Abstract
Background
Up to 15 per cent of colorectal cancers present with peritoneal metastases (CPM). Cytoreductive surgery and heated intraperitoneal chemotherapy (CRS + HIPEC) aims to achieve macroscopic tumour resection combined with HIPEC to destroy microscopic disease. CRS + HIPEC is a major operation with significant morbidity and effects on quality of life (QoL). Improving patient selection is crucial to maximize patient outcomes while minimizing morbidity and mortality. The aim of this study was to identify prognostic factors for patients with CPM undergoing CRS + HIPEC.
Methods
A systematic search of MEDLINE, Embase and Cochrane Library electronic databases was performed using terms for colorectal cancer, peritoneal metastasis and CRS + HIPEC. Included studies focused on the impact of prognostic factors on overall survival following CRS + HIPEC in patients with CPM.
Results
Twenty‐four studies described 3128 patients. Obstruction or perforation of the primary tumour (hazard ratio (HR) 2·91, 95 per cent c.i. 1·5 to 5·65), extent of peritoneal metastasis as described by the Peritoneal Carcinomatosis Index (PCI) (per increase of 1 PCI point: HR 1·07, 1·02 to 1·12) and the completeness of cytoreduction (CC score above zero: HR 1·75, 1·18 to 2·59) were associated with reduced overall survival after CRS + HIPEC.
Conclusion
Primary tumour obstruction or perforation, PCI score and CC score are valuable prognostic factors in the selection of patients with CPM for CRS + HIPEC.
Introduction
Colorectal peritoneal metastasis (CPM) occurs in up to 15 per cent of patients with colorectal cancer1, 2, 3. The prognosis of patients with CPM is poor: untreated median overall survival (OS) is just 6 months4. With systemic chemotherapy, median OS is improved to up to 20 months5, 6, 7. The standard for CPM is cytoreductive surgery and heated intraperitoneal chemotherapy (CRS + HIPEC), which may improve median OS by 20–63 months8, 9, 10, 11, 12, 13, 14, 15, 16.
CRS + HIPEC is a long, high‐cost operation associated with a protracted inpatient and high‐dependency or intensive care unit stay, and an associated mortality rate of 1–12 per cent and morbidity rate of 7–63 per cent11, 14, 16, 17, 18, 19, 20, 21, 22, 23. Improving patient selection is therefore crucial to maximize patient outcomes whilst minimizing morbidity and mortality.
Variation in outcomes for CRS + HIPEC can be explained in part by patient selection, for example necessitating the ability to achieve complete cytoreduction at CRS, the exclusion of patients with extensive CPM as assessed by Sugarbaker's Peritoneal Carcinomatosis Index (PCI), and selection of patients with minimal co‐morbidity and good performance status.
Several clinicopathological variables that impact on survival have been identified in the literature, including lymph node (LN) status, tumour differentiation and histological findings, the completeness of cytoreduction (CC score) and PCI. There is wide variation, however, in the variables reported by studies and selection criteria for centres performing CRS + HIPEC worldwide, and in turn in their outcomes.
Studies reporting prognostic factors for CRS + HIPEC are cohort in design with small samples, and each examines numerous and varying prognostic factors on differing scales of measurement. No consensus exists as to which prognostic factors contraindicate CRS + HIPEC, or predict a good outcome. A comprehensive evidence synthesis is therefore called for to determine relevant prognostic factors.
The aim of this systematic review and meta‐analysis was to analyse all prognostic factors affecting OS in patients with CPM undergoing CRS + HIPEC.
Methods
A comprehensive literature search was conducted in accordance with the PRISMA guidelines24. MEDLINE, Embase and Cochrane Library electronic databases, registers of clinical trials (ClinicalTrials.gov and the WHO International Clinical Trials Registry) and the Conference Proceedings Citation Index, Zetoc, were searched from inception to the present. The search strategy captured terms for colorectal cancer, peritoneal metastasis and CRS + HIPEC techniques, separated by the Boolean operator ‘AND’. For an example search strategy, see Appendix S1 (supporting information). Searches were supplemented by a hand search of selected journals and the reference lists of all included studies.
Study selection
English‐language articles were eligible for inclusion if they reported on the impact of prognostic factors on OS in patients with CPM undergoing CRS + HIPEC. Where multiple studies described the same cohort of patients, the largest and most complete data set was included. Review articles, case reports and case series of fewer than ten patients were excluded. Additional exclusion criteria included studies involving patients with a primary tumour other than colorectal cancer and studies in which a proportion of the cohort did not receive combined CRS + HIPEC.
After screening the titles and abstracts, articles fulfilling the eligibility criteria were identified and their full‐text publications reviewed. Literature search and study selection were done independently by two researchers, and any disagreements were resolved by discussion with senior reviewers. After qualitative assessment, articles were screened to ensure they presented adequate statistical information to be included in the meta‐analysis: hazard ratios (HRs) with confidence intervals or Kaplan–Meier curves with the number of events and patients at risk. When HRs, confidence intervals or P values were not provided directly, the methods of Tierney et al.25 were used to estimate them indirectly from Kaplan–Meier curves, when presented in adequate detail with the numbers of events and patients at risk26.
Assessment of risk of bias
The quality and risk of bias of individual studies was assessed using the Quality in Prognosis Studies (QUIPS) tool27. This tool reviews each study according to six criteria: study participation, attrition, prognostic factor measurement, outcome measurement, confounding factors, and statistical analysis and reporting. Two authors scored all articles independently.
Data extraction
Data were extracted independently by two reviewers using a dedicated and piloted data extraction form. The number of patients, study design, patient demographics, tumour characteristics, use of adjuvant and neoadjuvant regimens, CRS + HIPEC techniques, survival and prognostic factors were recorded.
The unadjusted HR and its 95 per cent c.i. and P value were extracted. When adjusted HRs (with confidence intervals and P values) were reported, these were extracted along with the set of adjustment factors used. If HRs, confidence intervals or P values were not provided directly, the methods of Tierney and colleagues25 were used to estimate them indirectly from Kaplan–Meier curves, when presented in adequate detail with the numbers of events and patients at risk26.
Prognostic factors reported on a continuous scale were extracted. If results were categorized into three or more categories, results for each comparison were extracted and, when clinically relevant, were grouped to form a binary comparison.
Prognostic factor selection
All prognostic factors described adequately and reported by two or more independent studies were included.
Statistical analysis
Owing to clinical and methodological heterogeneity, a random‐effects meta‐analysis was used (on the log(HR) scale) using the method of DerSimonian and Laird28. The combined effect size was described by the pooled HR, its confidence interval and P value, with P < 0·050 considered significant. For prognostic factors reported by more than two studies, a 95 per cent prediction interval (a measure of the variation in treatment effects) is presented29. Heterogeneity was also described by the I 2 statistic30. All analyses were performed in STATA® version 15 (StataCorp, College Station, Texas, USA).
Results
The final literature search was performed on 3 April 2018. Literature searches (after removal of duplicates) identified 1052 records. Titles and abstract screening identified 158 full‐text articles for review. Of these, 51 studies met the inclusion criteria. Twenty‐four unique studies11, 18, 20, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 reporting on 3128 patients with CPM presented adequate data to be included in the meta‐analysis (Fig. 1). Of the 24 cohort studies, six18, 34, 37, 40, 44, 47 were prospective and the remaining 1811,20,31–33,35,36,38,39,41–43,45,46,48–51 were retrospective (Table S1, supporting information).
Figure 1.
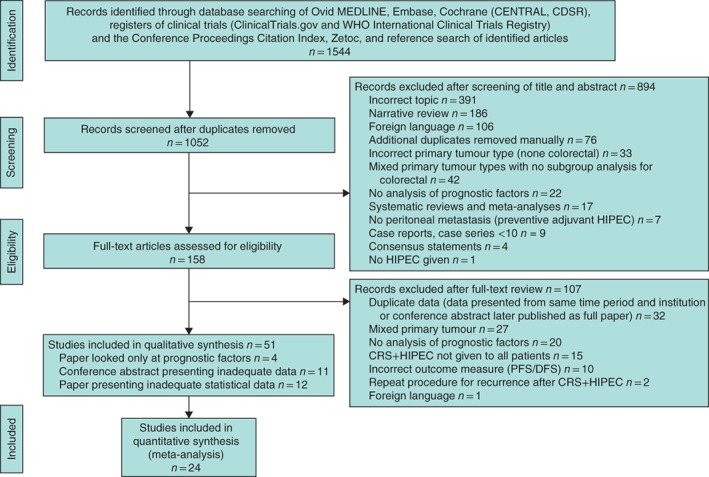
PRISMA diagram for the review CENTRAL, Cochrane Central Register of Controlled Trials; CDSR, Cochrane Database of Systematic Reviews; DARE, Database of Abstracts of Reviews of Effects; HIPEC, heated intraperitoneal chemotherapy; CRS, cytoreductive surgery; PFS, progression‐free survival; DFS, disease‐free survival.
A number of studies presented both an unadjusted and adjusted HR. As adjustment factors varied widely between studies (Table S2, supporting information), meta‐analysis was stratified according to whether the HR was unadjusted or adjusted.
Quality of research
There was a low risk of bias from study participation. The moderate risk of bias due to study attrition reflects the poor reporting of loss to follow‐up. There was a low risk of bias due to prognostic factor measurement. Prognostic factors were objective, clearly defined and clinically relevant. Reporting of all prognostic factors and treatment variations was incomplete in the majority of studies, resulting in a moderate risk of bias. The presentation of results and analytical strategy was sufficient in the majority of studies resulting in a low risk of bias (Table S3, supporting information).
Pooled median OS across all studies was 32 (range 12·2–51) months, with a pooled median follow‐up of 28·1 (13·3–62·4) months (Table S4, supporting information).
Patient factors
Age
The pooled median age of patients included was 54 (range 45·5–69·3) years. Eleven studies18, 20, 33, 34, 37, 40, 42, 48, 49, 50, 52 reported on age as a prognostic factor and ten presented adequate data to be included in the meta‐analysis. Five studies categorized age into binary outcomes that could not be combined meaningfully. The pooled unadjusted HR was 1·00 (95 per cent c.i. 0·98 to 1·03) (I 2 = 38·5 per cent) and adjusted HR was 1·00 (0·96 to 1·04) (I 2 = 69·3 per cent), for an increase in age of 1 year (Fig. 2; Fig. S1, supporting information). These data provide no evidence that age is a useful predictor of OS.
Figure 2.
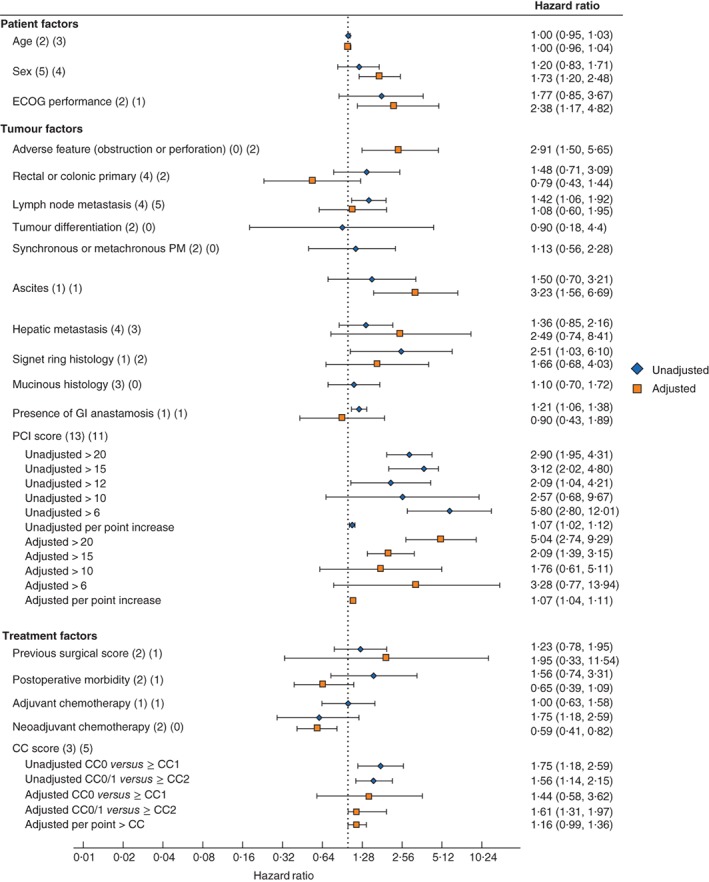
Effect of prognostic factors on overall survival Hazard ratios are shown with 95 per cent confidence intervals. Values in parentheses after each factor indicate the numbers of studies providing unadjusted and adjusted hazard ratios respectively. ECOG, Eastern Cooperative Oncology Group; PM, peritoneal metastasis; GI, gastrointestinal; PCI, Peritoneal Carcinomatosis Index; CC, completeness of cytoreduction.
Sex
Nine studies18, 20, 34, 36, 37, 40, 42, 51, 52 reported on the effect of sex on OS and eight presented adequate data to be included in the meta‐analysis. The pooled unadjusted HR for male sex was 1·20 (95 per cent c.i. 0·83 to 1·71) (I 2 = 33·8 per cent) and adjusted HR was 1·73 (1·20 to 2·48) (I 2 = 11·6 per cent) (Fig. 2; Fig. S1, supporting information). It is therefore unclear whether sex is a useful predictor of OS.
Eastern Cooperative Oncology Group performance status
Five studies18, 31, 43, 52, 53 reported on the influence of Eastern Cooperative Oncology Group (ECOG) status on OS, and three presented adequate data to be included in the meta‐analysis. The pooled unadjusted HR for an ECOG score of at least 2 was 1·77 (95 per cent c.i. 0·85 to 3·67) (I 2 = 0 per cent) (Fig. 2; Fig. S1, supporting information). These data provide no evidence that ECOG is a useful predictor of OS.
Tumour factors
Adverse primary tumour features
Three studies18, 33, 52 reported on the influence of adverse features of the primary tumour (obstruction or perforation) on OS; two presented adequate data to be included in the meta‐analysis. The pooled adjusted HR for adverse features of the primary tumour was 2·91 (95 per cent c.i. 1·5 to 5·64) (I 2 = 0 per cent) (Fig. 2; Fig. S1, supporting information). These data indicate that adverse primary features are a useful predictor of decreased OS (Table S5, supporting information).
Rectal or colonic primary
Eight studies20, 34, 36, 40, 43, 47, 53, 54 reported on the influence of a rectal or colonic primary on OS and six presented adequate data to be included in the meta‐analysis. The pooled unadjusted HR for rectal primary (compared with colonic primary) was 1·48 (95 per cent c.i. 0·71 to 3·09) (I 2 = 73·8 per cent) (Fig. 2; Fig. S1, supporting information). These data provide no evidence that a rectal primary is a useful predictor of OS.
Lymph node metastasis
Eleven studies11, 18, 33, 34, 35, 37, 48, 49, 50, 53, 54 reported on the effect of LN status on OS; nine presented adequate data to be included in the meta‐analysis. The pooled unadjusted HR for positive LNs (compared with negative LNs) was 1·42 (95 per cent c.i. 1·06 to 1·92) (I 2 = 0 per cent) and adjusted HR was 1·08 (0·60 to 1·95) (I 2 = 68·6 per cent) (Fig. 2; Fig. S1, supporting information). It is therefore unclear whether lymph nodes are a useful predictor of OS.
Tumour differentiation
Two studies34, 40 reported on the effect of primary tumour differentiation on OS. The pooled unadjusted HR for well differentiated tumours was 0·90 (95 per cent c.i. 0·18 to 4·40) (I 2 = 72·4 per cent) (Fig. 2; Fig. S1, supporting information). These data provide no evidence that primary tumour differentiation is a useful predictor of OS.
Timing of colorectal peritoneal metastasis (synchronous or metachronous)
Three studies11, 40, 54 reported on the effect of the timing of CPM (synchronous or metachronous) on OS. Two presented adequate data to be included in the meta‐analysis. The pooled unadjusted HR for synchronous CPM was 1·13 (95 per cent c.i. 0·56 to 2·28) (I 2 = 57·1 per cent) (Fig. 2; Fig. S1, supporting information), indicating that the timing of CPM is not a useful predictor of OS.
Ascites
Two studies18, 40 reported on the effect of malignant ascites on OS. One paper40 presented an unadjusted HR (1·50, 95 per cent c.i. 0·70 to 3·21) and one18 an adjusted HR (3·23, 1·56 to 6·69) (Fig. 2; Fig. S1, supporting information), so it was not possible to provide a pooled effect estimate.
Hepatic metastasis
Eleven studies18, 31, 34, 35, 36, 37, 38, 43, 44, 47, 50 reported on the effect of surgically treated hepatic metastasis on OS. Seven presented adequate data to be included in the meta‐analysis. The pooled unadjusted HR for surgically treated hepatic metastasis was 1·36 (95 per cent c.i. 0·85 to 2·16) (I 2 = 43·6 per cent) and the adjusted HR was 2·49 (0·74 to 8·41) (I 2 = 85·3 per cent) (Fig. 2; Fig. S1, supporting information). These data provide no evidence that the presence of surgically treated hepatic metastasis is a useful predictor of OS.
Signet ring histology
Three studies11, 42, 46 reported on the effect of signet ring histology on OS. Two included adequate data to be included in the meta‐analysis. The pooled adjusted HR for signet ring histology was 1·65 (95 per cent c.i. 0·68 to 4·03) (I 2 = 0 per cent) (Fig. 2; Fig. S1, supporting information), suggesting that signet ring histology is not a useful predictor of OS.
Mucinous histology
Four studies11, 43, 46, 49 reported on the influence of mucinous histology on OS. Three studies presented adequate data to be included in the meta‐analysis. The pooled unadjusted HR for mucinous histology was 1·10 (95 per‐cent c.i. 0·70 to 1·72) (I 2 = 0 per cent) (Fig. 2; Fig. S1, supporting information), suggesting that mucinous histology is not a useful predictor of OS.
Gastrointestinal anastomosis in CRS + HIPEC
Two studies18, 43 reported on the effect of one or more gastrointestinal anastomoses on OS. One paper43 presented an unadjusted HR (1·21, 95 per cent c.i. 1·06 to 1·38) and the other18 an adjusted HR (0·90, 0·43 to 1·89) (Fig. 2; Fig. S1, supporting information), so it was not possible to provide a pooled effect estimate.
Peritoneal Carcinomatosis Index
Eighteen studies11, 20, 31, 32, 33, 34, 35, 36, 37, 38, 40, 41, 43, 45, 49, 50, 54, 55 reported on the effect of the extent of CPM as described by the PCI; 16 studies presented adequate data to be included in the meta‐analysis. PCI score was reported as a continuous variable or condensed into categorical variables.
A PCI score greater than the following levels was predictive of reduced OS: PCI above 20 (unadjusted HR 2·90, 95 per cent c.i. 1·95 to 4·31, I 2 = 0 per cent; adjusted HR 5·04, 2·74 to 9·29, I 2 = 0 per cent); PCI above 15 (unadjusted HR 3·12, 2·02 to 4·80, I 2 = 0 per cent; adjusted HR 2·09, 1·39 to 3·15, I 2 = 0 per cent). PCI as a continuous variable was predictive of reduced OS: pooled unadjusted HR 1·07 (1·02 to 1·12) per increase of one PCI point (I 2 = 46·1 per cent); pooled adjusted HR 1·07 (1·04 to 1·11) per PCI point increase (I 2 = 67·1 per cent). Lower PCI levels were not predictive of OS: PCI above 10 (unadjusted pooled HR 2·57, 0·68 to 9·67; I 2 = 90 per cent); PCI above 6, adjusted pooled HR 3·28 (0·77 to 13·94; I 2 = 92 per cent) (Fig. 2; Fig. S1, supporting information).
Treatment factors
Previous surgical score
Three studies11, 34, 42 reported on the influence of the previous surgical score (PSS) on OS. The pooled unadjusted HR for a PSS of at least 2 was 1·23 (95 per cent c.i. 0·78 to 1·95) (I 2 = 0 per cent) (Fig. 2; Fig. S1, supporting information). These data provide no evidence that PSS is a useful predictor of OS.
Postoperative morbidity
Three studies40, 43, 50 reported on the association between postoperative morbidity and OS. The pooled unadjusted HR for a Clavien–Dindo complication of grade III or above was 1·56 (95 per cent c.i. 0·74 to 3·31) (I 2 = 48·7 per cent) (Fig. 2; Fig. S1, supporting information). These data provide no evidence that postoperative morbidity is a useful predictor of OS.
Neoadjuvant and adjuvant chemotherapy
Neoadjuvant and adjuvant treatment of the primary tumour was reported poorly (Table S6, supporting information). No study reported response to treatment, or whether it was completed as planned.
Two studies11, 43 reported on the effect of neoadjuvant chemotherapy before CRS + HIPEC on OS. The pooled unadjusted HR for the use of neoadjuvant chemotherapy was 1·00 (95 per cent c.i. 0·63 to 1·58) (I 2 = 0 per cent) (Fig. 2; Fig. S1, supporting information). These data provide no evidence that neoadjuvant chemotherapy is a useful predictor of OS.
Adjuvant chemotherapy
Four studies11, 35, 43, 50 reported on the effect of adjuvant chemotherapy after CRS + HIPEC on OS. The pooled unadjusted HR for adjuvant chemotherapy was 0·60 (95 per cent c.i. 0·29 to 1·21) (I 2 = 68·1 per cent) (Fig. 2; Fig. S1, supporting information), which suggests there is no evidence that use of adjuvant chemotherapy is predictive of OS.
Completeness of cytoreduction
Eight studies11, 32, 35, 40, 42, 43, 51, 55 reported on the effect of the completeness of cytoreduction on OS; seven presented adequate data to be included in the meta‐analysis. A CC score above zero was predictive of a reduction in OS (unadjusted HR 1·75, 95 per cent c.i. 1·18 to 2·59) (I 2 = 79·5 per cent), as was a CC score of 2 or more (HR 1·61, 1·31 to 1·97) (I 2 = 0 per cent) (Fig. 2; Fig. S1, supporting information).
Discussion
This systematic review and meta‐analysis examined the effect of prognostic factors on OS following CRS + HIPEC for CPM. Emergency presentation with obstruction or perforation of the primary tumour as well as the extent of CPM and the completeness of resection, as described by the PCI and the CC score respectively, were the only significant prognostic factors.
An emergency presentation of the primary tumour with obstruction or perforation was predictive of reduced OS (for both synchronous and metachronous CPM). A number of factors may contribute to this. In the primary setting, colorectal cancers presenting with obstruction or perforation are associated with decreased cancer‐specific survival and increased postoperative mortality56. Obstructed or perforated colorectal cancer is, by definition, advanced in stage and increases the risk of metastasis; additionally, emergency presentation limits the possibility of neoadjuvant treatment and may delay adjuvant treatment owing to postoperative morbidity. The extent of peritoneal metastasis as described by the PCI was predictive of reduced OS as a continuous variable and when the PCI score was 12 or above. A complete cytoreduction (CC0) was predictive of improved OS. Improving patient selection is therefore reliant on the ability to predict accurately the extent of peritoneal metastasis and the ability to resect it completely. Specialist radiologists have demonstrated good concordance between radiological and surgical PCI estimations, particularly when combining modalities; however, these tend to be most accurate in patients with high PCI scores57, 58. In some centres this is used in combination with diagnostic laparoscopy before CRS + HIPEC. This is feasible in the majority of patients and may help to reduce the laparotomy rate in patients for whom CRS + HIPEC may not be possible59.
Included patients were relatively young at 54 (range 45–69) years compared with the incident age of colorectal cancer (80–90 years)60 In addition, performance status was not reported by the majority of studies in the review, and limited to an ECOG score of less than 2 by a further nine. Within these limits, no other patient or tumour factor was predictive of OS.
Details of neoadjuvant and adjuvant treatments were reported poorly in the included studies. Within these limits, the use of neoadjuvant or adjuvant chemotherapy was not predictive of OS after CRS + HIPEC. One meta‐analysis, by Kwakman and colleagues61 from 2016, examined the effect of clinicopathological variables only on OS following CRS + HIPEC. Significant prognostic factors identified in the present review (adverse features of the primary tumour, PCI, CC score) were not comparable with those from Kwakman et al.61 as they were not examined. In contrast to the present study, Kwakman and co‐workers61 found performance status, the presence of lymph node or hepatic metastasis, tumour differentiation, signet ring histology, a rectal primary and the use of neoadjuvant chemotherapy to be predictive prognostic factors. A number of differences may explain this variation in findings: the exclusion of 73 papers described as unavailable in full text may introduce a potential selection bias61. In addition, a number of studies included by Kwakman and colleagues were excluded in the present study for the following reasons: presentation of inadequate data to estimate the HR accurately13, 52, 54, 62 the inclusion of mixed primary tumours48, 63, 64, and the inclusion of patients having repeat CRS + HIPEC procedures. Finally, the meta‐analysis61 combined all studies regardless of the presentation of unadjusted or adjusted HR.
The strengths of the present study include the comprehensive and systematic literature search including 3128 patients with CPM undergoing CRS + HIPEC, all potential prognostic factors were included, and a consistent association was found between predictive prognostic factors across different studies. The low heterogeneity associated with these factors adds to the strength and generalizability of the findings. The application of strict inclusion criteria limits the potential impact of factors such as mixed primary tumour origin. The present analysis takes into account the adjustment factors used in primary studies to ensure meta‐analysis of time to event data was performed only when data were comparable.
A number of limitations must, however, be acknowledged. This review is limited by the quality of primary studies and the heterogeneity of the population. Prognostic factor systematic reviews, by their nature, represent one of the most difficult categories due to their retrospective and observational nature. As CRS + HIPEC is performed at tertiary centres, data concerning the primary tumour and its treatment may not have been captured or reported fully. Additionally, the statistical analysis and presentation of data necessary for accurate meta‐analysis varied widely across primary studies, which may introduce a degree of inclusion bias.
There was a lack of molecular and genetic data concerning patients with CPM undergoing CRS + HIPEC in comparison with studies of primary colorectal cancer, and this is an area for future research. Discordance between primary genetic mutations and those in peritoneal metastases may identify novel therapeutic targets and prognostic markers in this metastatic group. Recent research by Schneider and colleagues65 found that RAS/RAF mutations impair survival after CRS/HIPEC, although this is the only study that has considered this prognostic factor and validation is required. Further large‐scale research is needed to account for these factors; this would require collaboration between CRS + HIPEC centres, standardization of the analysis and presentation of prognostic factor time to event data.
Disclosure
The authors declare no conflict of interest.
Supporting information
Appendix S1. Example search strategy: Medline
Fig. S1. Forest and funnel plots for each prognostic factor
Table S1. Patient demographics
Table S2. Adjustment factors used in multivariable analysis
Table S3. Risk‐of‐bias assessment results for each study using the Quality in Prognostic Studies (QUIPS) tool
Table S4. Outcomes
Table S5. Tumour factors
Table S6. Treatment factors
Funding information
No funding
References
- 1. Jayne DG, Fook S, Loi C, Seow‐Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002; 89: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 2. Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 2012; 99: 699–705. [DOI] [PubMed] [Google Scholar]
- 3. Quere P, Facy O, Manfredi S, Jooste V, Faivre J, Lepage C et al Epidemiology, management, and survival of peritoneal carcinomatosis from colorectal cancer: a population‐based study. Dis Colon Rectum 2015; 58: 743–752. [DOI] [PubMed] [Google Scholar]
- 4. Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989; 63: 364–367. [DOI] [PubMed] [Google Scholar]
- 5. Klaver YL, Simkens LH, Lemmens VE, Koopman M, Teerenstra S, Bleichrodt RP et al Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur J Surg Oncol 2012; 38: 617–623. [DOI] [PubMed] [Google Scholar]
- 6. van Oudheusden TR, Razenberg LG, van Gestel YR, Creemers GJ, Lemmens VE, de Hingh IH. Systemic treatment of patients with metachronous peritoneal carcinomatosis of colorectal origin. Sci Rep 2015; 5: 18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM et al Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 2012; 30: 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8‐year follow‐up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008; 15: 2426–2432. [DOI] [PubMed] [Google Scholar]
- 9. Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M et al Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi‐institutional study. J Clin Oncol 2004; 22: 3284–3292. [DOI] [PubMed] [Google Scholar]
- 10. Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L et al; French Surgical Association . Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi‐institutional study of 1290 patients. Cancer 2010; 116: 5608–5618. [DOI] [PubMed] [Google Scholar]
- 11. Cashin PH, Graf W, Nygren P, Mahteme H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: prognosis and treatment of recurrences in a cohort study. Eur J Surg Oncol 2012; 38: 509–515. [DOI] [PubMed] [Google Scholar]
- 12. Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM et al Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009; 27: 681–685. [DOI] [PubMed] [Google Scholar]
- 13. Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ III. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010; 116: 3756–3762. [DOI] [PubMed] [Google Scholar]
- 14. Hompes D, D'Hoore A, Van Cutsem E, Fieuws S, Ceelen W, Peeters M et al The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: a Belgian multicentre prospective phase II clinical study. Ann Surg Oncol 2012; 19: 2186–2194. [DOI] [PubMed] [Google Scholar]
- 15. Moran B, Cecil T, Chandrakumaran K, Arnold S, Mohamed F, Venkatasubramaniam A. The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. Colorectal Dis 2015; 17: 772–778. [DOI] [PubMed] [Google Scholar]
- 16. Cavaliere F, Valle M, De Simone M, Deraco M, Rossi CR, Di Filippo F et al 120 peritoneal carcinomatoses from colorectal cancer treated with peritonectomy and intra‐abdominal chemohyperthermia: a S.I.T.I.L.O. multicentric study. In Vivo 2006; 20: 747–750. [PubMed] [Google Scholar]
- 17. Glehen O, Cotte E, Schreiber V, Sayag‐Beaujard AC, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg 2004; 91: 747–754. [DOI] [PubMed] [Google Scholar]
- 18. Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA et al Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol 2004; 11: 178–186. [DOI] [PubMed] [Google Scholar]
- 19. Vaira M, Cioppa T, D'Amico S, de Marco G, D'Alessandro M, Fiorentini G et al Treatment of peritoneal carcinomatosis from colonic cancer by cytoreduction, peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Experience of ten years. In Vivo 2010; 24: 79–84. [PubMed] [Google Scholar]
- 20. Frøysnes IS, Larsen SG, Spasojevic M, Dueland S, Flatmark K. Complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastasis in Norway: prognostic factors and oncologic outcome in a national patient cohort. J Surg Oncol 2016; 114: 222–227. [DOI] [PubMed] [Google Scholar]
- 21. Desantis M, Bernard JL, Casanova V, Cegarra‐Escolano M, Benizri E, Rahili AM et al Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC). Langenbecks Arch Surg 2015; 400: 37–48. [DOI] [PubMed] [Google Scholar]
- 22. Simkens GA, van Oudheusden TR, Luyer MD, Nienhuijs SW, Nieuwenhuijzen GA, Rutten HJ et al Predictors of severe morbidity after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis. Ann Surg Oncol 2016; 23: 833–841. [DOI] [PubMed] [Google Scholar]
- 23. Simkens GA, van Oudheusden TR, Braam HJ, Luyer MD, Wiezer MJ, van Ramshorst B et al Treatment‐related mortality after cytoreductive surgery and HIPEC in patients with colorectal peritoneal carcinomatosis is underestimated by conventional parameters. Ann Surg Oncol 2016; 23: 99–105. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 25. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2012; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158: 280–286. [DOI] [PubMed] [Google Scholar]
- 28. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 29. IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta‐analysis. BMJ Open 2016; 6: e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baratti D, Kusamura S, Iusco D, Bonomi S, Grassi A, Virzì S et al Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long‐term outcome of patients with peritoneal metastases from colorectal cancer: a two‐center study of 101 patients. Dis Colon Rectum 2014; 57: 858–868. [DOI] [PubMed] [Google Scholar]
- 32. Benizri EI, Bernard JL, Rahili A, Benchimol D, Bereder JM. Small bowel involvement is a prognostic factor in colorectal carcinomatosis treated with complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2012; 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg 2006; 203: 878–886. [DOI] [PubMed] [Google Scholar]
- 34. Duraj FF, Cashin PH. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal and hepatic metastases: a case–control study. J Gastrointest Oncol 2013; 4: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B et al Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010; 28: 63–68. [DOI] [PubMed] [Google Scholar]
- 36. Elias D, Borget I, Farron M, Dromain C, Ducreux M, Goéré D et al Prognostic significance of visible cardiophrenic angle lymph nodes in the presence of peritoneal metastases from colorectal cancers. Eur J Surg Oncol 2013; 39: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 37. Elias D, Mariani A, Cloutier AS, Blot F, Goéré D, Dumont F et al Modified selection criteria for complete cytoreductive surgery plus HIPEC based on peritoneal cancer index and small bowel involvement for peritoneal carcinomatosis of colorectal origin. Eur J Surg Oncol 2014; 40: 1467–1473. [DOI] [PubMed] [Google Scholar]
- 38. Faron M, Macovei R, Goéré D, Honoré C, Benhaim L, Elias D. Linear relationship of peritoneal cancer index and survival in patients with peritoneal metastases from colorectal cancer. Ann Surg Oncol 2016; 23: 114–119. [DOI] [PubMed] [Google Scholar]
- 39. Franko J, Gusani NJ, Holtzman MP, Ahrendt SA, Jones HL, Zeh HJ III et al Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol 2008; 15: 3065–3072. [DOI] [PubMed] [Google Scholar]
- 40. Huang CQ, Yang XJ, Yu Y, Wu HT, Liu Y, Yonemura Y et al Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for patients with peritoneal carcinomatosis from colorectal cancer: a phase II study from a Chinese center. PLoS One 2014; 9: e108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang Y, Alzahrani NA, Chua TC, Liauw W, Morris DL. Impacts of peritoneal cancer index on the survival outcomes of patients with colorectal peritoneal carcinomatosis. Int J Surg 2016; 32: 65–70. [DOI] [PubMed] [Google Scholar]
- 42. Ihemelandu C, Sugarbaker PH. Management for peritoneal metastasis of colonic origin: role of cytoreductive surgery and perioperative intraperitoneal chemotherapy: a single institution's experience during two decades. Ann Surg Oncol 2017; 24: 898–905. [DOI] [PubMed] [Google Scholar]
- 43. Lorimier G, Linot B, Paillocher N, Dupoiron D, Verrièle V, Wernert R et al Curative cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis and synchronous resectable liver metastases arising from colorectal cancer. Eur J Surg Oncol 2017; 43: 150–158. [DOI] [PubMed] [Google Scholar]
- 44. Maggiori L, Goéré D, Viana B, Tzanis D, Dumont F, Honoré C et al Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case–control study. Ann Surg 2013; 258: 116–121. [DOI] [PubMed] [Google Scholar]
- 45. Ng JL, Ong WS, Chia CS, Tan GH, Soo KC, Teo MC. Prognostic relevance of the peritoneal surface disease severity score compared to the peritoneal cancer index for colorectal peritoneal carcinomatosis. Int J Surg Oncol 2016; 2016: 2495131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simkens GA, Razenberg LG, Lemmens VE, Rutten HJ, Creemers GJ, de Hingh IH. Histological subtype and systemic metastases strongly influence treatment and survival in patients with synchronous colorectal peritoneal metastases. Eur J Surg Oncol 2016; 42: 794–800. [DOI] [PubMed] [Google Scholar]
- 47. Simkens GA, van Oudheusden TR, Braam HJ, Wiezer MJ, Nienhuijs SW, Rutten HJ et al Cytoreductive surgery and HIPEC offers similar outcomes in patients with rectal peritoneal metastases compared to colon cancer patients: a matched case control study. J Surg Oncol 2016; 113: 548–553. [DOI] [PubMed] [Google Scholar]
- 48. Sluiter NR, de Cuba EM, Kwakman R, Meijerink WJ, Delis‐van Diemen PM, Coupé VM et al Versican and vascular endothelial growth factor expression levels in peritoneal metastases from colorectal cancer are associated with survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Clin Exp Metastasis 2016; 33: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Teo MC, Ching Tan GH, Lim C, Chia CS, Tham CK, Soo KC. Colorectal peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: the experience of a tertiary Asian center. Asian J Surg 2015; 38: 65–73. [DOI] [PubMed] [Google Scholar]
- 50. Ung L, Chua TC, David LM. Peritoneal metastases of lower gastrointestinal tract origin: a comparative study of patient outcomes following cytoreduction and intraperitoneal chemotherapy. J Cancer Res Clin Oncol 2013; 139: 1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Winer J, Zenati M, Ramalingam L, Jones H, Zureikat A, Holtzman M et al Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2014; 21: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 52. Varban O, Levine EA, Stewart JH, McCoy TP, Shen P. Outcomes associated with cytoreductive surgery and intraperitoneal hyperthermic chemotherapy in colorectal cancer patients with peritoneal surface disease and hepatic metastases. Cancer 2009; 115: 3427–3436. [DOI] [PubMed] [Google Scholar]
- 53. Votanopoulos KI, Swett K, Blackham AU, Ihemelandu C, Shen P, Stewart JH et al Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from rectal cancer. Ann Surg Oncol 2013; 20: 1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rivard JD, McConnell YJ, Temple WJ, Mack LA. Cytoreduction and heated intraperitoneal chemotherapy for colorectal cancer: are we excluding patients who may benefit? J Surg Oncol 2014; 109: 104–109. [DOI] [PubMed] [Google Scholar]
- 55. Füzün M, Sökmen S, Terzi C, Canda AE. Cytoreductive approach to peritoneal carcinomatosis originated from colorectal cancer: Turkish experience. Acta Chir Iugosl 2006; 53: 17–21. [DOI] [PubMed] [Google Scholar]
- 56. Chen HS, Sheen‐Chen SM. Obstruction and perforation in colorectal adenocarcinoma: an analysis of prognosis and current trends. Surgery 2000; 127: 370–376. [DOI] [PubMed] [Google Scholar]
- 57. Dohan A, Hoeffel C, Soyer P, Jannot AS, Valette PJ, Thivolet A et al Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br J Surg 2017; 104: 1244–1249. [DOI] [PubMed] [Google Scholar]
- 58. Flicek K, Ashfaq A, Johnson CD, Menias C, Bagaria S, Wasif N. Correlation of radiologic with surgical peritoneal cancer index scores in patients with pseudomyxoma peritonei and peritoneal carcinomatosis: how well can we predict resectability? J Gastrointest Surg 2016; 20: 307–312. [DOI] [PubMed] [Google Scholar]
- 59. Iversen LH, Rasmussen PC, Laurberg S. Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br J Surg 2013; 100: 285–292. [DOI] [PubMed] [Google Scholar]
- 60. Office for National Statistics . Cancer Registration Statistics, England: 2014 https://cy.ons.gov.uk/releases/cancerregistrationstatisticsengland2014 [accessed 3 May 2019].
- 61. Kwakman R, Schrama AM, van Olmen JP, Otten RH, de Lange‐de Klerk ES, de Cuba EM et al Clinicopathological parameters in patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer metastases: a meta‐analysis. Ann Surg 2016; 263: 1102–1111. [DOI] [PubMed] [Google Scholar]
- 62. Chua TC, Yan TD, Ng KM, Zhao J, Morris DL. Significance of lymph node metastasis in patients with colorectal cancer peritoneal carcinomatosis. World J Surg 2009; 33: 1488–1494. [DOI] [PubMed] [Google Scholar]
- 63. Elias D, Glehen O, Pocard M, Quenet F, Goéré D, Arvieux C et al; Association Française de Chirurgie . A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann Surg 2010; 251: 896–901. [DOI] [PubMed] [Google Scholar]
- 64. Baumgartner JM, Tobin L, Heavey SF, Kelly KJ, Roeland EJ, Lowy AM. Predictors of progression in high‐grade appendiceal or colorectal peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2015; 22: 1716–1721. [DOI] [PubMed] [Google Scholar]
- 65. Schneider MA, Eden J, Pache B, Laminger F, Lopez‐Lopez V, Steffen T et al Mutations of RAS/RAF proto‐oncogenes impair survival after cytoreductive surgery and PEC for peritoneal metastasis of colorectal origin. Ann Surg 2018; 268: 845–853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Example search strategy: Medline
Fig. S1. Forest and funnel plots for each prognostic factor
Table S1. Patient demographics
Table S2. Adjustment factors used in multivariable analysis
Table S3. Risk‐of‐bias assessment results for each study using the Quality in Prognostic Studies (QUIPS) tool
Table S4. Outcomes
Table S5. Tumour factors
Table S6. Treatment factors


