Abstract
Monoclonal antibody inhibitors of the epidermal growth factor receptor (EGFR) have been shown to improve outcomes for patients with metastatic colorectal cancer (mCRC) without RAS gene mutations. However, treatment with anti-EGFR agents can be associated with toxicities of the skin, nails, hair, and eyes. Because these dermatologic toxicities can result in treatment discontinuation and affect patient quality of life, their management is an important focus when administering anti-EGFR monoclonal antibodies. The present systematic review describes the current data reporting the nature and incidence of, and management and treatment options for, dermatologic toxicities occurring during anti-EGFR treatment of mCRC. A search of the National Library of Medicine PubMed database from January 1, 2009, to August 18, 2016, identified relevant reports discussing dermatologic toxicity management among patients with mCRC receiving anti-EGFR therapy. The studies were grouped by type and rated by level of evidence using the GRADE approach developed by the Agency for Healthcare Research and Quality. Overall, 269 reports were reviewed (nonrandomized trials, n = 120; randomized trials, n = 31; retrospective studies, n = 15; reviews, n = 39). Dermatologic toxicity of any grade occurs in most patients who receive anti-EGFR therapy; approximately 10% to 20% of patients experienced grade 3/4 toxicity. The most common dermatologic toxicities include papulopustular/acneiform rash, xerosis, and pruritus; however, nail changes, hair abnormalities, and ocular conditions also occur. Guidance for managing these toxicities includes the use of inexpensive emollient ointments and moisturizers, avoidance of sun exposure, avoidance of irritants, and the use of short showers. Several studies also found that preemptive treatment was more effective than reactive treatment at limiting the incidence and severity of skin toxicity. With appropriate treatment, the dermatologic toxicities associated with anti-EGFR monoclonal antibody therapy can be managed, minimizing patient discomfort and the need for therapy interruption and/or discontinuation. Additionally, preemptive treatment can reduce dermatologic toxicity severity, ultimately yielding better quality of life.
Keywords: Dermatologic toxicity management, Epidermal growth factor receptor, Patient outcomes, Skin toxicity
Introduction
Activation of the epidermal growth factor receptor (EGFR), a cell-surface, tyrosine kinase receptor, results in receptor dimerization and tyrosine autophosphorylation, which mediates cell survival, proliferation, angiogenesis, and tumor invasiveness in colorectal cancer (CRC).1 Monoclonal antibody inhibitors of the EGFR have been shown to improve outcomes in patients with CRC.2–5 Two monoclonal anti-EGFR antibodies, panitumumab and cetuximab, have been approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of certain patients with metastatic CRC (mCRC).6–9 Others are currently under investigation (eg, nimotuzumab,10 necitumumab,11 imgatuzumab12). Panitumumab, a fully human anti-EGFR monoclonal antibody, and cetuximab, a chimeric anti-EGFR monoclonal antibody, have demonstrated efficacy in patients with wild-type KRAS CRC in the first-, second-, and third-line settings as monotherapies and combined with chemotherapy.4,5,13–16 Determining KRAS status and, more recently, RAS status (ie, KRAS exon 2, 3, 4, and NRAS exons 2, 3, 4), is extremely important in CRC because patients with mutated, constitutively active RAS will not respond to panitumumab or cetuximab therapy.5,14–20 The current guidelines from the National Comprehensive Cancer Network and European Society of Medical Oncology recommend the use of panitumumab and cetuximab as appropriate options for patients with wild-type RAS mCRC and recommend the use of extended RAS testing for all patients before receiving treatment with these anti-EGFR antibodies.21,22
Treatment with anti-EGFR agents has been associated with a number of dermatologic toxicities (including skin rash, abnormal hair growth, ocular abnormalities). These toxicities can occur frequently: ~90% of patients will experience skin toxicity of any grade during treatment with panitumumab or cetuximab monotherapy, although most events will be grade 1 or 2 in severity2,23 and rarely life-threatening. In a systematic review of 8998 patients with cancer, no deaths were attributed to dermatologic toxicity.24 However, because these toxicities can result in treatment discontinuation and can potentially affect a patient’s emotional and physical well-being, their management should be an important focus when administering these agents.25 Guidance on the management of skin toxicity occurring during treatment with EGFR inhibitors in patients with cancer was reported in 2009.25
Since 2009, the methods for treatment of mCRC with anti-EGFR antibodies have changed because of important developments in the management of such skin toxicity and changes in the clinical use of anti-EGFR monoclonal antibodies. Anti-EGFR therapy was initially approved as third-line therapy2,23; however, subsequent approvals for use as first- and second-line therapy and combined with chemotherapy have occurred in the United States, Europe, Canada, and other localities.7,9,26,27 Evidence has also shown that the incidence and severity of dermatologic toxicity can be influenced by the addition of chemotherapy.2,4,5,23,28 New approaches to the management of skin toxicity have been used, such as the introduction of novel therapeutic agents and the use of preemptive treatment approaches based on the regimens evaluated in the STEPP (Skin Toxicity Evaluation Protocol with Panitumumab) and J-STEPP (randomized controlled trial on the skin toxicity of panitumumab in Japanese patients with metastatic colorectal cancer: HGCSG1001 study) randomized studies, which showed that preemptive treatment resulted in a reduced incidence of skin toxicity compared with reactive treatment.29,30 Furthermore, new evidence has shown associations between skin toxicity and both efficacy outcomes31 and patient quality of life.32,33 Given these changes, an updated report providing information on the management of dermatologic toxicity during treatment with anti-EGFR inhibitors in patients with mCRC would be of significant value. To address this need, we conducted a systematic review of recent data to examine the types and frequencies of dermatologic toxicities associated with anti-EGFR therapies and to explore the management and treatment options for patients with CRC currently used by clinicians.
Patients and Methods
Search Parameters
A search of the National Library of Medicine PubMed database was performed to identify relevant data discussing the management of dermatologic toxicities associated with the use of anti-EGFR therapies in the treatment of mCRC and the association between toxicity and patient outcomes. The lower limit date of the search was set at January 1, 2009, to capture studies reported after the 2009 Journal of the National Comprehensive Cancer Network publication. The upper limit date was set at August 18, 2016. Search terms were selected that would capture studies addressing anti-EGFR therapies, CRC, dermatologic toxicity, or treatment of dermatologic toxicity (Table 1).
Table 1.
Literature Review Search Terms
| (Vectibix [tiab] OR panitumumab [tiab] OR ABX-EGF [tiab] OR Panitumumab [nm] OR Erbitux [tiab] OR cetuximab [tiab] OR IMC-C225 [tiab] OR Cetuximab [nm] OR nimotuzumab [tiab] OR Theracim [tiab] OR Theraloc [tiab] OR “BIOMAb EGFR” [tiab] OR matuzumab [tiab] OR “EMD 72000” [tiab] OR zalutumumab [tiab] OR EGFR [tiab] OR HuMax-EGFR [tiab]) |
| AND |
| (Colorectal [tiab] OR colon [tiab] OR rectum [tiab] OR rectal [tiab] OR colonic [tiab]) |
| AND |
| (skin [tiab] OR rash [tiab] OR integument [tiab] OR dermatitis [tiab] OR stomatitis [tiab] OR pruritus [tiab] OR papulopustular [tiab] OR acne [tiab] OR “dry skin” [tiab] OR eczema [tiab] OR nail [tiab] OR erythrodysesthesia [tiab] OR erythrodysaesthesia [tiab] OR erythema [tiab] OR paronychia [tiab] OR eye [tiab] OR retina [tiab] OR Acanthosis [tiab] OR acne pustular [tiab] OR angiokeratoma [tiab] OR blister [tiab] OR capillaritis [tiab] OR cataract [tiab] OR cellulitis [tiab] OR chorioretinitis [tiab] OR conjunctivitis [tiab] OR corneal [tiab] OR acneiform [tiab] OR herpetiformis [tiab] OR psoriasiform [tiab] OR eosinophilia [tiab] OR dry eye [tiab] OR dry skin [tiab] OR endophthalmitis [tiab] OR exfoliative [tiab] OR eye pruritus [tiab] OR eyelash thickening [tiab] OR eyelids pruritus [tiab] OR hyperkeratosis [tiab] OR keratitis [tiab] OR nail bed inflammation [tiab] OR nail psoriasis [tiab] OR optic neuropathy [tiab] OR palmar erythema [tiab] OR palmar-plantar erythrodysaesthesia syndrome [tiab] OR palmar-plantar erythrodysesthesia syndrome [tiab] OR perivascular dermatitis [tiab] OR petechiae [tiab] OR plantar erythema [tiab] OR psoriasis [tiab] OR pustular psoriasis [tiab] OR rash papular [tiab] OR papulosquamous [tiab] OR pruritic [tiab] OR rash pustular [tiab] OR xeroderma [tiab] OR retinitis [tiab] OR exfoliation [tiab] OR photosensitivity [tiab] OR onycholysis [tiab] OR maceration [tiab] OR erythematous [tiab] OR hair [tiab] OR ulceration [tiab] OR alopecia [tiab] OR folliculitis [tiab] OR maculo-papular [tiab] OR “vision blurred” [tiab] OR “blurred vision” [tiab] OR cheilitis [tiab] OR excoriation [tiab] OR hypertrichosis [tiab] OR hyperpigmentation [tiab] OR urticaria [tiab] OR conjunctival [tiab] OR “eye swelling” [tiab] OR hirsutism [tiab] OR “ingrowing nail” [tiab] OR “lip dry” [tiab] OR onychoclasis [tiab] OR papule [tiab] OR pigmentation [tiab] OR scab [tiab] OR xerosis [tiab] OR abscess [tiab] OR fissure [tiab] OR eyelid [tiab] OR eyes [tiab] OR nails [tiab] OR eyelashes [tiab] OR hordeolum [tiab] OR dermal [tiab] OR intertrigo [tiab] OR macular [tiab] OR ocular [tiab] OR onychomadesis [tiab] OR onychomycosis [tiab] OR otitis [tiab] OR periorbital [tiab] OR seborrhoea [tiab] OR seborrhoeic [tiab] OR purpura [tiab] OR sunburn [tiab] OR retinopathy [tiab] OR dermatologic [tiab] OR dermatology [tiab] OR dermatologist [tiab] OR soap [tiab] OR cream [tiab] OR “topical steroid” [tiab] OR hydrocortisone [tiab] OR moisturizer [tiab] OR sunscreen [tiab] OR Lubriderm [tiab] OR PABA [tiab] OR SPF [tiab] OR doxycycline [tiab] OR “hair changes” [tiab] OR) |
| AND |
| (“2009/01/01”[PDAT]: ”2016/8/18“[PDAT]”) |
Abbreviations: nm = supplementary concept; PDAT = publication date; Tiab = title or abstract.
Data Collection and Analysis
Studies were selected for inclusion in the systematic review if they reported the incidence of dermatologic toxicity, treatment options, guidelines or recommendations for managing dermatologic toxicity, or an association between dermatologic toxicity and patient outcomes. All of us participated in the study selection and review. The studies were grouped by type (eg, randomized trials, nonrandomized trials, case reports, medical record reviews or case studies, economic analyses, letters to the editor, systematic reviews or meta-analyses, observational studies, preclinical studies, retrospective reviews, and reviews). The reports were rated by the level of evidence using the GRADE approach developed by the Agency for Healthcare Research and Quality.34
Review
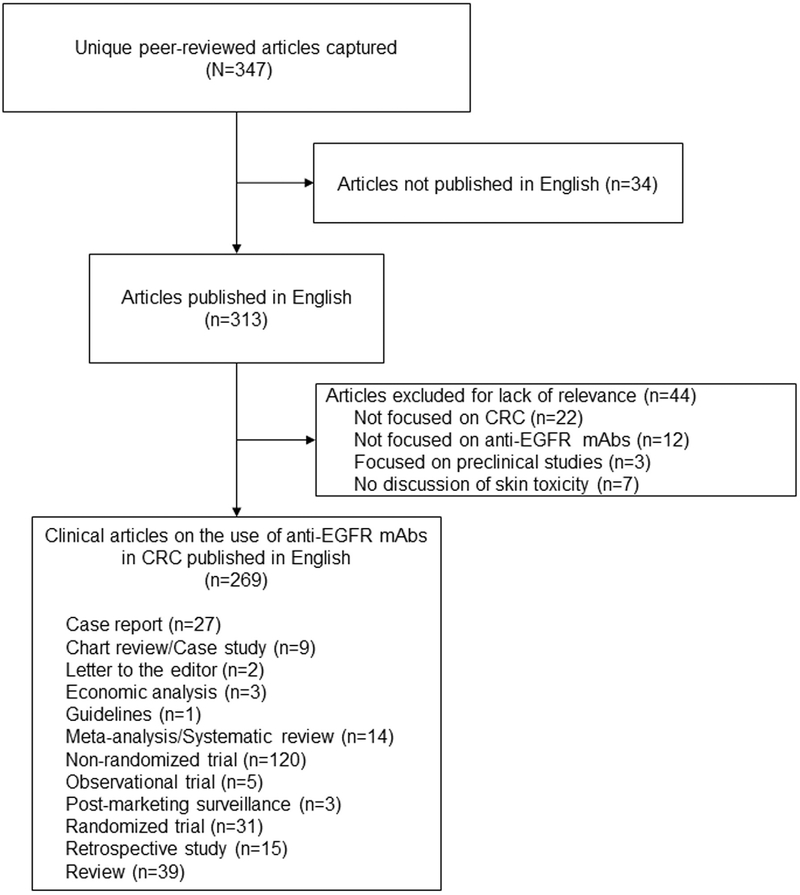
Using the defined criteria, a total of 347 reports were obtained for review. We excluded 34 studies because they had been reported in a language other than English and 44 because they did not meet the article inclusion criteria. Overall, 269 reports were reviewed for the present analysis (Figure 1).
Figure 1.
Types of Studies Included in the Systematic Review
Abbreviations: CRC = colorectal cancer; EGFR = epidermal growth factor receptor; mAbs = monoclonal antibodies.
Incidence of Dermatologic Toxicities
Dermatologic toxicity of any grade occurs in most patients who receive anti-EGFR therapy, and ~10% to 20% of patients will experience grade 3/4 toxicity.4,15,35,36 The overall incidence of grade 3/4 skin toxicity was greater in phase III studies of anti-EGFR therapy combined with either 5-fluorouracil, leucovorin, and irinotecan (FOLFIRI; CRYSTAL, 20050181) or 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX; Panitumumab Randomized Trial in Combination with Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy [PRIME], Oxaliplatin and Cetuximab in First-line Treatment of mCRC [OPUS]; 18%–35%) compared with EGFR inhibitor monotherapy (20020408, A Study of Panitumumab Efficacy and Safety Compared to Cetuximab [ASPECCT], 20100007; < 15%).3,4,28,36,37 The results from the ASPECCT study indicated that the incidence, severity, and nature of dermatologic toxicity occurring with either panitumumab or cetuximab treatment are generally similar.36 The most frequent grade 1/2 skin-related toxicities that occurred in the panitumumab and cetuximab arms were skin rash (45.4% vs. 47.4%), dermatitis acneiform (24.4% vs. 24.3%), dry skin (16.5% vs. 15.7%), pruritus (ie, severe itching; 15.9% vs. 17.3%), paronychia (9.5% vs. 12.9%), and acne (9.9% vs. 12.7%). Few patients had grade ≥3 skin-related toxicities (Table 2).
Table 2.
Incidence of Dermatologic Toxicity Among Patients Receiving Panitumumab or Cetuximab in the ASPECCT Study
| Variable | Panitumumab (n = 496) | Cetuximab (n = 503) | ||||
|---|---|---|---|---|---|---|
| Grades 1/2 | Grade 3 | Grade 4 | Grades 1/2 | Grade 3 | Grade 4 | |
| Patient incidence of skin and subcutaneous tissue toxicitya | 368 (74.2) | 60 (12.1) | 2 (0.4) | 392 (77.9) | 48 (9.5) | 0 (0.0) |
| Skin toxicity adverse events in > 5% of patients in either treatment arm | ||||||
| Rash | 225 (45.4) | 23 (4.6) | 1 (0.2) | 239 (47.5) | 18 (3.6) | 0 (0.0) |
| Dermatitis acneiform | 121 (24.4) | 17 (3.4) | 0 (0.0) | 122 (24.3) | 14 (2.8) | 0 (0.0) |
| Dry skin | 82 (16.5) | 1 (0.2) | 0 (0.0) | 79 (15.7) | 0 (0.0) | 0 (0.0) |
| Pruritus | 79 (15.9) | 4 (0.8) | 0 (0.0) | 87 (17.3) | 1 (0.2) | 0 (0.0) |
| Paronychia | 47 (9.5) | 11 (2.2) | 0 (0.0) | 65 (12.9) | 10 (2.0) | 0 (0.0) |
| Acne | 49 (9.9) | 3 (0.6) | 0 (0.0) | 64 (12.7) | 5 (1.0) | 0 (0.0) |
| Skin fissures | 41 (8.3) | 1 (0.2) | 0 (0.0) | 40 (8.0) | 3 (0.6) | 0 (0.0) |
| Nail disorder | 25 (5.0) | 1 (0.2) | 0 (0.0) | 29 (5.8) | 2 (0.4) | 0 (0.0) |
Data presented as n (%).
Including adverse events in the “Skin and Subcutaneous Tissue Disorders” system organ class of the Medical Dictionary for Regulatory Activities, version 15.1.
Skin Rash and Dermatitis.
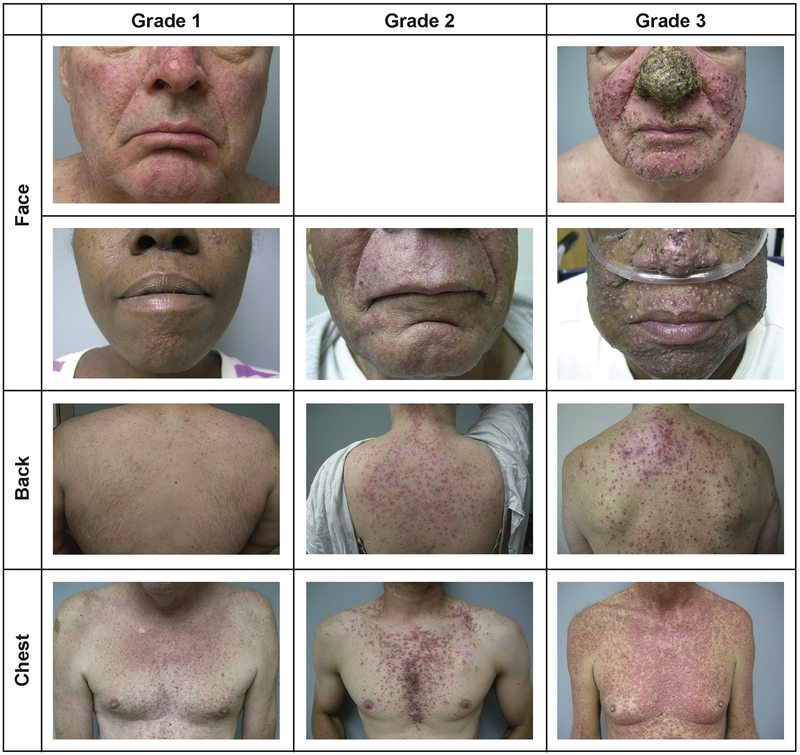
The most common dermatologic toxicity associated with the use of anti-EGFR inhibitors is a papulopustular/acneiform rash, which, if it occurs, will usually appear within the first 1 to 2 weeks of initiating anti-EGFR therapy.25 The Common Terminology Criteria for Adverse Events (CTCAE), version 4.0, grades skin rash using a scale from 1 to 4.38 Grading the severity of skin toxicity using the CTCAE considers the physical manifestations of these events, in addition to their psychosocial impact, effect on activities of daily living, and the need for intravenous antibiotics (Figure 2). In addition to rash, xerosis (ie, rough, dry skin; Figure 3A) and pruritus (ie, severe itching sensation of the skin that provokes the need to scratch) are common occurrences. The first symptoms of xerosis typically occur within 1 to 2 months of the initiation of therapy, and pruritus usually develops 2 to 3 weeks after the initiation of anti-EGFR therapy.39 In most cases, pruritus will be mild or localized and can be managed with topical intervention, with very few patients experiencing grade ≥3 pruritus. However, even in these instances, pruritus can affect patients’ quality of life and activities of daily living (Table 2).36
Figure 2.
Photographs of Skin Rash Occurring During Anti-epidermal Growth Factor Receptor Monoclonal Antibody Treatment According to Body Location and Grade. Grade 1 Skin Rash Is Defined as Papules/Pustules Covering < 10% of the Body. Grade 2 Skin Rash Is Defined as Papules/Pustules Covering 10% to 30% of the Body and Is Associated With Psychosocial Effects and Limiting Daily Life. Grade 3 Rash Is Defined as Papules/Pustules Covering > 30% of the Body That Limit Daily Life and Are Associated With Local Superinfection Requiring Oral Antibiotics
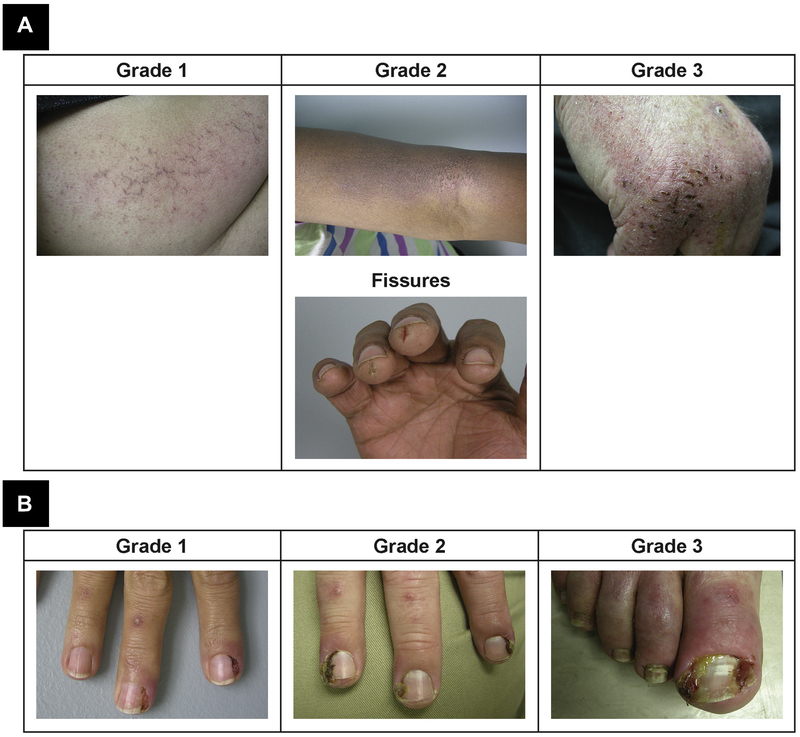
Figure 3.
Photographs of (A) Xerosis and (B) Paronchyia Occurring During Anti-Epidermal Growth Factor Receptor (EGFR) Therapy by Grade. The First Symptoms for Xerosis (ie, Rough, Dry Skin) Typically Occur Within 1 to 2 Months of Initiation of anti-EGFR Therapy. Paronchyia (ie, Inflammation of the Nail Folds of the Fingernails and Toenails) Can Lead to Infection and Swelling/Tenderness and Usually Develops After Skin Reactions, Within 20 Days to 6 Months After Treatment Initiation
Nail Changes.
Paronychia, an inflammation of the nail folds of the fingernails and toenails, can lead to infection, and the consequent swelling and tenderness often affect patients’ activities of daily living (Figure 3B). Paronychia typically develops after skin reactions, usually within 20 days to 6 months of initiating anti-EGFR treatment.25 Approximately 10% to 30% of patients experience paronychia during anti-EGFR therapy.40–44
Hair Abnormalities.
Because the EGFR is expressed in both the keratinocytes of the epidermis and at the root of hair follicles, anti-EGFR therapies can also affect hair growth.25 Most hair-growth abnormalities associated with anti-EGFR therapies occur on the scalp or eyelashes; however, hair abnormalities vary with body location and among individuals.25 Treatment with anti-EGFR therapies can cause both scalp and body alopecia and can also cause trichomegaly, a rare condition in which the eyelashes grow long and curl inward. Trichomegaly will occur in approximately 30% of patients.40
Ocular Conditions.
The EGFR is also expressed on the eye surfaces and in the tear and sebaceous glands; thus, ≤ 15% of patients receiving anti-EGFR therapy can experience ocular toxicity.45 Among the patients who develop these issues, the most frequent include foreign body sensations (38%), dryness (32%), itchiness (28%), rash (22%), redness (14%), eyelash changes (12%), blurry vision (7%), tearing (6%), burning (3%), and photophobia (3%).45 Additionally, conjunctivitis has been reported in ~6% to 20% of patients.40
Management of Dermatologic Toxicities
A variety of treatment options and approaches have been shown to be effective in the management of the dermatologic toxicities associated with anti-EGFR therapy in patients with mCRC. Guidance for the management of specific dermatologic toxicities, considering both the nature and severity of the event, is provided in the subsequent sections.
In general, for patients who develop mild to moderate (ie, grade 1/2) skin reactions, these skin-related toxicities are commonly managed with inexpensive emollient ointments and moisturizers, the avoidance of sun exposure,46–48 avoidance of the use of irritants,47–51 and the use of short showers. Patients should avoid alcohol-based or perfumed products because they can dry the skin47–51 and limit the skin’s ability to heal by keeping it in a state of stress. However, the use of alcohol-free products that contain esterified alcohols (eg, cetyl, stearyl, and cetaryl alcohols) that do not irritate the skin can be effective.52 In patients with moderate to severe skin toxicities, application of topical steroid creams, such as hydrocortisone (0.5%–2.5%), alone or combined with topical emollients and moisturizers, can be required.29,48,53,54 In instances in which the dermatologic skin toxicity leads to infection, topical antibiotic ointments or systemic antibiotics must also be given.53,54
In circumstances in which the toxicity is moderate to severe (grade 3/4 events), referral of the patient to a dermatologist is recommended because the dermatologist will have more knowledge and experience treating the more severe dermatologic ailments. However, if this is not practical or expedient, the patient’s primary provider might be able to determine the type of treatment required to manage dermatologic toxicity. Referral to a dermatologist is also recommended in cases in which the toxicity does not improve within 1 to 2 weeks, if the patient is having difficulty managing more severe skin toxicity and is considering stopping EGFR inhibitor treatment, and in cases in which the patient is severely symptomatic (ie, if necrosis, bleeding, or petechial or purpuric lesions are present or if the skin toxicity has an uncharacteristic appearance or distribution).48
Preemptive Versus Reactive Management of Skin Toxicity.
Measures for managing dermatologic toxicity can be taken either before administering anti-EGFR monoclonal antibody therapy (ie, preemptive treatment) or after symptoms have occurred (ie, reactive treatment). Several studies have examined the effect of preemptive versus reactive treatment regimens on the incidence and severity of skin toxicity.29,30,53 The largest of these trials was the STEPP (Skin Toxicity Evaluation Protocol with Panitumumab) and HGCSG1001 (J-STEPP) studies.
The STEPP trial was a phase II, open-label, randomized trial that evaluated the effect of preemptive versus reactive treatment regimens on skin toxicity management for patients treated with panitumumab combined with irinotecan or FOLFIRI.30 Preemptive treatment was administered beginning on day −1 and continued for weeks 1 to 6. Preemptive treatment consisted of the use of a skin moisturizer (applied to the face, hands, feet, neck, back, and chest daily in the morning), sunscreen (applied before going outdoors), topical steroids (1% hydrocortisone cream applied to the face, hands, feet, neck, back, and chest daily in the evening), and oral doxycycline (100 mg, taken twice daily).30 Reactive treatment consisted of any treatment deemed appropriate by the investigator and could be administered during weeks 1 to 6.30 The results from the STEPP trial showed the incidence of grade ≥2 skin toxicities was 29% among patients who received preemptive treatment compared with 62% in the reactive group (odds ratio, 0.3; 95% confidence interval [CI], 0.1–0.6).30 Six percent of patients in the preemptive arm developed grade 3/4 skin toxicity compared with 21% in the reactive group. Efficacy was similar between the 2 groups: 7 patients (15%) in the preemptive group had a partial response compared with 5 patients (11%) in the reactive group. Also, the stable disease rate was similar between the 2 groups (50% vs. 53%).30 Furthermore, the median progression-free survival (PFS) time was 4.7 months (95% CI, 2.9–6.0) in the preemptive group and 4.1 months (95% CI, 2.9–6.2) in the reactive group (hazard ratio [HR], 1.0; 95% CI, 0.6–1.6).30
The J-STEPP study was a phase III, open-label, randomized trial that evaluated the differences between preemptive and reactive treatment in skin toxicity management in Japanese patients with mCRC. A central review of skin toxicities was also performed by a single dermatologist, using photographs that were taken at every office visit.29 The treatments used were similar to those in the STEPP study. Preemptive treatment consisted of a skin moisturizer and topical steroid (0.5% hydrocortisone cream applied to the face, hands, feet, neck, back, and chest twice daily morning and evening), sunscreen (applied to sun-exposed areas before going outside), and minocycline (100 mg, once daily).29 Reactive treatment consisted solely of a skin moisturizer, although the use of sunscreen was permitted if requested by the patient.29 The results indicated the cumulative incidence of grade ≥2 skin toxicities in 6 weeks was 21.3% (95% CI, 9.6–33.0) in the preemptive treatment group compared with 62.5% (95% CI, 48.8–76.2) in the reactive treatment group (relative risk, 0.34; 95% CI, 0.19–0.62; P < .001). Similar incidence trends were observed at 8 and 12 weeks.29 Two percent of patients in the preemptive arm and 15% in the reactive arm developed grade 3/4 skin toxicity.29 No statistically significant differences were found in PFS (HR, 1.20; 95% CI, 0.78–1.84; P = .413), overall survival (OS; HR, 1.19; 95% CI, 0.75–1.90; P = .469), or the objective response rate (ORR; pre-emptive, 13.3%; reactive, 18.2%; P = .530) were observed between the 2 groups.29
In both the STEPP30 and J-STEPP29 studies, skin toxicity symptoms and treatment compliance were documented by patients using daily diaries, and this information was used by the investigators to complete case report forms. Because these data were self-reported daily by patients, the possibility exists that an inherent bias could have been introduced and that the patients’ mental state or level of overall physical health could have affected their perception of the severity of their symptoms. Thus, this approach might have resulted in a greater recorded overall incidence of skin toxicity compared with the more objective investigator assessments of toxicity typically used in clinical trials. The results from these studies indicated that preemptive treatment can reduce the incidence of skin toxicity during treatment with panitumumab without altering antitumor efficacy. Together, these data indicate that the use of a preemptive management regimen can decrease the occurrence and severity of dermatologic toxicity resulting from anti-EGFR therapy with no significant effects on treatment efficacy. The recommended preemptive treatments for dermatologic toxicity include avoidance of sun exposure, hydrocortisone combined with moisturizers, the daily use of sunscreen, doxycycline, minocycline, oral antibiotics, corticosteroid creams, and/or oatmeal baths.29,30,46,47,53,54
Skin Rash and Dermatitis.
The treatments commonly used for the management of skin rash are detailed in Table 3. The reported data have described treatment of patients with grade 1 or 2 skin rash to include petrolatum emollients, medium- to high-potency topical corticosteroids, oral minocycline or doxycycline, 0.5% to 2.5% hydrocortisone cream, H1 antihistaminic loratadine, and saline/boric acid compresses.40,51,55–59 Topical antibiotics such as clindamycin, erythromycin, and metronidazole and benzoyl peroxide can be used to treat skin toxicities; however, these are usually avoided in patients with papulopustular or acneiform eruption because these treatments have drying properties that can induce rosacea and can be irritating.40,60 Additionally, the use of topical retinoids is typically avoided because of their greater potential for irritation.57 Patients are cautioned to avoid sunbathing, direct sunlight, hot temperatures, and humidity.48,58,59,61 Patients should avoid manipulation of skin or hot blow-drying of the hair because these can increase the risk of infection.39 Although the use of greasy creams such as petroleum jelly is highly effective, the treatment can cause folliculitis owing to its occlusive properties.
Table 3.
Management of Skin Rash Associated With Anti-EGFR Therapies
| Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Corticosteroids | Minocycline/doxycycline | Minocycline/doxycycline | Minocycline/doxycycline |
| Minocycline/doxycycline | Low-dose isotretinoin | Low-dose isotretinoin | Anti-EGFR discontinuation |
| Antibiotics (clindamycin, erythromycin) | Menthol cream | Oral or IV antihistamines | |
| Benzoyl peroxide | Oral antihistamines | High-dose tetracycline | |
| Metronidazole | Antibiotics | Clindamycin | |
| Avoidance of sun, heat, humidity | Wet compresses | IV antibiotics | |
| Hydrocortisone cream | Hydrocortisone cream | ||
| Metronidazole | Anti-EGFR dose reduction | ||
| Saline/boric acid compresses |
Abbreviations: EGFR = epidermal growth factor receptor; IV = intravenous.
For patients with grade 3/4 skin rash, the reports most commonly suggested temporary dose interruption and/or reduction of anti-EGFR therapy, depending on the severity of the rash and the patient’s tolerance level.47,54,56,61,62 In addition to the suggested reductions, other possible recommended treatment methods include the administration of doxycycline; minocycline; oral; intramuscular; intravenous antihistamine; high-dose tetracycline; oral corticosteroids (methylprednisolone, prednisone); oral retinoids (low-dose isotretinoin); or intravenous antibiotics.47,56,57,61
Treatment of xerosis and/or pruritus often includes the use of moisturizers or oral antihistamines.57,58 Emollients and cyanoacry-late tissue adhesives can effectively treat xerosis but must be used with care because their occlusive properties can lead to folliculitis.63,64 For patients who experience eczema, treatment with cortico steroids is recommended; for those who experience eczema with blisters (wet eczema), cultures should be conducted to ensure no bacterial or viral infection is present.64
Nail Changes.
When monitoring for paronychia or fissures, it is important to begin careful inspections of the hands and feet early during treatment and to continue these inspections at every clinic visit. If nail abnormalities are identified, the treatments described included antibacterial emollient applied regularly, corticosteroid ointments, or a white vinegar in water solution (1 part vinegar, 1 part water).61,65 Other suggested treatments for paronychia include the use of gentamycin ointment for 4 to 5 weeks, bathing the hands and/or feet in a diluted chloramine bath, and wearing loose-fitting shoes to avoid pressure to the nail beds.40,50 Preemptive daily treatment with corticosteroids was shown to reduce the incidence of paronychia in both the J-STEPP and STEPP studies.29,30
Hair Abnormalities.
For the treatment of trichomegaly, the reported data suggest trimming eyelashes that are long or curled (usually by an ophthalmologist) and removing eyelashes that have misdirected growth (again, usually by an ophthalmologist).40,45,61 For other hair and scalp problems, emollients, such as those used for skin rash, and erythromycin ophthalmic ointments can be used.40,51 Patients who experience an acneiform eruption on the scalp can be treated with an oil bath and topical steroid therapy.40 If the eruption is infected, an oil bath, followed by treatment with oral antibiotics, has been recommended; topical antibiotics, such as neomycin and bacitracin, are not effective in the treatment of scalp infections.40,66 Hair stickiness can also be treated with an oil bath or using mild shampoos.40
Ocular Conditions.
The treatments for ocular conditions are outlined in Table 4. For patients with a mild case of dry eye, treatment with supplemental tears 4 to 6 times daily has been suggested.45,54 For cases that are moderate to severe, the use of tear film or anti-inflammatory medication could be needed.45 For patients who experience blepharitis (ie, eyelid margin inflammation), recent reports suggested treatment with lid scrubs and warm compresses for 5 minutes twice daily for those with mild cases.45,54 Moderate cases might require treatment with an eye ointment,45 and severe cases could require treatment with doxycycline 50 mg twice daily for 2 weeks, followed by 50 mg once daily for 4 weeks.45
Table 4.
Management of Ocular Conditions Associated With Anti-EGFR Therapies
| Severity | Dry Eye | Blepharitis | Eyelid Hyperemia | Conjunctivitis | Telangiectasias |
|---|---|---|---|---|---|
| Mild or acute | Supplemental tears 4–6 times QD | Lid scrubs and warm compresses for 5 min BID | Fluorometholone (0.1%) 1–3 times daily (1 wk) | Ophthalmic suspension of neomycin, polymyxin B sulfates, and dexamethasone (14 days) | Laser therapy |
| Moderate/severe or chronic | Tear film | Eye ointment | Tacrolimus (0.03%) ointment BID | Ophthalmic suspension of neomycin, polymyxin B sulfates, and dexamethasone (14 days) | Laser therapy |
| Anti-inflammatory medication | Doxycycline 50 mg BID (2 wk), 50 mg QD (4 wk) | Pimecrolimus cream BID |
Abbreviations: BID = twice daily; EGFR = epidermal growth factor receptor; QD = once daily.
For the treatment of eyelid hyperemia (vascular engorgement of the eyelid), the reports suggested treating acute instances with fluorometholone (0.1%) 1 to 3 times daily for 1 week.45 For chronic cases, treatment with tacrolimus (0.03%) ointment or pimecrolimus cream twice daily might be required.45 For patients who contract conjunctivitis, treatment of the eye with an ophthalmic suspension of neomycin and polymyxin B sulfates and dexamethasone for 14 days can be used.40 To treat patients who develop telangiectasias (ie, dilation of the capillaries in the eye, causing the appearance of small red or purple clusters), laser therapy is advised.40
For all instances of ocular toxicity, patients should be referred to an ophthalmologist if they experience persistent ocular pain, significant loss of vision or decreased acuity of vision, or severe redness of the eye or light sensitivity.67 Patients should also be referred to a specialist if they fail to respond within 1 week of treatment initiation for squamous blepharitis, meibomitis, or dysfunctional tear syndrome.67
Treatments Currently Under Investigation.
Numerous new options are currently under investigation for the management of dermatologic toxicities, and most of these treatments are targeted for the treatment of skin rash. One treatment option, Abound, which is a mixture of β-hydroxyl β-methyl butyrate, glutamine, and arginine, is under investigation in Japan.68 Previously, Abound showed activity for increasing the lean body mass in patients with cachexia caused by cancer or rheumatoid arthritis.69,70 In a case report, the use of Abound on the lower limbs of a patient receiving anti-EGFR therapy resulted in a profound reduction in the extent and severity of dermatologic toxicity after 1 month of continued use.68
The use of phytomenadione cream (vitamin K1) for the treatment of skin rash associated with anti-EGFR therapy is also under investigation.59,71–73 A small case series (n = 20) investigated the application of phytomenadione cream as a pretreatment for anti-EGFR therapy.71 Patients applied the cream twice daily during the first month of therapy, beginning the day before infusion, and daily during the second month.71 Most patients (75%) only experienced mild grade 1 acneiform rash with pretreatment; the remaining 25% experienced a grade 2 rash.71 No signs of toxicity or intolerance were observed after the topical application, and no changes in blood coagulation occurred.71 Another study (n = 41) found the application of phytomenadione cream twice daily resulted in a low proportion of patients with grade 2 (25%) and grade 3 (15%) rash.72 Furthermore, a recent study (n = 60) found that applying the cream 3 times daily on the day of anti-EGFR administration improved the skin itch and dry skin symptoms for patients with mCRC.73
The use of bittim soap (made from the oil extracted from Pistacia terebinthus fruits) for the treatment of skin toxicity is also being assessed.74 In a small study (n = 15) that evaluated the use of bittim soap in the treatment of grade 2 or 3 toxicity, patients applied the soap twice daily for 2 minutes and then rinsed for 1 week; the use of topical or oral antibiotics, corticosteroids, or moisturizers was not permitted.74 The complete response rate (ie, disappearance of all toxicity) for patients with grade 2 or 3 toxicity was 100% and 33%, respectively; the remaining patients with grade 3 toxicity improved to grade 1 with treatment.74 Skin toxicity recurred when the use of the soap was discontinued.74
Associations Among Skin Toxicity, Clinical Outcomes, and Quality of Life
Association With Clinical Outcomes.
The appearance of dermatologic toxicity is an on-target event for patients receiving anti-EGFR therapy and direct evidence that EGFR is being inhibited to a biologically meaningful extent. Because this inhibition is required for an antitumor effect to occur, it has been hypothesized that the appearance of dermatologic toxicity might be evidence of antitumor activity. Early studies observed a correlation between better outcomes and patients who developed dermatologic toxicity early after treatment.75,76 Subsequently, numerous other studies (which have been extensively reviewed previously31,48,77–81) also described positive associations between the severity of skin toxicity and outcomes, such as the ORR, OS, PFS, and time to tumor progression, among patients receiving cetuximab or panitumumab.82,83
Association With Quality of Life.
The appearance of dermatologic toxicity has the potential to severely affect a patient’s quality of life and activities of daily living, possibly resulting in missed anti-EGFR therapy doses, dose reductions, and/or the complete cessation of therapy.
Using a dermatology-specific quality-of-life questionnaire (Skindex-16) to evaluate the domains of symptoms, emotion, and function, Rosen et al33 found that patients with advanced cancer who experienced rash or pruritus associated with an anti-EGFR therapy (cetuximab, panitumumab, erlotinib, gefitinib, or lapatinib) had higher scores across all 3 domains compared with patients who had not received anti-EGFR therapy and did not experience these events. In a subsequent study that used Skindex-16 to evaluate the quality of life of patients who received anti-EGFR therapy, the rash grade was significantly associated with greater Skindex-16 scores, suggesting that the National Cancer Institute CTCAE grade represents an appropriate tool for the assessment of skin toxicity severity on patient quality of life.32
A number of other studies have evaluated the quality of life of patients with and without skin toxicity but did not demonstrate a direct association between skin toxicity and quality of life.76,84–86 However, most of the quality-of-life instruments used in these studies were not designed to evaluate the influence of skin toxicity on quality of life and might have lacked sensitivity for this outcome.32,33 One study reported that 41% of patients treated with anti-EGFR therapy showed psychological distress; however, no significant correlation was found between the appearance of skin rash and psychological distress. However, the study also noted that 47% of the patients avoided social situations and going out, that patients with a longer history of disease considered skin rash to be part of coping with advanced cancer, and that patients can be encouraged to continue treatment because the presence of a skin rash is indicative of the response.87 Another study found that the perceived severity of the skin reaction had a significant influence on the dermatologic health-related quality of life, as measured using the Deutsches Instrument zur Erfassung der Lebensqualität bei Hauterkrankungen, and this perception remained stable throughout the course of treatment.88 No significant correlation was found between the objective severity of the skin reaction and the Deutsches Instrument zur Erfassung der Lebensqualität bei Hauterkrankungen score.88 Furthermore, in 1 study, increased skin toxicity severity during panitumumab treatment was associated with better quality of life, as measured using the modified Dermatology Quality of Life Index (mDLQI).76 This plainly paradoxical outcome might have resulted from an association between skin toxicity severity and the duration of therapy or an association between the biologic effect of panitumumab and skin toxicity, with such associations resulting in improved patient outcomes that potentially result in improvements in aspects of quality of life that outweigh the influence of skin toxicity.
Because maintaining patient morale is an important part of cancer therapy, every opportunity should be taken to minimize the potential effects of dermatologic toxicity on patients’ quality of life. A few studies have indicated that the use of preemptive treatment for managing dermatologic toxicity can improve patient quality of life. In 1 study, after preemptive treatment, only 3 patients of 51 reported a moderate effect of skin toxicity on their overall quality of life, as measured using the mDLQI.89 Similarly, the results from the STEPP trial indicated a reduced mean mDLQI score for patients who received preemptive treatment for dermatologic toxicity compared with those receiving reactive treatment, indicating that reducing the appearance of early skin reactions could have a significant effect on patient perceptions and morale.30
Costs Associated With Dermatologic Toxicity
Relatively few studies thus far have evaluated the costs associated with developing dermatologic toxicity during anti-EGFR treatment. However, 1 economic analysis noted that dermatologic toxicity requiring inpatient treatment (eg, hospitalization) resulted in substantially greater costs (~$4500/event) than if the toxicity could be treated in the outpatient setting ($185/event).90 Furthermore, a retrospective analysis found that little difference was present in the costs of treatment for a grade 2 (range, 200€–295€) versus grade 3 (range, 159€–234€) rash; however, the cost for grade 1 rash was minimal (no treatments were initiated).91 Together, these studies suggest that appropriate management to reduce the severity of dermatologic reactions (ie, using preemptive treatment) could translate into substantial cost reductions for the patient, in addition to the other benefits of such an approach.
Discussion
In the present review, we described the management and treatment options for dermatologic toxicity, which generally consist of the application of topical moisturizers or corticosteroid creams for lesser grade occurrences or the use of systemic treatments such as oral antibiotics for more severe occasions. In addition to these treatment options, patients should be counseled to avoid sun exposure and excessive heat, to wear loose fitting clothing and shoes, and to use alcohol-free products that do not irritate the skin.
Recent studies have suggested that these treatment methods appear to be more effective when applied as part of a preemptive regimen rather than after skin toxicity has occurred. The results from the STEPP and J-STEPP studies have indicated that the patients who underwent preemptive treatment for dermatologic toxicity had a lower incidence of grade ≥2 skin toxicity compared with those who received reactive treatment (20%–30% vs. 60%), with no significant effect on efficacy outcomes, including PFS, OS, and ORR.29,30 Furthermore, preemptive therapy can also reduce the incidence of diarrhea, dehydration, and neutropenia. Decreased recruitment of neutrophils to the skin and maintenance of the integrity of the skin might minimize the incidence of neutropenia and dehydration.29,30 EGFR-induced diarrhea also has an inflammatory or infectious component that can be improved through use of doxycycline therapy. Preemptive treatment with doxycycline therapy might also play a role in reducing secondary dermatologic infections.92
Recent retrospective analyses have shown that patients who experienced more severe dermatologic toxicity or dermatologic toxicity that occurred early after treatment had improved outcomes compared with those who had less severe reactions or reactions that occurred later after treatment, suggesting that the development of skin toxicity might be indicative of a positive response to anti-EGFR therapy. This association between skin toxicity and tumor outcomes suggests potential predictive value exists for dermatologic toxicity in patients with mCRC; however, this association is potentially confounded by exposure. Patients who have received more anti-EGFR inhibitor exposure are more likely to develop dermatologic toxicity and also to have a tumor response; therefore, it is impossible to be certain whether dermatologic toxicity is truly predictive of the response or just correlated with the outcomes. Although the development of dermatologic toxicity might be indicative of a positive response and the development of toxicity has not been directly associated with a poorer quality of life for these patients, the proper management of this toxicity remains essential to minimize patient discomfort during therapy.
The present systematic review was subject to limitations. The analysis was limited to studies of CRC; reports of other cancer types for which anti-EGFR therapies are used or those for which the cancer type was not specified were not included. Additionally, studies that were not reported in peer-reviewed journals (ie, have only been presented at conferences) or that were reported in journals that are not indexed in PubMed were not included. Furthermore, this search was limited to studies reported in English.
Conclusion
With appropriate treatment, dermatologic toxicities associated with anti-EGFR therapies can be managed, minimizing patient discomfort and reducing the need for therapy interruption or discontinuation. Furthermore, the preemptive treatment of patients can reduce the severity of dermatologic toxicities that result from anti-EGFR therapy, ultimately leading to a better patient quality of life.
Acknowledgments
The authors thank Meghan Johnson, PhD (Complete Healthcare Communications, LLC, West Chester, PA), whose work was funded by Amgen Inc., and Emily Plummer, PhD (Amgen Inc), for medical writing assistance in the preparation of this manuscript. This work was supported by Amgen Inc. M.E.L. is partially funded through the National Institutes of Health/National Cancer Institute Cancer Center Support (grant P30 CA008748).
Disclosure
M.E.L. has been a consultant to Quintiles, Boehringer Ingelheim, AstraZeneca Pharmaceuticals, Genentech, Foamix, Infinity Pharmaceuticals, Janssen, and Novartis and has received research funding from Bristol-Myers Squibb and Berg. M.A. has been a consultant for Best Doctors, Inc, Amgen, and Biogen. A.J. has received research funding from Boston Biologics, Aveo Pharmaceuticals, and Entera Health. T.G. and C.B. are employees of, and stockholders in, Amgen Inc. E.M. has been a consultant for Novartis, Bristol-Myers Squibb, and Amgen.
References
- 1.Weng W, Feng J, Qin H, et al. Molecular therapy of colorectal cancer: progress and future directions. Int J Cancer 2015; 136:493–502. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007; 25:1658–64. [DOI] [PubMed] [Google Scholar]
- 3.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009; 27:663–71. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360:1408–17. [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010; 28: 4697–705. [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency (EMA). Erbitux (cetuximab). Summary of product characteristics. Darmstadt, Germany: Merck; 2014. [Google Scholar]
- 7.Erbitux ®, cetuximab. Indianapolis, IN: Eli Lilly and Company; 2015. [Google Scholar]
- 8.European Medicines Agency (EMA). Vectibix (panitumumab). Summary of product characteristics. Thousand Oaks, CA: Amgen; 2013. [Google Scholar]
- 9.Vectibix ®, panitumumab. Thousand Oaks, CA: Amgen Inc.; 2015. [Google Scholar]
- 10.Jin T, Zhu Y, Luo JL, et al. Prospective phase II trial of nimotuzumab in combination with radiotherapy and concurrent capecitabine in locally advanced rectal cancer. Int J Colorectal Dis 2015; 30:337–45. [DOI] [PubMed] [Google Scholar]
- 11.Elez E, Hendlisz A, Delaunoit T, et al. Phase II study of necitumumab plus modified FOLFOX6 as first-line treatment in patients with locally advanced or metastatic colorectal cancer. Br J Cancer 2016; 114:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delord JP, Tabernero J, Garcia-Carbonero R, et al. Open-label, multicentre expansion cohort to evaluate imgatuzumab in pre-treated patients with KRAS-mutant advanced colorectal carcinoma. Eur J Cancer 2014; 50:496–505. [DOI] [PubMed] [Google Scholar]
- 13.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:1626–34. [DOI] [PubMed] [Google Scholar]
- 14.Peeters M, Price TJ, Cervantes A, et al. Final results from a randomized phase 3 study of FOLFIRI {+/−} panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol 2014; 25:107–16. [DOI] [PubMed] [Google Scholar]
- 15.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011; 22:1535–46. [DOI] [PubMed] [Google Scholar]
- 16.Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 2014; 32:2240–7. [DOI] [PubMed] [Google Scholar]
- 17.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013; 369:1023–34. [DOI] [PubMed] [Google Scholar]
- 18.Peeters M, Kafatos G, Taylor A, et al. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: a pooled analysis of randomised controlled trials. Eur J Cancer 2015; 51:1704–13. [DOI] [PubMed] [Google Scholar]
- 19.Bokemeyer C, Kohne CH, Ciardiello F, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer 2015; 51:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Cutsem E, Lenz HJ, Kohne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015; 33:692–700. [DOI] [PubMed] [Google Scholar]
- 21.NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers, v2.2014. Fort Washington, PA: National Comprehensive Cancer Network; 2014. [Google Scholar]
- 22.Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(suppl 3):iii1–9. [DOI] [PubMed] [Google Scholar]
- 23.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007; 357:2040–8. [DOI] [PubMed] [Google Scholar]
- 24.Jatoi A, Nguyen PL. Do patients die from rashes from epidermal growth factor receptor inhibitors? A systematic review to help counsel patients about holding therapy. Oncologist 2008; 13:1201–4. [DOI] [PubMed] [Google Scholar]
- 25.Burtness B, Anadkat M, Basti S, et al. NCCN task force report: management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw 2009; 7(suppl 1):S5–21, quiz S22eS24. [DOI] [PubMed] [Google Scholar]
- 26.European Medicines Agency. Erbitux European Public Assessment Report, Summary of Product Characteristics 2015. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000558/human_med_000769.jsp&mid=WC0b01ac058001d124. Accessed: April 21, 2016.
- 27.European Medicines Agency. Vectibix European Public Assessment Report, Summary of Product Characteristics 2016. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000741/human_med_001128.jsp&mid=WC0b01ac058001d124. Accessed: April 21, 2017.
- 28.Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010; 28:4706–13. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi Y, Komatsu Y, Yuki S, et al. Randomized controlled trial on the skin toxicity of panitumumab in Japanese patients with metastatic colorectal cancer: HGCSG1001 study; J-STEPP. Future Oncol 2015; 11:617–27. [DOI] [PubMed] [Google Scholar]
- 30.Lacouture ME, Mitchell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010; 28:1351–7. [DOI] [PubMed] [Google Scholar]
- 31.Petrelli F, Borgonovo K, Barni S. The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: a systematic review and meta-analysis of published trials. Target Oncol 2013; 8:173–81. [DOI] [PubMed] [Google Scholar]
- 32.Joshi SS, Ortiz S, Witherspoon JN, et al. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer 2010; 116:3916–23. [DOI] [PubMed] [Google Scholar]
- 33.Rosen AC, Case EC, Dusza SW, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. Am J Clin Dermatol 2013; 14:327–33. [DOI] [PubMed] [Google Scholar]
- 34.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nie F, Shen J, Tong JL, et al. Meta-analysis: the efficacy and safety of monoclonal antibody targeted to epidermal growth factor receptor in the treatment of patients with metastatic colorectal cancer. J Dig Dis 2009; 10:247–57. [DOI] [PubMed] [Google Scholar]
- 36.Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 2014; 15:569–79. [DOI] [PubMed] [Google Scholar]
- 37.Douillard J, Cassidy J, Jassem J, et al. Randomized, open-label, phase III study of panitumumab (pmab) with FOLFOX4 versus FOLFOX4 alone as first-line treatment (tx) for metastatic colorectal cancer (mCRC): efficacy by skin toxicity (ST) [abstract]. J Clin Oncol 2010; 28:3528. [Google Scholar]
- 38.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Bethesda, MD: National Institutes of Health; 2010. [Google Scholar]
- 39.Potthoff K, Hofheinz R, Hassel JC, et al. Interdisciplinary management of EGFR-inhibitor-induced skin reactions: a German expert opinion. Ann Oncol 2011; 22: 524–35. [DOI] [PubMed] [Google Scholar]
- 40.Ocvirk J, Cencelj S. Management of cutaneous side-effects of cetuximab therapy in patients with metastatic colorectal cancer. J Eur Acad Dermatol Venereol 2010; 24: 453–9. [DOI] [PubMed] [Google Scholar]
- 41.Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009; 27: 672–80. [DOI] [PubMed] [Google Scholar]
- 42.Hecht JR, Cohn A, Dakhil S, et al. SPIRITT: a randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin Colorectal Cancer 2015; 14:72–80. [DOI] [PubMed] [Google Scholar]
- 43.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15:1065–75. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell EP, Piperdi B, Lacouture ME, et al. The efficacy and safety of panitumumab administered concomitantly with FOLFIRI or irinotecan in second-line therapy for metastatic colorectal cancer: the secondary analysis from STEPP (skin toxicity evaluation protocol with panitumumab) by KRAS status. Clin Colorectal Cancer 2011; 10:333–9. [DOI] [PubMed] [Google Scholar]
- 45.Borkar DS, Lacouture ME, Basti S. Spectrum of ocular toxicities from epidermal growth factor receptor inhibitors and their intermediate-term follow-up: a five-year review. Support Care Cancer 2013; 21:1167–74. [DOI] [PubMed] [Google Scholar]
- 46.Addeo R, Caraglia M, Cerbone D, et al. Panitumumab: a new frontier of target therapy for the treatment of metastatic colorectal cancer. Expert Rev Anticancer Ther 2010; 10:499–505. [DOI] [PubMed] [Google Scholar]
- 47.Gobbo M, Ottaviani G, Mustacchi G, et al. Acneiform rash due to epidermal growth factor receptor inhibitors: high-level laser therapy as an innovative approach. Lasers Med Sci 2012; 27:1085–90. [DOI] [PubMed] [Google Scholar]
- 48.Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol 2009; 16:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol 2009; 4:107–19. [DOI] [PubMed] [Google Scholar]
- 50.Ouwerkerk J, Boers-Doets C. Best practices in the management of toxicities related to anti-EGFR agents for metastatic colorectal cancer. Eur J Oncol Nurs 2010; 14: 337–49. [DOI] [PubMed] [Google Scholar]
- 51.Pinto C, Barone CA, Girolomoni G, et al. Management of skin toxicity associated with cetuximab treatment in combination with chemotherapy or radiotherapy. Oncologist 2011; 16:228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.US Food and Drug Administration. “Alcohol Free.” 2014. Available at: http://www.fda.gov/Cosmetics/Labeling/Claims/ucm2005201.htm. Accessed: March 14, 2016.
- 53.Dascalu B, Kennecke HF, Lim HJ, et al. Prophylactic versus reactive treatment of acneiform skin rashes from epidermal growth factor receptor inhibitors in metastatic colorectal cancer. Support Care Cancer 2016; 24:799–805. [DOI] [PubMed] [Google Scholar]
- 54.Fuloria J. Safety profiles of current antiangiogenic therapies for metastatic colorectal cancer. Onco Targets Ther 2012; 5:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergman H, Walton T, Del Bel R, et al. Managing skin toxicities related to panitumumab. J Am Acad Dermatol 2014; 71:754–9. [DOI] [PubMed] [Google Scholar]
- 56.Fakih M, Vincent M. Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr Oncol 2010; 17(suppl 1): S18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Noronha e Menezes NM, Lima R, Moreira A, et al. Description and management of cutaneous side effects during erlotinib and cetuximab treatment in lung and colorectal cancer patients: a prospective and descriptive study of 19 patients. Eur J Dermatol 2009; 19:248–51. [DOI] [PubMed] [Google Scholar]
- 58.Jaka A, Gutierrez-Rivera A, Lopez-Pestana A, et al. Predictors of tumor response to cetuximab and panitumumab in 116 patients and a review of approaches to managing skin toxicity. Actas Dermosifiliogr 2015; 106:483–92. [DOI] [PubMed] [Google Scholar]
- 59.Pinto C, Barone CA, Girolomoni G, et al. Management of skin reactions during cetuximab treatment in association with chemotherapy or radiotherapy: update of the Italian expert recommendations. Am J Clin Oncol 2016; 39:407–15. [DOI] [PubMed] [Google Scholar]
- 60.Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol 2005; 16:1425–33. [DOI] [PubMed] [Google Scholar]
- 61.Eng C. Toxic effects and their management: daily clinical challenges in the treatment of colorectal cancer. Nat Rev Clin Oncol 2009; 6:207–18. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Foncillas J, Diaz-Rubio E. Progress in metastatic colorectal cancer: growing role of cetuximab to optimize clinical outcome. Clin Transl Oncol 2010; 12:533–42. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto H. Superglue for the treatment of heel fissures. J Am Podiatr Med Assoc 1999; 89:434–5. [DOI] [PubMed] [Google Scholar]
- 64.Galimont-Collen AF, Vos LE, Lavrijsen AP, et al. Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer 2007; 43:845–51. [DOI] [PubMed] [Google Scholar]
- 65.Rockwell PG. Acute and chronic paronychia. Am Fam Physician 2001; 63:1113–6. [PubMed] [Google Scholar]
- 66.Wiznia LE, Choi JN. Unique presentations of epidermal growth factor receptor inhibitor-induced papulopustular eruption related to bacterial superinfection. Dermatol Online J 2013; 19:8. [PubMed] [Google Scholar]
- 67.Basti S. Ocular toxicities of epidermal growth factor receptor inhibitors and their management. Cancer Nurs 2007; 30:S10–6. [DOI] [PubMed] [Google Scholar]
- 68.Matsuhashi N, Takahashi T, Nonaka K, et al. A case report on efficacy of Abound for anti-EGFR antibody-associated skin disorder in metastatic colon cancer. World J Surg Oncol 2014; 12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marcora S, Lemmey A, Maddison P. Dietary treatment of rheumatoid cachexia with beta-hydroxy-beta-methylbutyrate, glutamine and arginine: a randomised controlled trial. Clin Nutr 2005; 24:442–54. [DOI] [PubMed] [Google Scholar]
- 70.Berk L, James J, Schwartz A, et al. A randomized, double-blind, placebo-controlled trial of a β-hydroxyl β-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer 2008; 16:1179–88. [DOI] [PubMed] [Google Scholar]
- 71.Tomkova H, Pospiskova M, Zabojnikova M, et al. Phytomenadione pre-treatment in EGFR inhibitor-induced folliculitis. J Eur Acad Dermatol Venereol 2013; 27: 514–9. [DOI] [PubMed] [Google Scholar]
- 72.Pinta F, Ponzetti A, Spadi R, et al. Pilot clinical trial on the efficacy of prophylactic use of vitamin K1-based cream (Vigorskin) to prevent cetuximab-induced skin rash in patients with metastatic colorectal cancer. Clin Colorectal Cancer 2014; 13:62–7. [DOI] [PubMed] [Google Scholar]
- 73.Li AM, Miao JH, Liu H, et al. Drug-induced skin toxicity and clinical nursing of VitK cream on colorectal cancer patients. Pak J Pharm Sci 2015; 28:1499–503. [PubMed] [Google Scholar]
- 74.Tastekin D, Tambas M, Kilic K, et al. The efficacy of Pistacia terebinthus soap in the treatment of cetuximab-induced skin toxicity. Invest New Drugs 2014; 32: 1295–300. [DOI] [PubMed] [Google Scholar]
- 75.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351:337–45. [DOI] [PubMed] [Google Scholar]
- 76.Peeters M, Siena S, Van Cutsem E, et al. Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer 2009; 115:1544–54. [DOI] [PubMed] [Google Scholar]
- 77.Abdullah SE, Haigentz M Jr, Piperdi B. Dermatologic toxicities from monoclonal antibodies and tyrosine kinase inhibitors against EGFR: pathophysiology and management. Chemother Res Pract 2012; 2012:351210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bouche O, Beretta GD, Alfonso PG, et al. The role of anti-epidermal growth factor receptor monoclonal antibody monotherapy in the treatment of metastatic colorectal cancer. Cancer Treat Rev 2010; 36(suppl 1):S1–10. [DOI] [PubMed] [Google Scholar]
- 79.Dienstmann R, Brana I, Rodon J, et al. Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist 2011; 16:1729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giovannini M, Gregorc V, Belli C, et al. Clinical significance of skin toxicity due to EGFR-targeted therapies. J Oncol 2009; 2009:849051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kubo A, Hashimoto H, Takahashi N, et al. Biomarkers of skin toxicity induced by anti-epidermal growth factor receptor antibody treatment in colorectal cancer. World J Gastroenterol 2016; 22:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kogawa T, Doi A, Shimokawa M, et al. Early skin toxicity predicts better outcomes, and early tumor shrinkage predicts better response after cetuximab treatment in advanced colorectal cancer. Target Oncol 2015; 10:125–33. [DOI] [PubMed] [Google Scholar]
- 83.Saridaki Z, Tzardi M, Papadaki C, et al. Impact of KRAS, BRAF, PIK3CA mutations, PTEN, AREG, EREG expression and skin rash in >/=2 line cetuximab-based therapy of colorectal cancer patients. PLoS One 2011; 6:e15980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ooki A, Ando M, Sakamoto J, et al. A prospective observational study to examine the relationship between quality of life and adverse events of first-line chemotherapy plus cetuximab in patients with KRAS wild-type unresectable metastatic colorectal cancer: QUACK Trial. Jpn J Clin Oncol 2014; 44:383–7. [DOI] [PubMed] [Google Scholar]
- 85.Sommeijer DW, Karapetis CS, Zalcberg JR, et al. The relationship between rash, tumour KRAS mutation status and clinical and quality of life outcomes in patients with advanced colorectal cancer treated with cetuximab in the NCIC CTG/AGITG CO.17. Acta Oncol 2014; 53:877–84. [DOI] [PubMed] [Google Scholar]
- 86.Thaler J, Karthaus M, Mineur L, et al. Skin toxicity and quality of life in patients with metastatic colorectal cancer during first-line panitumumab plus FOLFIRI treatment in a single-arm phase II study. BMC Cancer 2012; 12:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Romito F, Giuliani F, Cormio C, et al. Psychological effects of cetuximab-induced cutaneous rash in advanced colorectal cancer patients. Support Care Cancer 2010; 18:329–34. [DOI] [PubMed] [Google Scholar]
- 88.Unger K, Niehammer U, Hahn A, et al. Treatment of metastatic colorectal cancer with cetuximab: influence on the quality of life. Z Gastroenterol 2013; 51:733–9. [DOI] [PubMed] [Google Scholar]
- 89.Grande R, Narducci F, Bianchetti S, et al. Pre-emptive skin toxicity treatment for anti-EGFR drugs: evaluation of efficacy of skin moisturizers and lymecycline: a phase II study. Support Care Cancer 2013; 21:1691–5. [DOI] [PubMed] [Google Scholar]
- 90.Burudpakdee C, Zhao Z, Munakata J, et al. Economic burden of toxicities associated with metastatic colorectal cancer treatment regimens containing monoclonal antibodies. J Med Econ 2012; 15:371–7. [DOI] [PubMed] [Google Scholar]
- 91.Giuliani J, Indelli M, Marzola M, et al. The management of skin toxicity during cetuximab treatment in advanced colorectal cancer: how much does it cost? A retrospective economic assessment from a single-center experience. Tumori 2012; 98:408–12. [DOI] [PubMed] [Google Scholar]
- 92.Eilers RE Jr, Gandhi M, Patel JD, et al. Dermatologic infections in cancer patients treated with epidermal growth factor receptor inhibitor therapy. J Natl Cancer Inst 2010; 102:47–53. [DOI] [PubMed] [Google Scholar]