Abstract
Yersinia pestis is the causative agent of plague and is a re-emerging pathogen that also has the potential as a biological weapon, necessitating the development of a preventive vaccine. Regardless of intense efforts for the last several decades, there is currently not a vaccine approved by FDA. The rF1-V vaccine adjuvanted with Alhydrogel is a lead candidate subunit vaccine for plague and generates a strong Th2-mediate humoral response with a modest Th1 cellular response. As immune protection against Y. pestis requires both humoral and Th1 cellular responses, modifying the rF1-V subunit vaccine formulation to include a robust inducer of Th1 responses may improve efficacy. Thus, we reformulated the subunit vaccine to include SA-4-1BBL, an agonist of the CD137 costimulatory pathway and a potent inducer of Th1 response, and assessed its protective efficacy against pneumonic plague. We herein show for the first time a sex bias in the prophylactic efficacy of the Alhydrogel adjuvanted rF1-V vaccine, with female mice showing better protection against pneumonic plague than male. The sex bias for protection was irrespective of the generation of comparable levels of rF1-V-specific antibody titers and Th1 cellular responses in both sexes. The subunit vaccine reformulated with SA-4-1BBL generated robust Th1 cellular and humoral responses. A prime-boost vaccination scheme involving prime with rF1-V + Alhydrogel and boost with the rF1-V + SA-4-1BBL provided protection in male mice against pneumonic plague. In marked contrast, prime and boost with rF1-V reformulated with both adjuvants resulted in the loss of protection against pneumonic plague, despite generating high levels of humoral and Th1 cellular responses. While unexpected, these findings demonstrate the complexity of immune mechanisms required for protection. Elucidating mechanisms responsible for these differences in protection will help to guide the development of better prophylactic subunit vaccines effective against pneumonic plague.
Keywords: Yersinia pestis, Plague, Subunit vaccine, SA-4-1BBL, Th1-mediated immunity
Introduction
Yersinia pestis is a zoonotic pathogen most frequently transmitted to humans as an accidental host through the bite of an infected flea, but can also be transmitted through inhalation of a relatively small number of the bacteria in aerosols [1, 2]. Transmission through a flea bite results in the bubonic form of the disease, characterized by rapid dissemination to, and replication of bacteria, in the lymph nodes, causing development of painfully swollen buboes, an identifying feature of the disease. Bubonic plague can develop into septicemic or secondary pneumonic plague by hematological spread if the infection is not treated within days of the onset of the first symptoms, and results in death in 30 to 90% of untreated individuals [3, 4]. When bacteria are contracted through aerosol transmission, they cause a severe, rapidly-developing form of pneumonia referred to as primary pneumonic plague. This manifestation has an incubation period of only two to three days and is associated with high mortality even when treated with antibiotics [5].
Although many antibiotics are effective against Y. pestis infection, the rapidity of disease development [6, 7], and the potential acquisition of antibiotic resistance either naturally [8] or as part of bio-weaponization [9], underscore the need for development of an effective vaccine for disease prevention. Attempts to develop plague vaccines have been going on for many decades, but there is currently no FDA approved vaccine. Vaccines based on live, attenuated Y. pestis strains and whole-cell killed formulations have been developed [4]. Those generated through inactivation of virulent Y. pestis strains by either heating or chemical treatment were relatively safe and provided good protection against bubonic plague, but failed to provide efficient protection against pneumonic plague [10]. Formalin-killed bacterial vaccines further proved to be reactogenic and showed poor protection against the pneumonic disease [11, 12]. Therefore, although a formalin-killed vaccine was used in the U.S., it has not gained wide acceptance due primarily to safety concerns, and is currently not approved by the FDA [13].
Subunit vaccines against plague are a promising alternative for balancing efficacy and safety concerns. Genome sequencing, algorithmic antigenic predictions, and recombinant DNA technology have provided valuable tools for identifying immunodominant and protective antigens that may be purified and incorporated either alone or in combination in vaccines formulations [4, 14]. One promising subunit vaccine originally developed at the U.S. Army Medical Research Institute for Infectious Diseases (USAMRIID) utilizes an antigen consisting of a recombinant fusion protein of the F1 capsular protein and the LcrV protein (rF1-V). A formulation of this antigen with the Alhydrogel adjuvant is currently under clinical evaluation [15]. However, while displaying an excellent safety profile in humans and providing promising protection against pneumonic plague in cynomolgus macaques, this formulation provided only inconsistent protection in African green monkeys, raising questions about translation into human efficacy [16, 17].
One possible limitation of the rF1-V subunit vaccine is the tendency of Alhydrogel adjuvant in generating a T helper 2 (Th2)-regulated humoral response that may prove less effective than a more balanced humoral and cellular responses for protection [16–18]. A widening body of evidence supports the importance of a potent T helper 1 (Th1) cell-mediated immunity for providing an optimal response under more stringent bacterial challenge, such as pneumonic plague [16, 19–26]. Cellular Th1 immunity relies upon the cytolytic activity and potent cytokine production of responding T cells to effectively eradicate pathogens with the capacity to infect host cells. In addition, these cells also have an important function in the development of antigen-specific B cell responses, improving the potency and specificity of the antibody response. For instance, exogenous administration of the Th1 cell-derived cytokines IFNγ and TNFα protects mice against Y. pestis challenge [27], while neutralization of these cytokines during challenge abrogates protection [28]. Therefore, the hypothesis has been made that augmenting the rF1-V vaccine formulation to stimulate a more robust Th1 response may increase the efficacy of the vaccine.
SA-4-1BBL, a recombinant oligomeric form of the native CD137 (4–1BB) costimulatory receptor ligand, has previously been shown to boost the Th1 response to vaccine antigens with therapeutic efficacy in various cancer models [29, 30]. Capitalizing on this activity of SA-4-1BBL, we recently reported that the addition of SA-4-1BBL also increases the efficacy of the lead rF1-V + Alhydrogel subunit vaccine formulation in the bubonic plague mouse model [31]. This effect was mediated by an increased Th1 response induced by SA-4-1BBL to balance the humoral response generated by Alhydrogel. Based on the success of the vaccine formulation in the bubonic plague model, this study was conducted to test whether the combined formulation of SA-4-1BBL and Alhydrogel as adjuvant components of the rF1-V subunit vaccine could provide increased protective efficacy in the more stringent pneumonic plague model.
We herein report for the first time a sex bias in protection conferred by the lead rF1-V + Alhydrogel subunit vaccine against pneumonic plaque, with male animals being less responsive than female. Furthermore, prime and boost with SA-4-1BBL-reformulated subunit vaccine did not protect against pneumonic plague, irrespective of generating robust Th1 and humoral responses. In marked contrast, prime with the rF1-V + Alhydrogel vaccine and boost with rF1-V + SA-4-1BBL resulted in strong Th1 cellular and humoral responses and protection against pneumonic plaque. These findings show the complexity of protective immune responses against pneumonic plague and caution against using high levels of Th1 and humoral responses as reliable predictors of the efficacy of prophylactic vaccines.
Materials and methods
Mice
Male and female, 6 to 8-wks-old C57BL/6 and BALB/c mice were purchased from the Jackson Laboratory or bred within the barrier facility at the University of Louisville. Animals were housed in accordance with NIH guidelines and all procedures were approved by the University of Louisville IACUC (Protocol No. 15275 and 16577). Three days prior to challenge with Y. pestis CO92, animals were transferred to University of Louisville’s Center for Predictive Medicine Regional Biocontainment Laboratory ABSL-3 facilities to acclimate to the facility. Mice remained in ABSL-3 after challenge for up to 14 days.
Vaccinations
Recombinant Y. pestis rF1-V protein (NR-4526) was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH maintained by BEI Resources. Aluminum hydroxide gel adjuvant (Alhydrogel) with an aluminum content of 10 mg/ml was purchased from InvivoGen (San Diego, USA). SA-4-1BBL recombinant protein was expressed and purified as previously published [31, 32]. Vaccine formulations were prepared by mixing on a rotator at 4°C overnight to allow for adsorption of the antigen to aluminum hydroxide gel. Mice were vaccinated subcutaneously (s.c.) at the base of the tail with 0.2 ml of the vaccine on day zero (0). When indicated, mice were boosted with a second s.c. injection of 0.2 ml of the indicated vaccine formulation on day 21. Seven days prior to challenge, serum samples were harvested to determine anti-rF1-V IgG titers, and a subset of vaccinated animals were euthanized to isolate peripheral lymph nodes (inguinal, axillary and brachial) to measure antigen-specific cellular responses.
Anti-rF1-V Ab analysis
96-well titer plates were coated with 1 µg/ml of rF1-V antigen overnight at 4◦C, blocked with PBS + 5% dry fat free milk + 0.05% Tween-20 for 1 h at room temperature, and then washed twice with PBS + 0.05% Tween-20 (PBST). Two-fold serial dilutions of serum were added and incubated for 90 min at room temperature. Wells were washed three times with PBST and incubated with goat anti-mouse IgG-HRP (Jackson ImmunoResearch Laboratories; Cat. No. 115– 035-003) for 60 min, and washed. For subytpe analysis, the wells were incubated with goat anti-mouse IgG2c- or IgG1- HRP Abs (Jackson ImmunoResearch Laboratories; Cat. No. 115-035-208 and 115-035-205, respectively). TMB substrate (BD biosciences) was added, and the reaction was stopped with 2 N sulfuric acid. Absorbance was measured at 450 nm and anti-rF1-V Abs were reported as log10 titers of the greatest serial dilution with a mean OD450 value >two-fold the OD450 value of naïve serum at the same dilution. Samples with an Ab titer of log10 1.5 or less were considered negative. For subtype analysis, the ratio of IgG2c /IgG1 were calculated.
Intracellular cytokine analysis
4 × 106 lymphocytes from peripheral lymph nodes were plated in 0.5 ml in a 48 well-plate and stimulated with rF1-V protein (20 µg) and SA-4-1BBL (25 ng) and 20 U IL-2 for 24 hours. Wells without proteins served as negative controls. 1 µl/ml Golgi Plug (BD Pharmingen) was added during the last 4 h of incubation. Cells were surface stained with anti-CD4-Alexa700 (BD Biosciences; Cat. No. 557956), anti-CD8-APC-Cy7 (BD Biosciences; Cat. No. 557654) and anti-CD44-APC (eBiosciences; Cat. No. 17–0441-83) antibodies and fixed with 4% paraformaldehyde for 15 min. Following permeabilization, cells were stained with anti-IFN-γ-PE-Cy7 (eBiosciences; Cat. No. 25–7311-82) or isotype controls (eBiosciences; Cat. No. 12–4301-82, 25–4301-82, and 17–4031-82) and analyzed using flow cytometry (BD FACS LSR-II and FACSDiva software).
Bacterial challenge and in vivo imaging
Vaccinated mice were challenged with 10x LD50 of fully virulent Y. pestis CO92 LuxPcysZK [33]. Briefly, mice were anesthetized with ketamine/xylazene and inoculated intranasally with 20 µl of bacterial suspension as previously described [33]. Infected mice were monitored for the development of disease symptoms twice daily and moribund animals were humanely euthanized. At each health check, bacterial proliferation and dissemination as a function of Y. pestis CO92 LuxPcysZK bioluminescence was assessed by optical imaging using the IVIS Spectrum Imaging System (PerkinElmer, Waltham, MA) as previously described [31, 33]. Regions of interest were generated using Living Image 4.5 (Perkin Elmer) to calculate the average radiance (photons/sec/cm2) at the site of infection.
Statistical analysis
One-way analysis of variance (ANOVA) was used to compare total IgG Ab titers and CD4+ and CD8+ effector IFN-γ responses. The Student t-test (2-tail) was used to compare titers of IgG subclasses and IgG2c/IgG1ratios. Kaplan–Meier log-rank test was used to generate survival curves. Statically significant p values are represented as: *** p<0.005; **p<0.01; * p<0.05.
Results
Single dose vaccination with rF1-V + Alhydrogel reveals potential sex bias in vaccine efficacy.
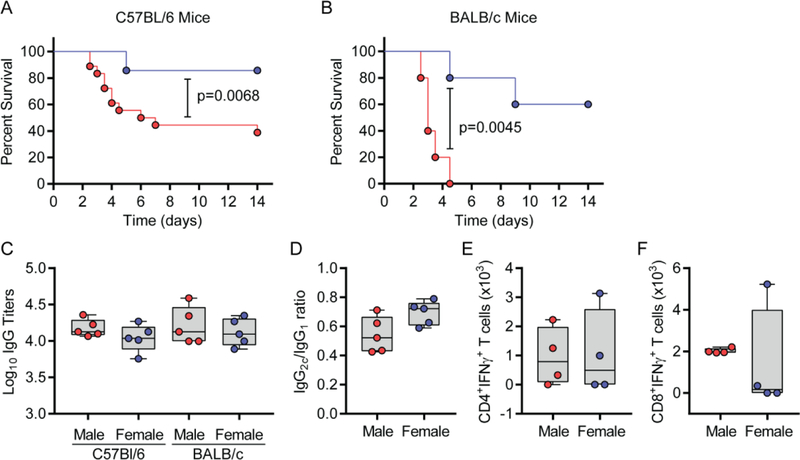
Several studies have shown that vaccination with the recombinant protein rF1-V adjuvanted with Alhydrogel results in a robust anti-rF1-V antibody response that directly correlates with protection against plague in mice [34–36]. While many of these studies used a multiple dose “prime-boost” protocol, we recently demonstrated that a single dose of 1 µg rF1-V + Alhydrogel could protect 80% of C57BL/6 female mice from lethal Y. pestis infection in the bubonic plague model [31]. Protection could be improved further with the addition of SA-4-1BBL to the vaccine platform [31]. In an attempt to translate these initial findings to pneumonic plague, C57BL/6 mice were immunized with a single dose of 1 µg rF1-V + 50 µg Alhydrogel and challenged intranasally with Y. pestis thirty-five days later. Similar to the bubonic plague model, rF1-V + Alhydrogel prevented the development of lethal pneumonic plague in 80% of female mice (Fig. 1A). However, protection in male mice following the same protocol was significantly lower (~40%, p=0.0068), indicating a potential sex bias. To determine if this sex bias was mouse strain specific, the same vaccination and challenge protocol was performed using BALB/c mice. The sex difference in BALB/c was much more pronounced as compared with C57BL/6 mice as none of males were protected, versus 60% protection in females against lethal pneumonic plague (Fig. 1B; p=0.0045). Together, these data demonstrate a sex bias in the efficacy of the rF1-V + Alhydrogel subunit vaccine against pneumonic plague in mice.
Figure 1. Single dose of rF1-V + Alhydrogel vaccine better protects female mice against pneumonic plague.
Male and female mice were vaccinated with rF1-V + Alhydrogel and challenged 35 days later intranasally with 10 LD50 of virulent Y. pestis CO92 LuxPcysZK. Survival was monitored for 14 days. (A) Survival of C57BL/6 mice (compiled data from 4 independent experiments; n=18 and 14 for male and female mice, respectively). (B) Survival of BALB/c mice (data from one experiment; n=5). (C) Anti- rF1-V IgG titers from subset of mice in A and B 7 d prior to infection. (D) Ratio of IgG2c to IgG1 antibody subtypes from C57BL/6 mice. Absolute numbers (E) CD4+ T cells and (F) CD8+ T cells secreting IFN-γ from subset of C57BL/6 mice 35 d post vaccination. Survival data were analyzed by Kaplan-Meier and log-rank test and antibody and T cell phenotypes were analyzed by one-way ANOVA with Tukey post hoc analysis or student’s T-test.
Male and female mice develop similar biomarkers of rF1-V + Alhydrogel vaccine efficacy.
The sex bias in vaccine efficacy may reflect potential differences in immune responses generated by male and female against the subunit vaccine. Previous studies have identified a direct correlation between anti-rF1-V IgG antibody titers and protection against plague in mice [37, 38]. Indeed, passive transfer of serum from immunized humans and nonhuman primates into mice resulted in protection against plaque, confirming the importance of antibodies [39, 40]. Thus, we tested whether the decreased protective efficacy of rF1-V + Alhydrogel in male mice could be a result of lower antibody titers compared to females. Surprisingly, no significant differences in total IgG antibody titers to rF1-V were observed between male and female mice for both strains (Fig. 1C). Indeed, there was a trend towards better Ab titers in males of both strains as compared with females. Furthermore, in C57BL/6 mice no significant differences were apparent in the ratios of the predominant IgG isotypes (IgG1 and IgG2c) between the sexes (Fig. 1D). These data indicate that differences in antibody titers are not responsible for the observed sex bias in vaccine protection.
A Th1 cellular response has also been shown to contribute to protection against plague infection [41, 42]. We next explored whether male and female mice develop differential Th1 responses to the vaccine. Twenty-eight days after vaccination with rF1-V + Alhydrogel, T cells were isolated from male and female C57BL/6 mice, and antigen-specific CD4+ and CD8+ responses were assessed using flow cytometry for intracellular IFNγ as a Th1 signature cytokine. As we previously reported [31], vaccination of female mice with rF1-V + Alhydrogel generated only a moderate IFNγ response in CD4+ (Fig. 1E) and CD8+ T cells (Fig. 1F). However, there was no significant sex difference in antigen-specific IFNγ response, indicating that improved antigen- specific cellular responses by females in response to the vaccine were not a correlate of improved protection. Taken together, these data do not implicate a differential Th1 or antibody response as correlates of the observed sex bias for the protective efficacy of the lead rF1-V + Alhydrogel subunit vaccine in mice.
Addition of SA-4-1BBL improves the generation of a Th1 response, but does not increase protection of male mice in a prime only setting.
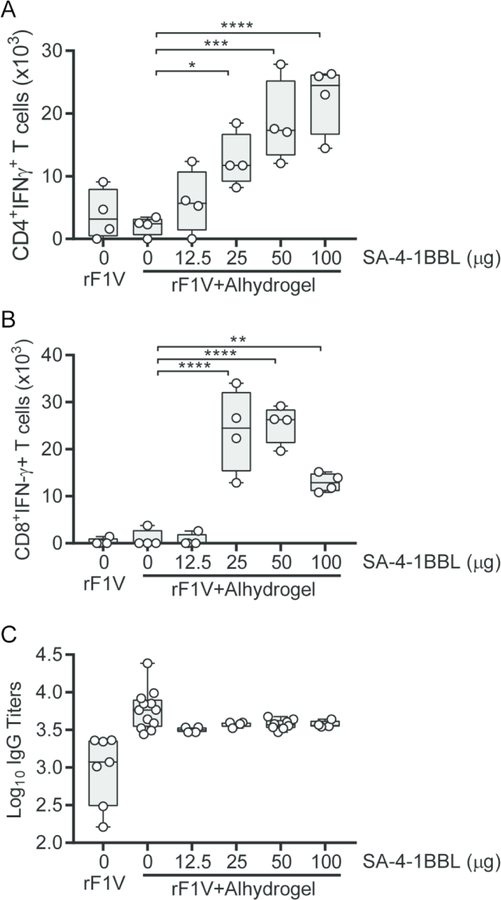
The inability of rF1-V + Alhydrogel subunit vaccine to generate a strong Th1 response combined with its reduced protective efficacy in male mice led us to use this model to assess the impact of SA-4-1BBL as a strong Th1 adjuvant on rF1-V vaccine efficacy. To establish a vaccine formulation that generates a balanced humoral and Th1 responses, male C57BL/6 mice were vaccinated with rF1-V alone, rF1-V + Alhydrogel, or rF1-V + Alhydrogel with increasing doses of SA-4-1BBL. As we have previously observed ([31] and Fig. 1E, vaccination with rF1-V alone or in combination with Alhydrogel resulted in only a modest number of rF1-V-specific CD4+ and CD8+ T cells expressing IFNγ (Fig. 2A and B). However, addition of SA-4-1BBL to the vaccine formulation resulted in a significant dose-dependent increase in the absolute numbers of CD4+ T cells expressing IFNγ (Fig. 2A). A similar pattern was also observed for CD8+ T cells, except that at the highest dose of 100 µg, SA-4-1BBL appeared to decrease the absolute number of cells expressing IFNγ (Fig. 2B). We also determined the impact of SA-4-1BBL on antibody titers, and while mean titers decreased on average with the addition of SA-4-1BBL compared to Alhydrogel alone (mean titers of 3.6 × 103 vs. 5.89 × 103, respectively; Fig. 2C), the variation in individual responses did not yield statistically significant differences. These data were consistent with our previous observations in female mice [31]. Taken together, these results allowed us to establish 50 µg of SA-4-1BBL as the optimum dose for the rF1-V + Alhydrogel vaccine formulation to generate a balanced antigen-specific Th1 cellular and humoral responses in male mice.
Figure 2. Addition of SA-4-1BBL improves the Th1 cellular response of the rF1-V + Alhydrogel vaccine.
C57BL/6 male mice were vaccinated with rF1-V or rF1-V + Alhydrogel with increasing concentrations of SA-4-1BBL. Absolute numbers of (A) CD4+ T cells and (B) CD8+ T cells secreting IFN-γ were determined 35 d post vaccination. (C) Anti-rF1-V IgG titers of vaccinated mice were determined 28 post-vaccination. Data was analyzed by one-way ANOVA with Dunnett’s post hoc analysis compared to 0 µg of SA-4-1BBL: ****= p<0.0001; *** = p<0.001; ** = p<0.01; * = p<0.05.
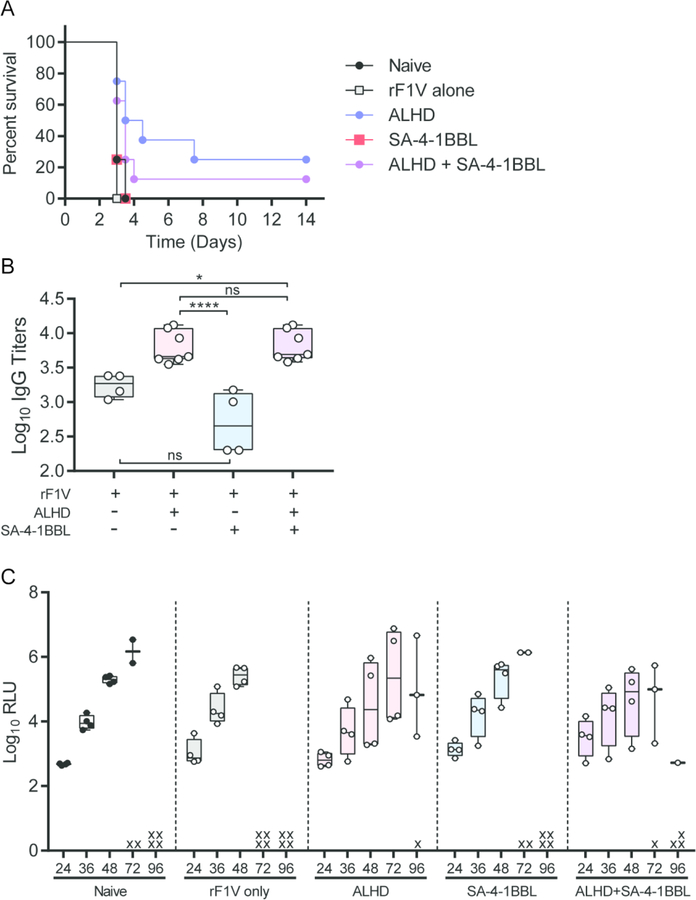
To determine if increased rF1-V- Th1 cellular responses could improve the efficacy of prime only vaccination against pneumonic plague, male mice were immunized with rF1-V alone, rF1-V with single adjuvants (either 50 µg SA-4-1BBL or 50 µg Alhydrogel), or rF1-V with both adjuvants. Thirty-five days later, mice were challenged intranasally with a lethal dose of Y. pestis. As expected, all mice immunized with rF1-V without an adjuvant succumbed to infection at the same rate as naïve animals (Fig. 3A). All mice that received vaccine adjuvanted with only SA-4-1BBL also succumbed to infection within 96 h of exposure. This directly correlated with significantly lower rF1-V-specific IgG titers in the SA-4-1BBL only animals (Fig. 3B). In contrast, vaccination with rF1-V + Alhydrogel resulted in 25% of the animals surviving the infection (Fig. 3A). Surprisingly, despite the significant increase in antigen-specific Th1 cellular responses in the animals receiving Alhydrogel+SA-4-1BBL (Fig. 2B and C), there was not a significant change in vaccine protection from lethal infection in animals receiving both adjuvants (Fig. 3A). Also, we did not observe any differences in the rate of bacterial proliferation in the lungs between animals that succumbed to infection (Fig. 3C). These data demonstrate that while increased rF1-V-specific Th1 responses could improve the efficacy of a single dose of rF1-V + Alhydrogel vaccine against bubonic plague [31], a similar improvement in efficacy was not observed against pneumonic plague.
Figure 3. Improved Th1 immune response in SA-4-1BBL+ rF1-V + Alhydrogel does not increase protection against pneumonic plague.
C57BL/6 male mice (data from one representative experiment; n=4) were vaccinated with rF1-V antigen alone or with Alhydrogel (ALHD), SA-4-1BBL, or Alhydrogel and SA-4-1BBL (ALHD + SA-4-1BBL) and challenged 35 days later with 10 LD50 of virulent Y. pestis CO92 LuxPcysZK. (A) Survival was monitored for 14 d. (B) Anti- rF1-V IgG titers from animals in A were determined 7 d prior to Y. pestis challenge. (C) Bacterial proliferation in the lungs as a function of bacterial luminescence of infected mice was monitored by real time live animal optical imagining every 12 h from 24 to 96 h post-infection. Animals that succumbed to infection are represented by x. Survival data were analyzed by Kaplan-Meier and log-rank test and antibody titers by one-way ANOVA with Tukey post hoc analysis: **** = p<0.0001; * = p<0.05; ns = not significant.
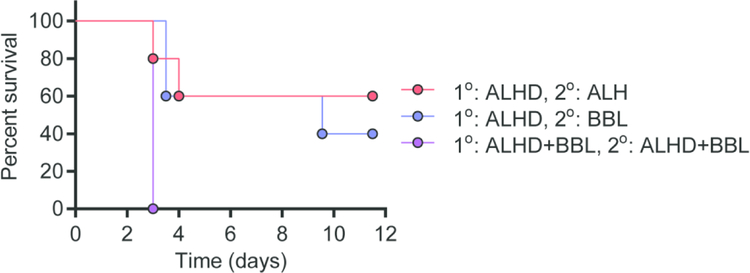
A vaccination scheme consisting of priming with the rF1-V + Alhydrogel and boosting with rF1-V + SA-4-1BBL generates protection against pneumonic plague.
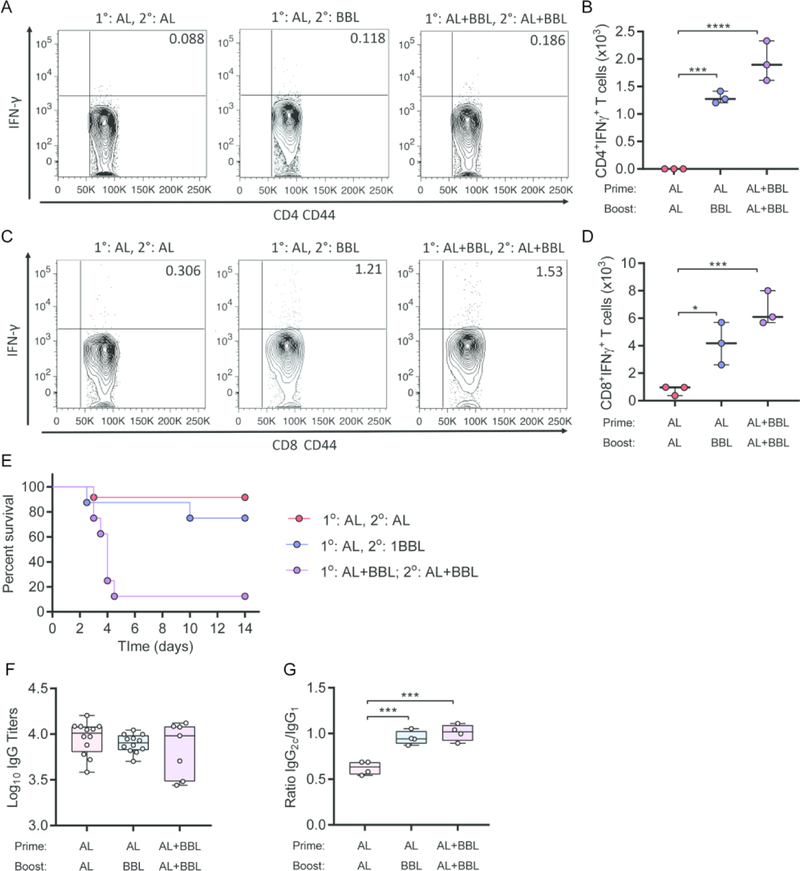
Lack of protective efficacy in spite of a balanced Th1 and humoral responses generated by SA-4-1BBL-adjuvanted lead subunit vaccine in the prime only setting led us to assess protective efficacy in a prime-boost vaccination scheme. Towards this end, we tested various vaccine formulations and timing of SA-4-1BBL administration in a prime-boost setting. Two cohorts of male C57BL/6 mice were primed with rF1-V + Alhydrogel alone, while a third group received rF1-V + Alhydrogel + SA-4-1BBL. Animals were boosted 21 days later with rF1-V + Alhydrogel alone, rF1-V + SA-4-1BBL alone, or rF1-V + Alhydrogel + SA-4-1BBL. Analysis of Th1 responses 60 days post-priming demonstrated significantly higher absolute numbers of antigen-specific CD4+ and CD8+ T cells expressing IFNγ with the addition of SA-4-1BBL either alone in the boost or in a combination adjuvant platform at prime and boost (Fig. 4A–D). Animals primed with rF1-V + Alhydrogel alone and boosted either rF1-V + Alhydrogel alone or rF1-V + SA-4-1BBL had robust protection against pneumonic plague (~90% and 75%, respectively). Surprisingly, animals that received prime and boost vaccination with the rF1-V + Alhydrogel + SA-4-1BBL vaccine formulation were not protected against lethal infection (Fig. 4E), despite robust antigen-specific Th1 cell responses (Fig. 4B and D).
Figure 4: Boost with vaccine containing Alhydrogel or SA-4-1BBL improves survival of male mice against pneumonic plague.
Male C57BL/6 mice were primed with rF1-V + Alhydrogel alone (AL) or rF1-V + Alhydrogel + SA-4-1BBL (AL+BBL). 21 d later, mice were boosted with rF1-V + Alhydrogel (AL; n=12), rF1-V + SA-4-1BBL (BBL; n=8), or rF1-V + Alhydrogel + SA-4-1-BBL (AL+BBL; n=8). Absolute numbers of (A and B) CD4+ T cells and (C and D) CD8+ T cells secreting IFN-γ were determined 60 d post prime vaccination from a subset of animals. A and C are representative flow cytometer data from one animal in each group. (E) Mice were challenged intranasally 60 d post prime vaccination with 10 LD50 of virulent Y. pestis CO92 LuxPcysZK and survival was monitored for 14 d (compiled data from 3 independent experiments). (F) Anti- rF1-V IgG titers 7 d prior to infection. (G) Ratio of IgG2c to IgG1 antibody subtypes from subset of mice in D. Survival data were analyzed by Kaplan-Meier and log-rank test and cell numbers and antibody titers by one-way ANOVA with Dunnett’s post hoc analysis compared to the AL/AL prime boost group: *** = p<0.001; * = p<0.05.
Repeated stimulation of CD137 receptor using agonist antibodies was shown to result in the inhibition of humoral responses in a CD4+ T cell-dependent mechanism [44]. Therefore, it is possible that multiple vaccinations with SA-4-1BBL could have decreased the overall anti-rF1-V antibody response, resulting in the observed decrease in protection. However, analysis of serum IgG titers 60 days post-priming showed that all three vaccination schemes generated robust and comparable IgG titers (Fig. 4F). Furthermore, the lack of protection was not due to a significant shift from Th2-regulated humoral immune responses (IgG1) to Th1-regulated responses (IgG2C), as the ratio of IgG1/IgG2C were similar between both vaccine formulations adjuvanted with SA-4-1BBL (Fig. 4G). Together, these data indicate that a boost with either rF1- V + Alhydrogel or rF1-V + SA-4-1BBL can protect male mice from lethal pneumonic plague, but only a vaccine formulation with SA-4-1BBL improves the Th1 cellular response.
Prime-boost vaccination provides long term protection against pneumonic infection.
A short-term (60 days) prime-boost vaccination improved efficacy over prime only vaccination in male mice. To gauge if prime-boost vaccination establishes long-term protection against pneumonic plague, separate groups of animals receiving the same vaccination schedule were challenged intranasally with a lethal dose of Y. pestis 150 days post-priming. Priming with rF1-V + Alhydrogel and boosting with rF1-V + SA-4-1BBL had similar level of protection against pneumonic as using rF1-V + Alhydrogel in both the prime and boost vaccinations (40% and 60%, respectively; Fig. 5). As predicted by data from the short-term prime-boost setting, all animals that received SA-4-1BBL in both the prime and boost vaccine succumbed to infection within three days of challenge (Fig. 5). These data demonstrate that equivalent long-term immunity was induced via boosting with either Alhydrogel or SA-4-1BBL.
Figure 5: Prime-boost vaccination provides long term protection against pneumonic infection.
Male C57BL/6 mice were primed with rF1-V + Alhydrogel alone (AL) or rF1-V + Alhydrogel + SA-4-1BBL (AL+BBL). 21 d later, mice were boosted with rF1-V + Alhydrogel (ALH; n=5), rF1-V + SA-4-1BBL (BBL; n=5), or rF1-V + Alhydrogel + SA-4-1-BBL (AL+BBL; n=5). Mice were challenged 150 d after prime vaccination with 10 LD50 of virulent Y. pestis CO92 LuxPcysZK and monitored for survival for 14 d. Data was analyzed using Kaplan-Meier survival curve and log-rank test.
Discussion
Although several plague vaccines based on attenuated Y. pestis strains and whole-cell killed formulations have shown some efficacy and are currently being used in several countries, these formulations have raised safety concerns based on reported incidences of relatively severe fever, headache, and injection-site inflammation [47]. Importantly, they showed limited efficacy and lacked long-term memory, requiring repeated booster vaccinations [4, 13, 48]. Therefore, they are currently not approved for use in the United States. A subunit vaccine based on a fusion of the F1 capsular and the LcrV virulence proteins is the most promising vaccine currently in clinical trials. This antigen combination formulated with Alhydrogel adjuvant has shown efficacy in generating humoral immune responses with protective efficacy in mice [4, 34, 41] and an excellent safety profile in humans [49]. However, the efficacy of this lead subunit vaccine against pneumonic plague in NHPs have been inconsistent, providing protection in cynomolgus macaques, but not African green monkeys [16, 17]. Although the underlying immune mechanisms responsible for these variable NHP outcomes remain to be investigated, the inability of the vaccine to generate a balanced humoral and cellular responses against plague may provide an explanation. This notion is consistent with the demonstrated function of Alhydrogel adjuvant in generating a humoral response derived by Th2 cells [18].
Accumulating evidence in rodent models implicate both humoral and cellular, particularly Th1, responses in protection conferred by the recombinant rF1-V vaccine [41, 50, 51]. For example, passive transfer of immune serum from humans, NHPs, or rodents was shown to protect mice against lethal infection [38] and µM transgenic mice (which are B-cell deficient) lacking humoral response vaccinated with a live vaccine were protected against pneumonic plague [42]. Importantly, IFNγ, TNFα, and IL-17 cytokine responses were also shown to be critical to protection against plague [50–53]. Indeed, the IFNγ and TNFα responses were also required in the setting of passive immune serum transfer, providing direct evidence for a dominant role of a Th1 cellular immune response in protection against plague[41]. Therefore, we hypothesized that the efficacy of the lead rF1-V + Alhydrogel vaccine could be improved by SA-4-1BBL, a strong inducer of Th1 responses [54] and used the more stringent pneumonic plague model to test this notion.
We show for the first time that the rF1-V + Alhydrogel vaccine has better prophylactic efficacy against pneumonic plague in female than in male mice. This sex bias was conserved between both C57BL/6 and BALB/c strains but appeared to be more pronounced in the BALB/c strain. The reasons behind this sex bias are not clear, but do not appear to be directly related to differences in overall antigen-specific humoral or Th1 cellular immune responses generated by the vaccine. These data raise concerns about past and future plague vaccine studies that rely on only female mice. As such, especially in the context of single administration vaccine protocols, male cohorts may be more predictive of vaccine success with regards to new plague vaccine candidates.
Based on these observations, we decided to use the more sensitive male mice to assess the efficacy of SA-4-1BBL in improving rF1-V + Alhydrogel vaccine protection against pneumonic plague. Dose-response studies established 50 µg of SA-4-1BBL as the most effective dose in generating robust Th1 CD4+ and CD8+ T cell responses without compromising the Alhydrogel regulated humoral responses. These findings are consistent with the demonstrated role of CD137 signaling in the generation of IFNγ producing CD8+ T cells [55]. We also demonstrated that SA-4-1BBL drives the expansion of both CD4+ and CD8+ T cells producing IFNγ that translated in therapeutic efficacy against cancer in various preclinical models [56–58] as well as protection against bubonic plaque [59]. Importantly, CD4+ T cells have been found to be essential for boosting the protection provided by CD8+ T cells in an adoptive transfer pneumonic plague model [60]. In addition, CD4+ T cells help facilitate Ab isotype switching. As shown here, animals that received SA-4-1BBL in adjuvant formulations demonstrated a stronger IgG2c -biased Ab response associated with a Th1-type response (Fig. 3B and 4G), suggesting that IFNγ-producing CD4+ T cells stimulated by SA-4-1BBL may significantly alter the humoral response, similar to what has been reported for CpG ODN adjuvant added to rF1-V + Alhydrogel previously [61].
Unexpectedly, and differing from our results in the bubonic plague model [31], our ability to increase the Th1 cellular and IgG2c responses by adding SA-4-1BBL to the subunit vaccine formulation did not improve protection relative to rF1-V + Alhydrogel in either a prime-only or prime-boost model. More strikingly, prime-boost vaccination with SA-4-1BBL formulated into rF1-V + Alhydrogel provided minimal protection against pneumonic plague. It is currently unclear why the combined use of SA-4-1BBL and Alhydrogel in the prime-boost setting blocks the protection conferred by the subunit vaccine. Repeated stimulation of CD137 receptor using agonist antibodies was shown to result in the inhibition of humoral responses in a CD4+ T cell-dependent mechanism [44]. Also, T cells upregulate CD137 receptor within 24 h after antigen-induced activation [43] and repeated stimulation with the ligand may distort homeostasis of immune cells [44, 45]. Importantly, in an acute viral infection it was demonstrated that the protective efficacy of CD137 stimulation was time dependent as administration of an agonistic anti-CD137 antibody at the time of challenge with lymphocytic choriomeningitis virus resulted in the abrogation of protection while a 3-day delayed treatment has protective efficacy [46]. However, our data show strong humoral and Th1 cellular responses generated by the SA-4-1BBL-adjuvanted rF1-V + Alhydrogel independent of prime or prime-boost vaccinations, arguing against dysregulation in CD4+ T cells or immune homeostasis.
These findings are surprising and imply that a strong systemic balanced cellular and humoral response alone is not protective against pneumonic plague, at least in mice. Importantly, they implicate unknown critical immune molecular or cellular elements negatively regulated with the combination of Alhydrogel and SA-4-1BBL that are important to protection against a pneumonic plague. Investigation and identification of these immune elements will provide critical mechanistic information that will contribute to the development of effective prophylactic vaccines against pneumonic plague. The negative impact in the protective efficacy of the lead rF1-V vaccine was not observed when SA-4-1BBL was used alone as a booster following rF1-V + Alhydrogel prime vaccination. This finding is important, as prime with rF1-V + Alhydrogel and boost with SA-41BL-adjuvanted rF1-V generated a more balanced Th1/Th2 response that may be required in humans for long-term immune memory and consistent protective efficacy. The importance of cellular responses for immune protection against pneumonic plague in nonhuman primates and by extension, humans, have previously been suggested by other investigators [16, 62].
In summary, this study demonstrates for the first time a sex bias for the lead rF1-V+ Alhydrogel vaccine against pneumonic plague, male being more sensitive than females irrespective of generating comparable humoral and cellular immune responses. Prime with rF1- V +Alhydrogel and boost with SA-4-1BBL adjuvanted rFV1 generated a balanced humoral and Th1 cellular immune response that was protective against pneumonic plague. A complete mechanistic understating of the SA-4-1BBL adjuvanted vaccine may lead to further improvement of the lead rF1-V vaccine for protection against pneumonic plague.
Highlights:
Sex bias observed for mice vaccinated with rF1-V subunit plague vaccine
Sex bias appears independent of antigen-specific antibody or cellular responses
SA-4-1BBL addition to vaccine formulation increases Th1 cellular responses
Boosting with the rF1-V vaccine with SA-4-1BBL protects against pneumonic plague
Balanced cellular/humoral response alone may not predict plague vaccine efficacy
Acknowledgements
We would like to acknowledge the University of Louisville Center for Predictive Medicine Shared Resources Staff for technical support for these studies. This work was supported by funding support from the National Institutes of Health NIAID (AI112618 to MBL and HS; AI120353 to WB, MBL, and HS) and NIGMS (GM125504 to MBL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Dennis DT and Chow CC, Plague. Pediatr Infect Dis J, 2004. 23(1): p. 69–71. [DOI] [PubMed] [Google Scholar]
- 2.Pechous RD, et al. , Pneumonic Plague: The Darker Side of Yersinia pestis. Trends Microbiol, 2016. 24(3): p. 190–197. [DOI] [PubMed] [Google Scholar]
- 3.Prentice MB and Rahalison L, Plague. Lancet, 2007. 369(9568): p. 1196–207. [DOI] [PubMed] [Google Scholar]
- 4.Verma SK and Tuteja U, Plague Vaccine Development: Current Research and Future Trends. Front Immunol, 2016. 7: p. 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisen RJ, Dennis DT, and Gage KL, The Role of Early-Phase Transmission in the Spread of Yersinia pestis. J Med Entomol, 2015. 52(6): p. 1183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koster F, et al. , Milestones in progression of primary pneumonic plague in cynomolgus macaques. Infect Immun, 2010. 78(7): p. 2946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price PA, Jin J, and Goldman WE, Pulmonary infection by Yersinia pestis rapidly establishes a permissive environment for microbial proliferation. Proc Natl Acad Sci U S A, 2012. 109(8): p. 3083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch TJ, et al. , Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One, 2007. 2(3): p. e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zilinskas RA, A brief history of biological weapons programmes and the use of animal pathogens as biological warfare agents. Rev Sci Tech, 2017. 36(2): p. 415–422. [DOI] [PubMed] [Google Scholar]
- 10.Russell P, et al. , A comparison of Plague vaccine, USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine, 1995. 13(16): p. 1551–6. [DOI] [PubMed] [Google Scholar]
- 11.Meyer KF, et al. , Plague immunization. I. Past and present trends. J Infect Dis, 1974. 129: p. Suppl:S13–8. [DOI] [PubMed] [Google Scholar]
- 12.Titball RW and Williamson ED, Yersinia pestis (plague) vaccines. Expert Opin Biol Ther, 2004. 4(6): p. 965–73. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, et al. , Live-attenuated Yersinia pestis vaccines. Expert Rev Vaccines, 2013. 12(6): p. 677–86. [DOI] [PubMed] [Google Scholar]
- 14.Sabhnani L, et al. , Developing subunit immunogens using B and T cell epitopes and their constructs derived from the F1 antigen of Yersinia pestis using novel delivery vehicles. FEMS Immunol Med Microbiol, 2003. 38(3): p. 215–29. [DOI] [PubMed] [Google Scholar]
- 15.Morris SR, Development of a recombinant vaccine against aerosolized plague. Vaccine, 2007. 25(16): p. 3115–7. [DOI] [PubMed] [Google Scholar]
- 16.Smiley ST, Current challenges in the development of vaccines for pneumonic plague. Expert Rev Vaccines, 2008. 7(2): p. 209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson ED, et al. , Recombinant (F1+V) vaccine protects cynomolgus macaques against pneumonic plague. Vaccine, 2011. 29(29–30): p. 4771–7. [DOI] [PubMed] [Google Scholar]
- 18.Brewer JM, et al. , Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol, 1999. 163(12): p. 6448–6454. [PubMed] [Google Scholar]
- 19.Ali R, et al. , Multiple antigen peptide containing B and T cell epitopes of F1 antigen of Yersinia pestis showed enhanced Th1 immune response in murine model. Scand J Immunol, 2013. 77(5): p. 361–71. [DOI] [PubMed] [Google Scholar]
- 20.Gupta G, et al. , Evaluation of CD4+/CD8+ T-cell expression and IFN-gamma, perforin secretion for B-T constructs of F1 and V antigens of Yersinia pestis. Int Immunopharmacol, 2012. 12(1): p. 64–73. [DOI] [PubMed] [Google Scholar]
- 21.Gupta G, Khan AA, and Rao DN, Cell-mediated immune response and Th/Th cytokine profile of B-T constructs of F1 and V antigen of Yersinia pestis. Scand J Immunol, 2010. 71(3): p. 186–98. [DOI] [PubMed] [Google Scholar]
- 22.Lin JS, et al. , Yersinia pestis YopE contains a dominant CD8 T cell epitope that confers protection in a mouse model of pneumonic plague. J Immunol, 2011. 187(2): p. 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rai R, et al. , MAP of F1 and V antigens from Yersinia pestis astride innate and adaptive immune response. Microb Pathog, 2015. 87: p. 13–20. [DOI] [PubMed] [Google Scholar]
- 24.Shreewastav RK, et al. , Cell-mediated immune response to epitopic MAP (multiple antigen peptide) construct of LcrV antigen of Yersinia pestis in murine model. Cell Immunol, 2012. 278(1–2): p. 55–62. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, et al. , Involvement of CD8+ T cell-mediated immune responses in LcrV DNA vaccine induced protection against lethal Yersinia pestis challenge. Vaccine, 2011. 29(39): p. 6802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zvi A, et al. , Novel CTL epitopes identified through a Y. pestis proteome-wide analysis in the search for vaccine candidates against plague. Vaccine, 2017. 35(44): p. 5995–6006. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima R and Brubaker RR, Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun, 1993. 61(1): p. 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kummer LW, et al. , Antibodies and cytokines independently protect against pneumonic plague. Vaccine, 2008. 26(52): p. 6901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schabowsky RH, et al. , A novel form of 4-1BBL has better immunomodulatory activity than an agonistic anti-4–1BB Ab without Ab-associated severe toxicity. Vaccine, 2009. 28(2): p. 512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma RK, et al. , 4–1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines. Cancer Res, 2010. 70(10): p. 3945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinc G, et al. , Improving the Th1 cellular efficacy of the lead Yersinia pestis rF1-V subunit vaccine using SA-4-1BBL as a novel adjuvant. Vaccine, 2014. 32(39): p. 5035–40. [DOI] [PubMed] [Google Scholar]
- 32.Elpek KG, et al. , Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol, 2007. 179(11): p. 7295–304. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, et al. , Development of bioluminescent bioreporters for in vitro and in vivo tracking of Yersinia pestis. PLoS One, 2012. 7(10): p. e47123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williamson ED, et al. , A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against pneumonic plague. Vaccine, 1997. 15(10): p. 1079–1084. [DOI] [PubMed] [Google Scholar]
- 35.Heath DG, et al. , Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine, 1998. 16(11–12): p. 1131–1137. [DOI] [PubMed] [Google Scholar]
- 36.Green M, et al. , The SCID/Beige mouse as a model to investigate protection against Yersinia pestis. FEMS Immunol. Med. Microbiol, 1999. 23(2): p. 107–113. [DOI] [PubMed] [Google Scholar]
- 37.Williamson ED, et al. , A single dose sub-unit vaccine protects against pneumonic plague. Vaccine, 2000. 19(4–5): p. 566–71. [DOI] [PubMed] [Google Scholar]
- 38.Bashaw J, et al. , Development of in vitro correlate assays of immunity to infection with Yersinia pestis. Clin. Vaccine Immunol, 2007. 14(5): p. 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson ED, et al. , Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect Immun, 2005. 73(6): p. 3598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson ED, et al. , Immunogenicity of the rF1+rV vaccine for plague with identification of potential immune correlates. Microb Pathog, 2007. 42(1): p. 11–21. [DOI] [PubMed] [Google Scholar]
- 41.Lin JS, et al. , TNFalpha and IFNgamma contribute to F1/LcrV-targeted immune defense in mouse models of fully virulent pneumonic plague. Vaccine, 2010. 29(2): p. 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parent MA, et al. , Cell-mediated protection against pulmonary Yersinia pestis infection. Infect. Immun, 2005. 73(11): p. 7304–7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawicki W and Watts TH, Expression and function of 4-1BB during CD4 versus CD8 T cell responses in vivo. Eur. J Immunol, 2004. 34(3): p. 743–751. [DOI] [PubMed] [Google Scholar]
- 44.Mittler RS, et al. , Anti-4–1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp. Med, 1999. 190(10): p. 1535–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’ Reilly LA, et al. , Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature, 2009. 461(7264): p. 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B, et al. , Immune suppression or enhancement by CD137 T cell costimulation during acute viral infection is time dependent. J Clin Invest, 2007. 117(10): p. 3029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hart MK, et al. , Advanced Development of the rF1V and rBV A/B Vaccines: Progress and Challenges. Adv. Prev. Med, 2012. 2012: p. 731604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu K, et al. , Immunogenicity and safety of subunit plague vaccine: A randomized phase 2a clinical trial. Hum Vaccin Immunother, 2016. 12(9): p. 2334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price JL, et al. , Preclinical safety assessment of a recombinant plague vaccine (rF1V). Int J Toxicol, 2013. 32(5): p. 327–35. [DOI] [PubMed] [Google Scholar]
- 50.Kummer LW, et al. , Antibodies and cytokines independently protect against pneumonic plague. Vaccine, 2008. 26(52): p. 6901–6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parent MA, et al. , Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect. Immun, 2006. 74(6): p. 3381–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakajima R and Brubaker RR, Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun, 1993. 61(1): p. 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin JS, et al. , IL-17 contributes to cell-mediated defense against pulmonary Yersinia pestis infection. J. Immunol, 2011. 186(3): p. 1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma RK, Yolcu ES, and Shirwan H, SA-4-1BBL as a novel adjuvant for the development of therapeutic cancer vaccines. Expert. Rev. Vaccines, 2014. 13(3): p. 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Croft M, The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol, 2009. 9(4): p. 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma RK, et al. , Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel from of 4-1BBL eradicates established tumors. Cancer Res, 2009. 69(10): p. 4319–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma RK, et al. , 4–1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines. Cancer Res, 2010. 70(10): p. 3945–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srivastava AK, et al. , SA-4-1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines. Cancer Res, 2014. 74(22): p. 6441–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dinc G, et al. , Improving the Th1 cellular efficacy of the lead Yersinia pestis rF1-V subunit vaccine using SA-4-1BBL as a novel adjuvant. Vaccine, 2014. 32(39): p. 5035–5040. [DOI] [PubMed] [Google Scholar]
- 60.Philipovskiy AV and Smiley ST, Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect Immun, 2007. 75(2): p. 878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amemiya K, et al. , CpG oligodeoxynucleotides augment the murine immune response to the Yersinia pestis F1-V vaccine in bubonic and pneumonic models of plague. Vaccine, 2009. 27(16): p. 2220–9. [DOI] [PubMed] [Google Scholar]
- 62.Williamson ED, et al. , An IgG1 titre to the F1 and V antigens correlates with protection against plague in the mouse model. Clin Exp Immunol, 1999. 116(1): p. 107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]