Abstract
Gastrointestinal Stromal Tumor (GIST) is the most common human sarcoma, and can form along the entire GI tract. Over the last 20 years, considerable advances have been made in our understanding of the biology of GIST. The advent of tyrosine kinase inhibitors has provided effective medical therapy for the first time. In fact, given that GIST typically is driven by either a KIT or PDGFRA gene mutation, it has become a paradigm of targeted molecular therapy. In addition, diagnostic and surgical techniques have been refined. Here, we summarize the critical aspects of primary GIST and how it is now managed with an integrated approach. Treatment plans are developed based on specific pathologic and molecular features of the tumor. We outline the general principles of therapy and highlight some of the nuances. Particular focus is given to diagnosis, surgical considerations, and use of preoperative and postoperative tyrosine kinase inhibitors.
Keywords: Gastrointestinal stromal tumor, Imatinib, Surgery, primary, adjuvant
Precis:
The multidisciplinary management of primary gastrointestinal stromal tumor has evolved considerably over the last 20 years. Here, we review the surgical and medical considerations.
Introduction
Gastrointestinal stromal tumors (GISTs) are mesenchymal neoplasms with variable malignant potential that can form along the entire length of the gastrointestinal (GI) tract, including some extra-intestinal sites. They are thought to arise from the interstitial cells of Cajal, smooth muscle-derived pacemaker cells in the wall of the luminal GI tract.1 GIST is the most common human sarcoma subtype,2 but has only been recognized as a distinct entity since the late 20th century.3 In the late 1990s, GISTs were shown to highly express mutated oncogenic forms of KIT, a receptor tyrosine kinase (RTK) protein responsible for cell survival and proliferation.4, 5 This discovery ignited a rapid expansion of knowledge and literature on GIST for the past two decades. Most (70–80%) of GISTs contain mutations in KIT, but about 10% contain mutations in PDGFRA (the gene for platelet-derived growth factor receptor alpha, another RTK),6 and the remaining 10% has defects in succinate dehydrogenase or a mutation in BRAF or other oncogenes.7 The most common tumor location is the stomach (50–70%), followed by small intestine (25–35%), rectum (5–10%), and esophagus (<5%).8 GISTs are extraordinarily rare in the colon. Extra-intestinal GISTs (~6%) are found most commonly in the omentum, but can also arise in the mesentery or retroperitoneum. The most common sites of metastasis are the liver and peritoneum.9 GISTs are diagnosed by morphology and immunohistochemical staining for the KIT receptor (CD117) and DOG1, a highly expressed chloride channel protein.10
Imatinib mesylate (IM) is a small molecule tyrosine kinase inhibitor (TKI) that is active against most mutated forms of KIT and PDGFRα oncoproteins. IM has prolonged 5-year overall survival in advanced GIST from a historical 16–19 months9, 11, 12 to 5 years.13–17 IM is the first line therapy for advanced GIST. Sunitinib and regorafenib are multikinase inhibitors that are FDA-approved for IM resistance or intolerance.18, 19
How do patients typically present?
GISTs are discovered in a variety of ways. About 70% of patients have symptoms. The remainder have incidental findings on imaging, endoscopy, or during surgery for other conditions. Patients can have intraluminal bleeding that ranges from occult to massive. Tumor rupture can result in an acute abdomen with or without massive bleeding, necessitating an emergency operation. Some patients have vague abdominal pain or are found to have a palpable abdominal mass. Rarely, GISTs may erode into the gastrointestinal tract with the subsequently infected tumor resulting in fever. Strangely, intestinal obstruction almost never occurs in primary GIST.
Is preoperative tissue confirmation mandatory?
Proving the diagnosis of GIST preoperatively is recommended but can be problematic. Cross-sectional imaging may be highly suggestive of GIST. We prefer to use computed tomography (CT) with oral and intravenous contrast of the abdomen and pelvis. The classic features of GIST are an enhancing mass arising from the wall of the GI tract with exophytic or endophytic extension (Figure 1).20 Air-fluid levels indicate the presence of a fistula with the lumen of the GI tract (Figure 1A). Magnetic resonance imaging (MRI) scan is an alternative to CT. It is preferable for defining the anatomy in rectal GIST, slightly better at detecting and characterizing small liver lesions, but less sensitive in demonstrating peritoneal metastasis. PET scans are almost never necessary.
Figure 1 -. Examples of primary GISTs.
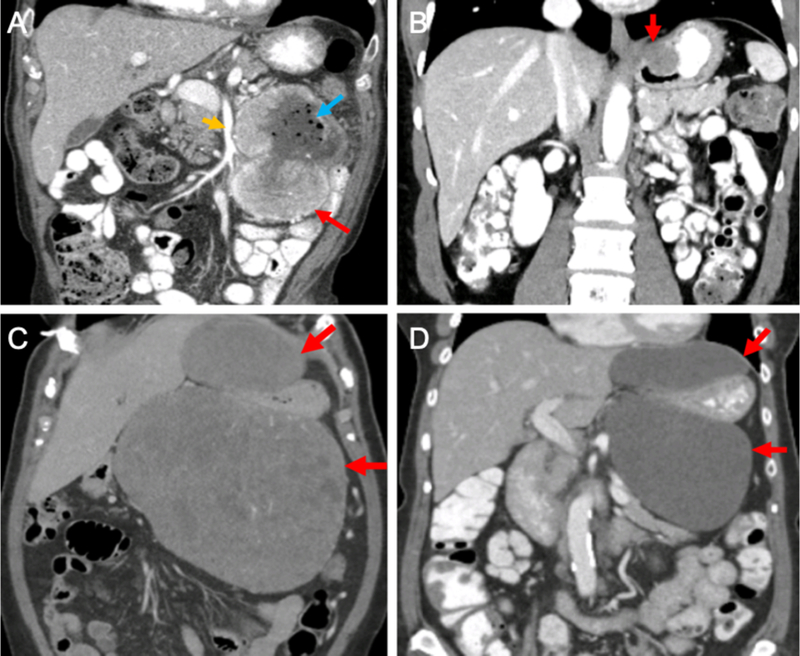
(A) Coronal CT image of a proximal jejunal GIST (red arrow) near the superior mesenteric artery (orange arrow). Note the air bubbles within the tumor (blue arrow), indicating the existence of a sinus tract between the bowel and the tumor. (B) Coronal CT image of a GIST (red arrow) located at the gastroesophageal junction. (C&D) Coronal CT images of a massive gastric GIST (C) before and (D) after 6 months of neoadjuvant imatinib. Red arrows indicate the bilobed tumor surrounding the stomach. Note the decreased density of the tumor after therapy. At operation, the tumor arose from the gastric cardia and was removed with splenectomy and a limited partial gastrectomy. CT – computed tomography
A tissue diagnosis can be obtained with an endoscopic or percutaneous biopsy. The feasibility of these techniques depends on the tumor location and operator skill. Endoscopy for esophageal, gastric, proximal small intestinal, and rectal GISTs will typically reveal a submucosal mass with normal overlying mucosa (unless ulceration has occurred) and well-defined margins. Endoscopic ultrasonography (EUS) provides further clarification, showing a hypoechoic lesion that most commonly arises from the muscularis propria layer. EUS-guided fine needle aspiration or core biopsy may not yield a definitive diagnosis as there may be no actual tumor tissue or only enough to conclude that spindle cells are present, which could represent GIST or leiomyoma. Percutaneous fine needle aspiration or biopsy is reserved for masses not reachable by endoscopy, but this approach has a small risk for hemorrhage or perforation. In patients without histologic proof of a GIST, especially those who have already undergone an inconclusive biopsy, we do not necessarily pursue another biopsy if the radiographic features are highly suggestive of GIST. The patient is counseled about the possibility of a “GIST masquerade”, such as schwannoma, leiomyoma, leiomyosarcoma, synovial sarcoma, or even ectopic pancreas.
When should neoadjuvant therapy be used?
Several factors contribute to decision-making for a patient with a proven or suspected GIST (Figure 2). The first priority is to stage the patient with cross-sectional imaging to identify the presence of metastases, which would lead to IM therapy and then surgery in the future only if all the tumor could be removed. (Note: Metastatic disease would mandate lifelong medical therapy and is not the focus of this review.) Gastric tumors less than 2 cm in diameter can be observed.21 For tumors 2 cm or greater, the patient is taken directly to surgery unless sacrifice of a lot of normal tissue is necessary because of large tumor size or difficult location.22
Figure 2 -. Management of primary GIST.
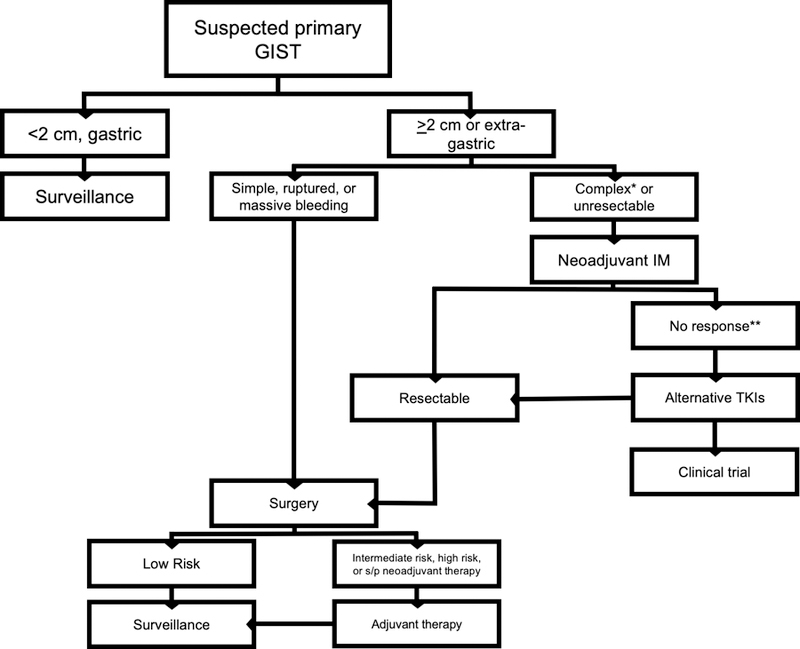
*requiring multivisceral resection due to technically challenging location or massive size **for complex but resectable tumors that do not respond to neoadjuvant IM, one can alternatively proceed directly to surgery IM – imatinib mesylate; TKI – tyrosine kinase inhibitor
If neoadjuvant therapy is being considered it is generally standard practice to confirm the diagnosis via biopsy. Some institutions can even perform a tumor mutation analysis on small samples, although this usually takes several weeks. We generally perform a CT scan 2–3 weeks after starting IM to assess tumor response. A reduction in tumor density or size indicates response, as defined by the Choi criteria.23 If the tumor has responded, we generally obtain the next scan 4 months later and operate between 6–9 months on IM, the time of maximal tumor shrinkage. IM can be safely continued up until the day before operation. If the tumor has grown on the early scan after IM initiation, the diagnosis may be incorrect or the specific GIST mutation may not be sensitive. Additional pathologic analysis of the biopsy may be indicated.
There have been a number of single-arm and non-controlled clinical trials demonstrating the safety and feasibility of neoadjuvant IM.24, 25 However, whether it improves the rate of R0 resection or overall survival is not proven. IM can be useful to arrest slow intraluminal bleeding. It is clear that responsive tumors have much less blood flow and usually reduced tumor size, rendering surgery easier to perform. This is particularly relevant in challenging anatomic locations. Reducing the size of a tumor at the gastroesophageal junction can convert a total gastrectomy to a local resection with primary closure. Downsizing of a duodenal GIST near the ampulla may avoid the need for pancreatoduodenectomy. GISTs at the duodenal/jejunal junction (Figure 1A) are typically close to the superior mesenteric artery and tumor shrinkage is often desirable. Neoadjuvant IM can facilitate sphincter preservation in certain rectal GISTs. Indeed, patients with GISTs in all of these sites have been commonly selected for neoadjuvant treatment in retrospective series.26–28
The current recommendation for those at moderate to high risk of recurrence is 3 years of adjuvant therapy, whether neoadjuvant therapy was used or not. The problem is that in many patients, risk cannot be fully evaluated since pre-treatment mitotic rate is often not available. The large, multi-institutional experiences suggest that 1–3 years of adjuvant therapy after neoadjuvant IM is safe and replicates the standard of RFS seen in the randomized trials of adjuvant IM. 25, 29, 30 With the emergence of data supporting increasingly longer durations of TKI therapy in the adjuvant setting, we may ultimately find that chronic adjuvant therapy is indicated for some patients who received neoadjuvant therapy.31
As our familiarity with specific primary KIT and PDGFRA mutations increases and preoperative tumor sequencing becomes faster and more widespread, neoadjuvant therapy will become more precise. For instance, though the most common KIT exon 11 mutants will respond to IM, PDGFRA D842V mutation is resistant to IM, KIT exon 9 mutations may respond better to higher doses of IM,32, 33 and SDH and NF1 mutations do not respond to TKIs. Primary KIT exon 13 and 14 mutations are rare and data is lacking, however it is known that secondary exon 13 and 14 mutations exhibit IM resistance and respond better to sunitinib.34
What are the fundamentals of GIST surgery?
There are several specific considerations in GIST surgery. Foremost, the tumors are soft and friable compared to adenocarcinomas and therefore easy to rupture, which risks peritoneal dissemination. Tumor rupture is an independent predictor of poor prognosis.35, 36 Meticulous dissection is necessary as GISTs are particularly vascular tumors and prone to bleed during resection. It is rare for GISTs to grow into adjacent structures, but rather they tend to push them aside. However, if a GIST is adherent to a nearby structure, the involved tissue should be removed en bloc. GISTs rarely metastasize to lymph nodes, so routine lymphadenectomy is unnecessary. However, when nearby nodes are enlarged, they should be removed and alternative diagnoses should be considered. The exception to this rule is patients with SDH-deficient GIST, who commonly have nodal metastasis.7
The optimal margin of tumor resection in GIST is not well defined. Since GISTs do not tend to spread submucosally, a grossly negative margin is generally sufficient and a 1 cm margin should be adequate. The prognostic impact of an R1 (microscopically positive) resection was unclear in the pre-IM era.9, 37 The American College of Surgeons Oncology Group (ACOSOG) Z9000 and Z9001 trials of adjuvant IM failed to show a significant effect of R1 resection on recurrence-free survival (RFS).36 It should be pointed out that true local recurrence is rare in GIST. Instead, peritoneal metastasis occurs. In the setting of an R1 resection, a discussion with the pathologist should take place since the resection edges can retract after surgery or staple lines are removed during tumor processing, which can make an initially negative margin appear either grossly or microscopically involved. Ultimately, the management of a patient with an R1 resection should involve a multidisciplinary approach with discussion of patient-specific risk factors and consideration of the risks of reoperation versus watchful waiting or IM therapy.
When should minimally invasive surgery be performed?
Laparoscopy is indicated for diagnosis and resection of smaller GISTs. We performed a size-matched comparison and found that laparoscopic resection was oncologically safe.38 In contrast, the limited published series of endoscopic resection for small GISTs have mixed results with perforation rates up to 13%.39 There are insufficient data to recommend routine endoscopic resection at this time. However, endoscopy can be helpful during a laparoscopic operation to facilitate identification of small, endophytic GISTs that may not be otherwise readily apparent at laparoscopy.40
What are the technical considerations for specific resections?
Because GISTs occur in diverse locations, the surgeon has to be familiar with a variety of techniques. GISTs arise most frequently in the stomach. Laparoscopic resection is favored for small to medium-sized gastric GISTs, especially those that are exophytic. Small tumors that grow endophytically may require concomitant intraoperative endoscopy. Posterior tumors are more difficult to remove as they may require extensive mobilization of the stomach, which may include division of the greater omentum and preservation of the gastroepiploic arteries to enter the lesser sac. Sometimes, the short gastric arteries must be sacrificed. Partial gastrectomy can often be achieved with surgical staplers, taking care to avoid narrowing the gastric lumen. A majority of the time a formal anatomic gastrectomy is not required, even for proximal tumors. We prefer an open approach for tumors near the gastroesophageal junction (Figure 1B). If there is concern about gastric lumen compromise, a gastrotomy can be made and the GIST is resected with a 1cm radial margin circumferentially with cautery. Although some patients present with massive gastric tumors, total gastrectomy is rarely required if neoadjuvant IM is utilized (Figure 1C, D). Preoperative discussion should include the possibility of total gastrectomy for certain tumors and also include the potential for removing nearby organs if they are attached to the tumor, such as the distal pancreas, spleen, or splenic flexure of the colon.
Many small bowel GISTs of the jejunum and ileum can be removed laparoscopically with an approach that emphasizes segmental resection with minimal direct handling of the tumor. The tumor is identified by laparoscopically running the small bowel from the ligament of Treitz to the ileocecal junction. Large tumors should be removed via an open approach to minimize risk of rupture. Duodenal GISTs are more complex and their management depends on which portion of the duodenum is affected. Periampullary tumors of the second portion may be treated with neoadjuvant IM but may still require pancreatoduodenectomy due to their medial location, whereas those of the lateral duodenal wall, if small or exophytic enough, may be resected locally with either primary closure or Roux-en-Y reconstruction using an isoperistaltic duodenojejunostomy.28 Large tumors of the more distal duodenum/proximal jejunum may be in close proximity to the SMA and neoadjuvant IM may be of benefit (Fig. 1A).
Rectal GISTs can be particularly challenging. Typically, neoadjuvant IM is employed to facilitate a sphincter-preserving operation and avoid an abdominoperineal resection and permanent end-colostomy. A full thickness resection of the rectal wall is desirable and may sometimes be performed via the transanal approach if distal enough.27
There are also select mutation-specific surgical considerations. SDH-deficient41 and NF142 associated GIST can both present as multifocal disease, and their management is not well defined. These tumors have malignant potential, but because of their multifocality the role of surgical resection must be balanced with the extent of potential tissue loss and potential long-term surgical morbidity. Therefore, surgery is often indicated when, on surveillance imaging, one or a few tumors are found to be growing faster than the others, or have become symptomatic. For example, partial gastrectomy may be performed for a dominant tumor in favor of total gastrectomy, leaving other smaller, indolent tumors in situ. Also, as noted above, regional lymphadenectomy should be performed for SDH-deficient GISTs, due to their ability to metastasize to lymph nodes.
How is the risk of recurrence determined?
It should be emphasized that we now know that over 70% of patients with primary GIST are cured by surgery alone, as demonstrated by the ACOSOG Z9001 placebo arm.43 Prognosis after resection of a primary, localized GIST depends on tumor size, location, and mitotic rate. There are 2 main prognostic systems based on these variables. Miettinen and Lasota originally used mitotic rate (>5 or ≤5 mitoses per 50 hpf), tumor size (cutoffs of 2cm, 5cm and 10cm), and tumor location (stomach is more favorable than small intestine or rectum).21, 44 Subsequently, we created an online nomogram to estimate 2- and 5- year RFS after resection (http://www.mskcc.org/mskcc/html/98103.cfm).45 The nomogram has been validated by others.46, 47 The Miettinen criteria are the basis for the most recent version of the American Join Commission on Cancer (AJCC) Staging system, which incorporates mitotic rate and anatomic location into their standard TNM system.48
Mutation status of the primary tumor should also be considered in assessing patient prognosis. In the pre-IM era, patients did worse if their tumor had a KIT exon 11 deletion or insertion versus a point mutation.49 Moreover, deletions specific to KIT amino acids 557 and 558 indicated poor prognosis.50, 51 However, there is less clarity regarding the impact of these mutations in the IM era. One analysis of the Scandinavian Sarcoma Group XVIII (SSGXVIII) multicenter clinical trial concluded that the KIT exon 11 deletions were associated with poorer RFS in patients treated with 1 year of IM, but that effect was ameliorated with 3 years of treatment.52 Additionally, ACOSOG Z9001 data determined that exon 11 deletions were associated with improved RFS in the 1 year IM arm, compared to placebo.53 Less common KIT exon mutation sites include exons 9, 13, 14, and 17. Exon 9 mutations may respond better to higher doses of IM in metastatic GIST,33 but there are inadequate data for this in the adjuvant setting. Primary mutations in exons 13 and 14 are rare, though they may still respond to IM treatment. However, secondary mutations in exons 13, 14, and 17 are usually responsible for IM resistance54 and heightened awareness of this fact should guide surveillance practice and transition to sunitinib or another appropriate TKI upon progression. Of note, the D842V subset of PDGFRA mutant GISTs is resistant to IM therapy. GISTs without mutations in KIT or PDGFRA are unlikely to benefit from adjuvant IM therapy. Lastly, the Complexity Index in Sarcoma (CINSARC) profile of 67 genes related to chromosome integrity, mitotic control, and genome complexity has been applied to GIST and was shown to further help stratify patients of intermediate risk.55
Adjuvant Therapy – Who, why, and how long?
While it is now clear that adjuvant IM increases RFS in patients with intermediate or high-risk primary GIST (Table 1), the optimal duration of treatment is unclear. ACOSOG Z9001, a phase 3 randomized placebo controlled trial of adjuvant IM versus placebo in patients with complete gross removal of GISTs ≥3cm showed prolonged RFS for IM compared to placebo with a median follow-up of 19.7 months, which led to FDA and European Medicines Agency approval for adjuvant IM in GIST.43 Subsequent long term follow-up (median of 74 months) showed persistently prolonged RFS, even though many patients in the placebo arm did not experience recurrence.56 However, patients in the treatment arm only received 1 year of adjuvant IM, and some developed recurrence after drug discontinuation.
Table 1 –
Summary of adjuvant imatinib trials in primary GIST.
| Trial | Phase | Entry Criteria | Treatment Dose/Duration | Relevant Findings |
|---|---|---|---|---|
| ACOSOG Z900151 | III | Tumor ≥ 3cm | 400 mg daily for 1 year vs placebo | 1-year RFS 98% vs 83% No difference in OS |
| SSG XVIII52, 53 | III | High-risk tumor | 400 mg daily for 1 year vs 3 years | 3-year RFS 73% vs 55% 3-year OS 92% vs 85% 3-year DSS 95% vs 89%¶ |
| EORTC 6202454 | III | Intermediate and high-risk tumor | 400 mg daily for 2 years vs no treatment | 5-year IFFS 87% vs 84%¶ 3-year RFS 84 vs 66% 5-year RFS 69% vs 63% |
| PERSIST-555 | II | Intermediate or high-risk tumor | 400 mg daily for 5 years | 5-year RFS 90% 5-year OS 95% 50% in treatment arm discontinued imatinib |
These findings were not statistically significant. ACOSOG, American College of Surgeons Oncology Group; EORTC, European Organization for the Research and Treatment of Cancer; SSG, Scandinavian Sarcoma Group; PERSIST-5, Postresection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors With 5 Years of Adjuvant Imatinib; RFS, Recurrence Free Survival; OS, Overall Survival; IFFS, Imatinib-Failure Free Survival
The SSG XVIII trial, a large Scandinavian prospective randomized phase 3 trial demonstrated superior RFS with three years of adjuvant IM compared to one year. There was significant improvement in overall survival (OS), but not disease-specific survival (DSS).57, 58 Another European trial by the EORTC randomized 2 years of adjuvant IM versus observation in intermediate or high-risk GIST and evaluated time to IM resistance, finding that in high risk patients there was improved RFS in the IM arm but no difference in IM failure-free survival.59 The PERSIST-5 trial, a phase 2 study of five years of adjuvant IM therapy was recently reported. It showed that no patient with an IM-sensitive mutation had a tumor recurrence while on IM, but 49% of patients stopped treatment early, mostly due to patient choice rather than adverse events.31
Our practice is to clarify the goals of adjuvant IM with intermediate and high-risk patients. There is no doubt that RFS is prolonged. Since recurrence can develop in some patients after IM is stopped at 1 or 3 years, chronic therapy (>5 years) may be indicated for select patients. One example is patients with intraoperative tumor rupture. These GISTs, regardless of tumor location, fall into a high-risk category.60 These patients should generally be considered to have metastatic disease61 and therefore treated with chronic IM.
Whether adjuvant IM increases DSS has not been proven. DSS may not be altered since patients are followed closely with serial radiologic scans, resulting in recurrence usually being detected when there is minimal disease that usually responds to initiation of IM. The potential benefits of prolonged RFS have to be balanced against the side effects and cost of the drug. IM is generally well tolerated, but it can be associated with nausea/vomiting, edema of the face, hands and feet, diarrhea, skin rashes, and fever, many of which subside with time. Dose reduction is required in some patients.
There are additionally some mutation-specific considerations to bear in mind when prescribing adjuvant IM. Although patients with a KIT exon 9 mutation may benefit from a higher IM dose (i.e., 800 mg daily) in the metastatic setting, there are no data in the adjuvant setting and so 400 mg daily is recommended. PDGFRA D842V-mutant GISTs are resistant to IM, as are those with SDH-deficient or NF1 related GISTs, and so adjuvant IM is not used. There are newer targeted therapies emerging for some of these IM resistant primary mutations, though no formal treatment guidelines exist yet.
Conclusions and Future Directions.
The management of primary localized GIST has evolved significantly over the past 2 decades, and now there is an excellent understanding of patient presentation, diagnostic methods, surgical and anatomic considerations, prognostication, and role for neoadjuvant and adjuvant therapy. Nevertheless, there is still room for improvement. Novel RTKs are being developed to treat IM-resistant mutations. Other approaches may include immunotherapy, or combination therapies, with the ultimate goal of eradicating residual, microscopic disease. New advances will lead to further personalization of therapy.
Acknowledgments
Funding: This work was supported in part by NIH grant R01 CA102613 (RPD)
Footnotes
Conflicts of Interest Statement:
This statement certifies that the authors Mark S. Etherington and Ronald P. DeMatteo have no conflicts of interest to declare regarding this manuscript entitled “Tailored Management of Primary Gastrointestinal Stromal Tumors.” The authors certify that there are no conflicts of interest to report.
Contributor Information
Mark S. Etherington, Department of Surgery, Perelman School of Medicine, University of Pennsylvania.
Ronald P. DeMatteo, Department of Surgery, Perelman School of Medicine, University of Pennsylvania.
References.
- 1.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152: 1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 2.Mastrangelo G, Coindre JM, Ducimetière F, et al. Incidence of soft tissue sarcoma and beyond: a population-based prospective study in 3 European regions. Cancer 2012;118: 5339–5348. [DOI] [PubMed] [Google Scholar]
- 3.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7: 507–519. [DOI] [PubMed] [Google Scholar]
- 4.Nakahara M, Isozaki K, Hirota S, et al. A novel gain-of-function mutation of c-kit gene in gastrointestinal stromal tumors. Gastroenterology 1998;115: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 5.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279: 577–580. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299: 708–710. [DOI] [PubMed] [Google Scholar]
- 7.Boikos SA, Pappo AS, Killian JK, et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol 2016;2: 922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001;438: 1–12. [DOI] [PubMed] [Google Scholar]
- 9.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 2004;165: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 1992;215: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005;103: 821–829. [DOI] [PubMed] [Google Scholar]
- 13.van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 2001;358: 1421–1423. [DOI] [PubMed] [Google Scholar]
- 14.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001;344: 1052–1056. [DOI] [PubMed] [Google Scholar]
- 15.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347: 472–480. [DOI] [PubMed] [Google Scholar]
- 16.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26: 626–632. [DOI] [PubMed] [Google Scholar]
- 17.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364: 1127–1134. [DOI] [PubMed] [Google Scholar]
- 18.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368: 1329–1338. [DOI] [PubMed] [Google Scholar]
- 19.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics 2003;23: 283–304, 456; quiz 532. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23: 70–83. [DOI] [PubMed] [Google Scholar]
- 22.Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 2007;5 Suppl 2: S1–29; quiz S30. [PubMed] [Google Scholar]
- 23.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25: 1753–1759. [DOI] [PubMed] [Google Scholar]
- 24.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 2009;99: 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blesius A, Cassier PA, Bertucci F, et al. Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial. BMC Cancer 2011;11: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013;20: 2937–2943. [DOI] [PubMed] [Google Scholar]
- 27.Cavnar MJ, Wang L, Balachandran VP, et al. Rectal Gastrointestinal Stromal Tumor (GIST) in the Era of Imatinib: Organ Preservation and Improved Oncologic Outcome. Ann Surg Oncol 2017;24: 3972–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SY, Goh BK, Sadot E, et al. Surgical Strategy and Outcomes in Duodenal Gastrointestinal Stromal Tumor. Ann Surg Oncol 2017;24: 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutkowski P, Debiec-Rychter M, Nowecki ZI, et al. Different factors are responsible for predicting relapses after primary tumors resection and for imatinib treatment outcomes in gastrointestinal stromal tumors. Med Sci Monit 2007;13: CR515–522. [PubMed] [Google Scholar]
- 30.Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol 2012;19: 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raut CP, Espat NJ, Maki RG, et al. Efficacy and Tolerability of 5-Year Adjuvant Imatinib Treatment for Patients With Resected Intermediate- or High-Risk Primary Gastrointestinal Stromal Tumor: The PERSIST-5 Clinical Trial. JAMA Oncol 2018: e184060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widmer N, Decosterd LA, Csajka C, et al. Imatinib plasma levels: correlation with clinical benefit in GIST patients. Br J Cancer 2010;102: 1198–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol 2008;26: 5360–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 2008;26: 5352–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutkowski P, Bylina E, Wozniak A, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur J Surg Oncol 2011;37: 890–896. [DOI] [PubMed] [Google Scholar]
- 36.McCarter MD, Antonescu CR, Ballman KV, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg 2012;215: 53–59; discussion 59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langer C, Gunawan B, Schuler P, Huber W, Fuzesi L, Becker H. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg 2003;90: 332–339. [DOI] [PubMed] [Google Scholar]
- 38.Karakousis GC, Singer S, Zheng J, et al. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol 2011;18: 1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: Advantages and hurdles. World J Gastrointest Endosc 2015;7: 192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 2008;22: 1729–1735. [DOI] [PubMed] [Google Scholar]
- 41.Miettinen M, Wang ZF, Sarlomo-Rikala M, Osuch C, Rutkowski P, Lasota J. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol 2011;35: 1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson J, Sihto H, Meis-Kindblom JM, Joensuu H, Nupponen N, Kindblom LG. NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol 2005;29: 1170–1176. [DOI] [PubMed] [Google Scholar]
- 43.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373: 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130: 1466–1478. [DOI] [PubMed] [Google Scholar]
- 45.Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 2009;10: 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chok AY, Goh BK, Koh YX, et al. Validation of the MSKCC Gastrointestinal Stromal Tumor Nomogram and Comparison with Other Prognostication Systems: Single-Institution Experience with 289 Patients. Ann Surg Oncol 2015;22: 3597–3605. [DOI] [PubMed] [Google Scholar]
- 47.Belfiori G, Sartelli M, Cardinali L, et al. Risk stratification systems for surgically treated localized primary Gastrointestinal Stromal Tumors (GIST). Review of literature and comparison of the three prognostic criteria: MSKCC Nomogram, NIH-Fletcher and AFIP-Miettinen. Ann Ital Chir 2015;86: 219–227. [PubMed] [Google Scholar]
- 48.DeMatteo RP, Maki RG, Agulnik M, et al. Gastrointestinal Stromal Tumor. In: AJCC Cancer Staging Manual 8th Edition. Amin MB, editor. Chicago: AJCC, 2017: 523 Corrected at 4th printing, 2018. [Google Scholar]
- 49.Singer S, Rubin BP, Lux ML, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 2002;20: 3898–3905. [DOI] [PubMed] [Google Scholar]
- 50.Wardelmann E, Losen I, Hans V, et al. Deletion of Trp-557 and Lys-558 in the juxtamembrane domain of the c-kit protooncogene is associated with metastatic behavior of gastrointestinal stromal tumors. Int J Cancer 2003;106: 887–895. [DOI] [PubMed] [Google Scholar]
- 51.Martin J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol 2005;23: 6190–6198. [DOI] [PubMed] [Google Scholar]
- 52.Joensuu H, Wardelmann E, Sihto H, et al. Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial. JAMA Oncol 2017;3: 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014;32: 1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 2005;11: 4182–4190. [DOI] [PubMed] [Google Scholar]
- 55.Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res 2012;18: 826–838. [DOI] [PubMed] [Google Scholar]
- 56.DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg 2013;258: 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 58.Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol 2016;34: 244–250. [DOI] [PubMed] [Google Scholar]
- 59.Casali PG, Le Cesne A, Poveda A, et al. Time to definitive failure to the first tyrosine kinase inhibitor in localized gastrointestinal stromal tumors (GIST) treated with imatinib as an adjuvant: Final results of the EORTC STBSG, AGITG, UNICANCER, FSG, ISG, and GEIS randomized trial. Annals of Oncology 2017;28. [Google Scholar]
- 60.Joensuu H Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39: 1411–1419. [DOI] [PubMed] [Google Scholar]
- 61.Nishida T, Cho H, Hirota S, et al. Clinicopathological Features and Prognosis of Primary GISTs with Tumor Rupture in the Real World. Ann Surg Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


