Abstract
Although only a few stem cell-based therapies are currently available to patients, stem cells hold tremendous regenerative potential, and several exciting clinical applications are on the horizon. Biomaterials with tuneable mechanical and biochemical properties can preserve stem cell function in culture, enhance survival of transplanted cells and guide tissue regeneration. Rapid progress with three-dimensional hydrogel culture platforms provides the opportunity to grow patient-specific organoids, and has led to the discovery of drugs that stimulate endogenous tissue-specific stem cells and enabled screens for drugs to treat disease. Therefore, bioengineering technologies are poised to overcome current bottlenecks and revolutionize the field of regenerative medicine.
Stem cell therapies have the potential to transform medicine by enabling patient-specific regeneration of injured or diseased tissues, providing cures for some of humanity’s most intractable diseases, such as muscular dystrophies, diabetes and neurodegeneration. The rapid expansion of stem cell research over the past two decades has uncovered methods that use a patient’s own cells to form mature cell types and even miniature organs, or organoids, in the laboratory. These strategies can harness the native regenerative capacity of somatic stem cells that reside in the patient’s own tissues, such as the bone marrow or skeletal muscle. Alternatively, the advent of induced pluripotent stem (iPS) cells allows researchers to take mature cells from a patient’s skin or blood and reprogram these cells into an immature, embryonic state. These iPS cells can then be differentiated into any cell type of any given adult tissue, providing an avenue to achieve the goals of personalized medicine. Such patient-specific cells can be used to repair damaged tissues or as diagnostic tools to screen for drugs or inform treatment decisions made by physicians.
Successful reports of translating stem cell therapies to patients over the past several years have fostered hope that strategies for regenerative medicine may one day cure some of the most challenging illnesses. Recently, genetically modified keratinocyte cultures containing epidermal stem cells restored more than 80% of the surface area of the skin of a young patient suffering from a deadly blistering disorder1. In other examples, embryonic stem (ES) cells or patient-derived iPS cells that were differentiated into retinal pigment epithelial cells and transplanted into the eye improved the sight of patients at risk of becoming blind due to macular degeneration2-4. Despite such highly publicized and exciting cases of success, the majority of stem cell clinical trials to date have not yet achieved regulatory approval and commercialization as stem cell therapies5. Although hundreds of clinical trials are registered with the US FDA (Food and Drug Administration) on the clinical trials website (https://clinicaltrials.gov/), the only FDA-approved stem cell products consist of umbilical cord blood-derived haematopoietic progenitors6. World-wide, rigorous clinical trials have led to approval of only a handful of therapies based on adult stem cells5. This is not only because of lengthy regulatory hurdles, but also due to biological obstacles.
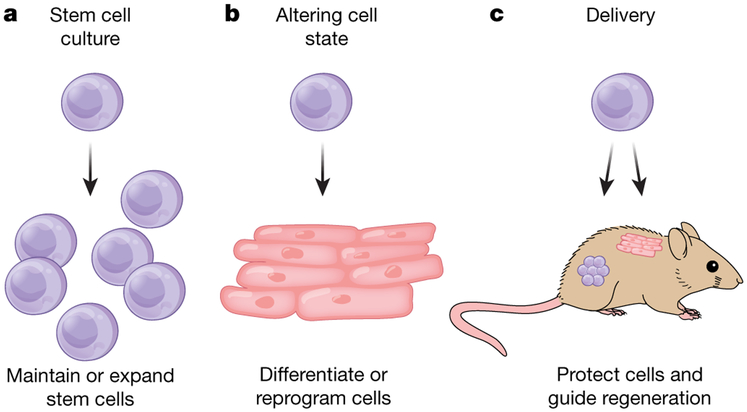
Despite substantial advances in our understanding of stem cell biology, several challenges remain that limit the widespread clinical use of stem cell therapies. Current hurdles to the clinical translation of stem cell therapies include maintenance of the stem cell state, reproducible expansion of large numbers of stem cells for transplantation, efficient control of the cell state both pre- and post-transplantation, and protection of the cells during and after delivery to patients (Fig. 1). Another bottleneck is exemplified by a failure of clinical-grade neural stem cells to replicate the regenerative effects of research-grade cells in pre-clinical animal models, highlighting the difficulties associated with stem cell production and transplantation for use in patients7,8. Engineering approaches offer solutions to overcome current limitations. In particular, advances in materials science have enabled unprecedented control over the biochemical and biophysical properties of materials used for stem cell therapies. Material properties can be tuned to create an artificial niche to both expand naive stem cells and efficiently differentiate stem cells into mature cell types (Fig. 2). Material carriers can improve the survival and engraftment of transplanted stem cells, and controlling the properties of these carriers can promote an enhanced regenerative response from the delivered cells. Innovative material design can aid in meeting regulatory standards and facilitate the increase in scale necessary for commercialization. Here we describe how bioengineered materials have already substantially contributed to stem cell advances and discuss how novel material design can overcome remaining difficulties, accelerating and expanding clinical applications of stem cell-based therapies.
Fig. 1 ∣. Challenges in translating stem cell therapies with potential bioengineered solutions.
a, Present challenges culturing stem cells include maintenance of the stem cell state ex vivo16,18,20,21,23-25 and efficient expansion of naive stem cells9,10,12,27. b, To fully realize the potential of stem cells, reliable protocols for altering cell state must be developed, including differentiation of stem cells to mature cell types35-45 and reprogramming of somatic cells to pluripotent stem cells47,48. c, Conventional cell delivery approaches do not address crucial obstacles in cell transplantation therapies, including maintaining the viability and potency of stem cells during injection49-51, providing a supportive microenvironment for the cells after implantation50,52-55, and controlling the fate of the cells by providing cues to guide regeneration in vivo56,59. Engineering approaches are being applied to design materials to address these challenges.
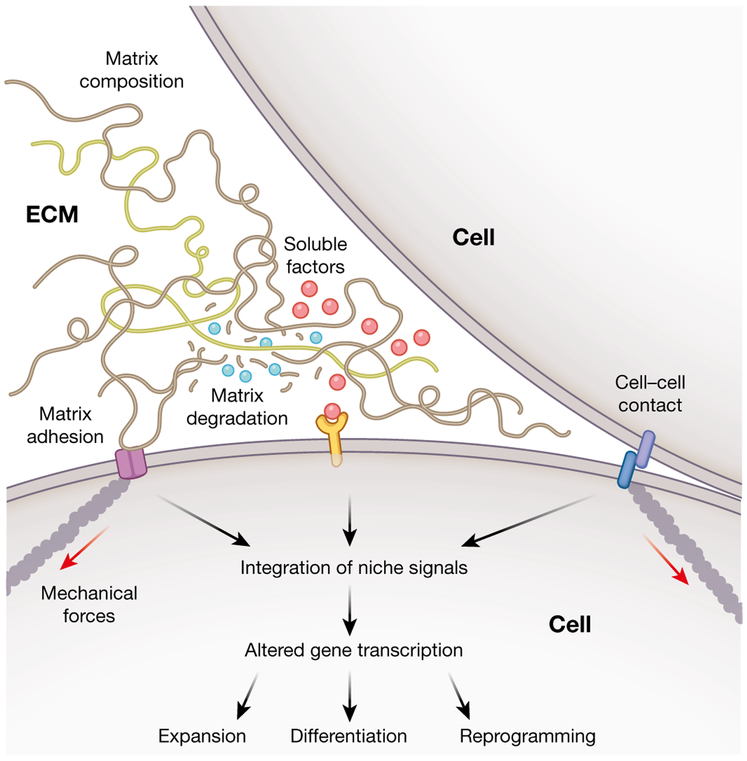
Fig. 2 ∣. Recapitulating niche interactions to direct stem cell fate.
Various biochemical and biophysical factors within the stem cell microenvironment combine to modulate cellular behaviours. Careful design of materials for stem cell culture and transplantation can effectively control matrix properties, such as biochemical composition, mechanics and degradation, as well as soluble factor signalling and cell-cell contact to regulate stem cell fate.
Expanding stem cells
One of the major bottlenecks in translating stem cell therapies to the clinic has been expansion of the large numbers of cells that are required for transplantation, typically tens to hundreds of millions of cells per patient. Cells to be used for human therapies must be cultured under fully defined conditions to meet regulatory requirements and exhibit minimal batch-to-batch variation for consistent therapeutic efficacy. Furthermore, the platforms used for stem cell expansion must be amenable to industrial scale-up.
Historically, the most common techniques used to culture pluripotent stem cells (ES and iPS cells) contained animal-derived components, such as a layer of live mouse embryonic fibroblasts or coatings of Matrigel, a mixture of extracellular matrix (ECM) proteins derived from mice. The use of such animal-derived components hinders regulatory approval, and ECM extracts such as Matrigel can be highly variable in their composition, potentially impacting the reproducibility of cells expanded by using this material. To address these issues, coatings for tissue culture substrates have been designed to recapitulate specific aspects of the native biochemistry. Fully recombinant ECM proteins9, surfaces grafted with peptides that promote cell adhesion10,11, and synthetic polymer coatings12 have all been used to facilitate expansion of pluripotent stem cells. Additionally, high-throughput techniques have enabled screening for matrix-bound protein components and their effects on stem cell state, further refining the presentation of microenvironmental cues from biomimetic materials. For instance, micro-well arrays spotted with proteins led to identification of niche factors that promoted neural stem cell (NSC) proliferation13.
In addition to the biochemical composition, the physical properties of the matrix on which stem cells reside can also alter stem cell expansion. One crucial physical regulator of stem cell fate is matrix stiffness, a measure of how easily the matrix deforms under an applied load. The stiffness of biological materials is often reported as an elastic modulus (also known as the Young’s modulus), which is an inherent material property, independent of material geometry. In the body, stem cells and their progeny experience stiffness spanning several orders of magnitude, from relatively compliant brain tissue (elastic moduli of approximately 102 Pa)14 to rigid calcified bone (elastic moduli of approximately 1010 Pa)15. Systems with a tunable stiffness that encompasses a physiological range have typically used materials known as hydrogels, which are water-swollen polymer networks.
The profound role of substrate stiffness in regulating the self-renewal of somatic stem cells is clear from studies using hydrogel substrates. For example, muscle stem cells (MuSCs), which are responsible for the maintenance and repair of skeletal muscle tissue, need to be cultured on hydrogels with an equivalent stiffness to native muscle tissue to maintain their regenerative potential during expansion in culture and after transplantation in vivo16. Moreover, substrate elasticity has had a crucial role in the ability to ‘rejuvenate’ MuSCs derived from aged mice to improve regenerative function17. With ageing, MuSCs acquire intrinsic defects that make them less potent than cells derived from young mice17, hampering the much-needed therapeutic function of native MuSCs in elderly individuals. However, a combination of culturing aged MuSCs on compliant substrates with muscle-like stiffness and pharmacological inhibition of p38 MAP kinase resulted in expansion of a stem cell pool with improved engraftment and regenerative capacity, culminating in a marked increase in strength17.
The observation that substrate stiffness in culture can regulate the function of expanded stem cells even after transplantation in vivo suggests that the stem cells are capable of ‘remembering’ the mechanical environment in which they were cultured. A noteworthy study, which used hydrogels that dynamically soften in response to controlled light exposure, has shown that mesenchymal stem cells (MSCs), which are bone, cartilage and fat-forming cells derived from the bone marrow, possessed a ‘mechanical memory’18. Stiff hydrogels biased the MSCs towards differentiation over stem cell maintenance, and prolonged culture on stiff substrates resulted in an irreversible loss of stem cell potential18. Identification of the molecular mechanisms that are responsible for this mechanical memory may help to restore function in stem cells that have acquired defects from fibrotic stiffening due to disease and ageing. One such memory molecule in MSCs is the microRNA miR-21. Resetting expression levels of miR-21 effectively ‘erased’ the memory of being cultured on a stiff substrate19.
Other stem cell types are similarly sensitive to stiffness and expand optimally when cultured on substrates of a particular elasticity. Culture on compliant substrates enhanced the ex vivo expansion of haematopoietic stem cells20,21, which are responsible for reconstituting blood and immune cells. Human embryonic stem cells were best maintained on relatively compliant substrates, which led to the expression of high levels of pluripotency genes and retention of the capacity of these cells to differentiate into all three germ layers22.
The two-dimensional (2D) nature of traditional cell culture often does not adequately replicate the three-dimensional (3D) environment experienced by stem cells in the body. Hydrogels have proven to be a useful material platform to culture cells in a more native-like 3D microenvironment. Studies of the native ECM revealed that cell-secreted enzymes, such as metalloproteases, remodel the matrix to permit cell spreading and migration through their surrounding material. Incorporating this principle, hydrogel systems for 3D culture have been engineered to permit degradation and remodelling. Matrix remodelling has recently been demonstrated to have significant and diverse impacts on the expansion of stem cells ex vivo. NSCs embedded within 3D hydrogels must remodel the surrounding matrix in order to maintain cell-cell contacts and retain their stem cell state, irrespective of matrix stiffness23. By contrast, maintenance and proliferation of intestinal stem cell cultures is decreased upon culture in hydrogels susceptible to degradation by cell-secreted enzymes24. However, to facilitate maturation of intestinal organoid cultures, gradual, passive degradation of the matrix is necessary24.
Microstructural variation is an additional parameter provided by the native ECM that is not replicated in traditional 2D cultures or homogeneous 3D hydrogels. The ECM of many tissues consists of fibrous components spanning the nano- to micrometre scales. Cell culture substrates presenting features along these length scales can alter cellular behaviour. In one example, nanoscale-patterned surfaces with a square lattice geometry promoted enhanced maintenance of a stem cell phenotype in cultured MSCs25. ES cells are also acutely sensitive to nanoscale topography. Culture on nanoscale smooth surfaces promoted ES cell self-renewal and maintenance of pluripotency, whereas culture on nanoscale rough surfaces induced spontaneous differentiation26.
Engineered materials can address processing concerns related to industrial scale-up of stem cell production. Traditional 2D culture methods have high space and nutrient costs. Transitioning to 3D culture platforms can decrease the amount of surface area required for cell culture by stacking cells in the z-dimension. To this end, temperature-responsive 3D hydrogel systems have been developed for easy encapsulation and expansion of pluripotent stem cells27. In addition to saving space, these hydrogels facilitate the collection of the expanded stem cells, which can be triggered by simply lowering the temperature to dissolve the polymers comprising the gel27. Successful commercialization of stem cell therapies will ultimately require large-scale cell culture technologies, such as bioreactors28,29. Many stem cells need to adhere to surfaces to maintain their stem cell state, requiring materials that can serve as microcarriers that provide both crucial chemical and mechanical cues to cells cultured in large reactors. Good examples of these microcarriers are polymeric microbeads coated with matrix proteins as supports in stirred reactors30,31 and hydrogel microbeads that facilitate adhesion and expansion of pluripotent stem cells in reactor-compatible 3D microenvironments32.
Altering cell state
Many of the proposed therapeutic applications of stem cells require controlled methods of altering cell state. For tissue-replacement therapies using stem cells differentiated into mature cell types, highly efficient differentiation into the target cell population is required to limit potential deleterious effects of co-transplanting either highly proliferative naive stem cells or other potentially antagonistic differentiated cells. This concern also applies to in vitro studies of cells derived from stem cells, as the presence of improperly differentiated cells can skew the results of bulk biochemical assays. Just as physical matrix properties can be used to preserve stem cell phenotype, these matrix properties can be tuned to direct and augment the differentiation of stem cells.
The first demonstration that physical interactions with the matrix could mediate changes in cell state arose from seminal studies of malignant transformation in breast cancer. In 3D cultures of malignant breast cancer cells, reversion of the cells to a non-malignant phenotype was achieved by blocking specific integrins, the cell-surface receptors that connect the intracellular force-generation mechanisms of the cytoskeleton to the ECM33. Conversely, increasing matrix stiffness resulted in transformation of cells from a benign to a malignant phenotype34. These studies pointed to force generation by cells as a means for sensing and responding to the mechanical properties of the matrix.
In a ground-breaking study, the differentiation of MSCs was demonstrated to be biased according to the stiffness of their underlying substrate35. MSCs cultured on stiff substrates similar to pre-calcified bone preferentially differentiated into bone cells, whereas MSCs cultured on intermediate stiffness similar to muscle tissue displayed a more muscle-like phenotype35. MSCs cultured on the most compliant matrices, reminiscent of brain tissue, exhibited a neuron-like phenotype35.
The premise that matrix stiffness can alter cell state through force generation was corroborated in 3D materials, with an optimal stiffness mediating bone differentiation of MSCs through integrin clustering36. NSCs are also sensitive to matrix mechanics, preferentially differentiating into neurons on very compliant substrates similar to the elasticity of brain tissue and into supporting glial cells on stiff substrates37. Furthermore, matrix stiffness may have a crucial role in developmental processes, as compliant substrates have been shown to enhance the mesodermal differentiation potential of embryonic stem cells38. Beyond materials with a fixed stiffness, recent studies have implicated the time-dependent mechanical properties of viscoelastic materials as regulators of MSC differentiation39-41 (Box 1). At a fixed stiffness, MSC differentiation into a bone lineage was markedly enhanced in materials with a greater viscous character40.
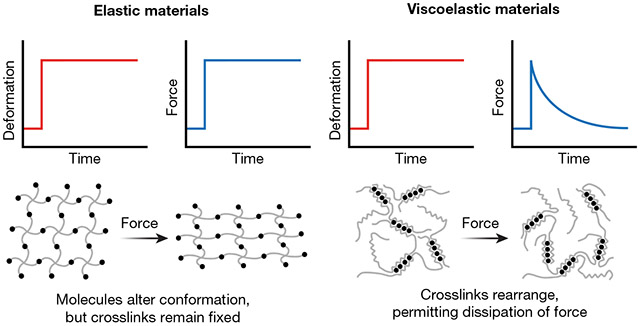
BOX 1. Elasticity versus viscoelasticity.
The term stiffness is commonly used to describe materials that exhibit elastic mechanical properties. When a force is applied to an elastic material, the force is retained in the material over time, similar to the way in which a strained rubber band provides constant resistance (see figure). The native ECM is not a purely elastic material, but rather exhibits both elastic (solid-like) and viscous (liquid-like) behaviour. Viscoelastic materials have time-dependent mechanical properties. For instance, natural ECM exhibits stress-relaxation, where resistance to an applied load is dissipated over time by rearrangement of the molecules that comprise the ECM (see Figure). This is analogous to a ball of putty deforming over time after a force is applied. Thus, characterizing materials used in cell culture by only stiffness may oversimplify the mechanisms by which cells can interact with bioengineered materials. Recent studies have highlighted the importance of accounting for viscoelasticity in stem cell differentiation. At a given stiffness, both in 2D and 3D, tuning the viscous characteristics of the material can enhance the differentiation of MSCs into bone.39-41
Various other matrix parameters have been implicated in regulating how stem cells alter their cell state. For instance, matrix degradation by encapsulated MSCs was required for force generation and subsequent differentiation into a bone lineage42. Cell–cell contacts can alter how MSCs respond to mechanical cues43. The cell-adhesive ligands presented by the matrix also play an important part in directing differentiation. By controlling the temporal presentation of adhesive cues, MSC differentiation into cartilage44 and NSC differentiation into neurons45 were enhanced. Because the interactions among multiple types of cell-adhesive ligands are often complicated and nonlinear, combinatorial studies that include statistical approaches have been used to optimize ligand composition to promote differentiation46.
The reprogramming of somatic cells to generate patient-specific iPS cells is also highly sensitive to matrix interactions that modulate cell state. Traditional iPS cell reprogramming protocols use standard 2D tissue culture techniques. However, the properties of the culture substrate can have a substantial impact on the efficiency of iPS cell colony generation. Forcing alignment of fibroblasts on substrates with aligned microgrooves mediated epigenetic modifications that increased reprogramming efficiency47. Transitioning to a 3D hydrogel platform also resulted in an increase in efficiency, with optimal matrix properties identified via high-throughput screens48. Such technologies have the potential to decrease the variability and cost associated with generating patient-specific stem cell therapies.
Improving cell delivery
Efficient transplantation and engraftment into host tissues remains a notable barrier to therapeutic success. Many cells die from the mechanical damage that is caused by the injection process or fail to engraft in the relatively inhospitable microenvironment of damaged tissue. Recent advances in material-based cell delivery systems show promise in overcoming these difficulties.
The simple act of injecting stem cells through a needle significantly reduces the viability of the injected cells49,50. As the solution in which the cells are suspended transitions from the syringe barrel to the needle, the fluid undergoes an increase in velocity of two orders of magnitude, exposing the cells to substantial extensional flows that can damage cell membranes49. The porous and highly hydrated nature of hydrogels is ideally suited to encapsulation of small molecules, growth factors or proteins together with stem cells. Injectable hydrogels have been developed to limit the membrane damage experienced by cells49,51. In these systems, the bulk of the hydrogel moves through the needle as a solid; only the edges near the needle wall flow like a liquid51. Therefore, the vast majority of the cells pass through the needle without experiencing damaging shear deformation through extensional flow.
Once injected into tissue, hydrogel carriers can also serve to retain the cells at the target location. Very few cells that are delivered via commonly used saline injections are retained in tissues for an extended period. Rather, the immune response of the host in the damaged tissue and the lack of adhesive sites lead to cell death and clearance. Accordingly, by increasing the stability of injected hydrogel carriers, cell retention was increased at the injection site in a mouse model50. Moving towards therapeutic applications, injectable hydrogel-mediated delivery of endothelial progenitor cells to ischaemic rat hearts increased cell engraftment and decreased fibrosis compared to cells injected in saline52. Injectable hydrogels have also significantly improved the survival of iPS cell-derived oligodendrocytes delivered to injured rat spinal cords53.
Hydrogels can serve as immuno-protective barriers to shield the transplanted cells from host inflammation, overcoming a major difficulty for the use of allogeneic cells for transplantation. A particularly poignant case in point is the treatment of diabetes by pancreatic cells that comprise the β-islets. The ideal material would protect the transplanted cells from immune clearance while permitting sustained insulin secretion. Early approaches met with limited clinical success in part because of immune responses to both animal-derived cells and the materials intended to protect these cells. Islets derived from human ES cells and hydrogel materials that elicit minimal inflammatory responses have provided new hope that islet transplantation can be used to cure type I diabetes54. The immuno-protective effect of hydrogels may also facilitate cell-mediated tissue regeneration, as hydrogel delivery of iPS cell-derived neural progenitors has been shown to decrease inflammation and improve neuronal differentiation compared to saline delivery55.
To enhance the regenerative phenotypes of the delivered cells, some of the same matrix properties used to modulate cell state in culture can be incorporated into hydrogel delivery vehicles. For instance, differentiation of MSCs towards a bone lineage is known to be mechano-sensitive in vitro35,36, and transplanting MSCs in hydrogels of optimal stiffness enhanced bone regeneration in a critical-sized cranial defect model in vivo56. Furthermore, the recent observation that MSC differentiation in vitro depends not only on the time-independent elasticity of the material, but also on the time-dependent viscoelasticity of the material39-41 (Box 1), also holds true when viscoelastic hydrogels are used to transplant MSCs in vivo. Cells delivered in hydrogels with a more viscous character exhibited increased bone regeneration in vivo compared to hydrogels of a comparable stiffness that were predominantly elastic57.
Hydrogel microstructure can also profoundly impact the fate of transplanted stem cells. Peptide amphiphile hydrogels are a classic example of a 3D cell culture material with a characteristic nanoscale fibrous architecture58. When these materials are subjected to heating and cooling, the peptides self-assemble to form noodles of aligned nanofibres that, when mixed in a calcium-rich suspension, can encapsulate cells together with growth factors59. These scaffolds increase cell viability, mediate cell alignment parallel to the hydrogel nanofibres, and have degradation rates that fit the time course of regeneration59. These features are ideally suited to MuSC delivery and have led to improved MuSC engraftment and muscle repair59.
Successful regeneration of functional tissue requires integration of transplanted stem cells with the host vasculature and innervation of the newly formed tissue. Neovascularization is critical for long-term survival of transplanted cells, as oxygen and nutrient transport requirements dictate that, in general, cells must be located within 100–200 μm of a capillary60. Classical strategies for vascularization have taken a bottom-up approach starting from the individual cellular components of blood vessels, relying on self-assembly of either host or exogenous endothelial cells and smooth muscle cells. Sequential delivery of angiogenic factors from biomaterial scaffolds resulted in initial vascular sprouting followed by vessel maturation culminating in a more robust vasculature61. Alternative approaches have used co-cultures of endothelial cells, supporting stromal cells and tissue progenitor cells to generate vascularized tissues that were perfused by host vasculature when transplanted62. More recently, top-down techniques starting from the viewpoint of the finalized tissue, including 3D printing63 and two-photon lithography64, have been used to produce engineered constructs with user-defined vasculature. Innervation of engineered tissues can be achieved through delivery of growth factors, including classical neurotrophins such as nerve growth factor65 and angiogenic factors such as vascular endothelial growth factor66.
Improving human cell culture models
Cell transplantation for tissue regeneration is just one facet of personalized medicine made possible by advances in stem cell biology. Patient-derived iPS cells that can give rise to a myriad of differentiated cell types have provided researchers unprecedented access to diverse healthy and diseased samples that can help to inform basic biology, drug screens and toxicology studies. A challenge has been the degree of differentiation, as the differentiated cell types obtained are notoriously immature. Engineered cellular microenvironments offer hope.
Engineered matrices have been developed to support the complex 3D architecture of organotypic cultures that are used to study developmental and disease processes. Synthetic hydrogels can replace the highly variable Matrigel substrate in primary24,67 as well as ES and iPS cell-derived68 intestinal organoids. Microfilament scaffolds improved cortical development in human brain organoids69, and controlling matrix stiffness and 2D versus 3D dimensionality permitted generation of amnion-like structures70.
Engineered human tissue constructs provide a novel platform to study disease progression and test potential therapeutic interventions. In a noteworthy example, human iPS cell-derived brain organoids produced by bioreactor culture and infected with Zika virus exhibited reduced NSC proliferation, suggestive of a microcephaly-like phenotype71. Engineered tissues also enable the study of patient-specific genetic diseases. Filamentous matrices were used to generate cardiomyocytes from healthy and diseased patients to study contractile abnormalities in congenital cardiomyopathy72.
The patient-specific nature of iPS cell-derived cells makes them attractive platforms for screening drugs for potential toxicity on an individual patient level. For example, cardiomyocytes generated from patient-derived iPS cells recapitulate the heightened toxicity in response to chemotherapy seen in specific cancer patients73. Advances in engineered microsystems have provided platforms to investigate the effects of drug treatment on iPS cell-derived cardiomyocyte function74,75. In addition to cardiac models, synthetic matrices have been used to improve the sensitivity of vascular toxicity screens76, and microphysiological systems using iPS cell-derived kidney cells recapitulate drug-induced kidney toxicity77.
Future outlook
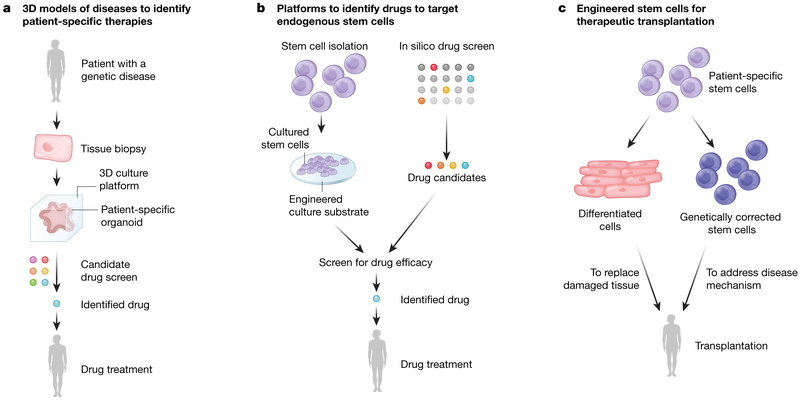
Although only a handful of stem cell therapies have currently been approved for use in patients, several exciting clinical applications are on the horizon that have benefitted from bioengineered materials (Fig.3). Rapid progress is being made in the use of organotypic cultures from patient-derived stem cells or tissue-specific stem cells in hydrogels24,67,68,70,78,79. This advance has led to the discovery of drugs to treat disease. A striking example highlights how patient-specific organoid cultures can profoundly impact clinical outcome. Intestinal organoids derived from patients with cystic fibrosis were grown in 3D hydrogels and used to screen drugs that could reverse the effects of the disease78. These culture models have uncovered life-changing therapies for patients suffering from very rare mutations, or orphan diseases, by rapidly and effectively assessing potential efficacy of costly drugs80. As a result, patients with cystic fibrosis who once had no treatment options have now been matched with drugs that address their disease in culture, ameliorate their symptoms, and markedly improve their quality of life80. In another example, 3D organoid cultures of cochlear stem cells enabled identification of a combination of small molecules that can stimulate expansion of these cells, which in turn differentiate into hair cells responsible for hearing79. This drug combination may enable the activation of endogenous stem cells to reverse hearing loss in patients81, a problem that confronts our increasingly aged population. Combining advances in hydrogel stem cell culture techniques with in silico screens can further increase the success rate of identifying new drugs targeting endogenous stem cells. Such an in silico approach was instrumental in identifying prostaglandin E2 as a natural inflammatory modulator capable of potently inducing of skeletal muscle stem cell expansion in vitro and muscle regeneration in vivo82. This approach capitalizes on the quiescent stem cells resident in muscle tissues throughout life that are dedicated to skeletal muscle repair. The function of these cells declines with age17. Identification of agents capable of rejuvenating the function of these endogenous stem cells opens the door to therapies that counter muscle wasting and restore strength, countering frailty, a major cause of morbidity with ageing.
Fig. 3 ∣. Impact of bioengineering on stem cell advances currently in the clinic or on the horizon.
a, b, Hydrogel-based culture systems, such as intestinal organoid cultures, have enabled identification of promising drugs to treat cystic fibrosis78,80 (a), while others are used to target endogenous stem cells within tissues to restore hearing79,81 and augment strength17,82 (b). c, Treatments in clinical trials that could achieve greater efficacy by using engineered scaffolds to culture and transplant cells include ES and iPS cell-derived retinal epithelial cells to restore vision to macular degeneration patients2-4 and skin grafts of genetically corrected epidermal stem cells to save patients from a deadly skin blistering disease1.
Fully realizing the potential of personalized medicine provided by stem cells will require advances in bioengineered materials. The native stem cell microenvironment is highly dynamic, with temporally varying biochemical and biophysical properties. For example, tissue dysfunction in ageing and disease is often characterized by fibrosis, which reflects an increase in deposition and crosslinking of ECM proteins that leads to changes in the stiffness and composition of the cellular microenvironment. Given that cells are acutely sensitive to these signals, biomimetic materials are needed that permit incorporation of this dynamism to enable improved in vitro models of fibrosis. Recent studies have shown promise using light- and enzyme-mediated approaches to alter substrate stiffness and presentation of bioactive factors18,44,83-87. In particular, photo-mediated degradation of hydrogel crosslinks has enabled dynamic softening of cell culture substrates to study how stem cells respond to changes in their mechanical environment18. Conversely, photo-initiated polymerization has enabled in situ stiffening of hydrogels87, reminiscent of fibrotic disease states. Combining these approaches with genetic reporters commonly used in cell biology settings may enable real-time investigation of signalling changes as a result of changes in matrix properties. However, existing chemical approaches to dynamically modulate matrix mechanics are commonly limited by the use of potentially mutagenic UV light, although chemistries that are compatible with two-photon and blue-light illumination have been developed44,83. Furthermore, the free radicals generated during photo-initiated polymerization and stiffening may be toxic to sensitive stem cells. Future work directed at refining these approaches should focus on using less perturbative stimuli, such as visible light and exploring fully biocompatible chemistries, to permit completely orthogonal tuning of the cellular microenvironment both in vitro and in vivo. Such dynamically tunable systems may improve the accuracy of preclinical models by better mimicking the native cellular niche and potentially enable modulation of tissue-engineered constructs in vivo to facilitate precise spatiotemporal control of morphogenic cues.
Furthermore, we must increase our understanding of the critical role of endogenous tissue-specific cell modifications of delivered materials. In addition to potential effects on endogenous stem cells, impacts on immune cells, which are crucial to efficacious regeneration, are of paramount importance. Many existing strategies for material design have focused on minimizing the immune response of the host at the site of delivery. This is particularly important for the transplantation of allogeneic, as opposed to patient-specific, stem cell therapies. Initial widespread application of iPS cell therapies may utilize libraries of reprogrammed stem cell lines to enable close genetic matching between donor and recipient, similar to the way that organ donations are screened for a close antigenic match88. Such allogeneic approaches do not guarantee successful transplant engraftment and may still require immunosuppression, but may provide earlier access to stem cell therapies until systems for generating and validating patient-specific stem cells are implemented on a large scale. Lessons learned from the development of biomaterials for immuno-isolated tissue engineering, for instance in islet transplantation for diabetes treatment54, can be applied to generate materials that enhance stem cell engraftment and function by limiting deleterious local immune responses.
The direct participation of immune cells in the process of regeneration is increasingly recognized as essential to proper restoration of tissue function. Thus, in contrast to materials designed to evade an immune response, well-designed immunomodulatory materials could aid in the process of regeneration89,90. Although studies to date have mostly focused on using materials to direct the immune response to existing disease states, such as targeting cancer91,92 or induction of tolerance in autoimmune disorders93,94, lessons learned from programming immune cells with biomaterials can be harnessed to improve stem cell therapeutic outcomes by orchestrating the immune response during regeneration. For instance, self-assembling peptide scaffolds have been used to modulate presentation of T cell epitopes, resulting in a dose-dependent response to activate different immune cell populations95. Similar material strategies may be used in tandem with stem cell-targeting factors to simultaneously regulate the immunological response during tissue regeneration. Initial results using materials to control cytokine delivery enabled temporal control over recruitment of different macrophage subtypes, which in turn secreted different angiogenic factors at appropriate morphogenic time points to enhance vascularization of tissue engineered constructs96.
As novel materials are developed for stem cell therapies, regulatory requirements must also be considered. Competing interests in designing materials that sufficiently recapitulate the complexities of the native matrix to control cell fate must be balanced with the need to develop scalable, cost-effective platforms for commercialization. For clinical use, materials must be fully defined and free of animal-derived components. By synthesizing knowledge from fields such as materials science, chemistry, bioengineering and cell biology, the groundwork has been laid to propel stem cell-based therapies into the clinic.
Acknowledgements
C.M.M. is supported by the Stanford ChEM-H Interdisciplinary Postdoctoral Training Program in Quantitative Mechanobiology. S.C.H. acknowledges support from the National Institutes of Health (NIH) (U19 AI116484 and R21 HL13804201), the National Science Foundation (DMR 1508006) and the California Institute for Regenerative Medicine (CIRM) (RT3-07948). H.M.B. acknowledges support from the NIH (R01 AG020961, R01 AR063963, R01 NS089533, and R01 HG00967401), CIRM (DISC1-10036), the American Heart Association (17CSA33590101), the Baxter Foundation, and the Li Ka Shing Foundation.
Footnotes
Competing interests The authors declare no competing interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hirsch T et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 551, 327–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz SD et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Schwartz SD et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385, 509–516 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Mandai M et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038–1046 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Trounson A & McDonald C Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17, 11–22 (2015). [DOI] [PubMed] [Google Scholar]
- 6.FDA warns about stem cell therapies. US Food & Drug Administration https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm286155.htm (FDA, 2017). [Google Scholar]
- 7.Anderson AJ, Piltti KM, Hooshmand MJ, Nishi RA & Cummings BJ Preclinical efficacy failure of human CNS-derived stem cells for use in the pathway study of cervical spinal cord injury. Stem Cell Reports 8, 249–263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh SE et al. HuCNS-SC Human NSCs fail to differentiate, form ectopic clusters, and provide no cognitive benefits in a transgenic model of Alzheimer’s disease. Stem Cell Reports 8, 235–248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodin S et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 28, 611–615 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Melkoumian Z et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol. 28, 606–610 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Klim JR, Li L, Wrighton PJ, Piekarczyk MS & Kiessling LL A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat. Methods 7, 989–994 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villa-Diaz LG et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol. 28, 581–583 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gobaa S et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods 8, 949–955 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Gefen A & Margulies SS Are in vivo and in situ brain tissues mechanically similar? J. Biomech. 37, 1339–1352 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Rho JY, Ashman RB & Turner CH Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J. Biomech. 26, 111–119 (1993). [DOI] [PubMed] [Google Scholar]
- 16.Gilbert PM et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081 (2010).This study demonstrated that muscle stem cells best maintained their stem cell phenotype and regenerative potential when cultured on substrates with stiffness approximating that of healthy muscle.
- 17.Cosgrove BD et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Tibbitt MW, Basta L & Anseth KS Mechanical memory and dosing influence stem cell fate. Nat. Mater 13, 645–652 (2014).This study used hydrogel substrates that were dynamically softened by light to demonstrate that mesenchymal stem cells can ‘remember’ the stiffness of the substrates on which they were cultured.
- 19.Li CX et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat. Mater. 16, 379–389 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Holst J et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol. 28, 1123–1128 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Choi JS & Harley BAC Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci. Adv. 3, e1600455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhury F et al. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS ONE 5, e15655 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madl CM et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 16, 1233–1242 (2017).These studies23,24,42 identified mechanisms by which matrix degradation can modulate stem cell fate.
- 24.Gjorevski N et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016). [DOI] [PubMed] [Google Scholar]
- 25.McMurray RJ et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 10, 637–644 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Chen W et al. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano 6, 4094–4103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei Y & Schaffer DV A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl Acad. Sci. USA 110, E5039–E5048 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zweigerdt R, Andree B, Kropp C & Kempf H in Bioreactors: Design, Operation and Novel Applications (ed. Mandenius C-F) (Wiley-VCH, Weinheim, 2016). [Google Scholar]
- 29.Li Y et al. Engineering-derived approaches for iPSC preparation, expansion, differentiation and applications. Biofabrication 9, 032001 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Nie Y, Bergendahl V, Hei DJ, Jones JM & Palecek S P Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol. Prog. 25, 20–31 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kehoe DE, Jing D, Lock LT & Tzanakakis ES Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue Eng. Part A 16, 405–421 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabata Y, Horiguchi I, Lutolf MP & Sakai Y Development of bioactive hydrogel capsules for the 3D expansion of pluripotent stem cells in bioreactors. Biomater. Sci. 2, 176–183 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Weaver VM et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137, 231–245 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).This study identified substrate stiffness as a potent regulator of stem cell differentiation in 2D culture systems.
- 36.Huebsch N et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9, 518–526 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha K et al. Substrate modulus directs neural stem cell behavior. Biophys. J. 95, 4426–4438 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Przybyla L, Lakins JN & Weaver VM tissue mechanics orchestrate Wnt-dependent human embryonic stem cell differentiation. Cell Stem Cell 19, 462–475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cameron AR, Frith JE & Cooper-White JJ The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979–5993 (2011).These studies39-41 demonstrated that the viscoelastic properties of engineered extracellular matrices can modulate stem cell differentiation.
- 40.Chaudhuri O et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das RK, Gocheva V, Hammink R, Zouani OF & Rowan AE Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater 15, 318–325 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Khetan S et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12, 458–465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosgrove BD et al. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat. Mater. 15, 1297–1306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kloxin AM, Kasko AM, Salinas CN & Anseth KS Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freeman R et al. Instructing cells with programmable peptide DNA hybrids. Nat. Commun. 8, 15982 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam J, Carmichael ST, Lowry WE & Segura T Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv. Healthc. Mater. 4, 534–539 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Downing TL et al. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 12, 1154–1162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caiazzo M et al. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater. 15, 344–352 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Aguado BA, Mulyasasmita W, Su J, Lampe KJ & Heilshorn SC Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 18, 806–815 (2012).This study identified shear-thinning hydrogels as material carriers to protect cells from mechanical damage during injection.
- 50.Cai L, Dewi RE & Heilshorn SC Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 25, 1344–1351 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan C et al. Injectable solid peptide hydrogel as a cell carrier: effects of shear flow on hydrogels and cell payload. Langmuir 28, 6076–6087 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaffey AC et al. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J. Thorac. Cardiovasc. Surg. 150, 1268–1277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Führmann T et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials 83, 23–36 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Vegas AJ et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 22, 306–311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lam J, Lowry WE, Carmichael ST & Segura T Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv. Funct. Mater. 24, 7053–7062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huebsch N et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 14, 1269–1277 (2015).This study demonstrated that hydrogel stiffness can modulate stem cell behaviour in vivo.
- 57.Darnell M et al. Substrate stress-relaxation regulates scaffold remodeling and bone formation in vivo. Adv. Healthc. Mater. 6, 1601185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silva GA et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Sleep E et al. Injectable biomimetic liquid crystalline scaffolds enhance muscle stem cell transplantation. Proc. Natl Acad. Sci. USA 114, E7919–E7928 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lovett M, Lee K, Edwards A & Kaplan DL Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 15, 353–370 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richardson TP, Peters MC, Ennett AB & Mooney DJ Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19, 1029–1034 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Levenberg S et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23, 879–884 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Miller JS et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arakawa CK, Badeau BA, Zheng Y & DeForest CA Multicellular vascularized engineered tissues through user-programmable biomaterial photodegradation. Adv. Mater. 29, 1703156 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suuronen EJ et al. Functional innervation in tissue engineered models for in vitro study and testing purposes. Toxicol. Sci. 82, 525–533 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Shvartsman D et al. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol. Ther. 22, 1243–1253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DiMarco RL, Dewi RE, Bernal G, Kuo C & Heilshorn SC Protein-engineered scaffolds for in vitro 3D culture of primary adult intestinal organoids. Biomater. Sci. 3, 1376–1385 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cruz-Acuna R et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19, 1326–1335 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lancaster MA et al. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 35, 659–666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shao Y et al. Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat. Mater. 16, 419–425 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qian X et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Z et al. Three-dimensional filamentous human diseased cardiac tissue model. Biomaterials 35, 1367–1377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burridge P W. et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 22, 547–556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lind JU et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 16, 303–308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ribeiro AJS et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl Acad. Sci. USA 112, 12705–12710 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen EH et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed. Eng. 1, 0096 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Musah S et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng. 1, 0069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dekkers JF et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 8, 344ra84 (2016). [DOI] [PubMed] [Google Scholar]
- 79.McLean WJ et al. Clonal expansion of Lgr5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep. 18, 1917–1929 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saini A Cystic fibrosis patients benefit from mini guts. Cell Stem Cell 19, 425–427 (2016). [Google Scholar]
- 81.Lyon J Hearing restoration: a step closer? J. Am. Med. Assoc. 318, 319–320 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Ho ATV et al. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc. Natl Acad. Sci. USA 114, 6675–6684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosales AM, Vega SL, DelRio FW, Burdick JA & Anseth KS Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angew. Chem. Int Ed. 56, 12132–12136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeForest CA & Tirrell DA A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat. Mater. 14, 523–531 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Lee TT et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater. 14, 352–360 (2015).This study demonstrated the feasibility of using light as a stimulus to dynamically modify biomaterial properties in vivo.
- 86.Cambria E et al. Covalent modification of synthetic hydrogels with bioactive proteins via sortase-mediated ligation. Biomacromolecules 16, 2316–2326 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guvendiren M & Burdick JA Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 3, 792 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Turner M et al. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell 13, 382–384 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Rice JJ et al. Engineering the regenerative microenvironment with biomaterials. Adv. Healthc. Mater. 2, 57–71 (2013). [DOI] [PubMed] [Google Scholar]
- 90.Vishwakarma A et al. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol. 34, 470–482 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Ali OA, Emerich D, Dranoff G & Mooney DJ In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci. Transl. Med. 1, 8ra19 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hori Y, Stern P J., Hynes, R. O. & Irvine, D. J. Engulfing tumors with synthetic extracellular matrices for cancer immunotherapy. Biomaterials 30, 6757–6767 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Getts DR et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 30, 1217–1224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoon YM et al. A combination hydrogel microparticle-based vaccine prevents type 1 diabetes in non-obese diabetic mice. Sci. Rep. 5, 13155 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pompano RR et al. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Adv. Healthc. Mater. 3, 1898–1908 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spiller KL et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 37, 194–207 (2015).This study demonstrated that regulation of the host immune response can enhance regeneration in response to engineered constructs.