mutation. Total RNA was extracted from one half brain by homogenization in RNAzol B. Messenger RNA was selected on Oligotex columns (Qiagen, Valencia, CA). Equal volumes of mRNA were mixed with loading buffer (NorthernMax-Gly; Ambion, Austin, TX), heated to 50°C for 30 min, separated on 0.7% agarose gels, and transferred to nylon membranes. PS-1 mRNA was detected with a [32P]dUTP-labeled riboprobe representing the 3′ end of human PS-1 [nucleotides 1083–1428 cloned into a pGEM-T (Promega, Madison, WI) vector]. The same blots were hybridized with a GAPDH probe (Ambion) to control for RNA loading. To visualize mRNA, the membranes were exposed to PhosphorImager (Molecular Dynamics, Sunnyvale, CA) screens and examined on a Molecular Dynamics Storm unit. Band densities were determined with ImageQuant software.
Primary neurons. Cell cultures were obtained from the cerebral cortex of embryonic day 16 mice using standard techniques (Banker and Goslin, 1998). For cell dissociation, minced cortical tissues were briefly trypsinized, washed in the presence of soybean trypsin inhibitor and bovine serum albumin, and triturated through narrow-tip pipettes. For analyses of neuronal vulnerability, cells were plated on polyornithine/laminin-coated 96-, 24-, or 6-well plates at 6 × 104/cm2 in Neurobasal medium with B27 supplement (Life Technologies, Rockville, MD) and maintained at 37°C in humidified 95% air and 5% CO2. Serum and antibiotics were not used at any time during the culture preparation. The culture medium was changed 3 hr after plating and every other day thereafter. Experiments were performed after 7–8 d in vitro. The cellular composition of the cultures was evaluated at this time by immunohistochemical analysis using antibodies to neuron- and glial-specific markers and was found to consist of >95% neurons. For evaluation of Aβ secretion, cells were plated at a 10-fold higher density.
Cell viability analyses. Neuronal apoptosis was assessed after 18–48 hr treatment with either staurosporine (100–600 nm; Sigma, St. Louis, MO), Aβ1–42 (5–25 μm; Bachem, Torrance, CA), or DMSO vehicle (0.1%), or brief 15 min exposure to hydrogen peroxide (3–300 μm), followed by 24 hr recovery. The Aβ1–42 was prepared as a 1 mm stock solution in Neurobasal medium and subjected to one freeze–thaw cycle before use to induce the formation of neurotoxic aggregates. Cultures were fixed in 4% paraformaldehyde (Sigma) in 0.1m sodium phosphate, pH 7.4, at 4°C 60 min, permeabilized in 0.05% Triton X-100 in PBS, pH 7.4, at 4°C 15 min, then rinsed in PBS. Cultures were incubated in Hoechst 33342 (1 μg/ml PBS; Sigma) at 37°C for 10 min, rinsed twice in PBS, and examined with a Nikon fluorescence microscope at 400× under UV illumination. Cells with bright, condensed chromatin were scored as apoptotic. More than 400 cells were counted in each experimental group (at least four cultures per group). Neuronal necrosis was evaluated 24 hr after addition of l-glutamate (10–100 μm). Cultures were incubated with 0.1% Trypan blue in PBS 5 min at 22°C, rinsed with PBS, then fixed in 4% paraformaldehyde in 0.1m sodium phosphate. Trypan blue-positive and -negative cells were counted by an observer blinded to the treatment group. More than 300 cells were evaluated in each experimental group (three cultures per group).
Caspase activity. Neuronal extracts were prepared from cultures treated 6 hr with staurosporine (125–500 nm) or DMSO vehicle (0.1%). Four cultures were evaluated for each experimental condition. Cultures were rinsed in PBS, and cells were collected by scraping into ice-cold assay buffer (0.5 ml/35 mm well) consisting of 25 mm HEPES, pH 7.5, 5 mmβ-mercaptoethanol, 5 mm EDTA, 50 μmleupeptin, and 2.5 μm pepstatin A. Cells were briefly sonicated, then centrifuged at 10,000 × g 20 min at 4°C. Supernatants were brought to 10% glycerol, snap-frozen, and stored at −80°C. Caspase 3-like proteolytic activity was quantified by hydrolysis of acetyl-Asp-Glu-Val-Asp-aminofluorocoumarin (20 μm; Biomol, Plymouth Meeting, PA). Reactions of 200 μl were run at 30°C for 90 min in 96-well plates in assay buffer supplemented with 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid. Substrate hydrolysis was measured in a Cytofluor fluorimeter (Millipore, Bedford, MA) every 5 min at 420 nm excitation/530 nm emission, under conditions whereby fluorescence intensity (F) changed in linear proportion with incubation time and extract concentration. An aliquot of each extract was used to determine total protein content (Bradford method; Bio-Rad, Burlingame, CA). Caspase activity is represented as ΔF (arbitrary units) per hour per microgram of protein.
Immunoblot. Western blot analyses were performed on vehicle-, staurosporine-, and l-glutamate-treated cultures grown in 6-well plates. Cultures were rinsed twice in ice-cold PBS containing 5 mm EDTA, then cells were collected by scraping and brief sonication in 0.1 ml/well Laemmli sample buffer. Polypeptides were separated by SDS-PAGE and detected by immunoblotting as previously described (Roberts-Lewis et al., 1994; Siman et al., 1999), using the Renaissance ECL kit (DuPont, Boston, MA). The following primary antibodies were used: anti-α-spectrin-Ab212 (1:5000; Roberts-Lewis et al., 1994); calpain-derived COOH-terminal α-spectrin fragment-Ab41 (1:3000; Roberts-Lewis et al., 1994); caspase-3-MAb46 (1:2000; Transduction Laboratories, Lexington, KY); poly(ADP-ribose) polymerase-MAbC2–10 (1:1000; Transduction Laboratories); GDEVD-containing caspase substrates-Ab127 (1:5000; Siman et al., 1999). To quantify l-glutamate-stimulated spectrin degradation, blots were scanned, and the density of intact α-spectrin and its calpain-derived fragments determined using ImageQuant software (Molecular Dynamics). An antiserum reactive with the p17 subunit of activated caspase-3 was prepared using the peptide CGIETD (Infinity, Upland, PA), which corresponds to the COOH terminus of the free p17 subunit. The peptide was conjugated through its cysteine residue to keyhole limpet hemocyanin by the heterobifunctional coupling agent maleimidobenzoyl-N-hydroxysuccinimide ester (Pierce, Rockford, IL), and the conjugate was used to immunize rabbits and generate the Ab206 antiserum. On Western blots, Ab206 labels an ∼17 kDa polypeptide present in apoptotic, but not control, neuronal extracts, but does not detect the ∼32 kDa procaspase-3. The immunoreactive ∼17 kDa polypeptide comigrates with p17 detected using MAb46, and appearance of both ∼17 kDa polypeptides is blocked when cells are pretreated with a caspase inhibitor.
ELISA. Aβx-42 and Aβx-40 were quantified from conditioned media and mouse brain extracts using antibodies, extraction methods, and immunoassays described previously (Savage et al., 1998;Durkin et al., 1999). The rate of secretion of the Aβ variants from cultured cortical neurons was determined by analyzing media conditioned for 2, 4, or 7 hr (n = 3 per condition and time point), and represented as picograms of Aβ per milliliter per milligram of protein per hour. Levels of the Aβ forms in mouse brain are represented as nanograms of Aβ per milligram of protein and are the mean values from 4–10 animals per group.
Immunohistochemistry. Under deep pentobarbital anesthesia, mice were perfused with Ringer's solution, and the brains were removed. Half of each brain was frozen for ELISA, and the other half was immersed in 70% ethanol and 0.15 m NaCl for 48 hr. Paraffin-embedded sections were cut at 10 μm. Sections were reacted with a rabbit antibody directed at Aβ1–28 (Ab1153 at 1/1000; Siman et al., 1995) and labeled using the SuperSensitive kit for HRP (Biogenex, San Ramon, CA) and nickel intensification of the diaminobenzidine chromagen. For double immunolabeling, a mouse antibody to glial fibrillary acidic protein (GFAP; Dako, Carpinteria, CA) was diluted 1:1000 and visualized using nickel-intensified diaminobenzidine (purple), followed by rabbit Ab1153 and detection using diaminobenzidine (brown). Adjacent sections were stained with thioflavin S.
RESULTS
Presenilin mutant knock-in mouse
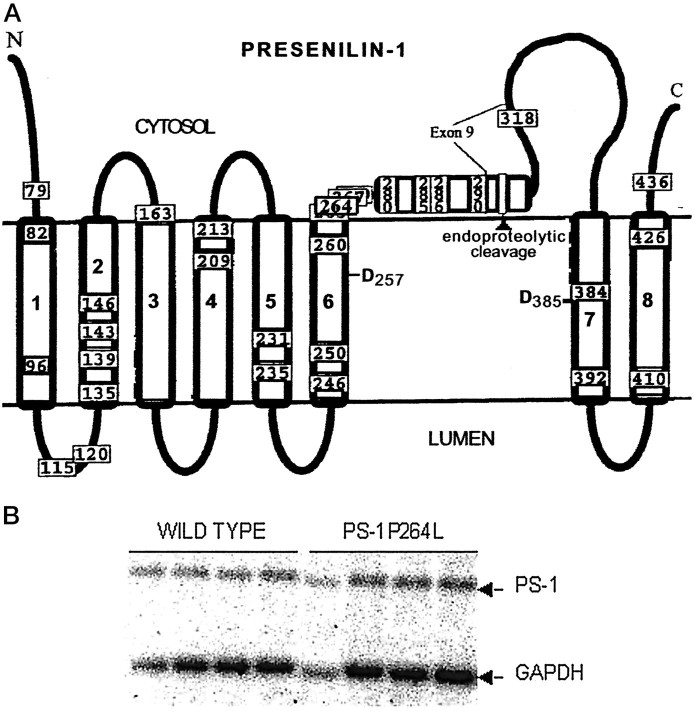
An exon replacement strategy was used to generate mouse lines carrying a targeted mutation in their endogenouspresenilin-1 gene. A proline-to-leucine substitution was targeted to codon 264 in exon 8 by homologous recombination in embryonic stem (ES) cells (Dorfman et al., 1998; Flood, Reaume, Dorfman, Lin, Lang, Trusko, Savage, Siman, and Scott, unpublished observations). As shown schematically in Figure1A, this FAD-linked mutation (Campion et al., 1995; Wasco et al., 1995) is located in the large cytoplasmic loop region near the interface with the putative sixth transmembrane domain (Doan et al., 1996), is predicted to alter profoundly the local secondary structure, and is in proximity to one of the transmembrane aspartate residues required for γ-secretase processing of APP and Notch-1 (De Strooper et al., 1999; Wolfe et al., 1999). This PS-1 mutation reportedly increases the secretion of Aβ42 from transfected cells (Murayama et al., 1999). We found that the neomycin resistance-encoding cassette in the upstream intron substantially lowered PS-1 mRNA levels (Dorfman et al., 1998; Flood, Reaume, Dorfman, Lin, Lang, Trusko, Savage, Siman, and Scott, unpublished observations), and so used the cre-loxP recombinase system to excise the cassette. Targeted ES cells were used to generate chimeric founder mice and, after germline transmission of the targeted allele and removal of the neomycin cassette, the PS-1 P264L knock-in mouse line. Southern blot and PCR analyses demonstrated germline transmission of the targeted allele and confirmed that codon 264 contains the FAD-linked mutation.
Fig. 1.
Location of P264L knock-in mutation introduced into PS-1 by gene-targeting. A, The eight transmembrane model for the topology of PS-1 is shown (Doan et al., 1996), along with mutations that have been linked to FAD (Hardy, 1997). Note that codon 264 is within a cluster site for FAD-linked mutations in the cytoplasmic loop region and is in proximity to one of the transmembrane aspartates (D257) implicated in regulating proteolytic function of γ-secretase. B, Northern analysis of PS-1 mRNA expression in brains of PS-1 wild-type and PS-1P264L/P264L mice. Levels of PS-1 and GAPDH mRNA were quantified by densitometry. Relative to GAPDH expression levels, knock-in of the P264L mutation did not alter expression of PS-1 mRNA.
For the PS-1 wild-type and PS-1 P264L/P264L homozygous knock-in mouse lines, we performed Northern blot analysis to quantify expression of PS-1 in the adult brain. Blots were reprobed for GAPDH mRNA to control for RNA loading. PS-1 mRNA levels, normalized to those of GAPDH, were equivalent between the wild-type and knock-in genotypes (Fig.1B). Western blot analysis demonstrated that the mutant PS-1 was subjected to endoproteolytic processing and was present in the mouse brain predominantly as ∼30 kDa N-terminal and ∼20 kDa COOH-terminal fragments (Flood, Reaume, Dorfman, Lin, Lang, Trusko, Savage, Siman, and Scott, unpublished observations), similar to the processing of endogenous PS-1 (Thinakaran et al., 1996), but different from transgenic overexpression, which leads to accumulation of the unprocessed PS-1 holoprotein (Borchelt et al., 1996; Duff et al., 1996; Thinakaran et al., 1996).
Effect of PS-1 P264L knock-in mutation on morphological apoptosis in cortical neurons
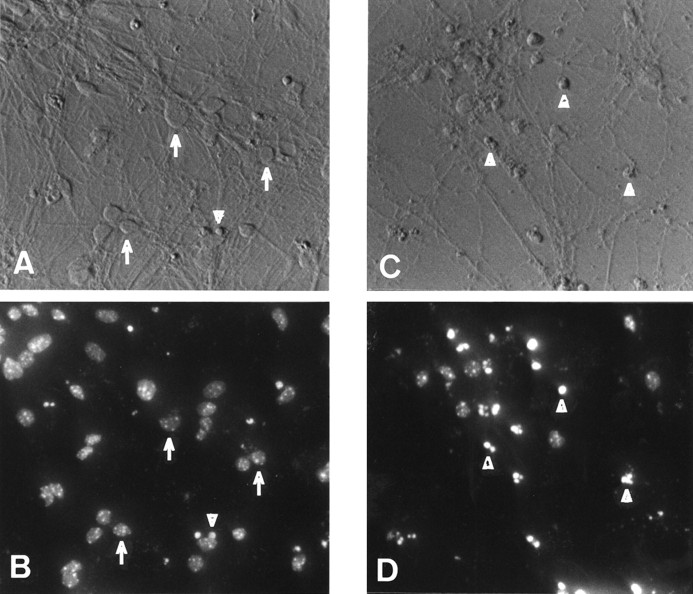
Because the PS-1 P264L knock-in mutation is expressed at normal levels and under endogenous cell- and development-specific regulatory control, cultured neurons from the knock-in mouse brain are an excellent experimental system for analyzing the potential endangering effects of this FAD-linked mutation. We evaluated primary neocortical neurons, derived from wild-type and PS-1 P264L/P264L mice and cultured for 7 d, for their sensitivity to apoptosis. After 18–48 hr treatments with either staurosporine (STS; 100–600 nm), Aβ1–42 (5–25 μm), or the DMSO vehicle, nuclear morphology was assessed after Hoechst 33342 staining. For vehicle-treated or untreated cultures, a small but detectable proportion of cortical neurons degenerated during the 7 d culture period. The dead cells exhibited shrunken perikarya, beaded or retracted neurites, and condensed, fragmented chromatin indicative of apoptosis (Fig. 2). Based on chromatin condensation, there was no difference in the background level of apoptosis between the two genotypes (Table1). STS induced chromatin condensation and apoptosis in a dose-dependent manner, with up to 70% of the neurons dying within 24 hr. Neither the dose dependence nor maximal extent of STS-induced apoptosis differed as a function of PS-1 genotype. A smaller proportion of cortical neurons were apoptotic 24 hr after treatment with 25 μm Aβ1–42 treatment, and the extent of cell death was not changed by the PS-1 P264L mutation (Table1). Similar results were obtained after 48 hr exposure to Aβ1–42 (5–25 μm) or brief treatment with hydrogen peroxide (3–300 μm) as apoptotic stimulus (data not shown). Thus, neither the basal level of morphological apoptosis nor the response to three different apoptogenic agents was altered significantly in primary cortical neurons by the PS-1 P264L knock-in mutation.
Fig. 2.
Assessment of apoptosis in primary cortical neurons. Cultures were treated for 18 hr with either vehicle (A, B) or 600 nm STS (C, D). Shown inA and C are photomicrographs taken with modulation contrast optics, whereas B andD are fluorescence images of the same fields of chromatin staining with Hoechst 33342. Note the detectable but modest background level of apoptosis in vehicle-treated cultures, with some of the healthy neurons denoted by the arrows and an apoptotic profile indicated by the arrowhead. STS treatment caused abundant neuronal shrinkage, degeneration of processes, and condensation of chromatin indicative of apoptosis (arrowheads) in >70% of the neurons.
Table 1.
Apoptosis in cultured cortical neurons either wild-type for PS-1 or homozygous for the PS-1 P264L knock-in mutation
| Condition | Genotype | Apoptotic index |
|---|---|---|
| Control | WT/WT | 14 ± 1.3 |
| P264L/P264L | 10 ± 0.4 | |
| Staurosporine, 150 nm | WT/WT | 33 ± 1.7 |
| P264L/P264L | 28 ± 2.9 | |
| Staurosporine, 300 nm | WT/WT | 52 ± 3.8 |
| P264L/P264L | 46 ± 2.2 | |
| Aβ1-42, 25 μm | WT/WT | 25 ± 2.1 |
| P264L/P264L | 24 ± 2.0 |
Cortical neurons in primary culture were treated with the indicated agents and apoptotic nuclear morphology evaluated as described in Materials and Methods. The percentage of apoptotic profiles is represented as the apoptotic index (mean ± SEM). The basal level of apoptosis did not differ across PS-1 genotype, nor did the stimulation of apoptosis by STS or Aβ1-42 treatment.
Biochemical hallmarks of apoptosis in primary cortical neurons
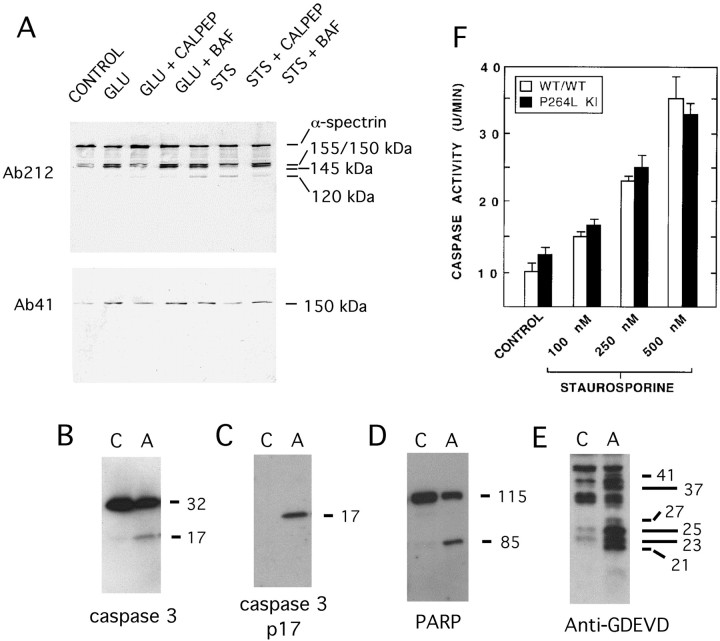
As another means of assessing the influence of the PS-1 P264L knock-in mutation on cortical neuronal vulnerability to degeneration, we examined activation of the apoptotic effector caspase 3 and quantified caspase 3-like proteolytic activity in neuronal extracts. STS was the toxin of choice for these experiments because it induces a rapid, constitutive apoptosis (Weil et al., 1996) that is especially amenable to biochemical analyses. Caspase 3 activation and caspase substrate degradation were analyzed by Western blotting 6–24 hr after STS treatment. Spectrin, an actin-binding protein that is an established high-affinity substrate for caspases and calpains activated during cell death (Wang, 2000), was degraded during STS-induced cortical neuronal apoptosis to produce several breakdown products. As shown in Figure 3A, STS treatment increased the level of immunoreactive fragments of the α-subunit of spectrin of ∼155/150 kDa (a doublet) and ∼120 kDa. The latter α-spectrin derivative has been described previously as a caspase-derived fragment accumulating in apoptotic cells (Wang et al., 1998). Consistent with its derivation from caspase, formation of the ∼120 kDa α-spectrin fragment was markedly reduced by the broad-spectrum caspase inhibitor benzyloxycarbonyl-Asp(OMe)-fluoromethylketone (BAF); (50 μm), but was unaffected by the calpain inhibitor calpeptin (50 μm). On the other hand, appearance of the ∼150 kDa α-spectrin fragments (migrating slightly faster than the ∼155 kDa polypeptide) was attenuated by calpeptin but not by BAF. STS-induced activation of calpain was confirmed by immunoblotting with Ab41, a cleavage site-specific antibody that reacts preferentially with a calpain-derived ∼150 kDa COOH-terminal α-spectrin fragment (Siman and Noszek, 1988; Roberts-Lewis et al., 1994). The Ab41-immunoreactive fragment was reduced by treatment with calpeptin, but not with BAF. Collectively, the findings indicate that STS causes activation of both caspase and calpain in cortical neurons, leading to appearance of an ∼120 kDa caspase-derived α-spectrin fragment and an ∼150 kDa calpain derivative.
Fig. 3.
Cysteine protease activation during STS-induced apoptosis and l-glutamate-induced necrosis.A, Immunoblot detection of α-spectrin and its proteolytic fragments from cortical neurons treated withl-glutamate (100 μm) or STS (300 nm) in the presence or absence of the calpain inhibitor calpeptin (calpep, 50 μm) or the caspase inhibitor Boc-Asp(OMe)-FMK (BAF, 50 μm). An antibody to α-spectrin (Ab212) detects the intact ∼250 kDa polypeptide, as well as proteolytic fragments of ∼120–155 kDa. l-glutamate stimulates production of calpain-derived ∼150 and ∼145 kDa spectrin fragments, but not the ∼120 kDa caspase derivative. Calpain-mediated α-spectrin degradation is also detectable with Ab41, specific for a calpain-derived COOH-terminal ∼150 kDa fragment. B–E, Immunoblot detection of caspase-3 activation and caspase substrate degradation in apoptotic (A) but not control (C) cortical neurons. B, Procaspase-3 and the p17 subunit of activated caspase-3 detected with MAb46. C, The p17 subunit of activated caspase-3 detected with the neoepitope-specific antibody Ab206. D, Poly(ADP-ribose) polymerase (PARP; ∼115 kDa) and the caspase-derived ∼85 kDa fragment. E, Caspase substrate fragments containing the GDEVD caspase recognition motif, labeled with the neoepitope-specific antibody Ab127. F, Effect of PS-1 P264L/P264L on caspase activity in cortical neurons. Hydrolysis of the caspase substrate Ac-DEVD-AFC was quantified as described in Materials and Methods, and is represented as the time-dependent change in fluorescence per culture. Neither the basal level of caspase activity nor the STS-stimulated activity differed between the two PS-1 genotypes.
The caspase 3 zymogen is activated by proteolytic processing into self-associating large and small subunits (Thornberry and Lazebnik, 1998). We obtained evidence for activation of caspase 3 in apoptotic cortical neurons by immunoblot detection of formation of the proteolytically active p17 large subunit from the 32 kDa proenzyme (Fig. 3B). Identification of the 17 kDa polypeptide as the large subunit of activated caspase 3 was confirmed by labeling with a cleavage site-specific antibody (Ab206) reactive with the COOH terminus of the p17 subunit (Finn et al., 2000), which was detected in the apoptotic but not the control extract (Fig. 3C). As further evidence for activation of caspase 3 or a closely related caspase in cortical neuronal apoptosis, the caspase substrate poly(ADP-ribose) polymerase (PARP) was cleaved to a characteristic ∼85 kDa COOH-terminal fragment (Fig. 3D). Additionally, caspase proteolysis was detected by immunoblotting with a cleavage site-specific antibody (Ab127) reactive with the GDEVD caspase cleavage site motif (Siman et al., 1999). Whereas Ab127 immunolabeled only a small number of polypeptides in control neuronal extracts, immunoreactive polypeptides ranging from ∼21 to 45 kDa were abundant in apoptotic extracts (Fig. 3E). Therefore, cortical neuronal apoptosis is accompanied by activation of caspase 3 and degradation of several caspase substrates.
Effect of PS-1 P264L knock-in mutation on caspase activity
Having linked caspase activation to STS-induced apoptosis in mouse cortical neurons, we measured basal- and STS-stimulated caspase activity from neurons wild-type for PS-1 or homozygous for the P264L knock-in mutation. Caspase activity was assessed by quantifying DEVDase activity in neuronal extracts using the specific fluorogenic caspase substrate Ac-DEVD-AFC. As shown in Figure 3F, the basal level of caspase 3-like activity did not differ between the two genotypes. STS (125–500 nm) caused a dose-dependent increase of up to 2.5-fold in DEVDase activity, and neither the dose dependence nor the maximal extent of caspase activity was altered significantly by the PS-1 P264L mutation. When coupled with our examination of nuclear morphology, the data indicate that knock-in of the FAD-linked P264L mutation into the mouse PS-1 gene does not generally alter cortical neuronal vulnerability to STS or other apoptogenic agents.
Cortical neuronal sensitivity to glutamate-induced necrosis
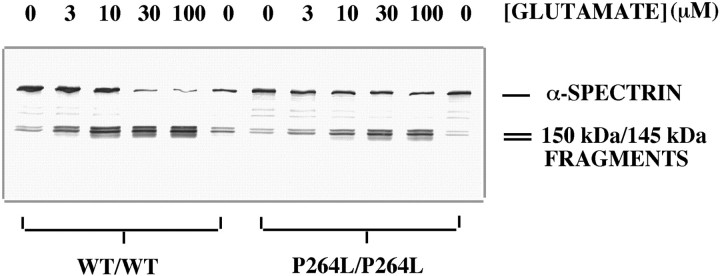
Next, we investigated the influence of the PS-1 P264L targeted mutation on vulnerability of primary cortical neurons tol-glutamate-induced necrosis. In the virtual absence of non-neuronal cells, glutamate caused rapid neuritic beading and neuronal death in the 10–100 μm concentration range. As shown in Figure 3A, glutamate treatment caused appearance of ∼150 kDa spectrin derivatives reactive with an antibody to the spectrin holoprotein, one of which was detected with the calpain cleavage site-specific Ab41. Appearance of these polypeptides was blocked by calpeptin, but not by BAF. The ∼120 kDa caspase-derived fragment was below the limit of detection. Therefore, glutamate stimulated the calpain-mediated degradation of α-spectrin, but did not lead to spectrin degradation by caspases. Moreover, the glutamate toxicity was not accompanied by chromatin condensation or fragmentation (data not shown). Given that calpain activation accompanies excitotoxic necrosis in a variety of cultured cell and in vivo systems (Siman et al., 1996), the morphological and biochemical observations indicate that glutamate toxicity under the current conditions is predominantly necrotic.
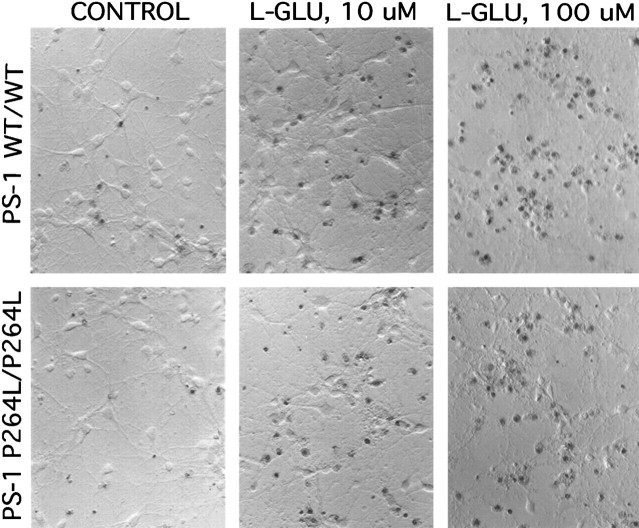
To quantify neuronal sensitivity to glutamate-induced necrosis, we counted the proportion of cortical neurons stained with Trypan blue, which measures the loss of plasma membrane integrity (Fig.4). A proportion of neurons were dead after culturing for 7 d and washing under basal conditions, and glutamate caused a dose-dependent increase in neuronal necrosis: 41% (±5) of the PS-1 wild-type neurons and 47% (±9%) of the PS-1 mutant neurons were dead after a 24 hr treatment with 10 μmglutamate, whereas with 100 μm glutamate, 71% (±9) and 57% (±13) were killed, respectively. There was no significant difference in either the basal- or glutamate-induced necrosis between the two PS-1 genotypes.
Fig. 4.
l-glutamate-induced necrosis is not altered by the PS-1 P264L/P264L knock-in mutation. Cortical neurons were treated for 24 hr with the indicated concentrations ofl-glutamate, then stained with Trypan blue to detect loss of plasma membrane integrity. Note that l-glutamate causes a dose-dependent neurotoxicity, and neither the basal- nor thel-glutamate-stimulated neuronal death was altered appreciably by the PS-1 point mutation. Hoffman modulation contrast optics, 200×.
As another measure of neuronal sensitivity to glutamate, we evaluated dose-dependent calpain activation as monitored by spectrin degradation. Under basal conditions, an antibody to the spectrin holoprotein labeled predominantly the ∼250 kDa intact α-subunit, and also small amounts of ∼150 kDa α-spectrin fragments. Glutamate caused the disappearance of intact α-spectrin and appearance of calpain-derived ∼150 kDa fragments in a dose-dependent manner. In the experiment shown in Figure 5, glutamate-stimulated spectrin degradation was actually modestly reduced from the PS-1 P264L/P264L neurons in comparison with neurons wild-type for PS-1. Although this difference was not observed in repeated experiments, the calpain-mediated spectrin degradation for PS-1 mutant neurons never exceeded that for wild-type neurons. The results of the biochemical analysis confirm those from cell counting, in demonstrating that neuronal sensitivity to glutamate was not enhanced by the PS-1 P264L knock-in mutation.
Fig. 5.
Calpain-mediated spectrin degradation is not enhanced by PS-1 P264L/P264L. Western blot analysis of α-spectrin degradation using Ab212, after 4 hr l-glutamate treatment. Under basal conditions, α-spectrin exists predominantly as an ∼250 kDa polypeptide, with minor levels of ∼150/145 kDa fragments.l-glutamate causes a dose-dependent disappearance of intact α-spectrin and appearance of calpain-derived fragments of ∼150/145 kDa (Fig. 3). In the experiment shown, the basal- and glutamate-stimulated levels of spectrin degradation were modestly reduced by the PS-1 P264L mutation. Although this difference was not observed in two repeat experiments, in none of the experiments did the PS-1 point mutation enhance spectrin degradation.
PS-1 P264L knock-in mutation enhances amyloidogenic APP processing in cortical neurons
To investigate the influence of the PS-1 targeted mutation on amyloidogenic APP processing, we measured secretion of Aβ derivatives from primary cortical neurons derived from PS-1 wild-type and PS-1 P264L/P264L mice. For this analysis, the PS-1 knock-in and wild-type mouse lines were cross-bred with a line homozygous for a targeted “Swedish” mutation in the APP gene, as well as a humanized Aβ domain (APPswe KI; Reaume et al., 1996), to generate mice carrying homozygous targeted mutations inAPP alone, or both APP and PS-1. We measured the rate of secretion of Aβx-40 and Aβx-42 into neuron-conditioned media using sandwich ELISAs, which are >500-fold selective for their respective Aβ variants and have been characterized extensively (Howland et al., 1998; Savage et al., 1998;Durkin et al., 1999). Cortical neurons wild-type for PS-1 secreted both Aβ variants into the medium, with secretion being linear with time for 7 hr for both derivatives, and approximately sixfold higher for Aβ40 than Aβ42 (Table 2). This is similar to the Aβ42/Aβ40 secretion ratios reported previously for numerous cultured cell lines under nonkinetic conditions (Suzuki et al., 1994; Scheuner et al., 1996; Citron et al., 1997). The PS-1 P264L mutation doubled the rate of Aβ42 secretion and the ratio of the secretion rates of Aβ42/total Aβ (p < 0.005). In contrast, the PS-1 mutation led to a modest 20% reduction in the rate of Aβ40 secretion (p < 0.05). The rate of total Aβ secretion from cortical neurons was unchanged by the PS-1 P264L knock-in mutation.
Table 2.
Reciprocal effects of PS-1 P264L targeted mutation on secretion of Aβ42 and Aβ40 by cortical neurons
| Genotype | Secretion rate (pg/ml/mg/hr) | Aβ42/Total Aβ | ||
|---|---|---|---|---|
| Aβx−40 | Aβx−42 | Aβ40+42 | ||
| APPswe KI/PS-1WT/WT | 470 ± 14 | 75.8 ± 3.2 | 546 ± 16 | 0.140 ± .01 |
| APPswe KI/PS-1P264L/P264L | 389 ± 11* | 153 ± 5.92-160 | 542 ± 13 | 0.283 ± .012-160 |
Primary cortical neuronal cultures were derived from APPswe knock-in mice, either wild-type for PS-1 or homozygous for the PS-1 P264L knock-in mutation. Aβ variants were measured in media conditioned for 2, 4, or 7 hr, and the secretion rates determined by ELISA as described in Materials and Methods. Mean ± SEM.
p < 0.05;
F2-160: p < 0.005
PS-1 P264L knock-in mutation elevates Aβ42 in the brain and accelerates amyloid deposition
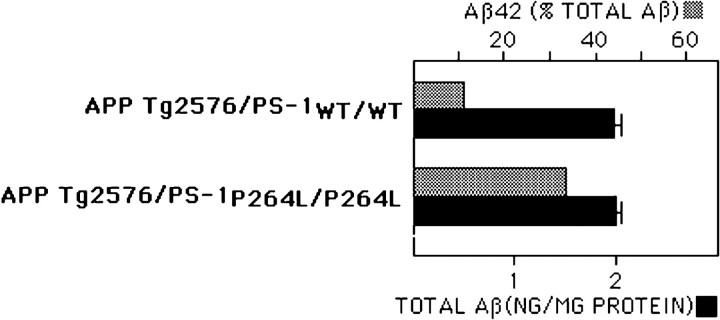
To determine whether the PS-1 targeted mutation alters Aβ levels in the mouse brain and influences the development of AD-type neuropathology, we bred the PS-1 mutation into an APP695swe transgenic mouse line that develops a well characterized aging-dependent amyloid deposition (Hsiao et al., 1996; Holcomb et al., 1998). First, we measured levels of Aβx-42 and Aβx-40 in mouse brain. For the APP695swetransgenic mouse wild-type for PS-1, Aβ42 comprised ∼12% of the total Aβ (Fig.6; Borchelt et al., 1996). Homozygous knock-in of the PS-1 P264L mutation did not alter significantly the total Aβ or Aβx-40 levels, but increased Aβ42 nearly threefold. The elevation in Aβ42/Aβ40 was evident in 1-month-old mice that were presymptomatic for amyloid deposition, based on immunohistochemical staining for Aβ (see below). Knock-in of one mutant PS-1 allele caused a smaller, ∼50% increase in brain Aβ42 content (data not shown).
Fig. 6.
PS-1P264L/P264L knock-in mutation elevates selectively Aβ42 level in the mouse brain. Whole brain extracts taken from 1-month-old mice were evaluated by ELISAs for Aβx-42 and Aβx-40 and normalized to total protein content. Although the total Aβ content does not differ between the two PS-1 genotypes, Aβ42 levels increased from 12% of the total Aβ to >30%. The difference is significant (p < 0.01).
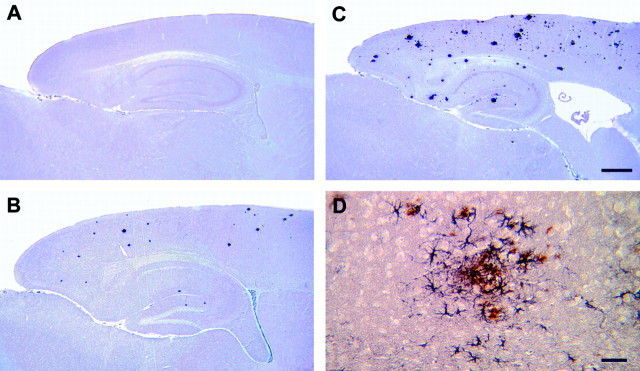
The PS-1 P264L-induced increase in amyloidogenic APP processing was accompanied by a marked acceleration in the onset of amyloid deposition and its attendant reactive gliosis. Based on immunohistochemistry using either of two Aβ antibodies, sparse amyloid deposits first appeared in the APP695swe mouse telencephalon between 6 and 9 months of age (Fig. 7A), an onset very similar to that reported by other investigators (Hsiao et al., 1996; Holcomb et al., 1998), and increased in frequency with aging. Deposits were commonly observed through most of the telencephalon, although the striatum was relatively devoid of amyloid. Relatively modest numbers of amyloid deposits were present in the thalamus and colliculus, whereas the hypothalamus, cerebellum, and brainstem contained very few core-containing deposits. APP695swe mice heterozygous for the PS-1 P264L mutation did not have amyloid deposits at 2 months of age, but had numerous deposits by 4 months (Fig. 7B). By 6 months of age, APP695swe/PS-1 P264L heterozygotes had even higher levels of deposition (Fig. 7C), comparable with those seen in APP695swe mice at 15 months. Many of the deposits had amyloid cores, stained with thioflavin S, and were surrounded by GFAP-immunopositive reactive astroglia (Fig.7D). A similar acceleration of the amyloid neuropathology by the PS-1 mutation has been observed for the APPswe knock-in mouse line (Flood, Reaume, Dorfman, Lin, Lang, Trusko, Savage, Siman, and Scott, unpublished observations). Despite its marked effect on the onset and frequency of amyloid deposition, the PS-1 P264L heterozygous knock-in mutation did not alter appreciably the restricted regional distribution of the deposits or reactive astrogliosis.
Fig. 7.
PS-1P264L knock-in mutation accelerates amyloid deposition and reactive astrogliosis even as a single mutant allele. Sagittal sections immunostained for Aβ from 6-month-old APP695swe/PS-1wt/wt (A), 4-month-old APP695swe/PS-1 P264L/wt (B), and 6-month-old APP695swe/PS-1 P264L/wt (C, D). D is a higher magnification of double-labeling for Aβ (brown) and GFAP (purple) from parietal cortex. Scale bars:A–C, 500 μm; D, 50 μm.
DISCUSSION
We have used gene targeting to introduce an FAD-linked point mutation into the endogenous mouse PS-1 gene and to evaluate two potential pathogenic mechanisms in primary cortical neurons and the mouse brain: vulnerability to degeneration and enhanced amyloidogenic processing of APP. Because FAD is inherited with autosomal dominance, a PS-1 mutation-induced phenotype should be displayed in a mouse experimental model in the heterozygous state to be of potential pathogenic significance. We find that even as a homozygous mutation, the PS-1 P264L knock-in does not alter basal- or toxin-stimulated cortical neuronal death, whereas a single mutant PS-1 allele is sufficient to elevate the concentration of Aβ42 in brain and speed the onset of amyloid deposition and reactive astrogliosis. Transgenic overexpression of PS-1 bearing FAD-linked mutations has been shown previously to increase Aβ42 concentration in the brain and accelerate the onset of amyloid deposition and its attendant reactive gliosis (Borchelt et al., 1996, 1997; Duff et al., 1996; Holcomb et al., 1998). Our findings indicate that these effects in the mouse do not require PS-1 overexpression and are transmitted with autosomal dominance. Similarly, knock-in of the FAD-causing Swedish double mutation into theAPP gene and an I213T mutation into the PS-1gene increase the amyloidogenic processing of APP as single mutant alleles (Reaume et al., 1996; Nakano et al., 1999). Collectively, the results provide further support for the amyloid cascade hypothesis, in which enhanced Aβ42 production is a key triggering event in the pathogenesis of FAD.
The present finding that the PS-1 P264L knock-in mutation does not alter the sensitivity of primary cortical neurons to apoptotic and necrotic insults contrasts with numerous reported examples of increased vulnerability and death of many types of cells expressing mutant PS-1 (for review, see Mattson et al., 1998), including hippocampal neurons (Guo et al., 1999a,b; Weihl et al., 1999). There are several distinctions between the present work and previous studies that could account for the different results. By using gene-targeting coupled with the Cre-lox system to remove the neomycin selection cassette from the targeted gene, knock-in of the PS-1–264L mutation created a faithful mouse genetic model of FAD in which the mutant PS-1 is expressed at normal levels, and with endogenous regulatory controls over gene splicing, cell-, development-, and tissue-specific expression. Many of the previous cell culture studies depended on the overexpression of PS-1, which could interfere functionally with intracellular secretory compartments (Kovacs et al., 1996; Lah et al., 1997), and key signaling pathways (Levitan et al., 1996; Zhang et al., 1998; Imafuku et al., 1999; Passer et al., 1999; Weihl et al., 1999). Given that overexpression of wild-type presenilins promotes apoptosis (Wolozin et al., 1996; Czech et al., 1998), an endangering effect of nonphysiological expression cannot be excluded. Gene targeting has been used to introduce a double point mutation into PS-1 without resorting to overexpression, and hippocampal neurons bearing the double mutation are reportedly more sensitive to multiple apoptotic and necrotic insults (Guo et al., 1999a,b). In contrast to our targeted mouse line, however, the double mutant contained a drug selection cassette in the upstream intron, and quantitation of PS-1 expression in the brain was not reported. We (Reaume et al., 1996; Flood, Reaume, Dorfman, Lin, Lang, Trusko, Savage, Siman, and Scott, unpublished observations) and others (McDevitt et al., 1997) have found that the drug selection cassette reduces expression of the targeted gene, probably through impaired transcription. Underexpression of presenilins is known to increase cell vulnerability (Vito et al., 1996; Roperch et al., 1998; Ye and Fortini, 1999), and so could account for the discrepancy in results. It is also possible that neuronal sensitivity to degeneration may be influenced by differences in the PS-1 mutations, neuronal populations, or culture conditions. Consistent with our findings, another study failed to observe enhanced neuronal vulnerability for primary neurons expressing mutant presenilin (Bursztajn et al., 1998). Moreover, overexpression of mutant presenilins in the transgenic mouse does not result, in most cases, in overt abnormal cell death during brain development or maturation (Borchelt et al., 1996; Duff et al., 1996; Oyama et al., 1998; but seeChui et al., 1999). To evaluate the endangerment hypothesis critically, we used several apoptotic and necrotic insults and evaluated neuronal degeneration using both nuclear morphology and cysteine protease activation as endpoints. Our results indicate that FAD-linked mutant presenilins, when expressed at normal levels and under endogenous control mechanisms, do not invariably increase morphological or biochemical indices of neurodegeneration.
Despite the lack of neuronal endangerment observed in the present study, it remains to be determined whether FAD-linked mutations alter neuronal vulnerability in a manner relevant to the aging-dependent, regionally restricted neurodegeneration of AD. The findings reported here are for relatively immature cortical neurons maintained in primary culture for only 7 d, leaving open the possibility that a property of neuronal aging, which has not been identified yet, could promote sensitivity to mutant presenilins. Although our study examined Aβ1–42 and glutamate, toxic insults that have been implicated previously in the neurodegeneration of AD (Yankner, 1996; Olney et al., 1997; Mattson et al., 1998), presenilin mutations could sensitize neurons to other relevant stressors. In this regard, signaling through the Notch, β-catenin, c-jun, Akt/PKB, and unfolded protein response pathways are reportedly modified by mutant presenilins (Levitan et al., 1996; Zhang et al., 1998; Imafuku et al., 1999; Kang et al., 1999;Katayama et al., 1999; Nishimura et al., 1999; Weihl et al., 1999). The factors initiating neuronal degeneration in the AD brain are not known, and the roles played by survival and death signaling pathways potentially regulated by the presenilins will require further study. An identification of insults preferentially responsive to the PS-1 P264L knock-in mutation may provide clues to the processes that trigger neurodegeneration in AD.
The differential effect of the PS-1 P264L knock-in mutation on secretion of Aβ variants reported here for cortical neurons supports a model for APP processing and its regulation by presenilins that emerged from studies of other cell types. The PS-1 P264L mutation causes reciprocal changes in the secretion of Aβ42 and Aβ40 by cortical neurons (Table 2) and elevates the concentration only of Aβ42 in the mouse brain (Fig. 6). Ultrastructural and cell biological studies have demonstrated that the two Aβ variants are produced, at least in part, in distinct compartments within the secretory pathway, with Aβ42 generation preceding that of Aβ40 (Cook et al., 1997;Hartmann et al., 1997; Greenfield et al., 1999). The parallel increases in Aβ and its biosynthetic precursor, C99, caused by the Swedish double mutation in APP (Citron et al., 1992; Cai et al., 1993; Reaume et al., 1996), coupled with the elevation in C99 levels induced by γ-secretase inhibitors (Durkin et al., 1999; Zhang et al., 1999) indicate that C99 availability for γ-secretase processing limits Aβ formation. Reciprocal effects of the PS-1 P264L point mutation on Aβ42 and Aβ40 in cortical neurons could result, therefore, from the stepwise processing of a limiting quantity of C99 first to Aβ42, and subsequently to Aβ40. This model is consistent with mounting evidence that PS-1 is essential for γ-secretase processing and Aβ production (De Strooper et al., 1998), and may be the γ-secretase itself (Wolfe et al., 1999). Our findings do not distinguish between the possibilities that PS-1 mutations could increase Aβ42 directly by promoting selectively γ-secretase formation of this Aβ variant or indirectly by altering recruitment of C99 to an Aβ42-forming cellular compartment. Investigating these possibilities is complex using transfected cells overexpressing mutant PS-1, because such cells coexpress wild-type PS-1. Neurons bearing the homozygous P264L targeted mutation completely lack wild-type PS-1, and so should be a useful alternative experimental system for further delineating how presenilin mutations regulate Aβ production.
Introducing the PS-1 P264L knock-in mutation into APP695swe transgenic mice or APPswe KI gene-targeted mice leads not only to a large increase in brain levels of Aβ42, but also to a marked acceleration of the age-dependent onset of amyloid pathology, and shares a number of features with PS-1-linked FAD. The elevation in Aβ42 concentration in mouse brain precedes its deposition, making it likely that enhanced amyloidogenic APP processing is responsible for the acceleration of deposition. As is true for older APP695swe mice wild-type for PS-1 and the AD brain, many of the amyloid deposits in younger APP695swe/PS-1 P264L mice contain dense cores that are thioflavin-positive and surrounded by hypertrophic astroglia. Even in the older double mutant mice bearing a large amyloid burden, the deposits and reactive astrocytes are concentrated in brain regions that preferentially exhibit pathology in AD (Fig. 7; Flood et al., unpublished observations). This resembles PS-1-linked FAD, which is characterized by an accelerated age-of-onset and increased amyloidosis, but involves restricted, stereotypical brain regions and a duration of disease very similar to late-onset AD (Hardy, 1997; Lopera et al., 1997). Our observations of the PS-1 P264L knock-in mutation support the hypothesis that amyloid fibrillogenesis is a key pathogenic factor in the progression of AD, but are also consistent with reports that nonfibrillar oligomers of Aβ (Lambert et al., 1998; Hartley et al., 1999), and insoluble Aβ42 accumulating within vulnerable neurons (LaFerla et al., 1997; Skovronsky et al., 1998; Gouras et al., 2000) could trigger neurodegeneration. The rapid onset of amyloid pathology in the APP695swe/PS-1 P264L mouse line is accelerated even further by the presence of a second mutant PS-1 allele (Flood, Reaume, Dorfman, Lin, Lang, Trusko, Savage, Siman, and Scott, unpublished observations), which may facilitate studies directed at the localization, inhibition, and reversibility of Aβ aggregation and amyloid fibril formation, and their respective effects on the surrounding neuropil. The mouse models described here should be valuable for studying the evolution of AD-type neuropathologies and their cause-and-effect relationships, and may provide a clearer understanding of critical factors initiating the progressive cognitive and behavioral syndrome of AD.
Footnotes
This work was supported by a grant from the Neurosciences Education and Research Foundation (R.S.) and Cephalon. We thank Diane Lang and Karen Dorfman for excellent technical assistance, Dr. Jim Hirsch for performing the genotyping analyses, Ed McCabe, Renee Simmons, and the vivarium staff at Cephalon for maintaining the mouse colonies, and Dr. Randy Pittman for support and encouragement.
Correspondence should be addressed to Dr. Robert Siman, Department of Pharmacology, University of Pennsylvania School of Medicine, 3620 Hamilton Walk, Philadelphia, PA 19104-6084. E-mail:siman@pharm.med.upenn.edu.
Dr. Reaume's present address: Pfizer Central Research, Groton, CT.
REFERENCES
- 1.Banker G, Goslin K. Culturing nerve cells. MIT; Cambridge, MA: 1998. [Google Scholar]
- 2.Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitski T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Aβ1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 3.Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 4.Bursztajn S, DeSouza R, McPhie DL, Berman SA, Shioi J, Robakis NK, Neve RL. Overexpression in neurons of human presenilin-1 or a presenilin-1 familial Alzheimer disease mutant does not enhance apoptosis. J Neurosci. 1998;18:9790–9799. doi: 10.1523/JNEUROSCI.18-23-09790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai XD, Golde TE, Younkin SG. Release of excess amyloid β protein from a mutant amyloid β-protein precursor. Science. 1993;259:514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- 6.Campion D, Flaman J-M, Brice A, Hannequin D, Dubois B, Martin C, Moreau V, Charbonnier F, Didierjean O, Tardieu S, Penet C, Puel M, Pasquier F, Le Doze F, Bellis G, Calenda A, Heilig R, Martinez M, Mallet J, Bellis M, Clerget-Darpoux F, Agid Y, Frebourg T. Mutations of the presenilin 1 gene in families with early-onset Alzheimer's disease. Hum Mol Genet. 1995;4:2373–2377. doi: 10.1093/hmg/4.12.2373. [DOI] [PubMed] [Google Scholar]
- 7.Chui DH, Tanahashi H, Ozawa K, Ikeda S, Checler F, Ueda O, Suzuki H, Araki W, Inoue H, Shirotani K, Takahashi K, Gallyas F, Tabira T. Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat Med. 1999;5:560–564. doi: 10.1038/8438. [DOI] [PubMed] [Google Scholar]
- 8.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 9.Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George Hyslop P, Selkoe DJ. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- 10.Cook DG, Forman MS, Sung JC, Leight S, Kolson DL, Iwatsubo T, Lee VM-Y, Doms RW. Alzheimer's Aβ(1–42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat Med. 1997;3:1021–1024. doi: 10.1038/nm0997-1021. [DOI] [PubMed] [Google Scholar]
- 11.Czech C, Lesort M, Tremp G, Terro F, Blanchard V, Schombert B, Carpentier N, Dreisler S, Bonici B, Takashima A, Moussaoui S, Hugon J, Pradier L. Characterization of human presenilin 1 transgenic rats: increased sensitivity to apoptosis in primary neuronal cultures. Neuroscience. 1998;87:325–336. doi: 10.1016/s0306-4522(98)00162-6. [DOI] [PubMed] [Google Scholar]
- 12.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 13.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 14.Doan A, Thinakaran G, Borchelt DR, Slunt HH, Ratovitski T, Podlisny M, Selkoe DJ, Seeger M, Gandy SE, Price DL, Sisodia SS. Protein topology of presenilin 1. Neuron. 1996;17:1023–1030. doi: 10.1016/s0896-6273(00)80232-9. [DOI] [PubMed] [Google Scholar]
- 15.Dorfman KS, Reaume AG, Lang DM, Trusko SP, Flood DG, Siman R, Scott RW. Creation and characterization of two distinct types of mutations in PS-1 using gene targeting in mice. Soc Neurosci Abstr. 1998;24:472. [Google Scholar]
- 16.Duff K, Eckman C, Zehr C, Yu X, Prada C-M, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 17.Durkin JT, Murthy S, Husten EJ, Trusko SP, Savage MJ, Rotella DP, Greenberg BD, Siman R. Rank-order of potencies for inhibition of the secretion of Aβ40 and Aβ42 suggests that both are generated by a single γ-secretase. J Biol Chem. 1999;274:20499–20504. doi: 10.1074/jbc.274.29.20499. [DOI] [PubMed] [Google Scholar]
- 18.Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC. Evidence that Wallerian degeneration and axon degeneration induced by localized neurotrophin deprivation do not involve caspases. J Neurosci. 2000;20:1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- 20.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 21.Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Ab42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenfield JP, Tsai J, Gouras GK, Hai B, Thinakaran G, Checler F, Sisodia SS, Greengard P, Xu H. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer β-amyloid peptides. Proc Natl Acad Sci USA. 1999;96:742–747. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999a;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 24.Guo Q, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid β-peptide toxicity: central roles of superoxide production and caspase activation. J Neurochem. 1999b;72:1019–1029. doi: 10.1046/j.1471-4159.1999.0721019.x. [DOI] [PubMed] [Google Scholar]
- 25.Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 26.Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmann T, Bieger SC, Bruhl B, Tienari P, Ida N, Allsop D, Roberts GW, Masters CL, Dotti CG, Unsicker K, Beyreuther K. Distinct sites of intracellular production for Alzheimer's disease Aβ40/42 amyloid peptides. Nat Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- 28.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 29.Howland DS, Trusko SP, Savage MJ, Reaume AG, Lang DM, Hirsch JD, Maeda N, Siman R, Greenberg BD, Scott RW, Flood DG. Modulation of secreted β-amyloid precursor protein and amyloid β-peptide in brain by cholesterol. J Biol Chem. 1998;273:16576–16582. doi: 10.1074/jbc.273.26.16576. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole GM. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:177–178. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 31.Imafuku I, Masaki T, Waragai M, Takeuchi S, Kawabata M, Hirai S, Ohno S, Nee LE, Lippa CF, Kanazawa I, Imagawa M, Okazawa H. Presenilin 1 suppresses the function of c-jun homodimers via interaction with AM/Jif-1. J Cell Biol. 1999;147:121–134. doi: 10.1083/jcb.147.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, Leibowitz G, Levine F, Koo EH. Presenilin 1 facilitates the constitutive turnover of β-catenin: differential activity of Alzheimer's disease-linked PS1 mutants in the β-catenin-signaling pathway. J Neurosci. 1999;19:4229–4237. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, Hitomi J, Morihara T, Yoneda T, Gomi F, Mori Y, Nakano Y, Takeda J, Tsuda T, Itoyama Y, Murayama O, Takashima A, St Goerge-Hyslop P, Takeda M, Tohyama M. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol. 1999;1:479–485. doi: 10.1038/70265. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs DM, Fausett JH, Page KJ, Kim T-W, Moir RD, Merriam DE, Hollister RD, Hallmark OG, Mancini R, Felsenstein KM, Hyman BT, Tanzi RE, Wasco W. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med. 1996;2:224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- 35.LaFerla FM, Troncoso JC, Strickland DK, Kawas CH, Jay G. Neuronal cell death in Alzheimer's disease correlates with apoE uptake and intracellular Aβ stabilization. J Clin Invest. 1997;100:310–320. doi: 10.1172/JCI119536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lah JJ, Heilman CJ, Nash NR, Rees HD, Yi S, Counts SE, Levey AI. Light and electron microscopic localization of presenilin-1 in primate brain. J Neurosci. 1997;17:1971–1980. doi: 10.1523/JNEUROSCI.17-06-01971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Iosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusable, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levitan D, Doyle TG, Brousseau D, Lee MK, Thinakaran G, Slunt HH, Sisodia SS, Greenwald I. Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1996;93:14940–14944. doi: 10.1073/pnas.93.25.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopera F, Ardilla A, Martinez A, Madrigal L, Arango-Viana JC, Lemere CA, Arango-Lasprilla JC, Hincapie L, Arcos-Burgos M, Ossa JE, Behrens IM, Norton J, Lendon C, Goate AM, Ruiz-Linares A, Rosselli M, Kosik KS. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA. 1997;277:793–799. [PubMed] [Google Scholar]
- 40.Mattson MP, Guo Q, Furukawa K, Pedersen WA. Presenilins, the endoplasmic reticulum, and neuronal apoptosis in Alzheimer's disease. J Neurochem. 1998;70:1–14. doi: 10.1046/j.1471-4159.1998.70010001.x. [DOI] [PubMed] [Google Scholar]
- 41.McDevitt MA, Shivdasani RA, Fujiwara Y, Yang H, Orkin SH. A “knockdown” mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc Natl Acad Sci USA. 1997;94:6781–6785. doi: 10.1073/pnas.94.13.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murayama O, Tomita T, Nihonmatsu N, Murayama M, Sun X, Honda T, Iwatsubo T, Takashima A. Enhancement of amyloid β42 secretion by 28 different presenilin 1 mutations of familial Alzheimer's disease. Neurosci Lett. 1999;265:61–63. doi: 10.1016/s0304-3940(99)00187-1. [DOI] [PubMed] [Google Scholar]
- 43.Nakano Y, Kondoh G, Kudo T, Imaizumi K, Kato M, Miyazaki JI, Tohyama M, Takeda J, Takeda M. Accumulation of murine amyloid β42 in a gene-dosage-dependent manner in PS1 “knock-in” mice. Eur J Neurosci. 1999;11:2577–2581. doi: 10.1046/j.1460-9568.1999.00698.x. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura I, Uetsuki T, Dani SU, Ohsawa Y, Saito I, Okamura H, Uchiyama Y, Yoshikawa K. Degeneration in vivo of rat hippocampal neurons by wild-type Alzheimer amyloid precursor protein overexpressed by adenovirus-mediated gene transfer. J Neurosci. 1998;18:2387–2398. doi: 10.1523/JNEUROSCI.18-07-02387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimura M, Yu G, Levesque G, Zhang DM, Ruel L, Chem F, Milman P, Holmes E, Liang Y, Kawarai T, Jo E, Supala A, Rogaeva E, Xu DM, Janus C, Levesque L, Bi Q, Duthie M, Roxmahel R, Mattila K, Lannfelt L, Westaway D, Mount HTJ, Woodgett J, Fraser PE, St George-Hyslop P. Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of β-catenin, a component of the presenilin protein complex. Nat Med. 1999;5:164–169. doi: 10.1038/5526. [DOI] [PubMed] [Google Scholar]
- 46.Olney JW, Wozniak DF, Farber NB. Excitotoxic neurodegeneration in Alzheimer's disease. Arch Neurol. 1997;54:1234–1240. doi: 10.1001/archneur.1997.00550220042012. [DOI] [PubMed] [Google Scholar]
- 47.Oyama F, Sawamura N, Kobayashi K, Morishima-Kawashima M, Kuramochi T, Ito M, Tomita T, Maruyama K, Saido TC, Iwatsubo T, Capell A, Walter J, Grunberg J, Ueyama Y, Haass C, Ihara Y. Mutant presenilin 2 transgenic mouse: effect on an age-dependent increase of amyloid β-protein 42 in the brain. J Neurochem. 1998;71:313–322. doi: 10.1046/j.1471-4159.1998.71010313.x. [DOI] [PubMed] [Google Scholar]
- 48.Passer BJ, Pellegrini L, Vito P, Ganjei JK, D'Adamio L. Interaction of Alzheimer's presenilin-1 and presenilin-2 with Bcl-XL. J Biol Chem. 1999;274:24007–24013. doi: 10.1074/jbc.274.34.24007. [DOI] [PubMed] [Google Scholar]
- 49.Phinney AL, Deller T, Stalder M, Calhoun ME, Frotscher M, Sommer B, Staufenbiel M, Jucker M. Cerebral amyloid induces aberrant axonal sprouting and ectopic terminal formation in amyloid precursor protein transgenic mice. J Neurosci. 1999;19:8552–8559. doi: 10.1523/JNEUROSCI.19-19-08552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Price DL, Tanzi RE, Borchelt DR, Sisodia SS. Alzheimer's disease: genetic studies and transgenic models. Annu Rev Genet. 1998;32:461–493. doi: 10.1146/annurev.genet.32.1.461. [DOI] [PubMed] [Google Scholar]
- 51.Reaume AG, Howland DS, Savage MJ, Trusko SP, Lang DM, Greenberg BD, Siman R, Scott RW. Enhanced amyloidogenic processing of the β-amyloid precursor protein in gene-targeted mice bearing the Swedish Familial Alzheimer's Disease mutations and a “humanized” Aβ sequence. J Biol Chem. 1996;271:23380–23388. doi: 10.1074/jbc.271.38.23380. [DOI] [PubMed] [Google Scholar]
- 52.Roberts-Lewis JM, Savage MJ, Marcy V, Pinsker L, Siman R. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci. 1994;16:3934–3944. doi: 10.1523/JNEUROSCI.14-06-03934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roperch J-P, Alvaro V, Prieur S, Tuynder M, Nemani M, Lethrosne F, Piouffre L, Gendron M-C, Israeli D, Dausset J, Oren M, Amson R, Telerman A. Inhibition of presenilin 1 expression is promoted by p53 and p21WAF-1 and results in apoptosis and tumor suppression. Nat Med. 1998;4:835–838. doi: 10.1038/nm0798-835. [DOI] [PubMed] [Google Scholar]
- 54.Savage MJ, Trusko SP, Howland DS, Pinsker LR, Mistretta S, Reaume AG, Greenberg BD, Siman R, Scott RW. Turnover of mouse brain amyloid β-protein in vivo and acute reduction of its level by phorbol ester. J Neurosci. 1998;18:1743–1752. doi: 10.1523/JNEUROSCI.18-05-01743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Vittanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 56.Selkoe DJ. The cell biology of β-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 57.Siman R, Durkin JT, Husten EJ, Savage MJ, Murthy S, Mistretta S, Chatterjee S, Rotella DP, Dembofsky B, Poorman R, Greenberg BD. Genesis and degradation of Aβ protein by cultured human neuroblastoma cells. In: Iqbal K, Mortimer J, Winblad B, Wisniewski H, editors. Research advances in Alzheimer's disease and related disorders. Wiley; Sussex, UK: 1995. pp. 675–684. [Google Scholar]
- 58.Siman R, Noszek JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- 59.Siman R, Bozyczko-Coyne D, Savage MJ, Roberts-Lewis JM. The calcium-activated protease calpain I and ischemia-induced neurodegeneration. Adv Neurol. 1996;71:167–176. [PubMed] [Google Scholar]
- 60.Siman R, Bozyczko-Coyne D, Meyer SL, Bhat RV. Immunolocalization of caspase proteolysis in situ: evidence for widespread caspase-mediated apoptosis of neurons and glia in the postnatal rat brain. Neuroscience. 1999;92:1425–1442. doi: 10.1016/s0306-4522(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 61.Skovronsky DM, Doms RW, Lee VM. Detection of a novel intraneuronal pool of insoluble amyloid β protein that accumulates with time in culture. J Cell Biol. 1998;141:1031–1039. doi: 10.1083/jcb.141.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid β-protein secreted by familial amyloid β-protein precursor (β-APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 64.Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitski T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 65.Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 66.Uetsuki T, Takemoto K, Nishimura I, Okamoto M, Niinobe M, Momoi T, Miura M, Yoshikawa K. Activation of neuronal caspase-3 by intracellular accumulation of wild-type Alzheimer amyloid precursor protein. J Neurosci. 1999;19:6955–6964. doi: 10.1523/JNEUROSCI.19-16-06955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vito P, Wolozin B, Ganjei JK, Iwasaki K, Lacana E, D'Adamio L. Requirement of the familial Alzheimer's disease gene PS2 for apoptosis. Opposing effect of ALG-3. J Biol Chem. 1996;271:31025–31028. doi: 10.1074/jbc.271.49.31025. [DOI] [PubMed] [Google Scholar]
- 68.Wang KKW, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV, Glantz SB, Morrow JS. Simultaneous degradation of αII- and βII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem. 1998;273:22490–22497. doi: 10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- 69.Wang KKW. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- 70.Wasco W, Pettingell WP, Jondro PD, Schmidt SD, Gurubhagavatula S, Rodes L, DiBlasi T, Romano DM, Guienett SY, Kovacs DM, Growdon JH, Tanzi RE. Familial Alzheimer's chromosome 14 mutations. Nat Med. 1995;1:848. doi: 10.1038/nm0995-848a. [DOI] [PubMed] [Google Scholar]
- 71.Weihl CC, Ghadge GD, Kennedy SG, Hay N, Miller RJ, Roos RP. Mutant presenilin-1 induces apoptosis and down-regulates Akt/PKB. J Neurosci. 1999;19:5360–5369. doi: 10.1523/JNEUROSCI.19-13-05360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weil M, Jacobson MD, Coles HS, Davies TJ, Gardner RL, Raff KD, Raff MC. Constitutive expression of the machinery for programmed cell death. J Cell Biol. 1996;133:1053–1059. doi: 10.1083/jcb.133.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 74.Wolozin B, Iwasaki K, Vito P, Ganjei JK, Lacana E, Sunderland T, Zhao B, Kusiak JW, Wasco W, D'Adamio L. Participation of presenilin 2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutation. Science. 1996;274:1710–1713. doi: 10.1126/science.274.5293.1710. [DOI] [PubMed] [Google Scholar]
- 75.Yamatsuji T, Matsui T, Okamoto T, Domatsuzaki K, Takeda S, Fukumoto H, Iwatsubo T, Suzuki N, Asami-Odaka A, Ireland S, Kinane TB, Giambarella U, Nishimoto I. G protein-mediated neuronal DNA fragmentation induced by familial Alzheimer's disease-associated mutants of APP. Science. 1996;272:1349–1352. doi: 10.1126/science.272.5266.1349. [DOI] [PubMed] [Google Scholar]
- 76.Yankner BA. Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 77.Ye Y, Fortini ME. Apoptotic activities of wild-type and Alzheimer's disease-related mutant presenilins in Drosophila melanogaster. J Cell Biol. 1999;146:1351–1364. doi: 10.1083/jcb.146.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Z, Hartmann H, Do VM, Abranmowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, De Strooper B, He X, Yankner BA. Destabilization of β-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395:698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- 79.Zhang L, Song L, Parker EM. Calpain inhibitor I increases β-amyloid peptide production by inhibiting the degradation of the substrate of γ-secretase: evidence that substrate availability limits β-amyloid peptide production. J Biol Chem. 1999;274:8966–8972. doi: 10.1074/jbc.274.13.8966. [DOI] [PubMed] [Google Scholar]