Abstract
Acinetobacter baumannii, has been developing resistance to even the last line of drugs. Antimicrobial peptides (AMPs) to which bacteria do not develop resistance easily may be the last hope. A few independent experimental studies have designed and studied the activity of AMPs on A. baumannii, however the number of such studies are still limited. With the goal of developing a rational approach to the screening of AMPs against A. baumannii, we carefully curated the drug activity data from 75 cationic AMPs, all measured with a similar protocol, and on the same ATCC 19606 strain. A quantitative model developed and validated with a part of the data. While the model may be used for predicting the activity of any designed AMPs, in this work, we perform an in silico screening for the entire database of naturally occurring AMPs, to provide a rational guidance in this urgently needed drug development.
Introduction
Acinetobacter baumannii (A. baumannii) [1] is mainly implicated in hospital infections and is responsible for 80% of the Acinetobacter infections. A. baumannii can also be found on normal human skin, but it generally does not pose a threat to a healthy person [2–5], besides the not-so-frequent skin and soft tissue infections, infections in the surgical site, urinary tract infection, etc [6, 7]. In the past 30 years, A. baumannii has evolved into a multidrug resistant (MDR) [8–10] opportunistic pathogen that selectively infects seriously ill patients in intensive care unit (ICU), trauma or burn patients [2–4, 11]. The presence of intrinsic efflux pump and high rates of genetic adaptation, contributes to adaptation against the antibiotics [12–14]. Besides, it also possesses several beta-lactamase genes which offer resistance against beta-lactam antibiotics [15, 16]. A. baumannii has also been developing resistance against carbapenem [17] which had been one of the last line of drugs against it. Combination therapies such as of colistin, polymixin B, and tigecycline are used to treat MDR strains, but these are complex compared to a single drug when it comes to quantification of the effect and the validation of their safety [18–20]. Due to the growing concern about MDR, new types of antimicrobial agents are needed.
Antimicrobial peptides (AMP) are a fundamental part of the innate defense system and are reportedly present in organisms from bacteria and fungus to humans [21, 22]. Although several modes of AMP activity, including DNA damage [23], RNA damage [24] and targeting ribosomes [25–27] regulatory enzymes [28] or other proteins [29] have been proposed, it is generally believed that the positively charged AMPs act by disrupting the bacterial membrane [30–32] and the membrane disruption is one of the key factor for the AMP activity [29, 33, 34]. Because of this fundamental difference in the mechanism compared to the traditional drugs, it is believed that the bacteria do not develop resistance easily against AMPs [21]. The low toxicity of AMPs towards human cells and their tendency not to result in resistant strains makes them an ideal rational choice as the next generation antimicrobial agents [35–37], possibly eventually becoming effective drugs for A. baumannii.
Quantitative Structure and Activity Relationship (QSAR) [38] is an approach in computer aided rational drug design, which uses biophysical or biochemical parameters of the molecules to develop a quantitative relation with the measured activities. Once validated, the computational model can be used for predicting the activities of the possible drug candidates and for pre-screening them. Recent studies have developed a QSAR relation using 29 small molecule drug candidates which act on the oxphos metabolic path of A. baumannii [39]. As noted above, since bacteria are less likely to develop resistance against AMP based drugs, we focus on QSAR for AMPs against A. baumannii.
The present work has three major objectives. Several experimental groups have independently evaluated the activity of AMP against A. baumannii. We curated these experimental results against a single, well studied target, ATCC 19606 strain, whose activity is quantified using Clinical and Laboratory Standards Institute (CLSI) or related protocols. [40] We developed a computational model using neural networks to rationally predict the activity from the biochemical attributes of the AMP. Since A. baumannii is a growing threat, while realizing the potential limitations of training on 75 peptides, we also predict the activity of all the naturally occurring AMPs in the AMP database to enable a rational screening of AMPs against A. baumannii.
Methods
Curation of data
Training QSAR models with data from multiple sources, obtained with different protocols and on different strains can lead to poor predictive capabilities. [41] In order to standardize the data used in the analysis, we used three criteria for inclusion- the tests should be on ATCC 19606 strain, with cationic antimicrobial peptides and studied according to the CLSI or equivalent guidelines. With these inclusion criteria, we believed that the mechanism of antibiotic action will be similar and the data curated from different sources can be compared. Since data availability was limited, we had to include data from different groups. AMP sequence and activity data against A. Baumannii was curated from different sources [42–52] and is presented in Table A in S1 File. The curated AMP data set had the activity of 75 AMPs with their length ranging from 10 to 43 amino acids and charges in the range +1 to +12. Of these, for 63 AMPs the MIC was available (referred to as quantitative data), and for the remaining 12, only the lower bound of minimum inhibitory concentration (MIC) (refered to as the qualitative data).
Parameter computation
in vivo aggregation propensity is calculated by using a web-based software AGGRESCAN [53]. Where the aggregation propensity is calculated on the basis of aggregation- propensity scale of amino acids. in vitro aggregation propensity is calculated by using TANGO software (with ionic strength 0.02M, pH 7.0 and T = 298K) [54], where we only consider the β-sheet aggregation term. Aliphatic index of the peptides is calculated as described by Ikai [55]. Grand average hydropathy is calculated on the scale given by Kyte-Doolittle [56] and the hydrophobic moment is calculated by using HELIQUEST software [57]. The toxicity of the AMPs was predicted using ToxinPred (http://crdd.osdd.net/raghava/toxinpred/). [58] The method allows for the prediction of toxicity of peptides shorter than 50 amino acids. However, this was not a limitation as peptides longer than that are anyways complicated to synthesize and may not be ideal drug candidates.
Artificial neural network
Since the available data is limited, we used used both the quantitative and the qualitative data, albeit with different proportions, to train and test the models. We used 63 of the MIC values from the quantitative data and 3 from the qualitative data for which the cited lower bound was treated as the MIC for the purpose of this analysis. We performed a 10-fold cross validation to check the robustness of our models. To do the 10-fold cross validation, we divide the data set into 10 different test sets, each contains 7 data points. We performed the artificial neural network (ANN) calculation for each test set by taking 53 data points for training and 6 data points for validation. Rest of the 9 points from the qualitative data are used for an independent qualitative test. The activity of the AMPs was predicted by ANN model with an open module for machine learning called Scikit-learn [59] in Python. For the activation function, logistic function was used and low memory BFGS optimization algorithm was used a solver. Three independent neural network calculations have been performed to do the 10-fold cross validation, by using a hidden layer of 6 neurons, 8 neurons and 10 neurons. 2500 trial runs in each case were made by taking 50 different random initializations for the input biases and 50 random choices for the training and validation sets. We screened the results of these 2500 trials with and . Two best models were selected based on the result obtained from the 10-fold cross validation. The models were expected to perform with for the quantitative data and at least 5 predictions for the qualitative data set. These models were then used to predict the MIC values of a complete AMP database [60–62] (https://aps.unmc.edu).
Results
Curated data for AMPs and their effectiveness
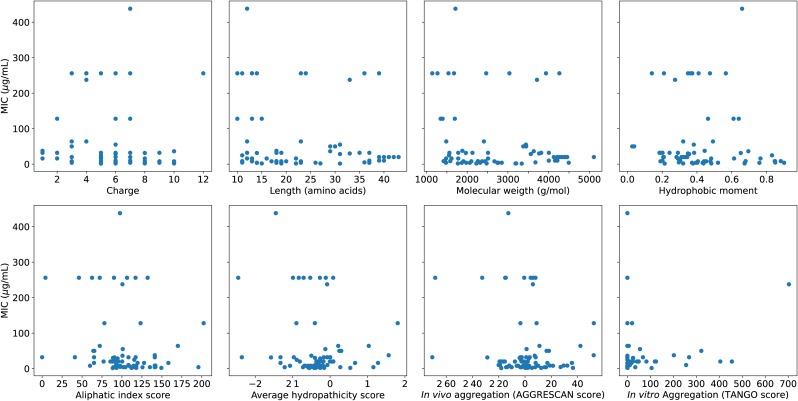
The data on the activity of AMPs on A. baumannii is scattered in literature. We curated the data mainly with the goal of developing a quantitative model, and hence restricted the focus to the most commonly studied ATCC 19606 strain. To maintain uniformity of standards, we included studies which were performed according to CLSI or equivalent guidelines. The sequence data and the antimicrobial activity of these peptides measured as the MIC was gathered (Table A in S1 File). Overall, the comprehensive collection of the data on AMP activity allowed a classification based on the various biophysical parameters which are commonly used for developing a quantitative relation with activity: (1) charge, which draws the AMPs selectively to anionic membrane, (2) length, reflecting how it has to be commensurate with the membrane thickness for an improved activity [63, 64] (3) molecular weight, which gives an idea of the bulkiness and membrane penetration efficiency (4) hydrophobic moment (μH), [57] which quantifies the amphipathic characters required to form pores in the membrane, (5) aliphatic index [55], which indicates the volume of aliphatic content (A, V, I and L) of the peptide, (6) grand average of hydropathy (GRAVY) based on Kyte-Doolittle hydropathicity scale [56], (7) in vivo aggregation propensity, calculated by using a web-based software AGGRESCAN [53] and (8) in vitro (β-sheet) aggregation propensity, calculated by using TANGO software (with ionic strength 0.02 M, pH 7.0 and temperature 298 K) [54]. The in vitro aggregation, before interaction with the membrane can at times stop proteolytic degradation [65] by the bacteria but in many other cases reduce the drug potency [66, 67]. Further, the aggregation propensity affects the barrel-stave [68] and carpet mechanisms [63] of action differently. Toxicity of peptides obtained from ToxinPred [58] was categorical, and it was used only to classify the AMPs from the database as potential drug candidates, and not for the activity prediction. The distribution of the eight parameters for all the curated AMPs are given in Fig A in S1 File and their individual relation with MIC in Fig 1, which shows that each of the parameters individually is not sufficient to describe the activity.
Fig 1. MIC versus different parameters.
The AMPs used in the analysis along with the sequences and biophysical parameters are given in Table A in S1 File.
Quantitative models for AMP activity
ANN model was used to obtain the relationship between the various above-mentioned parameters and MIC values (Methods). A schematic of how we developed the model is shown in Fig B in S1 File. The first step was to create a model with the activity data from 75 AMPs, of which some were used for an internal assessment of the quality of predictions. The second step was to use the test set in the 75 AMP data analysis as a secondary validation for refining the choice of model that can be used for making the predictions for the AMP database. The details are as follows. Out of the 75 AMPs curated, for 12 of them a lower bound of MIC, as being greater than a certain value (Table B in S1 File), rather than a precise number was cited. To include them in the analysis, and not to reduce the data size which is already small (75 AMPs), we created two independent test sets, one in which a quantitative MIC comparison was made (referred to as quantitative data) and another qualitative one in which the calculated MIC was checked if it was more than the experimental lower bound (referred to as qualitative data). The combined data set with quantitative and qualitative data was used to construct training, validation and test sets (Methods). We performed a 10-fold cross validation with three different architectures with 6, 8 and 10 hidden neurons respectively. The overall error in the architecture with 8 neurons was optimal, thus justifying a small sampling around it with 6 and 10 neurons (Table C in S1 File). However, all three architectures were satisfactory in their predictions (Figs C, D and E respectively in S1 File), resulting in many models, which qualify for the criteria ( & ). Several of these models also had good predictions for the test sets, which are about 10% of the data.
Selecting the best model
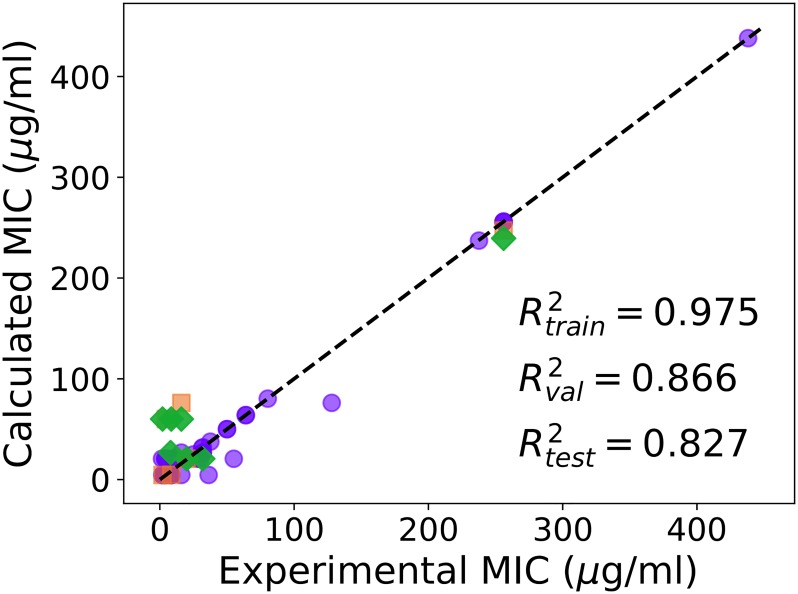
In a traditional QSAR analysis, the choice of the best model would be guided by the combination of the best and , following which on a small fraction of the data, in our case 7 data points, comes as a consequence. Since the goal of screening through the large set of potential AMPs whose activities against an extremely important pathogen are not yet available is more ambitious than performing well on these 7 points, we performed a secondary validation check to select the best models. We used two additional criteria: for the quantitative and that at least 5 predictions in qualitative data set were correct to within a factor of 2 (Table B in S1 File). Two models satisfied these conditions, with and they were selected. The best among these models (referred to as Model-1) obtained from the calculation with 8 hidden neurons, had good predictions (, and ). The experimental MIC for the quantitative data set versus MIC values predicted from Model-1 is shown in Fig 2. Results obtained from another model (Model-2) are given in Fig F in S1 File.
Fig 2. Comparison of the experimental and calculated MIC (μg/ml) of curated AMPs on A. baumannii obtained from Model-1, calculated by using 8 hidden neurons.
Training (purple circles), validation (orange squares) and test (green diamonds) sets are shown. The data used in the analysis is shown in Table 1 in S1 File.
Predicting the results for naturally occurring AMPs
Considering the health threat A. baumannii is posing, and the potential of AMPs for antibiotic-resistance-free activity, we propose a rational basis for an in silico screening of AMPs active against A. baumannii. Our models were used to predict the MIC values of the 2338 AMPs obtained from database [60–62] (https://aps.unmc.edu) of naturally occurring AMPs. We made the predictions from Model-1 and Model-2 (S2 File). In order to reduce the risk of a poorly trained ANN model with limited data, we filtered these results for a consistent prediction that is within ΔMIC ≤ 5 μg/ml for both the models (Table 1). Despite the potential statistical limitations of training and validating on 75 AMPs, a pre-screening to rationally sort multiple AMPs with their predicted activity, in vitro and in vivo aggregation potential, toxicity and length (a surrogate for synthetic complexity), all are provided in Table 1 and in the S2 File. The computational scripts and the predictions are made accessible (S3 File), to provide an immediate access to a pool of rational choices that can help progress towards large scale experimental testing, considering the extreme urgency of developing effective strategies to combat the superbug, A. baumannii
Table 1. Using the 2 different models, we predicted the activity of 2338 naturally occurring AMPs documented in the AMP database.
The complete list of predictions are given in the S2 File. However, of these the AMPs which had consistent predictions from both the models (ΔMIC ≤ 5 μg/ml) were selected and presented in this table. All of these were peptides listed below were non-toxic according to the predictions from ToxinPred (http://crdd.osdd.net/raghava/toxinpred/) [58].
| Peptide | Sequence | Length | Model-1 MIC(μg/ml) |
Model-2 MIC(μg/ml) |
|---|---|---|---|---|
| AP01466 | VNWKKILGKIIKVAK | 15 | 2.84 | 6.20 |
| AP00143 | KKLLKWLKKLL | 11 | 9.08 | 4.59 |
| AP01456 | VGKTWIKVIRGIGKSKIKWQ | 20 | 9.34 | 4.60 |
| AP00708 | GFKRIVQRIKDFLRNLV | 17 | 9.38 | 4.59 |
| AP00161 | GLWSKIKTAGKSVAKAAAKAAVKAVTNAV | 29 | 14.24 | 10.44 |
| AP00577 | GLFTLIKGAAKLIGKTVAKEAGKTGLELMACKITNQC | 37 | 14.24 | 15.64 |
| AP00608 | KRIVQRIKDFLR | 12 | 14.40 | 14.25 |
| AP01525 | SWLSKTYKKLENSAKKRISEGIAIAIQGGPR | 31 | 16.38 | 20.78 |
| AP00869 | ILPLVGNLLNDLL | 13 | 17.60 | 20.67 |
| AP00425 | GCWSTVLGGLKKFAKGGLEAIVNPK | 25 | 18.23 | 20.86 |
| AP01388 | GLLSGILNSAGGLLGNLIGSLSN | 23 | 21.02 | 20.67 |
| AP00733 | LLGDFFRKAREKIGEEFKRIVQRIKDFLRNLVPRTES | 37 | 21.70 | 19.43 |
| AP01387 | GLLSGILNTAGGLLGNLIGSLSN | 23 | 22.83 | 20.67 |
| AP00061 | GIGGVLLSAGKAALKGLAKVLAEKYAN | 27 | 23.57 | 20.66 |
| AP00210 | GMASKAGAIAGKIAKVALKAL | 21 | 25.07 | 20.26 |
| AP00006 | GNNRPVYIPQPRPPHPRI | 18 | 27.10 | 26.77 |
| AP00007 | GNNRPVYIPQPRPPHPRL | 18 | 27.10 | 26.77 |
| AP00024 | GVSGHGQHGVHG | 12 | 27.10 | 27.98 |
| AP00025 | HGVSGHGQHGVHG | 13 | 27.10 | 26.77 |
| AP00141 | RKKWFW | 6 | 27.10 | 26.77 |
| AP00150 | ILPWKWPWWPWRR | 13 | 27.10 | 26.77 |
| AP00152 | VRRFPWWWPFLRR | 13 | 27.10 | 26.77 |
| AP00169 | GRPNPVNTKPTPYPRL | 16 | 27.10 | 26.77 |
| AP00170 | VDKGSYLPRPTPPRPIYNRN | 20 | 27.10 | 26.77 |
| AP00172 | GKPRPYSPRPTSHPRPIRV | 19 | 27.10 | 26.79 |
| AP00190 | HPLKQYWWRPSI | 12 | 27.10 | 26.77 |
| AP00191 | ECRRLCYKQRCVTYCRGR | 18 | 27.10 | 26.77 |
| AP00211 | RRWCFRVCYRGFCYRKCR | 18 | 27.10 | 26.77 |
| AP00212 | RRWCFRVCYKGFCYRKCR | 18 | 27.10 | 26.77 |
| AP00213 | KWCFRVCYRGICYRKCR | 17 | 27.10 | 26.77 |
Parameter importance in model
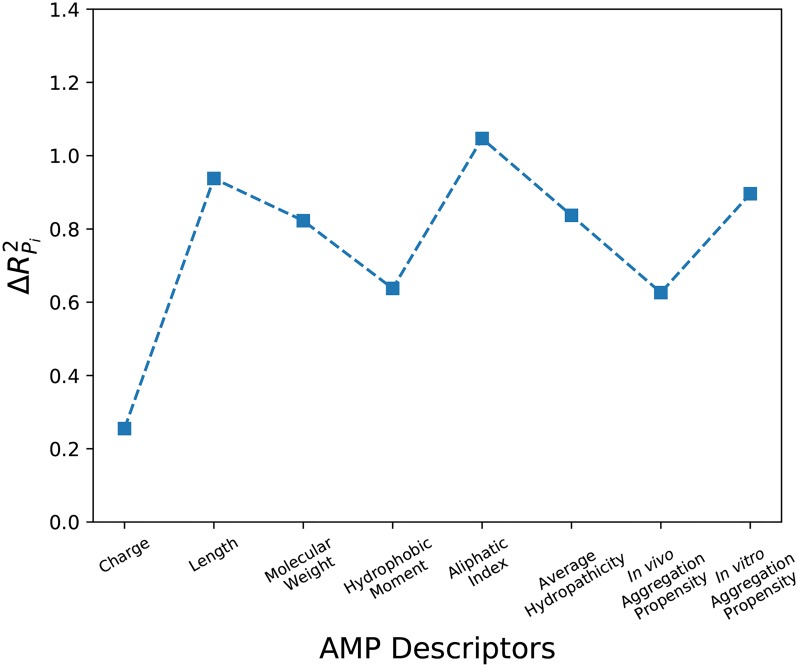
It is important to know which are the parameters (Pi) that are most responsible for the activity on A. baumannii. In the combined training and validation set used for accepting the models, we replaced (Pi) with its average <Pi> and measure the difference . is treated as reflecting the importance of the parameter. The results obtained from Model-1 are given in Fig 3 and the result obtained from another model is given in Fig G in S1 File. From our calculations, we found out that the aliphatic index is the most important parameter in both the models.
Fig 3. The relative importance of the different parameters in Model-1 is shown.
Aliphatic index influences the outcomes of the predictions the most in this model.
Relevance of predictions for MDR strains
In order to reduce the uncertainties, our computational model was trained on data standardized in three ways, A. baumannii strain used, choice of cationic AMPs and measurements by CLSI method. However, considering the threat that A. baumannii MDR strains are posing, it is important to ask whether our calculations have any relevance to these clinical variants. The two limitations of this work are the smaller data size used for training, and it was based on ATCC 19606 strain. Interestingly, in the limited studies that we found the activity of cationic AMPs against ATCC 19606 and other MDR strains of A. baumannii are comparable [43, 46], thus potentially removing the latter strain specific data limitation for A. baumannii, although for other bacteria, such as S. aureus the activity changes quite significantly with the strain [69, 70]. Drawing confidence from this fact, we used our models to predict the activity for a few MDR strains [71–73]. The results reported in Table D in S1 File are encouraging at this stage, although more such validations will be helpful in establishing the utility of the screening models we proposed.
Conclusions
To our knowledge, the present work is the only QSAR study for predicting AMP activity against A. baumannii. The present work is different from the only other QSAR in two different ways, using AMPs instead of small molecules for a better tolerance to antibiotic resistance and a slightly larger set (75 AMPs compared to 29 small molecules). Using the ANN models we developed, we could make quantitative predictions for the entire database of naturally occuring AMPs. We hope that our work will inspire the further studies quantifying the activity of AMPs on A. baumannii, some of which may follow the activity predictions and others that differ offer an opportunity to retrain the ANN models.
Supporting information
S1 File contains Table A, details of 75 curated AMPs, Fig A, histogram of all parameters corresponding to AMPs, Table B, experimental and predicted MIC values of 9 qualitative data that were used for additional test, Table C, 10 fold cross validation analysis with different number of hidden neurons, Fig B, Fig C, Fig D, comparison of experimental and predicted MIC obtained from 10 fold cross validation using 6, 8 and 10 neurons respectively, Fig E, comparison of experimental and predicted MIC for Model-2, calculated using 6 neurons, Fig F, importance of different parameters used in Model-2, Fig G, flow chart showing the schematic of how the ANN model were developed, Table D, comparison of the experimental and predicted MIC values for the MDR A. baumannii strains.
(PDF)
These contains the prediction for the 75 different curated peptides and prediction for the 2338 natural occurring AMPs.
(XLSX)
(ZIP)
Acknowledgments
A.M. wants to thank Swagatam Barman for scientific discussions. M.K.P. wants to thank JNCASR for stipend.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
M.K.P. gratefully acknowledges funding from DBT-JNCASR “Life Science Research, Education and Training at JNCASR” (BT/INF/22/SP27679/2018).
References
- 1. Nemec A, Musilek M, Maixnerova M, De Baere T, van der Reijden TJ, Vaneechoutte M, et al. Acinetobacter beijerinckii sp. nov. and Acinetobacter gyllenbergii sp. nov., haemolytic organisms isolated from humans. International Journal of Systematic and Evolutionary microbiology. 2009;59(1):118–124. 10.1099/ijs.0.001230-0. [DOI] [PubMed] [Google Scholar]
- 2. Towner K. Acinetobacter: an old friend, but a new enemy. Journal of Hospital Infection. 2009;73(4):355–363. 10.1016/j.jhin.2009.03.032 [DOI] [PubMed] [Google Scholar]
- 3. Struelens M, Carlier E, Maes N, Serruys E, Quint WG, Van Belkum A. Nosocomial colonization and infection with multiresistant Acinetobacter baumannii: outbreak delineation using DNA macrorestriction analysis and PCR-fingerprinting. Journal of Hospital infection. 1993;25(1):15–32. 10.1016/0195-6701(93)90005-k [DOI] [PubMed] [Google Scholar]
- 4. Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerging infectious diseases. 2005;11(8):1218–1224. 10.3201/1108.050103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young LS, Sabel AL, Price CS. Epidemiologic, clinical, and economic evaluation of an outbreak of clonal multidrug-resistant Acinetobacter baumannii infection in a surgical intensive care unit. Infection Control & Hospital Epidemiology. 2007;28(11):1247–1254. [DOI] [PubMed] [Google Scholar]
- 6. Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infection Control & Hospital Epidemiology. 2013;34(1):1–14. [DOI] [PubMed] [Google Scholar]
- 7. Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infection Control & Hospital Epidemiology. 2016;37(11):1288–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gordon NC, Wareham DW. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. International Journal of Antimicrobial Agents. 2010;35(3):219–226. 10.1016/j.ijantimicag.2009.10.024 [DOI] [PubMed] [Google Scholar]
- 9. Falagas ME, Karageorgopoulos DE. Pandrug resistance (PDR), extensive drug resistance (XDR), and multidrug resistance (MDR) among Gram-negative bacilli: need for international harmonization in terminology. Clinical Infectious Diseases. 2008;46(7):1121–1122. 10.1086/528867 [DOI] [PubMed] [Google Scholar]
- 10. Pachón J, Vila J. Treatment of multiresistant Acinetobacter baumannii infections. Current Opinion in Investigational Drugs (London, England: 2000). 2009;10(2):150–156. [PubMed] [Google Scholar]
- 11. Öncül O, Keskin Ö, Acar HV, Küçükardalı Y, Evrenkaya R, Atasoyu EM, et al. Hospital-acquired infections following the 1999 Marmara earthquake. Journal of Hospital Infection. 2002;51(1):47–51. 10.1053/jhin.2002.1205 [DOI] [PubMed] [Google Scholar]
- 12. Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiology and Molecular Biology Reviews. 2010;74(3):417–433. 10.1128/MMBR.00016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan SYY, Chua SL, Liu Y, Høiby N, Andersen LP, Givskov M, et al. Comparative genomic analysis of rapid evolution of an extreme-drug-resistant Acinetobacter baumannii clone. Genome Biology and Evolution. 2013;5(5):807–818. 10.1093/gbe/evt047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, et al. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathogens. 2012;8(12):e1003068 10.1371/journal.ppat.1003068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genetics. 2006;2(1):e7 10.1371/journal.pgen.0020007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sahl JW, Gillece JD, Schupp JM, Waddell VG, Driebe EM, Engelthaler DM, et al. Evolution of a pathogen: a comparative genomics analysis identifies a genetic pathway to pathogenesis in Acinetobacter. PloS One. 2013;8(1):e54287 10.1371/journal.pone.0054287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nemec A, Krizova L, Maixnerova M, van der Reijden TJ, Deschaght P, Passet V, et al. Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus–Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov.(formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov.(formerly Acinetobacter genomic species 13TU). Research in Microbiology. 2011;162(4):393–404. 10.1016/j.resmic.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 18. Fan B, Guan J, Wang X, Cong Y. Activity of colistin in combination with meropenem, tigecycline, fosfomycin, fusidic acid, rifampin or sulbactam against extensively drug-resistant Acinetobacter baumannii in a murine thigh-infection model. PloS One. 2016;11(6):e0157757 10.1371/journal.pone.0157757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim WY, Moon JY, Huh JW, Choi SH, Lim CM, Koh Y, et al. Comparable efficacy of tigecycline versus colistin therapy for multidrug-resistant and extensively drug-resistant Acinetobacter baumannii pneumonia in critically ill patients. PLoS One. 2016;11(3):e0150642 10.1371/journal.pone.0150642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rao GG, Ly NS, Diep J, Forrest A, Bulitta JB, Holden PN, et al. Combinatorial pharmacodynamics of polymyxin B and tigecycline against heteroresistant Acinetobacter baumannii. International Journal of Antimicrobial Agents. 2016;48(3):331–336. 10.1016/j.ijantimicag.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. 10.1038/415389a [DOI] [PubMed] [Google Scholar]
- 22. Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnology. 2006;24(12):1551–1557. 10.1038/nbt1267 [DOI] [PubMed] [Google Scholar]
- 23. Ghosh A, Kar RK, Jana J, Saha A, Jana B, Krishnamoorthy J, et al. Indolicidin targets duplex DNA: structural and mechanistic insight through a combination of spectroscopy and microscopy. ChemMedChem. 2014;9(9):2052–2058. 10.1002/cmdc.201402215 [DOI] [PubMed] [Google Scholar]
- 24. Hao G, Shi YH, Tang YL, Le GW. The intracellular mechanism of action on Escherichia coli of BF2-A/C, two analogues of the antimicrobial peptide Buforin 2. Journal of microbiology. 2013;51(2):200–206. [DOI] [PubMed] [Google Scholar]
- 25. Krizsan A, Volke D, Weinert S, Sträter N, Knappe D, Hoffmann R. Insect-Derived Proline-Rich Antimicrobial Peptides Kill Bacteria by Inhibiting Bacterial Protein Translation at the 70 S Ribosome. Angewandte Chemie International Edition. 2014;53(45):12236–12239. 10.1002/anie.201407145 [DOI] [PubMed] [Google Scholar]
- 26. Seefeldt AC, Nguyen F, Antunes S, Pérébaskine N, Graf M, Arenz S, et al. The proline-rich antimicrobial peptide Onc112 inhibits translation by blocking and destabilizing the initiation complex. Nature structural & molecular biology. 2015;22(6):470. [DOI] [PubMed] [Google Scholar]
- 27. Roy RN, Lomakin IB, Gagnon MG, Steitz TA. The mechanism of inhibition of protein synthesis by the proline-rich peptide oncocin. Nature structural & molecular biology. 2015;22(6):466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dangel A, Ackermann N, Abdel-Hadi O, Maier R, Önder K, Francois P, et al. A de novo-designed antimicrobial peptide with activity against multiresistant Staphylococcus aureus acting on RsbW kinase. The FASEB Journal. 2013;27(11):4476–4488. 10.1096/fj.13-234575 [DOI] [PubMed] [Google Scholar]
- 29. Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends in Biotechnology. 2011;29(9):464–472. 10.1016/j.tibtech.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 30. Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacological Reviews. 2003;55(1):27–55. 10.1124/pr.55.1.2 [DOI] [PubMed] [Google Scholar]
- 31. Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature Reviews Microbiology. 2005;3(3):238–250. 10.1038/nrmicro1098 [DOI] [PubMed] [Google Scholar]
- 32. Lohner K, Blondelle S. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Combinatorial Chemistry & High Throughput Screening. 2005;8(3):241–256. [DOI] [PubMed] [Google Scholar]
- 33. Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clinical microbiology reviews. 2006;19(3):491–511. 10.1128/CMR.00056-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yeung AT, Gellatly SL, Hancock RE. Multifunctional cationic host defence peptides and their clinical applications. Cellular and Molecular Life Sciences. 2011;68(13):2161 10.1007/s00018-011-0710-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oyston P, Fox M, Richards S, Clark G. Novel peptide therapeutics for treatment of infections. Journal of Medical Microbiology. 2009;58(8):977–987. 10.1099/jmm.0.011122-0 [DOI] [PubMed] [Google Scholar]
- 36. Poole K. Overcoming multidrug resistance in gram-negative bacteria. Current Opinion in Investigational Drugs (London, England: 2000). 2003;4(2):128–139. [PubMed] [Google Scholar]
- 37. Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clinical Immunology. 2010;135(1):1–11. 10.1016/j.clim.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 38. Jenssen H, Fjell CD, Cherkasov A, Hancock RE. QSAR modeling and computer-aided design of antimicrobial peptides. Journal of Peptide Science: An Official Publication of the European Peptide Society. 2008;14(1):110–114. [DOI] [PubMed] [Google Scholar]
- 39. Sayiner HS, Abdalrahm AA, Basaran MA, Kovalishyn V, Kandemirli F. The Quantum Chemical and QSAR Studies on Acinetobacter Baumannii Oxphos Inhibitors. Medicinal Chemistry. 2018;14(3):253–268. 10.2174/1573406413666171002124408 [DOI] [PubMed] [Google Scholar]
- 40. Wikler MA. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. Clinical and Laboratory Standards Institute; 2006. [Google Scholar]
- 41. Zhao L, Wang W, Sedykh A, Zhu H. Experimental errors in QSAR modeling sets: What we can do and what we cannot do. ACS omega. 2017;2(6):2805–2812. 10.1021/acsomega.7b00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vila-Farres X, De La Maria CG, López-Rojas R, Pachón J, Giralt E, Vila J. In vitro activity of several antimicrobial peptides against colistin-susceptible and colistin-resistant Acinetobacter baumannii. Clinical Microbiology and Infection. 2012;18(4):383–387. 10.1111/j.1469-0691.2011.03581.x [DOI] [PubMed] [Google Scholar]
- 43. Peng SY, You RI, Lai MJ, Lin NT, Chen LK, Chang KC. Highly potent antimicrobial modified peptides derived from the Acinetobacter baumannii phage endolysin LysAB2. Scientific reports. 2017;7(1):11477 10.1038/s41598-017-11832-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jayamani E, Rajamuthiah R, Larkins-Ford J, Fuchs BB, Conery AL, Vilcinskas A, et al. Insect-derived cecropins display activity against Acinetobacter baumannii in a whole-animal high-throughput Caenorhabditis elegans model. Antimicrobial agents and chemotherapy. 2015; p. 1728–1737. 10.1128/AAC.04198-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jaśkiewicz M, Neubauer D, Kazor K, Bartoszewska S, Kamysz W. Antimicrobial activity of selected antimicrobial peptides against planktonic culture and biofilm of Acinetobacter baumannii. Probiotics and Antimicrobial Proteins. 2018; p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mohamed MF, Brezden A, Mohammad H, Chmielewski J, Seleem MN. A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Scientific Reports. 2017;7(1):6953 10.1038/s41598-017-07440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Andrä J, Monreal D, de Tejada GM, Olak C, Brezesinski G, Gomez SS, et al. Rationale for the design of shortened derivatives of the NK-lysin derived antimicrobial peptide NK-2 with improved activity against Gram-negative pathogens. Journal of Biological Chemistry. 2007. 10.1074/jbc.M608920200 [DOI] [PubMed] [Google Scholar]
- 48. Kohn EM, Shirley DJ, Arotsky L, Picciano AM, Ridgway Z, Urban MW, et al. Role of cationic side chains in the antimicrobial activity of C18G. Molecules. 2018;23(2):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li D, Yang Y, Tian Z, Lv J, Sun F, Wang Q, et al. Synergistic antibiotic effect of looped antimicrobial peptide CLP-19 with bactericidal and bacteriostatic agents. Oncotarget. 2017;8(34):55958–55966. 10.18632/oncotarget.18124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Almaaytah A, Ya’u A, Abualhaijaa A, Tarazi S, Alshar’i N, Al-Balas Q. Peptide consensus sequence determination for the enhancement of the antimicrobial activity and selectivity of antimicrobial peptides. Infection and drug resistance. 2017;10:1–17. 10.2147/IDR.S118877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thomsen TT. Peptide Antibiotics for ESKAPE Pathogens: Past, Present and Future Perspectives of Antimicrobial Peptides for the Treatment of Serious Gram-Negative and Gram-Positive Infections. Department of Biology, Faculty of Science, University of Copenhagen; 2016. [Google Scholar]
- 52. Almaaytah A, Qaoud MT, Abualhaijaa A, Al-Balas Q, Alzoubi KH. Hybridization and antibiotic synergism as a tool for reducing the cytotoxicity of antimicrobial peptides. Infection and Drug Resistance. 2018;11:835–847. 10.2147/IDR.S166236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Conchillo-Solé O, de Groot NS, Avilés FX, Vendrell J, Daura X, Ventura S. AGGRESCAN: a server for the prediction and evaluation of “hot spots” of aggregation in polypeptides. BMC Bioinformatics. 2007;8(1):65 10.1186/1471-2105-8-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fernandez-Escamilla AM, Rousseau F, Schymkowitz J, Serrano L. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nature Biotechnology. 2004;22(10):1302–1306. 10.1038/nbt1012 [DOI] [PubMed] [Google Scholar]
- 55. Ikai A. Thermostability and aliphatic index of globular proteins. The Journal of Biochemistry. 1980;88(6):1895–1898. [PubMed] [Google Scholar]
- 56. Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. Journal of Molecular Biology. 1982;157(1):105–132. 10.1016/0022-2836(82)90515-0 [DOI] [PubMed] [Google Scholar]
- 57. Gautier R, Douguet D, Antonny B, Drin G. HELIQUEST: a web server to screen sequences with specific α-helical properties. Bioinformatics. 2008;24(18):2101–2102. 10.1093/bioinformatics/btn392 [DOI] [PubMed] [Google Scholar]
- 58. Gupta S, Kapoor P, Chaudhary K, Gautam A, Kumar R, Raghava GP, et al. In silico approach for predicting toxicity of peptides and proteins. PLoS One. 2013;8(9):e73957 10.1371/journal.pone.0073957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: Machine learning in Python. Journal of Machine Learning Research. 2011;12(Oct):2825–2830. [Google Scholar]
- 60. Wang Z, Wang G. APD: the antimicrobial peptide database. Nucleic Acids Research. 2004;32(suppl_1):D590–D592. 10.1093/nar/gkh025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang G, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Research. 2008;37(suppl_1):D933–D937. 10.1093/nar/gkn823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Research. 2015;44(D1):D1087–D1093. 10.1093/nar/gkv1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shai Y. Mode of action of membrane active antimicrobial peptides. Peptide Science: Original Research on Biomolecules. 2002;66(4):236–248. [DOI] [PubMed] [Google Scholar]
- 64. Deslouches B, Phadke SM, Lazarevic V, Cascio M, Islam K, Montelaro RC, et al. De novo generation of cationic antimicrobial peptides: influence of length and tryptophan substitution on antimicrobial activity. Antimicrobial Agents and Chemotherapy. 2005;49(1):316–322. 10.1128/AAC.49.1.316-322.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochemical Journal. 1999;341(Pt 3):501–513. [PMC free article] [PubMed] [Google Scholar]
- 66. Kustanovich I, Shalev DE, Mikhlin M, Gaidukov L, Mor A. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. Journal of Biological Chemistry. 2002;277(19):16941–16951. 10.1074/jbc.M111071200 [DOI] [PubMed] [Google Scholar]
- 67. Feder R, Dagan A, Mor A. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. Journal of Biological Chemistry. 2000;275(6):4230–4238. 10.1074/jbc.275.6.4230 [DOI] [PubMed] [Google Scholar]
- 68. Ehrenstein G, Lecar H. Electrically gated ionic channels in lipid bilayers. Quarterly reviews of biophysics. 1977;10(1):1–34. [DOI] [PubMed] [Google Scholar]
- 69. Majumder A, Biswal MR, Prakash MK. One drug multiple targets: An approach to predict drug efficacies on bacterial strains differing in membrane composition. ACS Omega. 2019;4(3):4977–4983. [Google Scholar]
- 70. Mishra NN, McKinnell J, Yeaman MR, Rubio A, Nast CC, Chen L, et al. In vitro cross-resistance to daptomycin and host defense cationic antimicrobial peptides in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrobial agents and chemotherapy. 2011;55(9):4012–4018. 10.1128/AAC.00223-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. das Neves RC, Mortari MR, Schwartz EF, Kipnis A, Junqueira-Kipnis AP. Antimicrobial and Antibiofilm Effects of Peptides from Venom of Social Wasp and Scorpion on Multidrug-Resistant Acinetobacter baumannii. Toxins. 2019;11(4):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hirsch R, Wiesner J, Marker A, Pfeifer Y, Bauer A, Hammann PE, et al. Profiling antimicrobial peptides from the medical maggot Lucilia sericata as potential antibiotics for MDR Gram-negative bacteria. Journal of Antimicrobial Chemotherapy. 2018;74(1):96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Di Bonaventura I, Baeriswyl S, Capecchi A, Gan BH, Jin X, Siriwardena TN, et al. An antimicrobial bicyclic peptide from chemical space against multidrug resistant Gram-negative bacteria. Chemical communications. 2018;54(40):5130–5133. 10.1039/c8cc02412j [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 File contains Table A, details of 75 curated AMPs, Fig A, histogram of all parameters corresponding to AMPs, Table B, experimental and predicted MIC values of 9 qualitative data that were used for additional test, Table C, 10 fold cross validation analysis with different number of hidden neurons, Fig B, Fig C, Fig D, comparison of experimental and predicted MIC obtained from 10 fold cross validation using 6, 8 and 10 neurons respectively, Fig E, comparison of experimental and predicted MIC for Model-2, calculated using 6 neurons, Fig F, importance of different parameters used in Model-2, Fig G, flow chart showing the schematic of how the ANN model were developed, Table D, comparison of the experimental and predicted MIC values for the MDR A. baumannii strains.
(PDF)
These contains the prediction for the 75 different curated peptides and prediction for the 2338 natural occurring AMPs.
(XLSX)
(ZIP)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.