In the midst of rampant tuberculosis plaguing Europe, René Laennec invented the stethoscope in 1816 as a result of both his genius and respect for patient modesty (1, 2). The auscultation method of ear-on-chest was replaced by a new technology that eventually brought clearer and more uniform sound quality. Since that time, the stethoscope has evolved into the universal symbol of clinical medicine. The World Health Organization estimates 10 million physicians and 20 million nurses worldwide are involved in direct patient care (3). Equipped with stethoscopes, tens of millions of lungs are likely examined every day. For its 200-year history, the stethoscope has been an invaluable tool for pulmonologists to diagnose diseases that produce sounds like crackles and wheezes, which are well known signs of disease. In this note, however, I will explore a novel proposal: that the mechanical events creating these sounds also cause disease.
How do mechanical stresses and strains cause disease or injury? This question falls into the general topic of mechanotransduction (4). Mechanically perturbed cells respond in many possible ways: growth, remodeling, altered metabolism, modified gene expression, release of bioactive molecules, membrane rupture, tissue failure, and death. For example, endothelial cells respond to blood flow shear stresses (5), osteocytes to force loading (6), and lung epithelium to inflation and deflation (7). Often, the forces and their responses are part of the natural function, as in newly laid-down matrix in bone or production of surfactant in the lung. However, the forces can also create disease or injury, as in formation of blood clots promoted through endothelium responses or ventilator-induced lung injury (8). In the lung, mechanical perturbations can also create sound, and this poses a particularly interesting thought reversal. Can crackles and wheezes both indicate and cause disease? Let’s take a look at the possibilities.
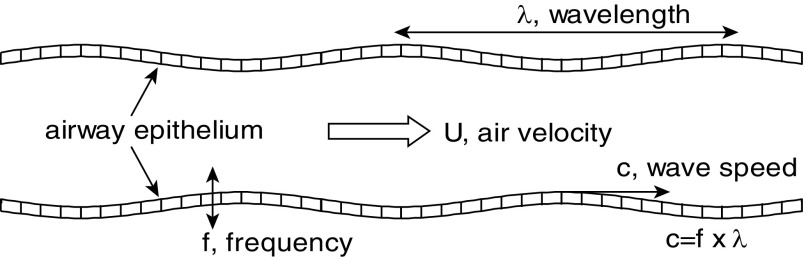
Expiratory wheezes are a sign of obstructed airways, as occur in asthma and emphysema. The airflow couples with the elastic airway walls to create flow-induced oscillations. Mechanically, this is very much like blowing up a balloon, then releasing the air while stretching the outlet and making it squeal. As a reference point, in Figure 1, air flowing at a critical speed, U, through a model flexible airway causes wall oscillations of frequency, f, and wavelength, λ, which travel at wave speed c = f × λ (9). The oscillations shake the epithelial cells up and down, as indicated in Figure 1. It is f that we hear, and it covers a wide audible frequency range. For an airway, wheezing also can signify local flow limitation (10).
Figure 1.
Wheezing as flow-induced oscillations of an airway. Air flows at critical velocity, U, the walls and cells oscillate at frequency, f, for wavelength, λ, creating a traveling wave at speed c = f × λ.
Can these oscillations of the epithelial cells injure them? In a study aimed at snoring and sleep apnea, cultured human bronchial epithelial cells were vibrated at 60 Hz to assess their response (11). The vibratory stimulus triggered an inflammatory cascade, as measured by an increase in IL-8 (interleukin-8) release. In a following study, vibrations were applied to the upper airway of an in vivo rat model in a snoring pattern: 1 second of vibration followed by 3 seconds of no vibration (12). Examining the soft palate tissue, it was shown that vibration significantly increased the gene expression of the proinflammatory cytokine TNF-α (tumor necrosis factor-α) and neutrophil attractant chemokine MIP-2 (macrophage inflammatory protein-2), which is a rodent equivalent of human IL-8. So the mechanical stimulus of vibration triggers an early proinflammatory process in respiratory epithelium. For an obstructed airway, vibratory stimulation of epithelial cells already inflamed can contribute to a vicious cycle, because increased inflammation exacerbates bronchoconstriction, provoking more wheezing.
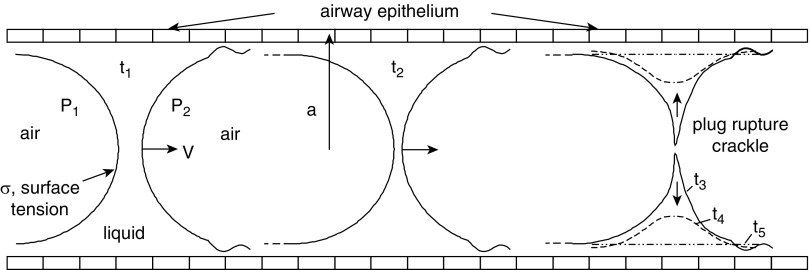
Turning to inspiration, airway-related crackles are considered the rupturing of liquid plugs, or menisci, in small airways as they “pop open.” The sound mechanism is very similar to drinking through a straw when you get down to the last sips at the bottom of the cup. The gurgling is a mixture of liquid and air with popping bubbles and rupturing plugs. They can occur with excess airway liquid, like that found in pulmonary edema or pneumonia, and in mechanical ventilation become more prevalent with low end-expiratory volumes, or low regional volumes, in a process of small airway closure and reopening. Figure 2 shows a typical sequence at five times. At time t1, inspiration starts the liquid plug propagating to the right at speed V in a tube of radius a. It is pushed because of the air pressure difference, P1 − P2. Surface tension, σ, is present at the air–liquid interfaces. At time t2, the plug has traveled farther downstream but is thinner because it is losing volume to the airway surface liquid lining the wall. At time t3 into the inspiration, the thinning has finally caused the plug to rupture. Rupture is an explosive event, which equalizes the air pressure, while the surface tension snaps liquid back onto the cells. Both of those actions contribute to the acoustical signature of a crackle. The rupture sequence t3-t4-t5 is a time span measured in milliseconds. An additional feature would be wall flexibility, so that the radius of the tube suddenly increases at rupture (i.e., pops open).
Figure 2.
Liquid plug propagation and rupture causing crackle sounds. The plug travels at speed V in a tube of radius a, because of the air pressure difference P1 − P2. Inspiration starts at time t1; the plug thins at t2 and ruptures at t3 to t5, reopening the airway. The surface tension is σ.
Can crackles cause disease? In a microfluidic system mimicking a small airway, similar to Figure 2, liquid plugs were forced to flow over cultured human airway epithelial cells (13). The cells in general were damaged by plug propagation, t1 to t3. The rupture event, however, registered acoustically as a crackle, and the underlying cells had a very high death rate. During the rupture sequence, t3 to t5, the snapped flow creates very high local stresses calculated from computational fluid dynamics simulations (14). The ratio σ/a scales the stress levels, so higher surface tension and smaller airways promote larger potential for damage. Surface tension injuries have been shown in ex vivo rat lungs that received a 3-ml instillation of normal saline, partially filling the lung. It was followed by air cycling, an arrangement of mixed air and liquid that creates many liquid plugs (15). Injury was evaluated by measuring cell membrane defects. For comparison, normal air-filled lungs and completely saline-filled lungs, which are free of air, were also studied. By far the greatest damage was in the partially filled saline group, where surface tension is so prevalent, leading to the conclusion that surface tension forces are responsible for the injury.
An additional study was done for in vivo, open-chest rabbits undergoing low end-expiratory volume ventilation using zero end-expiratory pressure (16). Under these circumstances, airways close near end-expiration and then reopen during inspiration. Histologic indexes of bronchiolar injury were present, but not in an additional group that received a tracheally instilled surfactant. Airway reopening is accompanied by crackles (17), and here is why. Because the airways are liquid lined, closure is ultimately due to a liquid plug formation, made easier for decreasing airway radius or collapse during expiration. Then reopening expands the airway but also propagates the liquid plug, as shown in Figure 2, leading to rupture. Surfactants reduce surface tension, so that the stress scale σ/a is reduced. Consequently, they are protective for this kind of reopening injury. As a follow-up to the surfactant-free studies of Huh and colleagues (13), plug propagation and rupture with surfactants showed protection of the underlying cells (18). These surface tension injuries promote an inflammatory response in small airways, which could also exacerbate the development of acute respiratory distress syndrome. Often crackles occur in sequentially related groups called an “avalanche” (19), which can potentially damage a distributed pathway in the lung.
Now, is any of this clinically relevant? A first good response to that important question might be: “Is anyone looking?” Pulmonary acoustics are inherently linked to lung mechanical perturbations. Lung air is confluent with room air, which lends to the sounds being an indicator of mechanical events. As an extreme example, the loudest sound a lung generates is a severe cough, which can cause a pneumothorax (i.e., tissue failure) (20). Cough sends a large stress wave through the lung on a global scale, which can cause shear injury to tissue, including the pleura. The sound we hear correlates to this very large mechanical perturbation. At a smaller stress scale, crackles and wheezes are part of this continuum. The literature shows that mechanical stresses caused by perturbations found in crackles and wheezes certainly can injure cells, damage their membranes, promote inflammatory responses, and cause cell death.
If there are clinical injuries from crackles and wheezes, for example, how would those be measured? To what degree is their effect, if any? Do they simply make bad conditions more challenging to treat, like asthmatic wheezing? Can they create injury in an otherwise healthy small airway, like crackles from pulmonary edema due to congestive heart failure? Mechanically, those two situations are not very different from the experiments described above. In general, what may be the implications of hearing crackles and wheezes, knowing they are not only a sign of underlying pathology but also a cause of inflammation and injury? Could that insight drive a more aggressive treatment approach to stop the sounds? If there is injury from congestive heart failure–related crackles, would they someday be treated with diuretics and antiinflammatory agents? Is there a role for exogenous surfactants?
I think a hopeful answer to the question “Is anyone looking?” might be “No, not yet!” Investigative journeys, even paradigm shifts, sometimes need a starting point, often coming from a different perspective. My laboratory has focused for more than 35 years on the intersection of pulmonary function with fundamental biofluid mechanics in experimental and computational formats. Perhaps this note will stimulate a productive conversation. Dr. Grotberg supported by NIH HL136141
Supplementary Material
Footnotes
Supported by National Institutes of Health HL 136141.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Laennec RTH. De l’auscultation médiate ou traité du diagnostic des maladies des poumon et du coeur, 1st ed. Paris: Brosson & Chaudé; 1819. [Google Scholar]
- 2.Roguin A. Rene Theophile Hyacinthe Laënnec (1781-1826): the man behind the stethoscope. Clin Med Res. 2006;4:230–235. doi: 10.3121/cmr.4.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global strategy on human resources for health: Workforce 2030. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 4.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol. 2000;119:1–17. doi: 10.1016/s0034-5687(99)00092-4. [DOI] [PubMed] [Google Scholar]
- 8.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 9.Grotberg JB, Gavriely N. Flutter in collapsible tubes: a theoretical model of wheezes. J Appl Physiol (1985) 1989;66:2262–2273. doi: 10.1152/jappl.1989.66.5.2262. [DOI] [PubMed] [Google Scholar]
- 10.Gavriely N, Palti Y, Alroy G, Grotberg JB. Measurement and theory of wheezing breath sounds. J Appl Physiol. 1984;57:481–492. doi: 10.1152/jappl.1984.57.2.481. [DOI] [PubMed] [Google Scholar]
- 11.Puig F, Rico F, Almendros I, Montserrat JM, Navajas D, Farre R. Vibration enhances interleukin-8 release in a cell model of snoring-induced airway inflammation. Sleep. 2005;28:1312–1316. doi: 10.1093/sleep/28.10.1312. [DOI] [PubMed] [Google Scholar]
- 12.Almendros I, Acerbi I, Puig F, Montserrat JM, Navajas D, Farré R. Upper-airway inflammation triggered by vibration in a rat model of snoring. Sleep. 2007;30:225–227. doi: 10.1093/sleep/30.2.225. [DOI] [PubMed] [Google Scholar]
- 13.Huh D, Fujioka H, Tung YC, Futai N, Paine R, III, Grotberg JB, et al. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci USA. 2007;104:18886–18891. doi: 10.1073/pnas.0610868104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan EA, Uzgoren E, Fujioka H, Grotberg JB, Shyy W. Adaptive Lagrangian-Eulerian computation of propagation and rupture of a liquid plug in a tube. Int J Numer Methods Fluids. 2011;67:1373–1392. [Google Scholar]
- 15.Hussein O, Walters B, Stroetz R, Valencia P, McCall D, Hubmayr RD. Biophysical determinants of alveolar epithelial plasma membrane wounding associated with mechanical ventilation. Am J Physiol Lung Cell Mol Physiol. 2013;305:L478–L484. doi: 10.1152/ajplung.00437.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Angelo E, Pecchiari M, Gentile G. Dependence of lung injury on surface tension during low-volume ventilation in normal open-chest rabbits. J Appl Physiol (1985) 2007;102:174–182. doi: 10.1152/japplphysiol.00405.2006. [DOI] [PubMed] [Google Scholar]
- 17.Munakata M, Homma Y, Matsuzaki M, Ogasawara H, Tanimura K, Kusaka H, et al. Production mechanism of crackles in excised normal canine lungs. J Appl Physiol (1985) 1986;61:1120–1125. doi: 10.1152/jappl.1986.61.3.1120. [DOI] [PubMed] [Google Scholar]
- 18.Tavana H, Zamankhan P, Christensen PJ, Grotberg JB, Takayama S. Epithelium damage and protection during reopening of occluded airways in a physiologic microfluidic pulmonary airway model. Biomed Microdevices. 2011;13:731–742. doi: 10.1007/s10544-011-9543-5. [DOI] [PubMed] [Google Scholar]
- 19.Suki B, Barabási AL, Hantos Z, Peták F, Stanley HE. Avalanches and power-law behaviour in lung inflation. Nature. 1994;368:615–618. doi: 10.1038/368615a0. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Nugent WC. Cough-induced bilateral spontaneous pneumothorax. Ann Thorac Surg. 2010;90:1363–1365. doi: 10.1016/j.athoracsur.2010.04.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.