Abstract
Background:
Thyroid cancer incidence is the most rapidly increasing malignancy; rates are three times higher in women than men. Thyroid-hormone disrupting flame-retardant chemicals, including polybrominated diphenyl ethers (PBDE) and polybrominated biphenyls (PBB), may contribute to this trend.
Methods:
We investigated the relationship between PBDE/PBB exposure and papillary thyroid cancer (PTC) in 250 incident female papillary thyroid cancer cases and 250 female controls frequency-matched on age. Interviews and post-diagnostic serum samples were collected from 2010–2013. Serum samples were analyzed for 11 congeners. We calculated odds ratios (OR) and 95% confidence intervals (95%CI) using single-pollutant logistic regression models for continuous and categorical lipid-adjusted serum concentrations of PBDE/PBB, adjusted for age, alcohol consumption, and education. We applied three multi-pollutant approaches (standard multi-pollutant regression models, hierarchical Bayesian modeling [HBM], principal components analysis [PCA]) to investigate associations with PBDE/PBB mixtures.
Results:
In single pollutant models, a decreased risk was observed at the highest (>90th percentile) versus lowest (<median) category of BDE-209 concentrations (OR: 0.47; 95%CI: 0.23–0.98); an elevated PTC risk was observed at the highest versus lowest category of BB-153 concentrations (OR: 1.81; 95%CI: 0.96–3.39). In standard multi-pollutant models, an interquartile range increase in BDE-100 concentrations was associated with increased PTC risk (OR: 1.18; 95%CI: 1.01–1.38). HBM and PCA yielded no statistically significant results.
Conclusions:
Our results using single and multi-pollutant modeling do not generally support a positive association with PBDE/PBB and PTC risk.
Impact:
Prospective studies with more advanced statistical approaches to analyze mixtures and populations with higher exposures could reveal new insights.
Introduction
Thyroid cancer rates have been increasing rapidly worldwide for the past decades; in the United States (US), age-adjusted thyroid cancer incidence rates have increased three-fold from 4.8 cases/100,000 in 1975 to 15.0/100,000 in 2015 such that approximately 1.2% of adults will be diagnosed with thyroid cancer in their lifetime (1, 2). Incidence rates in women are three times those in men (3, 4). Papillary thyroid cancer (PTC) is the most common subtype, comprising approximately 80% of new cases (5).
The increasing trend can be partially explained by improved diagnostic imaging methods, such as ultrasounds, positron emission tomography, and computerized tomography, which more accurately detect small thyroid nodules for early medical attention (6). However, this is unlikely to be the sole driver, as incidence rates are also increasing for more easily detectable larger tumors and among younger individuals less likely to be targeted for screening (7, 8). Some risk factors for thyroid cancer have been identified, such as ionizing radiation exposure, family history, reduced or excess iodine consumption, and obesity (6, 9); however, the etiology generally remains poorly understood. Exposures to thyroid hormone-disrupting environmental chemicals have been suggested as another potential risk factor (10, 11).
Polybrominated diphenyl ethers (PBDE) are flame retardants used in commercial and household products such as plastics, electronics, foam furniture padding, and upholstery (12, 13). PBDE are not chemically bound to their products and therefore can leach into the environment, partitioning into dust particles and other media, where they can accumulate overtime (13–15). Human exposures to these compounds increased 1–2 orders of magnitude from the mid-1970s to mid-2000s (14, 16). Studies had initially reported subsequent declines in serum concentrations of several PBDE in the US and other nations due to manufacturing restrictions resulting from concern about widespread exposure, persistence, and potential toxicities (17, 18); however, recent publications indicate that serum concentrations of several congeners have plateaued or increased from 2011–2015 (19, 20), indicating continued exposure. Further, these compounds remain ubiquitous in currently available consumer products, the environment, the food supply, and the human body due to their stability and lipophilicity (21–23). Exposures may also remain elevated in certain developing countries, where regulations pertaining to production and use are less stringent (24). Polybrominated biphenyls (PBB), structurally similar flame retardants, were added to plastics used in a variety of household products in the US until 1976 (25). Decades later, PBB exposure remains common throughout the United States (26).
Several mechanisms of thyroid carcinogenicity have been proposed. PBDE have been demonstrated to disrupt thyroid hormone homeostasis in animals and humans (27). Because they are structurally analogous to thyroid hormones triiodothyronine (T3) and thyroxine (T4), PBDE and their hydroxylated metabolites may competitively bind with thyroid hormone transport proteins, resulting in reduced circulation of thyroid hormones. This has been hypothesized to cause abnormal proliferation in the thyroid, which may result in tumorigenesis (10). Other research has shown that PBDE hydroquinone metabolites are capable of forming DNA adducts (28, 29), possibly leading to mutations or chromosomal aberrations which could result in carcinogenicity. Another possible mechanism is PBDE-mediated upregulation of cytochrome-P450 enzymes, which generate more reactive oxygen species and oxidative stress, which in turn may promote tumor development and progression (30). PBBs have been linked to thyroid hormone disruption via similar mechanisms (30–33).
In toxicology studies, increased thyroid follicular adenomas have been observed in rats exposed to commercial flame retardant mixtures including deca-BDE (consisting primarily of the congener BDE-209) (34) and penta-BDE (consisting primarily of BDE-99, BDE-47, BDE-100, BDE-153, and BDE-154) (30). PBB have been linked to carcinogenic effects in male and female rats and mice, particularly hepatocellular carcinomas (33, 35).
There is a paucity of epidemiological evidence on the relationship between PBDE exposure and risk of thyroid cancer, with only two published studies on this topic, to our knowledge. A nested case-control study carried out in multiple locations in the US found no significant association between serum levels of four PBDE congeners (BDE-47, BDE-99, BDE-100, and BDE-153) and increased risk of PTC (36). As noted by the authors, this analysis had a few important limitations, including relatively low case numbers (104 cases, 208 controls), a population generally older than peak age of diagnosis (median age of 62 yr), and few congeners with sufficient detection frequencies for analysis. A case-control study in North Carolina that measured PBDE in both serum and house dust (37) observed statistically significant higher odds of PTC among those with house dust BDE-209 concentrations greater than the median compared to below, particularly for smaller tumors (<2 cm). No associations were observed for serum concentrations of the two congeners with sufficient detection frequency for analysis (BDE-47 and BDE-153). This study also had small case numbers (70 cases, 70 controls). Though the authors applied a principal components analysis to examine effects of joint exposures, this was constrained by the small sample size and did not reveal any new information. To our knowledge, no epidemiologic studies of PBB exposure and thyroid cancer have been conducted.
The objective of the current case-control study is to examine the association of PBDE/PBB serum levels and risk of papillary thyroid cancer in Connecticut women with a larger number of cases and numerous congeners using single-pollutant and multi-pollutant approaches. This represents the largest study to date and the first study to present results for the relationship between individual and joint exposures to several PBDE and thyroid cancer.
Methods
Study Population
This analysis was conducted within a previously described population-based case-control study of thyroid cancer in Connecticut (38, 39). Briefly, eligible cases were those aged 21 to 84 years at diagnosis with no previous cancer diagnosis except non-melanoma skin cancer. Histologically confirmed (papillary, follicular, medullary, and anaplastic) incident thyroid cancer cases diagnosed between 2010 and 2011 were identified through the Yale Cancer Center’s Rapid Case Ascertainment Shared Resource, part of Connecticut Tumor Registry. A total of 462 cases participated (65.9% participation rate). Controls were Connecticut residents identified through random digit dialing (61.5% participation rate) and frequency-matched to cases by age (+/− 5 yr). We necessarily conducted our analysis on a subset of the parent study population due to resource constraints. We focused on female Caucasians, as they were more likely to be cases compared to individuals of differing demographics. In the parent study, cases were 81% female and 90% White (39). Therefore, we conducted the current analysis in 250 randomly selected female Caucasian cases of papillary thyroid cancer and 250 female Caucasian controls.
Collection of personal data, covariates, and potential confounders
Procedures were performed in accordance with protocols approved by the Human Investigation Committees at Yale University and Connecticut Department of Public Health; the Centers for Disease Control and Prevention (CDC) determined that the agency was not engaged in human subjects’ research. All participants provided informed written consent. In-person interviews were conducted in participant homes by trained interviewers using a standardized, structured questionnaire including questions about demographic characteristics, radiation exposure, smoking and alcohol use, medical history, lifetime occupational history, and lifetime residential history.
Serum collection and analysis
Blood samples were collected by a trained phlebotomist at the in-person interviews. Most cases had their serum sampled within 6 months of diagnosis (median: 174 days, interquartile range [IQR]): 121, 238 days). After separation from whole blood, serum samples were aliquoted and stored at –20°C until shipment to the CDC (Atlanta, Georgia) for analysis. Samples were analyzed for ten PBDE congeners and one PBB congener: BDE-17, BDE-28, BDE-47, BDE-85, BDE-99, BDE-100, BDE-153, BDE-154, BDE-183, BDE-209, BB-153, using gas chromatography isotope dilution high resolution mass spectrometry (GC-IDHRMS) employing a DFS instrument (Thermo DFS, Fremen, Germany); the analytical method has been described in detail (40, 41). Total serum lipid concentrations were measured to normalize the concentrations of PBDE in serum, quantified using commercially available enzymatic methods (Roche Diagnostics Corp; Indianapolis, IN) for total triglycerides and total cholesterol on a Hitachi 912 Chemistry Analyzer (Hitachi; Tokyo, Japan). All concentration data were reported as ng/g lipid weight and were background-corrected by subtracting the average concentration in blank samples (42). Three blanks and three quality control/quality assurance samples prepared internally by the laboratory were included in every set of 30 samples; additionally, 25 laboratory-blind quality control (QC) samples were included across the batches. Laboratory personnel were blinded to case-control status. The coefficient of variation (CV) from laboratory-blind QCs (n=25) ranged from 2.76% (BDE-99) to 7.23% (BDE-85), indicating a high level of reproducibility.
Statistical Methods
Seven congeners (BDE-28, 47, 99, 100, 153, 209, BB-153) were measured in >80% of samples; concentrations were right-skewed. For these compounds, we used a single imputation method to assign a value to samples below the method detection limit (DL) using a maximum likelihood procedure that assumed a lognormal distribution defined by the distribution of measurements above the DL (43); no covariates were used in the imputation process. The use of a single imputation generally yields unbiased risk estimates and accurate measures of variance when the percent missing is ≤30% (43). In our analyses of these highly detected compounds, we examined both the continuous PBDE concentrations and categories of PBDE concentrations based on distributions among controls. All continuous PBDE concentrations were standardized for regression analyses by subtracting the mean and dividing by the standard deviation to reduce the influence of outliers and to improve computational stability during model fitting. This achieves the same benefits as the more commonly used natural log transformation while retaining interpretability on the arithmetic scale. To address the low variability in PBDE/PBB serum concentrations across the population, we assigned categories corresponding to ≤ median, >median and ≤90th percentile, and >90th percentile.
Three congeners (BDE-85, 154, 183) had detection frequencies of approximately 30% and were therefore modeled as detected versus non-detect; continuous concentrations were not modeled. PBDE-17 was detected in only 3.6% of the samples and therefore was excluded from statistical analyses.
In traditional, single-pollutant models, odds ratios (OR) and 95% confidence intervals (95% CIs) were calculated using logistic regression in separate models for each congener. We considered the following variables as potential confounders of the association of serum PBDE levels and risk of PTC based on a review of the literature: frequency of dental x-ray exposure (never to more than once/year), diagnostic x-ray exposure (ever have diagnostic x-rays such as chest x-rays or mammograms), tobacco use (ever smoked ≥100 cigarettes), alcohol use (ever consumed ≥12 servings of alcoholic beverages), family history of cancer among first-degree relatives, educational attainment, family income per capita, age, and body mass index (BMI). All potential confounders were included in the logistic regression models for each congener and removed via backward elimination if their removal yielded a ≥10% change in the OR. Years of education, age, and alcohol consumption met inclusion criteria in most models and therefore we included them in all final models. We conducted stratified analyses to examine whether there was any difference in relationships between PBDE exposure and PTC based on tumor size using two size cut-points: tumors with diameter ≤1 cm [microcarcinoma] and >1 cm and tumors ≤2 cm and > 2 cm. Papillary microcarcinoma are generally considered of lower clinical significance, because they often remain indolent and have a positive prognosis (44). However, some microcarcinoma have been reported to have aggressive and metastatic behavior (45). A cut-point of 2 cm was also examined because of its application in the assessment of clinical or pathologic stage.
We applied three multipollutant methods to examine the association between concurrent exposures to multiple PBDE/PBB and risk of PTC. First, we used a multiple logistic regression to jointly analyze the impact of all 10 congeners within a single model. This is preferable to the common practice of summing all congeners, because the sum is often correlated with the pollutant present at the highest concentration and signals may be masked if effects of individual chemical trend in different directions, as commonly occurs with endocrine disrupting chemicals (46). However, a multiple logistic regression model with all 10 congeners may be unstable due to high correlations between certain congeners (e.g., rSpearman=0.95 between PBDE 47 and PBDE 99; Supplemental Table 1), leading to inflated standard errors and potentially misleading risk parameter estimates.
Second, we applied a hierarchical Bayesian statistical approach similar to method “P2” presented in (47). In this method, the regression parameters corresponding to the different congeners are assumed to follow a normal distribution, centered at zero, with a common variance parameter (to be estimated). By incorporating this prior distribution structure, we carried out data-driven shrinkage of the individual risk parameters towards zero, thereby leading to more stable parameter estimates and statistical inference that may be less impacted by the high correlations between exposures. Full details are presented in the Supplemental Material.
Finally, we applied a principal component analysis (PCA) on the raw PBDE concentrations paired with a logistic regression analysis to investigate possible interactive effects between multiple chemicals. Using the factor loadings, we created new exposure metrics that represent linear combinations of individual congeners. Based on the Kaiser rule (i.e., only keeping the principal components (PCs) with eigenvalues of at least one (48), we identified the most important PCs and investigated their association with cancer risk using a multiple logistic regression analysis.
As a sensitivity analysis, we refit each of these multipollutant methods to the subset of cases with and without microcarcinoma separately to determine the impact of tumor size on associations with exposure. Single-pollutant modeling was done with SAS (Version 8.4, SAS Institute Inc., Cary, NC, US) and multi-pollutant methods were applied within the R statistical software package (R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/). All multipollutant models were adjusted for the same covariates as the single-pollutant models.
Results
Compared to controls, cases tended to be younger, less educated, have higher BMI, be less likely to consume alcohol, and be more likely to have a family history of thyroid cancer (p≤0.1) (Table 1). Cases and controls were similar with respect to income, smoking status, receipt of dental x-rays, and diagnostic medical radiation.
Table 1.
Distribution of selected characteristics of the papillary thyroid cancer cases and controls.
| Cases (n=250) | Controls (n=250) | p-valuea | |
|---|---|---|---|
| Age | 0.10 | ||
| <40 | 51 | 34 | |
| 40–49 | 69 | 76 | |
| 50–59 | 78 | 72 | |
| 60–69 | 40 | 45 | |
| ≥70 | 12 | 23 | |
| Years of Education | 0.02 | ||
| High school or less | 72 | 41 | |
| Technical school | 14 | 14 | |
| College | 99 | 123 | |
| Graduate/Professional school | 54 | 66 | |
| Other | 9 | 5 | |
| Poverty Level | 0.14 | ||
| Below Poverty Level | 10 | 2 | |
| Above poverty level | 173 | 171 | |
| Unknown | 69 | 75 | |
| Family Income | 0.65 | ||
| <24,999 | 17 | 20 | |
| 25,000–49,999 | 29 | 23 | |
| 50,000–89,999 | 45 | 37 | |
| >90,000 | 90 | 95 | |
| Refused | 66 | 74 | |
| Missing | 3 | 1 | |
| BMI | 0.06 | ||
| <25 | 90 | 111 | |
| 25–29.99 | 75 | 76 | |
| ≥ 30 | 85 | 63 | |
| Family History of Cancer | 0.05 | ||
| No | 76 | 77 | |
| Thyroid Cancer | 42 | 24 | |
| Other Cancer | 132 | 149 | |
| Thyroid Disease | <0.0001 | ||
| Yes | 38 | 4 | |
| No | 212 | 246 | |
| Smoking | 0.34 | ||
| Yes | 75 | 85 | |
| No | 175 | 165 | |
| Alcohol Consumption | 0.001 | ||
| Yes | 95 | 127 | |
| No | 155 | 123 | |
|
Dental X-Rays |
0.37 | ||
| Never | 9 | 5 | |
| Less than every few years | 90 | 105 | |
| Every few years | 35 | 41 | |
| Once a year | 97 | 89 | |
| More than once a year | 14 | 8 | |
| Unknown | 5 | 2 | |
| Prior Diagnostic Medical Radiation Exposure | 0.35 | ||
| Yes | 233 | 233 | |
| No | 17 | 15 | |
| Unknown | 0 | 2 | |
| Tumor Diameter | - | ||
| ≤ 1 cm | 140 | - | |
| >1cm | 110 | - |
P-value corresponds to χ2 test.
The distributions of the lipid-adjusted concentrations of the different congeners in serum for cases and controls are presented in Table 2. BDE-47 was present at the highest concentrations, with a median (IQR) lipid-adjusted concentration among controls of 7.33 ng/g lipid (4.10–15.3), followed by BDE-153 (3.08 ng/g lipid [2.02–5.86]), BDE-209 (1.73 ng/g lipid [1.28–2.67]), BB-153 1.49 ng/g lipid ([1.01–2.45]), BDE-100 ng/g lipid (1.48 [0.81–2.94]), and BDE-99 (1.23 ng/g lipid [0.72–2.76]). Median concentrations of BDE-17, BDE-28, BDE-85, and BDE-183 were all <1 ng/g lipid. Concentrations in cases were similar or lower than in controls. Comparison of the 25th and 75th percentiles indicates that the variability in congener exposures was relatively small.
Table 2.
Distributions of serum concentrations of PBDE flame retardants in female papillary thyroid cancer cases and controls.
| Serum Concentrations (ng/g lipd) | |||||||
|---|---|---|---|---|---|---|---|
| Chemical name (abbreviation) | Estimated Half-Life (yr)a | Median Limit of Detection (LOD) | % > LOD | Cases (n=250) | Controls (n=250) | ||
| Median | 25th–75th percentile | Median | 25th–75th percentile | ||||
| 2,2’,4-Tribromodiphenyl ether (BDE-17) | NI | 0.17 | 4 | <LODb | <LODb | <LODb | <LODb |
| 2,4,4’-Tribromodiphenyl ether (BDE-28) | 3.0 | 0.20 | 82 | 0.44 | 0.22 – 0.73 | 0.51 | 0.26 – 1.0 |
| 2,2’,4,4’-Tetrabromodiphenyl ether (BDE-47) | 1.4–3.0 | 0.39 | 99 | 6.36 | 3.45 – 10.94 | 7.28 | 4.04 – 15.33 |
| 2,2’,3,4,4’-Pentabromodiphenyl ether (BDE-85) | NI | 0.18 | 33 | <LODa | <LODa | <LODa | <LODa |
| 2,2’,4,4’,5-Pentabromodiphenyl ether (BDE-99) | 5.4 | 0.22 | 95 | 1.04 | 0.54 – 1.74 | 1.21 | 0.66 – 2.74 |
| 2,2’,4,4’,6-Pentabromodiphenyl ether (BDE-100) | 1.8–2.9 | 0.17 | 98 | 1.27 | 0.69 – 2.33 | 1.47 | 0.78 – 2.86 |
| 2,2’,4,4’,5,5’-Hexabromodiphenyl ether (BDE-153) | 7.4–11.7 | 0.17 | 100 | 3.04 | 1.77 – 6.01 | 3.08 | 2.02 – 5.86 |
| 2,2’,4,4’,5,6’-Hexabromodiphenyl ether (BDE-154) | 5.8 | 0.16 | 37 | <LODa | <LODa | <LODa | <LODa |
| 2,2’,3,4,4’,5’,6-Heptabromodiphenyl ether (BDE-183) | 0.26–0.30 | 0.16 | 28 | <LODa | <LODa | <LODa | <LODa |
| Decabromodiphenyl ether (BDE-209) | 0.04–0.07 | 0.83 | 84 | 1.47 | 1.04 – 2.14 | 1.55 | 1.00 – 2.41 |
| 2,2’,4,4’,5,5’-Hexabromobiphenyl (BB-153) | 13–29 | 0.17 | 97 | 1.40 | 0.82 – 2.33 | 1.45 | 0.94 – 2.44 |
Half-lives compiled from Geyer et al. 2004 (50), McDonald et al. 2005 (62), Thuresson et al. 2006 (51), Blanck et al. 2000 (63). NI indicates a half-life was not identified for that congener in the literature.
Median and quartile limits not calculated because <60% detection frequency for this compound.
Spearman correlation coefficients (rSpearman) between PBDE concentrations ranged from −0.09 to 0.95 with a median of 0.20 (Supplemental Table 1). Stronger correlations were observed between congeners with similar degrees of bromination or PBDE present in the same commercial products. For example, the following six pairs of congeners had Spearman correlation coefficients >0.8: BDE-28 and BDE-47 (rSpearman =0.92), BDE-28 and BDE-99 (rSpearman =0.83), BDE-28 and BDE-100 (rSpearman =0.85), BDE-47 and BDE-99 (rSpearman =0.95), BDE-47 and BDE-100 (rSpearman =0.92), BDE-99 and BDE-100 (rSpearman =0.88). The commercial mixture penta-PBDE is comprised primarily of BDE-47 and BDE-99.
In adjusted single-pollutant models, BB-153 had an elevated but non-statistically significant association with PTC risk when comparing the highest exposure category (>90th percentile) to the reference (≤median) (OR: 1.81, 95%CI: 0.96–3.39) (Table 3). A decreased risk was observed at the highest category of BDE-209 exposure (>90th percentile) compared to the reference (≤median) (OR: 0.47, 95%CI: 0.23–0.98). No other statistically significant associations were observed. Results stratified by tumor sizes demonstrated statistically significantly inverse associations with risk of microcarcinomas for six PBDE congeners (generally when comparing the highest exposure category to the reference), while effect sizes were near one for the larger tumor sizes (Table 4). Results stratified by tumor sizes using the 2-cm cut-point were similar to those for the microcarcinoma, yielding no statistically significant associations for the larger tumors and some statistically significant inverse associations for the smaller tumors (Supplemental Table 2). However, some of the effect estimates for tumors >2 cm were elevated compared to those for tumors >1 cm.
Table 3.
Associations between PBDE serum concentrations and risk of papillary thyroid cancer in 250 cases and 250 controls.
| PBDE Congener (ng/g)a | Controls | Cases | Unadjusted OR (95%CI) | Adjusted ORb (95%CI) |
|---|---|---|---|---|
| BDE-28 | ||||
| ≤0.51 | 125 | 147 | 1 | 1 |
| >0.51 to ≤1.65 | 100 | 78 | 0.66 (0.45–0.97) | 0.67 (0.45–1.00) |
| >1.65 | 25 | 25 | 0.85 (0.47–1.56) | 0.87 (0.46–1.65) |
| Continuous | 250 | 250 | 0.96 (0.81–1.15) | 0.94 (0.78–1.13) |
| BDE-47 | ||||
| ≤7.28 | 125 | 139 | 1 | 1 |
| >7.28 to ≤24.91 | 100 | 90 | 0.81 (0.56–1.18) | 0.80 (0.54–1.18) |
| >24.91 | 25 | 21 | 0.76 (0.40–1.42) | 0.66 (0.34–1.28) |
| Continuous | 250 | 250 | 0.91 (0.74–1.18) | 0.89 (0.72–1.10) |
| BDE-85 | ||||
| <LOD | 161 | 176 | 1 | 1 |
| ≥LOD | 89 | 74 | 0.76 (0.52–1.11) | 0.71 (0.48–1.05) |
| Continuousc | - | - | - | - |
| BDE-99 | ||||
| ≤1.21 | 125 | 138 | 1 | 1 |
| >1.21 to ≤5.00 | 100 | 94 | 0.85 (0.59–1.23) | 0.83 (0.56–1.21) |
| >5.00 | 25 | 18 | 0.65 (0.34–1.25) | 0.57 (0.29–1.12) |
| Continuous | 250 | 250 | 0.93 (0.76–1.14) | 0.91 (0.74–1.12) |
| BDE-100 | ||||
| ≤1.47 | 125 | 138 | 1 | 1 |
| >1.47 to ≤5.43 | 100 | 91 | 0.82 (0.57–1.20) | 0.78 (0.53–1.15) |
| >5.43 | 25 | 21 | 0.76 (0.41–1.43) | 0.73 (0.38–1.41) |
| Continuous | 250 | 250 | 1.07 (0.89–1.29) | 1.05 (0.87–1.26) |
| BDE-153 | ||||
| ≤3.08 | 125 | 130 | 1 | 1 |
| >3.08 to ≤14.02 | 100 | 88 | 0.85 (0.58–1.23) | 0.85 (0.58–1.26) |
| >14.02 | 25 | 32 | 1.23 (0.69–2.19) | 1.19 (0.66–2.16) |
| Continuous | 250 | 250 | 1.08 (0.90–1.29) | 1.08 (0.90–1.30) |
| BDE-154 | ||||
| <LOD | 152 | 164 | 1 | 1 |
| ≥LOD | 98 | 86 | 0.81 (0.57–1.17) | 0.78 (0.53–1.13) |
| Continuousc | - | - | - | - |
| BDE-183 | ||||
| <LOD | 176 | 185 | 1 | 1 |
| ≥LOD | 74 | 65 | 0.84 (0.57–1.24) | 0.74 (0.49–1.12) |
| Continuousc | - | - | - | - |
| BDE-209 | ||||
| ≤1.55 | 125 | 136 | 1 | 1 |
| >1.55 to ≤4.08 | 100 | 100 | 0.92 (0.64–1.33) | 0.90 (0.61–1.32) |
| >4.08 | 25 | 14 | 0.52 (0.26–1.03) | 0.47 (0.23–0.98) |
| Continuous | 250 | 250 | 0.90 (0.75–1.09) | 0.87 (0.71–1.06) |
| BB-153 | ||||
| ≤1.45 | 125 | 129 | 1 | 1 |
| >1.45 to ≤3.51 | 100 | 89 | 0.86 (0.59–1.26) | 1.16 (0.76–1.76) |
| > 3.51 | 25 | 32 | 1.24 (0.70–2.21) | 1.81 (0.96–3.39) |
| Continuous | 250 | 250 | 1.05 (0.87–1.27) | 1.15 (0.88–1.52) |
Categories established based on distributions among controls and correspond to either (i) ≤median, >median and ≤90th percentile, and >90th percentile for congeners with detection frequency ≥80% (ii) undetected versus detected samples for congeners with detection frequency <80%.
Adjusted for age, alcohol consumption, and years of education.
Continuous models not run when detection frequency was <80%.
LOD, limit of detection
Table 4.
Associations between PBDE serum concentrations and papillary thyroid cancer risk stratified by tumor size.
| Microcarcinomas < 1 cm (n=138 cases) | Tumor size ≥ 1 cm (n=110 cases) | |||||
|---|---|---|---|---|---|---|
| PBDE Congener (ng/g)a | Controls | Cases | Adjusted ORb (95% CI) | Controls | Cases | Adjusted ORb (95% CI) |
| BDE-28 | ||||||
| ≤0.51 | 125 | 90 | 1 | 125 | 57 | 1 |
| >0.51 to ≤1.65 | 100 | 33 | 0.43 (0.26–0.70) | 100 | 43 | 1.00 (0.61–1.65) |
| >1.65 | 25 | 15 | 0.74 (0.36–1.55) | 25 | 10 | 0.94 (0.40–2.19) |
| Continuous | 250 | 138 | 0.84 (0.65–1.09) | 250 | 110 | 1.00 (0.80–1.25) |
| BDE-47 | ||||||
| ≤7.28 | 125 | 85 | 1 | 125 | 54 | 1 |
| >7.28 to ≤24.91 | 100 | 41 | 0.57 (0.35–0.91) | 100 | 47 | 1.09 (0.66–1.78) |
| >24.91 | 25 | 12 | 0.56 (0.25–1.22) | 25 | 9 | 0.73 (0.31–1.74) |
| Continuous | 250 | 138 | 0.85 (0.64–1.14) | 250 | 110 | 0.91 (0.68–1.22) |
| BDE-85 | ||||||
| <LOD | 161 | 107 | 1 | 161 | 68 | 1 |
| ≥LOD | 89 | 31 | 0.46 (0.28–0.76) | 89 | 42 | 1.08 (0.66–1.75) |
| Continuousc | - | - | - | - | - | - |
| BDE-99 | ||||||
| ≤1.21 | 125 | 84 | 1 | 125 | 53 | 1 |
| >1.21 to ≤5.00 | 100 | 44 | 0.59 (0.37–0.95) | 100 | 49 | 1.09 (0.67–1.78) |
| >5.00 | 25 | 10 | 0.48 (0.21–1.10) | 25 | 8 | 0.69 (0.28–1.69) |
| Continuous | 250 | 138 | 0.94 (0.75–1.16) | 250 | 110 | 0.77 (0.41–1.47) |
| BDE-100 | ||||||
| ≤1.47 | 125 | 86 | 1 | 125 | 52 | 1 |
| >1.47 to ≤5.43 | 100 | 43 | 0.54 (0.34–0.86) | 100 | 46 | 1.09 (0.67–1.80) |
| >5.43 | 25 | 9 | 0.48 (0.21–1.10) | 25 | 12 | 1.14 (0.52–2.51) |
| Continuous | 250 | 138 | 1.04 (0.85–1.27) | 250 | 110 | 1.06 (0.81–1.39) |
| BDE-153 | ||||||
| ≤3.08 | 125 | 77 | 1 | 125 | 52 | 1 |
| >3.08 to ≤14.02 | 100 | 45 | 0.72 (0.45–1.14) | 100 | 42 | 1.01 (0.61–1.68) |
| >14.02 | 25 | 16 | 1.06 (0.52–2.16) | 25 | 16 | 1.31 (0.63–2.74) |
| Continuous | 250 | 138 | 1.06 (0.86–1.30) | 250 | 110 | 1.11 (0.88–1.39) |
| BDE-154 | ||||||
| <LOD | 152 | 98 | 1 | 152 | 65 | 1 |
| ≥LOD | 98 | 40 | 0.57 (0.36–0.91) | 98 | 45 | 1.02 (0.63–1.64) |
| Continuousc | - | - | - | - | - | - |
| BDE-183 | ||||||
| <LOD | 176 | 109 | 1 | 176 | 74 | 1 |
| ≥LOD | 74 | 29 | 0.55 (0.33–0.93) | 74 | 36 | 1.05 (0.63–1.74) |
| Continuousc | - | - | - | - | - | - |
| BDE-209 | ||||||
| ≤1.55 | 125 | 77 | 1 | 125 | 58 | 1 |
| >1.55 to ≤4.08 | 100 | 54 | 0.85 (0.54–1.33) | 100 | 45 | 0.94 (0.58–1.54) |
| >4.08 | 25 | 7 | 0.40 (0.16–0.99) | 25 | 7 | 0.52 (0.20–1.34) |
| Continuous | 250 | 138 | 0.75 (0.55–1.03) | 250 | 110 | 0.94 (0.75–1.18) |
| BB-153 | ||||||
| ≤1.45 | 125 | 66 | 1 | 125 | 63 | 1 |
| >1.45 to ≤3.51 | 100 | 53 | 1.14 (0.70–1.85) | 100 | 35 | 0.95 (0.55–1.63) |
| > 3.51 | 25 | 19 | 1.60 (0.79–3.25) | 25 | 12 | 1.46 (0.65–3.29) |
| Continuous | 250 | 138 | 1.00 (0.63–1.58) | 250 | 110 | 1.19 (0.89–1.59) |
Categories established based on distributions among controls and correspond to either (i) ≤median, >median and ≤90th percentile, and >90th percentile for congeners with detection frequency ≥80% (ii) undetected versus detected samples for congeners with detection frequency <80%.
Adjusted for age, alcohol consumption, and years of education.
Continuous models not run when detection frequency was <80%.
LOD, limit of detection
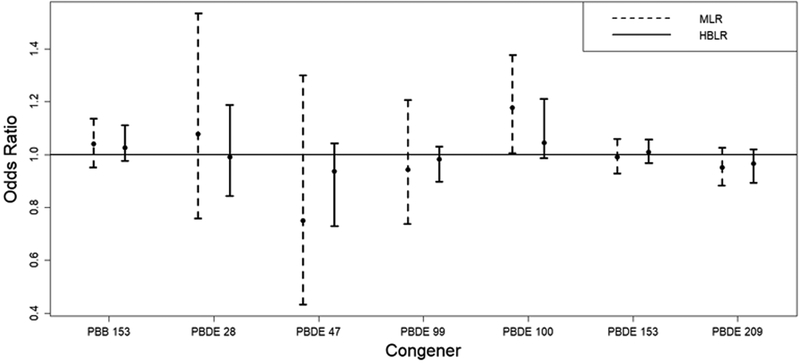
Multiple logistic regression analysis risk parameter estimates (OR scale) and confidence/credible intervals from the standard regression model including all continuously modeled PBDE/PBB and the hierarchical Bayesian method are presented in Figure 1. A statistically significant positive association was observed between BDE-100 and thyroid cancer risk (OR per interquartile range increase in exposure: 1.18; 95% CI: 1.01–1.38; p-value: 0.04). However, this finding is inconsistent with the single-pollutant, the hierarchical Bayesian, and the PCA modeling results.
Figure 1.
Parameter estimates and 95% confidence/credible interval results from the standard multiple logistic regression (MLR) analysis and the hierarchical Bayesian multiple logistic regression (HBLR) analysis. Posterior means and quantile-based credible intervals are displayed for HBLR. The odds ratios represent the increase in odds for an interquartile range increase in exposure.
No statistically significant associations were observed in the Bayesian modeling. The shrinkage that results from the hierarchical Bayesian method can be clearly observed as the credible intervals are much narrower than the corresponding CIs from the standard multipollutant regression analysis and the point estimates are pulled towards zero (Figure 1).
Two principal components (PCs) met the criteria of the Kaiser method (48). The results indicate an elevated OR with increased exposure as defined by PC 2, though not statistically significant (OR: 1.25, 95% CI: 0.94–1.66; p=0.13) (Table 5). The factor loadings in Table 5 indicate that PC 2 represents a mixture component that is positively weighted on BB-153 and BDE-153 and negatively weighted on BDE-209, suggesting that exposure to higher levels of BB-153 and/or BDE-153 while being less exposed to BDE-209 may have an adverse impact on cancer risk. Stratification by tumor size did not yield any statistically significant associations for any of the multi-pollutant methods.
Table 5.
Principal component (PC) analysis factor loadings and regression results. Only factor loadings larger than 0.10 in absolute value are displayed for ease of interpretability.
| Compound | PC 1 | PC 2 |
|---|---|---|
| BB-153 | 0.933 | |
| BDE-28 | 0.432 | |
| BDE-47 | 0.478 | |
| BDE-99 | 0.461 | |
| BDE-100 | 0.471 | |
| BDE-153 | 0.387 | 0.205 |
| BDE-209 | −0.273 | |
| OR (95%CI)a | ||
| 0.98 (0.09–1.08) | 1.25 (0.94–1.66) | |
| P=0.72 | P=0.13 | |
PC, principal component
Adjusted for age, alcohol consumption, and years of education.
Discussion
The results from this case-control study do not provide evidence of an increased risk of thyroid cancer in relation to PBDE exposure. Further, some evidence of inverse associations is reported. This work, representing the largest epidemiologic study population to date and reporting on the greatest number of PBDE congeners, is consistent with the null results from the only two published studies on this topic which used serum biomarkers for the exposure assessment. However, the inverse findings for BDE-209 contrast with a positive association between house dust BDE-209 concentrations and PTC and reported by Hoffmann et al. (2017) (37). We do report the first epidemiologic evidence suggesting a positive association between BB-153 exposure and PTC risk.
Detection frequencies were higher for many congeners in our study samples compared to other studies (Supplemental Table 3), because our relatively large quantity of serum available for measurement (median: 2.00 g; 1st −99th percentile: 0.96–2.01 g) resulted in lower analytical detection limits. However, the median and 75th percentiles of serum BDE exposures in our population tended to be lower compared to these other populations (Supplemental Table 3). The relatively low levels and limited contrast of exposures within our study population may have impeded our analyses and contributed to the generally null results. Further inquiry into explanations for the lower exposures in this population of Connecticut women as compared to a nationally representative sample or women living in other states could provide some valuable insights into exposure determinants or exposure mitigation.
The inconsistent relationships with thyroid cancer risk with respect to BDE-209 in serum in our study compared to those in dust reported in Hoffman et al. (2017) could reflect differences in geographic region (Connecticut vs. North Carolina), the study population (women only with mean age of 51 yr vs. men and women with mean age of 45 yr), sample size (250 vs. 70 cases), or the exposure assessment methods (serum biomarker vs. dust) (37). The two exposure assessment methods reflect different sources and exposure scenarios. PBDE biomarkers are a direct measure of dose which aggregate exposure across all pathways and routes PBDE concentrations in indoor dust are a useful surrogate for personal exposure within the home, and house dust is considered the major exposure source in US populations (13). House dust PBDE concentrations are also correlated with other measures of exposure, such as handwipes or biomonitoring (21, 49). However, dust levels do not capture exposures in other microenvironments, such as vehicles or workplaces, and they omit the dietary exposure pathway. The conflicting results with respect to BDE-209 may also reflect its different behaviors compared to other PBDEs. Most PBDE are readily absorbed internally and have long half-lives (on the order of years), and therefore serum PBDE concentrations are a reasonable proxy for longer-term exposures (Table 2) (50, 51). In contrast, BDE-209, the fully brominated, larger, bulky molecule, has limited absorptive capacity and a short half-life. House dust concentrations of PBDE are correlated over time (52, 53), indicating that they provide a reasonable representation of past exposures; however, BDE-209 has been shown to photodegrade and debrominate into other congeners in house dust (54). Therefore, these two exposure assessment methods may be capturing different exposure time windows or may be reflecting some differences related to the kinetics and chemistry of BDE-209. Additional studies measuring BDE-209 in both serum and dust are needed to clarify its relationship with thyroid cancer risk.
We conducted some multi-pollutant modeling to reflect more realistic exposure scenarios, as these congeners co-exist in mixtures and may exhibit synergistic, antagonistic, or other interactions (55). The multi-pollutant models were generally consistent with the single-pollutant models. Both the PCA analysis and the single pollutant model provided evidence for an inverse association with BDE-209 and a positive association between BB-153 exposure and PTC risk. An association between BDE-100 and PTC risk was only observed in the standard multi-pollutant regression model. Though use of BB-153 was discontinued in 1976, our finding is relevant, as people continue to be exposed to BB-153, and in combination with other thyroid hormone-disrupting chemicals (56, 57). Therefore, these results suggest some relationships to explore in future analyses. More advanced approaches to mixtures, such as a Bayesian statistical approach using toxicology data or kernel regression could reveal some new insights.
Several inverse associations between PBDE congeners and PTC microcarcinoma were observed in single-pollutant models but not in the multipollutant models. While chance findings cannot be ruled out, equivocal or inverted associations between structurally similar endocrine disrupting compounds and hormone-related cancers have been observed before, such as with serum concentrations of PCBs and breast cancer (58, 59); effect estimates less than 1 are consistent with the thyroid cancer study using pre-diagnosis serum PBDE concentrations to assign exposure. Finally, a relatively small proportion of microcarcinoma are considered clinically significant, and therefore information on stage could bring more precision to this outcome.
These results should be interpreted in the context of some important limitations. A single sample was used to represent exposure during the relevant window of susceptibility. One-time serum measurements of persistent pollutants are generally considered to be a reasonable proxies for past exposures due to the long biological half-lives of most of these congeners (i.e., 1 to 12 years; Table 2) (51) and the correlation in repeated measures of structurally similar compounds measured within the same women over time (60, 61). However, there are possible variations over time due to changes in exposures, weight loss, and dietary changes (61). The assumption of representativeness of BDE-209 is less robust, given the relatively short half-life in the blood. Another limitation is that the blood sample was collected post-diagnosis. Though biomarker concentrations in retrospective studies could be subject to bias if influenced by the disease state, we consider thyroid cancer unlikely to modify serum PBDE or lipid concentrations if the individual was maintained on thyroid hormones, because the treatment is relatively specific to the thyroid gland (i.e., surgical removal and radioactive iodine treatment). Additionally, the few positive/elevated relationships observed could have been due to chance or multiple comparisons. Another limitation is that the results may only be generalizable to Caucasian females. We also did not have information on tumor stage, which could indicate the aggressiveness of the tumor, and we lacked data on genetics, which could be important in relation to genetic polymorphisms that influence activity of enzymes that metabolize endogenous hormones or detoxify PBDEs (10)).
In summary, this study overcame some methodological limitations of prior studies by having a larger population, higher detection frequencies across a range of congeners including BDE-209, and accounting for mixtures. Limitations included a single post-diagnostic serum sample to assess exposure and a population with relatively low PBDE exposure. While some inverse associations were observed in single pollutant models, a few positive associations were also observed in single and multi-pollutant models. Overall, there is limited evidence to date in support of a positive relationship between PBDE exposure and thyroid cancer risk. However, there is also insufficient evidence to conclude no risk from PBDE, and therefore these relationships warrant follow-up, given the biological plausbility derived from their chemical structures, toxicological evidence in human hormone studies, in vitro assays, and animal experiments, and a previously reported positive association for BDE-209 in dust. A prospective study with application of more advanced statistical approaches to analyze mixtures, incorporation of genetic polymorphisms, a population with higher exposure levels and increased exposure variability, and collection of both dust and serum samples could reveal new insights.
Supplementary Material
Acknowledgments:
This research was supported by the American Cancer Society (ACS) grant RSGM-10-038-01-CCE and 127509-MRSG-15-147-01-CNE, and the National Institutes of Health (NIH) grant R01ES020361. Certain data used in this study wereobtained from the Connecticut Tumor Registry located in the Connecticut Department of Public Health. The authors assume full responsibilityfor analyses and interpretation of these data. The cooperation of the Connecticut hospitals, including Charlotte Hungerford Hospital, Bridgeport Hospital, Danbury Hospital, Hartford Hospital, Middlesex Hospital, Hospital of Central Connecticut, Yale/New Haven Hospital, St. Francis Hospital and Medical Center, St. Mary’s Hospital, Hospital of St. Raphael, St. Vincent’s Medical Center, Stamford Hospital, William W. Backus Hospital, Windham Hospital, Eastern Connecticut Health Network, Griffin Hospital, Bristol Hospital, Johnson Memorial Hospital, Greenwich Hospital, Lawrence and Memorial Hospital, New Milford Hospital, Norwalk Hospital, MidState Medical Center, John Dempsey Hospital and Waterbury Hospital, in allowing patient access, is gratefully acknowledged. Rajni Mehta from the Yale Comprehensive Cancer Center’s RCA provided great help in both IRB approvals and field implementation of the study. Helen Sayward, Anna Florczak, and Renee Capasso from Yale School of Public Health did an exceptional work in study subject recruitment.
Abbreviations:
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CV
coefficient of variation
- DL
detection limit
- IQR
interquartile range
- PBB
polybrominated biphenyls
- PBDE
polybrominated diphenyl ethers
- PC
principal components
- PCA
principal component analysis
- PTC
papillary thyroid cancer
- QC
quality control
- T3
triiodothyronine
- T4
thyroxine
- US
United States
Footnotes
Conflict of interest:
The authors declare no conflicts of interest.
DISCLAMER: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
References
- 1.Howlader NNA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2014. Bethesda, MD: National Cancer Institute; 2017. [Google Scholar]
- 2.Noone AMHN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2015. Bethesda, MD: National Cancer Institute; April 2018. [Google Scholar]
- 3.Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA(eds). SEER Cancer Statistics Review, 1975–2011, Bethesda, MD: 2014. [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians 2019, 69, (1), 7–34. [DOI] [PubMed] [Google Scholar]
- 5.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017;317:1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12:646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr. 2014;164:1481–5. [DOI] [PubMed] [Google Scholar]
- 8.Cho YY, Jang HW, Joung JY, Park SM, Jeong DJ, Kim SW, et al. Trends in Thyroid Cancer Incidence in Korean Children (1999–2012) Based on Palpation and Nonpalpation Detection Methods. Eur Thyroid J. 2015;4:252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res. 2015;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Guo GL, Han X, Zhu C, Kilfoy BA, Zhu Y, et al. Do Polybrominated Diphenyl Ethers (PBDEs) Increase the Risk of Thyroid Cancer? Bioscience hypotheses. 2008;1:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman K, Sosa JA, Stapleton HM. Do flame retardant chemicals increase the risk for thyroid dysregulation and cancer? Curr Opin Oncol. 2017;29:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs--a review of levels and sources. Int J Hyg Environ Health 2009;212:109–34. [DOI] [PubMed] [Google Scholar]
- 13.Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere. 2009;76:542–8. [DOI] [PubMed] [Google Scholar]
- 14.Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005;47:199–211. [DOI] [PubMed] [Google Scholar]
- 15.Harrad S, Ibarra C, Robson M, Melymuk L, Zhang X, Diamond M, et al. Polychlorinated biphenyls in domestic dust from Canada, New Zealand, United Kingdom and United States: implications for human exposure. Chemosphere. 2009;76:232–8. [DOI] [PubMed] [Google Scholar]
- 16.Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE 3rd, et al. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect. 2004;112:654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W, Holden A, Smith SC, Gephart R, Petreas M, Park JS. PBDE levels in breast milk are decreasing in California. Chemosphere. 2016;150:505–13. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Kang JH, Choi SD, Zhu J, Chang YS. Levels of polybrominated diphenyl ethers in the Korean metropolitan population are declining: A trend from 2001 to 2013. Environ Toxicol Chem. 2018;37:2323–30. [DOI] [PubMed] [Google Scholar]
- 19.Parry E, Zota AR, Park J-S, Woodruff TJ. Polybrominated diphenyl ethers (PBDEs) and hydroxylated PBDE metabolites (OH-PBDEs): A six-year temporal trend in Northern California pregnant women. Chemosphere. 2018;195:777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurley S, Goldberg D, Nelson DO, Guo W, Wang Y, Baek H-G, et al. Temporal Evaluation of Polybrominated Diphenyl Ether (PBDE) Serum Levels in Middle-Aged and Older California Women, 2011–2015. Environ Sci Technol. 2017;51:4697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammel SC, Hoffman K, Lorenzo AM, Chen A, Phillips AL, Butt CM, et al. Associations between flame retardant applications in furniture foam, house dust levels, and residents’ serum levels. Environ Int. 2017;107:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjodin A, et al. Exposure to PBDEs in the office environment: evaluating the relationships between dust, handwipes, and serum. Environ Health Perspect. 2011;119:1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu F, Giovanoulis G, van Waes S, Padilla-Sanchez JA, Papadopoulou E, Magner J, et al. Comprehensive Study of Human External Exposure to Organophosphate Flame Retardants via Air, Dust, and Hand Wipes: The Importance of Sampling and Assessment Strategy. Environ Sci Technol. 2016;50:7752–60. [DOI] [PubMed] [Google Scholar]
- 24.Jinhui L, Yuan C, Wenjing X. Polybrominated diphenyl ethers in articles: a review of its applications and legislation. Environmental Science and Pollution Research. 2017;24:4312–21. [DOI] [PubMed] [Google Scholar]
- 25.Agency for Toxic Substances and Disease Registry(ATSDR). Toxicological profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2004. [Google Scholar]
- 26.Sjödin A, Wong L-Y, Jones RS, Park A, Zhang Y, Hodge C, et al. Serum Concentrations of Polybrominated Diphenyl Ethers (PBDEs) and Polybrominated Biphenyl (PBB) in the United States Population: 2003–2004. Environ Sci Technol. 2008;42:1377–84. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Zhao G, Li J, Zhao H, Wang Y, Chen J, et al. Association of polybrominated diphenylethers (PBDEs) and hydroxylated metabolites (OH-PBDEs) serum levels with thyroid function in thyroid cancer patients. Environ Res. 2017;159:1–8. [DOI] [PubMed] [Google Scholar]
- 28.Lai Y, Lu M, Gao X, Wu H, Cai Z. New evidence for toxicity of polybrominated diphenyl ethers: DNA adduct formation from quinone metabolites. Environ Sci Technol. 2011;45:10720–7. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Lai Y, Li C, Qiu B, Cai Z. Formation and characterization of glutathione adducts derived from polybrominated diphenyl ethers. Chemosphere. 2015;120:365–70. [DOI] [PubMed] [Google Scholar]
- 30.Dunnick JK, Pandiri AR, Merrick BA, Kissling GE, Cunny H, Mutlu E, et al. Carcinogenic activity of pentabrominated diphenyl ether mixture (DE-71) in rats and mice. Toxicol Rep. 2018;5:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibhazehiebo K, Iwasaki T, Okano-Uchida T, Shimokawa N, Ishizaki Y, Koibuchi N. Suppression of thyroid hormone receptor-mediated transcription and disruption of thyroid hormone-induced cerebellar morphogenesis by the polybrominated biphenyl mixture, BP-6. Neurotoxicology. 2011;32:400–9. [DOI] [PubMed] [Google Scholar]
- 32.Byrne JJ, Carbone JP, Hanson EA. Hypothyroidism and abnormalities in the kinetics of thyroid hormone metabolism in rats treated chronically with polychlorinated biphenyl and polybrominated biphenyl. Endocrinol. 1987;121:520–7. [DOI] [PubMed] [Google Scholar]
- 33.(NTP) NTP. Toxicology and NTP Carcinogenesis Studies of a Polybrominated Biphenyl Mixture (Firemaster FF-1) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl Toxicol Program Tech Rep Ser. 1983;244:1–106. [PubMed] [Google Scholar]
- 34.(NTP) NTP. Toxicology and carcinogenesis studies of decabromodiphenyl toxide in F344/N rats and B6C3F1 mice 1986.
- 35.Linares V, Bellés M, Domingo JL. Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol. 2015;89:335–56. [DOI] [PubMed] [Google Scholar]
- 36.Aschebrook-Kilfoy B, DellaValle CT, Purdue M, Kim C, Zhang Y, Sjodin A, et al. Polybrominated diphenyl ethers and thyroid cancer risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial cohort. Am J Epidemiol. 2015;181:883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffman K, Lorenzo A, Butt CM, Hammel SC, Henderson BB, Roman SA, et al. Exposure to flame retardant chemicals and occurrence and severity of papillary thyroid cancer: A case-control study. Environ Int. 2017;107:235–42. [DOI] [PubMed] [Google Scholar]
- 38.Deziel NC, Yi H, Stapleton HM, Huang H, Zhao N, Zhang Y. A case-control study of exposure to organophosphate flame retardants and risk of thyroid cancer in women. BMC Cancer. 2018;18:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Chen Y, Huang H, Sandler J, Dai M, Ma S, et al. Diagnostic radiography exposure increases the risk for thyroid microcarcinoma: a population-based case-control study. Eur J Cancer Prev. 2015;24:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones REE, Anderson S, Zhang Y, Sjödin A. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Comp. 2012;74:97–8. [Google Scholar]
- 41.Sjodin A, McGahee EE 3rd, Focant JF, Jones RS, Lapeza CR, Zhang Y, et al. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers and polybrominated and polychlorinated biphenyls in breast milk. Anal Chem. 2004;76:4508–14. [DOI] [PubMed] [Google Scholar]
- 42.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: Effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. [DOI] [PubMed] [Google Scholar]
- 43.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arora N, Turbendian HK, Kato MA, Moo TA, Zarnegar R, Fahey TJ 3rd. Papillary thyroid carcinoma and microcarcinoma: is there a need to distinguish the two? Thyroid. 2009;19:473–7. [DOI] [PubMed] [Google Scholar]
- 45.Karatzas T, Vasileiadis I, Kapetanakis S, Karakostas E, Chrousos G, Kouraklis G. Risk factors contributing to the difference in prognosis for papillary versus micropapillary thyroid carcinoma. Am J Surg. 2013;206:586–93. [DOI] [PubMed] [Google Scholar]
- 46.Lazarevic N, Barnett AG, Sly PD, Knibbs LD. Statistical Methodology in Studies of Prenatal Exposure to Mixtures of Endocrine-Disrupting Chemicals: A Review of Existing Approaches and New Alternatives. Environ Health Perspect. 2019;127:26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacLehose RF, Dunson DB, Herring AH, Hoppin JA. Bayesian methods for highly correlated exposure data. Epidemiology. 2007;18:199–207. [DOI] [PubMed] [Google Scholar]
- 48.Kaiser HF. The Application of Electronic Computers to Factor Analysis. Educational and psychological measurement. 1960;20:141–51. [Google Scholar]
- 49.Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring Indoor Exposure to Organophosphate Flame Retardants: Hand Wipes and House Dust. Environ Health Perspect. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geyer HJ SK, Darnerud PO, Aune M, Feicht EA, Fried KW, Henkelmann B, Lenoir D, Schmid P, McDonald TA. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Comp. 2004;66. [Google Scholar]
- 51.Thuresson K, Hoglund P, Hagmar L, Sjodin A, Bergman A, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitehead TP, Brown FR, Metayer C, Park JS, Does M, Petreas MX, et al. Polybrominated diphenyl ethers in residential dust: sources of variability. Environ Int. 2013;57–58:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, et al. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;46:13056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stapleton HM, Dodder NG. Photodegradation of decabromodiphenyl ether in house dust by natural sunlight. Environ Toxicol Chem. 2008;27:306–12. [DOI] [PubMed] [Google Scholar]
- 55.Braun JM, Gennings C, Hauser R, Webster TF. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ Health Perspect. 2016;124:A6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pumarega J, Gasull M, Lee DH, Lopez T, Porta M. Number of Persistent Organic Pollutants Detected at High Concentrations in Blood Samples of the United States Population. PLoS One. 2016;11:e0160432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Porta M, Pumarega J, Gasull M. Number of persistent organic pollutants detected at high concentrations in a general population. Environ Int. 2012;44:106–11. [DOI] [PubMed] [Google Scholar]
- 58.Holford TR, Zheng T, Mayne ST, Zahm SH, Tessari JD, Boyle P. Joint effects of nine polychlorinated biphenyl (PCB) congeners on breast cancer risk. Int J Epidemiol. 2000;29:975–82. [DOI] [PubMed] [Google Scholar]
- 59.Leng L, Li J, Luo X-m, Kim J-y, Li Y-m, Guo X-m, et al. Polychlorinated biphenyls and breast cancer: A congener-specific meta-analysis. Environ Int. 2016;88:133–41. [DOI] [PubMed] [Google Scholar]
- 60.Gammon MD, Wolff MS, Neugut AI, Terry MB, Papadopoulos K, Levin B, et al. Temporal variation in chlorinated hydrocarbons in healthy women. Cancer epidemiology, biomarkers & prevention. 1997;6:327–32. [PubMed] [Google Scholar]
- 61.Vo TT, Gladen BC, Cooper GS, Baird DD, Daniels JL, Gammon MD, et al. Dichlorodiphenyldichloroethane and polychlorinated biphenyls: intraindividual changes, correlations, and predictors in healthy women from the southeastern United States. Cancer EpidemiolBiomarkersPrev. 2008;17:2729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDonald TA. Polybrominated diphenylether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Integr Environ Assess Manag. 2005;1:343–54. [PubMed] [Google Scholar]
- 63.Blanck HM, Marcus M, Hertzberg V, Tolbert PE, Rubin C, Henderson AK, et al. Determinants of polybrominated biphenyl serum decay among women in the Michigan PBB cohort. Environ Health Perspect. 2000;108:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.