Abstract
Modafinil is an increasingly popular wake-promoting drug used for the treatment of narcolepsy, but its precise mechanism of action is unknown. To determine potential pathways via which modafinil acts, we administered a range of doses of modafinil to rats, recorded sleep/wake activity, and studied the pattern of neuronal activation using Fos immunohistochemistry. To contrast modafinil-induced wakefulness with spontaneous wakefulness, we administered modafinil at midnight, during the normal waking period of rats. To determine the influence of circadian phase or ambient light, we also injected modafinil at noon on a normal light/dark cycle or in constant darkness. We found that 75 mg/kg modafinil increased Fos immunoreactivity in the tuberomammillary nucleus (TMN) and in orexin (hypocretin) neurons of the perifornical area, two cell groups implicated in the regulation of wakefulness. This low dose of modafinil also increased the number of Fos-immunoreactive (Fos-IR) neurons in the lateral subdivision of the central nucleus of the amygdala. Higher doses increased the number of Fos-IR neurons in the striatum and cingulate cortex. In contrast to previous studies, modafinil did not produce statistically significant increases in Fos expression in either the suprachiasmatic nucleus or the anterior hypothalamic area. These observations suggest that modafinil may promote waking via activation of TMN and orexin neurons, two regions implicated in the promotion of normal wakefulness. Selective pharmacological activation of these hypothalamic regions may represent a novel approach to inducing wakefulness.
Keywords: modafinil, tuberomammillary nucleus, lateral hypothalamic area, perifornical area, orexin, hypocretin, striatum, amygdala, suprachiasmatic nucleus, anterior hypothalamic area, Fos, dopamine, stimulant, narcolepsy
Modafinil has been used for the treatment of excessive sleepiness for over a decade, but its precise mechanism of action is unknown. Traditional stimulants such as amphetamine appear to promote wakefulness by facilitating neural transmission by catecholamines, particularly dopamine (Nishino and Mignot, 1997). Treatment of animals with inhibitors of catecholamine synthesis such as α-methyl-p-tyrosine (αMPT) substantially blocks the effects of amphetamine (Lin et al., 1992). In contrast, the wake-promoting effects of modafinil are not blocked by αMPT (Lin et al., 1992), suggesting that modafinil may promote wakefulness via novel mechanisms.
To determine potential neuronal targets for modafinil, Lin et al. (1996) treated cats with modafinil or amphetamine and studied the pattern of neuronal activation as indicated by the expression of Fos. The transcription factor Fos is expressed in physiologically activated brain regions after a variety of stimuli, thus serving as an indicator of neuronal activation (Sagar et al., 1988). In contrast to the Fos pattern induced by amphetamine, Lin found that a comparable wake-promoting dose of modafinil induced very little Fos in dopamine-responsive areas such as the striatum. However, modafinil strongly induced the expression of Fos in the anterior hypothalamic area (AHA) and the dorsolateral portion of the suprachiasmatic nuclei (SCN). Lin hypothesized that these regions play an important role in coordinating the activity of hypothalamic and basal forebrain regions that promote wakefulness. Subsequent work in rats treated with modafinil also demonstrated increased Fos immunoreactivity in the SCN and AHA (Engber et al., 1998a).
Although these previous studies provide many important observations, they are limited in several ways. Neither of these studies combined anatomic observations with sleep/wake recordings, thereby hindering correlations between patterns of Fos expression and behavior. Additionally, the researchers administered modafinil via gavage or acute intraperitoneal injection, techniques that could produce stress and confound the results. Finally, animals in these studies were treated with only a single dose of modafinil at approximately noon, but because sleep/wake behavior varies considerably over the day, the neural and behavioral responses to modafinil may also vary.
The purpose of our experiments was to define the pattern of neuronal activation induced by modafinil in rats. We administered modafinil via chronic intraperitoneal catheters at different doses and at different times of day in combination with physiological recordings of sleep/wake behavior. We then used immunohistochemistry to identify Fos-immunoreactive (Fos-IR) neurons. We also used double immunohistochemistry for Fos and orexin (also known as hypocretin), because we have found previously that modafinil increases Fos expression in orexin neurons of mice (Chemelli et al., 1999). We found that modafinil strongly induced Fos in neurons of the tuberomammillary nucleus (TMN) and in orexin neurons of the perifornical area (PFx), two cell groups implicated in the control of wakefulness.
MATERIALS AND METHODS
Experimental design
To gain insight into modafinil's wake-promoting mechanism, we performed three experiments. In all experiments, rats were killed 2 hr after treatment, and the brains were analyzed by the use of immunohistochemistry for Fos. In experiments 1 and 2, rats were instrumented for electroencephalogram (EEG) and electromyogram (EMG) recordings as well as telemetric measurement of body temperature. To avoid the stress of handling during administration of modafinil or vehicle, chronic catheters were placed in the peritoneal cavity for administration of modafinil or vehicle.
Experiment 1. To determine the pattern of neuronal activation induced by modafinil, we administered modafinil (75 or 150 mg/kg) or vehicle at midnight to contrast modafinil-induced wakefulness with spontaneous wakefulness.
Experiment 2. In contrast to previous studies, experiment 1 did not demonstrate an increase in SCN or AHA Fos expression with modafinil. Because these differences may have been caused by time-of-day effects, we administered modafinil (150 mg/kg) at noon as done in the previous studies. These experiments still did not show any changes in these regions, and we were concerned that morning light might induce Fos in the SCN, masking any drug-induced changes. Thus, additional groups of animals were maintained in constant darkness on the day of the experiment, and a wider range of doses (75, 150, and 300 mg/kg) was studied.
Experiment 3. Because experiments 1 and 2 did not demonstrate any modafinil-induced change in SCN or AHA Fos expression, we replicated the protocol of Engber et al. (1998a). Uninstrumented rats were handled daily and received direct intraperitoneal injections of saline at noon for 4 d. On the fifth day, they received modafinil (300 mg/kg) or vehicle at noon and were killed 2 hr later.
Animals and recording environment
Sixty-five male Sprague Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) weighing 270–330 gm were housed individually in a pathogen-free barrier facility in a room maintained at 21.5–22.5°C with lights on at 7 A.M. and off at 7 P.M. Rats had food and water available ad libitum. At least 3 d before each experiment, rats were placed into a light-tight, sound-attenuated recording chamber (Biocube; Hartford Systems) in an isolated room. Light intensity was 100–150 lux inside each cage during the light period and <0.5 lux during the dark period. The Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center and Harvard Medical School approved all procedures.
Animal surgery
Under chloral hydrate anesthesia (350 mg/kg, i.p.), the rats of experiments 1 and 2 were surgically implanted with four EEG screws (anteroposterior, +3, −4; lateral, +2, −2 from bregma) lightly contacting the dura and two EMG wires (Plastics One, Roanoke, VA) below the nuchal muscles. The leads were connected to a six-channel connector (Plastics One) that was affixed to the skull with dental acrylic. A telemetric temperature transmitter (TA10TA-F40; Data Sciences International, St. Paul, MN) was placed in the peritoneal cavity of all but five rats. To administer drugs without handling the rats, an 80 cm SILASTIC catheter (1 mm inner diameter; Baxter Scientific Products) was inserted into the peritoneal cavity, subcutaneously tunneled to the scalp, cemented in place with dental acrylic, and protected externally by a spring. This intraperitoneal catheter was filled with heparinized, pyrogen-free saline and flushed weekly and 3 d before the experiment. Animals recovered at least 14 d and then acclimated to recording cables for 3 d before the start of physiological recordings.
Drug administration
Modafinil (lot #PA 008; Cephalon, Inc., West Chester, PA) was suspended in a solution of 0.25% methylcellulose, pH 7.4 (Dow Chemical, Inc., Midland, MI), in 0.9% pyrogen-free saline. The drug was administered in a volume of 2.0 ml/kg at doses of 75, 150, or 300 mg/kg. Control animals received an equal volume of methylcellulose vehicle. Catheters were then flushed with 1 ml of 0.9% saline to ensure delivery of drug into the peritoneal cavity. A dim, red flashlight (8 lux at 25 cm) was used during injections performed in the dark.
Histology and immunohistochemistry
Two hours after drug injections, animals were deeply anesthetized with chloral hydrate (600 mg/kg, i.p.) and transcardially perfused with 100 ml of 0.9% saline followed by 500 ml of phosphate-buffered 10% formalin, pH 7.0 (Sigma, St. Louis, MO). Brains were removed, post-fixed for 4 hr in formalin, and then allowed to equilibrate in 20% sucrose in 0.1 m PBS with 0.02% sodium azide (Sigma) at 4°C. Brains were sectioned (1:5 series; 30 μm) on a freezing microtome and stored in PBS-azide at 4°C. One series from each brain was stained for Fos by the use of previously described methods (Elmquist et al., 1996). Briefly, sections were incubated for 48 hr at 4°C in anti-Fos rabbit polyclonal antiserum (Ab-5; Oncogene Research Products; 1:100,000 dilution), 3% donkey serum (Jackson ImmunoResearch, West Grove, PA), and PBS-azide with 0.25% Triton X-100 (PBT-Az). Tissue was then rinsed in PBS, incubated in biotinylated donkey anti-rabbit IgG (1:1000; Jackson ImmunoResearch) for 1 hr at room temperature, and incubated with peroxidase-conjugated avidin–biotin complex (ABC; Vector Laboratories, Burlingame, CA) for 1 hr, followed by 0.05% diaminobenzidine tetrahydrochloride (DAB) and 0.01% H2O2 with 1% NiSO4 and 0.5% CoCl2, to produce a black reaction product in cell nuclei.
Double staining for Fos and orexin was performed on the tissue of experiment 1 by the use of an antiserum directed against orexin-A (M.Y., University of Texas Southwestern Medical Center at Dallas). After the Fos staining, sections were rinsed in PBS-azide and incubated in rabbit anti-orexin antiserum (1:5000) with 3% normal donkey serum and PBT-Az overnight at room temperature. Sections were then incubated in donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch) for 1 hr at room temperature, and the ABC and DAB steps were followed as above with the omission of the NiSO4 and CoCl2 in the DAB step to yield a brown cytoplasmic product in orexin-IR neurons. All tissue was mounted on gelatin-coated slides, dehydrated in ascending concentrations of ethanol, dilipidated in xylenes, and coverslipped. The orexin immunostaining was blocked by preadsorption with 50 μg/ml orexin-A, and omission of the primary antisera resulted in no specific staining.
Physiological recordings
Intraperitoneal temperature and EEG and EMG signals were recorded for at least 30 hr before and 2 hr after drug or vehicle injections. EEG and EMG signals were amplified using Grass model 12 amplifiers and digitally acquired using ICELUS software (Mark Opp; University Texas at Galveston). EEG signals were amplified 5000×, bandpass filtered at 0.3–30 Hz, and sampled at 128 Hz. EMG signals were amplified 5000×, bandpass filtered at 10–100 Hz, sampled at 128 Hz, and integrated into 1 sec bins. Arousal states were scored in 12 sec epochs by a single examiner blinded to the treatment group of the animals. Intraperitoneal temperature was monitored every 5 min using Dataquest software (Data Sciences International) and integrated into 30 min bins.
Cell counts
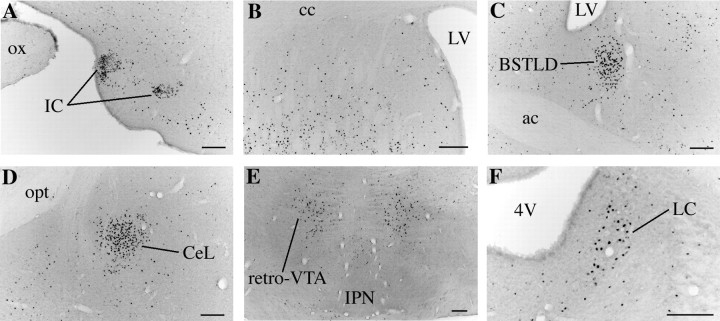
The pattern of Fos immunoreactivity was examined throughout the entire brain, and regions in which modafinil-treated rats appeared to differ from controls were identified. To quantify these differences, Fos-IR neurons were counted in regions with possible modafinil-induced Fos by an examiner blinded to experimental conditions. For all nuclei, bilateral counts were taken on three consecutive sections, 120 μm apart, that contained the largest nuclear areas, and these six counts were averaged. In experiment 1, Fos-IR nuclei were counted in regions implicated in behavioral state control: the ventrolateral preoptic area (VLPO), SCN, AHA, PFx, TMN, ventral tegmental area, dorsal raphe nucleus, and locus coeruleus (LC). Traditional stimulants often increase sympathetic activity, and therefore Fos-IR nuclei were also counted in putative autonomic regulatory regions: the paraventricular nucleus of the hypothalamus, laterodorsal subnucleus of the bed nucleus of the stria terminalis (BSTLD), the lateral subdivision of the central nucleus of the amygdala (CeL), the central and external lateral subnuclei of the parabrachial nucleus, and the nucleus of the solitary tract. Counts were also performed in other areas of interest: the cingulate cortex and a region just posterior to the ventral tegmental area referred to as the retro-ventral tegmental area. In most of these regions, counts were performed within the region demarcated by the borders of the nucleus as seen in dark field. Fos-IR nuclei were counted in the VLPO and several other regions without clear borders by the use of predetermined counting boxes as described previously (Scammell et al., 1998). AHA nuclei were counted within a vertically oriented 0.5 × 0.8 mm box with the medial edge along the ventricular wall and the ventral edge bounded by the optic chiasm; these counts began at the most caudal section that included the SCN, but the SCN was excluded from AHA counts. In the cingulate cortex, labeled nuclei were counted within a horizontally oriented 1 × 0.5 mm box with the ventral edge along the corpus callosum and the medial edge at the pial surface of the cortex at the level of the decussation of the anterior commissure. In the same sections, striatal Fos-IR nuclei were counted within a 0.5 × 0.5 mm box with the dorsomedial corner of the box 0.5 mm below and 0.5 mm lateral to the dorsomedial corner of the striatum. PFx Fos-IR nuclei, orexin-IR neurons, and double-labeled neurons were counted in sections beginning 360 μm caudal to the paraventricular nucleus of the hypothalamus using a horizontally oriented 1 × 0.4 mm box centered just dorsal to the fornix. Fewer regions were counted in experiments 2 and 3.
Statistical analysis
Mann–Whitney rank-sum tests with a Bonferroni correction were used to compare physiological variables between the modafinil and vehicle groups during the 2 hr after injection; Kruskal–Wallis tests were used for comparisons of three or more groups. Data were analyzed in 1 hr intervals, and p was considered significant if <0.025 because two time points were studied. These same tests were used for comparisons of Fos-IR cell counts; p was considered significant if <0.05. In experiments 1 and 2 in which multiple doses of modafinil were tested, a post hoc Scheffe test was used to identify significant differences from vehicle with p< 0.05 considered significant. Counts of Fos-IR nuclei were not corrected for double-counting errors (Guillery and Herrup, 1997) because there was no change in sizes of labeled nuclei across groups and only relative, not absolute, values were sought.
RESULTS
Experiment 1: injections of modafinil at midnight
In experiment 1, we administered modafinil (75 or 150 mg/kg) or vehicle during the rat's normal waking period at midnight to contrast spontaneous wakefulness with modafinil-induced wakefulness.
Behavioral effects of modafinil
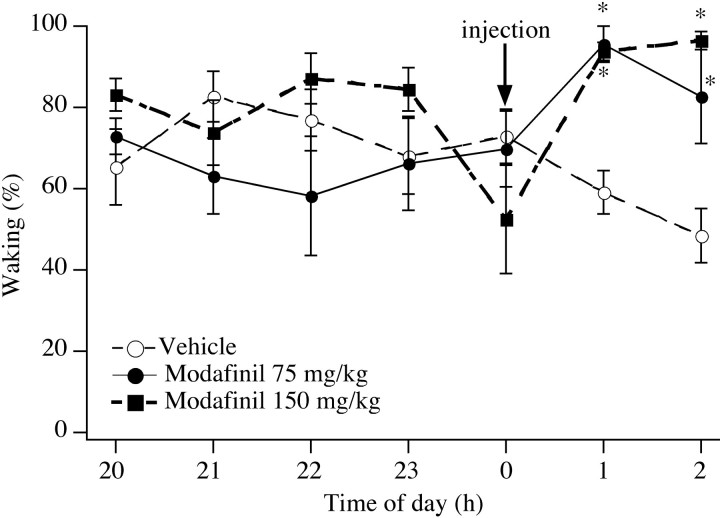
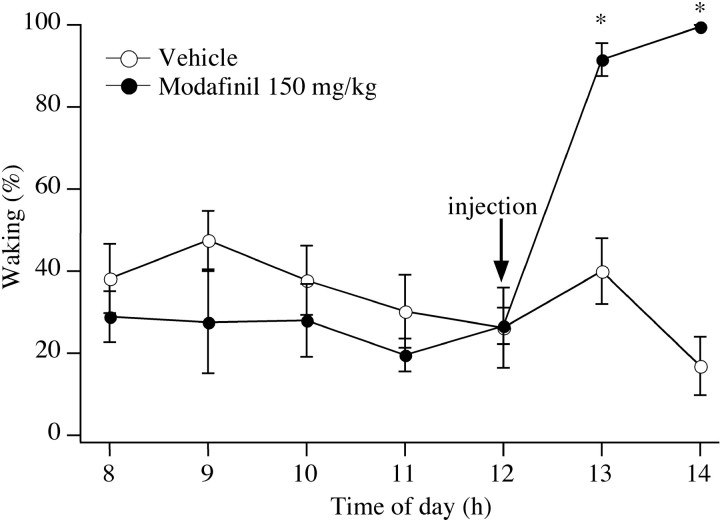
During the baseline night, no differences in the amounts of wake, nonrapid eye movement (NREM), or REM sleep were evident across the three groups. After injection of vehicle on the night of the experiment, control rats continued to have the same amount of each behavioral stage that they had on the previous night (Fig.1). However, rats treated with either 75 or 150 mg/kg modafinil spent >95% of the first postinjection hour awake (p < 0.0001 for either dose). Low-dose modafinil appeared to promote waking less effectively over time; during the second hour after injection, 75 mg/kg modafinil increased waking to 80% (p = 0.02), whereas the 150 mg/kg dose increased waking to 96% (p < 0.001). Neither dose of modafinil significantly affected body temperature.
Fig. 1.
Effects of modafinil on rats treated at midnight. Modafinil produces clear increases in waking, whereas rats treated with vehicle have no increase in waking. *p < 0.001.
The pattern of neuronal activation induced by modafinil
Treatment with modafinil consistently increased Fos expression in many brain regions, with 75 mg/kg modafinil inducing Fos in fewer neurons compared with 150 mg/kg (Table1).
Table 1.
Cell counts for rats treated with modafinil or vehicle at midnight
| Vehicle | Modafinil (75 mg/kg) | Modafinil (150 mg/kg) | Kruskal–Wallis p value | |
|---|---|---|---|---|
| n | 6 | 5 | 6 | — |
| Cingulate cortex | 52 ± 13 | 156 ± 22 | 191 ± 38* | 0.008 |
| Accumbens nucleus, core | 15 ± 4 | 24 ± 6 | 23 ± 3 | NS |
| Accumbens nucleus, shell | 55 ± 13 | 80 ± 12 | 65 ± 7 | NS |
| BSTLD | 15 ± 3 | 53 ± 8* | 62 ± 4* | 0.003 |
| CeL | 15 ± 4 | 80 ± 17* | 103 ± 11* | 0.004 |
| Striatum | 4 ± 1 | 37 ± 12 | 114 ± 23* | 0.002 |
| VLPO | 29 ± 4 | 30 ± 5 | 19 ± 5 | NS |
| SCN | 40 ± 5 | 36 ± 8 | 40 ± 5 | NS |
| AHA | 120 ± 17 | 100 ± 12 | 132 ± 11 | NS |
| PVH | 66 ± 11 | 98 ± 44 | 157 ± 41 | NS |
| TMN | 13 ± 7 | 58 ± 6* | 46 ± 4* | 0.005 |
| Ventral tegmental area | 8 ± 2 | 10 ± 1 | 12 ± 3 | NS |
| Retro-ventral tegmental area | 12 ± 2 | 44 ± 11 | 92 ± 11* | 0.004 |
| Dorsal raphe nucleus | 11 ± 3 | 18 ± 3 | 18 ± 5 | NS |
| Locus coeruleus | 4 ± 1 | 8 ± 2 | 21 ± 4* | 0.006 |
| PBel | 29 ± 3 | 40 ± 10 | 79 ± 18 | NS |
| PBcl | 29 ± 4 | 28 ± 10 | 38 ± 7 | NS |
| Nucleus of the solitary tract | 53 ± 12 | 43 ± 5 | 92 ± 10 | NS |
| PFx | ||||
| Orexin-IR neurons | 47 ± 5 | 53 ± 4 | 57 ± 4 | NS |
| Fos-IR neurons | 21 ± 2 | 39 ± 5 | 54 ± 7* | 0.003 |
| Fos-IR, orexin-IR neurons | 7 ± 2 | 23 ± 4* | 28 ± 2* | 0.003 |
| Fos-IR, non-orexin-IR neurons | 14 ± 1 | 16 ± 2 | 26 ± 7 | NS |
Values are means ± SEs.
Statistically significant from vehicle by the use of apost hoc Scheffe test.
PBel and PBcl, External lateral and central lateral subnuclei of the parabrachial nucleus; PVH, paraventricular nucleus of the hypothalamus.
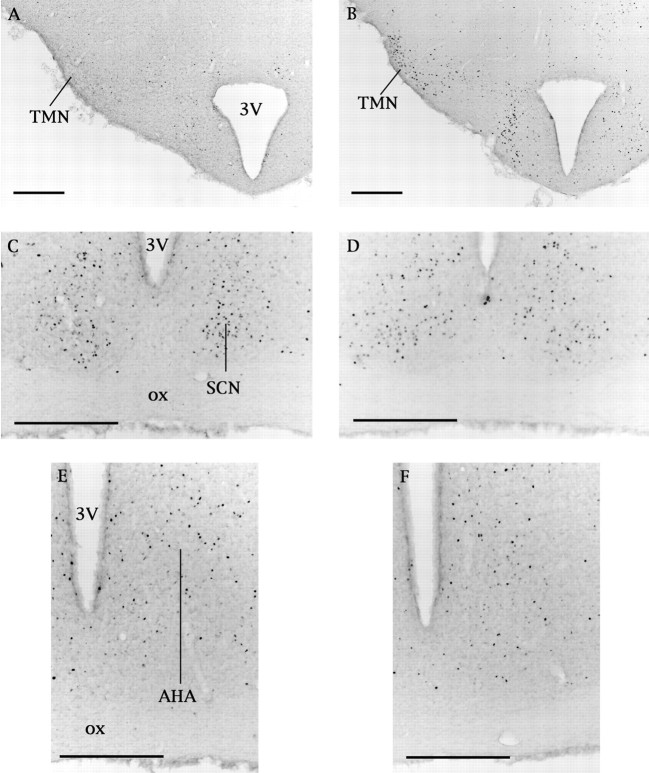
Some of the most striking changes in Fos expression occurred within specific hypothalamic areas implicated in the control of wakefulness (Figs. 2,3). After either dose of modafinil, the TMN had four times as many Fos-IR neurons as seen in the controls (Fig.3). Neurons of the VLPO are active during sleep (Sherin et al., 1996;Szymusiak et al., 1998), and because all animals were mainly awake, it was not surprising that Fos-IR VLPO neurons were uncommon in all rats. No changes in Fos expression were evident in the SCN or AHA (Fig.3).
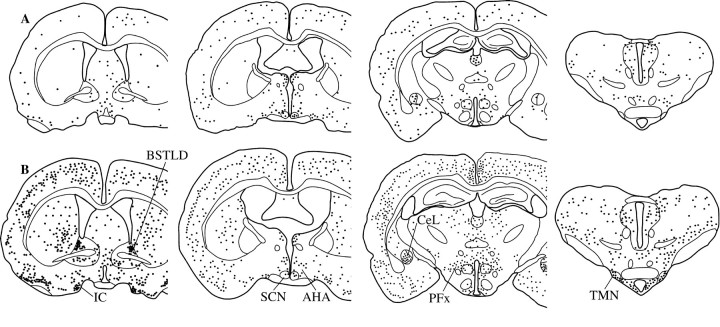
Fig. 2.
A series of line drawings illustrating the distribution of Fos-IR neurons in individual rats after administration of vehicle (A) or modafinil (75 mg/kg;B) at midnight. Modafinil-treated rats have substantial increases in Fos-IR neurons in hypothalamic arousal regions such as theTMN and PFx, as well as in theBSTLD and the CeL. Increased numbers of Fos-IR neurons can also be seen in the cortex, islands of Calleja (IC), and striatum, but not in the SCN orAHA.
Fig. 3.
Effects of vehicle (A, C, E) or modafinil (B, D, F) on Fos expression in putative sleep/wake regulatory regions. Administration of modafinil (150 mg/kg) at midnight substantially increases Fos expression in the TMN, but little change is evident in the SCN or AHA. Scale bars, 100 μm.3V, Third ventricle; ox, optic chiasm.
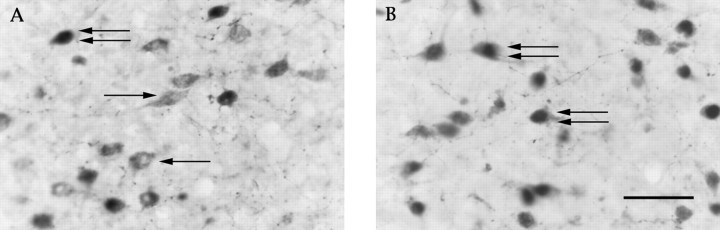
In the PFx at the tuberal level of the hypothalamus, modafinil-treated rats had twice as many Fos-IR neurons as controls. To determine whether this Fos expression occurred in orexin neurons, we used double immunohistochemistry for Fos and orexin. The number of PFx orexin-IR neurons with Fos-IR nuclei was increased threefold by modafinil (Fig.4). Modafinil slightly increased the number of nonorexin-IR, Fos-IR neurons (total PFx Fos-IR neurons minus double-labeled neurons), but this change did not reach statistical significance. The number of orexin-IR neurons did not differ among groups.
Fig. 4.
Few orexin-IR neurons contain Fos in rats treated with vehicle at midnight (A), but Fos-IR nuclei are common in orexin-IR neurons of rats treated with modafinil (150 mg/kg; B). Single arrow, Orexin-IR neuron; double arrows, Fos-IR and orexin-IR neuron. Scale bar, 30 μm.
In other hypothalamic areas, modafinil produced a moderate increase in Fos expression within the paraventricular nucleus, but only small increases were seen in the ventromedial preoptic nucleus, median preoptic nucleus, supraoptic nucleus, and dorsomedial nucleus. No consistent changes were evident in the arcuate nucleus, ventromedial nucleus, or supramammillary region.
Modafinil-treated rats had more Fos immunoreactivity in the cortex than did the controls. This Fos induction was evident across much of the cortex but was often more pronounced in cingulate and pyriform cortex with moderate amounts in frontal and parietal cortex; the spontaneously awake control animals usually had a more diffuse and less intense pattern of cortical Fos. Fos-IR neurons were always rare in the hippocampus.
Fos-IR neurons were uncommon in the basal ganglia of vehicle-treated rats but common in modafinil-treated rats, especially in the caudate and putamen (Fig. 5). Few Fos-IR neurons were present in the ventral striatum, and none were seen in the globus pallidus. Modafinil induced small but statistically insignificant increases in Fos expression in the shell and core of the accumbens nucleus. The olfactory tubercle of modafinil-treated rats had striking increases in the number of Fos-IR neurons in the islands of Calleja. No change in Fos expression was evident in the basal forebrain.
Fig. 5.
Photomicrographs of several regions with notable increases in Fos-IR neurons after treatment with modafinil (150 mg/kg) at midnight. A, The IC have marked increases in the number of Fos-IR cells, but Fos expression is considerably less in other parts of the olfactory tubercle.B, The striatum has large increases in Fos expression.C, D, The BSTLD (C) and CeL (D) also show intense Fos expression that is not seen in other parts of the extended amygdala.E, F, Modafinil also induces Fos immunoreactivity at the mesopontine junction in the retro-ventral tegmental area (retro-VTA; E) and in theLC (F). Scale bars, 100 μm.ac, Anterior commissure; cc, corpus callosum; IPN, interpeduncular nucleus;LV, lateral ventricle; opt, optic tract;4V, fourth ventricle.
In the thalamus, Fos-IR neurons were common in the paraventricular nucleus of all animals with small increases in modafinil-treated rats. Low-to-moderate numbers of Fos-IR cells were present in other midline thalamic nuclei regardless of treatment. No consistent changes were evident in the ventromedial or reticular nuclei. The lateral habenular nuclei contained low levels of Fos expression in all rats, with small increases in modafinil-treated animals.
Modafinil induced striking increases in the number of Fos-IR neurons in the extended amygdala, particularly the BSTLD and the CeL. Modafinil produced approximately a fourfold increase in Fos immunoreactivity in the BSTLD and sixfold increases in the CeL. Subtle increases were apparent in the ventral part of the bed nucleus of the stria terminalis and in the medial subdivision of the central nucleus of the amygdala with little change in other regions.
In the brainstem, the dorsal raphe nucleus and ventral tegmental area contained only rare Fos-IR neurons with no significant differences after treatment with modafinil. No qualitative differences were evident in the pedunculopontine or laterodorsal tegmental nuclei. However, modafinil consistently produced large increases in Fos expression in a region that we refer to as the retro-ventral tegmental area (Fig. 5); these Fos-IR neurons were clustered dorsolateral to the most caudal portion of the interpeduncular nucleus, along the lateral margin of the caudal linear and median raphe nuclei. The 150 mg/kg dose of modafinil produced consistent increases in Fos expression in the LC (Fig. 5). Fos-IR neurons were abundant among the transverse fibers of the pons in all groups. Modafinil-treated rats had a small increase in Fos-IR neurons within the caudal portion of the ventrolateral periaqueductal gray. In the parabrachial nucleus, modafinil tended to increase Fos expression in the external lateral subnucleus. After 150 mg/kg modafinil, the nucleus of the solitary tract had a slight increase in the number of Fos-IR neurons mainly in the medial subnuclei. No changes were evident in the ventrolateral medulla.
Experiment 2: injections of modafinil at noon in light/dark or constant dark conditions
In animals maintained on a normal light/dark (LD) cycle, administration of modafinil (150 mg/kg) at noon markedly increased wakefulness over the next 2 hr (Fig. 6), whereas vehicle had no effect compared with the baseline day. Baseline recordings over the previous day revealed no differences between the groups in the amounts of wake, NREM, or REM sleep. Neither modafinil nor vehicle had any effect on body temperature.
Fig. 6.
Effects of modafinil on rats treated at noon on a 12:12 hr LD cycle. Modafinil markedly increases waking, whereas vehicle has no effect. *p < 0.01.
The pattern of Fos immunoreactivity was very similar to that seen in experiment 1. In brief, modafinil-treated rats had clear increases in the number of Fos-IR neurons in the TMN, with decreases in the VLPO (Table 2). Brain sections were not double stained for Fos and orexin, but the total number of Fos-IR PFx neurons had a small but statistically insignificant increase. Modafinil treatment increased expression of Fos in frontal and cingulate cortex, the striatum, the CeL, and the BSTLD. The numbers of Fos-IR SCN and AHA neurons were slightly increased but did not reach statistical significance.
Table 2.
Cell counts for rats treated with modafinil or vehicle at noon under LD or constant dark conditions
| LD | Constant dark | |||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | Modafinil (150 mg/kg) | Mann–Whitney pvalue | Vehicle | Modafinil (75 mg/kg) | Modafinil (150 mg/kg) | Modafinil (300 mg/kg) | Kruskal–Wallis pvalue | |
| n | 6 | 7 | 7 | 6 | 6 | 4 | ||
| VLPO | 52 ± 7 | 26 ± 3* | 0.007 | 44 ± 5 | 20 ± 5* | 24 ± 4* | 21 ± 3* | 0.007 |
| SCN | 56 ± 19 | 89 ± 23 | NS | 11 ± 3 | 17 ± 11 | 28 ± 7 | 6 ± 2 | NS |
| AHA | 84 ± 15 | 103 ± 6 | NS | 75 ± 10 | 51 ± 5 | 72 ± 15 | 67 ± 8 | NS |
| PFx | 45 ± 9 | 72 ± 10 | NS | 33 ± 7 | 39 ± 7 | 52 ± 9 | 58 ± 14 | NS |
| TMN | 8 ± 4 | 43 ± 5* | 0.003 | 3 ± 1 | 20 ± 8 | 18 ± 7 | 22 ± 5 | 0.04 |
| BSTLD | 20 ± 4 | 74 ± 9* | 0.003 | 19 ± 3 | 45 ± 5* | 62 ± 9* | 65 ± 9* | 0.008 |
| CeL | 42 ± 13 | 133 ± 12* | 0.008 | 28 ± 6 | 74 ± 15 | 149 ± 25* | 142 ± 18* | 0.007 |
| Striatum | 1 ± 0 | 116 ± 19* | 0.003 | 1 ± 0 | 22 ± 13 | 94 ± 24* | 102 ± 21* | 0.002 |
Statistically different from vehicle by the use of apost hoc Scheffe test.
To examine the extent to which this pattern might be influenced by morning light exposure, additional animals were kept in darkness on the day of the experiment and treated at noon. These rats also were treated with lower (75 mg/kg) and higher (300 mg/kg) doses of modafinil to explore dose–response effects on Fos expression.
In comparison with the previous baseline day during which the animals were on a regular LD cycle, rats housed in constant darkness had no change in the hourly amounts of waking. However, this dark exposure moderately increased REM sleep across the morning to 15% from an average baseline of 8.5% (p < 0.01 for each hour between 8 A.M. and noon) as has been described previously (Lisk and Sawyer, 1966; Benca et al., 1991). NREM sleep during this morning period was mildly reduced to an average of 56.7% from 65.3% on the baseline day with statistically significant reductions only during the 8–9 A.M. and 9–10 A.M. periods (p < 0.005).
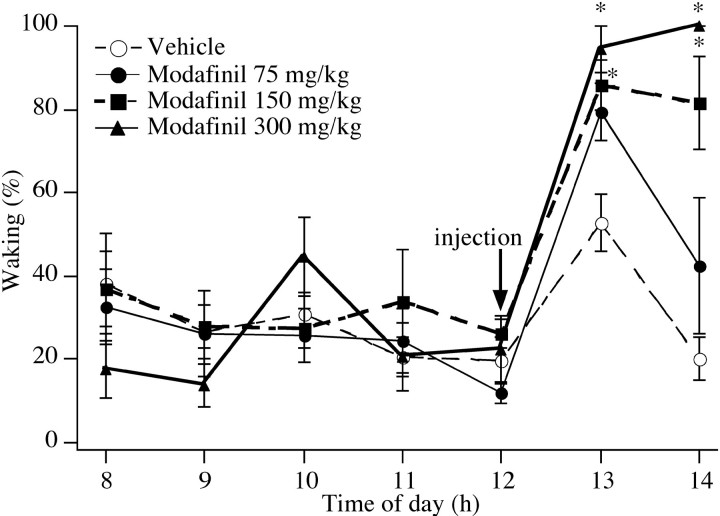
Injections of modafinil produced dose-dependent increases in wakefulness (Fig. 7). During the first and second hours after injection, 150 or 300 mg/kg modafinil significantly increased waking (p < 0.01), but the wake-promoting effect of the 75 mg/kg dose was marginal during the first hour (p = 0.06) and not evident during the second hour (p = 0.55). None of the three doses produced a significant change in body temperature.
Fig. 7.
Effects of modafinil on rats housed in constant dark and treated at noon. Rats treated with vehicle have a small and transient increase in waking, but those treated with modafinil have a dose-dependent increase in waking. *p < 0.01.
In most respects, the pattern of Fos immunoreactivity induced by injections during the dark was similar to that described above. The modafinil-treated groups had more Fos-IR neurons in the TMN and fewer in the VLPO (Table 2). The PFx had a small but statistically insignificant increase in Fos expression. Constant darkness reduced the number of Fos-IR neurons in the SCN, but modafinil still did not produce a statistically significant increase in the number of Fos-IR neurons in either the SCN or AHA. The modafinil-treated rats had a marked, dose-dependent increase in Fos-IR neurons in the striatum, CeL, and BSTLD.
Experiment 3: injections of modafinil at noon in uninstrumented animals
To ensure that the pattern of Fos immunoreactivity was not influenced by the instrumentation or tethering of animals for physiological recordings, we followed the experimental protocol used byEngber et al. (1998a). As found in our other experiments, modafinil substantially increased Fos expression in the TMN and decreased Fos in the VLPO (Table 3). Fos expression was increased in the striatum, CeL, and BSTLD. Modafinil also produced a modest, statistically insignificant increase in PFx and SCN Fos expression, but there was no change in the AHA.
Table 3.
Cell counts for uninstrumented rats treated with modafinil or vehicle at noon
| Vehicle | Modafinil (300 mg/kg) | Mann–Whitney p value | |
|---|---|---|---|
| n | 6 | 6 | |
| VLPO | 31 ± 3 | 16 ± 2 | 0.004 |
| SCN | 24 ± 4 | 68 ± 21 | NS |
| AHA | 84 ± 10 | 79 ± 10 | NS |
| PFx | 79 ± 9 | 120 ± 20 | NS |
| TMN | 2 ± 1 | 34 ± 2 | 0.006 |
| BSTLD | 13 ± 3 | 50 ± 3 | 0.004 |
| CeL | 22 ± 6 | 89 ± 18 | 0.02 |
| Striatum | 2 ± 1 | 61 ± 12 | 0.004 |
DISCUSSION
Across a variety of conditions, we found that modafinil promoted wakefulness and significantly increased Fos expression in the TMN and in orexin neurons of the PFx, two areas implicated in the regulation of wakefulness. We also found that higher doses of modafinil induced Fos immunoreactivity in neurons of the striatum and several other regions. In contrast to previous studies, we did not detect any statistically significant changes in the number of Fos-IR neurons in the SCN or AHA.
Technical considerations
Our experiments demonstrate a pattern of Fos immunoreactivity that differs from that described previously in cats (Lin et al., 1996) or rats (Engber et al., 1998a), and some technical aspects of our study deserve comment. Although modafinil sometimes produced small increases in Fos within the SCN and AHA, this increase was variable and never close to the 20-fold increase reported by Lin. We were initially concerned that this might be caused by differences in the treatment of our animals, but we were unable to detect any substantial changes in these regions despite administration of modafinil at different times of day, under light or darkness. Because exposure to dim light during nighttime injections, instrumentation, or tethering might have influenced our results, we replicated the methods of Engber and colleagues in experiment 3, but we still saw no significant change in the SCN or AHA. Engber described modafinil-induced Fos expression within the SCN of rats, but their qualitative analysis of a small number of rats may not be as reliable as our quantitative cell counts in larger groups of rats. Direct comparison of our results with the cat studies of Lin and colleagues is difficult because species differences may exist and they did not describe their cell-counting technique. The AHA lacks distinct borders, and our AHA counting box may not have been homologous to the region described by Lin. Alternatively, if modafinil induces Fos only in a subregion of the SCN or AHA, an increase could be obscured by dilution with counts from the entire nucleus. Still, we found no increase in Fos in any preoptic or anterior hypothalamic regions. Finally, these previous studies used a different Fos antiserum (Ab-2), and subtle differences in antibody specificity might contribute to dissimilar results. These differences in staining and counting techniques may explain some of the seeming discrepancies between studies.
Modafinil-induced neuronal activation
We found that even low doses of modafinil strongly increased Fos expression in TMN and orexin neurons, and activation of these neurons may be an essential component of modafinil's wake-promoting mechanism. These cell groups are anatomically well positioned to facilitate wakefulness with ascending projections to the cortex, basal forebrain, and midline thalamus and descending projections to other arousal-promoting regions such as the dorsal raphe nucleus, LC, and pontine cholinergic regions (Inagaki et al., 1988; Peyron et al., 1998;Chemelli et al., 1999). Both the TMN and PFx regions contain wake-active neurons as demonstrated with extracellular recordings (Vanni-Mercier et al., 1984; Steininger et al., 1996), and Fos immunoreactivity is common in orexin and TMN neurons during spontaneous wakefulness (Scammell et al., 1998) (I. Estabrooke, M. McCarthy, E. Ko, T. Chou, R. Chemelli, M. Yanagisawa, C. Saper, and T. Scammell, unpublished observations). The TMN is the sole neuronal source of histamine in brain, and these cells are probably necessary for promoting wakefulness because antihistamines or inhibitors of histamine synthesis decrease wakefulness (Lin et al., 1988, 1994). Orexin may play a related role in behavioral state control because orexin knock-out mice or dogs with mutations in the orexin type-2 receptor gene have a narcolepsy-like phenotype with poor maintenance of wakefulness (Chemelli et al., 1999; Lin et al., 1999). Orexin increases the firing rate of LC neurons (Hagan et al., 1999) and may have similar excitatory effects on other arousal regions including the TMN (K. Eriksson, personal communication). Thus, the combined activity of orexin and TMN neurons may play a key role in generating wakefulness and the response to modafinil.
Modafinil generally did not induce Fos within brainstem arousal regions such as the dorsal raphe nucleus or ventral tegmental area, although a small increase was evident in the LC after 150 mg/kg modafinil. Slightly lower doses of modafinil do not alter the firing rates of LC neurons (Akaoka et al., 1991), and this LC activation may only play a role with higher doses. Still, because the absence of Fos does not exclude activation of specific neurons, we cannot determine whether these brainstem arousal regions contribute to modafinil's wake-promoting effects.
Increased activity of TMN neurons should inhibit preoptic sleep-active neurons. The VLPO contains sleep-promoting neurons (Lu et al., 2000), and microinjection of histamine into this region decreases sleep (Lin et al., 1994). In agreement with this model, we found that during the day, modafinil often decreased the activity of VLPO neurons. This decrease was not evident among rats treated at midnight when rats from all groups were mainly awake; most likely Fos immunohistochemistry is unable to resolve differences in low VLPO activity in predominantly awake rats.
These experiments demonstrate modafinil-induced activation of wake-promoting regions and inhibition of a sleep-promoting region, but Fos immunohistochemistry can only provide correlative observations; we cannot determine whether this activation of TMN and orexin neurons helps promote wakefulness or is simply a consequence of sustained wakefulness. Further experiments to study the effects of modafinil in orexin knock-out mice or TMN-lesioned animals are needed to test the necessity of these regions.
Modafinil increases CeL metabolic activity as measured with the 2-deoxyglucose technique (Engber et al., 1998b), and we found that low doses of modafinil strongly increased Fos expression in the CeL and BSTLD. These two regions have very similar cytoarchitecture, neurotransmitters, afferents, and efferents (Moga et al., 1989; Alheid et al., 1995), so it is not surprising that they are jointly activated. The CeL projects to the LHA, dorsal raphe nucleus, and distal dendrites of the LC (Alheid et al., 1995) and may influence the activity of these arousal regions. Although lesions of the central nucleus of the amygdala do not alter the wake-promoting effects of modafinil (Silvestri et al., 2000), functionally similar neurons in the BSTLD may still promote wakefulness.
In contrast to amphetamines, modafinil has little potential to produce addiction or increase blood pressure or heart rate (Broughton et al., 1997; Mitler et al., 1998). Amphetamine increases Fos expression in the accumbens nucleus (Johansson et al., 1994), but we found no increase with modafinil. Modafinil (75 mg/kg) also induced little Fos immunoreactivity in brain regions critical for autonomic control. At higher doses, modafinil can slightly increase blood pressure, and 150 mg/kg modafinil produced small increases in Fos expression within the paraventricular nucleus, external lateral subnucleus of the parabrachial nucleus, and nucleus of the solitary tract. Although these increases were not statistically significant, they may reflect subtle autonomic activation.
Does modafinil act via dopaminergic or GABAergic mechanisms?
The pattern of Fos immunoreactivity induced by modafinil provides some useful insights into neurochemical mechanisms via which modafinil may activate neurons. The striatum and all other regions in which we found increases in Fos-IR neurons receive moderate-to-substantial dopaminergic afferents. However, little evidence indicates that modafinil increases dopaminergic transmission. Modafinil does not increase locomotor activity (Edgar and Seidel, 1997), bind to dopamine receptors (Mignot et al., 1994), or alter the firing rates of dopaminergic neurons in the ventral tegmental area (Akaoka et al., 1991). Even high doses of modafinil fail to elicit ipsilateral rotation in rats with unilateral lesions of the nigrostriatal pathway (Contreras et al., 1997). Still, modafinil might increase dopaminergic transmission by binding weakly to the dopamine transporter (Mignot et al., 1994). All regions in which we found modafinil-induced Fos have increased Fos after treatment with dopamine agonists (LaHoste et al., 1993; Eaton et al., 1996; Wirtshafter, 1998) or drugs that promote dopaminergic transmission such as amphetamine (Cole et al., 1992; Lin et al., 1996).
All regions activated by modafinil in our study also receive at least moderate GABAergic innervation. Modafinil reduces the outflow of GABA in cortex, striatum, and posterior hypothalamus as measured by microdialysis (Tanganelli et al., 1995; Ferraro et al., 1996, 1998). GABA release in the posterior hypothalamus may promote sleep via inhibition of the TMN (Yang and Hatton, 1997; Sherin et al., 1998) or PFx (Lin et al., 1989), and a reduction in GABAergic activity could disinhibit these hypothalamic regions resulting in increased wakefulness. Currently, modafinil's neurochemical mechanisms remain unclear, but increased dopaminergic or decreased GABAergic transmission may play critical roles in promoting wakefulness.
Conclusions
Administration of modafinil to rats produced wakefulness in association with activation of TMN and orexin neurons, two cell groups implicated in the control of normal wakefulness. Activation of specific arousal regions may underlie the wakefulness produced by modafinil in people with narcolepsy or other forms of excessive sleepiness. Selective pharmacological modulation of these regions may represent a novel approach to producing wakefulness that is devoid of amphetamine-like subjective effects.
Footnotes
This study was supported by grants from Cephalon, Inc., and by United States Public Health Service Grants MH 01507 and HL 60292. We are grateful to Thomas C. Chou for his expert advice and to Courtney Sears and Quan Hue Ha for excellent technical assistance.
Correspondence should be addressed to Dr. T. E. Scammell, Department of Neurology, Beth Israel Deaconess Medical Center, 77 Avenue Louis Pasteur, Boston, MA 02115. E-mail:tscammel@caregroup.harvard.edu.
REFERENCES
- 1.Akaoka H, Roussel B, Lin JS, Chouvet G, Jouvet M. Effect of modafinil and amphetamine on the rat catecholaminergic neuron activity. Neurosci Lett. 1991;123:20–22. doi: 10.1016/0304-3940(91)90148-m. [DOI] [PubMed] [Google Scholar]
- 2.Alheid GF, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. Academic; San Diego: 1995. pp. 495–578. [Google Scholar]
- 3.Benca RM, Bergmann BM, Leung C, Nummy D, Rechtschaffen A. Rat strain differences in response to dark pulse triggering of paradoxical sleep. Physiol Behav. 1991;49:83–87. doi: 10.1016/0031-9384(91)90235-g. [DOI] [PubMed] [Google Scholar]
- 4.Broughton RJ, Fleming JA, George CF, Hill JD, Kryger MH, Moldofsky H, Montplaisir JY, Morehouse RL, Moscovitch A, Murphy WF. Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy. Neurology. 1997;49:444–451. doi: 10.1212/wnl.49.2.444. [DOI] [PubMed] [Google Scholar]
- 5.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 6.Cole AJ, Bhat RV, Patt C, Worley PF, Baraban JM. D1 dopamine receptor activation of multiple transcription factor genes in rat striatum. J Neurochem. 1992;58:1420–1426. doi: 10.1111/j.1471-4159.1992.tb11358.x. [DOI] [PubMed] [Google Scholar]
- 7.Contreras PC, Edgar DM, Mignot E, Engber T, Vaught JL (1997) A comparison of the preclinical pharmacology of modafinil and amphetamine-like drugs. Paper presented at the Congress on Problems in Drug Dependence, Nashville, TN, June.
- 8.Eaton MJ, Cheung S, Moore KE, Lookingland KJ. Dopamine receptor-mediated regulation of corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 1996;738:60–66. doi: 10.1016/0006-8993(96)00765-2. [DOI] [PubMed] [Google Scholar]
- 9.Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J Pharmacol Exp Ther. 1997;283:757–769. [PubMed] [Google Scholar]
- 10.Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J Comp Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 11.Engber TM, Koury EJ, Dennis SA, Miller MS, Contreras PC, Bhat RV. Differential patterns of regional c-Fos induction in the rat brain by amphetamine and the novel wakefulness-promoting agent modafinil. Neurosci Lett. 1998a;241:95–98. doi: 10.1016/s0304-3940(97)00962-2. [DOI] [PubMed] [Google Scholar]
- 12.Engber TM, Dennis SA, Jones BE, Miller MS, Contreras PC. Brain regional substrates for the actions of the novel wake-promoting agent modafinil in the rat: comparison with amphetamine. Neuroscience. 1998b;87:905–911. doi: 10.1016/s0306-4522(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 13.Ferraro L, Tanganelli S, O'Connor WT, Antonelli T, Rambert F, Fuxe K. The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci Lett. 1996;220:5–8. doi: 10.1016/s0304-3940(96)13212-2. [DOI] [PubMed] [Google Scholar]
- 14.Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert FA, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett. 1998;253:135–138. doi: 10.1016/s0304-3940(98)00629-6. [DOI] [PubMed] [Google Scholar]
- 15.Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Hagan J, Leslie R, Patel S, Evans M, Wattam T, Holmes S, Benham C, Taylor S, Routledge C, Hemmati P, Munton R, Ashmeade T, Shah A, Hatcher J, Hatcher P, Jones D, Smith M, Piper D, Hunter A, Porter R, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inagaki N, Yamatodani A, Ando-Yamamoto M, Tohyama M, Watanabe T, Wada H. Organization of histaminergic fibers in the rat brain. J Comp Neurol. 1988;273:283–300. doi: 10.1002/cne.902730302. [DOI] [PubMed] [Google Scholar]
- 18.Johansson B, Lindstrom K, Fredholm BB. Differences in the regional and cellular localization of c-fos messenger RNA induced by amphetamine, cocaine and caffeine in the rat. Neuroscience. 1994;59:837–849. doi: 10.1016/0306-4522(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 19.LaHoste GJ, Yu J, Marshall JF. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc Natl Acad Sci USA. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JS, Sakai K, Jouvet M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology. 1988;27:111–122. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- 21.Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 22.Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M. Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res. 1992;591:319–326. doi: 10.1016/0006-8993(92)91713-o. [DOI] [PubMed] [Google Scholar]
- 23.Lin JS, Sakai K, Jouvet M. Hypothalamo-preoptic histaminergic projections in sleep-wake control in the cat. Eur J Neurosci. 1994;6:618–625. doi: 10.1111/j.1460-9568.1994.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci USA. 1996;93:14128–14133. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 26.Lisk RD, Sawyer CH. Induction of paradoxical sleep by lights-off stimulation. Proc Soc Exp Biol Med. 1966;123:664–667. doi: 10.3181/00379727-123-31571. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mignot E, Nishino S, Guilleminault C, Dement WC. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–437. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- 29.Mitler M, Guilleminault C, Harsh J, Hishkowitz M. Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. Ann Neurol. 1998;43:88–97. doi: 10.1002/ana.410430115. [DOI] [PubMed] [Google Scholar]
- 30.Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283:315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- 31.Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 32.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 34.Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc Natl Acad Sci USA. 1998;95:7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 36.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silvestri AJ, Pavlock AM, Sanford LD, Ross RJ, Morrison AR. The central nucleus of the amygdala (ACE) does not mediate the effects of modafinil. Soc Neurosci Abstr. 2000;26:566.16. [Google Scholar]
- 38.Steininger TL, Alam N, Szymusiak R, McGinty D. State-dependent discharge of neurons in the rat posterior hypothalamus. Soc Neurosci Abstr. 1996;22:273.6. [Google Scholar]
- 39.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 40.Tanganelli S, Perez de la Mora M, Ferraro L, Mendez-Franco J, Beani L, Rambert FA, Fuxe K. Modafinil and cortical gamma-aminobutyric acid outflow. Modulation by 5-hydroxytryptamine neurotoxins. Eur J Pharmacol. 1995;273:63–71. doi: 10.1016/0014-2999(94)00675-w. [DOI] [PubMed] [Google Scholar]
- 41.Vanni-Mercier G, Sakai K, Jouvet M. Specific neurons for wakefulness in the posterior hypothalamus in the cat. C R Acad Sci III. 1984;298:195–200. [PubMed] [Google Scholar]
- 42.Wirtshafter D. D1 dopamine receptors mediate neuroleptic-induced Fos expression in the islands of Calleja. Synapse. 1998;28:154–159. doi: 10.1002/(SICI)1098-2396(199802)28:2<154::AID-SYN5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 43.Yang QZ, Hatton GI. Electrophysiology of excitatory and inhibitory afferents to rat histaminergic tuberomammillary nucleus neurons from hypothalamic and forebrain sites. Brain Res. 1997;773:162–172. doi: 10.1016/s0006-8993(97)00932-3. [DOI] [PubMed] [Google Scholar]