Abstract
Background
The aim of this systematic review and meta-analysis was to evaluate the benefit of platelet rich plasma (PRP) in oral surgery.
Materials and methods
We performed a systematic search of the literature. The GRADE system was used to assess the certainty of the body of evidence.
Results
We found 21 randomised controlled trials that met our inclusion criteria: 12 studies included patients with periodontal defects, five studies focused on healing of extraction sockets, three studies on sinus lift augmentation, and one study on periapical osseous defects. However, for the quantitative synthesis (meta-analysis), we evaluated “periodontal defects” studies only, since for other clinical contexts the number of studies were too low and the procedural heterogeneity was too high to allow pooling of data. PRP-containing regimens were compared to non-PRP-containing regimens. Primary outcomes for the evaluation of periodontal defects were probing depths, clinical attachment level, gingival recession, and radiographic bone defect. It is not usually clear whether or not the use of PRP compared to controls affects “probing depth” at long-term follow up; the between group differences were small and unlikely to be of clinical importance (i.e., very low quality of evidence). For the other outcomes analysed (“clinical attachment levels”, “gingival recession”, “bony defect”), we observed a very slight marginal clinical benefit of PRP compared to controls. The available evidence for these comparisons was rated as low quality as most of the studies selected showed inconsistency, imprecision, and risk of bias.
Discussion
Evidence from a comparison between the use in oral surgery of PRP-containing regimens compared to other regimens not-containing PRP was of low quality. The results of the meta-analysis, limited to studies in patients with periodontal defects, document that PRP was slightly more effective compared to controls not-containing PRP.
Keywords: platelet-rich plasma, oral surgery, periodontal, implant
Introduction
Platelet-rich plasma (PRP) is defined as an autologous concentration of platelets in a small volume of plasma. It is considered to be a rich source of autologous growth factors1. It is produced from a patient’s whole blood through a 2-phase centrifugation process: the first centrifugation for the separation of blood components and the second for the final PRP production. There are currently more than 40 commercial systems that have been developed to concentrate autologous whole blood into a platelet-rich substance1. Besides platelets, PRP contains some inflammatory cells (i.e., monocytes and polymorphonuclear neutrophils) and large numbers of proteins, including platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), epithelial growth factor (EGF), and adhesion molecules (i.e., fibrin, fibronectin, and vitronectin). Such growth factors and cells have been shown to promote cell recruitment, proliferation and angiogenesis, which may be implicated in tissue regeneration and healing2–4. A number of investigators have studied the Patient Blood Management (PBM) strategies5–23 and thanks to the biological regenerative properties of PRP, it could be possible to hypothesise a role of PRP in the implementation of PBM strategies. On the other hand, the potential clinical benefit of PRP has been studed in a wide range of clinical conditions ranging from dermatological disorders to orthopaedic, oral and maxillofacial surgery24–29. The first report on the topical application of PRP in the field of dentistry dates back to 1998 and the work of Marx et al. who described the use of PRP in combination with autologous bone grafts for reconstruction of mandibular defects30. Since then, a large number of randomised clinical trials (RCTs) have been published on the use of PRP (alone or in combination with bone or bone substitutes) in different dental procedures, including periodontal, dentoalveolar, implant and reconstructive surgery31–51. A number of systematic reviews and meta-analyses52–57 have attempted to perform pooled analyses of the results from these RCTs. However, in spite of this, the possible effect of PRP in tissue healing and bone regeneration in this clinical setting remains unclear. In order to throw some light on this controversial issue, we carried out a systematic literature review and meta-analysis on the efficacy of PRP in oral surgery.
Materials and methods
Search strategy and search terms
A computer-assisted literature search of the MEDLINE (through PUBMED), EMBASE, SCOPUS, OVID and Cochrane Library electronic databases was performed (last access May 30, 2019) to identify studies on the use of autologous PRP in oral surgery. The focus was on the hypothesis that PRP could have a potentially positive effect on bone regeneration. A combination of the following text words was used to maximise search specificity and sensitivity: “platelet concentrate” AND “platelet-rich plasma” AND “platelet gel” AND “PRP” AND “oral surgery” AND “dentistry” AND “dental surgery” AND “periodontal surgery” AND “maxillofacial surgery” AND “dentoalveolar surgery” AND “intrabony defects” AND “bone regeneration” AND “bone healing” AND “tooth extraction” AND “socket preservation” AND “alveolar ridge preservation” AND “sinus floor augmentation” AND “dental implant”. In addition, a manual search was made of the bibliographies of the studies included and of other reviews in order to identify potentially eligible studies not captured by the initial electronic literature search.
Study selection and inclusion/exclusion criteria
Studies were selected independently by two reviewers (MF and MC), and a consensus was reached through discussion and the opinion of a third reviewer (CM). Eligibility assessment was based on the title or abstract and, if required, on the full text. Articles were considered eligible for this systematic review and meta-analysis if the use of PRP in oral surgery was reported either in the title or in the abstract. The other inclusion criteria required that the article should be: (i) original, (ii) concerned with RCTs performed in adult patients, (iii) published in full in English in the last 20 years (1999–2019). Studies enrolling less than 20 patients and with less than 2 months of follow up were excluded. In this systematic review, in order to render the collected data homogeneous, and to allow a comparison to be made between them, studies evaluating other platelet-derived concentrates (i.e., platelet-rich fibrin [PRF]) and the use of PRP in bisphosphonate-related osteonecrosis of the jaw (BRONJ) were not included.
Data extraction
The review was conducted according to the PRISMA statement for the reporting of systematic reviews and meta-analyses. For each RCT included in the systematic review31–51, the following data were extracted by two reviewers (MF and MC) independently: first author, year of publication, study design, sample size, median age and range, sex distribution, treatment modality, test group, control group, the follow-up period, and the main results of the study evaluated. As far as the evaluation of the main results of the study is concerned, clinical and radiological parameters were recorded for each study during the treatment period in order to allow pooling of data for meta-analysis. Disagreement was resolved by consensus and on the opinion of a third reviewer (CM), if necessary. The clinical context of the studies included in this systematic review included: periodontal defects, healing of extraction sockets, sinus lift augmentations, and periapical osseous defects. However, for the quantitative synthesis (meta-analysis), we evaluated “periodontal defects” studies only, since for other clinical contexts the number of studies was too low and the procedural heterogeneity was too high to allow pooling of data.
Outcomes
The unit of analysis was the defect. Measures of outcome for the evaluation of periodontal defects were the following:
- probing depths, a measure of the depth of a sulcus or periodontal pocket determined by measuring the distance from a gingival margin to the base of the sulcus or pocket with a calibrated periodontal probe;
- clinical attachment level, a measure of the position of the base of the pocket in relation to the cemento-enamel junction;
- gingival recession, the distance of the gingival margin from the cemento-enamel junction;
- radiographic bony defect, measured in various ways, implying procedural and numerical heterogeneity.
These measures were expressed in mm; a negative value for the differences of the means of these measures favoured the test treatment (PRP) arm compared to the control arm. All the outcomes we evaluated concerned bony defect healing. Other measures such as plaque index, gingival index, and bleeding on probing, healing of soft tissues after surgical intervention, pain, quality of life, and short-time outcomes were not considered for quantitative analysis.
Assessment of risk of bias in included studies
Two review authors (MF, MC) independently assessed the risk of bias of each study included following the domain-based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions58. They discussed any discrepancies and achieved consensus on the final assessment. The Cochrane “Risk of bias” tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting, and other issues relating to bias. For the selective reporting domain, we added an item for the outcome “adverse events” because reporting was inadequate only for this outcome. We have presented our assessment of risk of bias using two “Risk of bias” summary figures: 1) a summary of bias for each item across all studies; and 2) a cross-tabulation of each trial by all of the “Risk of bias” items.
“Summary of findings” tables
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes, and constructed a “Summary of findings” table using REVMAN 5. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of available data for the main outcomes. The “Summary of findings” tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach, which defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within-trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias.
When evaluating the “Risk of bias” domain, we down-graded the GRADE assessment when: 1) we classified a study as being at high risk of bias for one or more of the following domains: selection, attrition, performance, detection, reporting, and other bias; or 2) when the “Risk of bias” assessment for selection bias was unclear (either for the generation of the randomisation sequence or the allocation concealment domain). We have presented the following outcomes in the “Summary of findings” table: probing depths, clinical attachment, gingival recession, and radiographic bony defect.
Statistical analysis
All studies reporting a continuous outcome had to provide a mean and a standard deviation (SD). If the studies provided median and interquartile range, this was fixed using a pre-established procedure59. If the study provided median and range (minimum and maximum), the method of Hozo et al. was adopted60. When the outcome variables were continuous, the unstandardised (weighted) mean difference (MD) between the test arms and the control arm was calculated by meta-analytical pooling. The studies were weighted with the inverse variance method. The heterogeneity χ2-squared was also calculated, as the I2 for the variation due to heterogeneity. If significant heterogeneity was detected, a random effect (RE) method of study weight calculation was performed (DerSimonian-Laird method)61. The related forest plots were also generated. Since the measures of outcome and their definitions, and the acronyms employed for bony defect were heterogeneous, we also calculated the standardised mean differences (SMD).
Results
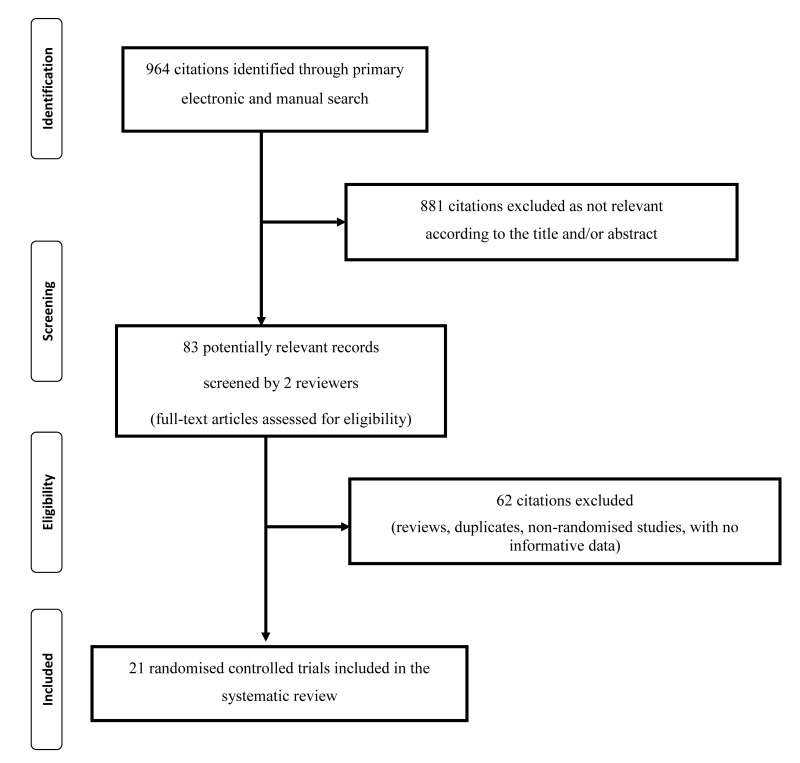
Twenty-one original RCTs were selected to perform a systematic review on the possible therapeutic applications of autologous PRP in dentistry. The main characteristics of the included studies are summarised in Table I. The study flow chart is summarised in Figure 1. Twelve studies31,34–36,38,40–42,45–48 were focused on periodontal defects, 5 studies32,33,37,39,44 on healing of extraction sockets, 3 studies49–51 on sinus lift augmentations, and 1 study on periapical osseous defects43. The clinical context and related surgical approach influenced the nature of the diagnostic work-up and the types of the outcome measures. Some studies used a split-mouth design, where the number of observations concerned the defects, whereas other studies counted the patients (one defect per patient). In the majority of the cases, both the control arm and the test arm were subjected to active modes of treatment (a surgical procedure plus, e.g., bone graft or β-tricalcium phosphate), but this did not hamper the evaluation of the PRP effect as the between-arm difference of the outcome variable after the post-surgical observation period. The majority of the studies concerning the periodontal defects reported probing depths (11 studies) and clinical attachment level (11 studies). Nine studies reported gingival recession, and 6 studies reported the bone defect. Outcomes were reported at a medium-/long-term follow-up period.
Table I.
Characteristics and main results of the included randomised controlled trials on the use of platelet-rich plasma in oral surgery.
| Study (year)ref | Study design | Patients (N) | Males/ females | Mean age, years (range) | Treatment | Test group (N) | Control group (N) | Follow-up | Main results |
|---|---|---|---|---|---|---|---|---|---|
| Agarwal (2014)31 | RCT (split-mouth) | 24 | 10/14 | NR (30–65) | Intrabony periodontal defects | FDBA, PRP (24) | FDBA (24) | 12 months | Statistically significant changes in all clinical and radiological parameters in the PRP-treated group |
| Alissa (2010)32 | RCT (parallel) | 23 | 8/15 | 30.5 (20–52) | Tooth extraction | PRP (12) | None (11) | 3 months | Statistically significant improvement in soft/bone tissue healing and reduction of post-operative pain and complications in PRP-treated group |
| Arenaz-Búa (2012)33 | RCT (split mouth) | 82 | 37/45 | 23 (18–45) | Tooth extraction | PRP (34) | None (34) | 3–6 months | PRP did not accelerate bone formation or improve clinical symptoms |
| Bajaj (2013)34 | RCT (parallel) | 42 | 22/20 | 39.4 (NR) | Treatment of furcation defects | PRP, OFD (14) | OFD (14) | 9 months | All clinical and radiographic parameters showed statistically significant improvement in PRP versus control group |
| Döri (2008)35 | RCT (parallel) | 26 | 12/14 | NR (32–56) | Intrabony periodontal defects | EMD, NBM, PRP (13) | EMD, NBM (13) | 12 months | No adjunct benefit with the use of PRP |
| Döri (2009)36 | RCT (parallel) | 30 | 9/21 | NR (28–65) | Intrabony periodontal defects | ABB, PRP (15) | ABBM (15) | 12 months | No adjunct benefit with the use of PRP |
| Dutta (2015)37 | RCT (parallel) | 60 | 29/31 | 34.5 (18–50) | Tooth extraction | PRP (30) | None (30) | 4 months | PRP improved bone regeneration and soft tissue healing |
| Eskan (2014)38 | RCT (parallel) | 28 | 14/14 | NR (19–75) | Alveolar ridge augmentation | CAN, PRP (14) | CAN (14) | 4 months | PRP enhanced bone regeneration |
| Geurs (2014)39 | RCT (parallel) | 41 | 12/29 | 52 (NR) | Tooth extraction | PRP, FDBA, TCP, collagen plug (12) | Collagen plug (9) | 2 months | Inclusion of PRP accelerated bone graft turnover |
| Harnack (2009)40 | RCT (split-mouth) | 22 | NR | NR | Intrabony periodontal defects | PRP, TCP (22) | TCP (22) | 6 months | PRP did not improve results in the treatment of intrabony defects |
| Keceli (2008)41 | RCT (parallel) | 40 | 10/30 | 38 (16–60) | Root coverage | CTG, PRP (20) | CTC (20) | 12 months | No difference between PRP and control groups |
| Menezes (2012)42 | RCT (split-mouth) | 60 | 30/30 | 37.7 (NR) | Intrabony periodontal defects | FDBA, PRP (60) | FDBA (60) | 48 months | Addition of PRP led to a statistically significant clinical improvement in intraosseous periodontal defects |
| Nakkeeran (2018)43 | RCT (parallel) | 20 | 12/8 | 24 (NR) | Osseous defects of the jaw | PRP, CS, ABG(10) | None (10) | 5 months | PRP use was associated with a more rapid bone formation |
| Ogundipe (2011)44 | RCT (parallel) | 60 | 25/35 | 24.7 (19–35) | Tooth extraction | PRP (30) | None (30) | 4 months | PRP group versus control group: statistically significant reduced pain, not statistically significant improvement in bone density, swelling, trismus |
| Okuda (2005)45 | RCT (parallel) | 70 | 21/49 | 55.5 (NR) | Intrabony periodontal defects | PRP, HA (35) | HA, saline (35) | 12 months | Statistically significant more favorable clinical improvement in PRP group |
| Piemontese (2008)46 | RCT (parallel) | 60 | 31/29 | NR (47–72) | Intrabony periodontal defects | PRP, FDBA (30) | FDBA, saline (30) | 12 months | No adjunctive benefit with the use of PRP |
| Pradeep (2009)47 | RCT (split-mouth) | 20 | 10/10 | 42.8 (NR) | Treatment of furcation defects | PRP (20) | OFD (20) | 6 months | PRP group versus control group: statistically significant difference in all the clinical and radiologic parameters. No complete closure of furcation defects |
| Saini (2011)48 | RCT (parallel) | 20 | 8/12 | 40.3 (22–50) | Intrabony periodontal defects | PRP, TCP (10) | TCP (10) | 9 months | Clinical and radiographic improvement with the use of PRP |
| Schaaf (2008)49 | RCT (split-mouth) | 53 | NR | NR | Maxillary sinus augmentation | PRP, ABG (34) | ABG (34) | 4 months | No positive effect of PRP in bone volume |
| Torres (2009)50 | RCT (split-mouth) | 87 | 40/47 | NR (52–78) | Maxillary sinus augmentation | PRP, ABB (87) | ABB (87) | 24 months | The use of PRP increase bone regeneration |
| Wiltfang (2003)51 | RCT (parallel) | 35 | 8/27 | 46 (32–64) | Maxillary sinus augmentation | PRP, TCP (17) | TCP (18) | 6 months | Statistically significant increased bone formation in the PRP group |
RCT: randomised clinical trials; FDBA: freeze-dried bone allograft; PRP: platelet-rich plasma; OFD: open flap debridement; EMD: enamel matrix protein derivative; NBM: natural bone mineral; ABB: anorganic bovine bone; CAN: cancellous allograft; TCP: tricalcium phosphate; CTG: connective tissue graft; CS: calcium sulfate; ABG: autologous bone graft; HA: hydroxyapatite; NR: not reported.
Figure 1.
Flow chart of the selection of the studies.
Risk of bias in included studies
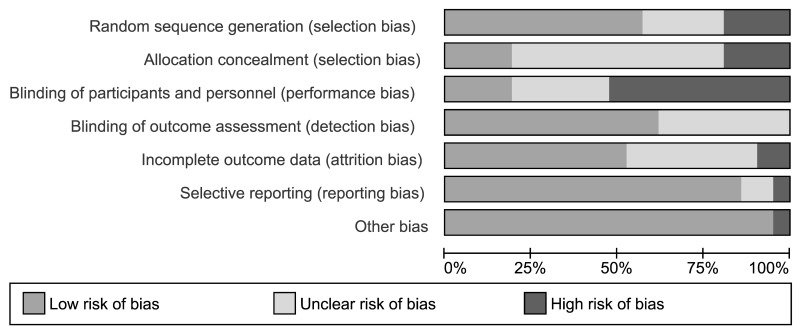
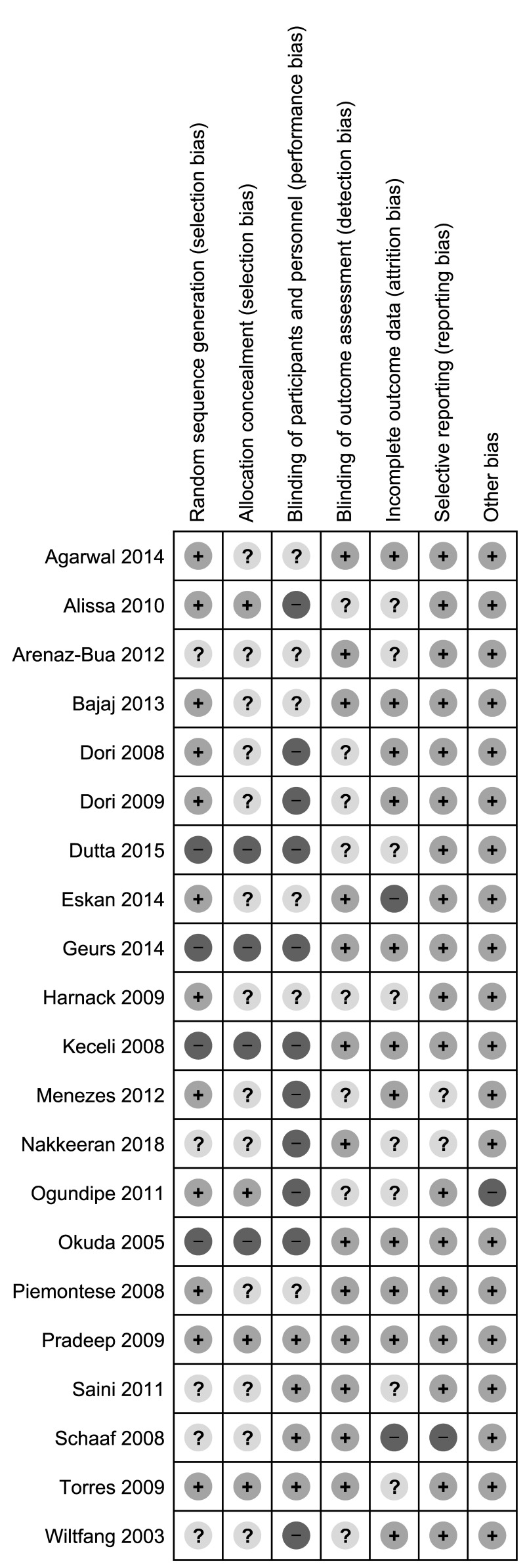
Thirteen (61.9%) studies were at high risk of bias for one or more domains, and 7 studies (33.3%) were at unclear risk of bias for 1 or more domains; one study47 was judged at low risk of bias in all the domains (Figures 2 and 3).
Figure 2.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Sequence generation and allocation concealment
Randomisation depends on two important aspects: adequate generation of the allocation sequence and concealment of the allocation sequence until assignment occurs. We assessed four studies as being at high risk of selection bias. For the random sequence generation, the reports of another 5 studies were at unclear risk of bias, while 11 studies were judged at low risk (Figure 3). For allocation concealment, 13 studies were judged at unclear risk of bias, 4 at high risk of bias, and 4 studies at low risk of bias.
Blinding
Eleven (52.3%) studies were open label, and they were graded as high risk of performance bias (blinding of participants and personnel). Six studies were graded at unclear risk of performance bias because they did not provide the information needed for “high” or “low” risk of bias related to the blinding of participants and personnel to be judged. Four studies were judged at low risk of performance bias since both patients and investigators were masked to group of intervention allocation. Thirteen studies were graded at low risk of detection bias because the assessor was blinded to treatment allocation; the remaining nine studies were graded at unclear risk of detection bias due because they did not provide the information needed for “high” or “low” risk of bias related to the blinding of outcome assessors to be judged.
Incomplete outcome data
Two studies were judged at high risk of attrition bias because there was a high proportion of withdrawals and/or missed data. Other eight studies were judged at unclear risk of bias. The remaining studies were judged at low risk of bias.
Selective reporting
Although the protocols of the studies were not always available on prospective registers of clinical trials, we judged the large majority of the included studies at low risk of reporting bias because the outcomes reporting was complete. Two studies were judged at unclear risk of reporting bias because reported information was not sufficient to allow review authors to extract usable data1.
Other potential sources of bias
We judged one study to be at high risk for other sources of bias because of unbalance at baseline44.
Effects of interventions
A summary of findings for the main comparison is presented in Table II, Figures 4–7 and Online Supplementary Figures S1–S4. As previously reported, a quantitative analysis was applied only to the 12 studies on periodontal defects.
Table II.
Platelet-rich plasma (PRP) in oral surger: summary of findings.
| Patient or population: with periodontal defects; Settings: outpatient; Unit of analysis: periodontal defect; Intervention: regimens containing PRP; Comparison: regimens not containing PRP. | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect: mean difference (95% CI) | N. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PRP | Controls | |||||
|
Probing depth (PD) in mm Follow-up: 6–48 months |
The mean score PD ranged across control groups from 1.52 to 5.85 | The mean score in the intervention groups was 0.39 lower (0.80 lower to 0.02 higher) | −0.39 (−0.80/0.02) | 566 (11 studies) | ⊕⊖⊖⊖1 very low |
On average, it is unclear whether or not use of PRP compared to controls affects the PD at long-term follow-up. Between group differences were small and unlikely to be of clinical importance. |
|
Clinical attachment level (CAL) Follow-up: 3–48 months |
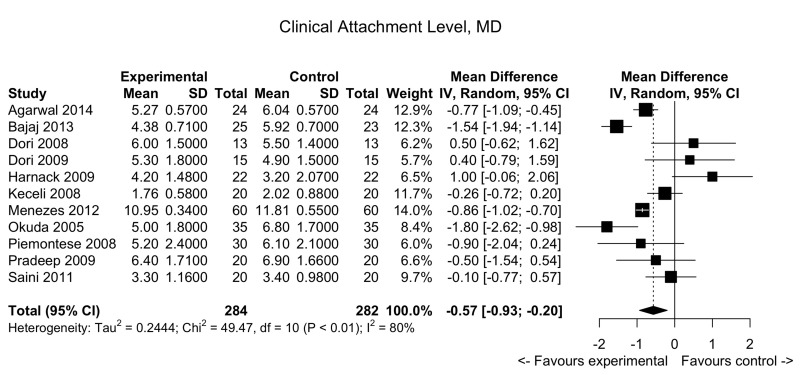
The mean score ranged across control groups from 2.02 to 11.81 | The mean score in the intervention groups was 0.57 lower (0.93 to 0.20 lower) | −0.57 (−0.93/−0.20) | 566 (11 studies) | ⊕⊕⊖⊖2 low |
Very marginal clinical benefit of PRP compared to controls. On average, compared to controls, PRP decreases CAL by 0.57. |
|
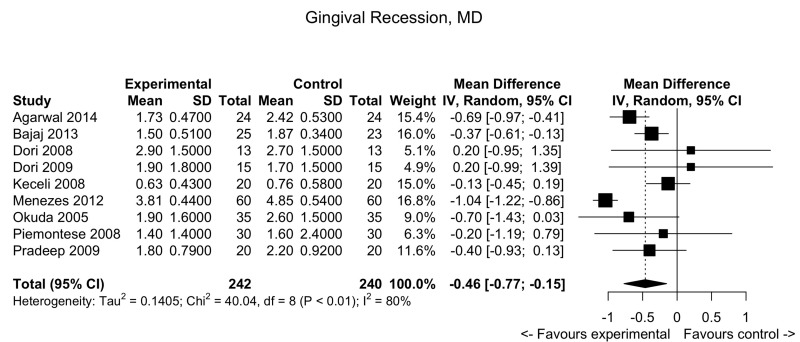
Gingival recession (GR) Follow-up: 6–48 months |
The mean score ranged across control groups from 0.76 to 4.75 | The mean score in the intervention groups was 0.46 lower (0.77 to 0.15 lower) | −0.46 (−0.77/−0.15) | 482 (9 studies) | ⊕⊕⊖⊖2 low |
Very marginal clinical benefit of PRP compared to controls. On average, compared to controls, PRP decreases GR by 0.57. |
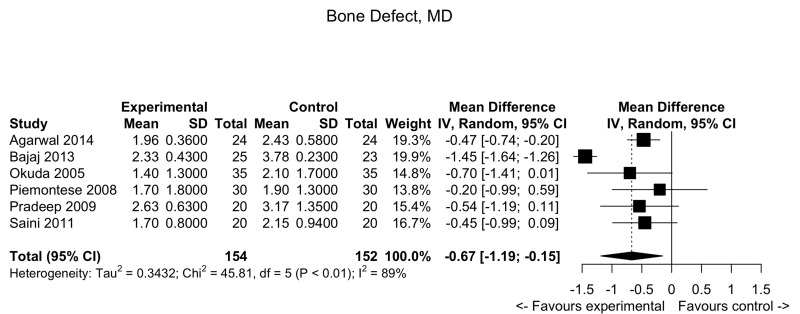
| Bone defect (BD) Follow-up: 9–12 months | The mean BD ranged across control groups from 1.90 to 3.78 | The mean score in PRP group was 0.67 lower (1.19 to 0.15 lower) | −0.67 (−1.19/−0.15) | 306 (6 studies) | ⊕⊕⊖⊖2 low |
Very marginal clinical benefit of PRP compared to controls. On average, compared to controls, PRP decreases BD by 0.67. |
The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval [CI]) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE: Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate.
Down-graded for imprecision (95% CI includes line of no effect), for inconsistency (due to substantial heterogeneity, I2 =80–89%) and because of high risk of bias or unclear risk of bias in some of the included studies.
Down-graded for inconsistency (due to substantial heterogeneity, I2=80–89%) and because of high risk of bias or unclear risk of bias in some of the included studies.
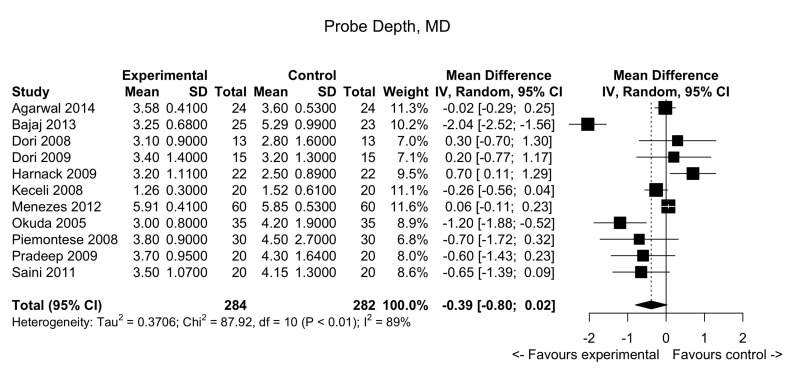
Figure 4.
Periodontal defects: forest plot for probing depths.
MD: mean difference; 95% CI: 95% confidence interval.
Figure 5.
Periodontal defects: forest plot for clinical attachment level.
MD: mean difference; 95% CI: 95% confidence interval.
Figure 6.
Periodontal defects: forest plot for gingival recession.
MD: mean difference; 95% CI: 95% confidence interval.
Figure 7.
Periodontal defects: forest plot for bone defects.
MD: mean difference; 95% CI: 95% confidence interval.
Probing depths
Pooled data from 11 trials showed no clear differences between the test study arm and the control arm: MD: −0.39; 95% confidence interval (CI): −0.80/0.02; p-value=not significant (very low quality evidence, down-graded for serious risk of bias, for inconsistency [due to substantial heterogeneity, I2=88.6%] and for imprecision [95% CIs include line of no effect]). (See summary of findings in Table II and Figure 4).
Clinical attachment level
Pooled data from 11 trials showed a slight decrease in clinical attachment level in the PRP group compared to the control arm: MD: −0.57; 95% CI: −0.93/−0.20; p=0.002 (low quality evidence, down-graded for serious risk of bias and for inconsistency [I2 = 79.8%]) (Figure 5).
Gingival recession
Pooled data from 9 trials showed a slight decrease in gingival recession in the PRP group compared to the control arm: MD: −0.46; 95% CI: −0.77/−0.15; p=0.0035 (low quality evidence, down-graded for serious risk of bias and for inconsistency [I2 = 80.0 %]) (Figure 6).
Bony defect
Pooled data from 6 trials showed a slight decrease in bony defects in the PRP group compared to the control arm: MD: −0.67; 95% CI: −1.19; −0.15; p=0.01 (low quality evidence, down-graded for serious risk of bias and for inconsistency [I2 = 89.1 %]) (Figure 7).
The SMD method was always inferentially consistent with MD (Supplementary Online Figures S1–S4).
Discussion
This systematic review aimed to assess the available scientific evidence for applying PRP in oral surgery. In the qualitative analysis, we included 21 RCTs that evaluated treatment regimens containing PRP (test group) vs treatment regimens not containing PRP (control group). The clinical context of the studies evaluated in this systematic review included: periodontal defects, healing of extraction sockets, sinus lift augmentations, and periapical osseous defects. However, we limited the quantitative synthesis (meta-analysis) to 11 studies evaluating PRP in the treatment of “periodontal defects” since for other clinical contexts the number of studies was too low and the procedural heterogeneity was too high to allow pooling of data.
The available evidence for all the comparisons was rated as low or very low quality due to inconsistency, imprecision, and risk of bias in most of the selected studies. The heterogeneity was high, probably because studies used different criteria for patient recruitment, different length of observation time after surgery, different devices to measure the periodontal defects, and because they included disease of variable severity. One study only was judged at low risk of bias in all the domains considered, while 13 (61.9%) studies were at high risk of bias for one or more domains, and 7 studies (33.3%) were at unclear risk of bias for 1 or more domains. As regards the quantitative analysis on periodontal defects, on average, it is unclear whether or not the use of PRP compared to controls affects “probing depth” at long-term follow up. The between group differences were small, and unlikely to be of clinical importance. For the other outcomes analysed (“clinical attachment levels”, “gingival recession”, “bony defect”), we observed a very marginal clinical benefit of PRP compared to controls (i.e., always <1 mm). This pooled analysis reflects the discordance arising from the evaluation of the single studies. Two RCTs42,48 investigating the efficacy of PRP combined with other graft materials in the treatment of intraosseous periodontal defects reported a significantly more favourable clinical improvement in periodontal sites treated with the combination of PRP and the graft material than in those treated with the graft material alone. In contrast, other studies concluded that the use of PRP failed to improve on the results obtained with the use of the graft material alone35,36,46.
Periodontal tissues have a hard tissue (bone) component and a soft tissue component. There is some evidence to suggest that PRP improves the intrabony periodontal defect, without affecting bone regeneration44. This would imply a net positive effect of PRP on soft tissue. On the contrary, other authors reported a bone gain under PRP37,43. One of these studies used a histomorphometric method not reported by other studies in the periodontal context38. On the whole, these statements are isolated cases and are not suitable for quantitative evaluation, but point to the need for further investigation. Future research in this field should be directed toward the implementation of well-designed, adequately powered RCTs. The results of such trials will help to elucidate the role of PRP in periodontal and other oral surgical settings.
Online supplementary content
Acknowledgements
The Authors thank Professor Marilyn Scopes (Italian Foundation for Research on Anaemia and Haemoglobinopathies, Genoa, Italy) for her precious assistance with language editing and proofreading.
Footnotes
Disclosure of conflicts of interest
GML is the Editor-in-Chief of Blood Transfusion and this manuscript has undergone additional external review as a result. The other Authors declare no conflicts of interest.
References
- 1.Piccin A, Di Pierro AM, Canzian L, et al. Platelet gel: a new therapeutic tool with great potential. Blood Transfus. 2017;15:333–40. doi: 10.2450/2016.0038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Martinez CE, Smith PC, Palma Alvarado VA. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Front Physiol. 2015;6:290. doi: 10.3389/fphys.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 5.Franchini M, Muñoz M. Towards the implementation of patient blood management across Europe. Blood Transfus. 2017;15:292–3. doi: 10.2450/2017.0078-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerra R, Velati C, Liumbruno GM, Grazzini G. Patient blood management in Italy. Blood Transfus. 2016;14:1–2. doi: 10.2450/2015.0171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a patient blood management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016;14:23–65. doi: 10.2450/2015.0172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaglio S, Gentili S, Marano G, et al. The Italian regulatory Guidelines for the implementation of patient blood management. Blood Transfus. 2017;15:325–8. doi: 10.2450/2017.0060-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muñoz M, Franchini M, Liumbruno GM. The post-operative management of anaemia: more efforts are needed. Blood Transfus. 2018;16:324–5. doi: 10.2450/2018.0036-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Der Linde R, Favaloro EJ. Tranexamic acid to prevent post-partum haemorrhage. Blood Transfus. 2018;16:321–3. doi: 10.2450/2018.0067-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchini M, Mengoli C, Cruciani M, et al. Safety and efficacy of tranexamic acid for prevention of obstetric haemorrhage: an updated systematic review and meta-analysis. Blood Transfus. 2018;16:329–37. doi: 10.2450/2018.0026-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liumbruno GM, Vaglio S, Biancofiore G, et al. Transfusion thresholds and beyond. Blood Transfus. 2016;14:123–5. doi: 10.2450/2016.0008-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franchini M, Liumbruno GM. The key role of tranexamic acid in Patient Blood Management programmes. Blood Transfus. 2018;16:471–2. doi: 10.2450/2018.0177-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchini M, Mengoli C, Marietta M, et al. Safety of intravenous tranexamic acid in patients undergoing major orthopaedic surgery: a meta-analysis of randomised controlled trials. Blood Transfus. 2018;16:36–43. doi: 10.2450//2017.0219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hourlier H, Fennema P. Tranexamic acid use and risk of thrombosis in regular users of antithrombotics undergoing primary total knee arthroplasty: a prospective cohort study. Blood Transfus. 2018;16:44–52. doi: 10.2450/2016.0160-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Jimeno N, Muñoz M, Mateo J, et al. Efficacy of topical tranexamic acid within a blood saving program for primary total hip arthroplasty: a pragmatic, open-label randomised study. Blood Transfus. 2018;16:490–7. doi: 10.2450/2018.0133-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz M, Gómez-Ramírez S, Besser M, et al. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus. 2017;15:422–37. doi: 10.2450/2017.0113-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girelli D, Marchi G, Busti F. Iron replacement therapy: entering the new era without misconceptions, but more research is needed. Blood Transfus. 2017;15:379–81. doi: 10.2450/2017.0152-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laso-Morales MJ, Vives R, Gómez-Ramírez S, et al. Intravenous iron administration for postoperative anemia management after colorectal cancer surgery in clinical practice: a single centre, retrospective study. Blood Transfus. 2018;16:338–42. doi: 10.2450/2018.0004-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basora M, Pereira A, Coca M, et al. Cost-effectiveness analysis of ferric carboxymaltose in pre-operative haemoglobin optimisation in patients undergoing primary knee arthroplasty. Blood Transfus. 2018;16:438–42. doi: 10.2450/2018.0031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suh DW, Han SB, Park JH, et al. Intravenous iron supplementation with intra-articular administration of tranexamic acid reduces the rate of allogeneic transfusions after simultaneous bilateral total knee arthroplasty. Blood Transfus. 2017;15:506–11. doi: 10.2450/2016.0051-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Cristofaro R. The use of viscoelastic haemostatic assays in non-cardiac surgical settings: a systematic review and meta-analysis. Blood Transfus. 2018;16:224–26. doi: 10.2450/2018.0040-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franchini M, Mengoli C, Cruciani M, et al. The use of viscoelastic haemostatic assays in non-cardiac surgical settings: a systematic review and meta-analysis. Blood Transfus. 2018;16:235–43. doi: 10.2450/2018.0003-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franchini M, Cruciani M, Mengoli C, et al. Efficacy of platelet-rich plasma as conservative treatment in orthopaedics: a systematic review and meta-analysis. Blood Transfus. 2018;16:502–13. doi: 10.2450/2018.0111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pupella S, Bianchi M, Ceccarelli A, et al. A cost analysis of public cord blood banks belonging to the Italian Cord Blood Network. Blood Transfus. 2018;16:313–20. doi: 10.2450/2017.0251-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacchi N. Is it time to re-think a sustainable banking model for the Italian Cord Blood Network? Blood Transfus. 2018;16:221–3. doi: 10.2450/2017.0040-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valle V, Screnci M, Murgi E, et al. Collection of umbilical cord blood for banking: collection rate and factors influencing collection. Blood Transfus. 2017;15:587–8. doi: 10.2450/2016.0262-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Corso M, Vervelle A, Simonpieri A, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr Pharm Biotechnol. 2012;13:1207–30. doi: 10.2174/138920112800624391. [DOI] [PubMed] [Google Scholar]
- 29.Simonpieri A, Del Corso M, Vervelle A, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol. 2012;13:1231–56. doi: 10.2174/138920112800624472. [DOI] [PubMed] [Google Scholar]
- 30.Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal A, Gupta ND. Platelet-rich plasma combined with decalcified freeze-dried bone allograft for the treatment of noncontained human intrabony periodontal defects: a randomized controlled split-mouth study. Int J Periodontics Restorative Dent. 2014;34:705–11. doi: 10.11607/prd.1766. [DOI] [PubMed] [Google Scholar]
- 32.Alissa R, Esposito M, Horner K, Oliver R. The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur J Oral Implantol. 2010;3:121–34. [PubMed] [Google Scholar]
- 33.Arenaz-Búa J, Luaces-Rey R, Sironvalle-Soliva S, et al. A comparative study of platelet-rich plasma, hydroxyapatite, demineralized bone matrix and autologous bone to promote bone regeneration after mandibular impacted third molar extraction. Med Oral Patol Oral Cir Bucal. 2010;15:e483–9. doi: 10.4317/medoral.15.e483. [DOI] [PubMed] [Google Scholar]
- 34.Bajaj P, Pradeep AR, Agarwal E, et al. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: a randomized controlled clinical trial. J Periodontal Res. 2013;48:573–81. doi: 10.1111/jre.12040. [DOI] [PubMed] [Google Scholar]
- 35.Döri F, Nikolidakis D, Húszár T, et al. Effect of platelet-rich plasma on the healing of intrabony defects treated with an enamel matrix protein derivative and a natural bone mineral. J Clin Periodontol. 2008;35:44–50. doi: 10.1111/j.1600-051X.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- 36.Döri F, Kovács V, Arweiler NB, et al. Effect of platelet-rich plasma on the healing of intrabony defects treated with an anorganic bovine bone mineral: a pilot study. J Periodontol. 2009;80:1599–605. doi: 10.1902/jop.2009.090058. [DOI] [PubMed] [Google Scholar]
- 37.Dutta SR, Singh P, Passi D, Patter P. Mandibular third molar extraction wound healing with and without platelet rich plasma: a comparative prospective study. J Maxillofac Oral Surg. 2015;14:808–15. doi: 10.1007/s12663-014-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eskan MA, Greenwell H, Hill M, et al. Platelet-rich plasma-assisted guided bone regeneration for ridge augmentation: a randomized, controlled clinical trial. J Periodontol. 2014;85:661–8. doi: 10.1902/jop.2013.130260. [DOI] [PubMed] [Google Scholar]
- 39.Geurs N, Ntounis A, Vassilopoulos P, et al. Using growth factors in human extraction sockets: a histologic and histomorphometric evaluation of short-term healing. Int J Oral Maxillofac Implants. 2014;29:485–96. doi: 10.11607/jomi.3408. [DOI] [PubMed] [Google Scholar]
- 40.Harnack L, Boedeker RH, Kurtulus I, et al. Use of platelet-rich plasma in periodontal surgery--a prospective randomised double blind clinical trial. Clin Oral Investig. 2009;13:179–87. doi: 10.1007/s00784-008-0223-7. [DOI] [PubMed] [Google Scholar]
- 41.Keceli HG, Sengun D, Berberoğlu A, Karabulut E. Use of platelet gel with connective tissue grafts for root coverage: a randomized-controlled trial. J Clin Periodontol. 2008;35:255–62. doi: 10.1111/j.1600-051X.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 42.Menezes LM, Rao J. Long-term clinical evaluation of platelet-rich plasma in the treatment of human periodontal intraosseous defects: a comparative clinical trial. Quintessence Int. 2012;43:571–82. [PubMed] [Google Scholar]
- 43.Nakkeeran KP, Saravanan K, Babu P, John RR. Evaluation of bone regeneration in periapical osseous defects with and without platelet rich plasma, combined calcium sulfate and autologous bone graft - A comparative study. J Stomatol Oral Maxillofac Surg. 2019;120:196–202. doi: 10.1016/j.jormas.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Ogundipe OK, Ugboko VI, Owotade FJ. Can autologous platelet-rich plasma gel enhance healing after surgical extraction of mandibular third molars? J Oral Maxillofac Surg. 2011;69:2305–10. doi: 10.1016/j.joms.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Okuda K, Tai H, Tanabe K, et al. Platelet-rich plasma combined with a porous hydroxyapatite graft for the treatment of intrabony periodontal defects in humans: a comparative controlled clinical study. J Periodontol. 2005;76:890–8. doi: 10.1902/jop.2005.76.6.890. [DOI] [PubMed] [Google Scholar]
- 46.Piemontese M, Aspriello SD, Rubini C, et al. Treatment of periodontal intrabony defects with demineralized freeze-dried bone allograft in combination with platelet-rich plasma: a comparative clinical trial. J Periodontol. 2008;79:802–10. doi: 10.1902/jop.2008.070436. [DOI] [PubMed] [Google Scholar]
- 47.Pradeep AR, Pai S, Garg G, et al. A randomized clinical trial of autologous platelet-rich plasma in the treatment of mandibular degree II furcation defects. J Clin Periodontol. 2009;36:581–8. doi: 10.1111/j.1600-051X.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 48.Saini N, Sikri P, Gupta H. Evaluation of the relative efficacy of autologous platelet-rich plasma in combination with β-tricalcium phosphate alloplast versus an alloplast alone in the treatment of human periodontal infrabony defects: a clinical and radiological study. Indian J Dent Res. 2011;22:107–15. doi: 10.4103/0970-9290.80008. [DOI] [PubMed] [Google Scholar]
- 49.Schaaf H, Streckbein P, Lendeckel S, et al. Topical use of platelet-rich plasma to influence bone volume in maxillary augmentation: a prospective randomized trial. Vox Sang. 2008;94:64–9. doi: 10.1111/j.1423-0410.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 50.Torres J, Tamimi F, Martinez PP, et al. Effect of platelet-rich plasma on sinus lifting: a randomized-controlled clinical trial. J Clin Periodontol. 2009;36:677–87. doi: 10.1111/j.1600-051X.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 51.Wiltfang J, Schlegel KA, Schultze-Mosgau S, et al. Sinus floor augmentation with beta-tricalciumphosphate: does platelet-rich plasma promote its osseous integration an degradation? Clin Oral Impl Res. 2003;14:213–8. doi: 10.1034/j.1600-0501.2003.140212.x. [DOI] [PubMed] [Google Scholar]
- 52.Stähli A, Strauss FJ, Gruber R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: a systematic review. Clin Oral Implants Res. 2018;29(Suppl 18):6–19. doi: 10.1111/clr.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esposito M, Felice P, Worthington HV. Interventions for replacing missing teeth: augmentation procedures of the maxillary sinus. Cochrane Database Syst Rev. 2014;5:CD008397. doi: 10.1002/14651858.CD008397.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Del Fabbro M, Bucchi C, Lolato A, et al. Healing of Postextraction Sockets Preserved With Autologous Platelet Concentrates. A Systematic Review and Meta-Analysis. J Oral Maxillofac Surg. 2017;75:1601–15. doi: 10.1016/j.joms.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Bae JH, Kim YK, Myung SK. Effects of platelet-rich plasma on sinus bone graft: meta-analysis. J Periodontol. 2011;82:660–7. doi: 10.1902/jop.2010.100529. [DOI] [PubMed] [Google Scholar]
- 56.Panda S, Doraiswamy J, Malaiappan S, et al. Additive effect of autologous platelet concentrates in treatment of intrabony defects: a systematic review and meta-analysis. J Investig Clin Dent. 2016;7:13–26. doi: 10.1111/jicd.12117. [DOI] [PubMed] [Google Scholar]
- 57.Zhou S, Sun C, Huang S, et al. Efficacy of adjunctive bioactive materials in the treatment of periodontal intrabony defects: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:8670832. doi: 10.1155/2018/8670832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 510 [up-dated March 2011] The Cochrane Collaboration; 2011. Available at: http://www.cochranehandbook.org. [Google Scholar]
- 59.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.