Abstract
Many of the principles established in adults with undifferentiated nasopharyngeal carcinoma (NPC) apply to children, adolescents and young adults. However, NPC in young patients should be distinguished from the adult form by several points. This review focuses mainly on differences between adult and pediatric NPC. The role of biology and genetics in pediatric NPC is discussed. Systemic treatment modalities including type of chemotherapy induction, timing of treatment, role of immunotherapy as adjuvant treatment, or in relapsing/ metastatic diseases are reported. Radiation modalities (doses, techniques…) in children are also reviewed. Long-term effects including secondary cancers are finally be discussed in this young NPC population.
Epidemiology
Nasopharyngeal carcinomas (NPC) represent less than 1% of all childhood cancers. The first peak of incidence is 10–20 years, with a median age of 13 years at diagnosis. In one of the largest pediatric Chinese report on 176 patients, 24% of the patients were under 14 year old1 European and American studies have reported an incidence rate nil before 10 years, 0.73 per million between 10 and 14 years, and one per million between 15 and 17 years of age.2–4 In contrast, the NPC incidence reaches 1/100,000 to more than 20/100,000 in endemic regions such as southern parts of China, Southeast Asia, Alaska, and in the Mediterranean Basin.1,5–8 Age distribution is different in these endemic regions. Children under 16 year old account for 1–2% of all NPC in China versus 10–18% in Mediterranean basin and in Africa. Sex ratio ranges from 1.7 to 4.8 in pediatric series.3,9–12 More than 95% of the patients have nodal involvement in the largest pediatric series although distant metastases remain quite rare (3–10%).3,4,10–14
Role of biology and genetic
EBV is especially associated with undifferentiated type which is the most frequent in children.15–17 High levels of immunoglobulin A (IgA) antibodies against EBV antigens are predictive of NPC in endemic areas.18 Elevated antibody titers of IgG and IgA against early antigen (EA) or viral capsid antigen (VCA) are also commonly seen in child with undifferentiated NPC.14,19 Elevated IgA against VCA and/or EA are less common in young patients from northern Africa than in adults.19 Higher expression of EBV-latent membrane protein 1 1, a major EBV oncogene involved in proliferation, survival and invasion has been observed in tumors from younger patients with NPC.20
Asian individuals who have HLA-A2, B46 and B18 types were found to have an approximately twofold increased risk of NPC while in Caucasians, the HLA-B5 allele was shown to be associated with NPC.21 More recently, genome-wide association studies have confirmed that genes within the major histocompatibility complex region on chromosome 6p21 that codes for the HLA genes are strongly associated with NPC.22 HLA plays an important role in presenting the viral antigens to the T cells. Alleles less efficient to induce immune response to viruses were shown to be more frequent in high-risk population.23 Other non-HLA genes are suspected in association such as the GABBR1 and HCG9 genes.23 Finally, familial clustering of NPC has been well described and suggests that the development of NPC may result from a complex interaction between multiple susceptibility genes and environmental factors.24 In low-incidence areas, younger cases of NPC tend usually to be familial cases and of undifferentiated type, suggesting that NPC may develop through exposure to environmental factors such as EBV infection in early life in genetically susceptible individuals.25
Specific clinical characteristics
The revealing symptom is often a neck mass reported in 60–90% of all the patients in major pediatric series.9,13,26–28 Nasal, auditory and neurological symptoms are correlated with the primary nasopharyngeal involvement. Nasal symptoms (obstruction, bleeding and discharge), auditory symptoms (otalgia, serous otitis and hearing loss) are respectively described for 30–70% and 20–45% of children9,13,26,28 and often non-symptomatic for a long time.29 Neurological symptoms (base skull involvement) include headaches (11–32%), cranial nerve deficit (5–22%).1,9,27 Other symptoms (trismus, taste disorders, dysphagia, difficult swallowing) are more rarely described in pediatric series.
Treatment modalities in non-metastatic NPC
The type of treatment depends on the tumor stage, according to the eighth Edition of the American Joint Committee on Cancer Staging System updated in 2017 (Table 1).30 Surgery is not part of the treatment of NPC except for initial biopsy. For Stage I and II (N0), the rare patients usually receive exclusive RT, leading to a 98% 10 year overall survival (OS) rate.31
Table 1. .
The eighth edition of American Joint Committee on Cancer staging system
| American Joint Committee on Cancer staging system30 | |
| Primary tumor | |
| T1 | Tumor confined to the nasopharynx or tumor extends to oropharynx and/or nasal cavity without parapharyngeal extension |
| T2 | Tumor with extension to parapharyngeal space and/or infiltration of the medial pterygoid, lateral pterygoid, and/or prevertebral muscles |
| T3 | Tumor invades bony structures of skull base cervical vertebra, pterygoid structures, and/or paranasal sinuses |
| T4 | Tumor with intracranial extension and/or involvement of cranial nerves, hypopharynx, orbit, parotid gland and/or infiltration beyond the lateral surface of the lateral pterygoid muscle |
| Nodes | |
| N1 | Unilateral metastasis, in cervical lymph node(s) above the caudal border of cricoid cartilage, and/or unilateral or bilateral metastasis in retropharyngeal lymph nodes, 6 cm or less, |
| N2 | Bilateral metastasis in cervical lymph node(s), 6 cm or less above the caudal border of cricoid cartilage |
| N3 | Metastasis in cervical lymph node(s) greater than 6 cm in dimension and/or extension below the caudal border of cricoid cartilage |
| Distant metastases | |
| MX | Distant metastases cannot be assessed |
| M0 | No distant metastases |
| M1 | Distant metastases |
| Stage | |
| I | T1 N0 M0 |
| II | T1-T2 N1 M0, T2 N0 M0 |
| III | T3 N0-1 M0, or T1-3 N2 M0 |
| IVA IVB |
T1-T4 N3 M0, T4 N0-2 M0 Any T, N, M1 |
In contrast, advanced-stages pediatric NPC treated historically by RT alone have demonstrated poor prognosis, with 4 year disease-free-survival (DFS) about 40% due to metastatic relapses.27,32 The positive impact of chemotherapy (CT) in addition of radiotherapy (RT) has been clearly demonstrated in adults.33 Several retrospective studies have reported a better survival after combined treatment in pediatric series: Cheuck et al reported 81% 15-year OS with the use of cisplatin as compared with 54% after RT alone.12 Combined treatment has become a standard in children considering the survival improvement reported in more recent prospective studies for Stages II (N1), III and IVa.3,4,11,14 CT modalities (induction CT and/or concomitant CT) are more debatable and discussed below. Metastatic patients (IVb) are treated with multimodal strategy with initial CT regimens, locoregional RT, whenever possible focal treatment of metastases and maintenance therapy.3,34
Induction chemotherapy regimen
Some recent large Phase III trials and meta-analysis comparing induction CT versus no neoadjuvant CT in adults did report an improvement in OS in favor of induction chemotherapy,35 while some others did not.35–39 Cisplatin-based regimen is a standard, but a recent meta-analysis could not determine the optimal neoadjuvant CT combination.40
In children, no prospective comparative studies have been performed to assess the role of neoadjuvant CT. A recent large retrospective study compared induction cisplatin-based CT followed by concurrent chemoradiotherapy (CT–RT) in 130 patients, versus only concurrent CT–RT in 64 patients with no difference in term of survival.41 A matched analysis identified 43 well-balanced patients in both two groups. With a median follow-up of 51.5 months, a trend in favor of induction CT was found in 5 year OS (83.7% vs 74.6%, p = 0.153), and PFS (79.2% vs 73.4%, p = 0.355) but induction CT was associated with increased rates of severe neutropenia.
However, non-randomized prospective pediatric studies leading to the most favorable outcomes include neo adjuvant CT in children NPC.3,4,11,14,42 A better outcome for patients in good response after neoadjuvant CT, as compared with patients in partial or minor response has been reported.13 The optimal type of induction CT is not consensual but all the prospective studies have reported optimal response using cisplatin-based CT in combination with 5-fluorouracil (FU).3,4,11,14 Methotrexate was included in the two oldest prospective reports4,14 but omitted in more recent protocols with the intention of reducing the rate of severe mucositis, without compromising neither response to induction CT nor survival.3,11
In children, the only prospective Phase II randomized study on 77 patients with NPC, median age 16 years, showed that the addition of docetaxel to cisplatin-5-FU induction therapy did not provide any benefit in terms of local control rate and outcomes in children and adolescents.43
Concomitant chemotherapy
In adults, a meta-analysis including eight randomized trials (1753 patients) compared cisplatin-based CT-RT versus RT alone in locally advanced NPC. A significant benefit was found for OS (6% at 5 years) and EFS (10% at 5 years) with the addition of CT.33 An update on 19 trials and 4806 patients, confirmed this benefit.44
In children, no randomized studies are available. Some retrospective studies have not demonstrated any impact of concomitant RT–CT in term of outcomes as compared with induction CT alone and RT.42,45 However, concomitant RT–CT have been used in recent prospective trials of NPC in children leading to very good outcomes, with DFS reaching more than 90% in the best series.3,11 As a consequence, most of the current protocol or guidelines consider concomitant RT–CT as a standard for the treatment of locally advanced pediatric NPC, in all the patients or only in selected patients after a poor response (stable disease or response <50%) to induction CT.34,46
RT–CT may, however, be responsible for additional acute toxic effects, especially in terms of mucositis (up to 42% Grade 3 and 4 in some series) and skin toxicity. Patients must have a close follow-up of nutritional status before and during the whole treatment.9,11,45,47
The role of interferon beta as maintenance therapy
In the large majority of studies, except in German series, patients did not receive systematic adjuvant therapy.11 Interferon β (IFN-ß) was shown to have antiproliferative effects, cytotoxic effects and enhancement of cell surface antigen expression. In a first single arm prospective GPOH trial, 59 young patients were treated with 6 months of IFN-ß. After RT–CT, 72% of the patients were in complete remission. At the end of IFN-ß therapy, 98% of the patients achieved complete remission.48 In another GPOH study, 45 young patients received IFN-ß for 6 months after completion of RT-CT. Two-third of the patients were in complete response or very good partial response after RT–CT, and 78% at the end of IFN-ß therapy respectively, with a good tolerance.11 A retrospective French review has finally reported, 17 patients out of 95 treated with maintenance IFN-ß. No relapse was interestingly observed in these patients.42 The preliminary results plead in favor of the prospective evaluation of this drug after the completion of initial treatment by CT and RT.11,42
radiation therapy
RT is crucial in the treatment of NPC, which are usually radiosensitive tumors. Every step in the RT delivery is highly important: immobilization device, target volume and critical organs definitions, choice of RT technique and RT dose
Immobilization, and planning-CT scan
Optimal immobilization is recommended in a supine position with a thermoplastic mask covering the head to the shoulders. The primary lesion and lymph nodes involvements are defined at diagnosis by conventional scan with contrast, axial contrast-enhanced MRI with thin slices (<3 mm), endoscopy examination and 18-fludeoxyglucose (FDG) positron emission tomography (PET)/scan when available. Its advantages are increasingly recognized in term of staging, especially to detect metastasis and clarify ambiguous MRI findings.11,49 In addition, 18F-FDG-PET/scan has been reported to change treatment volume delineation of the gross tumor volumes (GTV) in adult series.50,51 Dose escalation by using 18-FDG-PET/CT guided dose-painting IMRT has shown improved survival with no increased toxicities compared with CT-based IMRT in a retrospective large study in adults.50 No data are available in pediatric NPC concerning the interest of 18-FDG-PET/Scan in delineation optimization but should clearly be investigated in the future considering the potential interest. In pediatric NPC, at least axial contrast-enhance MRI with thin slices (<3 mm) and endoscopy examination should be performed again after two or three cycles of induction CT to evaluate tumor response that may allow RT dose adaptation. Target volumes and most of critical organs are delineated using both planning scan (with contrast) and fusion MRI, which is highly recommended. Fusion MRI should include at least three-dimensional (3D)-contrast-enhanced axial T1 with thin slices.
Target volume definition and critical organs
Target volumes are similar to those well-defined in adults.52 Briefly, the gross tumor volume 1 (GTV1) includes the primary nasopharyngeal carcinoma, retropharyngeal nodes, and gross nodal disease defined at diagnosis on scan, MRI and endoscopy examination. In childhood, a gross target volume 2 (GTV2) is often delineated as the residue (primary tumor or/and nodes) after induction CT and is considered as the higher-risk volume.
The clinical target volume 1 (CTV1) includes the GTV1 with a 3–5 mm margins, and must include the potential areas of microscopic spread of disease. CTV1 should include the entire nasopharynx, half (posterior) of nasal cavity, entire sphenoid sinus, posterior third of ethmoid sinuses, clivus, pterygoid fossae, parapharyngeal spaces, skull base. Sham et al have shown that the protection of pituitary gland did not lead to increase local failure if the skull base was not involved, which can be interesting in childhood.53 The CTV1 should also include lymph nodal groups involved at diagnosis. The CTV2 includes GTV2 (tumor and residual nodes after induction CT) and a 3 to 5 mm margin. Prophylactic RT is recommended in Level II, III, IV, V, VII, (and sometimes IB cervical lymph nodes) if they were not involved at diagnosis (and thus not included in CTV1 or CTV2). Finally, the planning target volumes PTV (PTV1 and 2) is defined as the CTV (CTV1 or CTV2) with a margin of 3–5 mm, to take into account setup margins and patient motion.
Critical organs to be delineated and doses to be respected as far as possible include : parotid glands (mean dose <25–30 Gy), sub maxillary glands (mean dose <35 Gy), brain stem (max. dose <54 Gy), upper spinal cord (max dose <45 Gy), hypophysis (max dose <45–50 Gy), chiasma (max dose <50–54 Gy), optic nerves (max dose <50–54 Gy), brain/temporal lobes (max dose <60 Gy), thyroid, eyes (mean dose <35 Gy), anterior chamber of the eyes, lens (mean dose <5–10 Gy), retina (max dose 45 Gy), internal ears and/or cochlea (mean dose <45 Gy), and larynx (mean dose <20 Gy), mouth cavity (max dose <55 Gy), temporomandibular joints (max dose <60 Gy).46,54
Total radiation doses in children an AYA patients
Historically, childhood NPC were treated with RT doses quite comparable with adult doses, up to 66 to 70 Gy to the high risk areas, around 60 Gy on standard-risk areas, and 50 to 54 Gy on prophylactic lymph node regions.55–58 Considering both the good prognosis and the high risk of severe late effects, several studies have more recently reported a RT dose reduction strategy in patients with good tumor response after induction chemotherapy in order to limit long-term RT toxicities in children or AYA NPC.4,11,13,48 In the POG 9486 study, patients received a limited dose of RT (50.4 gray Gy to the upper neck and 45.0 Gy to the lower neck, with a boost to the primary tumor and positive lymph nodes for a total dose of 61.2 Gy), in case of complete or partial response to neo adjuvant CT. 5-years OS and EFS were over 75%% despite the “limited” RT dose.4 In a French study, 34 children were treated for AJJC-TNM Stage IV NPC. After CT, cervical nodal RT was reduced (<50 Gy) in the 15 cases of a good response to chemotherapy (≥90% of initial tumor volume). The overall prognosis was not influenced by the dose of local RT delivered or response to the initial CT, but EFS was better in patients with a good response to CT. The cervical local failure rate was low despite RT dose reduction in the case of a good response to neo adjuvant chemotherapy.13
In the GPOH study, patients with Stage III/IV disease received 3 courses of induction CT. The cumulative RT dose was reduced to 54 Gy in five patients, who achieved complete remission after neoadjuvant CT, and 59.4 Gy in the remaining 40 patients, in combination with cisplatin, and followed by interferon. After a median follow-up of 30 months, the OS was excellent, over 97%.11
These results are in favor of RT dose reduction in children and young adults providing a good response to induction CT, in order to decrease the risk of severe late RT effects. Several national guidelines recommend such a strategy of decreasing RT dose after induction CT, providing a complete or good partial response to induction CT.3,34,42,46 Figure 1 represents the philosophy of current RT doses, recommended in pediatric NPC, depending both on the tumor/nodes involvement and on response to CT. Patients in minor response, stable disease or refractory disease (rare situations) are still treated with high RT doses, up to 66 Gy on tumor bed or involved nodes sites.
Figure 1. .
Representation of the RT doses levels according to the tumor and nodal involvements after induction chemotherapy. RT doses are adapted according to the risk of relapse, leading to several levels of doses (prophylactic = PTV0, standard risk = PTV1, high risk = PTV2). RT doses are also adapted to the chemotherapy response in several current guidelines. Chemo, chemotherapy; RT, radiotherapy; PTV, planning target volume
Fractionation
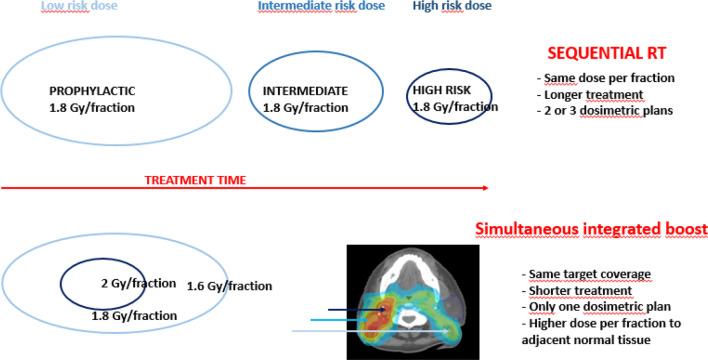
Historically, radiation oncologists prefer delivering RT using dose per fraction of 1.8 to 2 Gy in children and AYA, considering the risk of late effects is higher over this threshold. Randomized studies in adults have shown no significant difference between sequential boost and simultaneous integrated boosts (SIB) in terms of adverse events or tumor control.59 Only one retrospective small study on 34 patients is available in children with no evidence of more toxicity.60 Lots of clinicians consider SIB as an option even in pediatric practice but usually recommend a maximum dose per fraction of 2 Gy for high-risk volumes (Figure 2).
Figure 2. .
Principle of SIB as compared with sequential treatments (Gy: Grays). SIB, simultaneous integrated boost.
RT techniques
Randomized studies in adults have reported both increased local control and survival, as well as an improved quality of life of patients with NPC using intensity modulated radiotherapy (IMRT), as compared with conformal radiation therapy (3D-CRT).61–63 Several reports in childhood are also in favor of IMRT use in NPC, considering the decrease of acute severe toxicity (skin, mucous membrane, and pharynx mainly), late effects and a better prognosis in survival.1,56,64
The clinical benefit of protons in NPC is poorly known even in adults: the availability remains limited and the technique is complex to implement while volumes are large and highly complex. Most of the studies reporting dosimetric data, show similar adequate target coverage as compared with IMRT, while a better sparing of critical organs, especially parotid glands, cochlea, maxillary, and larynx.65,66 Clinical results after protons are still rare and awaited especially to evaluate the benefit of protons on late effects.67
Systemic treatment in distant metastatic patients
In metastatic situation and after relapse in adults, CT regimen based on gemcitabine/cisplatin has been defined as standard in a Phase III trial.68 No specific pediatric study has been conducted in children with Stage IVb NPC. The 5-year OS is poor, <20%.3,38 Since metastatic NPC is usually chemo sensitive at the beginning of the treatment, clinicians use a multimodal strategy with initial prolonged multidrugs cisplatin-based CT regimens, followed by locoregional head and neck RT, focal treatment of metastases lesions if feasible. Maintenance therapy with interferon-ß is finally suggested by some groups.34,42
An oxaliplatin-containing regimen in combination with gemcitabine was recently reported on 14 children with relapsed NPC, and shows that this combination is a reasonable choice for first-line salvage therapy.69 Immune-based therapy could be a promising treatment in case of relapses or refractory NPC and need to be evaluated in pediatric situations. In particular, some adult studies have reported both feasibility and safety of EBV-stimulated cytotoxic T-lymphocyte (EBV-CTLs) immunotherapy in EBV-related cancer including NPC, with or without previous lymphodepleting regimen.70–72
Results and survival in young patients
Despite more advanced disease in AYA patients as compared with adults,73,74 prognosis seems better: 5 year OS is over 75–80% (Table 2). Local and locoregional failures are rare (<8% in the vast majorities of the series), and distant relapse is the predominant mode of tumor failure. Relapses occur mainly within the first 2 years of follow-up, (Table 3). OS and DFS are very close : NPC relapses have a poor prognosis and the salvage gap after tumor events is low.76
Table 2. .
Results in recent studies in childhood and AYA NPC patients
| N | Type of study | Stage | Age (years) | Treatment | OS | DFS | |
| Jouin, 201942 | 95 | Retrospective | All M0 | Med. 15 y. | CT (90%) +RT/CT (59%) +IFN (18%) |
five y : 94% | five y : 91% |
| Qiu, 20171 | 176 | Retrospective | All M0 | 7–20 y. 24% < 14 y. |
CT +RT : 28% CT +RT/CT : 44% RT/CT : 23% |
five y : 76–90% | five y : 71–86% |
| Sahai, 20179 | 41 | Retrospective | 1/41 M1 (3%) | 6–20 y. Med. 14 y. 34% < 12 y. |
CT +RT/CT (68%)a | three y : 84% | three y : 56% |
| Guo, 201645 | 95 | Retrospective | All M0 | <25 y. 30% < 18 y. |
CT (100%) +RT/CT (52%) +adj. CT (30%) |
four y : 91% | 4y : 79% |
| Chen, 201528 | 32 | Retrospective | All M0 | 11 to 18 y. Mean 15 y. |
CT+ RT/CT (40%) or CT +RT + CT (31%)* |
five y : 86% III - 65% IV | NR |
| Liu, 201427 | 158 | Retrospective | All M0 | 8 to 20 y. Median 16 y. |
RT/CT 46% RT alone 54% |
five y : 83% | NR |
| Daoud, 201374 | 69 | Retrospective | All M0 | 10 to 20 y. | RT + CT 85% RT/CT 7% |
five y : 66% | five y : 66% |
| Hu, 2013 (78) | 95 | Retrospective | All M0 | 9 to 20 y. 16 y. |
CT + RT (38%)or RT alone (62%) |
five y : 54% | four y : 49% |
| Casanova, 20123 | 46 | Prospective | 5/46 M1 (10%) | 9 to 17 y. Med 13 y. |
CT +RT/CT | five y : 81% | five y 87% (M0) and 20% (M1) |
| Buerhlen, 201211 | 45 | Prospective | All M0 | 8 to 20 y. Med.15 y. |
CT + RT+IFN | 30 m : 97% | 30 m 92% |
| Cheuk, 201112 | 59 | Retrospective | 2/59 M1 (3%) | Med. 14 y. | CT +RT/CT (88%) RT alone (12%) |
15 y : 67% With cisplatin : 81% Without cisplatin : 54% |
15 y : 63% |
| Afqir, 200910 | 46 | Retrospective | 4/46 M1 (9%) | Med.16 y. | CT +RT | five y : 73% | five y : 41% |
| Orbach, 200813 | 34 | Retrospective | 1/34 M1 (3%) | Med.12 y. | RT +CT (91%) or RT 9% |
five y : 75% | five y : 73% |
| Rodriguez Galindo, 20054 | 17 | Prospective | All N2/N3 and/or M0/1 | Med. 13 y. | CT +RT | four y : 77% | four y : 75% |
| Mertens, 200514 | 59 | Prospective | All M0 | Med. 13 y. | CT +RT + IFN | nine y : 95% | nine y : 91% |
AYA, adolescents and young adults; CT, chemotherapy; DFS, disease-free survival; IFN, interferon; MO, no mets; N, number of patient; NPC, nasopharyngeal carcinoma; NR, not reported; OS, overall survival; RT, radiotherapy; adj CT, adjuvant chemotherapy.
other patients treated with various RT/CT sequences
Table 3. .
Sites and timing of relapses in young NPC patients
| Study | N | Local failure | Metastatic failure or combined | Time of relapse |
| Sahai, 20179 | 41 | 2.4% | 41% | Med. 9 months |
| Guo, 201645 | 95 | 5% | 16% | Med. 29 months |
| Greenwalt,201675 | 10 | 5% | 40% | NR |
| Liu, 201427 | 165 | 5% | 24% | All <15 months |
| Hu, 2013 (78) | 95 | 14% | 37% | NR |
| Daoud, 201374 | 69 | 1.4% | NR | All <24 months |
| Cheuk, 201112 | 59 | 5% | 25% | NR |
| Afqir, 200910 | 46 | 0% | 29% | NR |
N, number of patients; NPC, nasopharyngeal carcinoma;NR, not reported.
Late effects
The most frequent late radiation effects are shown in Table 4. IMRT was shown to decrease the hearing loss rates and xerostomia as compared with 3D-CRT. Protons should also limit some toxicities but in contrast with IMRT, this is not yet clinically proven.65–67 Protons may induce less xerostomia and as a consequence less cavities, as well as a potential gain on ear and endocrine toxicities. Other rare severe late complications include temporal necrosis (3–12%),38,74 neuropathy (3–8%),27 myelitis (<1%), osteonecrosis (1–5%)12,27 and hemorrhage.
Table 4. .
Most frequent late radiation effects of radiotherapy in childhood NPC, and impact of the IMRT use
| All grades (2D–3D CRT) |
All grades (IMRT) | Grade 3–4 (2D–3D CRT) |
Grade 3–4 (IMRT) |
Positive impact of IMRT vs 2D–3D CRT on late effect incidence | |
| Hearing loss | 40–54%1,12,27 | 22–50%1,27,45 | 5–10%12,27 | 027 | Yes1 No27 |
| Xerostomia | 52–97%1,74 | 34–48%1,45 | 0%27 | 0%27 | Yes1,27 |
| Neck fibrosis | 34–94%1,27 | 22–85%1,27,35 | 7–19%27,74) | 9%27 | Yes1 No27 |
| Hypothyroidism | 3–52%27,64,74 | 0–50%27,45 | 0% (27,80) | 0%27 | No1,27 |
| Growth retardation | 4–17%1,12 | 2–20%62,71 | NR | NR | No (1,45,80) |
| Trismus | 15–56%1,27,74 | <10%62,71 | 8%27 | 5%27 | Yes27 No1 |
| Caries | 22%4 | 2%7 | NR | NR | NR |
CRT, conformational radiation therapy; IMRT, intensity modulated radiation therapy; NPC, nasopharyngeal carcinoma; NR, not reported; positive impact, significative or trend (p < 0.
Secondary cancers are described in Table 5. In the future, the use of protontherapy which reduce integral dose, and may limit this risk.
Table 5. .
Risk of secondary cancer after NPC
| RT Technique (number of patients) | Second cancer (time of diagnosis since treatment when known) |
|
| Guo,45 | IMRT (95) | 0 % |
| Jouin, 201942 | 3D CRT38 IMRT57 |
3% (6–8 years) |
| Liu, 201427 | 3D CRT (103) IMRT55 |
3.8% (3–11 years) |
| Daoud, 201374 | 2D/3D CRT69 | 2.5 % |
| Casanova, 20123 | 2D/3D CRT30 IMRT18 |
3 % |
| Cheuk, 201112 | 2D/3D CRT52 IMRT6 |
8.5% (8–27 years) |
| Greenwalt, 201675 | IMRT10 | 10% (meningioma) |
CRT, conformational radiotherapy; IMRT, intensity modulated radiation therapy; NPC, nasopharyngeal carcinoma.
Conclusions
NPC in young patients are often advanced diseases but have a better prognosis than adults. 5 year OS is over 80% for non-metastatic disease, using combined strategies of induction CT, followed by concomitant CT and RT. Adapted-RT dose according to response to induction CT seems feasible but is still under larger evaluation. Adjuvant treatment such as IFN-ß may be of interest but need comparative studies. Protons should be more investigated to evaluate the long-term benefit in term regard of late effects, while they remain highly frequent and severe, especially after treatment in childhood and adolescence. International collaborations are clearly needed to pool the data and the knowledge.75
Footnotes
Acknowledgment: Ms Sophie KING for English corrections.
Contributor Information
Line Claude, Email: line.claude@lyon.unicancer.fr.
Emmanuel Jouglar, Email: Emmanuel.Jouglar@ico.unicancer.fr.
Loig Duverge, Email: loig.duverge@gmail.com.
Daniel Orbach, Email: daniel.orbach@curie.fr.
References
- 1.Qiu W-Z, Peng X-S, Xia H-Q, Huang P-Y, Guo X, Cao K-J. A retrospective study comparing the outcomes and toxicities of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy for the treatment of children and adolescent nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2017; 143: 1563–72. doi: 10.1007/s00432-017-2401-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pastore G, De Salvo GL, Bisogno G, Dama E, Inserra A, Cecchetto G, et al. Evaluating access to pediatric cancer care centers of children and adolescents with rare tumors in Italy: the TREP project. Pediatr Blood Cancer 2009; 53: 152–5. doi: 10.1002/pbc.22049 [DOI] [PubMed] [Google Scholar]
- 3.Casanova M, Bisogno G, Gandola L, Cecchetto G, Di Cataldo A, Basso E, et al. A prospective protocol for nasopharyngeal carcinoma in children and adolescents: the Italian rare tumors in pediatric age (TREP) project. Cancer 2012; 118: 2718–25. doi: 10.1002/cncr.26528 [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Galindo C, Wofford M, Castleberry RP, Swanson GP, London WB, Fontanesi J, et al. Preradiation chemotherapy with methotrexate, cisplatin, 5-fluorouracil, and leucovorin for pediatric nasopharyngeal carcinoma. Cancer 2005; 103: 850–7. doi: 10.1002/cncr.20823 [DOI] [PubMed] [Google Scholar]
- 5.Desandes E, Clavel J, Berger C, Bernard J-L, Blouin P, de Lumley L, et al. Cancer incidence among children in France, 1990-1999. Pediatr Blood Cancer 2004; 43: 749–57. doi: 10.1002/pbc.20148 [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Tang L-Q, Chen Q-Y, Liu H, Guo S-S, Liu L-T, et al. Plasma Epstein-Barr viral DNA complements TNM classification of nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy. Oncotarget 2016; 7: 6221–30. doi: 10.18632/oncotarget.6754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boussen H, Bouaouina N, Daldoul O, Benna F, Gritli S, Ladgham A. Update on medical therapies of nasopharyngeal carcinomas. Bull Cancer 2010; 97: 417–26. doi: 10.1684/bdc.2010.1091 [DOI] [PubMed] [Google Scholar]
- 8.Downing NL, Wolden S, Wong P, Petrik DW, Hara W, Le Q-T. Comparison of treatment results between adult and juvenile nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2009; 75: 1064–70. doi: 10.1016/j.ijrobp.2008.12.030 [DOI] [PubMed] [Google Scholar]
- 9.Sahai P, Mohanti BK, Sharma A, Thakar A, Bhasker S, Kakkar A, et al. Clinical outcome and morbidity in pediatric patients with nasopharyngeal cancer treated with chemoradiotherapy. Pediatr Blood Cancer 2017; 64: 259–66. doi: 10.1002/pbc.26240 [DOI] [PubMed] [Google Scholar]
- 10.Afqir S, Ismaili N, Alaoui K, Ahid S, Lotz J-P, Horn E, et al. Nasopharyngeal carcinoma in adolescents: a retrospective review of 42 patients. Eur Arch Otorhinolaryngol 2009; 266: 1767–73. doi: 10.1007/s00405-009-0911-1 [DOI] [PubMed] [Google Scholar]
- 11.Buehrlen M, Zwaan CM, Granzen B, Lassay L, Deutz P, Vorwerk P, et al. Multimodal treatment, including interferon beta, of nasopharyngeal carcinoma in children and young adults. Cancer 2012; 118: 4892–900. doi: 10.1002/cncr.27395 [DOI] [PubMed] [Google Scholar]
- 12.Cheuk DKL, Billups CA, Martin MG, Roland CR, Ribeiro RC, Krasin MJ, et al. Prognostic factors and long-term outcomes of childhood nasopharyngeal carcinoma. Cancer 2011; 117: 197–206. doi: 10.1002/cncr.25376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orbach D, Brisse H, Helfre S, Klijanienko J, Bours D, Mosseri V, et al. Radiation and chemotherapy combination for nasopharyngeal carcinoma in children: radiotherapy dose adaptation after chemotherapy response to minimize late effects. Pediatr Blood Cancer 2008; 50: 849–53. doi: 10.1002/pbc.21372 [DOI] [PubMed] [Google Scholar]
- 14.Mertens R, Granzen B, Lassay L, Bucsky P, Hundgen M, Stetter G, et al. Treatment of nasopharyngeal carcinoma in children and adolescents: definitive results of a multicenter study (NPC-91-GPOH. Cancer 2005; 104: 1083–9. doi: 10.1002/cncr.21258 [DOI] [PubMed] [Google Scholar]
- 15.Bogger-Goren S, Gotlieb-Stematsky T, Rachima M, Barkowsky E, Schlomo-David J. Nasopharyngeal carcinoma in Israel: epidemiology and Epstein-Barr virus-related serology. Eur J Cancer Clin Oncol 1987; 23: 1277–81. doi: 10.1016/0277-5379(87)90108-8 [DOI] [PubMed] [Google Scholar]
- 16.Bar-Sela G, Kuten A, Minkov I, Gov-Ari E, Ben-Izhak O. Prevalence and relevance of EBV latency in nasopharyngeal carcinoma in Israel. J Clin Pathol 2004; 57: 290–3. doi: 10.1136/jcp.2003.013094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sultan I, Casanova M, Ferrari A, Rihani R, Rodriguez-Galindo C. Differential features of nasopharyngeal carcinoma in children and adults: a seer study. Pediatr Blood Cancer 2010; 55: 279–84. doi: 10.1002/pbc.22521 [DOI] [PubMed] [Google Scholar]
- 18.Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, Chen CJ, et al. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 2001; 345: 1877–82. doi: 10.1056/NEJMoa011610 [DOI] [PubMed] [Google Scholar]
- 19.Karray H, Ayadi W, Fki L, Hammami A, Daoud J, Drira MM, et al. Comparison of three different serological techniques for primary diagnosis and monitoring of nasopharyngeal carcinoma in two age groups from Tunisia. J Med Virol 2005; 75: 593–602. doi: 10.1002/jmv.20310 [DOI] [PubMed] [Google Scholar]
- 20.Khabir A, Karray H, Rodriguez S, Rosé M, Daoud J, Frikha M, et al. Ebv latent membrane protein 1 abundance correlates with patient age but not with metastatic behavior in North African nasopharyngeal carcinomas. Virol J 2005; 2: 39. doi: 10.1186/1743-422X-2-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Fasano R, Wang E, Yao K-T, Marincola FM. Hla associations with nasopharyngeal carcinoma. Curr Mol Med 2009; 9: 751–65. doi: 10.2174/156652409788970698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SL, Cheng I, Haiman CA. Genome-Wide association studies of cancer in diverse populations. Cancer Epidemiol Biomarkers Prev 2018; 27: 405–17Available from. doi: 10.1158/1055-9965.EPI-17-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildesheim A, Wang C-P. Genetic predisposition factors and nasopharyngeal carcinoma risk: a review of epidemiological association studies, 2000–2011. Semin Cancer Biol 2012; 22: 107–16. doi: 10.1016/j.semcancer.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia W-H, Collins A, Zeng Y-X, Feng B-J, Yu X-J, Huang L-X, et al. Complex segregation analysis of nasopharyngeal carcinoma in Guangdong, China: evidence for a multifactorial mode of inheritance (complex segregation analysis of NPC in China. Eur J Hum Genet 2005; 13: 248–52. doi: 10.1038/sj.ejhg.5201305 [DOI] [PubMed] [Google Scholar]
- 25.Bray F, Haugen M, Moger TA, Tretli S, Aalen OO, Grotmol T. Age-Incidence curves of nasopharyngeal carcinoma worldwide: bimodality in low-risk populations and Aetiologic implications. Cancer Epidemiology Biomarkers & Prevention 2008; 17: 2356–65. doi: 10.1158/1055-9965.EPI-08-0461 [DOI] [PubMed] [Google Scholar]
- 26.Ayan I, Kaytan E, Ayan N. Childhood nasopharyngeal carcinoma: from biology to treatment. Lancet Oncol 2003; 4: 13–21. doi: 10.1016/S1470-2045(03)00956-2 [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Tang Y, Gao L, Huang X, Luo J, Zhang S, et al. Nasopharyngeal carcinoma in children and adolescents - a single institution experience of 158 patients. Radiat Oncol 2014; 9: 274. doi: 10.1186/s13014-014-0274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Hu F. Clinical and prognostic analysis in 32 pediatric nasopharyngeal carcinoma. J Cancer Res Ther 2015; 11: 226. [DOI] [PubMed] [Google Scholar]
- 29.Pua KC, Khoo ASB, Yap YY, Subramaniam SK, Ong CA, Gopala Krishnan G, et al. Nasopharyngeal carcinoma database. Med J Malaysia 2008; 63 Suppl C(Suppl C): 59–62 [PubMed] [Google Scholar]
- 30.Amin MB, Edge SB, Greene FL.et al. No Title : AJCC Cancer Staging Manual. 8th ed New York: Springer; 2017. [Google Scholar]
- 31.Chua DTT, Sham JST, Kwong DLW, Au GKH. Treatment outcome after radiotherapy alone for patients with stage I-II nasopharyngeal carcinoma. Cancer 2003; 98: 74–80. doi: 10.1002/cncr.11485 [DOI] [PubMed] [Google Scholar]
- 32.Lombardi F, Gasparini M, Gianni C, De Marie M, Molinari R, Pilotti S. Nasopharyngeal carcinoma in childhood. Med Pediatr Oncol 1982; 10: 243–50 10.1002/mpo.2950100304 [DOI] [PubMed] [Google Scholar]
- 33.Baujat B, Audry H, Bourhis J, Chan ATC, Onat H, Chua DTT, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 2006; 64: 47–56 10.1016/j.ijrobp.2005.06.037 [DOI] [PubMed] [Google Scholar]
- 34.Kontny U, Franzen S, Behrends U, Bührlen M, Christiansen H, Delecluse H, et al. Diagnosis and Treatment of Nasopharyngeal Carcinoma in Children and Adolescents - Recommendations of the GPOH-NPC Study Group. Klin Padiatr 2016; 228: 105–12. doi: 10.1055/s-0041-111180 [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Li W-F, Chen N-Y, Zhang N, Hu G-Q, Xie F-Y, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016; 17: 1509–20 10.1016/S1470-2045(16)30410-7 [DOI] [PubMed] [Google Scholar]
- 36.Chua DT, Sham JS, Choy D, Lorvidhaya V, Sumitsawan Y, Thongprasert S, et al. Preliminary report of the Asian-Oceanian clinical oncology association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Asian-Oceanian clinical oncology association nasopharynx cancer Study Group. Cancer 1998; 83: 2270–83. doi: [DOI] [PubMed] [Google Scholar]
- 37.He X, Xu K, Guo J, Zhu Y, Liang X. Liu L. a meta-analysis of neoadjuvant chemotherapy plus radiation in the treatment of locally advanced nasopharyngeal carcinoma. J Cancer Res Ther 2015; 2: C205–8. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y-P, Guo R, Liu N, Liu X, Mao Y-P, Tang L-L, et al. Efficacy of the additional neoadjuvant chemotherapy to concurrent chemoradiotherapy for patients with locoregionally advanced nasopharyngeal carcinoma: a Bayesian network meta-analysis of randomized controlled trials. J Cancer 2015; 6: 883–92 10.7150/jca.11814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hareyama M, Sakata K-ichi, Shirato H, Nishioka T, Nishio M, Suzuki K, et al. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer 2002; 94: 2217–23 10.1002/cncr.10473 [DOI] [PubMed] [Google Scholar]
- 40.Yuan C, X-H X, Luo S-W, Wang L, Sun M, L-H N. Which neoadjuvant chemotherapy regimen should be recommended for patients with advanced nasopharyngeal carcinoma?: A network meta-analysis. Medicine (Baltimore) [Internet].. 2018. Available from: http://insights.ovid.com/crossref?an=00005792-201808240-00082 [[cited 2019 Jan 28]]. [DOI] [PMC free article] [PubMed]
- 41.Li Y, Tang L-Q, Liu L-T, Guo S-S, Liang Y-J, Sun X-S, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma in children and adolescents: a matched cohort analysis. Cancer Res Treat 2018; 50: 1304–15 10.4143/crt.2017.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jouin A, Helfre S, Bolle S, Claude L, Laprie A, Bogart E, et al. Adapted strategy to tumor response in childhood nasopharyngeal carcinoma: the French experience. Strahlenther Onkol 2019; 195: 504–16. doi: 10.1007/s00066-019-01461-6 [DOI] [PubMed] [Google Scholar]
- 43.Casanova M, Özyar E, Patte C, Orbach D, Ferrari A, Veyrat-Follet C, et al. International randomized phase 2 study on the addition of docetaxel to the combination of cisplatin and 5-fluorouracil in the induction treatment for nasopharyngeal carcinoma in children and adolescents. Cancer Chemother Pharmacol 2016; 77: 289–98 10.1007/s00280-015-2933-2 [DOI] [PubMed] [Google Scholar]
- 44.Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015; 16: 645–55. doi: 10.1016/S1470-2045(15)70126-9 [DOI] [PubMed] [Google Scholar]
- 45.Guo Q, Cui X, Lin S, Lin J, Pan J. Locoregionally advanced nasopharyngeal carcinoma in childhood and adolescence: analysis of 95 patients treated with combined chemotherapy and intensity-modulated radiotherapy. Head Neck 2015 [DOI] [PubMed] [Google Scholar]
- 46.Orbach D. Orbach. 2018. Available from: http://sfce.sfpediatrie.com/content/recommandations-ucnt-2018.
- 47.Ribassin-Majed L, Marguet S, Lee AWM, Ng WT, Ma J, Chan ATC, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? an individual patient data network meta-analysis. JCO 2017; 35: 498–505. doi: 10.1200/JCO.2016.67.4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mertens R, Granzen B, Lassay L, Bucsky P, Hundgen M, Stetter G, et al. Treatment of nasopharyngeal carcinoma in children and adolescents. Cancer 2005; 104: 1083–9 10.1002/cncr.21258 [DOI] [PubMed] [Google Scholar]
- 49.Cheuk DK, Sabin ND, Hossain M, Wozniak A, Naik M, Rodriguez-Galindo C, et al. Positron emission tomography-computed tomography for staging and follow-up of pediatric nasopharyngeal carcinoma $watermark-text $watermark-text $watermark-text. Eur J Nucl Med Mol Imaging [Internet. 2012; 39: 1097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu F, Xi X-ping, Wang H, Han Y-qian, Xiao F, Hu Y, et al. PET/CT-guided dose-painting versus CT-based intensity modulated radiation therapy in locoregional advanced nasopharyngeal carcinoma. Radiat Oncol 2017; 12 10.1186/s13014-016-0739-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Zheng J, Tang T, Zhu F, Yao Y, Xu J. A Randomized Pilot Trial Comparing Position Emission Tomography (PET)-Guided Dose Escalation Radiotherapy to Conventional Radiotherapy in Chemoradiotherapy Treatment of Locally Advanced Nasopharyngeal Carcinoma. Thamm D, editor. PLoS One [Internet].. 2015. Available from: https://dx.plos.org/10.1371/journal.pone.0124018 [[cited 2019 May 15]]. [DOI] [PMC free article] [PubMed]
- 52.Fleury B, Biston MC, Montbarbon X, Pommier P. Nasopharyngeal cancer. Cancer Radiother 2010; 14 Suppl 1(Suppl 1): S23–33 10.1016/S1278-3218(10)70005-6 [DOI] [PubMed] [Google Scholar]
- 53.Sham J, Choy D, Kwong PWK, Cheng ACK, Kwong DLW, Yau CC, et al. Radiotherapy for nasopharyngeal carcinoma: shielding the pituitary may improve therapeutic ratio. Int J Radiat Oncol Biol Phys . 1994; 29: 699–704cited 2015 Oct 30[Internet]. doi: 10.1016/0360-3016(94)90556-8 [DOI] [PubMed] [Google Scholar]
- 54.Claude L, Laprie A. Quelles contraintes pour quels organes à risque en radiothérapie chez l’enfant ? Cancer/Radiothérapie 2015; 19(6–7): 484–8 10.1016/j.canrad.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 55.Wolden SL, Steinherz PG, Kraus DH, Zelefsky MJ, Pfister DG, Wollner N. Improved long-term survival with combined modality therapy for pediatric nasopharynx cancer. Int J Radiat Oncol Biol Phys 2000; 46: 859–64 10.1016/S0360-3016(99)00493-9 [DOI] [PubMed] [Google Scholar]
- 56.Laskar S, Sanghavi V, Muckaden MA, Ghosh S, Bhalla V, Banavali S, et al. Nasopharyngeal carcinoma in children: ten years' experience at the TATA Memorial Hospital, Mumbai. Int J Radiat Oncol Biol Phys 2004; 58: 189–95 10.1016/S0360-3016(03)00773-9 [DOI] [PubMed] [Google Scholar]
- 57.Ozyar E, Selek U, Laskar S, Uzel O, Anacak Y, Ben-Arush M, et al. Treatment results of 165 pediatric patients with non-metastatic nasopharyngeal carcinoma: a rare cancer network study. Radiother Oncol 2006; 81: 39–46 10.1016/j.radonc.2006.08.019 [DOI] [PubMed] [Google Scholar]
- 58.Guruprasad B, Tanvir P, Rohan B, Kavitha S, Naik SM, Appaji L. Paediatric nasopharyngeal carcinoma: an 8-year study from a tertiary care cancer centre in South India. Indian J Otolaryngol Head Neck Surg 2013; 65(Suppl 1): 131–4. doi: 10.1007/s12070-013-0622-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Songthong AP, Kannarunimit D, Chakkabat C, Lertbutsayanukul C. A randomized phase II/III study of adverse events between sequential (seq) versus simultaneous integrated boost (Sib) intensity modulated radiation therapy (IMRT) in nasopharyngeal carcinoma; preliminary result on acute adverse events. Radiat Oncol 2015; 10: 166. 10.1186/s13014-015-0472-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tao C-J, Liu X, Tang L-L, Mao Y-P, Chen L, Li W-F, et al. Long-Term outcome and late toxicities of simultaneous integrated boost-intensity modulated radiotherapy in pediatric and adolescent nasopharyngeal carcinoma. Chin J Cancer 2013; 32: 525–32[Internet]. doi: 10.5732/cjc.013.10124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng G, Wang T, Yang K-Y, Zhang S, Zhang T, Li Q, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012; 104: 286–93 10.1016/j.radonc.2012.08.013 [DOI] [PubMed] [Google Scholar]
- 62.Zhang M-X, Li J, Shen G-P, Zou X, Xu J-J, Jiang R, et al. Intensity-Modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer 2015; 51: 2587–95 10.1016/j.ejca.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 63.Klimstra D, Wenig B. Pancreatoblastoma A Clinicopathologic Study and Review of the Literature. … Surg Pathol [Internet].. 1995. Available from: http://journals.lww.com/ajsp/Abstract/1995/12000/Pancreatoblastoma_A_Clinicopathologic_Study_and.5.aspx [cited 2015 Sep 29]. [DOI] [PubMed]
- 64.Uzel O, Yörük SO, Sahinler I, Turkan S, Okkan S. Nasopharyngeal carcinoma in childhood: long-term results of 32 patients. Radiother Oncol 2001; 58: 137–41 10.1016/S0167-8140(00)00310-8 [DOI] [PubMed] [Google Scholar]
- 65.Taheri-Kadkhoda Z, Björk-Eriksson T, Nill S, Wilkens JJ, Oelfke U, Johansson K-A, et al. Intensity-Modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiat Oncol 2008; 3: 4. 10.1186/1748-717X-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Widesott L, Pierelli A, Fiorino C, Dell'oca I, Broggi S, Cattaneo GM, et al. Intensity-Modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and Ntcp evaluation. Int J Radiat Oncol Biol Phys 2008; 72: 589–96 10.1016/j.ijrobp.2008.05.065 [DOI] [PubMed] [Google Scholar]
- 67.Lewis GD, Holliday EB, Kocak-Uzel E, Hernandez M, Garden AS, Rosenthal DI, et al. Intensity-Modulated proton therapy for nasopharyngeal carcinoma: decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck 2016; 38 Suppl 1(S1): E1886–E1895 10.1002/hed.24341 [DOI] [PubMed] [Google Scholar]
- 68.Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. The Lancet 2016; 388: 1883–92 10.1016/S0140-6736(16)31388-5 [DOI] [PubMed] [Google Scholar]
- 69.DeRenzo C, Lam C, Rodriguez-Galindo C, Rapkin L, Gottschalk S, Venkatramani R. Salvage regimens for pediatric patients with relapsed nasopharyngeal carcinoma. Pediatr Blood Cancer 2019; 66e27469 10.1002/pbc.27469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Basso S, Zecca M, Merli P, Gurrado A, Secondino S, Quartuccio G, et al. T cell therapy for nasopharyngeal carcinoma. J Cancer 2011; 2: 341–6 10.7150/jca.2.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang J, Fogg M, Wirth LJ, Daley H, Ritz J, Posner MR, et al. Epstein-Barr virus-specific adoptive immunotherapy for recurrent, metastatic nasopharyngeal carcinoma. Cancer 2017; 123: 2642–50 10.1002/cncr.30541 [DOI] [PubMed] [Google Scholar]
- 72.Chia W-K, Teo M, Wang W-W, Lee B, Ang S-F, Tai W-M, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther 2014; 22: 132–9 10.1038/mt.2013.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu S-G, Liao X-L, He Z-Y, Tang L-Y, Chen X-T, Wang Y, et al. Demographic and clinicopathological characteristics of nasopharyngeal carcinoma and survival outcomes according to age at diagnosis: a population-based analysis. Oral Oncol 2017; 73: 83–7 10.1016/j.oraloncology.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 74.Daoud J, Ghorbal L, Siala W, Elloumi F, Ghorbel A, Frikha M. Résultats thérapeutiques du cancer du cavum : y a-t-il une différence entre enfants et adultes ? Cancer/Radiothérapie 2013; 17: 763–7 10.1016/j.canrad.2013.06.046 [DOI] [PubMed] [Google Scholar]
- 75.Ferrari A, Schneider DT, Bisogno G, Board EXPeRT, .EXPeRT Board . The founding of the European Cooperative Study Group on Pediatric Rare Tumors--EXPeRT. Expert Rev Anticancer Ther 2013; 13: 1–3 10.1586/era.12.155 [DOI] [PubMed] [Google Scholar]
- 76.Gioacchini FM, Tulli M, Kaleci S, Magliulo G, Re M. Prognostic aspects in the treatment of juvenile nasopharyngeal carcinoma: a systematic review. Eur Arch Otorhinolaryngol 2017; 274: 1205–14 10.1007/s00405-016-4154-7 [DOI] [PubMed] [Google Scholar]